Summary
Genetic investigations of Sjögren's syndrome (SS) have identified a susceptibility locus at p23.3 of chromosome 11, which contains the CXCR5 gene. C‐X‐C motif chemokine receptor 5 (CXCR5) is a chemokine receptor expressed on B and T cell subsets, and binds the chemotactic ligand C‐X‐C motif chemokine ligand 13 (CXCL13). In this study we aimed to link the genetic association with functional effects and explore the CXCR5/CXCL13 axis in SS. Expression quantitative trait loci analysis of the 11q23.3 locus was performed using B cell mRNA expression data from genotyped individuals. Lymphocyte surface markers were assessed by flow cytometry, and CXCL13 levels by a proximity extension assay. CXCR5+ and CXCL13+ cells in minor salivary glands were detected using immunohistochemistry. Our results demonstrated that SS‐associated genetic polymorphisms affected the expression of CXCR5 (P < 0·01). Notably, a decreased percentage of CXCR5+ cells, with lower CXCR5 expression, was observed for most circulating B and T cell subsets in SS patients, reaching statistical significance in CD19+CD27+immunoglobulin (Ig)D+ marginal zone (P < 0·001), CD19+CD27+IgD– memory (P < 0·05) and CD27‐IgD double‐negative (P < 0·01) B cells and CD4+CXCR3–CCR6+ Th17 cells (P < 0·05). CXCL13 levels were increased in patient plasma (P < 0·001), and immunohistochemical staining revealed expression of CXCL13 and higher numbers of CXCR5+ cells (P < 0·0001) within focal infiltrates and interstitially in salivary glands of SS patients. In conclusion, we link a genetic susceptibility allele for SS to a functional phenotype in terms of decreased CXCR5 expression. The decrease of CXCR5+ cells in circulation was also related to homing of B and T cells to the autoimmune target organ. Therapeutic drugs targeting the CXCR5/CXCL13 axis may be useful in SS.
Keywords: B cells, CXCR5, eQTL, Sjögren's syndrome, T cells
Introduction
Sjögren's syndrome (SS) is a systemic rheumatic autoimmune disease affecting exocrine glandular function, in which the salivary and lacrimal glands are the primary sites of inflammation 1. Here, focal mononuclear cell infiltration is seen in the glandular tissue, leading to glandular dysfunction and the common symptoms of dry mouth and dry eyes. Interestingly, the infiltrating cells in the salivary gland tissue may organize into B and T cell areas (zones) 2. This can, in turn, result in the formation of tertiary lymphoid structures, referred to as ectopic germinal centre (GC)‐like structures 3, 4, 5, 6. Structurally, these ectopic GCs in the salivary gland appear similar to conventional GCs observed in secondary lymphoid organs 7, and have been associated with an increased risk for developing non‐Hodgkin's lymphoma in SS 8. Patients with SS also have major disturbances in peripheral blood B cell homeostasis 9, 10, where elevated numbers of CD27– naive B cells coincide with diminished levels of CD27+ memory B cells in circulation 11.
SS is considered a multi‐factorial disease of mostly unknown aetiology, but where genetics play a central role. Genomewide association studies (GWAS) have been conducted, allowing the identification of a growing number of single nucleotide polymorphisms (SNPs) associated with disease susceptibility 12, 13, 14, 15. Several of the associated SNPs have been identified as expression quantitative trait loci (eQTLs), and considered likely to account for a substantial part of the genetic effects contributing to disease 16, 17. Previously identified genes for which SS‐linked genetic regulation of gene expression has been described include interferon (IFN) regulatory factor 5 (IRF5), interleukin (IL) 12A and tumour necrosis factor (TNF)AIP3 interacting protein 1(TNIP1), among other genes that are involved in both innate and adaptive immune responses 12.
C‐X‐C motif chemokine receptor 5 (CXCR5) is a chemokine receptor expressed on T follicular helper (Tfh) cells and B cells 18. It binds C‐X‐C motif chemokine ligand 13 (CXCL13), a ligand secreted by cells residing in lymphoid tissue, including follicular dendritic cells 4, 19, and contributes to the recruitment of naive B cells into the lymph nodes 20, 21. The CXCR5–CXCL13 interaction plays a central role in cell migration and GC reactions 22. In the follicles, Tfh cells provide survival signals to GC B cells via multiple pathways, including inducible T cell co‐stimulator (ICOS), programmed cell death protein 1 (PD‐1) and B cell activating factor (BAFF), which compete with Fas–FasL interactions 23, 24, 25, 26.
Polymorphisms close to the CXCR5 locus were recently linked genetically to SS 12. In this study we sought to identify functional impacts of the polymorphisms on CXCR5 expression. We therefore explored the expression pattern of the CXCR5–CXCL13 axis in SS in peripheral blood and minor salivary gland biopsies to gain a deeper understanding of the impact of these disease‐associated polymorphisms in the patients.
Materials and methods
Study population
The study included 53 patients with primary SS (pSS) and 35 controls. All patients fulfilled the American–European consensus group criteria (AECC) for pSS 27 and were diagnosed at the Rheumatology clinic at the Karolinska University Hospital, Stockholm, Sweden between 2007 and 2015. Peripheral blood was obtained from 15 patients without any immunomodulatory treatment and 15 age‐ and gender‐matched healthy donors. Genotype data generated on the Illumina OmniExpressExome‐8 version 1.4 chip (Illumina, San Diego, CA, USA) were available for 13 of the patients. Genotype quality control at the sample level was performed as described previously 28, and SNP probes fulfilling ≥ 98% call rate, a Hardy–Weinberg equilibrium test P > 1 × 10−4 and a minor allele frequency (MAF) of ≥ 1% were included in the quality‐controlled data set. After quality control, 12 patients remained for analysis. Minor salivary glands (MSG) from 38 patients were also included into the study. Twenty MSG biopsies from subjects evaluated for SS during the same period at the same department, but not fulfilling the criteria for primary or secondary SS, served as non‐SS tissue controls.
Clinical data were obtained from medical records, including focus score, anti‐Ro/SSA and anti‐La/SSB, summarized in Table 1. The Regional Ethics Committee approved the study and all participants gave written informed consent.
