Abstract
Aims
Previously, we have reported an association between clozapine use and elevated FL3 neutrophil fluorescence, a flow‐cytometric parameter for cell viability. Here, we developed and evaluated a pharmacokinetic–pharmacodynamic model relating FL3‐fluorescence to clozapine exposure and derived a nomogram for estimation of long‐term adherence.
Methods
Data from 27 patients initiating clozapine were analysed using nonlinear mixed effects modelling. A previously described pharmacokinetic model for clozapine was coupled to a FL3 fluorescence model. For this, an effect compartment with clozapine concentrations as input and a first order decay rate as output was linked with an Emax model to FL3‐fluorescence. FL3‐fluorescence was simulated for clozapine doses of 50, 150 and 400 mg daily (n = 10 000) to establish the nomogram. Finally, true simulated adherence (% of daily doses taken over 100 days) was compared to nomogram‐estimated adherence to evaluate the performance of the nomogram.
Results
The half‐life of FL3‐fluorescence was estimated at 228 h (coefficient of variation 35%). Median absolute prediction errors of the nomogram in case of fully random adherence for 50, 150 and 400 mg ranged from –0.193% to –0.525%. The nomogram performed slightly worse in case of nonrandom adherence (median prediction error up to 5.19%), but was still clinically acceptable. Compliance patterns containing longer drug holidays revealed that the nomogram adequately estimates compliance over approximately the last 3 weeks prior to FL3‐measurement.
Conclusion
Our nomogram could provide information regarding long‐term adherence based on prescribed clozapine dose and FL3‐fluorescence. Future studies should further explore the clinical value of this biomarker and nomogram.
Keywords: clozapine, FL3‐fluorescence, neutrophil granulocytes, biomarker, pharmacokinetics, pharmacodynamics, medication adherence, schizophrenia
What is Already Known about this Subject
Currently, therapeutic drug monitoring is an option to assess adherence to clozapine therapy, indicative for relatively short‐term adherence.
Elevated FL3‐fluorescence in neutrophil granulocytes was observed in clozapine users and might be a more optimal biomarker for treatment adherence.
What this Study Adds
Our study shows that FL3‐fluorescence could be an appropriate biomarker to estimate long‐term adherence given the relatively long half‐life of FL3‐fluorescence in relation to clozapine therapy.
Our developed nomogram could be used to estimate adherence to clozapine over a longer period based on the prescribed dose and FL3‐fluorescence, which may be an important aid in the treatment of patients.
Introduction
Schizophrenia is a severely disabling chronic form of mental illness with a prevalence of about 1% of the world population 1. Pharmacotherapy with antipsychotics forms the cornerstone of treatment. Within this group, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=38 is the only drug with demonstrated efficacy in treatment‐resistant schizophrenia 2, 3, 4, 5. Moreover, it is the only agent with demonstrated antisuicidal properties 6.
Known for its serious adverse effects including agranulocytosis, use of clozapine is limited to those suffering from treatment‐resistant schizophrenia. Nonadherence is a major issue in the treatment of schizophrenia. An estimated 25% of the patients are partially compliant within the first 7–10 days of treatment 7 and studies estimate the mean overall prevalence of nonadherence between 40 and 50% 8. Results of one study revealed that half of the ambulant schizophrenic patients take <70% of their medication 9. Another study showed that low treatment adherence is associated with a two‐fold increased risk for relapse in psychotic disorders within 6 months after discharge 10. More specifically, gaps in antipsychotics coverage of 10 days based on dispensing data were associated with an increased risk of psychiatric hospitalization 11, 12, reflecting the potential forgiveness of antipsychotic agents. Compared to other atypical antipsychotics, higher rates of adherence were observed with clozapine, explained by superior efficacy or the requirements for close monitoring 13. An earlier cohort study showed that 11% of patients registered to receive clozapine did not initiate therapy, while 30% discontinued therapy after having received clozapine for at least 1 week 14.
Given the strong relationship between serum concentrations and clinical effect of clozapine, therapeutic drug monitoring (TDM) is a valid tool to optimize dosing 15. TDM may also be used to assess adherence to therapy 16; however, due to relatively short elimination half‐life of clozapine of about 14 h 17 and of its main metabolite http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=333 (18 h 17), serum concentration monitoring only provides information on clozapine intake for a few days prior to blood sampling at most. Therefore, a biomarker providing long‐term information on adherence would be of great value.
Neutrophil granulocyte fluorescence measured at the FL3 channel on a flow cytometer, in this article termed FL3‐fluorescence, is used as a flow‐cytometric parameter that measures nucleated red blood cells and white blood cell viability by propidium iodide (PI) staining. Elevated FL3‐fluorescence has been serendipitously observed in patients using clozapine 18. In a previous study, our group assessed the association between clozapine use and FL3‐fluorescence 19. FL3‐fluorescence was significantly higher in clozapine users than in nonusers. FL3‐fluorescence was found to increase with increasing clozapine dose and at a given clozapine dose, this fluorescence intensity seemed to reach a maximum. Also, the degree of elevation in FL3‐fluorescence was most likely to be associated with duration of clozapine therapy. To determine whether FL3‐fluorescence could serve as a potential biomarker to estimate patients' long‐term adherence to clozapine, information regarding the time course of FL3‐fluorescence in relation to clozapine therapy is the next step. The objectives of the current study were to describe the association between FL3‐fluorescence and clozapine therapy in time using pharmacokinetic (PK)/pharmacodynamic (PD) modelling and to establish a nomogram for estimating long‐term adherence based on FL3 values.
Methods
Patient population and data collection
Data were extracted from the Utrecht Patient Oriented Database (UPOD), which is an infrastructure of relational databases comprising data on patient characteristics, hospital discharge diagnoses, medical procedures, medication orders and laboratory tests for all patients treated at the University Medical Center (UMC) Utrecht since 2004 20. UPOD data acquisition and data management were in line with current Dutch regulations concerning privacy and ethics. The data used for this cohort study were collected for patient care purposes and were used retrospectively. Because no extra material, for example, blood samples, is taken from patients, there is not a requirement to obtain informed consent from individual patients or seek institutional review board approval for every study protocol. The structure and content of UPOD have been described in detail elsewhere 20.
