Abstract
Being born very preterm is associated with later deficits in lung function and an increased rate of respiratory symptoms compared with term-born children. The rates of early respiratory infections are higher in very preterm-born subjects, which may independently lead to deficits in lung function in later life. As with very preterm-born children, deficits in lung function, increased respiratory symptoms and an increased risk of respiratory infections in early life are observed in late preterm-born children. However, the rates of respiratory symptoms are lower compared with very preterm-born children. There is some evidence to suggest that respiratory outcomes may be improving over time, although not all the evidence suggests improvements. Male sex appears to increase the risk for later adverse respiratory illness. Although not all studies report that males have worse long-term respiratory outcomes than females. It is essential that preterm-born infants are followed up into childhood and beyond, and that appropriate treatment for any lung function deficits and respiratory symptoms is prescribed if necessary. If these very preterm-born infants progress to develop chronic obstructive airway disease in later life then the impact, not only on the individuals, but also the economic impact on healthcare services, is immense.
Educational aims
To report the effect of the sex of the preterm baby on respiratory outcomes.
To explore the short- and long-term respiratory outcomes of preterm birth.
Short abstract
Preterm birth is associated with adverse long-term respiratory outcomes. Males appear to have increased risk for later adverse respiratory illness compared to females, although not all studies agree. http://ow.ly/bRmj30jvn4g
Introduction
Long-term outcomes, especially respiratory and neurodevelopmental outcomes are important after preterm birth. In this review, we shall report the epidemiology of preterm birth and discuss issues around changes in medical management and survival rates. We will then explore the available evidence on the long-term respiratory outcomes of very and late preterm-born infants. We shall also discuss the limited evidence on the impact of sex on the respiratory outcomes of preterm subjects.
Definitions and epidemiology of preterm birth
Preterm birth is defined as birth at <37 weeks’ gestation. In 2010, 11.1% of all live births worldwide were estimated to be preterm. However, rates varied from 5% to greater than 15% in different countries [1]. In the UK, the rate of preterm birth in 2016 was 7.8% of all live births [2]. Of the 7.8%, 21% were born at ≤32 weeks’ gestation, often known as “very preterm” birth; however, 79% were born at 33–36 weeks’ gestation, often called “late” or “moderately” preterm birth. Preterm birth, especially very preterm birth at ≤32 weeks’ gestation, is associated with long-term respiratory symptoms and lung function deficits; especially if the preterm-born infants had bronchopulmonary dysplasia (BPD) or chronic lung disease in infancy (CLD) [3, 4]. CLD or BPD is defined as dependency on supplemental oxygen at 28 days of life or at 36 weeks’ post-menstrual age. However, late preterm birth, which traditionally has been regarded as having a lower risk of long-term respiratory symptoms and lung function deficits, has recently been the focus of research and concerns regarding the long-term respiratory outcomes have been raised [5, 6].
Changing medical management of preterm-born infants
One of the difficulties with reporting of the long-term respiratory outcomes after preterm birth is that the medical management of premature infants is constantly progressing, e.g. the introduction of gentler ventilation, regular use of maternally administered antenatal corticosteroids and regular use of exogenous surfactant. A study of nearly 35 000 preterm infants born at ≤28 weeks’ gestation reviewed the 20-year trend in neonatal care between 1993 and 2012, noting a significant increase in the use of antenatal maternal corticosteroids administration (24% to 87%), and a significant decrease in delivery room intubation (80% to 65%) [7]. Therefore, the longer term outcomes may not reflect the outcomes of the current population of preterm-born infants being treated today.
