Abstract
Background
Transcranial magnetic motor evoked potentials (TMMEP) are associated with severity of clinical signs and magnetic resonance imaging (MRI) findings in dogs with spinal cord disease.
Hypothesis
That in initially paraplegic dogs with thoracolumbar intervertebral disc herniation (IVDH), MRI findings before surgery and TMMEPs obtained after decompressive surgery are associated with long‐term neurological status and correlate with each other.
Animals
Seventeen client‐owned paraplegic dogs with acute thoracolumbar IVDH.
Methods
Prospective observational study. TMMEPs were obtained from pelvic limbs and MRI (3T) of the spinal cord was performed at initial clinical presentation. Follow‐up studies were performed ≤ 2 days after reappearance of motor function and 3 months later. Ratios of compression length, intramedullary hyperintensities' length (T2‐weighted hyperintensity length ratio [T2WLR]), and lesion extension (T2‐weighted‐lesion extension ratio) in relation to the length of the 2nd lumbar vertebral body were calculated.
Results
TMMEPs could be elicited in 10/17 (59%) dogs at 1st and in 16/17 (94%) dogs at 2nd follow‐up. Comparison of TMMEPs of 1st and 2nd follow‐up showed significantly increased amplitudes (median from 0.19 to 0.45 mV) and decreased latencies (from 69.38 to 40.26 ms; P = .01 and .001, respectively). At 2nd follow‐up latencies were significantly associated with ambulatory status (P = .024). T2WLR obtained before surgery correlated with latencies at 2nd follow‐up (P = .04).
Conclusions
TMMEP reflect motor function recovery after severe spinal cord injury.
Keywords: canine, magnetic resonance imaging, spinal cord injury, therapy monitoring, transcranial magnetic stimulation
Abbreviations
- CLR
compression length ratio
- DPP
deep pain perception
- IVDH
intervertebral disc herniation
- MRI
magnetic resonance imaging
- mFFE
multi‐echo fast field echo
- SCI
spinal cord injury
- SD
standard deviation
- T
tesla
- T2W
T2‐weighted
- T2W‐LER
T2‐weighted‐lesion extension ratio
- T2WLR
T2‐weighted hyperintensity length ratio
- TE
echo time
- TMMEP
transcranial magnetic motor evoked potentials
- TMS
transcranial magnetic stimulation
- TR
repetition time
1. INTRODUCTION
Spinal cord injury (SCI) in dogs is frequently caused by thoracolumbar intervertebral disc herniation (IVDH) resulting in a broad range of clinical signs ranging from paraspinal hyperesthesia to paraplegia and loss of deep pain perception (DPP) with concomitant impairment of micturition and defecation.1, 2, 3, 4, 5, 6, 7, 8 Transcranial magnetic stimulation (TMS) generates transcranial magnetic motor evoked potentials (TMMEPs) that enable a noninvasive and fast evaluation of the functional integrity of descending motor pathways in the brain and spinal cord.9 TMS is well established in human medicine, providing information about corticospinal tract damage and lesion location in cervical SCI.10, 11, 12, 13 Moreover, it can be of prognostic value in stroke and SCI patients.14, 15 In veterinary medicine application of TMS has been described as an ancillary tool for evaluation of clinical signs in horses and dogs with spinal cord diseases.16, 17, 18 Associations of TMMEP data with severity of clinical signs and magnetic resonance imaging (MRI) findings has been described in cervical spondylomyelopathy in Great Danes and Dobermann Pinschers.18, 19
Various methods have been used to estimate prognosis after severe SCI in dogs. In particular, presence or absence of DPP is the most reliable prognostic indicator for recovery after severe SCI in dogs and is a frequently used reference for evaluating new prognostic approaches.1, 20, 21, 22, 23, 24 Potential cerebrospinal fluid biomarkers and imaging characteristics have been evaluated with respect to severity of SCI assessed by neurological examination and correlation with functional outcome.4, 25, 26, 27, 28, 29, 30 MRI allows accurate identification of characteristics of extruded disc material and parenchymal spinal cord damage.31, 32 In several previous studies, MRI measurements on T2‐weighted images have been associated with severity of clinical signs and long‐term ambulatory outcome.25, 33, 34
A comparison of TMMEP data with MRI findings and long‐term functional outcome has not previously been reported for dogs with thoracolumbar IVDH.
In this prospective study, initially paraplegic dogs with thoracolumbar IVDH diagnosed by MRI received decompressive surgery and were re‐evaluated after surgery by neurological examination, TMS and MRI during a 3 to 4 months lasting follow‐up period. It was hypothesized that TMMEPs obtained after surgery (1) are associated with long‐term neurological status and (2) correlate with MRI findings.
2. MATERIAL AND METHODS
2.1. Study design
For this prospective study, 17 client‐owned paraplegic dogs admitted to the Department of Small Animal Medicine and Surgery, University of Veterinary Medicine Hannover, Germany were recruited between November 2013 and May 2015. Before study enrollment, written owner consent was obtained. The study was conducted in accordance with the guidelines of the Animal Care Committee of the Government of Lower Saxony and national regulations for animal welfare (animal experiment number 33.14–42502‐04–13/1277). Dogs had to meet the inclusion criteria of: < 20 kg bodyweight, acute paraplegia (onset ≤ 7 days) with present or absent DPP, and SCI due to IVDH between T3‐L3 confirmed by MRI and surgery. Postoperative treatment consisted of analgetics/anti‐inflammatory drugs (methadone 0.2 mg/kg IV or fentanyl 2.7 mg/kg spot‐on during hospitalization and pregabalin 4 mg/kg PO, metamizole 25–50 mg/kg PO), gastroprotective drugs (omeprazole 1 mg/kg PO), alpha blockers if indicated (phenoxybenzamine 0.5 mg/kg PO), and parasympathomimetics (bethanechol 0.5 mg/kg PO) for control of urination and physical treatment at a physiotherapist on a defined weekly routine.
All dogs had a general physical and a neurological examination, complete blood cell count, routine serum biochemistry, and radiographs of the vertebral column. According to their neurological deficits, dogs were assigned to a scale published before with Grade I (spinal hyperesthesia without neurological deficits); Grade II (ambulatory paraparesis and ataxia); Grade III (non‐ambulatory paraparesis); Grade IV (paraplegia with DPP); and Grade V (paraplegia with loss of DPP).35
At initial clinical presentation, TMS was performed under deep sedation, as described in previously published studies.16, 36, 37, 38, 39 Afterward, MRI examinations were performed to definitely localize and characterize IVDH and the lesion within the spinal cord and subsequently all dogs underwent decompressive surgery during the same anesthesia. First follow‐up including a neurological examination and TMS was performed when motor function reappearance was observed at daily examination during hospitalization or after discharge either at serial weekly examinations by the investigators or by the instructed owners and confirmed at the hospital within 2 days (range 4–35 days after surgery). Second follow‐up was performed 3 months after the 1st follow‐up comprising repeated neurological examination, TMS and MRI (80–128 days after surgery; Figure 1).
Figure 1.

