Abstract
Introduction
Serodiscordant couples are a priority population for delivery of new HIV prevention interventions in Africa. An integrated strategy of delivering time‐limited, oral pre‐exposure prophylaxis (PrEP) to uninfected partners in serodiscordant couples as a bridge to long‐term antiretroviral treatment (ART) for infected partners has been implemented in East Africa, nearly eliminating new infections. We conducted a qualitative evaluation of the integrated strategy in Uganda, to better understand its success.
Methods
Data collection consisted of 274 in‐depth interviews with 93 participating couples, and 55 observations of clinical encounters between couples and healthcare providers. An inductive content analytic approach aimed at understanding and interpreting couples’ experiences of the integrated strategy was used to examine the data. Analysis sought to characterize: (1) key aspects of services provided; (2) what the services meant to recipients; and (3) how couples managed the integrated strategy. Themes were identified in each domain, and represented as descriptive categories. Categories were grouped inductively into more general propositions based on shared content. Propositions were linked and interpreted to explain “why the integrated strategy worked.”
Results
Couples found “couples‐focused” services provided through the integrated strategy strengthened partnered relationships threatened by the discovery of serodiscordance. They saw in services hope for “getting help” to stay together, turned joint visits to clinic into opportunities for mutual support, and experienced counselling as bringing them closer together.
Couples adopted a “couples orientation” to the integrated strategy, considering the health of partners as they made decisions about initiating ART or accepting PrEP, and devising joint approaches to adherence. A couples orientation to services, grounded in strengthened partnerships, may have translated to greater success in using antiretrovirals to prevent HIV transmission.
Conclusions
Various strategies for delivering antiretrovirals for HIV prevention are being evaluated. Understanding how and why these strategies work will improve evaluation processes and strengthen implementation platforms. We highlight the role of service organization in shaping couples’ experiences of and responses to ART and PrEP in the context of the integrated strategy. Organizing services to promote positive care experiences will strengthen delivery and contribute to positive outcomes as antiretrovirals for prevention are rolled out.
Keywords: HIV prevention, evaluation, mechanism of effect, service delivery, pre‐exposure prophylaxis (PrEP), antiretroviral treatment (ART), implementation, Uganda
1. Introduction
HIV serodiscordant couples – in which one partner is HIV‐infected and the other uninfected – are a priority population for delivery of new HIV‐prevention interventions in Africa. Oral antiretroviral medications are effective in preventing HIV infection, both when taken as treatment (ART) 1, 2, and when taken as pre‐exposure prophylaxis (PrEP) by uninfected persons 3, 4, 5.
The Partners Demonstration Project (Clinicaltrials.gov NCT02775929) was a prospective implementation study evaluating an integrated strategy of delivering ART and PrEP to serodiscordant couples in African public health settings 6, 7. The integrated strategy offered time‐limited PrEP to uninfected partners as a “bridge” to long‐term ART in the infected partner. Uninfected partners were offered PrEP at baseline and encouraged to discontinue once infected partners had used ART for six months. One thousand and thirteen (N = 1013) heterosexual HIV‐1 serodiscordant couples at high risk for HIV infection according to a validated risk score 8 participated. The research took place at four sites in Kenya and Uganda. The evaluation investigated: (1) uptake of and adherence to PrEP and ART, (2) continuing use of PrEP following initiation, and (3) the integrated strategy's effectiveness in preventing HIV infection.
Results revealed the integrated strategy to be highly successful. Rates of uptake of PrEP and ART were 97% and 91% respectively. PrEP adherence was high, as measured by return rates of dispensed pills, electronic medication event monitoring (MEMS) and levels of drug detected in blood plasma 6, 7, 9. Ninety‐five‐percent (95%) of uninfected partners initiating PrEP at enrolment were still receiving it after three months. Only four incident HIV infections occurred across the study population during the two‐year follow‐up period, for an observed HIV‐1 incidence of 0.24 per 100 person‐years. This represents a reduction of 95% in the rate of expected new infections, compared to a counterfactual simulation in which expected HIV incidence was calculated to be 4.9 per 100 person‐years 6, 7.
Outcomes of the Partners Demonstration Project indicate the integrated strategy is an effective, cost‐effective, and implementable approach to delivering PrEP and ART to African serodiscordant couples to prevent HIV infection 6, 7, 10. Once a new model of healthcare delivery has been shown to work, it becomes important to understand how and why it works, to facilitate future replication and scale‐up.
We evaluate the integrated strategy as implemented at two Partners Demonstration Project sites. The evaluation is based on qualitative data. It examines the organization of services and couples’ responses to address the question: Why did the integrated strategy of delivering PrEP and ART to East African discordant couples ‘work’ to prevent transmission of HIV?
2. Methods
2.1. Research setting
The qualitative evaluation was carried out at the Project's two Ugandan sites: the Infectious Diseases Institute – Kasangati, outside Kampala; and the Kabwohe Clinical Research Center, in Kabwohe.
2.2. Sampling and recruitment
Purposeful sampling, in which participants are deliberately selected to construct a varied study sample, supports in‐depth investigation in qualitative research 11. Here, purposeful sampling was used to identify couples with varying experiences of PrEP and ART. We also included couples where the HIV‐uninfected partners declined, as well as accepted, PrEP, and couples in which HIV‐infected partners were both ineligible and eligible for ART at enrolment. (At study outset, only HIV‐infected individuals with CD4 counts ≤350 were eligible for treatment; Ugandan national guidelines were revised in 2016) 12. Demonstration Project participants falling into these categories were referred by Ugandan study staff. Research assistants approached these individuals during clinic follow‐up visits for the Demonstration Project to describe the qualitative research, answer questions, and invite participation. Ninety‐three (N = 93) couples took part.