The study population comprised of all psychiatric inpatients of the UMC Utrecht aged 16 years or older having been prescribed clozapine between February 2004 and September 2013 with at least two FL3‐fluorescence measurements available (see below). Clozapine use was defined as having a medication order for clozapine. A medication order was defined as an order in the computerized physician order entry system. Clozapine medication orders, comedication, clozapine serum levels, indications for clozapine prescriptions and comorbidity were retrieved from the medical record.
Definition clozapine starters and stable users
Start of clozapine was defined as a first clozapine prescription with no evidence of a clozapine prescription for at least 1 month before, followed by a gradual dose escalation resulting in a dose of at least 50 mg once daily within the subsequent 7 days. Participants were followed until they were defined as stable users or stopped clozapine therapy. Stable clozapine users were defined as patients with a medication order for clozapine where the prescribed dose remained unchanged for at least 5 consecutive days or information in the patient records explicating continuing of same dose for at least 5 days, as it was expected that clozapine PK reached steady state after 5 days.
FL3‐fluorescence of neutrophil granulocytes
UPOD contains specific haematology data of automated blood cell analyses performed by the Abbott Cell‐Dyn Sapphire automated haematology analyser (Abbott Diagnostics, Santa Clara, CA, USA) 21, 22, 23. A feature of this analyser is that all parameters of the complete blood count are measured irrespective of the requested parameter. The analyser is equipped with an integrated 488 nm blue diode laser and uses spectrophotometry, electrical impedance and laser light scattering (multiangle polarized scatter separation), to measure the complete blood count and to classify the white blood cell (WBC) counts. For the WBC differential count the following five optical scatter signals are measured for each individual cell: cell size (0° scatter, axial light loss), cell complexity and granularity (7° scatter, intermediate angle scatter), nuclear lobularity (90° scatter, polarized side scatter), depolarization (90° depolarized side scatter) and red fluorescence [90° (FL3), 630 ± 30 nm]. This FL3‐fluorescence parameter measures nucleated red blood cells and WBC viability by PI staining. PI, one of the reagents used by the analyser, is capable of crossing the cell membrane of nonviable WBCs and stains nucleic acids (RNA and DNA). The WBC viability is reported by the analyser as the white cell viability factor. In the current analysis, only FL3‐fluorescence values associated with a white cell viability factor of ≥0.95 were included to differentiate between fresh and old samples 24. Because the clinical relevance of the FL3 parameter is unknown, this parameter was not reported to the physician but still included in the UPOD database.
PK/PD model development
Nonlinear mixed‐effect modelling was performed using NONMEM software (Version 7.2.0; ICON Development Solutions, Ellicott City, MD, USA). NONMEM runs were executed using Pirana modelling and simulation workbench for NONMEM 25. The first‐order conditional estimation with interaction (FOCE‐I) method was used. The minimum value of the objective function (OFV, –2 times the log likelihood), typical goodness‐of‐fit diagnostic plots, evaluation of the precision of model parameter and variability estimates were used for model selection during the model‐building process. Data processing and preparation of plots was performed in R (version 3.2.1).
Clozapine plasma concentration data were not available for this study so a previously published model was employed 26. Population PK parameters were fixed to those in the original model and population PK predictions were used as a driving force for the PD model.
A PK/PD model was built to describe the relation between clozapine plasma concentrations and FL3‐fluorescence over time. During PD model building‐process, different drug effect models and drug effect delay models were tested.
Interindividual variability for PD parameters was modelled assuming a log normal distribution:
where Pi is the individual parameter, θP is the typical value of the parameter and ηP, i is the random effect for that parameter with a mean of 0 and variance of ωP 2. Additive, proportional, additive–proportional and exponential error models were tested to describe residual error in FL3‐fluorescence prediction.
The evaluation of the model was carried out by means of an internal validation process. Nonparametric bootstrap resampling (n = 1000) was applied to the final model and the 95% confidence intervals (95%CI) for the estimates were calculated. The mean and 95%CI (2.5 and 97.5% percentiles) of these 1000 estimates were compared to those obtained in the original population set.
Development of adherence nomogram
The final PK/PD model was implemented in R as a set of ordinary differential equations. Interindividual and residual variability was taken into account in these simulations. A 100‐day study was simulated with 10 000 patients in each study arm. Three study‐arms were included: clozapine 50, 150 and 400 mg daily. The main outcome was FL3‐fluorescence values at the end of study period. A 100‐day period was chosen so that steady state was reached across model compartments. To simulate a wide variety of treatment adherence patterns, a random clozapine‐intake probability (between 0–1 from a uniform distribution) was generated for each patient. Then, this probability was used to determine whether each patient did or did not take their clozapine dose for each scheduled dose administration.
Simulation results were explored to find the relations between daily clozapine dose, treatment adherence and FL3‐fluorescence values at steady state. A graphical nomogram was then designed for estimation of predicted treatment adherence (PTA) expressed as proportion (%) daily doses taken over 100 days.
The ABC taxonomy, as described by the EMERGE steering committee 27, was followed for describing and defining adherence to clozapine use by means of: (i) initiation; (ii) implementation; and (iii) persistence of therapy.
