Men Are from Mars, Women Are from Venus. John Gray used this provocative title for his book to describe the fundamental psychological differences between the sexes. Many other controlled studies and brain scans demonstrate that men and women are physically and mentally different. The purpose of this physiology masterclass is to illustrate how sex-related differences are present in respiratory function and their possible clinical implications.
Short abstract
An overview of sex-related differences in respiratory function and their possible clinical implications http://ow.ly/106m30jqOSW
Men Are from Mars, Women Are from Venus. John Gray used this provocative title for his book to describe the fundamental psychological differences between the sexes. Many other controlled studies and brain scans demonstrate that men and women are physically and mentally different. The purpose of this physiology masterclass is to illustrate how sex-related differences are present in respiratory function and their possible clinical implications.
Anatomical differences
Airways
In the last 4 weeks of gestation, the female fetus shows lower specific airway resistance than the male. From the 26th to 36th weeks of gestation, female fetuses show a more mature phospholipid profile that reflects the production of surfactant. After birth, female neonates seem to be characterised by higher ratio of large to small airways. They tend to have higher flow rates and specific airway conductance than males. This has been attributed to the role of surfactant in maintaining patency of the smaller airway [1].
Men are characterised by larger nasal cavities, and longer, narrower and higher nasal floors than females of the same body size. Such sexual dimorphism in the human skull influences the morphology of the upper airways [2]. Male average skeletal cranial airways are larger, with taller piriform apertures and, more consistently, taller internal nasal cavities and choanae than females [3].
Absolute retropalatal cross-sectional area is larger in males during both wakefulness and sleep, but when it is corrected for body surface area there is no sex difference. Similarly, males’ absolute retropalatal compliance during sleep is higher than females, but this difference disappears after the correction for neck circumference [4]. Men are characterised by larger neck circumference both as an absolute value and after correction for body mass index. Neck circumference is considered a surrogate index of pharyngeal soft tissue volume and fat distribution. Men have higher fat deposition at the level of the palate. These non-neuromuscular properties of the upper airways are important determinants of retropalatal compliance [4].
There are also sex-related differences in the pharynx in terms of size and resistance. The cross-sectional area is higher in men than in women [5, 6]. Although age and percentage of ideal body weight are contributors, the strongest independent factor impacting on pharyngeal area is sex [6]. Differences also emerge dynamically with lung volume variations. The consequent changes in laryngeal area are larger in males both as absolute value and normalised for laryngeal size or expiratory reserve volume [6].
Males also demonstrate stronger volume dependence than females. The percentage change in pharyngeal area between total lung capacity (TLC) and residual volume (RV), and also between functional residual capacity (FRC) and RV are significantly higher in males than in females [5, 6]. Both the pharyngeal resistance, in the segment of upper airway between the choanae and epiglottis, and the supraglottic resistance are higher in men than women [7]. As pharyngeal resistance is higher in men, we can speculate that they also presumably have lower patency. The development of pharyngeal collapse and obstructive apnoea may be increased, explaining, at least in part, the male predominance in this syndrome [7].
By contrast, there is no sex effect on glottic cross-sectional area and epiglottal shape [8, 9]. Glottic area depends on and changes with lung volume. At any given lung volume, there is no difference in glottic area between men and women, and its reduction between TLC and RV is similar. However, this reduced glottic area among females occurs predominantly at low lung volumes, whereas it is more uniform throughout the vital capacity range in males [8]. Glottal closure in men is more complete, but briefer than in women [9].
The structure of the larynx shows significant sex-related differences in all absolute dimensions. They are particularly pronounced in the sagittal diameters and in the thyroid angles, and only to a lesser extent in the transverse diameters. However, the relative proportions are much more constant and are not sex-specific [10]. These differences are related to growth. In fact, while no differences between the sexes are present during infancy, in the phase from puberty to maturity differentiation between males and females takes place both in terms of morphology and size, due to the different curves of the body which are shaped during maturation [11]. The most evident change is in the thyroid cartilage that forms the shape of the Adam’s apple, which is more externally visible in men than in women, because of the higher acuteness of the angle [10, 11].
Finally, the tracheal cross-sectional area is 29% larger in men. Tracheal and body sizes are not correlated within each sex, and this sex effect is not affected by tonsillectomy and/or adenoidectomy in childhood [6, 12, 13]. Tracheal area shows a good correlation with flows in women, but much less so in men [13]. However, the differences in tracheal and mainstem bronchi size between men and women disappear when airways measurements are standardised for lung size [14].
In general, the luminal areas of both the larger and central airways are 14–31% larger in men even after matching for lung size [15].
Lung
Sex differences in lung growth and development start in the prenatal period. Lung maturation is more advanced in the female fetus. Between the 16th and 26th weeks of gestation, mouth movement starts, reflecting fetal breathing, and is considered a critical determinant for the development of the lung [1]. Other fundamental regulators of lung maturation are sex hormones, with androgens having mainly inhibitory effects and oestrogens stimulatory. Oestradiol is produced by the placenta, while testosterone is secreted also by fetal testes. While androgens delay the surge of surfactant lipid production, oestrogens have positive effects on both the production of fetal surfactant and on the alveologenesis during neonatal and pubertal periods [16, 17].
The different impacts of androgens and oestrogens on the production of surfactant may be one of the reasons why premature female neonates are at lower risk (1:1.7) of developing respiratory distress syndrome than males [18]. They also have a better response than male neonates do to hormone accelerators of surfactant production. As a result, premature males with respiratory distress syndrome show the highest incidence of morbidity [16].