Design of the prospective study. After naturally occurring thoracolumbar intervertebral disc herniation, dogs were presented at the clinic within 7 days. At initial presentation, a neurological examination (NE), transcranial magnetic stimulation (TMS) to obtain transcranial magnetic motor evoked potentials from the pelvic limbs and magnetic resonance imaging (MRI) of the spinal cord were performed before hemilaminectomy (surgery). First follow‐up included a NE and TMS within 2 days after observable motor function reappearance (range 4–35 days after surgery). Second follow‐up was performed 3 months after the 1st follow‐up (range 80–128 days after surgery) comprising repeated NE, TMS, and MRI
2.2. TMMEPs
Dogs were sedated with acepromazine (Vetranquil, CEVA Tiergesundheit GmbH, Düsseldorf, Germany; 0.02‐0.05 mg/kg) and levomethadone/fenpipramide (L‐Polamivet, Intervet Deutschland GmbH, Unterschleißheim, Germany; 0.2‐0.4 mg/kg) IV and TMS was performed in lateral recumbency or sternal positioning. TMMEPs were recorded as described in previous studies with minor modifications.19, 37, 39 A transcranial magnetic stimulator (Magstim 200², Magstim, Carmarthenshire, UK), capable of producing a maximum 4.0 Tesla (T) magnetic field (correlates with a 100% intensity) with a 50 mm ring coil was used. The coil was held tangentially to the skull in close contact to the skin with the center of the coil lateral to the vertex to stimulate the motor cortex. The current flow within the coil ran in clockwise direction and 4 consecutive stimulations were delivered for generation of TMMEPs. These resulting 4 potentials were recorded from pelvic limbs after contralateral magnetic stimulation by use of an electromyograph (Nicolet NicVue 2.9.1, Natus Medical Incorporated, Planegg, Germany). The recording muscle electrode was positioned in the middle of the muscle belly of the cranial tibial muscle. The reference electrode was positioned SC 1 cm distal to the muscle electrode, whereas the ground electrode was placed SC on the dorsal midline of the caudal lumbar region.
To display, measure, and save TMMEP waveforms commercially available computer software (VikingSelect‐Software Version 11.0, Viasys healthcare, CareFusion, Höchberg, Germany) was used. Graphic analysis was performed on the first 200 ms of recording time after stimulus offset. Onset latency and peak‐to‐peak amplitude were measured as described before by manually directed cursors on the oscilloscope.40 Onset latencies were measured in milliseconds (ms) and defined as interval between the stimulus to the start of the muscle response. Peak‐to‐peak amplitudes were measured in microvolts (mV) and calculated from the peak of the negative wave to the nadir of the 1st positive wave. Neuronal path length was measured starting at the vertex via the estimated course of nerve fibers to the muscle needle positioned within the cranial tibial muscle, contralateral to the stimulation site.19
2.3. Magnetic resonance imaging
All dogs were examined under general anesthesia using a 3.0 T magnet (Philips Achieva, Philips Medical Systems, PC Best, The Netherlands) with a phased array SENSE (sensitivity encoding)‐spine‐coil with 15 channels. The thoracolumbar spinal cord was scanned with the following sequences: T2‐weighted sequence was a turbo‐spin‐echo sequence with sagittal (repetition time [TR] = 3,100 ms, echo time [TE] = 120 ms, slice thickness 1.8 mm, interslice gap 0.2 mm) and transverse (TR = 8,418 ms, TE = 12 ms, slice thickness 1.8 mm, interslice gap 0.2 mm) planes. Images were complemented by transverse planes of T1‐weighted (TR = 491 ms, TE = 8 ms, slice thickness 1.8 mm, interslice gap 0.2 mm) and multi‐echo fast field echo (mFFE; TR = 21 ms, TE = 7 ms, slice thickness 1.8 mm, interslice gap 0.2 mm) sequences.
MRI data sets of all dogs were evaluated as DICOM formatted images by use of an image viewer and processing software (easyImage, easyVET, IFS GmbH, Hannover, Germany). T2‐weighted sequences were assessed by board certified neurologists (A. Tipold, VM. Stein, or both) in order to determine localization of SCI for subsequent surgical procedures. Identification of T2‐weighted intramedullary hyperintensities was performed upon assessment of sagittal images.25, 26, 33 Corresponding transverse T2‐weighted images were evaluated for detection of hyperintensities' length expansion to increase accuracy of measurements.41 T1‐weighted sequences were assessed in transverse planes to exclude presence of T1‐weighted hyperintensities.33 Quantification of spinal cord compression by extruded intervertebral disc material was achieved by measurements on sagittal T2‐weighted sequences, transverse T2‐, T1‐weighted, and mFFE sequences were used to confirm longitudinal extent of spinal cord compression. Analogously to previously published data, the maximal spinal cord compression, expressed as a ratio (SCCR) of the spinal cord diameter 1 vertebral articulation cranial to any compression was assessed.33 The sagittal height of vertebral canal at site of highest compression (most ventral aspect represented by dorsal border of extruded disc material) was used to calculate a ratio (VCCR) by division of sagittal vertebral canal height (ventral aspect represented by dorsal border of healthy disc) 1 vertebral articulation cranial to any compression.33 Total lesion extension evaluated on sagittal T2‐weighted sequences (T2W‐LE) was defined as overall length of spinal cord compression together with intramedullary hyperintense signal. This measurement was performed separately to account for possible overlapping of hyperintensities and regions of spinal cord compression. The length of L2 was used to calculate standardized T2‐weighted hyperintensity length ratio (T2WLR), spinal cord compression length ratio (CLR), and T2 weighted‐lesion extension ratio (T2W‐LER), as described before (Figure 2).25, 33, 34, 42 The same observers performed all measurements on initial and follow‐up MRI examinations and had access to a list of patient IDs, being blinded to neurological status throughout the study at the time of performing MRI measurements.
Figure 2.