Table 1.
Clinical characteristics of pSS patients and controls included in the study
| Flow cytometry and PEA | Immunohistochemistry | |||
|---|---|---|---|---|
| pSS patients | Healthy donors | pSS patients | Non‐SS | |
| Individuals included | n = 15 | n = 15 | n = 38 | n = 20 |
| Gender (female/male) | 15/0 | 15/0 | 27/11 | 20/0 |
| Mean age (years) | 56·9 years | 48·9 years | 59·9 years | 59·2 years |
| Ro/SSA | 15/15 (100%) | 0/15 | 32/38 (84%) | 0/20 |
| La/SSB | 12/15 (80%) | 0/15 | 20/38 (53%) | 0/20 |
| Mean focus score | 4·0 | – | 4·0 | 0 |
| GC formation | – | – | 18/38 (47%) | 0 |
| EGM | 4/15 (27%) | – | – | – |
PEA = proximity extension assay; GC = germinal centre; EGM = extra‐glandular manifestations [included lymphadenopathy and minor salivary glands (MSG) swelling]; pSS = primary Sjögren's syndrome.
Gene expression array data and CXCR5 eQTL analysis
Microarray‐based CD19+ B cell mRNA expression and genotype data from 287 healthy subjects (age range = 18–62 years; median age = 33·1 years) were obtained from a study published by Fairfax et al. 29. Detailed sampling and laboratory procedures have been described elsewhere 29. In short, the Illumina HumanHT‐12 version 4 BeadChip gene expression array platform was used for total RNA quantification, and the expression data were obtained as log2‐transformed values. The Illumina Human OmniExpress‐12 version 1.0 BeadChips, NCBI36 Build, were used for genomic DNA genotyping. Upon granted access, genotype data were downloaded from the European Genome–Phenome Archive as AGCT‐coded SNP data.
A polymorphism of the 11q23.3 locus analysed was chosen from the current literature of genetic association studies in SS, with the criterion of having the lowest P‐value for association with SS of those reaching genome‐wide significance (P < 5·0 × 10−8) 12. Proxy SNPs with high linkage disequilibrium (LD) (r 2 > 0·8) were identified using the SNAP Proxy search tool (SNP data set: 1000 Genomes Pilot 1; population panel: Caucasian (CEU); r 2 threshold = 0·8; distance limit = 500) 30.
The eQTL analysis was performed using the MatrixEQTL R package 31, employing an additive model.
Isolation of peripheral blood mononuclear cells (PBMCs) and plasma collection
Blood samples from patients and controls were collected in 10 ml ethylenediamine tetraacetic acid (EDTA) vacutainer tubes, centrifuged at 1440 g for 10 min at room temperature (RT), and plasma was removed and stored at −80°C. For PMBC isolation, 10 ml EDTA blood was diluted 1 : 2 in sterile phosphate‐buffered saline (PBS) (Sigma‐Aldrich, Munich, Germany) and transferred to 50 ml Sepmate tubes (Stemcell Technologies, Cambridge, UK) containing 15 ml of Ficoll (GE Healthcare Life Sciences, Uppsala, Sweden). The tubes were centrifuged at 1200 g for 15 min at room temperature, without break, and the upper phase was collected. PBMCs were then spun down for 7 min at 440 g and washed twice with PBS. To eliminate remaining erythrocytes, PBMCs were incubated with 10 ml lysis buffer (Becton, Dickinson, Franklin Lakes, NJ, USA) for 10 min at RT. Following washing twice with PBS, the PBMC pellet was dissolved in 2 ml PBS containing 2% fetal bovine serum.
Surface and intracellular staining and flow cytometry
For flow cytometry experiments, 1 × 106 PBMCs were labelled using the LIVE DEAD fixable IR Dead Cell Stain Kit (Invitrogen, Carlsbad, CA, USA) and stained further with fluorescent antibodies. For detection of CXCR5 expression on B cell subsets, PBMCs (106 cells per tube) were stained with allophycocyanin (APC)‐R700‐CXCR5 in combination with fluorescein isothiocyanate (FITC)‐CD5, phycoerythrin (PE)‐immunoglobulin D (IgD), PE‐cyanin 5 (Cy5)‐human leucocyte antigen D‐related (HLA‐DR), PE‐Cy7‐CD27, APC‐Cy7‐CD19 and PE‐CF594‐CD14. For detection of CXCR5 expression on T cell subsets, PBMCs (106 cells per tube) were stained with APC‐R700‐CXCR5 in combination with FITC‐CXCR3, PE‐Cy7‐CD4, APC‐CCR6, PB‐CD3, PE‐CF594‐CD14, APC‐PD‐1 and BB515‐ICOS. All antibodies were obtained from Becton Dickinson, except for PE‐Cy7‐CD4 and PB‐CD3, which were purchased from BioLegend (San Diego, CA, USA) (overview of antibodies in Supporting information, Table S1). The staining was performed for 30 min on ice in the dark. After washing with PBS, the cells were subjected to flow cytometry using a Gallios flow cytometer (Beckman Coulter, Brea, CA, USA) and analysed using FlowJo software version 10.3 (Tree Star, Inc., Ashland, OR, USA). At least 50 000 events per sample were analysed (gating strategy in Supporting information, Figs S1–S3).
Proximity extension assay
CXCL13 protein levels were measured in plasma samples using the proximity extension assay based on recognition of the target protein by two independent oligonucleotide‐labelled antibodies and a subsequent real‐time PCR‐amplification step (Olink Bioscience, Uppsala, Sweden), with the assay performed at the core clinical biomarkers facility (Science for Life Laboratory, Uppsala, Sweden), as described previously 32, 33. The data are presented as log2‐transformed arbitrary units (normalized protein expression, NPX).
Immunohistochemistry
Formalin‐fixed, paraffin‐embedded MSG biopsies obtained at patient diagnosis/evaluation were sectioned using a microtome (4–6 μm). The sections were placed on SuperFrost® Plus microscope slides (Fisher Scientific, Waltham, MA, USA) and incubated overnight at 56°C. This was followed by deparaffinization in xylene (Sigma‐Aldrich), and rehydration through a graded ethanol series (100, 96, 70%) and PBS (Sigma‐Aldrich). The sections were then subjected to epitope retrieval with citrate buffer (pH 6·0) (Dako, Carpinteria, CA, USA) at 98°C for 30 min, thereafter allowing the slides to cool, and washing in water for 5 min.