2.3. Data collection
Two types of qualitative data collection activities were used. Multiple, in‐depth individual and joint interviews were conducted with couples at different time points, to represent variation in experiences of the integrated strategy. Initial interviews were joint interviews, to allow for exploration of relationship dynamics. Subsequent interviews were a combination of individual and joint sessions, depending on the topic or topics to be discussed. Field observations focusing on services provided to couples were also carried out.
2.3.1. In‐depth Interviews
In‐depth interviews were conducted approximately one month after Partners Demonstration Project enrolment, at later points in the follow‐up period, and when key “transitions” in experiences of the integrated strategy occurred. Examples of events prompting “transition” interviews were: (1) ART initiation, (2) PrEP discontinuation, (3) separation from partner, and (4) missed clinic visits. Follow‐up interviews covered a variety of relevant topics, whereas transition interviews focused on examining a particular experience. Two hundred and seventy‐four (N = 274) interviews were completed: 93 initial interviews, 88 “follow‐up” interviews; and 93 “transition” interviews. One hundred and twenty‐six (N = 126) were individual interviews; 55 (44%) of individual interviewees were men; 71 (56%) were women.
Interviews were conducted by trained Ugandan research assistants in local languages (Luganda, Runyankore), using interview guides. Each interview type had a different guide, tailored to the experience being investigated. For example, baseline interview topics included: (1) discovery of serodiscordance, (2) decisions to accept or decline PrEP and ART, (3) early experiences of taking PrEP and ART, (4) understandings of PrEP, (5) impact of PrEP on the partnered relationship, (6) perceptions of the integrated strategy. A sampling of questions used in study interview guides appears in the Appendix.
Interviews were conducted in private settings in locations reflecting interviewee preferences. Participants gave written consent for interviews, which were audio‐recorded and lasted about an hour. Audio‐recordings were transcribed into English by the research assistants. Interview data were collected from November 2013 through December 2016.
2.3.2. Field observations
Field observations are a well‐established data collection technique in qualitative research that involve the presence of a researcher in a naturalistic setting, the witnessing of events and activities of interest, and sometimes a degree of participation 13. Observations provide a direct view of phenomena under study and thus complement the mediated perspective obtained through interview data.
Here, observations focused on clinical encounters between couples and health care providers implementing the integrated strategy, including interactions with counsellors, physicians and pharmacists. Screening/enrolment visits, quarterly follow‐up visits, and study exit visits were observed on randomly selected dates with permission from all parties. Research assistants observed interactions and took notes, but did not actively participate. Fifty‐five observations were completed, lasting 2½ hours on average. Consent for observations was confirmed verbally before each observation session. Observers wrote up what they had seen as field notes – complete narrative descriptions of the interactions. Observational data were collected from March 2014 to March 2016.
2.4. Data quality
Translated transcripts and field notes were reviewed for content and technique in weekly feedback phone calls and emails. Feedback focused on ensuring texts were rendered in clear, standard English. Harvard team members conducted periodic in‐person visits to study sites for additional support and “refresher” trainings.
2.5. Data analysis
An inductive, content analytic approach 14 was used for data analysis. Interview transcripts and field notes were reviewed as they were produced, to provide a general sense of data content and emerging themes. A coding scheme was developed based on the initial review; data were coded using Atlas.ti software. Coded data and field notes were re‐reviewed to formulate themes in three domains: (1) key aspects of services provided, from couples’ perspectives; (2) what these services meant to couples; and (3) how couples managed the integrated strategy. Themes formed the basis for category development. Resulting categories were labelled using statements summarizing their meaning, elaborated to specify their content, and illustrated using excerpts from the data. Categories were grouped inductively based on shared content into more general propositions. Propositions were linked and interpreted to propose an explanation of “why the integrated strategy worked.”
2.6. Ethical approvals
Ethical approvals were obtained from the Committee on Human Studies, Harvard Medical School, Boston MA, USA; the National HIV/AIDS Research Committee of the Ugandan National Council for Science and Technology, Kampala, Uganda; and the University of Washington Institutional Review Board, University of Washington, Seattle, WA, USA.
3. Results
3.1. Participant characteristics
Partners Demonstration Project eligible couples were ≥18 years of age, sexually active, and reported intending to remain together. Characteristics of couples participating in the qualitative study appear in Table 1.
Table 1.
Characteristics of couples participating in the qualitative evaluation study (N = 93 couples)
| Median (IQR) or N (%) | |
|---|---|
| Total | |
| Characteristics, uninfected partner | |
| (Female) sex | 43 (46%) |
| Age, years | 31 (26 to 37) |
| Education, years | 7 (5 to 10) |
| Monthly income, any | 91 (98%) |
| Initiated PrEP at Project enrolment | 82 (88%) |
|
Initiated PrEP at enrolment or during Project follow‐up period |
86 (92%) |
| PrEP Adherence, MEMS cap (MEMS cap bottle openings/expected openings) (N = 84)a | 91% (66 to 98) |
| Characteristics, infected partner | |
| Age, years | 31 (25 to 37) |
| Education, years | 7 (6 to 10) |
| Monthly income, any | 62 (66%) |
| ART eligible, Project enrolment | 61 (66%) |
| CD4 cell count/μL | 472 (293 to 708) |
| Initiated ART within 15 days of Project enrolment, eligible individuals (N = 60) | 40 (67%) |
| Time to ART for eligible individuals not initiating within 15 days of Project enrolment, days (N = 20) | 84 (33 to 168) |
|
Initiated ART at some point during Project follow‐up period (N = 91)b |
91 (100%) |
| Characteristics, couple | |
| Living together, years | 3 (1 to 9) |
| Married to each other | 91 (98%) |
| Children together | 1 (0 to 2) |
| Time since learning about serodiscordance, months | 2 (1 to 12) |
MEMS cap data are not available for 9 participants.
ART initiation data are not available for 2 participants.