Sagittal T2‐weighted MR image of an 11‐year‐old Shih Tzu with intervertebral disc herniation and marked spinal cord compression at the level of Th11/12. Solid lines indicate intramedullary hyperintensities (T2WL), whereas the dotted line indicates spinal cord compression length (CL). Summation of solid and dotted lines represents T2W‐LE; division of these lengths by the lengths of L2 (not depicted here) create the dimensionless ratios T2‐weighted hyperintensity length ratio, compression length ratio, and T2‐weighted‐lesion extension ratio. The latter of the 3 ratios was calculated separately to account for possible overlapping of hyperintensities and regions of spinal cord compression
2.4. Statistical methods
To compensate for the dogs' differing body sizes and therefore neural conduction pathway lengths onset latencies of each limb were normalized with the measured neuronal path‐length. Resulting stimulus conduction velocities (m/s) were used as a surrogate for normalized latencies in all calculations. TMMEP variables (normalized latencies and amplitudes) recorded in each dog from the right and left pelvic limbs were averaged to give a mean value for each dog and variable. Comparison of TMMEP data series was limited to dogs with recordable TMMEPs both at 1st and 2nd follow‐up (n = 9). Latencies and amplitudes were not normally distributed (determined by use of the Kolmogorov‐Smirnov test, histogram examination of outliers) and comparison between 1st and 2nd follow‐up TMMEP data was performed by use of non‐parametric tests (Wilcoxon signed‐rank test). Association between latencies and amplitudes with severity of neurological signs at 1st and 2nd follow‐up were examined by use of Wilcoxon rank‐sum test. For comparison of MRI data from initial presentation and 2nd follow‐up, non‐parametric methods (Wilcoxon signed‐rank test) were used, because all data sets were not normally distributed. Associations between initial MRI data with grades of neurological impairment at initial presentation and 2nd follow‐up examinations was calculated by Wilcoxon rank‐sum test. Correlation of CLR, T2WLR, and T2W‐LER obtained at initial presentation with TMMEPs at 1st follow‐up and 2nd follow‐up was calculated with a Pearson correlation test. Analogously, correlation was calculated of MRI findings with TMMEP data, both assessed at 2nd follow‐up. P values of < .05 were considered significant. The statistical analyses were performed with commercially available software programs (SAS Enterprise Guide 7.1, SAS Institute Inc, Cary, North Carolina; SPSS 24.0.0.0 for Windows, IBM SPSS Statistics, Chicago, Illinois).
3. RESULTS
3.1. Clinical data and neurological status
Seventeen paraplegic dogs with an SCI because of IVDH between T3‐L3 spinal cord segments were enrolled in this study. Onset of clinical signs was acute (duration of non‐ambulatory status median: 1 day; range, < 24 hours‐7 days). Dogs had a median age of 5.1 years (range, 2.6–10.7 years) and had a median bodyweight of 8.3 kg (range, 3.9–19.6 kg). The study population consisted of 5 sexually intact males, 7 neutered males, 4 sexually intact, and 5 spayed females. The study comprised Dachshunds (n = 4), mixed breed dogs (n = 4), 2 dogs of the following chondrodystrophic breeds: French Bulldog, Jack Russell Terrier, and Lhasa Apso and 1 dog of the following breeds: Shih Tzu, Bolognese, Bolonka Zwetna, and Havanese.
Thirteen of the 17 dogs were paraplegic with DPP and therefore classified as Grade IV, whereas 4/17 were paraplegic with absent DPP and classified as Grade V. All dogs showed reappearance of motor function and recovery of DPP after decompressive surgery. Dogs with and without DPP before surgery recovered voluntary motor function (confirmed by neurological examination) within 12.6 days (mean; range: 4–27 days) and 25.5 days (mean; range: 20–35 days), respectively. Fourteen of 17 dogs (82.35%) remained non‐ambulatory and 3/17 dogs (16.65%) regained ambulation at the 1st follow‐up. At the 2nd follow‐up (median 100; range, 80–128 days after surgery), 15/17 dogs (88.24%) became ambulatory, whereas 2/17 dogs (11.76%) with absent DPP before surgery remained non‐ambulatory, achieving Grade III (Table 1).
Table 1.
Course of motor function recovery and TMMEP data during follow‐up in 17 paraplegic dogs
| Initial presentation | 1st follow‐up examination a | 2nd follow‐up examination b | ||||
|---|---|---|---|---|---|---|
| Neurological grade According to Sharp and Wheeler (2005) c | Grade | Latencies [Link] , [Link] , [Link] (ms) {stimulus conduction velocity}(m/s) | Amplitudes [Link] , [Link] (mV) | Grade | Latencies [Link] , [Link] , [Link] (ms) {stimulus conduction velocity}(m/s) | Amplitudes [Link] , [Link] (mV) |
| (Number of dogs n = 17) g | (n = 17) | (TMMEPs in n = 10) | (n = 17) | (TMMEPs in n = 16) | ||
| V (4) | III (4) | 93.59 (35.98–151.20)(2) | 0.19 (0.10‐0.29)(2) | III (2) | 108.78 (99.55–118.4) (2) | 0.15 (0.10‐0.20) (2) |
| {13.17 (4.23‐22.10)} | {4.42 (2.58‐6.27)} | |||||
| II (2) | 36.08 (31.11–41.05) (2) | 0.36 (0.26‐0.45) (2) | ||||
| {20.58 (15.59‐25.57)} | ||||||
| IV (13) | III (10) | 84.87 (34.80–118.40)(5) | 0.25 (0.215‐0.74)(5) | II (6) | 40.26 (33.73–99.85) (6) | 0.64 (0.15‐1.43) (6) |
| {8.89 (5.66‐17.39)} | {15.97 (8.40‐22.40)} | |||||
| I (4) | 29.50 (28.28–61.36) (4) | 0.71 (0.22‐2.48) (4) | ||||
| {21.14 (13.41‐22.68)} | ||||||
| II (3) | 53.87 (48.95‐89.98)(4) | 0.19 (0.175‐0.20)(3) | II (1) | 39.73 (1) | 0.84 (1) | |
| {12.49 (6.59‐22.04)} | {28.20} | |||||
| I (2) | 44.98 (1) | 0.20 (1) | ||||
| {22.24} | ||||||
Abbreviation: TMMEP, transcranial magnetic motor evoked potentials.
aMedian 13 days; range, 4–35 days after surgery.
bMedian 101; range, 80–128 days after surgery.
Grade I = spinal hyperesthesia without neurological deficitsGrade II = ambulatory paraparesis and ataxiaGrade III = non‐ambulatory paraparesisGrade IV = paraplegia with deep pain perceptionGrade V = paraplegia with loss of deep pain perception
dMedian of onset latencies and peak‐to‐peak amplitudes.
eValues in round brackets represent range of onset latencies and peak‐to‐peak amplitudes.
fNumbers in curly brackets represent median and range of onset latencies normalized with neuronal path length.
gNumbers in square brackets represent number of dogs.
3.2. TMMEPs and comparison of TMMEP and neurological status
TMMEPs could not be generated in paraplegic dogs with Grade IV and V signs at initial presentation (n = 17). At 1st follow‐up, 1–2 days after reappearance of motor function TMMEPs were recorded in 10/17 (58,82%) dogs. In 4/10 paraparetic dogs (n = 1 with Grade II and n = 3 with Grade III) TMMEP generation was limited to 1 pelvic limb. The 7/17 dogs without measurable TMMEP were all still non‐ambulatory (Grade III). At the 2nd follow‐up examination, TMMEPs could be obtained in 16/17 (94.12%) dogs. In 15/17 dogs, TMMEPs were elicited from both pelvic limbs. In 1 non‐ambulatory dog (Grade III), TMMEPs could only be measured in 1 pelvic limb. TMMEPs could not be elicited in 1 dog that had ambulatory paraparesis (Grade II; Tables 1 and 2).
Table 2.