The EnVision system (Dako) was used to stain the sections for CXCR5 (R&D Systems, Minneapolis, MN, USA) and CXCL13 (R&D Systems), respectively, following the manufacturer's protocol. In brief, endogenous peroxidase activity was blocked using endogenous enzyme block for 10 min. Primary antibody CXCR5 (1 : 100, 10 μg/ml) or CXCL13 (1 : 10, 100 μg/ml) in antibody diluent (Dako) were added to the sections and incubated for 60 min in a humidified chamber. This was followed by incubation with horseradish peroxidase (HRP)‐conjugated anti‐mouse Envision secondary antibody (Dako) for 30 min. Thereafter, sections were incubated for 5–10 min with diaminobenzidine (DAB) (Dako). All incubations were performed at RT, and Tris‐buffered saline (TBS) containing 0·1% Tween was used as washing buffer (pH 7·6) between each step for 10 min. Finally, the sections were counterstained with Mayer's haematoxylin and mounted under coverslips using Mountex (HistoLab, Gothenburg, Sweden).
Evaluation of staining
The MSG sections were analysed by four investigators (K.S., J.R., L.A.A., M.W.H.). Both mononuclear cells in focal infiltrates and those located interstitially, i.e. in close proximity to the acinar or ductal epithelium, were examined. Morphometry was applied using a Polyvar II light microscope (Reichert‐Jung, Vienna, Austria) and the QWin Digital Image Analysis Software version 3 (Leica, Wetzlar, Germany) to calculate the ratio‐index of tissue stained in each gland. The ratio‐index, defined as the total stained area in the section divided by the total glandular tissue area, provides more accurate information on inflammation severity and staining pattern for each patient in addition to the focus score 34.
Statistical analysis
The Mann–Whitney U‐test was used for comparing two groups (non‐normal distribution of values). An additive model (0‐1‐2) was employed for the eQTL analysis. Spearman's non‐parametric correlation test was used to examine correlation between parameters. All calculations were performed using Prism 5 (GraphPad). A P‐value < 0·05 was considered significant.
Results
Carrying the CXCR5 allele with risk for SS coincides with decreased CXCR5 gene expression in B cells
To link the SS‐risk genetic polymorphisms of the q23.3 locus on chromosome 11 with functional outcome in terms of specific gene expression, we first performed an eQTL analysis for expression of the CXCR5 gene in the locus. As CXCR5 is expressed in most B cells 21, we based the analysis on gene expression of CXCR5 in purified CD19+ B cells in genotyped healthy individuals 29. Using the proxy SNP rs4938573 in high LD (r 2 = 0·865) with the top SS‐associated SNP rs7119038 12, which was not available in the data set, we observed a significantly decreased CXCR5 gene expression for the disease risk allele T (additive model, P < 0·01) (Fig. 1). These data associate the SS‐risk allele with a lower expression of CXCR5 in B cells.
Figure 1.
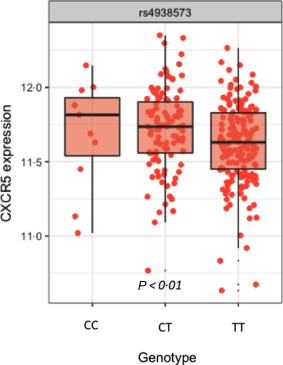
Carrying the disease risk allele for Sjögren's syndrome (SS) is associated with decreased C‐X‐C motif chemokine receptor 5 (CXCR5) gene expression. An expression quantitative trait loci (eQTL) analysis of microarray‐based CD19+ B cell mRNA expression and genotype data from 287 healthy subjects with regard to the disease risk allele T of rs4938573 in an additive model (0‐1‐2) demonstrates a significantly decreased CXCR5 gene expression (P < 0·01).
Decreased percentage of CXCR5+ B and T cell subsets as well as CXCR5 B cell surface expression in peripheral blood of SS patients
To determine the frequency of CXCR5+ B and T cell subsets and obtain semi‐quantitative values for the CXCR5 cell surface expression of CXCR5+ cells, flow cytometry was performed on PBMCs from patients with SS (n = 15) and sex‐ and age‐matched healthy donors (n = 15). Two samples originating from patients with SS were excluded due to low cell numbers, leaving 13 samples for analysis. Our analyses demonstrate that although the overall frequency of CD19+CD5– B cells was not different between patients and controls, there was a significant decrease in the percentage of CXCR5+ B cells (P < 0·05) (Fig. 2a). While there were no significant differences with regard to CXCR5+ expression in the CD19+CD27–IgD+ naive B cell population (Fig. 2b), a decrease in CXCR5+ frequency among CD19+CD27+IgD– memory B cells (P < 0·03) as well as a decrease in the cell surface expression of CXCR5 in the CXCR5+ memory B cells was observed (P < 0·05) (Fig. 2c). The same pattern was observed in the CD19+CD27+IgD+ marginal zone (MZ) B cells (P < 0·001) (Fig. 2d). Also, in CD27–IgD– double‐negative (DN) B cells, representing several small B cell populations 35, a decrease in circulating CXCR5+ cells (P < 0·01) and decreased expression levels of CXCR5 were noted in patients with SS (P < 0·05) (Fig. 2e).
Figure 2.