Infected and uninfected partners in the qualitative study were in their early thirties. Approximately half (46%) of uninfected partners were female. Eighty‐eight percent (N = 82) of uninfected partners initiated PrEP at enrolment; 66% (N = 61) of infected partners were eligible for ART at enrolment; 67% (N = 40) of eligible individuals initiated ART at enrolment. Median PrEP adherence, measured through electronic monitoring, was 91%.
3.2. Qualitative results
3.2.1. Overview
We propose an explanation for the success of the integrated strategy in preventing HIV transmission in Ugandan serodiscordant couples taking part in the Partners Demonstration Project. The explanation draws together analytic insights in three areas: (a) how services were organized to implement the integrated strategy; (b) how couples experienced this organization; and (c) how couples managed the integrated strategy. Essentially, we propose: (1) services were organized to be “couples‐focused” in ways that couples experienced as strengthening their partnered relationships, and as a result, (2) couples adopted a “couples orientation” to the integrated strategy. In the following Results section, these key findings are summarized as “propositions,” and elaborated using descriptive categories (Subsections of 3.2.2 and 3.2.3). Data excerpts illustrating category content appear in Tables 2 and 3. A summary linking the propositions interpretively completes our proposed answer to the question of “why the integrated strategy worked.”
Table 2.
Data excerpts illustrating proposition 1: couples‐focused services were experienced as strengthening serodiscordant relationships
| Category Summary | Elaboration | Data excerpts |
|---|---|---|
| A. In services designed for serodiscordant couples, couples saw hope for staying together. | “Getting help” to stay together |
“The idea of joining the project] “gave us courage because we expected to get help. When we reached [the Project clinic] we got counselling and got to know the world is like that [serodiscordance can happen]. They provided us with our first help.” –Female, HIV‐uninfected partner, Age 32 “… it is not like we were joining the project to get jobs or other benefits. We saw that joining this study would help us and also others in the world, because the research results might help other people to live together as discordant couples.” –Male, HIV‐infected partner, Age 48 |
| Serodiscordance as a condition of enrolment |
“…We work on serodiscordant couples only. If you are to continue with us, one of you has to be HIV positive and another one HIV negative…” –Field Observation, May 22, 2014 |
|
| B. Attending follow‐up appointments as a couple brought partners together, increasing mutual support. | Travelling and waiting room time provided couples with time for discussion, reflection and joint decision making. |
“…. We travelled together and talked together on the way there. I would say, ‘knowing what the clinic staff say, what do you really think?’ …I could ask her, ‘they told me this, but what should we do?’ We could advise each other.” –Male, HIV‐uninfected partner, Age 24 |
| Accompanying infected partners to clinic to ensure continued access to HIV care. |
“I go to [clinic] to help her. … if I terminate my participation there, …they may not help her.” –Male, HIV‐uninfected partner, Age 37 “… you put up a clinic …for discordant. Can you get medicine from there if you went as an individual? I think you have to go as a couple. [If] my husband goes to the clinic alone, they may … ask him ‘where is your wife?’ So I have to continue going to [name of clinic] to support my husband.” –Female, HIV‐uninfected partner, Age 21 |
|
| C. Partners experienced counselling for HIV prevention as bringing them closer to each other. | Counselling messages characterized HIV as a shared experience to be managed by partners together. |
A pharmacist urges an uninfected partner to take responsibility for reminding his wife to take her medication, as she initiates ART. “The pharmacist advised the participant to remind his wife to take the drugs. The participant replied, ‘But she told the counsellor that she will take them!’ The pharmacist added, ‘Yes, she can say that and then she forgets. It's normal. You remind her, since she is just starting.’” –Field observation, June 25, 2014 |
| Appreciation of guidance on managing the crisis of serodiscordance to avoid separation. |
Woman: “… since we started coming here, we have been getting counselling. When we go back [home] we put what we are taught… into practice. If it was not for counselling, we wouldn't be together. When the counsellor teaches you, you settle down.” Interviewer: [To male partner]: “What can you add, sir?” Man: “Yes, change is there. …. If it had not been for counselling, I think I wouldn't be having my wife up to this time. When we were tested and found out that we are discordant, I thought my wife was going to leave me, since she was negative. But because of counselling, we are together and she is very supportive up to now. She welcomes me when I come home, she cooks food and we eat and she is not discriminating and hating me because of my status.” – Male HIV‐infected partner, Age 28; Female HIV‐uninfected partner, Age 21 |
|
| Guidance on how to behave as a couple was gratefully received and had a positive impact. |
“We were handed to one lady, who welcomed us, then started asking us questions about what we go through as a couple.…We talked to her about our problems…and she counselled us, telling us how we were to conduct ourselves.[She taught us] how to cooperate as a couple because between us there was quarrelling….[She] said, ‘now each of you has to give the other respect. You have to love each other and should not say: why am I positive and you are negative?’…Going to [Demonstration Project clinic site] helped me so much because my home is now peaceful. We are one, we love each other, and we cooperate, compared to back before we went there. Those people have done a good job in our lives.” –Female, HIV‐uninfected partner, Age 44 |
|
| D. Simultaneous use of ARVs turned management of HIV into a shared experience. | Experience of taking antiretrovirals together helped couples “settle” … back into their relationship. |
Man: “I think it is better for a negative partner to be given PrEP at the same time a positive partner is taking ART …It has helped us; it introduced peace at home. … Because we both take medicine and we take it at the same time, we remind ourselves, sit together and take [the pills] and each one asks the other how he or she feels. It has continued to make us settle. Woman: By him seeing me taking PrEP without quarrelling, like ‘you brought HIV and you are making me take medicine,’ that made him settled and also to love me more. This was because I did not quarrel or treat him in a bad way. I could bring water and sometimes open the tin, remove medicine and give him to take whenever it was time to take medicine.” – Female, HIV‐uninfected partner, Age 32, Male, HIV‐infected partner, Age 33 |
Table 3.