Changes in survival rates and rates of BPD for preterm-born infants
The advances in medical treatment of preterm infants have led to improved survival rates of preterm-born children. Stoll et al. [7] reported a significantly increased rate of survival for infants born at 23 weeks’ gestation (27% to 33%) and 24 weeks’ gestation (63% to 65%), with smaller increases for infants born at 25 and 27 weeks’ gestation between 2009 and 2012. In England, the survival of preterm-born infants who were born at 22 to <32 weeks’ gestation and admitted to neonatal care has also been investigated. There was an overall increase in survival to discharge from 88% in 2008 to 91.3% in 2014 [8]. Whether the increase in survival is associated with an increase in BPD has also been studied. One study noted that the rate of BPD increased from 32% in 1993 to 45% in 2000 and to 40% in 2008 for infants born at ≤28 weeks’ gestation [7]. Trends for incidence of BPD in Canada between 1980 and 2008 showed an increase in the number of BPD cases, but lower mortality for preterm-born infants with respiratory distress syndrome (RDS) at <37 weeks’ gestation suggesting that the increase in BPD is most likely due to improved survival of extremely preterm infants [9]. Doyle et al. [10] studied extremely preterm-born children (<28 weeks’ gestation) in Australia, born in the three time periods 1991/92, 1997 and 2005. Over time, there was an increase in the use of nasal continuous positive airway pressure from a median of 5 days in 1991/92 to 31.5 days in 2005; however, the rate of oxygen dependency at 36 weeks’ post-menstrual age increased from 46% in 1991/92 to 56% in 2005 [10]. It has been observed that male, preterm-born infants have higher mortality and morbidity in infancy. Watkins et al. [11] reported that males born at all birthweights had higher mortality rates up to 18 years of age than females (figure 1).
Figure 1.
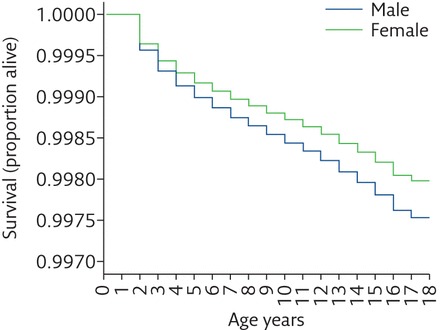
Association of sex with survival between 1 and 18 years of age for the UK population between 1993 and 2011. Reproduced from [11] with permission from the publisher.
Rates of CLD and RDS were increased in male, very low birthweight infants compared to female, very low birthweight infants in a study from Japan [12]. The sex difference appears to continue into childhood and beyond. Therefore, girls have an increased rate of survival compared with boys and a lower rate of respiratory disease, e.g. RDS in the neonatal period. The reasons for these differences are unknown, but hormonal differences, among other factors, may be important [13]. It should be borne in mind when studying rates of BPD that male preterm-born infants have a higher mortality rate than female infants. Collaco et al. [14] studied preterm-born children who had BPD in infancy who were attending an outpatient clinic up to 3 years of age. More males than females attended the clinic [14]. However, Zysman-Colman et al. [9] studied trends in BPD over three decades and reported that male sex was not associated with the development of BPD in preterm-born children born at <37 weeks’ gestation who had neonatal RDS. BPD diagnosis was confirmed using the National Institutes of Health consensus definition. When BPD was present, male sex was associated with increased severity of BPD in preterm-born infants who had RDS, adjusted OR 1.51 (1.14–2.00) [9]. Rutkowska et al. [15] reported an OR of 3.02 (95% CI, 1.3–7.5) for the risk factor between male sex and severe BPD (BPD was defined as oxygen dependency at 36 weeks’ gestation) in very preterm infants born at ≤32 weeks’ gestation with RDS when compared with females.
Conclusion
Improvements in the medical management of preterm-born infants have led to increased survival of very preterm-born infants, but appear to have also increased rates of BPD. The increase in survival rates of preterm-born infants means that a sizeable portion of the population has been born preterm. Even late preterm-born infants, the largest group of preterm-born infants, should be considered to be at risk of later complications. Female sex is associated with more survival and less risk for developing respiratory disease in the neonatal period. With increasing survival of preterm infants, a large population is at risk of respiratory disease. The respiratory deficits of this group of survivors are discussed in the second part of this review.
Long-term respiratory outcomes of preterm birth
Preterm-born children are at risk of later respiratory morbidity due to birth at an immature stage of lung development and also medical interventions such as mechanical ventilation. It is recognised that male infants and young children have a higher rates of respiratory disease than female infants even if they are term-born [13].