Results of TMMEP, MRI findings and the severity of neurological signs in 17 paraplegic dogs
| Course of study | Grade of neurological signs a (n = 17) b | Dogs with TMMEPs (n = 17) | Onset latency [Link] , [Link] , [Link] (ms) | Peak‐to‐peak amplitude [Link] , [Link] (mV) | T2WLR [Link] , [Link] (n = 17) | T2W‐LER [Link] , [Link] (n = 17) | CLR [Link] , [Link] (n = 17) | SCCR (n = 17) | VCCR (n = 17) |
|---|---|---|---|---|---|---|---|---|---|
| Initial presentation | V (4) IV (13) | 0/17 | – | – | 1.23 (0.0–4.15) | 2.48 (0.99‐9.13) | 1.68 (0.73‐4.06) | 0.77 (0.44‐0.94) | 0.72 (0.48‐0.92) |
| 1st follow‐up | III (14) II (3) | 10/17 f | 69.38 (34.8–151.2) | 0.19 (0.1‐0.74) | – | – | – | – | – |
| {10.69 (4.23‐22.10)} | |||||||||
| 2nd follow‐up | III (2) II (9) I (6) | 16/17g | 40.26 (28.28–118.0) | 0.45 (0.1–2.48) | 0.99 (0.0–3.21) | 1.76 (0.0–5.77) | 0.18 (0.0–1.29) | ||
| {18.20 (2.57‐28.20)} |
Abbreviations: TMMEP, transcranial magnetic motor evoked potentials; MRI, magnetic resonance imaging; T2W‐LER, T2‐weighted‐lesion extension ratio; T2WLR, T2‐weighted hyperintensity length ratio; CLR, compression length ratio; SCCR, spinal cord compression ratio; VCCR, vertebral canal compression ratio.
aAccording to Sharp and Wheeler (2005).35
bNumber in square brackets represents number of dogs.
cMedian of TMMEP and MRI data values.
dValues in round brackets represents range of TMMEP and MRI data values.
eNumbers in curly brackets represent median and range of onset latencies normalized with neuronal path length.
fIn 6/10 dogs recordable TMMEPs were limited to 1 pelvic limb.
gIn 16/17 dogs recordable TMMEPs were limited to 1 pelvic limb.
TMMEP values of 9 dogs could be measured both at 1st (latencies range: 34.80–151.20 ms; amplitudes range: 0.1‐0.74 mV) and 2nd (latencies range: 29.43–90.18 ms; amplitudes range: 0.1–2,43 mV) follow‐up and revealed a significant decrease of onset latency and a significant increase of peak‐to‐peak amplitudes during the course of therapy monitoring (Figure 3).
Figure 3.
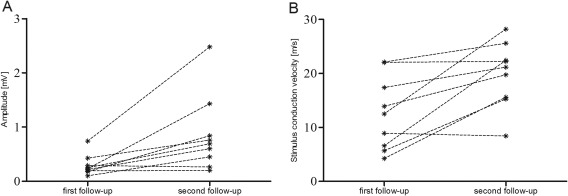
Comparison of peak‐to‐peak amplitudes and stimulus conduction velocity (normalized latencies) of transcranial magnetic motor evoked potentials (TMMEPs) recorded at 1st and 2nd follow‐up in 9 dogs. Comparison of these values revealed a significant increase of peak‐to‐peak amplitudes (3A; P = .01) and an increase of normalized latencies (3B; P = .001). Each plot represents 1 dog, dotted lines connect data of the same dog at 1st and 2nd follow‐up. At 1st follow‐up, TMMEPs could be obtained from the cranial tibial muscle in 16 pelvic limbs of 9 dogs; at 2nd follow‐up TMMEPs were recorded in 18 pelvic limbs of 9 dogs
Latencies were not significantly different between ambulatory (Grade II) and non‐ambulatory (Grade III) dogs at 1st follow‐up (Table 3). TMMEP latencies at 2nd follow‐up were significantly longer in non‐ambulatory (Grade III, range: 99.55–118.4) compared to ambulatory dogs (Grades I + II, range: 28.28–99.85; Tables 1 and 3).
Table 3.
Association between TMMEP results, MRI findings and the severity of neurological signs in 17 paraplegic dogs
| TMMEP and MRI findings obtained at: | Association with grade of neurological signs, assessed at: | Latencies a | Amplitudes | P values for: T2WLR | T2W‐LER | CLR |
|---|---|---|---|---|---|---|
| Initial presentation | Grade IV versus Grade V; at initial presentation | – | – | .017* | 0.33 | 0.43 |
| Ambulatory versus non‐ambulatory; at 1st follow‐up | – | – | .59 | 0.68 | 0.86 | |
| Ambulatory versus non‐ambulatory; at 2nd follow‐up | – | – | .02* | 0.058 | 0.500 | |
| 1st follow‐up | Ambulatory versus non‐ambulatory; at 1st follow‐up | 0.32 | 0.5 | – | – | – |
| Grade I versus Grade II; at 2nd follow‐up | 0.17 | 0.26 | – | – | – | |
| 2nd follow‐up | Ambulatory versus non‐ambulatory; at 2nd follow‐up | 0.024* | 0.05 | .078 | 0.052 | 0.24 |
Abbreviations: TMMEP, transcranial magnetic motor evoked potentials; MRI, magnetic resonance imaging; T2W‐LER, T2‐weighted‐lesion extension ratio; T2WLR, T2‐weighted hyperintensity length ratio; CLR, compression length ratio.
aCalculations are based on onset latencies normalized with neuronal path length (m/s).
*Indicates statistical significance (P <.05).
TMMEP amplitudes did not differ significantly between groups with various degrees of motor function impairment during follow‐up.
Onset latencies and amplitudes recorded at 1st follow‐up were not associated with neurological state at 2nd follow‐up, though median of onset latencies obtained at 1st follow‐up was higher in dogs achieving Grade II than in dogs recovering to Grade I at 2nd follow‐up (89.98 ms and 40.95 ms, respectively).
3.3. MRI data and comparison of MRI data and neurological status
Imaging data could be obtained in all dogs at initial presentation and at the 2nd follow‐up. At initial presentation intramedullary hyperintensities were detected on T2‐weighted images in 4/4 dogs with Grade V and 8/13 dogs with Grade IV. At the 2nd follow‐up, MRI 15/17 (88.23%) dogs showed T2‐weighted hyperintensities in the spinal cord. The 2 dogs without intramedullary hyperintensities in the follow‐up examination were initially presented with Grade IV.
Based on T2‐weighted images evaluated spinal cord compression ratio (SCCR) and vertebral canal compression ratio (VCCR) significantly increased, as expected in comparison between initial presentation (median: 0.772 range: 0.439‐0.939 and median: 0.722 range: 0.463‐0.921, respectively) and after decompressive surgery at 2nd follow‐up (median: 0.872 range: 0.651‐1.076; P = .005 and median: 0.907 range: 0.631‐1.022; P < .001, respectively). T2WLR was significantly associated with severity of neurological signs at initial presentation, as dogs with Grade V (median, 2.48; range, 1.78‐4.15) had significantly higher T2WLR (Table 3) than dogs with Grade IV (median 0.69; range, 0.00–3.16; Table 2). At initial presentation, dogs had a median CLR of 1.68 (range, 0.73‐4.06) and median T2W‐LER of 2.52 (range, 0.99‐9.13) with no significant difference between dogs with Grade IV and V.