Evaluation of the nomogram
The predictive performance of the nomogram was also investigated through simulations. The same study design as used in nomogram development was employed. For each simulated patient, nomogram‐based PTA values were compared to true treatment adherence (TA) to evaluate predictive performance. The median absolute prediction error (PE) and interquartile range (IQR) were calculated for each dosing level. PE was calculated as:
As an exploration of model robustness in adverse conditions, the nomogram was also tested assuming nonrandom adherence as might be expected in clinical practice. In these simulations, on the last day before FL3‐fluorescence analysis, patients with adherence levels equal or above 80% took the prescribed dose, and those patients with adherence below 80% took double the prescribed dose. In addition, we simulated three specific nonrandom adherence patterns (A, B and C) to evaluate the predictive performance of the nomogram. Details of these patterns are described in the legend of Figure 5.
Figure 5.
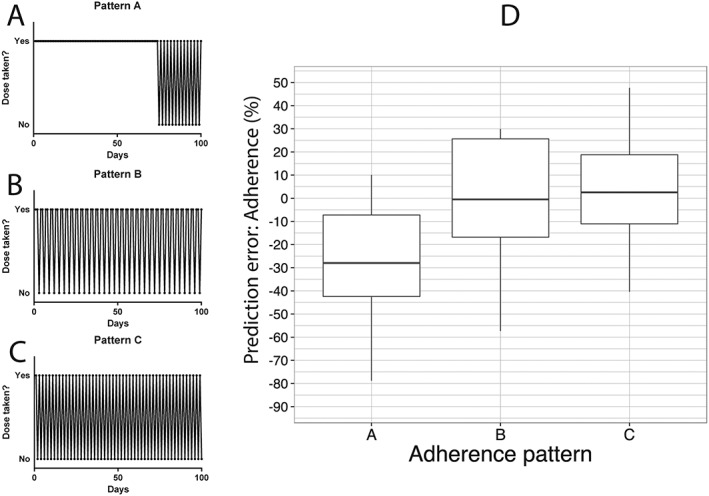
Nonrandom adherence patterns and prediction error of the nomogram. (A) Pattern A: until day 75 all doses were taken in. From day 75 a dose was not taken every 2 days. Thus, the overall adherence rate is nearly 90%. (B) Pattern B: throughout all 100 days, starting from day 1 a dose was not taken every 3 days. Thus, the overall adherence rate is nearly 66%. (C) Pattern C: throughout all 100 days, starting from day 1, a dose was not taken every 2 days. Thus, the overall adherence rate is nearly 50%. (D) Prediction error when the nomogram is tested in patients following patterns A, B and C
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 28, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.
Results
Data
A total of 27 patients (181 FL3 measurements) starting clozapine therapy were followed‐up after initiation of clozapine therapy. Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Included patients (n = 27) | |
|---|---|
| Sex, n (% male) | 19 (70.4) |
| Age (years), mean (SD) | 33.1 (15.4) |
| Weight (kg), mean (SD) | 70.5 (13.8) |
| BMI (kg m –2 ), mean (SD) | 22.1 (3.3) |
| Clozapine dose (mg day –1 ) during steady state, mean (SD): indication of clozapine therapy | 175.5 (90.6) |
| Schizophrenia, n (%) | 18 (66.7) |
| Schizoaffective disorder, n (%) | 3 (11.1) |
| Psychotic disorder NOS, n (%) | 3 (11.1) |
| Delirium in pre‐existing Lewy body dementia, n (%) | 1 (3.7) |
| Schizophreniform disorder, n (%) | 1 (3.7) |
| Depressive disorder with psychotic features, n (%) | 1 (3.7) |
BMI, body mass index; NOS, not otherwise specified; SD, standard deviation
PK/PD model development and evaluation
Among the tested PD model structures to describe FL3‐fluorescence, the final model (schematic representation in Figure 1) consisted of an effect compartment linked to FL3‐fluorescence by an Emax effect model. FL3‐fluorescence was described by:
in which, FL30 denotes the baseline value of FL3‐fluorescence, Emax denotes the maximal effect (maximal increase of FL3‐fluorescence relative to FL30), Ce50 denotes the concentration of clozapine in the effect compartment which produces 50% of maximal effect and Ce denotes the concentration of clozapine in the effect compartment, which was described by:
In this equation, Cp denotes clozapine plasma concentration and kout denotes the effect compartment elimination rate constant. kout was parameterized as:
where denotes elimination half‐life in effect compartment, which was estimated.
Figure 1.
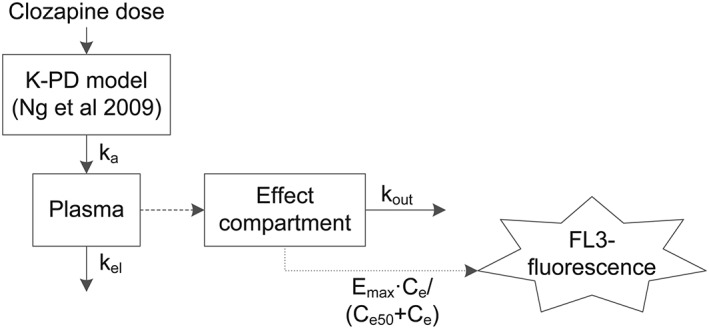
Schematic representation of the model
Residual error in FL3‐fluorescence was modelled using:
where FL3obs is the observed FL3‐fluorescence, FL3 is the model predicted FL3‐fluorescence and εexp,FL3 represents the exponential random error for FL3. It was assumed that ε is normally distributed with the mean 0 and variance σ2.
The model adequately described the data, as can be seen in the goodness‐of‐fit plots: Figures 2A shows the individual time course of observed (DV) and model‐derived individual predictions (IPRED) of FL3‐fluorescence values, and Figure 2B displays the relationship between DV and IPRED.
Figure 2.