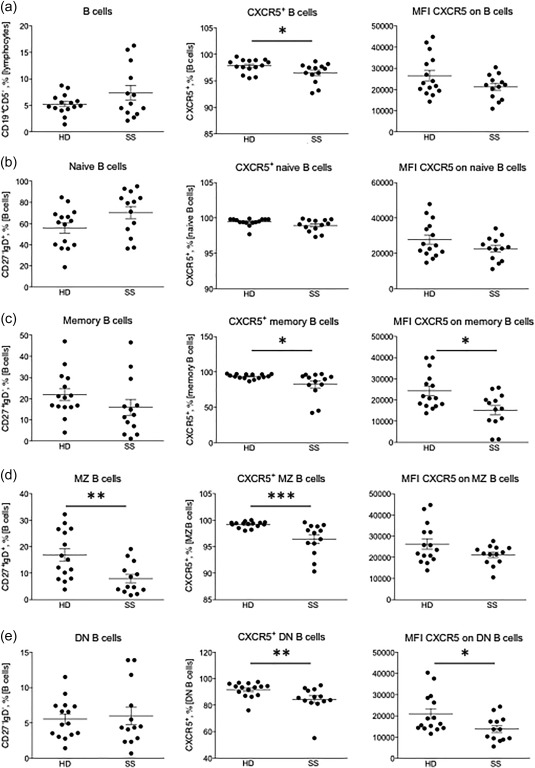
Decreased percentage and surface expression of C‐X‐C motif chemokine receptor 5 (CXCR5)+ B cells in peripheral blood of Sjögren's syndrome (SS) patients. (a) From left to right: the frequency of CD19+CD5– B cells, percentage of CXCR5+ B cells and CXCR5 cell surface expression on B cells. (b) From left to right: the frequency of CD27–immunoglobulin (Ig)D+ naive B cells, percentage of CXCR5+ naive B cells and CXCR5 expression on naive B cells. (c) From left to right: the frequency of CD27+IgD– memory B cells, percentage of CXCR5+ memory B cells and CXCR5 expression on memory B cells. (d) From left to right: the frequency of CD27+IgD+ marginal zone (MZ) B cells, percentage of CXCR5+ MZ B cells and CXCR5 expression on MZ B cells. (e) From left to right: the frequency of CD27–IgD– DN B cells, percentage of CXCR5+ DN B cells and CXCR5 expression on DN B cells. HD = healthy donors; SS = Sjögren's syndrome. CXCR5 median fluorescence intensity (MFI) is based on data from CXCR5+ cells only. Significant P‐values are indicated by: *P < 0·05; **P < 0·01; ***P < 0·001. CXCR5 protein expression is presented as MFI.
When screening for the T cell subsets we observed a slight, although not significant, decrease in CD3+CD4+ T cells in the SS patient group. The percentage of CXCR5+CD4+ T cells and CXCR5 cell surface expression appeared similar in both groups (Fig. 3a). The difference in amount of CXCR5+PD‐1+ Tfh cells was not significant between patients and controls, but a subgroup of SS patients presented with increased numbers (Fig. 3b). Moreover, a significantly decreased frequency of CXCR5+CD4+CXCR3–CCR6+ Th17 cells was observed in the patients (P < 0·05) (Fig. 3c).
Figure 3.
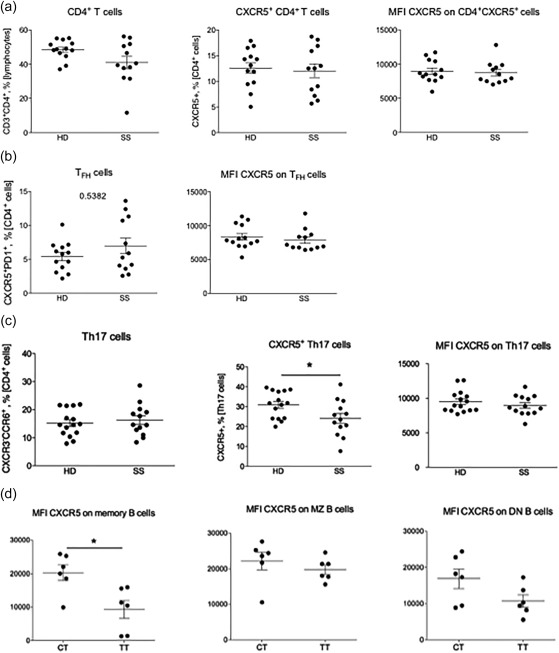
Low frequency and surface expression of C‐X‐C motif chemokine receptor 5 (CXCR5)+ T cells in peripheral blood of Sjögren's syndrome (SS) patients. (a) From left to right: the frequency of CD3+CD4+ T cells, percentage of CXCR5+ CD4+ T cells and CXCR5 cell surface expression on CD4+ T cells. (b) From left to right: the frequency of CXCR5+programmed death 1 (PD‐1)+ T follicular helper cells and CXCR5 cell surface expression on T follicular helper cells. (c) From left to right: the frequency of CXCR3–CCR6+ T helper type 17 (Th17) cells, CXCR5 cell surface expression on Th17 cells and CXCR5 cell surface expression on Th17 cells. (d) From left to right: CXCR5 cell surface expression related to the T risk allele on memory B cells, marginal zone (MZ) B cells (P = 0·1) and on DN B cells (P = 0·09) in patients with SS. HD = healthy donors; SS = Sjögren's syndrome. CXCR5 median fluorescent intensity (MFI) is based on data from CXCR5+ cells only. Significant P‐values are indicated by: *P < 0·05; **P < 0·01; ***P < 0·001. CXCR5 protein expression is presented as MFI.
We next stratified the percentage of CXCR5+ cell subsets and median fluorescence intensity (MFI) according to the genotype of rs4938573 in the patients to understand if the cell surface expression related to the T risk allele. In this small material with genotype data, available for 12 of the 13 patients, no patient was homozygous for C. However, a significantly lower cell surface expression in terms of CXCR5 in CD19+CD27+IgD– memory B cells was observed in patients homozygous for the T disease risk allele. A trend towards lower CXCR5 cell surface expression on MZ and DN B cells was also observed (Fig. 3d). The B cell population frequencies did not differ between genotypes, and no significant difference was detected for the T cell subsets, either in frequencies or cell surface expression (data not shown).
In all, the frequency of CXCR5+ subsets of most B cell populations is decreased in the circulation of patients with SS, and furthermore, the cell surface expression levels of CXCR5 is lower in these cells from SS patients, with the lowest CXCR5 expression in patients homozygous for the genetic CXCR5 polymorphism associated with disease risk.