Respiratory infections
There is no doubt that preterm-born infants and children are prone to more respiratory infections than term-born controls. Preterm-born infants who had CLD and who had an admission to hospital with respiratory syncytial virus (RSV), had higher rates of healthcare usage than preterm infants without RSV hospital admissions, up to the age of 2 years [16]. The children who had hospital admissions for RSV had larger deficits in lung function at 8–10 years than children who had hospital admissions for reasons other than RSV [17]. Since respiratory infections in childhood can impact on lung function in adult life [18], it is important to identify if prematurity and infection in infancy or both are independent risk factors for later respiratory dysfunction. A registry-based study from Finland reported on over 1 000 000 infants born between 1991 and 2008. The infants were classified into four groups, <32, 32–33, 34–36 and ≥ 37 weeks’ gestation, and data on hospital admissions for respiratory reasons up to the age of 7 years were collated. All preterm-born groups had higher rates of admissions to hospital for lower respiratory tract infections and for asthma, with hospital admissions increasing with decreasing prematurity (figure 2) [19, 20]. In addition, overall, boys had higher odds ratios for risk of hospital admissions for bronchiolitis/bronchitis and pneumonia (ORs 1.61 (1.58–1.64) and 1.13 (1.10–1.16), respectively) compared with girls up to 7 years of age. Paranjothy et al. [21] reached similar conclusions stating that: “The risk of respiratory admission during childhood decreased with each successive week in gestation up to 40 to 42 weeks. The increased risk is small for late preterm infants, but the number affected is large and has an impact on health care services”.
Figure 2.
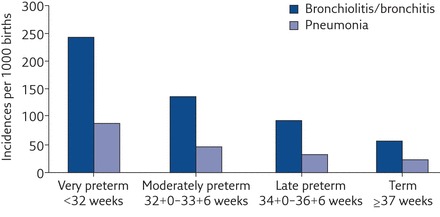
Hospital admissions for lower respiratory tract infections in children born moderately/late preterm. Reproduced from [19] with permission from the publisher.
In the study by Collaco et al. [14] more males than females attended the BPD clinic but there were no differences in respiratory morbidity, e.g. hospital admissions, antibiotics and steroid use, between males and females. By contrast, in early childhood, boys born at <32 weeks’ gestation had higher rates of hospital admissions between 3 and 5 years of age than girls. The most common reasons for readmissions were respiratory infection at 3 and 4 years of life [22].
Respiratory symptoms
The largest systematic review, thus far, by Been et al. [4] investigated respiratory symptoms resulting after preterm birth. Preterm-born children had an increased risk of wheezing disorders with an unadjusted OR of 1.71 (95% CI 1.57–1.87) compared with term-born children. Very preterm-born children <32 weeks’ gestation had an unadjusted OR of 3.00 (95% CI 2.61–3.44). Asthma is traditionally regarded as being associated with atopy. However, there is debate as to whether or not preterm-born children have higher rates of atopy. One study reported that preterm-born children had lower rates of atopy than term-born children [23]. Interestingly, in atopic preterm-born children, prematurity had a strong association with asthma (adjusted OR 4.7, 95% CI 1.5–14.3); however, this association was not observed in non-atopic preterm-born children [24]. By contrast, Edwards et al. [25] noted that respiratory symptoms increased with increasing prematurity, but the risk of wheeze in preterm-born subjects was independent of family history of atopy. These observations suggest that symptoms in preterm-born subjects are likely to be a separate disease entity to asthma.