Data excerpts illustrating proposition 2: couples adopted a “couples‐orientation” to the integrated strategy
| Category summary | Elaboration | Data excerpts |
|---|---|---|
| E. Concern for partner wellbeing was a reason for initiating ART. | Desire to protect one's partner as a reason for initiating ART |
“I told her, ‘I am going to use ARVs purposely for you because I want you to be protected.’ That is what I told her. I was not to take it for myself. I was not going to start ARVs if it was for me.” –Male, HIV‐infected partner, Age 30 M: “…I told them that I want to be the first security towards my wife.” I: “What do mean by security?” M: “Although she is swallowing [PrEP], it is I who has to be her first security. … I have to be her security and know how I handle her so that she does not get the virus.” –Male, HIV‐infected partner, Age 40 |
| F. Reinforcing the partnered relationship was a reason to accept PrEP. | Resolving the serodiscordance crisis as a reason for accepting PrEP |
“… we started loving each other when we were still young. We promised each other love until death, so when I suggested separation after I found out that we are discordant, she did not want to breach our agreement. … She told me ‘Let me take PrEP to protect myself from HIV other than separating from you because I still love you.’”
– Male, HIV‐infected partner, Age 24 |
| G. Couples devised joint strategies for adhering to PrEP and ART. | Mutual reminders |
“… when I am with her in the house and the time to swallow her medicine arrives, I remind her. She also reminds me when time arrives to swallow my medicine, because we help each other and we agree with each other.” –Male, HIV‐uninfected partner, Age 36 |
|
Emotional and material support for adherence |
“He takes ART well and … I also take good care of him. I do not worry him, I give him food in time, I make sure I give him drinks like passion juice, fruits and that helps him.” –Female, HIV‐uninfected partner, Age 32 |
3.2.2. Proposition 1: Couples‐focused services were experienced as strengthening serodiscordant relationships
In services designed for serodiscordant couples, couples saw hope for staying together
Testing for HIV and learning that one partner was HIV negative and the other HIV positive came as a shock to most couples, who until that moment may not have known serodiscordance was possible. Typically, discovery of serodiscordance created a crisis for partnered relationships. Neither partner wished to abandon the relationship, but no alternative to separation seemed available.
Couples struggling with the crisis of serodiscordance saw in the Partners Demonstration Project hope for avoiding separation. Knowing little before their first visit, they understood only that the Project represented an opportunity for people “like them.” Serodiscordance was poorly understood in local communities, and the use of antiretrovirals for prevention was unknown. Couples were initially drawn to the idea of participation as a way of “getting help” to stay together (Table 2, A).
The Project was perceived in local communities as a service for serodiscordant couples, as well as a research study. That couples, and not individuals, were to be the “unit of service” was consistently communicated. During introductory visits with couples who might participate, project staff made it clear that serodiscordance was a condition of enrolment, and made the meaning of the term explicit (Table 2, A). Word spread through the community that new services designed specifically for serodiscordant couples were available.
Attending follow‐up appointments as a couple brought partners together, increasing mutual support
Couples were asked to attend follow‐up appointments jointly. During these visits, couples sat together in consultations with clinicians, counsellors, and pharmacists. In this way, receiving healthcare became a shared experience: partners heard the same information at the same time, and asked and had their questions answered as a couple.
Joint visits represented for couples an opportunity to spend time together. Visits afforded time to talk, a luxury not routinely available during busy days filled with work and child care. Travelling to and from clinic, and waiting together between consultations, couples had time to discuss what they were hearing, reflect, and make decisions together (Table 2, B).
Being together at clinic visits was an occasion for mutual support. Infected persons interpreted their partner's presence at clinic visits as a sign of continued interest and willingness to invest in the relationship. Uninfected partners saw themselves as supporting their infected spouses during visits by ‘distracting’ them with conversation. Some uninfected partners – believing the visit was more for the “sick” partner than for them – felt they were showing support simply by being there. These individuals saw themselves as accompanying their partners to clinic to ensure continued access to HIV care (Table 2, B).
Partners experienced counselling for HIV prevention as bringing them closer to each other
Counselling for participating couples took place at enrolment and follow‐up visits. Through standardized messages developed for the Project, counselling provided education on serodiscordance, use of PrEP and ART for prevention, and the integrated strategy of delivery 15. Counsellors presented PrEP as an HIV prevention tool for use during periods of greatest HIV risk, rather than as a life‐long intervention 15. Messages were communicated across the various components of follow‐up visits, that is, not only in interactions with Project counsellors, but also in consultations with physicians and pharmacists.
The serodiscordant relationship was treated as a resource for HIV prevention in developing counselling messages 15. In practice, we see how particular messages that at one level target HIV prevention, at another level may function to bring partners closer together. Explanations that serodiscordance is real, but need not signal the end of a relationship eliminated the need for separation to prevent HIV transmission. Cautions against sexual relations with outside partners encouraged partners to turn toward each other. Recommending that partners take responsibility for reminding each other to practice HIV prevention – through regular condom use, and daily medication adherence – characterized HIV infection as a shared experience, to be managed by partners together (Table 2, C).
Couples appreciated and came to rely on the counselling they received during follow‐up visits. They experienced project staff as caring individuals who treated them with respect, listened to their concerns, and continuously provided encouragement and support. High praise was offered, in particular, for what couples experienced as guidance on managing the crisis of serodiscordance to avoid separation (Table 2, C).
Interviewees also credited other forms of counselling support with strengthening, even saving, their relationships. Reassurance that with treatment, HIV could be managed as a chronic illness, alleviated concerns about early death and disability, calming fears and ending quarrels. Encouraging shared responsibility for dosing with PrEP and ART turned adherence into a joint enterprise. Finally, counsellors’ clear and specific instructions on how to behave as a couple – what to do to get along – were gratefully received and had a positive impact (Table 2, C).