At birth, females have smaller lungs than males with fewer respiratory bronchioles [1]. The sex-related differences in lung growth persist from childhood to adulthood. They are present also during the brief period of adolescence (from 11 to 13 years) when females are taller than males, because of the onset of the pubertal growth spurt [1, 19, 20].
The fact that men have bigger lungs than women have been shown using different approaches: standard morphometric methods [21], chest radiographs [22] and three-dimensional geometric morphometric methods on computed tomography scans [23]. However, the number of alveoli per unit area, the number of alveoli per unit area volume, individual lung units and alveolar dimensions do not differ between males and females. Because boys have bigger lungs per unit of stature, they have a larger total number of alveoli and a larger alveolar surface area for a given age and stature [21].
The intrinsic elasticity of lung parenchyma is similar between sexes, whereas the recoil pressure differs because of the differences in lung size and in maximum distending forces [24, 25].
The shape of the lung differs between males and females, being more pyramidal in the former and more prismatic in the latter [23].
Dysanapsis
Green et al. [26] showed that there is a relatively loose coupling between lung and airways size. In other words, large lungs are not necessarily associated with a larger airways size than in a person with smaller lungs. For the first time, they introduced the term dysanapsis (from the Greek: dys meaning unequal and anaptixy meaning growth) to indicate the disproportionately growing pattern between the constituent parts of an organ that allows normal physiological function of the whole [26]. Later, Mead [27] quantified dysanapsis as the ratio of maximal expiratory flow divided by static recoil pressure at 50% of vital capacity. The former is sensitive to airway size, the latter to lung size. He found that lung size and airway length are not associated [27].
As airways and lung dimensions are significantly different between males and females, what about the relationship between their sizes?
Mead [27] showed that females are characterised by smaller ratios at a given size than adult males of comparable age. Women, therefore, have smaller airways relative to lung size than men. He also showed that these sex differences develop late on in growth [27].
Similar results were found based on a direct measurement of tracheal area using an acoustic reflection technique or chest radiograph [13, 14]. A different sex-related dysanaptic pattern emerged. It seems that in males the airways–parenchymal dysanapsis starts in childhood and persists into adulthood. By contrast, tracheal and lung volume grow proportionally during childhood in females, but then the airways start to grow faster than the lung and women show dysanapsis [13]. More recently, the dysanapsis ratio was found to be similar between the sexes [25, 28], but after correction for vital capacity, the results were smaller in woman [25].
Dysanapsis results, therefore, strongly depend on the methods used to quantify airways and lung sizes and whether the data have been normalised for some parameter (height, lung volume, lung recoil pressure etc.) [29]. There is also the need to understand if there is a cut-off between physiological and pathological conditions [30], but this is not the purpose of the present masterclass.
Chest wall
Important sex differences are present in both volume and configuration of the ribcage. Women are characterised by a disproportionately smaller rib cage size than males [22, 31]. Specifically, the cross-sectional area, the internal anterior–posterior and the lateral diameters are lower at different lung volumes. The thoracic index (i.e. ratio of anterior–posterior/lateral diameters of the rib cage) is similar between males and females at RV and FRC. At TLC, women show a rounder rib cage than men. The different thoracic configuration in females is also evidenced by a different relationship between rib cage cross-sectional area and the height of the diaphragm dome [22].
These results were obtained from chest radiographs [22], but higher ribcage dimensions in men were also found using other techniques.
Opto-electronic systems for motion analysis, based on infrared TV cameras, reconstruct the geometry of the ribcage using the three-dimensional coordinates of external, passive, reflecting markers placed on anatomical points. Results from this technique demonstrate the male ribcage is not only characterised by higher antero–posterior diameter, but also by larger perimeters, cross-sectional area and volume [32]. More recently, the use of semi-landmark methods on computed tomography reconstructions has allowed a more accurate morphometric quantification of the three-dimensional structure of the ribcage. This method adds more details on the sex-related differences in ribcage size. Rib cages are wider in men, particularly at the caudal part, whereas the sternum is in a higher position in females [2]. Males’ rib cages are also deeper than those of females of the same stature, and this is linked to a greater rib cage volume in males [33].
Finally, an important sex-related difference characterises the inclination of the ribs, with men’s ribs being more horizontally oriented than those of females. This emerges both from quantification of the angle formed by the lower border of the sixth rib and the vertical on lateral films of chest radiographs and from the three-dimensional rib cage morphology using a semi-landmark approach for computed tomography reconstruction [2, 22, 33, 34]. This difference may be a consequence of the different orientation of the spinous processes, which are more horizontal in females and more caudal in males. Such greater dorsal orientation of the transverse processes of men may reorient the ribs leading to greater radial ribcage diameters [35]. Torres-Tamayo et al. [23] suggest the movement of the ribs to be predominantly “pump-handle” in women and “bucket-handle” type in men.
The rib cage has the dual role of accommodating both lung and abdominal volume displacements, in particular when the abdomen is distended. The higher volume capacity of the rib cage of females in relationship to the size of their lungs, therefore, is suggested to be well suited to accommodate the increased abdominal distension caused by pregnancy. In this way, the effects on lung function and abdominal pressure of the enlarging uterus may be minimised [22, 36–38].
The aforementioned sex-related differences in the ribcage ultimately also affect the chest wall, as shown by Romei et al. [32]. In general, all the dimensions of the chest wall are greater in males than in females. Only the antero–posterior diameter and the volume of the abdomen are higher in men. For this reason, the chest wall differences can be only ascribed to the ribcage. Interestingly, thoracic and abdominal volumes are higher in men only as absolute values, whereas they are similar to women when expressed as percentage of total chest wall volume [32].