Late-preterm born infants were traditionally regarded as having no greater risk of long-term lung function deficits than term-born infants, despite having an increased incidence of respiratory conditions in the neonatal period [26]; thus, longer term outcomes have not been well studied in this group. However, recently, increasing numbers of studies are examining the longer term respiratory outcomes of late preterm-born children. The systematic review by Been et al. [4], which investigated the respiratory symptom outcomes after preterm birth, also separately reported that preterm-born children born at 32–36 weeks’ gestation had an increased risk of wheezing with an unadjusted OR 1.49 (95% CI, 1.34–1.66) compared with term-born children. Harju et al. [27] also noted that late preterm-born children, born at 33–36 weeks’ gestation, had a higher adjusted OR 1.7 (95% CI 1.4–2.0) of developing asthma compared with children born at 39–40 weeks’ gestation. In a large, UK population study, Boyle et al. [28] studied 3- and 5-year-old children born at 32–33 and 34–36 weeks’ gestation and reported that they had an increased odds ratio of wheezing or whistling in the chest in the past 12 months compared with children born at 39–41 weeks’ gestation. In addition, at 5 years of age children born at 32–33 and 34–36 weeks’ gestation had increased odds ratios of being prescribed asthma drugs [28]. Edwards et al. [25] also confirmed there was a gradient of increasing rates of wheezing at any time in the child’s life with decreasing length of gestation in preterm born children; the children born at 33–34 and 35–36 weeks’ gestation had higher rates of wheezing at any time compared with term-born children.
At 8–11 years of age, male sex was a significant risk factor for the development of asthma in preterm-born children born at <37 weeks’ gestation [29]. In a follow-up of their longitudinal cohort study, Vrijlandt et al. [30] specifically investigated sex differences in respiratory symptoms in preterm-born subjects born at ≤32 weeks’ gestation at 19 years of age. Asthma, wheezing and dyspnoea during exercise were noted to be higher in ex-preterms when compared with term-born controls; however, the effects were more pronounced in women. The rates of asthma were higher in preterm-born females compared with term-born female controls, 13% versus 5% (p<0.001), respectively; the rate for preterm-born males versus controls was 9% versus 4% (p=0.007). Preterm-born women with BPD had an even higher rate of asthma than controls (24% versus 5%; p<0.001). Rates for preterm-born men with BPD compared with controls were 8% and 4%, respectively (p=0.28) [30].
Lung function outcomes
Many studies have investigated the short- and long-term lung function outcomes of preterm birth in studies of varying sizes over a number of decades. In addition, there is disparity in the demographics of the preterm-born infants studied and the reporting of respiratory outcomes.
Stocks et al. [31] reported the effect of sex on lung function in infancy. The results suggested that male sex led to lower lung function in healthy preterm-born infants born at <37 weeks’ gestation when compared with healthy preterm-born female infants [31]. In agreement, Bentsen et al. [32] also reported that male sex was associated with lower lung function at discharge or term equivalent age in extremely preterm-born infants born at <28 weeks’ gestation. Lung function in the first year of life has also been studied in preterm-born infants whose mothers did and did not have chorioamnionitis. Overall, female infants had higher lung function results compared with male infants. However, female infants had a significant reduction of their lung function if they had been exposed to chorioamnionitis compared with female infants who were not exposed to chorioamnionitis. This was not the case for male infants where the findings were similar, irrespective of chorioaminionitis (figure 3) [33]. This is interesting as there is a suggestion that exposure to chorioaminionitis leads to greater respiratory morbidity [34].
Figure 3.
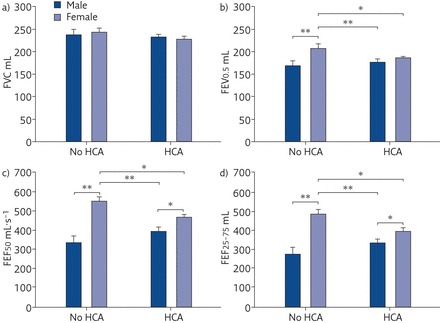
Lung function adjusted for body length and gestational age in male and female premature infants. Data are presented as the mean±sd. FVC: forced vital capacity; FEV0.5: forced expiratory volume in 0.5 s; FEF50: forced expiratory flow at 50% of FVC; FEF25–75: forced expiratory flow at 25–75% of FVC; HCA: histological chorioamnionitis. *: p<0.05; **: p<0.01. Reproduced from [33] with permission from the publisher.