T2WLR obtained at initial presentation was significantly associated with severity of neurological signs at the 2nd follow‐up, as dogs with persistent non‐ambulatory paraparesis (Grade III) had a significantly higher T2WLR at initial presentation (range, 2.99‐4.15) than dogs achieving ambulatory paraparesis (Grade II + I, median 0.89, range, 0–3.16; P = .02). Other MRI findings (T2W‐LER, CLR) were not significantly associated with postoperative ambulatory status at 1st and 2nd follow‐up.
No significant differences were found between T2WLR obtained at initial presentation compared to results at the 2nd follow‐up (P = .39). However, the CLR and T2W‐LER were significantly lower whereas the SCCR and VCCR were significantly higher at 2nd follow‐up examination compared to data obtained at initial presentation (P < .001; P = .002 and P = .005; P < .001 respectively).
3.4. Correlation of MRI data and TMMEP
There was a significant correlation of T2WLR at initial presentation and latencies obtained at 2nd follow‐up was detected (P = .045; r = −.507).
No further correlations were found for MRI data with TMMEP variables recorded in paraparetic dogs at 1st and 2nd follow‐up.
4. DISCUSSION
This study outlines that recording of TMMEPs is a feasible method to assess the course of motor function recovery after surgical intervention in dogs with thoracolumbar IVDH, reflecting a functional result of individual cellular recovery process.
In paraplegic dogs with thoracolumbar IVDH, therapeutic surgical intervention alleviates spinal cord compression and creates preconditions for motor function improvement.8, 23, 24, 43, 44 Therefore, decompressive surgery is a commonly recommended therapeutic regimen in paraplegic dogs with thoracolumbar IVDH, resulting in a motor function outcome which is up to now best predicted by presence or absence of DPP before surgery.4, 20, 24, 43 Findings of this study are in accordance with the aforementioned reports, as 15/17 dogs recovered ambulatory status during the approximately 4 month follow‐up, and dogs with initial Grade IV showed better motor function outcome than dogs with Grade V before surgery.
In the present study, TMMEPs could not be elicited in paraplegic limbs which is in accordance with a previously published study.16 During the course of the study all dogs recovered at least non‐ambulatory pelvic limb motor function at 1st follow‐up. Concurrent with motor function TMMEPs also reappeared in 59% (9/17) of the dogs. In a study in dogs with acute onset of thoracolumbar IVDH previously published only 3 of 9 dogs with voluntary movements of the pelvic limbs had recordable TMMEP (30%) after surgery.16 However, of these 9 dogs only 4 had been paraplegic before surgery of which none had reappearing TMMEPs limiting comparability of this and the present study. Furthermore, the period from surgery to 1st follow‐up TMS was much shorter compared to the present study (median: 2 days; range: 2–16 days and median: 13 days, range, 4–35 days, in the present study).16 Thus, healing mechanisms had more time to restore spinal cord motor function in the dogs enrolled in the study reported here. This assumption might be corroborated by a study of artificially induced SCI in rats; it reported about disappearance of motor evoked potentials after application of 60 g pressure for 10 seconds and reappearance of waves with lower amplitudes approximately 30 minutes later.45 TMMEP latency and amplitude are affected by changes in axonal myelination that result in altered conductivity and axonal integrity. Axonal demyelination is a prominent feature of SCI, and spontaneous remyelination of axons is considered to contribute to neurological recovery.46, 47, 48 Remyelination arises from resident glial progenitor cells, infiltrating the injured region, where they differentiate into oligodendrocytes producing myelin.49, 50 Additionally, PNS associated Schwann cells migrate to the spinal cord lesion, contributing to remyelination by expressing platelet derived growth factor receptor α.49, 51 These processes start immediately after injury. However, it takes weeks to achieve functional recovery.2 Therefore, the timing of TMS for an assessment of spinal cord recovery seems to be crucial. Hence, hospitalization and daily assessment of neurological status by specialists after surgery until reappearance of motor function would lead to more consistent state of recovery among dogs examined. This limitation of the present study seemed to be inevitable due to owner consent. Slightly varying state of motor function recovery based on the individual cellular recovery process at the day of 1st follow‐up examination could be a source of noise in this study. However, as TMMEPs might reflect cellular‐level changes in function not evident in the neurological exam, they might be considered as more sensitive early markers for recovery.
In this study, no TMMEPs could be evoked in 6/17 (35%) dogs from the pelvic limbs of dogs with motor function apparent at 1st follow‐up. This finding is in accordance with studies in human cervical spondylotic myelopathy, where TMMEPs could not be elicited in all individuals although motor function was present.11, 52 Additionally, similar findings were reported in Doberman Pinschers, Great Danes, and horses with cervical spinal cord lesions.17, 18, 19 In a previously published study, TMMEPs could be recorded only in ambulatory dogs after IVDH, which differs from findings in this study, as 7/14 non‐ambulatory dogs had detectable TMMEPs at least in 1 pelvic limb at 1st follow‐up.16 The reason that TMMEPs cannot be generated from limbs, although motor function is present, remains subject to assumption.17, 18, 19, 37 It is suspected that the propagating impulses could be insufficient to depolarize the postsynaptic membrane of the motor neuron and thus, propagation of impulses stops and no muscle contraction is detectable.17
Comparison of TMMEP data during therapy monitoring revealed a significant increase of peak‐to‐peak amplitude and a significant decrease of onset latencies at 2nd follow‐up, compared to the 1st follow‐up. Simultaneously, between 1st and 2nd follow‐up, 14 dogs recovered motor function of at least 1 Grade. In addition, all dogs improved motor function of at least 2 grades from initial presentation to 2nd follow‐up, which was approximately 3.5‐4.5 months after SCI. These findings point out that TMMEPs are capable to reflect motor function improvement in individuals that recover from paraplegia and emphasize the value of TMS as a supportive tool for recovery monitoring in such cases. At 2nd follow‐up, TMMEP latencies and amplitudes even in dogs with extensive recovery of motor function (that reached Grade I) did not achieve median values comparable to data of healthy dogs of similar size and bodyweight which were recently reported.39 However, follow‐up period in the present study (median 100; range, 80–128 days) could be still too short to detect the potential recovery of motor function and further approximation of TMMEP latencies and amplitudes to normal values.
An association between onset latencies and amplitudes obtained at 1st follow‐up and severity of neurological signs at the 2nd follow‐up did not reach level of significance. The low number of dogs that could be included for calculation could be a reason for non‐significant differences and over‐estimation of differences between these groups at the same time, limiting validity of these calculations.
At 2nd follow‐up, onset latencies were significantly longer in non‐ambulatory compared to ambulatory dogs. However, the number of dogs with Grade III in comparison to Grade II and I at 2nd follow‐up was very low, thus the high impact of single values could bias this analysis, as these findings are in conflict with recently published data.53 The normalization of onset latencies in the present study, eliminating the effect of differing body size between patients could explain the differing results of these 2 studies as well.