Increased CXCL13 protein expression in plasma and CXCL13‐producing cells at site of inflammation of SS patients
Soluble CXCL13 was measured in plasma, demonstrating significantly higher CXCL13 levels in patients with SS compared to healthy donors (P < 0·001) (Fig. 4a). Moreover, these elevated plasma levels of CXCL13 correlated inversely to the percentages of CXCR5+ memory B cells (Spearman's r = –0·47, P < 0·01), MZ B cells (Spearman's r = –0·60, P < 0·001) and double‐negative B cells (Spearman's r = –0·48, P < 0·01) in circulation (Fig. 4b). Hypothesizing that production of CXCL13 by tissue resident cells could give rise to the elevated plasma levels and further attract CXCR5 expressing cells to the target organ, we employed immunohistochemistry of paraffin‐embedded MSG biopsies to detect CXCL13+ cells. We observed CXCL13+ cells in the target organ of the patients with pSS, located predominantly within the focal infiltrates at the site of inflammation, but also interstitially (Fig. 4c). Here, mouse IgG1 was used as an isotype control (Fig. 4d).
Figure 4.
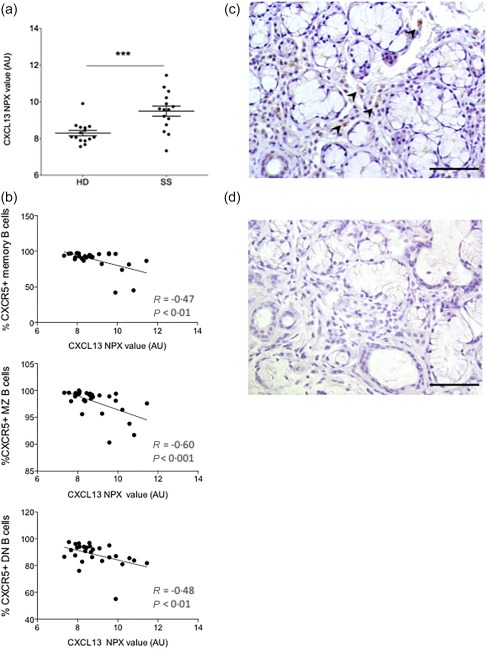
Increased C‐X‐C motif chemokine ligand 13 (CXCL13) expression in plasma of Sjögren's syndrome (SS) patients and CXCL13+ cells at site of inflammation. (a) CXCL13 protein levels in plasma of SS patients compared to healthy donors. (b) Correlation between CXCL13 levels in plasma and decrease in C‐X‐C motif chemokine receptor 5 (CXCR5)+ B cell subsets frequency (Spearman's rank correlation). (c) CXCL13+ cells in the minor salivary glands (MSG) of a patient with SS. CXCL13+ cells located predominantly to the focal infiltrates and interstitially. Scale bar 100 μm. (d) Isotype control staining of a MSG of a patient with SS. ***P < 0·001. Scale bar 100 μm.
Accumulation of CXCR5+ cells at the site of inflammation in the salivary gland target organ of SS patients
To understand whether CXCR5+ cells were indeed homing to autoimmune target organs such as the MSG, biopsies from 38 SS patients and 20 non‐SS sicca controls obtained from non‐treated individuals at diagnosis were stained by immunohistochemistry for CXCR5. We observed abundant and high numbers of CXCR5+ cells in the target organ of pSS patients (Fig. 5a–d) compared to the non‐SS sicca controls (Fig. 5e,f). The CXCR5 expressing cells were located mainly within the focal infiltrates at the site of inflammation, and also interstitially (Fig. 5a,b). Interestingly, ductal epithelial staining was also observed (Fig. 5c,d).
Figure 5.
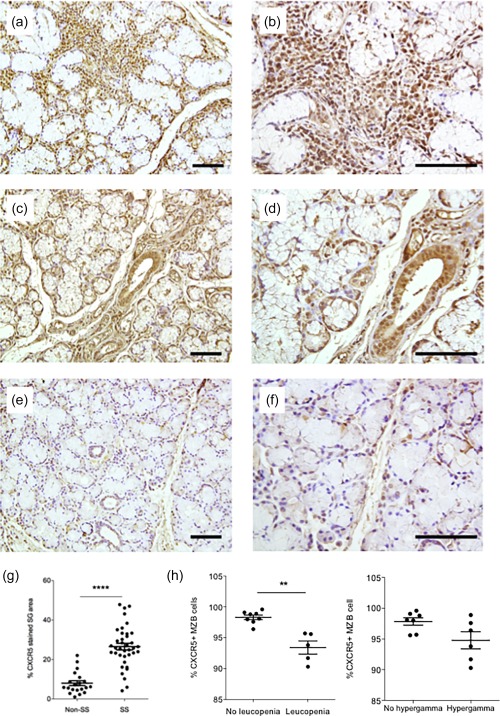
Accumulation of C‐X‐C motif chemokine receptor 5 (CXCR5)+ cells in the salivary gland target organ of Sjögren's syndrome (SS) patients. (a) Staining of CXCR5+ cells in SS minor salivary glands (MSG), visualizing staining within the focal infiltrates at the site of inflammation and showing high numbers of CXCR5+ cells. (b) Higher magnification of (a). (c) Staining of CXCR5+ cells in SS MSG, visualizing interstitial and ductal epithelial staining. (d) Higher magnification of (c). (e) Staining of CXCR5+ cells in non‐SS MSG, visualizing interstitial and ductal epithelial staining. (f) Higher magnification of (e). (g) Comparison of CXCR5+ staining in MSG of SS and non‐SS controls based on morphometry. (h) Relation of clinical parameters to frequency of circulating CXCR5+ marginal zone (MZ) B cells. **P < 0·01; ****P < 0·0001. Scale bar 100 μm.
In order to quantify the degree of CXCR5 staining we applied morphometry and scored the sections by calculating the ratio‐index and percentage of tissue stained in each gland. Our analysis demonstrated that there is a significant increase in percentage of CXCR5+ stained MSG tissue in the SS patients compared to the non‐SS sicca controls (P < 0·0001) (Fig. 5g). The amount of CXCR5‐positive cells in MSG was analysed for correlation with clinical parameters including focus score, presence of ectopic GCs and Ro/SSA and La/SSB autoantibody positivity, but no correlation with analysed parameters was detected (data not shown). Meanwhile, a decrease in circulating CXCR5+ MZ B cells was observed in patients with leucopenia (P < 0·001) and hypergammaglobulinaemia (Fig. 5h).