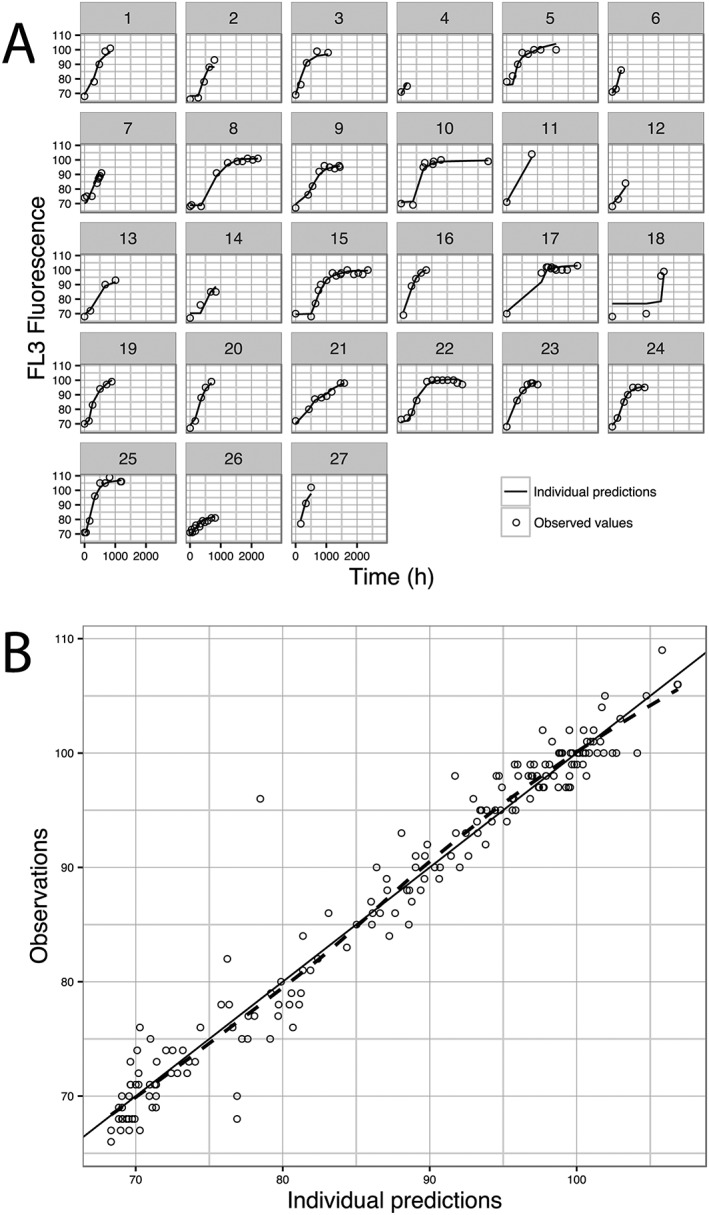
Goodness of fit plots. (A) individual time course of observed (DV) and model derived individual predictions (IPRED) for FL3‐fluorescence; (B) DV vs. IPRED, black line shows line of identity and blue line is the trend line showing the relation between DV and IPRED
Model parameter estimates, estimation errors and bootstrap results are shown in Table 2. All parameters were estimated with adequate precision and results of the bootstrap analysis were in close agreement with the final parameter estimates.
Table 2.
Final model parameter estimates and bootstrap results
| Parameter | Estimate | RSE (%) | Bootstrap results * | ||
|---|---|---|---|---|---|
| Median | 95% BI | ||||
| FL3 0 | 70.3 | 1 | 70.2 | 69.0–71.5 | |
| E max | 0.578 | 10 | 0.599 | 0.518–0.701 | |
| T e1/2 (h) | 228 | 35 | 205 | 131–400 | |
| C e50 | 57.0 | 14 | 58.2 | 45.9–78.7 | |
| CV% IIV FL3 0 | 3.08 | 22 | 2.98 | 0.732–4.50 | |
| CV% IIV E max | 9.05 | 49 | 10.07 | 2.07–18.2 | |
| CV% RSE exp | 3.32 | 25 | 2.98 | 1.92–4.24 | |
Bootstrap results are derived from 667 successful bootstrap runs.
RSE: relative estimation error; 95% CI: 95% confidence interval derived from the 2.5 and 97.5 percentiles of parameter estimates in successful bootstrap runs; FL30: baseline FL3‐fluorescence value; Emax: maximum increase in FL30 as a consequence of clozapine administration relative to FL30; Te1/2: elimination half‐life; Ce50: clozapine concentration in effect compartment necessary to achieve 50% of the maximum increase in FL3‐fluorescence; CV% IIV: parameter inter‐individual variability expressed as coefficient of variation (%); CV% RSEexp: residual exponential error expressed as coefficient of variation (%).
Development of nomogram
Results for the 100‐day simulation study with 10 000 patients in each study arm (clozapine 50, 150 and 400 mg daily, fully random adherence) are presented in Figure 3A. The relationship between FL3‐fluorescence and treatment adherence in patients receiving 50 mg clozapine four times daily was almost linear. At higher doses (150 and 400 mg), this relationship was strongly nonlinear.
Figure 3.