Diaphragm
The length of the diaphragm is ∼9% shorter in females than males at TLC, FRC and RV. The length of the diaphragm in the zone of apposition with the rib cage is smaller in females as well. While the dome-shape factor on lateral projections is greater in females, the dome-shape factors on anterior–posterior films and the height of the dome of the diaphragm below the first thoracic vertebra are similar between males and females. The dome-shape factor is defined as the ratio between the length of visible contours and the length of chords intersecting the contours end-points on chest radiographs [22].
Functional implications
Anatomical sex-related differences, summarised in table 1, are present at different levels of the respiratory system. Are these structural differences associated with functional differences emerging either during quiet spontaneous breathing and/or exercise in terms of volume variations, pressure swings and energetics?
Table 1.
Anatomy of the female respiratory system compared with males
| Airways | Lung | Chest wall | |||
| Pharynx | Volume | ↓ | Rib cage | ||
| Cross-sectional area | ↓ | Total alveolar number | ↓ | Cross-sectional area | ↓ |
|
Resistance |
↓ | Alveolar surface area | ↓ | Antero–posterior diameter | ↓ |
|
Patency |
↑ | Alveolar number per unit area | ≈ | Lateral diameter | ↓ |
| Larynx | Alveolar number per unit volume | ≈ | Thoracic index# | ≈ | |
| Glottis cross-sectional area | ≈ | Shape | ↓ pyramidal | Perimeter | ↓ |
| Absolute dimension | ↓ | Volume (absolute) | ↓ | ||
| Thyroid cartilage angle | ↑ | Volume (% chest wall volume) | ≈ | ||
| Trachea | Ribs inclination | ↑ | |||
| Cross-sectional area (absolute) | ↓ | Ribs movement | ↑ pump-handle | ||
| Cross-sectional area (standardised for lung size) | ≈ | Diaphragm | |||
| Total length | ↓ | ||||
| Length in the zone of apposition | ↓ | ||||
| Dome-shape factor on lateral projection | ↑ | ||||
| Dome-shape factor on antero–posterior projection | ≈ | ||||
| Height of the dome | ≈ | ||||
↑: increased compared with men; ↓: decreased compared with men; ≈ : no change between sexes. #: antero–posterior diameter/lateral diameter.
Males’ lungs are bigger not only in terms of absolute volume, but also in terms of their volume variations [25, 39–41]. Men, in fact, also have significantly larger mean values for all pulmonary variables, both volumes and flows, except resistance which is significantly lower in males [6, 12]. It is interesting to note how the flow measurements are indexes of smallest airways function in females [40]. For this reason, all the prediction equations for normal values include sex as discriminating factor.
The contraction of the inspiratory muscles produces negative oesophageal/pleural pressure swings that result in variations in chest wall volume. The ribcage expands because of contraction of the inspiratory ribcage muscles, while the abdomen expands because the piston-like movement of the diaphragm increases abdominal/gastric pressure [42, 43].
During resting breathing, the shorter diaphragm in females is associated with lower ratios of oesophageal to transdiaphragmatic pressure changes and of gastric to transdiaphragmatic pressure changes. End-expiratory oesophageal, gastric or transdiaphragmatic pressures are similar between the sexes. These suggest a greater contribution of inspiratory rib cage muscles in females than males, without different levels of tonic respiratory muscle activity [22]. In 1846, Dr. Hutchinson traced the shadows of various persons “under a strong light” during the different stages of respiration, to study the respiratory movements. He noted that “the ordinary breathing in the two sexes differs. In men it is chiefly by the diaphragm; in women chiefly by the ribs” [44]. A century and a half later, the analysis of thoraco–abdominal kinematics quantified the increased action of the inspiratory ribcage muscles in women. During quiet breathing and at vital capacity, the female ribcage contribution to tidal volume is higher than in men. This is particularly evident in the seated position and at different inclinations, but not in supine position [32, 45–47].
The greater inclination of the ribs in females may put the inspiratory rib cage muscles at a better mechanical advantage, being responsible for the prevalent contribution of these muscles to inspiratory pressure swings. The orientation of the ribs, in fact, affects the resultant contractile force of the intercostal muscles [48]. The more declined ribs of women make the intercostal muscles raise the ribs more efficiently and produce more pronounced thoracic breathing. The predominantly diaphragmatic breathing in men, therefore, could be the compensation for the inefficient action of intercostal muscles due to more horizontal ribs. The increased action of the diaphragm in men has been linked to the male thoracic morphology (wider at the caudal part), to the greater medio–lateral expansion of lower lungs and to their more pyramidal shape [2, 23].
Once more, it can be speculated that the greater contribution of the ribcage in women may be propaedeutic for the functional adaptation to the hormonal and anatomical changes induced by pregnancy [22, 38]. This had already been postulated in late 19th century: “it may be possible that this costal breathing is a provision against those periods when the abdomen contains the gravid uterus” [44].
Dynamics and energetics of breathing during exercise
The aforementioned sex-based differences in the structure and function of the respiratory system become critically important during dynamic exercise. The differences between women and men impact the development of flow, the regulation of lung volume, the pressure swings and the consequent work of breathing.
Females’ reduced airways diameter and lung volume result in lower peak expiratory flow and vital capacity. The most important consequence is women have a smaller maximal flow–volume loop. Their capacity to generate increased ventilation during exercise is, therefore, smaller with respect to men. This may predispose women to developing expiratory flow limitation (EFL). EFL occurs when the flow–volume loop of a tidal breath superimposes or exceeds the expiratory boundary of the maximum flow–volume curve. It consists of expiratory flow that cannot be further increased by increasing the effort of the expiratory muscles, being maximumal at that tidal volume [49]. McClaran et al. [50] first concluded that the smaller lung volumes and maximal flow rates in women causes increased prevalence of EFL, with tidal volume and minute ventilation being mechanically constrained at high workload. This is especially evident in highly fit women during the final stage of exercise [51, 52].