Simultaneous use of ARVs turned management of HIV into a shared experience
The integrated strategy meant both partners used antiretrovirals simultaneously for several months. Taking antiretrovirals during the same time period created a sense of HIV as a shared experience – a situation partners handled together. Over time, the experience of taking antiretrovirals together helped couples move past the shock and upset that accompanied discovery of serodiscordance, and “settle,” as they put it, back into their relationship (Table 2, D).
3.2.3. Proposition 2: Couples adopted a “couples orientation” to the integrated strategy
The integrated strategy required that infected partners initiate ART, uninfected partners “accept” PrEP, and both adhere to the medication. Couples could be seen prioritizing and drawing upon their relationship as they negotiated these expectations.
Concern for partner wellbeing was a reason for initiating ART
Ugandan national guidelines defining ART eligibility became more inclusive during the Partners Demonstration Project. This meant many infected persons had the option of beginning treatment before experiencing symptoms. At the same time, participating couples were also learning about the use of ART for prevention.
Accordingly, infected persons’ rationales for beginning treatment broadened beyond a focus on preserving personal health, to include a concern for partner wellbeing. When asked why they were initiating ART, interviewees not infrequently cited a desire to please and/or protect their partner (Table 3, E).
Reinforcing the partnered relationship was a reason to accept PrEP
A desire to reinforce the partnered relationship shaped HIV‐uninfected partners’ decisions to accept PrEP. Some saw PrEP use as a way of expressing solidarity with partners taking ART. Others embraced PrEP as a means of remaining uninfected while staying in the serodiscordant relationship. Resolving the serodiscordance crisis was a powerful motive for taking a chance with this unknown medication, in that it meant preserving emotional ties and honouring long‐standing commitments (Table 3, F).
Couples devised joint strategies for adhering to PrEP and ART
Couples understood the importance of good adherence, and they tried hard to take PrEP and ART as recommended – at the same time, every day. Typically, they approached adherence jointly – sharing responsibility for making sure each took his/her medication correctly. Shared responsibility for adherence took various forms. Three of these were: (a) dosing together, (b) mutual reminders and (c) emotional and material support.
Couples who dosed together took their pills in each other's presence. For these couples, dosing had a ritual quality. They would come together, set out the pills, bring water to take them with, and sit face‐to‐face while taking the medication, sometimes drinking from the same glass. Some couples experienced dosing together as bringing them closer as a couple.
Couples who did not dose together nevertheless made a point of knowing each other's dosing schedules and following each other's dosing behaviours. If one partner had not seen the other taking the medication at dosing time, s/he was likely to offer a reminder. Reminders were communicated in person, and via phone calls and/or texts (Table 3, G).
Partners also made efforts to provide each other with emotional and material support for adherence. They encouraged each other to continue taking pills daily, and worked to create a nurturing environment that would make the task of daily pill‐taking easier. Male partners provided money to ensure medications could be taken with food. Women prepared food, worked to create a stable daily routine, and tried to limit stress for partners taking ART (Table 3, G).
3.2.4. Linking propositions to explain “Why the integrated strategy worked”
We offer two propositions, drawn from qualitative data, to explain ‘why the integrated strategy worked’. The first is that couples experienced the integrated strategy's “couples‐focused” approach to service organization as strengthening serodiscordant relationships. The second is that couples tended to adopt a “couples orientation” to the integrated strategy.
We propose that this couples orientation arose, at least in part, from the new solidarity couples derived from couples‐focused services. Their management of the integrated strategy may have been more effective as a result. Better management of the integrated strategy may have translated to a favourable impact on ART initiation, PrEP acceptance, and PrEP and ART adherence – lowering rates of HIV transmission, and helping to make the integrated strategy a success (Figure 1).
Figure 1.
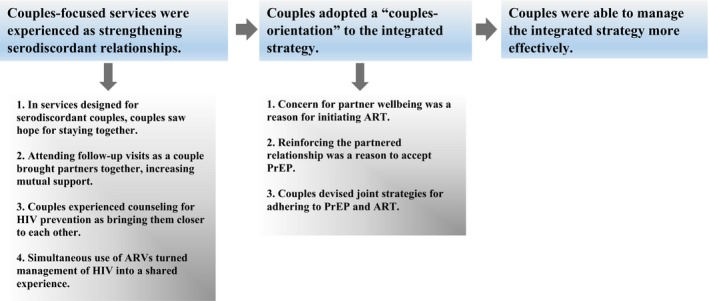
A proposed explanation of why integrated delivery of PrEP and ART to East African serodiscordant couples “worked” to prevent HIV transmission.
4. Discussion
The process of making PrEP and “immediate” ART available outside research settings is gaining momentum in Africa. Informing this process is currently a priority for research. The integrated strategy of delivering PrEP and ART to serodiscordant couples has been shown to be implementable and effective. We have unpacked the implementation process in Uganda to posit a mechanism of effect.
Much qualitative research on antiretrovirals for prevention in Africa reports user perspectives – attitudes, knowledge, preferences, and influences on the use of PrEP and immediate ART 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26. Qualitative methods have also been used to shed light on reasons underlying suboptimal PrEP adherence in the VOICE and FEM‐PrEP clinical trials 27, 28, 29, 30, 31, 32.
This study draws on user perspectives to look for insights into outcomes of the Partners Demonstration Project. We focus on service delivery in the integrated strategy, and on how participating couples responded. Other PrEP demonstration projects, as well as combination prevention and rapid treatment studies, employ diverse strategies for delivering interventions 33, 34, 35, 36, 37. This qualitative evaluation study is among the first to examine how these strategies are put into practice and what they mean to those involved, thereby helping to explain their mechanisms of effect 38.