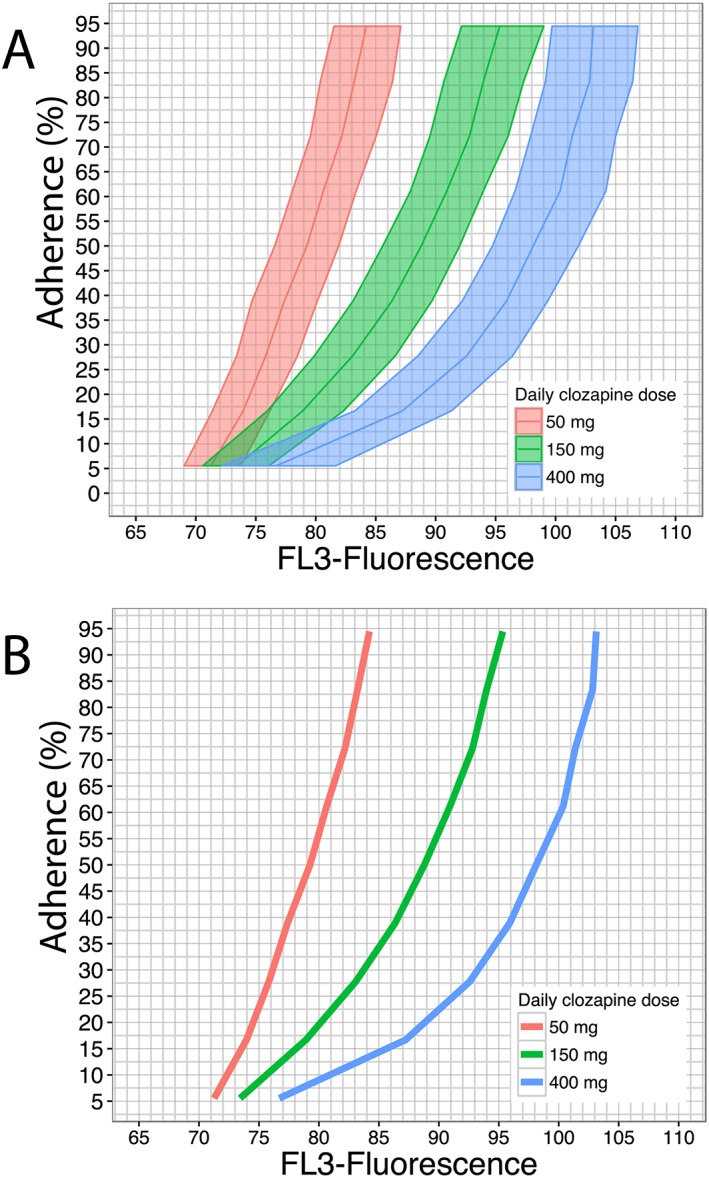
Simulation results and proposed nomogram. (A) Simulation results: 100‐day study with 10 000 patients in each study arm (clozapine 50, 150 and 400 mg daily). FL3‐fluorescence values at the end of study period (steady state) are plotted against treatment adherence values. Results have been stratified per dosing level [red: 50 mg daily (QD); green: 150 mg QD; blue: 400 mg QD]. Solid lines and shaded areas represent FL3‐fluorescence median and interquartile range, respectively, across treatment adherence values for different dosing arms. (B) Proposed nomogram for treatment adherence estimation, defined by a representation of solely median FL3‐fluorescence values as were also depicted in Figure 3A
To develop a nomogram for clozapine adherence prediction, both daily dose and FL3‐fluorescence values must be considered as shown in Figure 3A. Hence, the nomogram consisted of the representation of median FL3‐fluorescence values across adherence values, based on the simulation results derived from the developed PK/PD model (Figure 3B). The interpolation of FL3‐fluorescence value on the corresponding daily dose line outputs the PTA.
Evaluation of the nomogram
The performance of the nomogram for the 100‐day simulation study with 10 000 patients in each study arm (clozapine 50, 150 and 400 mg daily, fully random adherence) is presented in Figure 4A. Median absolute prediction errors (PE) and the IQR (%) for the 50, 150 and 400 mg arms were –0.193 (IQR –14.1–14.2), –0.193 (IQR –10.9–10.7 and –0.525 (IQR –12.1–11.8), respectively. No relevant bias was shown in nomogram predictions under these conditions.
Figure 4.
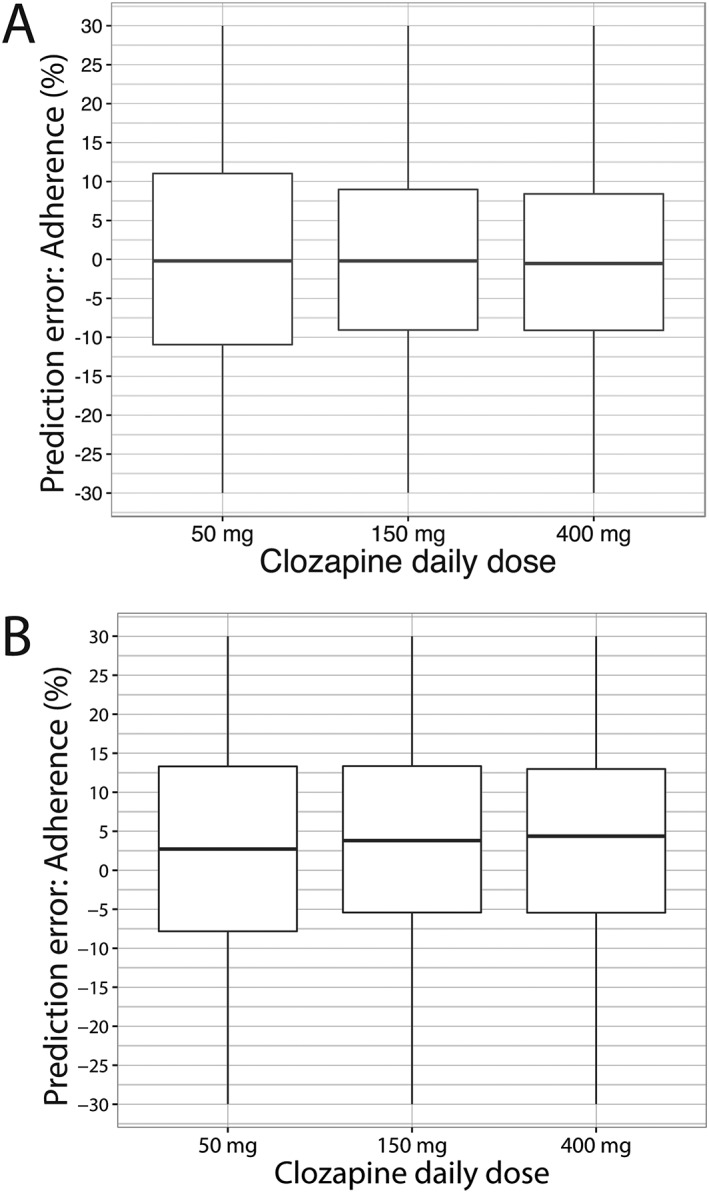
Prediction error of the nomogram. (A) Prediction error when nomogram is tested in patients having random adherence. (B) Prediction error when nomogram is tested with patients having nonrandom adherence (on the last day before FL3‐fluorescence analysis, patients with adherence levels equal or above 80% took the prescribed dose, and those patients with adherence below 80% took double the prescribed dose). Box and whisker plots depict the median and interquartile range of the prediction error
In case of nonrandom adherence (e.g. patients with low adherence take a double dose just before measurement) the performance of the nomogram is shown in Figure 4B. Under these conditions the median absolute PE and IQR (%) were 4.07 (IQR –9.50–18.7), 4.37 (IQR –7.05–16.6) and 5.19 (IQR –8.43–17.6). In addition, we tested a few typical adherence patterns to calculate the performance of the nomogram in each specific pattern in the 150 mg daily study arm, as depicted in Figure 5. Pattern 5A reflects a nonuniform pattern across the whole study period. Until day 75, all doses were taken and, subsequently, every 2 days, a dose was not taken, resulting in an overall adherence rate of nearly 90%. Pattern 5B depicts a uniform pattern across the study period, with one dose being omitted each 3 days (overall adherence rate of nearly 66%). Lastly, pattern 5C depicts also a uniform pattern, with one dose being omitted each 2 days (overall adherence rate of 50%). Median absolute PE and IQR (%) for pattern 5A, 5B and 5C were, respectively, –27.97 (IQR –42.42 to –7.23), –0.47 (IQR –16.79–25.65) and 2.54 (IQR –11.05–18.78).