Discussion
Among the previously suggested genes associated with SS, CXCR5 distinguishes itself as an interesting candidate for further analysis, due not least to the active involvement of this chemokine receptor of B and Tfh cells in the GC formation through the interaction with its CXCL13 ligand. As ectopic GC‐like formation is a prominent feature in SS pathogenesis, we wished to investigate the association and expression pattern of the CXCR5–CXCL13 axis in SS in order to gain a deeper understanding of the functional impact of these disease‐associated polymorphisms in the patients.
Considering that polymorphisms close to the CXCR5 locus have been described previously in SS 12, but that it was not known whether these associated with any difference in CXCR5 gene expression, we first conducted an eQTL analysis in an existing large data set derived from sorted CD19+ B cells 29 where CXCR5 is expressed readily. Our additive model showed that the disease risk allele is associated with a significantly decreased CXCR5 gene expression. Having confirmed a functional link of SS‐associated genetic polymorphisms with CXCR5 expression, we pursued studies of CXCR5 protein expression in PBMCs of SS patients. We observed a significantly decreased frequency of CXCR5+ B cells among memory B cells, MZ B cells and CD27–IgD– double‐negative B cells that contributed to a general decrease in circulating CXCR5+ B cells. Notably, even in CXCR5+ cells, the patients displayed a decrease in cell surface expression of this molecule.
When screening for CXCR5 protein expression in T cell subsets in peripheral blood, we observed a significant decrease in the frequency of CXCR5+ Th17 cells in PBMCs from SS patients. In addition to being IL‐17‐producing T helper (Th) cells 36, Th17 cells are active in recruitment of neutrophils and macrophages to the site of inflammation 37. The suggestive decrease in peripheral CXCR5+ Th17 cells could be the result of their retention in the salivary glands at the site of inflammation. In contrast to our results, others have reported increased levels of circulating Th17+ cells in SS patients compared to healthy donors 38, but they did not specifically examine CXCR5+ Th17 cells. Elevated levels of circulating Tfh cells, defined by CXCR5 expression, have been detected previously in SS patients with extra‐glandular manifestations 26. In the present study we did not observe a significant increase in circulating Tfh cells compared to controls, which may be explained by the fact that only four patients showed extra‐glandular manifestations.
The decrease in the frequency of CXCR5+ cells and diminished cell surface CXCR5 expression in SS patients could, potentially, be related to SS‐associated genetic polymorphisms. Even in our relatively small material, we observed a significantly lower cell surface expression of CXCR5 on memory B cells in patients who were homozygous carriers of the disease risk T allele, and a clear trend in MZ and DN B cells.
Expression of the CXCR5 ligand CXCL13 has been reported previously within the salivary gland target organ in SS 19; therefore, we next analysed CXCL13 protein levels in plasma. We found that SS patients exhibited significantly increased plasma levels of CXCL13, and that the levels of CXCL13 were correlated inversely with the percentage of CXCR5+ memory, MZ and DN B cells in circulation. We confirmed the presence of CXCL13‐producing cells in the salivary gland target organ of SS patients, and further observed significantly increased numbers and highly abundant CXCR5+ cells in the target organ of SS patients. These findings are in line with previous studies observing CXCR5 expression to be affected in B cells in both salivary gland tissue and peripheral blood 2, 39. The lesser expression of CXCR5 from the genetic variant associated with SS may appear difficult to connect with the pathogenic process. However, a possible scenario could be that early changes in the salivary gland due, for instance, to viral infection and the activation of innate immunity 40, initiates CXCL13 expression for recruitment of B cells. However, the lower cell surface expression of CXCR5 in SS susceptible individuals could lead to escalating chemokine production before recruitment is initiated. In the process, the threshold for recruitment of T cell subsets that generally express less CXCR5 may be passed, thereby bringing these cells into the glands in an untimely fashion and creating an unbalanced inflammatory state.
Such unbalanced regulation of cellular trafficking with high CXCL13 production may also underlie the generation of ectopic germinal centres in the salivary glands in SS. Increased levels of peripheral CXCL13 have been reported previously in patients with SS 41. It has also been demonstrated that these high serum levels of CXCL13 were associated with active disease, B cell activation and a history of lymphoma among SS patients 42. CXCL13‐expressing cells observed in our study were situated within the focal infiltrates, suggesting their active role in ectopic GC formation at the site of inflammation 43, 44. Overall, the patients showed relatively high focus score values, and 18 of them also displayed ectopic GC‐like formations in the salivary gland. The functionality of ectopic GC‐like structures that are formed in the salivary glands of SS patients has been investigated previously through immunohistochemical staining of functional GC markers, including CXCL13 and CXCR5 4, 45. Moreover, CXCR5–CXCL13 has also been associated with ectopic GC formation in other autoimmune and inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematosus 46, 47. Subsequently, increased levels of CXCL13 in plasma have also been correlated with the formation of ectopic lymphoid tissue 48. In accordance, our current findings also propose an association between elevated CXCL13 levels in plasma and the recruitment of CXCR5+ cells in SS, possibly giving rise to ectopic lymphoid tissue assembly. Indeed, CXCL13 has been considered likely to play a central role in other autoimmune diseases with an increased risk of lymphoma, including rheumatoid arthritis and systemic lupus erythematosus 49, 50.
In conclusion, we describe a novel eQTL for CXCR5 linking SS‐associated polymorphisms to this gene mechanistically. SS patients generally displayed decreased frequencies of CXCR5+ B cell subsets in peripheral blood, which concurred with increased CXCL13 protein levels in plasma. Furthermore, CXCL13+ cells were detected in the target organ, where extensive infiltration of CXCR5+ cells was observed at the site of inflammation. A link between genotype and CXCR5 cell surface expression in SS was also established. We suggest that the lower CXCR5 expression and decrease of CXCR5+ cells in the peripheral blood of SS patients could result from a combination of genotype‐regulated expression of CXCR5 and homing of peripheral B and T cells to the autoimmune target organs, related to the local expression of the CXCL13 ligand. Therapeutic drugs targeting the CXCR5–CXCL13 axis may therefore be useful in SS.