The regulation of lung volume during exercise is an important factor as it reflects the strategy by which tidal volume is achieved and it contributes to the work of breathing. Normally, the increased tidal volume during exercise is a consequence of an end-inspiratory lung volume increase and an end-expiratory lung volume (EELV) decrease with respect to the resting values. The reduction in EELV is similar between men and women throughout the majority of submaximal exercise and/or at a certain level of minute ventilation [52, 53]. By contrast, healthy fit women show a relative hyperinflation during heavy exercise and a higher rate of ventilation [50, 51]. This means that EELV increases at peak exercise. Specifically, dynamic hyperinflation occurs at the onset of significant EFL. It seems, therefore, that operational volume at maximal exercise depends on the presence or absence of EFL [51]. In fact, when EFL is reduced by He–O2 (79% He–21% O2) breathing EELV is maintained lower than baseline [50]. The presence of EFL during heavy exercise in healthy trained subjects, therefore, seems to trigger a reflex response that makes EELV increase to avoid dynamic compression of the airway downstream from the flow-limited segment [54]. The operational lung volume, therefore, shifts towards higher volume. Breathing, therefore, occurs: 1) where there is more expiratory flow reserve in the flow–volume loop; 2) towards the flatten part of the pressure–volume curve; and 3) distant from the optimal length of the length–tension relationship of inspiratory muscles.
Hyperinflation, therefore, may induce respiratory muscles fatigue because it makes the inspiratory muscles contract from a shorter length and in the presence of reduced lung compliance [50, 52].
The combination of EFL and dynamic hyperinflation makes the work of breathing (WOB) and oxygen cost of hyperpnoea increase. Women, in fact, show a higher WOB than men across a range of ventilations during progressive exercise. It even becomes twice that of men when ventilation is above 90 L⋅min−1 [51, 55]. However, no difference emerges between the sexes when WOB is compared to different percentages of maximal oxygen consumption (V′O2max), although women have ∼25% lower minute ventilation than their male counterparts. V′O2max is distributed among all the skeletal muscles, its relationship with the work performed being linear. Dominelli et al. [56] demonstrated that this is also valid for the respiratory muscles that are morphologically and functionally skeletal muscles. They computed the oxygen uptake of the respiratory muscles (V′O2RM) over a wide range of minute ventilations, showing that the greater WOB in women is linearly associated with higher V′O2RM with less efficiency than men at submaximal and maximal exercise intensities. Women, in fact, are characterised by greater V′O2RM for a given WOB and ventilation, with V′O2RM representing a significantly greater fraction of whole-body oxygen consumption in women (∼13.8%) than in men (∼9.4%) [56]. It can be speculated that a proportionally greater fraction of blood flow corresponds to the increased V′O2RM in women. This will possibly lead to an important competition for blood flow between respiratory and working muscles, particularly during heavy exercise [15, 50].
Collectively, these findings suggest that the physiological cost of moving a given amount of air in and out of the lungs is higher in women, because of a higher oxygen cost of breathing [15, 51, 52]. The greater oxygen cost of breathing in women means that a greater fraction of total oxygen uptake and cardiac output is directed to the respiratory muscles, influencing exercise performance [57].
The use of a Campbell diagram allows the two components of WOB to be distinguished: the elastic and the resistive work. The former is the work needed to overcome the elasticity of lung and chest wall tissues, the latter is the work required to overcome airflow resistance. While the former is similar between sexes, the latter is significantly higher in women [15, 25, 51, 52, 58]. Similar elastic work confirms the finding that there is no sex interaction in the intrinsic viscoelastic tissue and the static recoil pressures [24, 25], while the greater inspiratory resistive work is consistent with the smaller airway dimension. This may be explained when considering the principles of airflow. Airflow resistance is inversely proportional to radius to the fourth power. Larger airways are the main site of resistance (∼80%) while smaller airways contribute <20%. The anatomical sex difference in terms of airways size, therefore, makes women prone to have larger resistance and more turbulent flow at peak exercise, when both flow and ventilation are high. Dynamic hyperinflation during heavy exercise shifts the loop in the Campbell diagram upward therefore increasing the elastic work.
Finally, the smaller female vital capacity implies a lower maximal tidal volume to be achieved during exercise compensated by a higher respiratory rate to adequately ventilate the lungs. This would increase the dead space ventilation, resulting in reduced alveolar ventilation for a given ventilatory rate. The main consequences of such alveolar hypoventilation are a decrease in arterial oxygen tension and an increase in arterial carbon dioxide [50]. Women are, therefore, more vulnerability to hypoxaemia during exercise. Many active healthy young women experience significant exercise-induced arterial hypoxaemia (EIAH) at a lower V′O2max than their male contemporaries [59–61]. The presence of EFL, i.e. of mechanical ventilatory constraints, leads directly to EIAH because it also prevents adequate compensatory alveolar hyperventilation. The reduction in mechanical ventilatory constraints with Heliox inspiration partially reverses EIAH in those subjects who develop EFL. The cause of EIAH, of course, is multifactorial. One factor can be the inadequate pulmonary structure/function in women that contributes to limit maximal oxygen transport and utilisation during maximal exercise [59, 60].