Discussion
Previously, we performed a cross‐sectional observational study reporting a significant association between clozapine use and FL3‐fluorescence 19. FL3‐fluorescence was elevated in clozapine users and found to increase with increasing clozapine dose. However, the timing of onset and maximal increase of FL3‐fluorescence after initiation of clozapine therapy was not quantified. This information would contribute to the evaluation of FL3‐fluorescence being a potential long‐term adherence biomarker. For this, we developed a PK/PD model based on additional data. In the results of our data analysis, two essential factors arose.
First, a significant nonlinear relationship appears to exist between clozapine dose and FL3‐fluorescence evidenced by the Emax model that describes this relationship. Moreover, FL3‐fluorescence based estimations of adherence can only be performed taking the clozapine dose into account. As depicted in Figure 3, the association between adherence and FL3‐fluorescence at a clozapine dose of 50 mg day–1 displays an almost linear relationship, indicating that the nomogram becomes less discriminative at lower clozapine dose, resulting in increased prediction errors (Figure 4).
Second, the half‐life of FL3‐fluorescence elevation after clozapine exposure appeared to be approximately 230 h (CV 35%), which would imply that nearly 48 days (5 times the half‐life) are needed to reach steady state condition of elevated FL3‐fluorescence. This long half‐life makes FL3‐fluorescence an appropriate indicator for long‐term adherence.
As depicted in Figure 5, in case of nonrandom specific adherence patterns, the performance of the nomogram is not expected to change when the adherence pattern is more or less uniform across the whole study period of 100 days, evidenced by the patterns in Figure 5B and Figure 5C. More specifically, random omission of intake leading to short drug holidays (period of consecutively omitting clozapine intake) do not affect the performance of the nomogram, in accordance with the long half‐life of FL3‐fluorescence. This would also indicate that long drug holidays, thereby forcing clozapine intake to follow a nonuniform pattern across the study period, affect the performance of the nomogram. That is, the longer the drug holiday, the more time is given for enhanced FL3 to normalize towards baseline, eventually affecting the predictive performance of the nomogram negatively. In other words, if the patterns change considerably during the study period, results will depend mainly on the pattern in the period preceding the FL3 measurement as evidenced by Figure 5A.
Compared to the average completion over the 100‐day study period, the nomogram gives a biased estimate of adherence. However, an adequate estimation of adherence of the 3 weeks preceding the FL3 measurement is obtained. These results indicate that FL3 measurement could be considered as a potential mid‐term adherence marker estimating adherence over approximately 3 weeks.
In our dataset, we only had data from patients available who started clozapine therapy. Including patients who discontinued therapy would have provided an improved estimate of the half‐life of elevated FL3‐fluorescence, which is the key parameter for establishment of adherence based on this parameter. Unfortunately, FL3 measurements in patients discontinuing clozapine were scarce in our dataset, since, in clinical practice, blood counts are not routinely monitored after cessation of therapy. The findings described above are consistent with our previous cross‐sectional observational study, in which clozapine users who did not show elevated fluorescence appeared to be in the clozapine initiation phase and thus had not yet reached steady state of elevated FL3‐fluorescence.
Clozapine doses of 50, 150 and 400 mg day–1 were chosen for the simulation study, related to clinical practice, since these dosages distinguish geriatric and psychiatric indications, are prescribed as maintenance dose in initial clozapine therapy and prescribed as effective chronic maintenance dose.
The exact mechanism of FL3‐fluorescence increase on clozapine use remains unknown. Considering the half‐life and thus the period needed to reach steady state (approximately 48 days) of FL3‐fluorescence, the physiology of elevated FL3‐fluorescence might be linked to incorporation of this signal in the bone marrow. Indeed, FL3‐fluorescence is observed in neutrophil granulocytes and it is established that duration of myelopoiesis takes a matter of weeks 29.
A limitation of our study is our assumption that patients included in this study were fully adherent to clozapine. However, all participants were inpatients and clozapine intake took place under surveillance.
Another limitation is the lack of potential relevant covariates including smoking status and plasma concentrations. However, the smoking status might have an impact on clozapine exposure, but its influence on FL3‐fluorescence is unknown. Furthermore, it should be noted that the nomogram does not take parameter uncertainty into account. Full evaluation of the predictive performance can only be performed with additional data from patients with known and preferably also suboptimal adherence.