TMMEP amplitudes did not differ significantly between ambulatory and non‐ambulatory dogs during follow‐up. A possible explanation might be a high intra‐ and inter‐individual variability of TMMEP amplitudes.17
T2WLR was significantly associated with initial severity of neurological signs and with functional outcome, which is consistent with previously reported findings in a more heterogeneous dog population and another more recently published study in dogs with inclusion criteria similar to this study.33, 34 However, a significant difference of T2WLR between dogs with initial Grade IV and V as in our study was not reported in previously published works.33, 34 These findings might be related to slightly differing principles of T2W‐hyperintensity measurement between those and the present study, as the region of compression was spared according to this article's definition of T2WLR, which could differ to previously used definitions.33, 34 In addition, the aforementioned studies were performed with a 1 T MRI, thus, the current findings with a 3 T MRI could reflect higher resolution of spinal cord lesions, resulting in increased accuracy of hyperintensity detection. Hence, slight differences between extent of hyperintensities in dogs with Grade IV and V could become more pronounced with 3 T. These findings reflect association between severity of spinal cord functional impairment and extent of T2‐weighted hyperintensities, which emerge as a consequence of pathological processes such as myelomalacia, inflammation, edema, intramedullary hemorrhage, and necrosis.54, 55, 56 Restrictively, a low number of dogs initially presenting as Grade V were included in the study reported here, which could bias these findings. In the present study, a higher prevalence of T2‐weighted hyperintensities in dogs with absent DPP, compared to dogs with present DPP was detected, which is consistent with published findings.25, 34
As reported in 2 other studies before, in the present study no association was detected between extent (CLR) and degree (SCCR, VCCR) of compression and severity of neurological signs at initial presentation.25, 34 However, in one work such correlation was detected.33 These contradictory findings might be attributed to varying degree of neurological impairment at initial presentation, as in the present study only paraplegic dogs were enrolled and the aforementioned study included dogs with modified Frankel score ranging from 0 to 5.33 Assessment of lesion extension ratio did not reveal significant correlations and associations with severity of neurological signs.
Whereas CLR and T2W‐LER were significantly reduced and SCCR and VCCR significantly increased comparing initial and seco t9PoPp45‐Undond follow‐up examinations as expected due to successful decompressive surgery, T2WLR did not change significantly. As residual level decreased spinal cord and vertebral canal diameter at the level of former compression was very slight, leading to insignificant compression of the spinal cord a possible effect on functional outcome seems to be negligible. Compared to pre‐surgery MRI examinations at 2nd follow‐up an increased number of dogs presented spinal cord hyperintensities, this might be individually ascribed in some dogs to an early stage of chronic processes with edema and demyelination and later to formation of fluid filled cavities, as it has been described in earlier studies.57 As no histopathologic examination of these dogs has been performed these theories cannot be proven. This finding casts some doubt on the usefulness of MRI T2W‐hyperintensities as a prognostic tool as timing of MR imaging is critical; dogs might be imaged before relevant acute changes develop or potentially irrelevant chronic changes might be mistaken for relevant acute changes and differentiation cannot be achieved on the basis of pure T2W‐sequences.
MRI findings reflect severity of injury at initial state and TMMEPs correspondingly could not be elicited, thus, calculation of a correlation at that point in time was not possible. T2WLR was significantly associated with onset latencies assessed at 2nd follow‐up, which is consistent with the association of both variables with the severity of clinical signs at 2nd follow‐up. However, no further significant correlation was found of MRI findings obtained at initial presentation and 2nd follow‐up with TMMEP variables recorded in paraparetic dogs at 1st and 2nd follow‐up. In human and veterinary medicine, an association of TMMEPs and degree of chronic spinal cord compression assessed at the same point in time has been detected in chronic cervical spinal cord lesions.11, 19, 37 As in the present study, only dogs presenting acute severe motor function impairment due to a different pathogenesis were enrolled, the differences of CLR, T2W‐LER, and T2WLR values between Grade IV and V could be too low to detect a significant correlation with TMMEPs during therapy monitoring. A correlation of MRI measurements and TMMEPs both obtained at 2nd follow‐up examination was not expected, because absence of compression and persistence of focal spinal cord hyperintensities do not reflect microstructural processes, which contribute to functional motor recovery.46, 49, 51, 54, 57, 58
5. CONCLUSIONS
The present study revealed reappearance of TMMEPs in dogs with motor function recovery after severe SCI. During the course of motor function improvement, a significant increase of peak‐to‐peak amplitudes and decrease of onset latencies were detected. Thus, TMS is a valid method for therapy monitoring reflecting functional motor recovery. Additionally, onset latencies obtained during convalescence can reflect severity of motor function impairment and TMMEPs might provide information about further improvement of motor function to be expected. T2WLR obtained before surgery correlates with onset latencies 3 to 4 months after SCI.
CONFLICT OF INTEREST DECLARATION
Andrea Tipold serves as Associate Editor for the Journal of Veterinary Internal Medicine. She was not involved in review of this manuscript.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study was conducted in accordance with the guidelines of the Animal Care Committee of the Government of Lower Saxony and national regulations for animal welfare (animal experiment number 33.14–42502‐04–13/1277).
ACKNOWLEDGMENT
The study was supported by a grant of the Deutsche Forschungsgemeinschaft (DFG, FOR 1103, TI 309/4‐2).
Siedenburg JS, Wang‐Leandro A, Amendt H‐L, Rohn K, Tipold A, Stein VM. Transcranial magnetic motor evoked potentials and magnetic resonance imaging findings in paraplegic dogs with recovery of motor function. J Vet Intern Med. 2018;32:1116–1125. https://doi.org/10.1111/jvim.15058
Funding information Deutsche Forschungsgemeinschaft, Grant/Award Number: TI 309/4‐2
This work was performed at University of Veterinary Medicine Hannover, Foundation, Germany.
Preliminary results were presented as a poster at the 28th ECVN Annual Symposium, Amsterdam. 2015, and were published as part of a doctoral thesis entitled “Transcranial magnetic stimulation in dogs with functional motor recovery after thoracolumbar intervertebral disc herniation,” 2016, University of Veterinary Medicine Hannover, Germany.