Clinical implications
Dysanapsis or more generally the sex-related and/or the dimensional differences in the respiratory system are not just a fascinating anatomical and/or physiological curiosity; they do also have clinical implications or may influence the pathogenesis of diseases and exercise. Dysanapsis ratio per se, for example, is a good predictor of EFL at maximal exercise [28].
Influence of sex hormones, the menstrual cycle and diseases
Respiratory function is known to be influenced by the different phases of menstrual cycle and by common hormonal and metabolic conditions.
Early menarche seems to be associated with poorer general health later in life in terms of higher risk of asthma, lower lung function, cardiovascular disease and others. The menstrual cycle influences many diseases like migraine, epilepsy, bipolar disorder and rheumatoid arthritis [62]. Respiratory symptoms (i.e. wheeze, shortness of breath and cough) vary significantly with the menstrual cycle-induced hormonal changes. They tend to get worse during the mid-luteal to mid-follicular phases of the menstrual cycle (between days 10 and 22 of the cycle) [63]. Fluctuations in asthmatic symptoms are also reported during the menstrual period, possibly due to hormonal influence on airways. There are no consistent results on the effect of oral contraceptive pills on respiratory function, while irregular menstruation and polycystic ovary syndrome are associated with a higher risk of asthma and lower forced vital capacity (FVC). The relationship between menopause and lung health has still to be understood. What is clear is that smoking reduces the age at which menopause occurs [62].
Accumulating evidence suggests that sex hormones may either contribute to the pathogenesis of a disease or serve as protective factors. We have already shown that they regulate the development of the foetal lung and airways. Sex hormones are hypothesised to contribute to the sex differences in the adverse effects of antenatal smoking on lung function found into early adulthood. The lung function of male young adults exposed to smoking in utero is more adversely affected than females. Forced expiratory volume in 1 s (FEV1), in fact, is significantly reduced in men compared with women. It is possible that sex hormones modulate a different lung growth pathway to respond to the smoking exposure in utero [64]. It seems, therefore, that a sex-induced different susceptibility to the inflammatory process is present in the early stage of life. A different correlation between the inflammatory reaction and lung function decline is also present in adulthood. A stronger inverse association between C-reactive protein, a sensitive marker of systemic inflammation, and FVC and FEV1 declines characterises males. This suggests a sex difference in the mechanism of lung impairment, but its nature is unclear [65].
The sex difference in terms of retropalatal collapsibility can contribute to the prevalence of sleep disordered breathing in men. If airways are highly compliant, they are more likely to collapse; therefore, making men more prone to obstructive sleep apnoea (OSA). There is increasing evidence of the influence of testosterone in sleep disordered breathing [4]. The sex-related difference in OSA, however, reduces with age. After menopause, in fact, women experience a higher prevalence of sleep disordered breathing. Sex hormones are suggested to have a protective effect on both airways and ventilatory drive. Progesterone, which decreases after menopause, is known to increase the tone of the upper airways muscles and to stimulate respiration by increasing the chemoreceptor response to hypoxia and hypercapnia. A further contributor to increased OSA after menopause is the different distribution of body fat, which increases and is more likely to be concentrated in upper trunk area. Pregnancy per se increases the incidence (8.1%) of OSA because of the augmented neck circumference, the reduced nasal patency and the pharyngeal oedema. As a result, snoring is considered an important risk factor for pregnancy-induced hypertension [66].
Small airways are differently involved in males and females with asthma. The former show more methacholine-induced air trapping, while the latter have significantly higher bronchial fractions of exhaled nitric oxide. Men, therefore, demonstrate a larger degree of small airway collapsibility while inflammation in the airways characterises women [67]. Asthma is more prevalent in women among the general population, but the incidence changes with age. In childhood, asthma is more frequent among boys, but its incidence reverses thereafter becoming prevalently female until the sixth decade when there is no great sex-related difference in asthma occurrence. The influence of sex hormones is also confirmed by the fact that 20% of asthmatic females suffer an exacerbation during pregnancy and up to 40% of women with asthma report premenstrual worsening of the condition [62, 68–70].
Like asthma and allergic rhinitis, and also atopy, the tendency to show increased reactiveness to specific allergens, is predominant in boys before puberty. After puberty, the data are a bit contrasting, but it decreases with age for both males and females [70].
There is sex bias in the diagnosis of chronic obstructive pulmonary disease (COPD), with emphysema being more frequent in men whereas women show more reactive airways and more pronounced airway narrowing. While the male death rate for COPD in the USA has declined, no change has occurred among females. Women are more likely to be exposed to second-hand and environmental smoke. They develop COPD earlier, after a smaller pack-year-smoking history and have a faster decline in lung function than men. There seems to be a sex-related difference in the proteome analysis of bronchoalveolar lavage. Moreover, female nonsmokers or never-smokers (excluding α1-antitrypsin deficiency) are also more prone to develop COPD. Long-term oxygen therapy has a better outcome in women. They also have a 2.5-fold greater improvement in lung function once they quit smoking. It is interesting to note that while female infants show less susceptibility to maternal smoking, it becomes greater in adulthood. More studies are needed to understand whether sex hormones have a protective or detrimental effect in COPD [69, 70].
The rate of lung cancer mortality has been rising in women, but not in men. Every year the number of women who die from lung cancer is higher than those dying from breast, uterine and ovarian cancer combined. Among nonsmokers, women are also three times more likely to be diagnosed with lung cancer, therefore suggesting a possible hormonal component. Women have a more positive prognostic factor, in terms of surgical and chemotherapeutic response, and better 5-year survival than men do. It is still under investigation whether these differences can be attributed to biological, social, behavioural and/or environmental factors. There is a sex-related difference in the histological types of lung cancer, with men most likely to develop squamous cell carcinoma while women are mainly diagnosed by adenocarcinoma. More studies are needed to investigate the role of hormone receptors in lung pathology [68].