An advantage of FL3‐fluorescence is that this flow‐cytometric marker is determined automatically on the Abbott Cell‐Dyn haematology analyser when a blood count is being measured, which is often the case in clozapine users. For this reason, no additional costs or sample preparation are needed to obtain FL3‐fluorescence. Nevertheless, this specific flow‐cytometric analyser is not available in every clinical setting. Thus, signal output of other flow‐cytometry channels from other flow‐cytometry analysers should be examined and interpreted differently.
To date, as far as we know, a biomarker that could estimate long term clozapine adherence – similar to glycated haemoglobin providing long‐term information about glucose control – has not been established yet. Previously, other long half‐life markers are described in the assessment of adherence, including very low‐dose http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2804 (to investigate adherence in patients on reducing doses of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5458) 30, bromide in hypertensive patients 31 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4815 polyglutamates in juvenile idiopathic arthritis and juvenile dermatomyositis 32. In contrast to FL3 fluorescence, mentioned markers were quite time‐, cost‐ and work‐consuming, therefore hampering use in clinical practice. However, just like FL3 fluorescence, so called drug holidays cannot be assessed due to the long half‐life of the markers. Moreover, levels of such markers would not indicate when the last dose was taken or reflect irregular intake pattern exactly.
The developed nomogram could be of value in forensic psychiatry. Monitoring of adherence to clozapine in this setting is of high importance, especially in patients who can become a danger to their environment in case of nonadherence, including patients admitted under involuntary commitment or by authorization of the court. In these patients, assurance of adequate medication intake, supported by monitoring tools such as the developed nomogram, can contribute to better diagnosis and prognosis as clinicians are provided information on adherence patterns so that they can anticipate and prevent relapses. Moreover, this is valuable due to the lack of a depot formulation for clozapine.
As a suggestion for future studies, we propose to conduct a prospective validation study first comparing FL3 measurements with other forms of adherence measurements (e.g. MEMS). This would ultimately show the predictive performance of the current nomogram and will serve as external model evaluation. Eventually, this could be followed by a prospective validation in clinical settings, linking estimated adherence to clinical outcome measures.
Conclusion
With our proposed nomogram clinicians are offered an additional tool to obtain indicative information regarding long‐term clozapine adherence. Together with TDM, the use of the nomogram could depict any possible nonadherence in a broader context.
Future studies should point out the extent of uniformity in neutrophil fluorescence signal on several different analysers compared to FL3‐fluorescence signal obtained with the Abbott Cell‐Dyn. Also, a prospective follow‐up study comparing true adherence (measured with micro‐electromechanical systems or other adherence mechanisms) with the nomogram estimated adherence should be conducted to further evaluate our established model and nomogram for use in clinical practice.
Competing Interests
The division of Pharmacoepidemiology and Clinical Pharmacology, Utrecht Institute for Pharmaceutical Sciences, employing the authors I.W., E.H., W.S. and T.E., has received unrestricted funding for pharmacoepidemiological research from GlaxoSmithKline, private–public funded TopInstitute Pharma and includes co‐funding from universities, government, and industry, the Dutch Medicines Evaluation Board and the Dutch Ministry of Health.
UPOD is the result of the close collaboration between the Department of Pharmacoepidemiology and Clinical Pharmacology of the Utrecht Institute for Pharmaceutical Sciences of Utrecht University and several departments within UMC Utrecht. We are indebted to all our colleagues involved with the UPOD initiative. Thanks to Dr Mark de Groot for his contributions to the data management in UPOD.
Contributors
All authors participated in drafting this article, revising it critically for important intellectual content and gave final approval of the version to be submitted and any revised version.
Specifically, W.H.M. contributed to the acquisition of data and drafted the article primarily. T.E., E.R.H., I.W., W.v.S., A.P.P. and A.H. contributed to the conception and design of this work. A.P.P. and A.H. performed the data analysis and establishment of the nomogram.
Man, W. H. , Pérez‐Pitarch, A. , Wilting, I. , Heerdink, E. R. , van Solinge, W. W. , Egberts, A. C. G. , and Huitema, A. D. R. (2018) Development of a nomogram for the estimation of long‐term adherence to clozapine therapy using neutrophil fluorescence. Br J Clin Pharmacol, 84: 1228–1237. doi: 10.1111/bcp.13546.