Author contributions
The authors contributed as follows: M. W. H., M. I. and L. A. A. designed the study. M. K. and A. B. recruited the patients. L. A. A., M. I., A. B. and J. I. R. S. performed the experiments. L. A. A., M. I., A. B., J. I. R. S., J. I.‐K., G. N., K. C., K. S. and M. W. H. analysed the data. L. A. A. and M. W. H. wrote the manuscript. All authors critically evaluated the manuscript.
Disclosure
The authors declare no competing interests.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Overview of antibodies used in flow cytometry.
Fig. S1. Gating strategy for B cells.
Fig. S2. Gating strategy for T follicular helper (Tfh) cells.
Fig. S3. Gating strategy for T helper type 17 (Th17) cells.
Acknowledgements
We gratefully thank Amina Ossoinak for excellent technical assistance, and Professor Julian C. Knight and his research group at the University of Oxford for sharing genotype and expression data from primary B cells. The study was supported by grants from the Swedish Research Council, the Stockholm County Council, the Karolinska Institute, the Swedish Rheumatism association, the King Gustaf the Vth 80‐year foundation, and the Research Council of Norway through a FRIPRO Mobility Grant (co‐funded by the European Union's Seventh Framework Programme for research, technological development and demonstration under Marie Curie grant agreement no 608695).
References
- 1. Jonsson R, Vogelsang P, Volchenkov R, Espinosa A, Wahren‐Herlenius M, Appel S. The complexity of Sjögren's syndrome: novel aspects on pathogenesis. Immunol Lett 2011; 141:1–9. [DOI] [PubMed] [Google Scholar]
- 2. Hansen A, Lipsky PE, Dorner T. B cells in Sjögren's syndrome: indications for disturbed selection and differentiation in ectopic lymphoid tissue. Arthritis Res Ther 2007; 9:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amft N, Curnow SJ, Scheel‐Toellner D. Ectopic expression of the B cell‐attracting chemokine BCA‐1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center‐like structures in Sjögren's syndrome. Arthritis Rheum 2001; 44:2633–41. [DOI] [PubMed] [Google Scholar]
- 4. Salomonsson S, Jonsson MV, Skarstein K et al Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren's syndrome. Arthritis Rheum 2003; 48:3187–201. [DOI] [PubMed] [Google Scholar]
- 5. Jonsson MV, Skarstein K, Jonsson R, Brun JG. Serological implications of germinal center‐like structures in primary Sjögren's syndrome. J Rheumatol 2007; 34:2044–9. [PubMed] [Google Scholar]
- 6. Bombardieri M, Barone F, Humby F et al Activation‐induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjögren's syndrome. J Immunol 2007; 179:4929–38. [DOI] [PubMed] [Google Scholar]
- 7. Tew JG, DiLosa RM, Burton GF et al Germinal centers and antibody production in bone marrow. Immunol Rev 1992; 126:99–112. [DOI] [PubMed] [Google Scholar]
- 8. Theander E, Vasaitis L, Baecklund E et al Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren's syndrome. Ann Rheum Dis 2011; 70:1363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen A, Gosemann M, Pruss A et al Abnormalities in peripheral B cell memory of patients with primary Sjögren's syndrome. Arthritis Rheum 2004; 50:1897–908. [DOI] [PubMed] [Google Scholar]
- 10. Binard A, Le Pottier L, Devauchelle‐Pensec V, Saraux A, Youinou P, Pers JO. Is the blood B‐cell subset profile diagnostic for Sjögren syndrome? Ann Rheum Dis 2009; 68:1447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bohnhorst JO, Bjorgan MB, Thoen JE, Jonsson R, Natvig JB, Thompson KM. Abnormal B cell differentiation in primary Sjögren's syndrome results in a depressed percentage of circulating memory B cells and elevated levels of soluble CD27 that correlate with serum IgG concentration. Clin Immunol 2002; 103:79–88. [DOI] [PubMed] [Google Scholar]
- 12. Lessard CJ, Li H, Adrianto I et al Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren's syndrome. Nat Genet 2013; 45:1284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Zhang K, Chen H et al A genome‐wide association study in Han Chinese identifies a susceptibility locus for primary Sjögren's syndrome at 7q11.23. Nat Genet 2013; 45:1361–5. [DOI] [PubMed] [Google Scholar]
- 14. Song IW, Chen HC, Lin YF et al Identification of susceptibility gene associated with female primary Sjögren's syndrome in Han Chinese by genome‐wide association study. Hum Genet 2016; 135:1287–94. [DOI] [PubMed] [Google Scholar]
- 15. Taylor KE, Wong Q, Levine DM et al Genome‐wide association analysis reveals genetic heterogeneity of Sjögren's syndrome according to ancestry. Arthritis Rheumatol 2017; 69:1294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dixon AL, Liang L, Moffatt MF et al A genome‐wide association study of global gene expression. Nat Genet 2007; 39:1202–7. [DOI] [PubMed] [Google Scholar]
- 17. Maurano MT, Humbert R, Rynes E et al Systematic localization of common disease‐associated variation in regulatory DNA. Science 2012; 337:1190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morita R, Schmitt N, Bentebibel SE et al Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011; 34:108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salomonsson S, Larsson P, Tengner P, Mellquist E, Hjelmstrom P, Wahren‐Herlenius M. Expression of the B cell‐attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjögren's syndrome. Scand J Immunol 2002; 55:336–42. [DOI] [PubMed] [Google Scholar]
- 20. Cagigi A, Mowafi F, Phuong Dang LV et al Altered expression of the receptor‐ligand pair CXCR5/CXCL13 in B cells during chronic HIV‐1 infection. Blood 2008; 112:4401–10. [DOI] [PubMed] [Google Scholar]
- 21. Muller G, Hopken UE, Lipp M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol Rev 2003; 195:117–35. [DOI] [PubMed] [Google Scholar]
- 22. Carubbi F, Alunno A, Cipriani P et al Is minor salivary gland biopsy more than a diagnostic tool in primary Sjögren's syndrome? Association between clinical, histopathological, and molecular features: a retrospective study. Semin Arthritis Rheum 2014; 44:314–24. [DOI] [PubMed] [Google Scholar]