The EPICure study of extremely preterm-born infants at <26 weeks’ gestation concluded that at 11 years of age the preterm-born children have significantly lower lung function than term-born controls. The preterm-born children who had BPD had the worst lung function results [35]. Doyle et al. [36] reported that extremely preterm-born children have airway obstruction at 8 and 18 years of age compared with term-born controls and that the airway obstruction increases with age. At particular risk, were young preterm-born adults who smoked or who had BPD in infancy [36].
We have collated all the available evidence in the most comprehensive systematic review, to date, to identify deficits in forced expiratory volume in 1 s (FEV1) in both preterm-born subjects with and without BPD in comparison to term-born subjects [3]. The mean difference (95% CI) for predicted per cent FEV1 (%FEV1) deficits observed was −7.2% (−8.7% to −5.6%) for the preterm-born group without BPD in infancy when compared with term-born controls. For the preterm-born subjects with BPD, defined as either supplemental oxygen dependency at 28 days or as supplemental oxygen dependency at 36 weeks’ post-menstrual age, the differences for %FEV1 were −16.2% (−19.9% to −12.4%) and −18.9% (−21.1% to −16.7%), respectively. Perhaps of most interest was the data showing that preterm-born survivors with BPD (supplemental oxygen dependency at 28 days) had improvement in %FEV1 over the decades (figure 4). In agreement, Vollsaeter et al. [37] reported that 11-year-old children born at <28 weeks’ gestation or <1000 g in two separate cohorts, one in the early 1990s and one in the late 1990s to early 2000s, had deficits in lung function in both cohorts, but the deficits in general were lower in the more recent cohort especially for preterm-born children with BPD. By contrast, Doyle et al. [10] studied extremely preterm-born children at 8 years of age born in the three time periods 1991/92, 1997, and 2005 in Australia. Despite the changes in medical management over time, there were no significant improvements in lung function [10]. There is need for longitudinal studies to report the respiratory outcomes of cohorts of preterm-born subjects from infancy through to later life.
Figure 4.
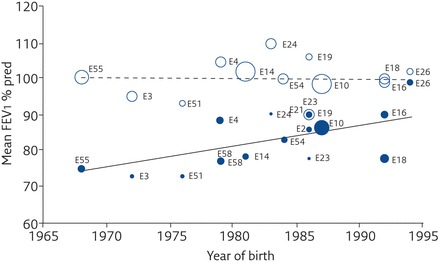
Effect of year of birth on percentage predicted FEV1 for the BPD group with supplemental oxygen dependency at 28 days (closed circles) and the term control group (open circles). Reproduced from [3] with permission from the publisher.
Deficits in lung function have been observed in late preterm-born children from infancy to adolescence. At term equivalent age, late preterm infants, born at 33–36 weeks’ gestation, were compared to term controls and lower respiratory compliance and expiratory flow ratio were noted [38]. Er et al. [39], using impulse oscillometry, concluded that 3–7-year-old children born at 34–36 weeks’ gestation have evidence of peripheral airway obstruction when compared with term-born controls. Todisco et al. [40] studied children, aged 11.6 years, who were born at 34–36 weeks’ gestation and who were not ventilated and did not have RDS in the neonatal period. Residual volume and residual volume/total lung capacity in the late preterm-born children were significantly increased when compared with their term-born siblings [40]. We reported that children, of 8–9 years of age, born at 33–34 weeks’ gestation have similar lung function deficits to children born at ≤32 weeks’ gestation, but improvements were seen in the lung function of the 33–34 weeks’ gestation group by 14–17 years of age [6]. By contrast, Thunqvist et al. [41], studied children who were born at 32–36 weeks’ gestation at eight and again at 16 years of age concluding that there was no improvement in lung function between the two ages. Interestingly, deficits were observed in FEV1 at 8 years of age in preterm-born girls when compared to term-born girls, but not between preterm-born boys and term-born boys. However, deficits in FEV1 were seen in both boys and girls at 16 years of age in comparison to term-born controls [41].