References
- 1. Bromet EJ, Bromet EJ, Fennig S, Fennig S. Epidemiology and natural history of schizophrenia. Biol Psychiatry 1999; 46: 871–881. [DOI] [PubMed] [Google Scholar]
- 2. Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B. Effectiveness of second‐generation antipsychotics in patients with treatment‐resistant schizophrenia: a review and meta‐analysis of randomized trials. Am J Psychiatry 2001; 158: 518–526. [DOI] [PubMed] [Google Scholar]
- 3. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment‐resistant schizophrenic: a double‐blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45: 789–796. [DOI] [PubMed] [Google Scholar]
- 4. Pickar D, Owen RR, Litman RE, Konicki E, Gutierrez R, Rapaport MH. Clinical and biologic response to clozapine in patients with schizophrenia. Crossover comparison with fluphenazine. Arch Gen Psychiatry 1992; 49: 345–353. [DOI] [PubMed] [Google Scholar]
- 5. Reid WH, Mason M, Hogan T. Suicide prevention effects associated with clozapine therapy in schizophrenia and schizoaffective disorder. Psychiatr Serv 1998; 49: 1029–1033. [DOI] [PubMed] [Google Scholar]
- 6. Meltzer HY, Fatemi H. Suicide in schizophrenia: the effect of clozapine. Clin Neuropharmacol 1995; 18: S18–S24. [Google Scholar]
- 7. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry 2003; 64: 1308–1315. [DOI] [PubMed] [Google Scholar]
- 8. Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry 2002; 63: 892–909. [DOI] [PubMed] [Google Scholar]
- 9. Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv 1998; 49: 196–201. [DOI] [PubMed] [Google Scholar]
- 10. Laan W, van der Does Y, Sezgi B, Smeets HM, Stolker JJ, de Wit NJ, et al Low treatment adherence with antipsychotics is associated with relapse in psychotic disorders within six months after discharge. Pharmacopsychiatry 2010; 43: 221–224. [DOI] [PubMed] [Google Scholar]
- 11. Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 2004; 55: 886–891. [DOI] [PubMed] [Google Scholar]
- 12. Caseiro O, Pérez‐Iglesias R, Mata I, Martínez‐Garcia O, Pelayo‐Terán JM, Tabares‐Seisdedos R, et al Predicting relapse after a first episode of non‐affective psychosis: a three‐year follow‐up study. J Psychiatr Res 2012; 46: 1099–1105. [DOI] [PubMed] [Google Scholar]
- 13. Valenstein M, Copeland LA, Blow FC, McCarthy JF, Zeber JE, Gillon L, et al Pharmacy data identify poorly adherent patients with schizophrenia at increased risk for admission. Med Care 2002; 40: 630–639. [DOI] [PubMed] [Google Scholar]
- 14. Taylor DM, Douglas‐Hall P, Olofmjana B, Whiskey E, Thomas A. Reasons for discontinuing clozapine: matched, case‐control comparison with risperidone long‐acting injection. Br J Psychiatry 2009; 194: 165–167. [DOI] [PubMed] [Google Scholar]
- 15. Spina E, Avenoso A, Facciolà G, Scordo MG, Ancione M, Madia AG, et al Relationship between plasma concentrations of clozapine and norclozapine and therapeutic response in patients with schizophrenia resistant to conventional neuroleptics. Psychopharmacology (Berl) 2000; 148: 83–89. [DOI] [PubMed] [Google Scholar]
- 16. Mennickent S, Sobarzo A, Vega M, de Diego M, Godoy G, Rioseco P, et al Determination of clozapine in serum of patients with schizophrenia as a measurement of medication compliance. Int J Psychiatry Clin Pract 2010; 14: 41–46. [DOI] [PubMed] [Google Scholar]
- 17. Flanagan RJ, Spencer EP, Morgan PE, Barnes TRE, Dunk L. Suspected clozapine poisoning in the UK/Eire, 1992–2003. Forensic Sci Int 2005; 155: 91–99. [DOI] [PubMed] [Google Scholar]
- 18. Hoffmann J, Lillholm A. Neutrophil fluorescence in patients using clozapine [abstract]. Ned Tijdschr Klin Chem 2000; 25 (108). [Google Scholar]
- 19. Man WH, ten Berg M, Wilting I, Huisman A, Cahn W, Douma JW, et al Fluorescence of neutrophil granulocytes as a biomarker for clozapine use. Eur Neuropsychopharmacol 2013; 23: 1408–1413. [DOI] [PubMed] [Google Scholar]
- 20. Ten Berg MJ, Huisman A, van den Bemt PM, Schobben AF, Egberts AC, van Solinge WW. Linking laboratory and medication data: new opportunities for pharmacoepidemiological research. Clin Chem Lab Med 2007; 45: 13–19. [DOI] [PubMed] [Google Scholar]
- 21. Groeneveld KM, Heeres M, Leenen LPH, Huisman A, Koenderman L. Immunophenotyping of posttraumatic neutrophils on a routine haematology analyser. Mediators Inflamm 2012; 2012: 509513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang SH, Kim HK, Ham CK, Lee DS, Cho HI. Comparison of four hematology analyzers, CELL‐DYN Sapphire, ADVIA 120, Coulter LH 750, and Sysmex XE‐2100, in terms of clinical usefulness. Int J Lab Hematol 2008; 30: 480–486. [PubMed] [Google Scholar]
- 23. Muller R, Mellors I, Johannessen B, Aarsand AK, Kiefer P, Hardy J, et al European multi‐center evaluation of the Abbott Cell‐Dyn sapphire hematology analyzer. Lab Hematol 2006; 12: 15–31. [DOI] [PubMed] [Google Scholar]
- 24. Huisman A, Stokwielder R, van Solinge WW. Mathematical correction of the invitro storage‐related increase in erythrocyte mean cell volume of an automated hematology analyzer – the Cell‐Dyn 4000. Lab Hematol 2004; 10: 68–73. [PubMed] [Google Scholar]
- 25. Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol 2013; 2: e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ng W, Uchida H, Ismail Z, Mamo DC, Rajji TK, Remington G, et al Clozapine exposure and the impact of smoking and gender: a population pharmacokinetic study. Ther Drug Monit 2009; 31: 360–366. [DOI] [PubMed] [Google Scholar]
- 27. Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012; 73: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cowland JB, Borregaard N. Isolation of neutrophil precursors from bone marrow for biochemical and transcriptional analysis. J Immunol Methods 1999; 232: 191–200. [DOI] [PubMed] [Google Scholar]
- 30. Wolff K, Hay AA, Raistrick D, Feely M. Use of “very low‐dose phenobarbital” to investigate compliance in patients on reducing doses of methadone (detoxification). J Subst Abuse Treat 1993; 10: 453–458. [DOI] [PubMed] [Google Scholar]
- 31. Braam RL, Van Uum SHM, Lenders JWM, Thien T. Bromide as marker for drug adherence in hypertensive patients. Br J Clin Pharmacol 2008; 65: 733–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hawwa AF, AlBawab A, Rooney M, Wedderburn LR, Beresford MW, McElnay JC. Methotrexate polyglutamates as a potential marker of adherence to long‐term therapy in children with juvenile idiopathic arthritis and juvenile dermatomyositis: an observational, cross‐sectional study. Arthritis Res Ther 2015; 17: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]