- 23. Chen M, Guo Z, Ju W, Ryffel B, He X, Zheng SG. The development and function of follicular helper T cells in immune responses. Cell Mol Immunol 2012; 9:375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Linterman MA, Rigby RJ, Wong RK et al Follicular helper T cells are required for systemic autoimmunity. J Exp Med 2009; 206:561–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deenick EK, Ma CS, Brink R, Tangye SG. Regulation of T follicular helper cell formation and function by antigen presenting cells. Curr Opin Immunol 2011; 23:111–8. [DOI] [PubMed] [Google Scholar]
- 26. Szabo K, Papp G, Barath S, Gyimesi E, Szanto A, Zeher M. Follicular helper T cells may play an important role in the severity of primary Sjögren's syndrome. Clin Immunol 2013; 147:95–104. [DOI] [PubMed] [Google Scholar]
- 27. Vitali C, Bombardieri S, Jonsson R et al Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis 2002; 61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berggren O, Alexsson A, Morris DL et al IFN‐alpha production by plasmacytoid dendritic cell associations with polymorphisms in gene loci related to autoimmune and inflammatory diseases. Hum Mol Genet 2015; 24:3571–81. [DOI] [PubMed] [Google Scholar]
- 29. Fairfax BP, Makino S, Radhakrishnan J et al Genetics of gene expression in primary immune cells identifies cell type‐specific master regulators and roles of HLA alleles. Nat Genet 2012; 44:502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O'Donnell CJ, de Bakker PI. SNAP: a web‐based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics 2008; 24:2938–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shabalin AA. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics 2012; 28:1353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Assarsson E, Lundberg M, Holmquist G et al Homogenous 96‐plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLOS ONE 2014; 9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lundberg M, Eriksson A, Tran B, Assarsson E, Fredriksson S. Homogeneous antibody‐based proximity extension assays provide sensitive and specific detection of low‐abundant proteins in human blood. Nucleic Acids Res 2011; 39:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature 1997; 388:133–4. [DOI] [PubMed] [Google Scholar]
- 35. Moura RA, Quaresma C, Vieira AR et al B‐cell phenotype and IgD–CD27– memory B cells are affected by TNF‐inhibitors and tocilizumab treatment in rheumatoid arthritis. PLOS ONE 2017; 12:e0182927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ambrosi A, Espinosa A, Wahren‐Herlenius M. IL‐17: a new actor in IFN‐driven systemic autoimmune diseases. Eur J Immunol 2012; 42:2274–84. [DOI] [PubMed] [Google Scholar]
- 37. Szodoray P, Nakken B, Barath S et al Altered Th17 cells and Th17/regulatory T‐cell ratios indicate the subsequent conversion from undifferentiated connective tissue disease to definitive systemic autoimmune disorders. Hum Immunol 2013; 74:1510–8. [DOI] [PubMed] [Google Scholar]
- 38. Li XY, Wu ZB, Ding J et al Role of the frequency of blood CD4(+) CXCR5(+) CCR6(+) T cells in autoimmunity in patients with Sjögren's syndrome. Biochem Biophys Res Commun 2012; 422:238–44. [DOI] [PubMed] [Google Scholar]
- 39. Gottenberg JE, Cagnard N, Lucchesi C et al Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren's syndrome. Proc Natl Acad Sci USA 2006; 103:2770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sisto M, Lorusso L, Lisi S. TLR2 signals via NF‐kappaB to drive IL‐15 production in salivary gland epithelial cells derived from patients with primary Sjögren's syndrome. Clin Exp Med 2017; 17:341–50. [DOI] [PubMed] [Google Scholar]
- 41. Kramer JM, Klimatcheva E, Rothstein TL. CXCL13 is elevated in Sjögren's syndrome in mice and humans and is implicated in disease pathogenesis. J Leukoc Biol 2013; 94:1079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nocturne G, Seror R, Fogel O et al CXCL13 and CCL11 serum levels and lymphoma and disease activity in primary Sjögren's syndrome. Arthritis Rheumatol 2015; 67:3226–33. [DOI] [PubMed] [Google Scholar]
- 43. Ekstrom Smedby K, Vajdic CM, Falster M et al Autoimmune disorders and risk of non‐Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood 2008; 111:4029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Theander E, Henriksson G, Ljungberg O, Mandl T, Manthorpe R, Jacobsson LT. Lymphoma and other malignancies in primary Sjögren's syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis 2006; 65:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao J, Kubo S, Nakayamada S et al Association of plasmacytoid dendritic cells with B cell infiltration in minor salivary glands in patients with Sjögren's syndrome. Mod Rheumatol 2016; 26:716–24. [DOI] [PubMed] [Google Scholar]
- 46. Bürkle A, Niedermeier M, Schmitt‐Gräff A, Wierda WG, Keating MJ, Burger JA. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B‐cell chronic lymphocytic leukemia. Blood 2007; 110:3316–25. [DOI] [PubMed] [Google Scholar]
- 47. Manzo A, Paoletti S, Carulli M et al Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol 2005; 35:1347–59. [DOI] [PubMed] [Google Scholar]
- 48. Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin‐dependent lymphoid neogenesis. Immunity 2000; 12:471–81. [DOI] [PubMed] [Google Scholar]
- 49. Greisen SR, Schelde KK, Rasmussen TK et al CXCL13 predicts disease activity in early rheumatoid arthritis and could be an indicator of the therapeutic ‘window of opportunity’. Arthritis Res Ther 2014; 16:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schiffer L, Worthmann K, Haller H, Schiffer M. CXCL13 as a new biomarker of systemic lupus erythematosus and lupus nephritis – from bench to bedside? Clin Exp Immunol 2015; 179:85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Overview of antibodies used in flow cytometry.
Fig. S1. Gating strategy for B cells.
Fig. S2. Gating strategy for T follicular helper (Tfh) cells.
Fig. S3. Gating strategy for T helper type 17 (Th17) cells.