Results of this study overlap with a conceptual model of health behaviour change proposed by Lewis et al. (2006) 39. The model lays out a mechanism explaining how being part of a couple may shape individual health behaviours. The logic is that a couple's interdependence leads partners to perceive threats to health as impacting the relationship. This perception prompts a “communal coping” response in which couples work together to reduce health threats. We have seen how HIV infection is experienced as weakening partnered relationships for serodiscordant couples in this and other studies 40. We have also seen partners thinking in relational terms when deciding to accept PrEP (“to stay together”) and initiate ART. “Communal coping” is reflected in the joint approach couples may adopt to adherence. In these ways, our findings corroborate Lewis et al.'s “interdependence and communal coping” model of health behaviour.
Serodiscordant couples participating in this study experienced integrated strategy services as strengthening their relationship. Nevertheless, not all couples remained together. Twenty‐one (23%) couples in the qualitative study reported ending their relationship after enrolling in the Partners Demonstration Project. Many of these couples struggled with additional challenges that may or may not have stemmed from discovery of serodiscordance. Among them were perceived infidelities, refusal to practice HIV prevention, and failure to provide material support.
Results of this qualitative evaluation amplify results of the Partners Demonstration Project itself. Several limitations should be recognized. First, our proposed explanation is intended as a partial explanation. We acknowledge the contributions of factors other than service organization (and medication efficacy) to the integrated strategy's success. Second, as qualitative research, this study does not claim generalizability of results. Results should, however, be transferable in ways that inform future implementation of the integrated strategy in African public health settings. Third, the proposed explanation should be understood as suggestive, rather than definitive. Framing the explanation as propositions deliberately sets the stage for critical examination through additional research. Fourth, our analysis is based on data from the Partners Demonstration Project's Ugandan sites only. Any differences in how the integrated strategy was implemented in Kenya are not captured here. Finally, member‐checking was not part of this qualitative research design.
5. Conclusions
Various strategies for delivering antiretrovirals for HIV prevention are being evaluated – in Africa and elsewhere. An understanding not only of whether, but how and why these strategies work (or do not work) should be part of the evaluation process, to create the strongest possible implementation platforms. Thorough understanding requires a broad focus that acknowledges the complexity of implementation processes and incorporates multiple intervention components.
We highlight the role of service organization as a component of effective strategies for delivering antiretrovirals for HIV prevention. In the integrated strategy, services organized to be “couples‐focused” strengthened relationships threatened by serodiscordance. We propose strengthened partnered relationships improved couples’ responses to and use of PrEP and ART by fostering a “couples orientation”. Organizing services to promote positive care experiences will strengthen delivery and contribute to positive outcomes as antiretrovirals for prevention are rolled out.
Competing interests
The authors have no competing interests to declare.
Authors’ contributions
NCW and MAW designed the qualitative research. NCW led the data analytic process and wrote and revised the manuscript. MAW and EEP provided general supervision for the data collection process in Uganda, contributed to the data analytic process and reviewed and commented on early drafts of the manuscript. TRM, ENJ, ETK, SBA and BT supervised data collection at the Ugandan sites. JMB, CLC and RAH provided feedback on emerging findings from the qualitative study and reviewed and provided comments on a preliminary draft of the manuscript. All authors critically reviewed and approved the current version of the manuscript.
Acknowledgements
We are grateful to the couples who contributed to this study by taking part in interviews and agreeing to observations. Justine Abenaitwe, Robert Baijuka, Brenda Kamusiime, Jackie Karuhanga, Vicent Kasiita, Grace Kakoola Nalukwago, Florence Nambi and John Bosco Tumuhairwe collected the qualitative data. Melanie Tam provided support to the staff, and assisted with coding the data. Katherine K. Thomas and Lara Kidoguchi provided data on characteristics of qualitative evaluation study participants. Andrew Mujugira gave comments and suggestions on the manuscript. We thank the Honorable Elioda Tumwesigye for his guidance and support. Research and clinical staff at the Infectious Diseases Institute – Kasangati, Kampala, Uganda, and the Kabwohe Clinical Research Center, Kabwohe, Uganda, provided general support for the research.
Funding
This work was funded by the National Institute of Mental Health of the US National Institutes of Health (R01 MH101027, Norma C. Ware, PI). The Partners Demonstration Project was funded by the National Institute of Mental Health of the US National Institutes of Health (grant R01 MH095507), the Bill & Melinda Gates Foundation (grant OPP1056051), and through the generous support of the American people through the US Agency for International Development (cooperative agreement AID‐OAA‐A‐12‐00023). Gilead Sciences donated the PrEP medication but had no role in data collection or analysis. The results and interpretation presented here do not necessarily reflect the views of the study funders.
Appendix 1.
1.1.