Pulmonary fibrosis is more prevalent in men than women. Its incidence ranges from 1.4:1 to 2.1:1 (males:females). Until 2003, the rate of mortality was higher in men, but it is now increasing in women too. There are no data on sex differences in pulmonary fibrosis in humans, and animal studies have not provided a clear explanation [69, 70].
Pulmonary hypertension is predominant in women in all its types (idiopathic, familiar, pulmonary artery hypertension and portopulmonary hypertension) with incidence ranging between 2:1 and 4:1 (females:males). On average, its first manifestation is in the third decade of life in women and 10 years later in men. Future research should focus on the role of sex steroids to understand if and how oestrogen makes women more susceptible and/or if there is a protective effect in men [70].
The main features of the female respiratory system are reported in figure 1.
Figure 1.
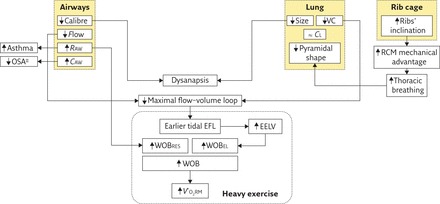
Schematic diagram summarising the main features of the female respiratory system compared with males. CAW: compliance of the airways; CL: lung compliance; RAW: airway resistance; RCM: ribcage respiratory muscles; VC: vital capacity; WOBEL: elastic component of work of breathing; WOBRES: resistive component of work of breathing. ↑: increment compared with men; ↓: decrement compared with men; ≈ : no change between sexes. #: until menopause.
Summary
Men are from Brobdingnag, women are from Lilliput (to paraphrase Gulliver’s Travels). This seems the obvious conclusion of this masterclass. Smaller diameter airways, lung volumes, maximum expiratory flow and diffusion surface characterise women compared with men and some of these anatomical differences seem to be propaedeutic for pregnancy. It is personal opinion of the authors that size, more than sex, is the main driving factor of the abovementioned functional implications. Often sex differences can, in fact, be attributed to scale, as women are generally smaller than men. The “outlier” women in some studies, i.e. those who were taller and similar in size to the men, confirm our hypothesis, as they mostly behaved like their male counterparts. Moreover, the sample sizes of the studies are very low and they consider only a specific portion of the population according to age, body mass index, physical training etc. For this reason, there is a discrepancy among different results and animal models are not always a solution because sometimes they are in contrast with human clinical data. In order to understand the real effect of sex and/or sex steroids on respiratory function there is a need for longitudinal cohort studies with specific selection criteria for the population.
Footnotes
Conflict of interest: None declared.
References
- 1.Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax 1999; 54: 1119–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García-Martínez D, Torres-Tamayo N, Torres-Sanchez I, et al. Morphological and functional implications of sexual dimorphism in the human skeletal thorax. Am J Phys Anthropol 2016; 161: 467–477. [DOI] [PubMed] [Google Scholar]
- 3.Bastir M, Godoy P, Rosas A. Common features of sexual dimorphism in the cranial airways of different human populations. Am J Phys Anthropol 2011; 146: 414–422. [DOI] [PubMed] [Google Scholar]
- 4.Rowley JA, Sanders CS, Zahn BR, et al. Gender differences in upper airway compliance during NREM sleep: role of neck circumference. J Appl Physiol 2002; 92: 2535–2541. [DOI] [PubMed] [Google Scholar]
- 5.Brown IG, Zamel N, Hoffstein V. Pharyngeal cross-sectional area in normal men and women. J Appl Physiol 1986; 61: 890–895. [DOI] [PubMed] [Google Scholar]
- 6.Brooks LJ, Strohl KP. Size and mechanical properties of the pharynx in healthy men and women. Am Rev Respir Dis 1992; 146: 1394–1397. [DOI] [PubMed] [Google Scholar]
- 7.White DP, Lombard RM, Cadieux RJ, et al. Pharyngeal resistance in normal humans: influence of gender, age, and obesity. J Appl Physiol 1985; 58: 365–371. [DOI] [PubMed] [Google Scholar]
- 8.Rubinstein I, England SJ, Zamel N, et al. Glottic dimensions in healthy men and women. Respir Physiol 1989; 77: 291–299. [DOI] [PubMed] [Google Scholar]
- 9.Sulter AM, Schutte HK, Miller DG. Standardized laryngeal videostroboscopic rating: differences between untrained and trained male and female subjects, and effects of varying sound intensity, fundamental frequency, and age. J Voice 1996; 10: 175–189. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich G, Lichtenegger R. Surgical anatomy of the larynx. J Voice 1997; 11: 345–355. [DOI] [PubMed] [Google Scholar]
- 11.Hirose H. Investigating the Physiology of Laryngeal Structures In: Hardcastle WJ, Laver J, Gibbon FE, eds. The Handbook of Phonetic Sciences. 2nd Edn Oxford, Wiley-Blackwell, 2010. [Google Scholar]
- 12.Brooks LJ, Byard PJ, Helms RC, et al. Relationship between lung volume and tracheal area as assessed by acoustic reflection. J Appl Physiol 1988; 64: 1050–1054. [DOI] [PubMed] [Google Scholar]
- 13.Hoffstein V. Relationship between lung volume, maximal expiratory flow, forced expiratory volume in one second, and tracheal area in normal men and women. Am Rev Respir Dis 1986; 134: 956–961. [DOI] [PubMed] [Google Scholar]