Conclusion
Male sex appears to increase the risk (due to greater hospital admissions for respiratory reasons, greater incidence of BPD, etc.) for later adverse respiratory illness. The physiological basis is unclear, but has somewhat been explained through comparison of airway growth between the sexes and the phenomenon known as dysynapsis. In boys, growth of the airways lags behind that of the lung parenchyma and introduces a disparity between airway calibre and lung size relative to girls. However, not all studies report that males have worse long-term respiratory outcomes than females. Puberty may be one contributing factor in the observed changes in lung function and symptomology during the teenage years, when females appear to fare worse.
Summary
In conclusion, being born very preterm is associated with later deficits in lung function, and those who had BPD in infancy have larger deficits in their lung function in later life. Respiratory symptoms are also increased after preterm birth compared with term-born children, the odds ratios of symptoms increase with increasing prematurity. The rates of early respiratory infections are higher in very preterm-born subjects, which may independently lead to deficits in lung function in later life. As with very preterm-born children, deficits in lung function, increased respiratory symptoms and an increased risk of respiratory infections in early life are observed in late preterm-born children. However, the rates of respiratory symptoms are lower compared with very preterm-born children. Nonetheless, late-preterm born children should be regarded as being at risk of long-term respiratory symptoms and deficits in lung function. There is some evidence to suggest that respiratory outcomes may be improving over time, although not all the evidence suggests improvements. Most of these children and adults are not followed up routinely, and worryingly, most physicians treating adults with respiratory illnesses do not routinely ask about early life factors, such as prematurity and neonatal admissions, which may impact on later outcomes [42]. Male sex appears to increase the risk (due to greater hospital admissions for respiratory reasons, greater incidence of BPD, etc.) for later adverse respiratory illness. The physiological basis is unclear, but has somewhat been explained through comparison of airway growth between sexes and the phenomenon known as dysynapsis. However, not all studies report that males have worse long-term respiratory outcomes than females. It is essential that preterm-born infants are followed up into childhood and beyond, and that appropriate treatment of any lung function deficits and respiratory symptoms is prescribed if necessary. If these, very preterm-born infants, progress to develop chronic obstructive airway disease in later life then the impact, not only on the individuals, but also the economic impact on healthcare services, is immense. In view of the larger number of late preterm-born infants born every year, this is a concern as they will place an unrecognised burden on health services throughout the world.
Self-evaluation questions
- All-cause mortality over the first 18 years of life is:
- a) Is higher in females than males
- b) Is no different between the sexes
- c) Is higher in males than females
- d) Is higher in males than females, but differences attenuate over time
- Which of the following is/are correct concerning preterm-born males during infancy?
- a) They have a higher rate of mortality and morbidity than preterm-born females
- b) They are more prone to hospital readmissions than preterm-born females
- c) They have a lower rate of respiratory distress syndrome than preterm-born females
- Regarding respiratory symptoms experienced by preterm-born children, which of the following is/are correct?
- a) These are increased in the most immature of preterm-born infants only
- b) Rates are higher in males than females
- c) The symptoms are independent of atopy
- Regarding respiratory and lung function outcomes of preterm-born subjects, which of the following is/are correct?
- a) Sex differences are not evident in the first years of life
- b) Contemporary cohorts have lower deficits in lung function when compared with historic cohorts
- c) One explanation for higher prevalence of symptoms in males is differential growth in lung tissue when compared to airway size
- d) There is strong evidence that the sex divide persists through into adulthood
Suggested answers
1. c.
2. a and b.
3. b and c.
4. b and c.
Footnotes
Conflict of interest: S.J. Kotecha has nothing to disclose.
Conflict of interest: J. Lowe has nothing to disclose.
Conflict of interest: S. Kotecha reports grants from the MRC, the NIHR HTA, the Welsh Government, and EU FP7, all outside the submitted work.