| Interview type | Sample questions from study interview guides |
|---|---|
| Initial interview: Joint | How did you find out about being serodiscordant? |
| How do you feel about serodiscordance? | |
| After you were given your [screening test] results, what did you tell the staff you decided to do about taking PrEP (and ART, if eligible)? How did you make this decision? | |
| In this study, HIV‐uninfected partners are offered PrEP to reduce the risk of HIV infection when the (HIV‐infected) partner is [waiting to start ART (for ineligible)] or [has just started ART (for ART eligible)]. What is your opinion of this? | |
| What do you think the possibility of [HIV‐uninfected partner] acquiring HIV now that you both are in the Demonstration Project? Why? What about before you joined the study? | |
| Transition interview: PrEP discontinuation | How do you feel about stopping PrEP? |
| How will you protect yourself from acquiring HIV? What changes will you make now that you've stopped PrEP, if any? | |
| If there was an opportunity for you to resume taking PrEP, would you? Why? | |
| When was the most recent time you missed taking a dose of your PrEP? What happened? | |
| Transition interview: separation | What led you and your partner to separate? |
| How has your separation from your partner changed your participation in the Demonstration Project? | |
| Since separating with your study partner, what changes have there been in the way you take your medicine? | |
| Final interview | While [participating in this project], you and your study partner were offered ART and PrEP as part of an HIV prevention strategy to prevent the uninfected partner from acquiring HIV. How do you feel this prevention strategy has worked for you and your partner? |
| Tell me how it was when your partner was taking ARVs and you were taking PrEP at the same time. | |
| Tell me about your last Demonstration Project follow‐up visit. Who did you see? What did you discuss? | |
| You continued attending Kasangati clinic for approximately two years. What were some of the reasons you continued to go to the clinic after you stopped taking PrEP? |
Ware, N. C. , Pisarski, E. E. , Nakku‐Joloba, E. , Wyatt, M. A. , Muwonge, T. R. , Turyameeba, B. , Asiimwe, S. B. , Heffron, R. A. , Baeten, J. M. , Celum, C. L. , Katabira, E. T. Integrated delivery of antiretroviral treatment and pre‐exposure prophylaxis to HIV‐1 serodiscordant couples in East Africa: a qualitative evaluation study in Uganda. J Int AIDS Soc. 2018; 21(5):e25113
References
- 1. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV‐1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV‐positive partner is using suppressive antiretroviral therapy. JAMA 2016;316(2):171–81. [DOI] [PubMed] [Google Scholar]
- 3. Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. [DOI] [PubMed] [Google Scholar]
- 5. Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baeten JM, Heffron RA, Kidoguchi L, Mugo N, Katabira E, Bukusi E, et al. Integrated delivery of antiretroviral treatment and pre‐exposure prophylaxis to HIV‐1‐serodiscordant couples: a prospective implementation study in Kenya and Uganda. PLoS Med. 2016;13(8):e1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heffron R, Ngure K, Odoyo J, Bulya N, Tindimwebwa E, Hong T, et al. Pre‐exposure prophylaxis for HIV‐negative persons with partners living with HIV: uptake, use, and effectiveness in an open‐label demonstration project in East Africa. Gates Open Res. 2017;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kahle EM, Hughes JP, Lingappa JR, John‐Stewart G, Celum C, Nakku‐Joloba E, et al. An empiric risk scoring tool for identifying high‐risk heterosexual HIV‐1‐serodiscordant couples for targeted HIV‐1 prevention. J Acquir Immune Defic Syndr. 2013;62(3):339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haberer JE, Kidoguchi L, Heffron R, Mugo N, Bukusi E, Katabira E, et al. Alignment of adherence and risk for HIV acquisition in a demonstration project of pre‐exposure prophylaxis among HIV serodiscordant couples in Kenya and Uganda: a prospective analysis of prevention‐effective adherence. J Int AIDS Soc. 2017;20(1):21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ying R, Sharma M, Heffron R, Celum CL, Baeten JM, Katabira E, et al. Cost‐effectiveness of pre‐exposure prophylaxis targeted to high‐risk serodiscordant couples as a bridge to sustained ART use in Kampala, Uganda. J Int AIDS Soc. 2015;18(4 Suppl 3):20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patton MQ. Qualitative research and evaluation methods, 3rd edn Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 12. Ministry of Health [Internet]. Consolidated guidelines for prevention and treatment of HIV in Uganda, December 2016. [cited 2017 June 21]. Available from: https://elearning.idi.co.ug/pluginfile.php/83/mod_page/content/50/CONSOLIDATED%20GUIDELINES%20FOR%20PREVENTION%20AND%20TREATMENT%20OF%20HIV%20IN%20UGANDA.PDF.
- 13. Adler PA, Adler P. Observational techniques In: Denzin NK, Lincoln YS, (editors). Handbook of qualitative research. Thousand Oaks, London, New Delhi: Sage Publications; 1994: pp. 377–92. [Google Scholar]
- 14. Hsieh HS, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277. [DOI] [PubMed] [Google Scholar]
- 15. Morton JF, Celum C, Njoroge J, Nakyanzi A, Wakhungu I, Tindimwebwa E, et al. Partners Demonstration Project team counseling framework for HIV‐serodiscordant couples on the integrated use of antiretroviral therapy and pre‐exposure prophylaxis for HIV prevention. J Acquir Immune Defic Syndr. 