- 14.Collins DV, Cutillo AG, Armstrong JD, et al. Large airway size, lung size, and maximal expiratory flow in healthy nonsmokers. Am Rev Respir Dis 1986; 134: 951–955. [DOI] [PubMed] [Google Scholar]
- 15.Sheel AW, Dominelli PB, Molgat-Seon Y. Revisiting dysanapsis: sex-based differences in airways and the mechanics of breathing during exercise. Exp Physiol 2016; 101: 213–218. [DOI] [PubMed] [Google Scholar]
- 16.Seaborn T, Simard M, Provost PR, et al. Sex hormone metabolism in lung development and maturation. Trends Endocrinol Metab 2010; 21: 729–738. [DOI] [PubMed] [Google Scholar]
- 17.Behan M, Kinkead R. Neuronal Control of Breathing: Sex and Stress Hormones Comprehensive Physiology, Volume 1. Hoboken, John Wiley & Sons, Inc., 2011; pp. 2101–2139. [DOI] [PubMed] [Google Scholar]
- 18.Farrell PM, Avery ME. Hyaline membrane disease. Am Rev Respir Dis 1975; 111: 657–688. [DOI] [PubMed] [Google Scholar]
- 19.Hibbert ME, Lannigan A, Landau LI, et al. Lung function values from a longitudinal study of healthy children and adolescents. Pediatr Pulmonol 1989; 7: 101–109. [DOI] [PubMed] [Google Scholar]
- 20.Hibbert M, Lannigan A, Raven J, et al. Gender differences in lung growth. Pediatr Pulmonol 1995; 19: 129–134. [DOI] [PubMed] [Google Scholar]
- 21.Thurlbeck WM. Postnatal human lung growth. Thorax 1982; 37: 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellemare F, Jeanneret A, Couture J. Sex differences in thoracic dimensions and configuration. Am J Respir Crit Care Med 2003; 168: 305–312. [DOI] [PubMed] [Google Scholar]
- 23.Torres-Tamayo N, García-Martínez D, Lois Zlolniski S, et al. 3D analysis of sexual dimorphism in size, shape and breathing kinematics of human lungs. J Anat 2018; 232: 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colebatch HJ, Greaves IA, Ng CK. Exponential analysis of elastic recoil and aging in healthy males and females. J Appl Physiol 1979; 47: 683–691. [DOI] [PubMed] [Google Scholar]
- 25.Dominelli PB, Molgat-Seon Y, Bingham D, et al. Dysanapsis and the resistive work of breathing during exercise in healthy men and women. J Appl Physiol 2015; 119: 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green M, Mead J, Turner JM. Variability of maximum expiratory flow-volume curves. J Appl Physiol 1974; 37: 67–74. [DOI] [PubMed] [Google Scholar]
- 27.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 1980; 121: 339–342. [DOI] [PubMed] [Google Scholar]
- 28.Smith JR, Rosenkranz SK, Harms CA. Dysanapsis ratio as a predictor for expiratory flow limitation. Respir Physiol Neurobiol 2014; 198: 25–31. [DOI] [PubMed] [Google Scholar]
- 29.Babb TG, Beck KC, Johnson BD. Dysanapsis. Med Sci Sport Exerc 2012; 44: 1194. [DOI] [PubMed] [Google Scholar]
- 30.Thompson BR. Dysanapsis – once believed to be a physiological curiosity – is now clinically important. Am J Respir Crit Care Med 2017; 195: 277–278. [DOI] [PubMed] [Google Scholar]
- 31.Bellemare JF, Cordeau MP, Leblanc P, et al. Thoracic dimensions at maximum lung inflation in normal subjects and in patients with obstructive and restrictive lung diseases. Chest 2001; 119: 376–386. [DOI] [PubMed] [Google Scholar]
- 32.Romei M, Mauro AL, D’Angelo MG, et al. Effects of gender and posture on thoraco-abdominal kinematics during quiet breathing in healthy adults. Respir Physiol Neurobiol 2010; 172: 184–191. [DOI] [PubMed] [Google Scholar]
- 33.Shi X, Cao L, Reed MP, et al. A statistical human rib cage geometry model accounting for variations by age, sex, stature and body mass index. J Biomech 2014; 47: 2277–2285. [DOI] [PubMed] [Google Scholar]
- 34.Weaver AA, Schoell SL, Stitzel JD. Morphometric analysis of variation in the ribs with age and sex. J Anat 2014; 225: 246–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bastir M, Higuero A, Ríos L, et al. Three-dimensional analysis of sexual dimorphism in human thoracic vertebrae: Implications for the respiratory system and spine morphology. Am J Phys Anthropol 2014; 155: 513–521. [DOI] [PubMed] [Google Scholar]
- 36.Contreras G, Gutiérrez M, Beroíza T, et al. Ventilatory drive and respiratory muscle function in pregnancy. Am Rev Respir Dis 1991; 144: 837–841. [DOI] [PubMed] [Google Scholar]
- 37.Gilroy RJ, Mangura BT, Lavietes MH. Rib cage and abdominal volume displacements during breathing in pregnancy. Am Rev Respir Dis 1988; 137: 668–672. [DOI] [PubMed] [Google Scholar]
- 38.LoMauro A, Aliverti A. Respiratory physiology of pregnancy. Breathe 2015; 11: 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quanjer PH, Brazzale DJ, Boros PW, et al. Implications of adopting the Global Lungs Initiative 2012 all-age reference equations for spirometry. Eur Respir J 2013; 42: 1046–1054. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz J, Katz SA, Fegley RW, et al. Sex and race differences in the development of lung function. Am Rev Respir Dis 1988; 138: 1415–1421. [DOI] [PubMed] [Google Scholar]
- 41.Quanjer PH, Hall GL, Stanojevic S, et al. Age- and height-based prediction bias in spirometry reference equations. Eur Respir J 2012; 40: 190–197. [DOI] [PubMed] [Google Scholar]