References
- 1.Tielsch JM. Global Incidence of Preterm Birth. Nestle Nutr Inst Workshop Ser 2015; 81: 9–15. [DOI] [PubMed] [Google Scholar]
- 2.Office for National Statistics Birth characteristics in England and Wales: 2016. Date last accessed: January 2018. Date last updated: October 16, 2017.
- 3.Kotecha SJ, Edwards MO, Watkins WJ, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax 2013; 68: 760–766. [DOI] [PubMed] [Google Scholar]
- 4.Been JV, Lugtenberg MJ, Smets E, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med 2014; 11: e1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotecha SJ, Dunstan FD, Kotecha S. Long term respiratory outcomes of late preterm-born infants. Semin Fetal Neonatal Med 2012; 17: 77–81. [DOI] [PubMed] [Google Scholar]
- 6.Kotecha SJ, Watkins WJ, Paranjothy S, et al. Effect of late preterm birth on longitudinal lung spirometry in school age children and adolescents. Thorax 2012; 67: 54–61. [DOI] [PubMed] [Google Scholar]
- 7.Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 2015; 314: 1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santhakumaran S, Statnikov Y, Gray D, et al. Survival of very preterm infants admitted to neonatal care in England 2008–2014: time trends and regional variation. Arch Dis Child Fetal Neonatal Ed 2018; 103: F208–F215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zysman-Colman Z, Tremblay GM, Bandeali S, et al. Bronchopulmonary dysplasia – trends over three decades. Paediatr Child Health 2013; 18: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle LW, Carse E, Adams AM, et al. Ventilation in extremely preterm infants and respiratory function at 8 years. N Engl J Med 2017; 377: 329–337. [DOI] [PubMed] [Google Scholar]
- 11.Watkins WJ, Kotecha SJ, Kotecha S. All-cause mortality of low birthweight infants in infancy, childhood, and adolescence: population study of England and Wales. PLoS Med 2016; 13: e1002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito M, Tamura M, Namba F. Role of sex in morbidity and mortality of very premature neonates. Pediatr Int 2017; 59: 898–905. [DOI] [PubMed] [Google Scholar]
- 13.Liptzin DR, Landau LI, Taussig LM. Sex and the lung: observations, hypotheses, and future directions. Pediatr Pulmonol 2015; 50: 1159–1169. [DOI] [PubMed] [Google Scholar]
- 14.Collaco JM, Aherrera AD, McGrath-Morrow SA. The influence of gender on respiratory outcomes in children with bronchopulmonary dysplasia during the first 3 years of life. Pediatr Pulmonol 2017; 52: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutkowska M, Hozejowski R, Helwich E, et al. Severe bronchopulmonary dysplasia – incidence and predictive factors in a prospective, multicenter study in very preterm infants with respiratory distress syndrome. J Matern Fetal Neonatal Med 2018; in press [ 10.1080/14767058.2017.1422711]. [DOI] [PubMed] [Google Scholar]
- 16.Greenough A, Cox S, Alexander J, et al. Health care utilisation of infants with chronic lung disease, related to hospitalisation for RSV infection. Arch Dis Child 2001; 85: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenough A, Alexander J, Boit P, et al. School age outcome of hospitalisation with respiratory syncytial virus infection of prematurely born infants. Thorax 2009; 64: 490–495. [DOI] [PubMed] [Google Scholar]
- 18.Barker DJ, Godfrey KM, Fall C, et al. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 1991; 303: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haataja P, Korhonen P, Ojala R, et al. Hospital admissions for lower respiratory tract infections in children born moderately/late preterm. Pediatr Pulmonol 2018; 53: 209–217. [DOI] [PubMed] [Google Scholar]
- 20.Haataja P, Korhonen P, Ojala R, et al. Asthma and atopic dermatitis in children born moderately and late preterm. Eur J Pediatr 2016; 175: 799–808. [DOI] [PubMed] [Google Scholar]
- 21.Paranjothy S, Dunstan F, Watkins WJ, et al. Gestational age, birth weight, and risk of respiratory hospital admission in childhood. Pediatrics 2013; 132: e1562–e1569. [DOI] [PubMed] [Google Scholar]