REFERENCES
- 1. Duval J, Dewey C, Roberts R, Aron D. Spinal cord swelling as a myelographic indicator of prognosis: A retrospective study in dogs with intervertebral disc disease and loss of deep pain perception. Vet Surg. 1996;25:6–12. [DOI] [PubMed] [Google Scholar]
- 2. Jeffery ND, Blakemore WF. Spinal cord injury in small animals. 1. Mechanisms of spontaneous recovery. Vet Rec. 1999;144:407–413. [DOI] [PubMed] [Google Scholar]
- 3. Ferreira AJA, Correia JHD, Jaggy A. Thoracolumbar disc disease in 71 paraplegic dogs: influence of rate of onset and duration of clinical signs on treatment results. J Small Anim Pract. 2002;43:158–163. [DOI] [PubMed] [Google Scholar]
- 4. Olby N, Levine J, Harris T, et al. Long‐term functional outcome of dogs with severe injuries of the thoracolumbar spinal cord: 87 cases (1996–2001). J Am Vet Med Assoc. 2003;222:762–769. [DOI] [PubMed] [Google Scholar]
- 5. Mayhew PD, McLear RC, Ziemer LS, et al. Risk factors for recurrence of clinical signs associated with thoracolumbar intervertebral disk herniation in dogs: 229 cases (1994–2000). J Am Vet Med Assoc. 2004;225:1231–1236. [DOI] [PubMed] [Google Scholar]
- 6. Cerda‐Gonzalez S, Olby NJ. Fecal incontinence associated with epidural spinal hematoma and intervertebral disk extrusion in a dog. J Am Vet Med Assoc. 2006;228:230–235. [DOI] [PubMed] [Google Scholar]
- 7. Fluehmann G, Doherr MG, Jaggy A. Canine neurological diseases in a referral hospital population between 1989 and 2000 in Switzerland. J Small Anim Pract. 2006;47:582–587. [DOI] [PubMed] [Google Scholar]
- 8. Aikawa T, Fujita H, Kanazono S, et al. Long‐term neurologic outcome of hemilaminectomy and disk fenestration for treatment of dogs with thoracolumbar intervertebral disk herniation: 831 cases (2000–2007). J Am Vet Med Assoc. 2012;241:1617–1626. [DOI] [PubMed] [Google Scholar]
- 9. Barker AT, Jalinous R, Freeston IL. Non‐invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. [DOI] [PubMed] [Google Scholar]
- 10. Maertens de Noordhout A, Remacle JM, Pepin JL, et al. Magnetic stimulation of the motor cortex in cervical spondylosis. Neurology. 1991;41:75–80. [DOI] [PubMed] [Google Scholar]
- 11. Lo YL, Chan LL, Lim W, et al. Systematic correlation of transcranial magnetic stimulation and magnetic resonance imaging in cervical spondylotic myelopathy. Spine. 2004;29:1137–1145. [DOI] [PubMed] [Google Scholar]
- 12. Shields CB, Ping Zhang Y, Shields LB, et al. Objective assessment of cervical spinal cord injury levels by transcranial magnetic motor‐evoked potentials. Surg Neurol. 2006;66:475–483; discussion 483. [DOI] [PubMed] [Google Scholar]
- 13. Kalupahana NS, Weerasinghe VS, Dangahadeniya U, Senanayake N. Abnormal parameters of magnetically evoked motor‐evoked potentials in patients with cervical spondylotic myelopathy. Spine J. 2008;8:645–649. [DOI] [PubMed] [Google Scholar]
- 14. Clarke CE, Modarres‐Sadeghi H, Twomey JA, Burt AA. Prognostic value of cortical magnetic stimulation in spinal cord injury. Paraplegia. 1994;32:554–560. [DOI] [PubMed] [Google Scholar]
- 15. Pennisi G, Rapisarda G, Bella R, et al. Absence of response to early transcranial magnetic stimulation in ischemic stroke patients: prognostic value for hand motor recovery. Stroke. 1999;30:2666–2670. [DOI] [PubMed] [Google Scholar]
- 16. Sylvestre AM, Cockshutt JR, Parent JM, et al. Magnetic motor evoked potentials for assessing spinal cord integrity in dogs with intervertebral disc disease. Vet Surg. 1993;22:5–10. [DOI] [PubMed] [Google Scholar]
- 17. Nollet H, Deprez P, Van Ham L, et al. The use of magnetic motor evoked potentials in horses with cervical spinal cord disease. Equine Vet J. 2010;34:156–163. [DOI] [PubMed] [Google Scholar]
- 18. De Decker S, Van Soens I, Duchateau L, et al. Transcranial magnetic stimulation in Doberman Pinschers with clinically relevant and clinically irrelevant spinal cord compression on magnetic resonance imaging. J Am Vet Med Assoc. 2011;238:81–88. [DOI] [PubMed] [Google Scholar]
- 19. Martin‐Vaquero P, Da Costa RC. Transcranial magnetic motor evoked potentials in Great Danes with and without clinical signs of cervical spondylomyelopathy: association with neurological findings and magnetic resonance imaging. Vet J. 2014;201:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scott HW, McKee WM. Laminectomy for 34 dogs with thoracolumbar intervertebral disc disease and loss of deep pain perception. J Small Anim Pract. 1999;40:417–422. [DOI] [PubMed] [Google Scholar]
- 21. Coates JR. Intervertebral disk disease. Vet Clin North Am Small Anim Pract. 2000;30:77–110. [DOI] [PubMed] [Google Scholar]
- 22. Davis GJ, Brown DC. Prognostic indicators for time to ambulation after surgical decompression in nonambulatory dogs with acute thoracolumbar disk extrusions: 112 cases. Vet Surg. 2002;31:513–518. [DOI] [PubMed] [Google Scholar]
- 23. Jeffery ND, Levine JM, Olby NJ, Stein VM. Intervertebral disk degeneration in dogs: consequences, diagnosis, treatment, and future directions. J Vet Intern Med. 2013;27:1318–1333. [DOI] [PubMed] [Google Scholar]
- 24. Jeffery ND, Barker AK, Hu HZ, et al. Factors associated with recovery from paraplegia in dogs with loss of pain perception in the pelvic limbs following intervertebral disk herniation. J Am Vet Med Assoc. 2016;248:386–394. [DOI] [PubMed] [Google Scholar]
- 25. Ito D, Matsunaga S, Jeffery ND, et al. Prognostic value of magnetic resonance imaging in dogs with paraplegia caused by thoracolumbar intervertebral disk extrusion: 77 cases (2000–2003). J Am Vet Med Assoc. 2005;227:1454–1460. [DOI] [PubMed] [Google Scholar]
- 26. Penning V, Platt SR, Dennis R, et al. Association of spinal cord compression seen on magnetic resonance imaging with clinical outcome in 67 dogs with thoracolumbar intervertebral disc extrusion. J Small Anim Pract. 2006;47:644–650. [DOI] [PubMed] [Google Scholar]
- 27. Royal AB, Chigerwe M, Coates JR, et al. Cytologic and histopathologic evaluation of extruded canine degenerate disks. Vet Surg. 2009;38:798–802. [DOI] [PubMed] [Google Scholar]
- 28. Srugo I, Aroch I, Christopher MM, et al. Association of cerebrospinal fluid analysis findings with clinical signs and outcome in acute nonambulatory thoracolumbar disc disease in dogs. J Vet Intern Med. 2011;25:846–855. [DOI] [PubMed] [Google Scholar]
- 29. Roerig A, Carlson R, Tipold A, Stein VM. Cerebrospinal fluid tau protein as a biomarker for severity of spinal cord injury in dogs with intervertebral disc herniation. Vet J. 2013;197:253–258. [DOI] [PubMed] [Google Scholar]