- 42.Macklem PT. Normal and abnormal function of the diaphragm. Thorax 1981; 36: 161–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Troyer A De, Kelly S, Macklem PT, et al. Mechanics of intercostal space and actions of external and internal intercostal muscles. J Clin Invest 1985; 75: 850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hutchinson J. On the capacity of the lungs, and on the respiratory functions, with a view of establishing a precise and easy method of detecting disease by the spirometer. Med Chir Trans 1846; 29: 137–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verschakelen JA, Demedts MG. Normal thoracoabdominal motions. Influence of sex, age, posture, and breath size. Am J Respir Crit Care Med 1995; 151: 399–405. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert R, Auchincloss JH, Peppi D. Relationship of rib cage and abdomen motion to diaphragm function during quiet breathing. Chest 1981; 80: 607–612. [DOI] [PubMed] [Google Scholar]
- 47.Binazzi B, Lanini B, Bianchi R, et al. Breathing pattern and kinematics in normal subjects during speech, singing and loud whispering. Acta Physiol 2006; 186: 233–246. [DOI] [PubMed] [Google Scholar]
- 48.Ratnovsky A, Elad D. Anatomical model of the human trunk for analysis of respiratory muscles mechanics. Respir Physiol Neurobiol 2005; 148: 245–262. [DOI] [PubMed] [Google Scholar]
- 49.Aliverti A. Lung and chest wall mechanics during exercise: Effects of expiratory flow limitation. Respir Physiol Neurobiol 2008; 163: 90–99. [DOI] [PubMed] [Google Scholar]
- 50.McClaran SR, Harms CA, Pegelow DF, et al. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol 1998; 84: 1872–1881. [DOI] [PubMed] [Google Scholar]
- 51.Guenette JA, Witt JD, McKenzie DC, et al. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol 2007; 581: 1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheel AW, Guenette JA. Mechanics of breathing during exercise in men and women. Exerc Sport Sci Rev 2008; 36: 128–134. [DOI] [PubMed] [Google Scholar]
- 53.Vogiatzis I, Aliverti A, Golemati S, et al. Respiratory kinematics by optoelectronic plethysmography during exercise in men and women. Eur J Appl Physiol 2004; 93: 581–587. [DOI] [PubMed] [Google Scholar]
- 54.Pellegrino R, Brusasco V, Rodarte JR, et al. Expiratory flow limitation and regulation of end-expiratory lung volume during exercise. J Appl Physiol 1993; 74: 2552–2558. [DOI] [PubMed] [Google Scholar]
- 55.Wanke T, Formanek D, Schenz G, et al. Mechanical load on the ventilatory muscles during an incremental cycle ergometer test. Eur Respir J 1991; 4: 385–392. [PubMed] [Google Scholar]
- 56.Dominelli PB, Render JN, Molgat-Seon Y, et al. Oxygen cost of exercise hyperpnoea is greater in women compared with men. J Physiol 2015; 593: 1965–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aliverti A. The respiratory muscles during exercise. Breathe 2016; 12: 165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guenette JA, Querido JS, Eves ND, et al. Sex differences in the resistive and elastic work of breathing during exercise in endurance-trained athletes. Am J Physiol Integr Comp Physiol 2009; 297: R166–R175. [DOI] [PubMed] [Google Scholar]
- 59.Harms CA, McClaran SR, Nickele GA, et al. Exercise-induced arterial hypoxaemia in healthy young women. J Physiol 1998; 507: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harms CA, McClaran SR, Nickele GA, et al. Effect of exercise-induced arterial O2 desaturation on VO2max in women. Med Sci Sports Exerc 2000; 32: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 61.Dominelli PB, Foster GE, Dominelli GS, et al. Exercise-induced arterial hypoxaemia and the mechanics of breathing in healthy young women. J Physiol 2013; 591: 3017– 3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Macsali F, Svanes C, Bjørge L, et al. Respiratory health in women: from menarche to menopause. Expert Rev Respir Med 2012; 6: 187–202. [DOI] [PubMed] [Google Scholar]
- 63.Macsali F, Svanes C, Sothern RB, et al. Menstrual cycle and respiratory symptoms in a general Nordic–Baltic population. Am J Respir Crit Care Med 2013; 187: 366–373. [DOI] [PubMed] [Google Scholar]
- 64.Hayatbakhsh MR, Sadasivam S, Mamun AA, et al. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax 2009; 64: 810–814. [DOI] [PubMed] [Google Scholar]
- 65.Ólafsdóttir IS, Gíslason T, Gudnason V, et al. CRP is associated with lung function decline in men but not women: a prospective study. Respir Med 2013; 107: 91–97. [DOI] [PubMed] [Google Scholar]
- 66.Wimms A, Woehrle H, Ketheeswaran S, et al. Obstructive sleep apnea in women: specific issues and interventions. Biomed Res Int 2016; 2016: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen J, Douma WR, ten Hacken NHT, et al. Physiology of the small airways: a gender difference? Respir Med 2008; 102: 1264–1271. [DOI] [PubMed] [Google Scholar]
- 68.Carey MA, Card JW, Voltz JW, et al. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab 2007; 18: 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sathish V, Martin YN, Prakash YS. Sex steroid signaling: implications for lung diseases. Pharmacol Ther 2015; 150: 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev 2012; 33: 1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]