- 22.Elisabeth R, Elke G, Vera N, et al. Readmission of preterm infants less than 32 weeks gestation into early childhood: does gender difference still play a role? Glob Pediatr Health 2014; 1: 2333794X14549621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siltanen M, Kajosaari M, Pohjavuori M, et al. Prematurity at birth reduces the long-term risk of atopy. J Allergy Clin Immunol 2001; 107: 229–234. [DOI] [PubMed] [Google Scholar]
- 24.Rosas-Salazar C, Ramratnam SK, Brehm JM, et al. Prematurity, atopy, and childhood asthma in Puerto Ricans. J Allergy Clin Immunol 2014; 133: 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards MO, Kotecha SJ, Lowe J, et al. Management of prematurity-associated wheeze and its association with atopy. PLoS One 2016; 11: e0155695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA 2010; 304: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harju M, Keski-Nisula L, Georgiadis L, et al. The burden of childhood asthma and late preterm and early term births. J Pediatr 2014; 164: 295–299. [DOI] [PubMed] [Google Scholar]
- 28.Boyle EM, Poulsen G, Field DJ, et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ 2012; 344: e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grischkan J, Storfer-Isser A, Rosen CL, et al. Variation in childhood asthma among former preterm infants. J Pediatr 2004; 144: 321–326. [DOI] [PubMed] [Google Scholar]
- 30.Vrijlandt EJ, Gerritsen J, Boezen HM, et al. Gender differences in respiratory symptoms in 19-year-old adults born preterm. Respir Res 2005; 6: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stocks J, Henschen M, Hoo AF, et al. Influence of ethnicity and gender on airway function in preterm infants. Am J Respir Crit Care Med 1997; 156: 1855–1862. [DOI] [PubMed] [Google Scholar]
- 32.Bentsen MH, Markestad T, Oymar K, et al. Lung function at term in extremely preterm-born infants: a regional prospective cohort study. BMJ Open 2017; 7: e016868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones MH, Corso AL, Tepper RS, et al. Chorioamnionitis and subsequent lung function in preterm infants. PLoS One 2013; 8: e81193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartling L, Liang Y, Lacaze-Masmonteil T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2012; 97: F8–F17. [DOI] [PubMed] [Google Scholar]
- 35.Bolton CE, Stocks J, Hennessy E, et al. The EPICure study: association between hemodynamics and lung function at 11 years after extremely preterm birth. J Pediatr 2012; 161: 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doyle LW, Adams AM, Robertson C, et al. Increasing airway obstruction from 8 to 18 years in extremely preterm/low-birthweight survivors born in the surfactant era. Thorax 2017; 72: 712–719. [DOI] [PubMed] [Google Scholar]
- 37.Vollsaeter M, Skromme K, Satrell E, et al. Children born preterm at the turn of the millennium had better lung function than children born similarly preterm in the early 1990s. PLoS One 2015; 10: e0144243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McEvoy C, Venigalla S, Schilling D, et al. Respiratory function in healthy late preterm infants delivered at 33-36 weeks of gestation. J Pediatr 2013; 162: 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Er I, Gunlemez A, Uyan ZS, et al. Evaluation of lung function on impulse oscillometry in preschool children born late preterm. Pediatr Int 2016; 58: 274–278. [DOI] [PubMed] [Google Scholar]
- 40.Todisco T, de Benedictis FM, Iannacci L, et al. Mild prematurity and respiratory functions. Eur J Pediatr 1993; 152: 55–58. [DOI] [PubMed] [Google Scholar]
- 41.Thunqvist P, Gustafsson PM, Schultz ES, et al. Lung function at 8 and 16 years after moderate-to-late preterm birth: a prospective cohort study. Pediatrics 2016; 137: e20152056. [DOI] [PubMed] [Google Scholar]
- 42.Bolton CE, Bush A, Hurst JR, et al. Are early life factors considered when managing respiratory disease? A British Thoracic Society survey of current practice. Thorax 2012; 67: 1110. [DOI] [PubMed] [Google Scholar]