- 30. Levine GJ, Cook JR, Kerwin SC, et al. Relationships between cerebrospinal fluid characteristics, injury severity, and functional outcome in dogs with and without intervertebral disk herniation. Vet Clin Pathol. 2014;43:437–446. [DOI] [PubMed] [Google Scholar]
- 31. Bos AS, Brisson BA, Nykamp SG, et al. Accuracy, intermethod agreement, and inter‐reviewer agreement for use of magnetic resonance imaging and myelography in small‐breed dogs with naturally occurring first‐time intervertebral disk extrusion. J Am Vet Med Assoc. 2012;240:969–977. [DOI] [PubMed] [Google Scholar]
- 32. Cooper JJ, Young BD, Griffin JF, et al. Comparison between noncontrast computed tomography and magnetic resonance imaging for detection and characterization of thoracolumbar myelopathy caused by intervertebral disk herniation in dogs. Vet Radiol Ultrasound. 2014;55:182–189. [DOI] [PubMed] [Google Scholar]
- 33. Levine JM, Fosgate GT, Chen AV, et al. Magnetic resonance imaging in dogs with neurologic impairment due to acute thoracic and lumbar intervertebral disk herniation. J Vet Intern Med. 2009;23:1220–1226. [DOI] [PubMed] [Google Scholar]
- 34. Boekhoff TM, Flieshardt C, Ensinger EM, et al. Quantitative magnetic resonance imaging characteristics: evaluation of prognostic value in the dog as a translational model for spinal cord injury. J Spinal Disord & Tech. 2012;25:E81–E87. [DOI] [PubMed] [Google Scholar]
- 35. Sharp NJH, Wheeler SJ. Diagnosis and differential diagnosis In: Small Animal Spinal Disorders Diagnosis and Surgery. Edinburgh, London, UK: Elsevier Mosby; 2005:35–39. [Google Scholar]
- 36. Van Ham LML, Nijs J, Mattheeuws DRG, Vanderstraeten GGW. Sufentanil and nitrous oxide anaesthesia for the recording of transcranial magnetic motor evoked potentials in dogs. Vet Rec. 1996;138:642–645. [DOI] [PubMed] [Google Scholar]
- 37. Da Costa RC, Poma R, Parent JM, et al. Correlation of motor evoked potentials with magnetic resonance imaging and neurologic findings in Doberman Pinschers with and without signs of cervical spondylomyelopathy. Am J Vet Res. 2006;67:1613–1620. [DOI] [PubMed] [Google Scholar]
- 38. Granger N, Blamires H, Franklin RJM, Jeffery ND. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double‐blinded trial in a canine translational model. Brain. 2012;135:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Amendt HL, Siedenburg JS, Steffensen N, et al. Transcranial magnetic stimulation with acepromazine or dexmedetomidine in combination with levomethadone/fenpipramide in healthy Beagle dogs. Vet J. 2016;217:40–42. [DOI] [PubMed] [Google Scholar]
- 40. Van Ham LML, Vanderstraeten GGW, Mattheeuws DRG, Nijs J. Transcranial magnetic motor evoked‐potentials in sedated dogs. Prog Vet Neurol. 1994;5:147–154. [Google Scholar]
- 41. Griffin JF, Davis MC, Ji JX, et al. Quantitative magnetic resonance imaging in a naturally occurring canine model of spinal cord injury. Spinal Cord. 2015;53:278–284. [DOI] [PubMed] [Google Scholar]
- 42. Wang‐Leandro A, Siedenburg JS, Hobert MK, et al. Comparison of preoperative quantitative magnetic resonance imaging and clinical assessment of deep pain perception as prognostic tools for early recovery of motor function in paraplegic dogs with intervertebral disk herniations. J Vet Intern Med. 2017;31:842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scott HW. Hemilaminectomy for the treatment of thoracolumbar disc disease in the dog: a follow‐up study of 40 cases. J Small Anim Pract. 1997;38:488–494. [DOI] [PubMed] [Google Scholar]
- 44. Ruddle TL, Allen DA, Schertel ER, et al. Outcome and prognostic factors in non‐ambulatory Hansen Type I intervertebral disc extrusions: 308 cases. Vet Comp Orthop Traumatol. 2006;19:29–34. [PubMed] [Google Scholar]
- 45. Patil AA, Nagaraj MP, Mehta R. Cortically evoked motor action potential in spinal cord injury research. Neurosurgery. 1985;16:473–476. [PubMed] [Google Scholar]
- 46. Jeffery ND, Blakemore WF. Locomotor deficits induced by experimental spinal cord demyelination are abolished by spontaneous remyelination. Brain. 1997;120:27–37. [DOI] [PubMed] [Google Scholar]
- 47. Ouyang H, Sun W, Fu Y, et al. Compression induces acute demyelination and potassium channel exposure in spinal cord. J Neurotrauma. 2010;27:1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fancy SP, Chan JR, Baranzini SE, et al. Myelin regeneration: a recapitulation of development? Annu Rev Neurosci. 2011;34:21–43. [DOI] [PubMed] [Google Scholar]
- 49. Zawadzka M, Rivers LE, Fancy SP, et al. CNS‐resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Powers BE, Sellers DL, Lovelett EA, et al. Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proc Natl Acad Sci USA. 2013;110:4075–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Papastefanaki F, Matsas R. From demyelination to remyelination: the road toward therapies for spinal cord injury. Glia. 2015;63:1101–1125. [DOI] [PubMed] [Google Scholar]
- 52. Lo YL. The role of electrophysiology in the diagnosis and management of cervical spondylotic myelopathy. Ann Acad Med Singapore. 2007;36:886–893. [PubMed] [Google Scholar]
- 53. Amendt HL, Siedenburg JS, Steffensen N, et al. Correlation between severity of clinical signs and transcranial magnetic motor evoked potentials in dogs with intervertebral disk herniation. Vet J. 2017;221:48–53. [DOI] [PubMed] [Google Scholar]
- 54. Falconer JC, Narayana PA, Bhattacharjee MB, Liu SJ. Quantitative MRI of spinal cord injury in a rat model. Magn Reson Med. 1994;32:484–491. [DOI] [PubMed] [Google Scholar]
- 55. Flanders AE, Spettell CM, Tartaglino LM, et al. Forecasting motor recovery after cervical spinal cord injury: value of MR imaging. Radiology. 1996;201:649–655. [DOI] [PubMed] [Google Scholar]
- 56. Narayana PA, Grill RJ, Chacko T, Vang R. Endogenous recovery of injured spinal cord: longitudinal in vivo magnetic resonance imaging. J Neurosci Res. 2004;78:749–759. [DOI] [PubMed] [Google Scholar]
- 57. Hu R, Zhou J, Luo C, et al. Glial scar and neuroregeneration: histological, functional, and magnetic resonance imaging analysis in chronic spinal cord injury. J Neurosurg Spine. 2010;13:169–180. [DOI] [PubMed] [Google Scholar]
- 58. Ryu HH, Lim JH, Byeon YE, et al. Functional recovery and neural differentiation after transplantation of allogenic adipose‐derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;10:273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]