2017;74(Suppl 1):S15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curran K, Ngure K, Shell‐Duncan B, Vusha S, Mugo NR, Heffron R, et al. ‘If I am given antiretrovirals I will think I am nearing the grave’: kenyan HIV serodiscordant couples’ attitudes regarding early initiation of antiretroviral therapy. AIDS. 2013;28(2):227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fowler N, Arkell P, Abouyannis M, James C, Roberts L. Attitudes of serodiscordant couples towards antiretroviral‐based HIV prevention strategies in Kenya: a qualitative study. J Int AIDS Soc. 2014;17(4 Suppl 3):19563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Namey E, Agot K, Ahmed K, Odhiambo J, Skhosana J, Guest G, et al. When and why women might suspend PrEP use according to perceived seasons of risk: implications for PrEP‐specific risk‐reduction counseling. Cult Healh Sex. 2016;18(9):1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ngure K, Heffron R, Curran K, Vusha S, Ngutu M, Mugo N, et al. ‘I knew I would be safer’: experiences of Kenyan HIV serodiscordant couples soon after pre‐exposure prophylaxis (PrEP) initiation. AIDS Patient Care & STDs. 2016;30(2):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel RC, Odoyo J, Anand K, Stanford‐Moore G, Wakhungu I, Bukusi EA, et al. Facilitators and barriers of antiretroviral therapy initiation among HIV discordant couples in Kenya: qualitative insights from a pre‐exposure prophylaxis implementation study. PLoS ONE. 2016;11(12):e0168057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel RC, Stanford‐Moore G, Odoyo J, Pyra M, Wakhungu I, Anand K, et al. ‘Since both of us are using antiretrovirals, we have been supportive to each other’: facilitators and barriers of pre‐exposure prophylaxis use in heterosexual HIV serodiscordant couples in Kisumu, Kenya. J Int AIDS Soc. 2016;19:21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karuga RN, Njenga SN, Mulwa R, Kilonzo N, Bahati P, O'reilley K, et al. ‘How I wish this thing was initiated 100 years ago!’: willingness to take daily oral pre‐exposure prophylaxis among men who have sex with men in Kenya. PLoS ONE. 2016;11(4):e0151716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. King R, Kim J, Nanfuka M, Shafic M, Nyonyitono M, Galenda F, et al. ‘I do not take my medicine while hiding’ ‐ a longitudinal qualitative assessment of HIV discordant couples’ beliefs in discordance and ART as prevention in Uganda. PLoS ONE. 2017;12(1):e0169088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Falcao J, Ahoua L, Zerbe A, di Mattei P, Baggaley R, Chivurre V, et al. Willingness to use short‐term oral pre‐exposure prophylaxis (PrEP) by migrant miners and female partners of migrant miners in Mozambique. Cult Health Sex. 2017;4:1–15. [DOI] [PubMed] [Google Scholar]
- 25. Amico KR, Wallace M, Bekker LG, Roux S, Atujuna M, Sebastian E, et al. Experiences with HPTN 067/ADAPT study‐provided open‐label PrEP among women in Cape Town: facilitators and barriers within a mutuality framework. AIDS Behav. 2017;21(5):1361–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van der Elst EM, Mbogua J, Operario D, Mutua G, Kuo C, Mugo P, et al. High acceptability of HIV pre‐exposure prophylaxis but challenges in adherence and use: ualitative insights from a phase I trial of intermittent and daily PrEP in at‐risk populations in Kenya. AIDS Behav. 2013;17(6):2162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Straten A, Stadler J, Luecke E, Laborde N, Hartmann M, Montgomery ET, et al. Perspectives on use of oral and vaginal antiretrovirals for HIV prevention: the VOICE‐C qualitative study in Johannesburg, South Africa. J Int AIDS Soc. 2014;17(3 Suppl 2):19146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Straten A, Stadler J, Montgomery E, Hartmann M, Magazi B, Mathebula F, et al. Women's experiences with oral and vaginal pre‐exposure prophylaxis: the VOICE‐C qualitative study in Johannesburg, South Africa. PLoS ONE. 2014;9(2):e89118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corneli A, Perry B, McKenna K, Agot K, Ahmed K, Taylor J, et al. Participants’ explanations for nonadherence in the FEM‐PrEP Clinical Trial. J Acquir Immune Defic Syndr. 2016;71(4):452–61. [DOI] [PubMed] [Google Scholar]
- 30. Corneli A, Perry B, Agot K, Ahmed K, Malamatsho F, Van Damme L. Facilitators of adherence to the study pill in the FEM‐PrEP clinical trial. PLoS One. 2015;10(4):e0125458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Corneli AL, McKenna K, Perry B, Ahmed K, Agot K, Malamatsho F, et al. The science of being a study participant: FEM‐PrEP participants’ explanations for overreporting adherence to the study pills and for the whereabouts of unused pills. J Acquir Immune Defic Syndr. 2015;68(5):578–84. [DOI] [PubMed] [Google Scholar]
- 32. Montgomery ET, van der Straten A, Stadler J, Hartmann M, Magazi B, Mathebula F, et al. Male partner influence on women's HIV prevention trial participation and use of pre‐exposure prophylaxis: the importance of ‘understanding’. AIDS Behav. 2015;19(5):784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gomez GB, Eakle R, Mbogua J, Akpomiemie G, Venter WD, Rees H. Treatment and prevention for female sex workers in South Africa: protocol for the TAPS demonstration project. BMJ Open. 2016;6(9):e011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. PrEP Watch [Internet]. Nigeria National Agency for the Control of AIDS Demonstration Project, [cited 2017 May 19]. Available from: http://www.prepwatch.org/naca-demo-project/
- 35. PEPFAR , Bill and Melinda Gates Foundation , GirlEffect , Johnson & Johnson , ViiV Healthcare , Gilead . DREAMS Core package of interventions summary. 2017. [cited July 16]. Available from: http://www.dreamspartnership.org/#welcome
- 36. Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On‐demand preexposure prophylaxis in men at high risk for HIV‐1 infection. N Engl J Med. 2015;373(23):2237–46. [DOI] [PubMed] [Google Scholar]
- 37. HPTN 067 The ADAPT study: a phase II, randomized, open‐label, pharmacokinetic and behavioral study of the use of intermittent oral Emtricitabine/Tenofovir Disoproxil Fumarate pre‐exposure prophylaxis (PrEP) [Internet]. [cited 2017 May 19]. Available from: https://www.hptn.org/sites/default/files/2016-05/067ProtocolV3_01Dec2011_0.pdf.
- 38. Semitala FC, Camlin CS, Wallenta J, Kampire L, Katuramu R, Amanyire G, et al. Understanding uptake of an intervention to accelerate antiretroviral therapy initiation in Uganda via qualitative inquiry. J Int AIDS Soc. 2017;20:e25033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lewis MA, McBride CM, Pollak KI, Puleo E, Butterfield RM, Emmons KM. Understanding health behavior change among couples: an interdependence and communal coping approach. Soc Sci Med. 2006;62:1369–80. [DOI] [PubMed] [Google Scholar]
- 40. Ware NC, Wyatt MA, Haberer JE, Baeten JM, Kintu A, Psaros C, et al. What's love got to do with it? Explaining adherence to oral antiretroviral pre‐exposure prophylaxis for HIV‐serodiscordant couples. J Acquir Immune Defic Syndr. 2012;59(5):463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


