Abstract
The “at risk mental state” for psychosis approach has been a catalytic, highly productive research paradigm over the last 25 years. In this paper we review that paradigm and summarize its key lessons, which include the valence of this phenotype for future psychosis outcomes, but also for comorbid, persistent or incident non‐psychotic disorders; and the evidence that onset of psychotic disorder can at least be delayed in ultra high risk (UHR) patients, and that some full‐threshold psychotic disorder may emerge from risk states not captured by UHR criteria. The paradigm has also illuminated risk factors and mechanisms involved in psychosis onset. However, findings from this and related paradigms indicate the need to develop new identification and diagnostic strategies. These findings include the high prevalence and impact of mental disorders in young people, the limitations of current diagnostic systems and risk identification approaches, the diffuse and unstable symptom patterns in early stages, and their pluripotent, transdiagnostic trajectories. The approach we have recently adopted has been guided by the clinical staging model and adapts the original “at risk mental state” approach to encompass a broader range of inputs and output target syndromes. This approach is supported by a number of novel modelling and prediction strategies that acknowledge and reflect the dynamic nature of psychopathology, such as dynamical systems theory, network theory, and joint modelling. Importantly, a broader transdiagnostic approach and enhancing specific prediction (profiling or increasing precision) can be achieved concurrently. A holistic strategy can be developed that applies these new prediction approaches, as well as machine learning and iterative probabilistic multimodal models, to a blend of subjective psychological data, physical disturbances (e.g., EEG measures) and biomarkers (e.g., neuroinflammation, neural network abnormalities) acquired through fine‐grained sequential or longitudinal assessments. This strategy could ultimately enhance our understanding and ability to predict the onset, early course and evolution of mental ill health, further opening pathways for preventive interventions.
Keywords: At risk mental state, psychosis, ultra high risk, transition, transdiagnostic psychiatry, clinical staging, CHARMS, prediction strategies, network theory, dynamical systems theory, joint modelling
Traditional approaches to psychiatric diagnosis have struggled to guide the care of patients and to illuminate the causes and mechanisms underlying mental ill health. Consequently and appropriately they are under constant critique. There has been very little innovation in over a century in how we conceptualize and classify mental illness, and what has passed for advances really only represent efforts to buttress a flawed paradigm.
How can we transcend a century of stagnation to pave the way for more effective mental health care which makes sense to clinicians, researchers and the public? A quarter of a century after the formulation of the concept of the “at risk mental state”, we could be on the cusp of transforming how we approach the challenge of defining and treating mental illnesses. In this paper we discuss how this transformational concept can point the way to a radical rethink with greater clarity, utility and validity.
“AT RISK MENTAL STATES”: ORIGINS AND BALANCED REVIEW
How did the at risk/clinical high risk/ultra high risk (UHR) concept originate, and what was the strategic intent behind it?
It had been well known for over a century that severe forms of mental disorder, notably schizophrenia, are typically preceded by a relatively non‐specific period of symptoms, which are subthreshold in nature and of insufficient severity and clarity to justify a diagnosis. Seen through the deterministic lens of 19th century nosology, the term “prodrome”, with its sense of inevitable progression, seemed to capture the concept well.
However, if a preventive approach to treatment of potentially serious mental disorders was going to be developed, this deterministic and fatalistic mindset had to change. The deadly nexus between diagnosis and prognosis within the concept of schizophrenia had to be severed or dramatically loosened. Prognosis had to be regarded as something that was malleable, and recovery as something possible. This objective was behind the decision to widen the focus to early psychosis and include the full spectrum of psychotic disorders, remaining agnostic about the future evolution of disorder1, 2.
This approach made further sense because so many clinical pictures were admixtures of mood and psychotic disorder and could only be arbitrarily assigned according to a binary schizophrenia/psychotic mood disorder system. Only around 60% of first episode psychosis patients met operational criteria for schizophrenia or schizophreniform disorder3. First episode psychosis was viewed as an early stage of psychotic illness which could have heterogeneous outcomes, from full remission to evolution in either direction along a spectrum from psychotic mood disorder to schizophrenia, with variable levels of associated functional impairment. The fact that overlapping and fluctuating outcomes did frequently occur supported the decision to select a wide boundary for entry.
This thinking extended to the re‐conceptualization of the prodromal period as an “at risk mental state” rather than as a fixed entity, one that might resolve fully, persist, or progress in several possible directions. This has been borne out by empirical data showing that approximately 36% of “at risk mental state” patients transition to psychosis within three years, approximately a third have persistent attenuated psychotic symptoms, and a third remit from symptoms4, 5. Transition was felt to be a crucial concept to operationally define the progression from subthreshold or inconsistent positive psychotic symptoms to sustained full threshold symptoms.
While our goal in treatment is optimizing functional outcomes, transition is a significant event connoting a likely more serious illness and certainly mandating a change in treatment, namely the use of antipsychotic medications. This was transition to psychosis, not schizophrenia, and it was felt to be important to define it in this particular way, to link it to a critical treatment decision. It is a potentially different question whether there might be a qualitative change in underlying neurobiology at that same specific point or any other6. Once again, only 60% of those transitioning would attract a diagnosis of schizophrenia or schizophreniform disorder.
As it later developed, however, the early psychosis field remained somewhat split as to whether to broaden the focus to the full spectrum of psychosis, with many, especially in North America and parts of Europe, still adhering to faith in the validity of the schizophrenia concept. Hence, many first episode psychosis programs were essentially aiming to be first episode schizophrenia programs, which had a flow on effect when they later embraced the UHR paradigm.
The target of the UHR strategy is not schizophrenia, but psychosis. The tenacity of the schizophrenia focus has fuelled in part some recent critiques, including that by van Os and Guloksuz7 in a previous issue of this journal. We support the main thrust of that critique, and most of its conclusions. However, in their intent to accelerate the demise of the increasingly fragile schizophrenia concept, those authors seem to have misinterpreted some aspects of the evidence in relation to the UHR field. A more balanced critique and synthesis is needed to highlight the real value of what has emerged from two decades of heuristic research and pave the way for genuine and exciting progress in pre‐emptive care. We do not wish to mount a line by line defence of the UHR field here, but do need to clarify some issues.
First, transition has been robustly and operationally defined, based on the generally agreed (though arbitrary) timing of a key treatment change. This definition has received significant validation through studies showing that a range of neurobiological markers differ at baseline in those who make a transition vs. those who do not, and sometimes longitudinally change in those who make the transition compared to those who do not6, 8, 9, 10, 11. These studies, however, do not allow us to define the optimal transition point from a neurobiological point of view. While functional outcome is worse in those who make the transition, this is not the only predictor or correlate of this point in the evolution of disorder12, 13.
If the sample is enriched to at least the level of 20% “true positives” for subsequent first episode psychosis, it is statistically possible to predict who is at especially high risk for transition14 and even assign individuals to different “risk classes”15. Indeed, the UHR research paradigm has been a very productive approach, illuminating risk factors, predictive markers and pointing towards aetiological mechanisms involved in onset of psychotic disorders, albeit with some limitations that might now be effectively addressed and a translation into clinical care that might be more effectively implemented (see below)14, 16, 17.
Interventions during the UHR stage of disorder are effective in not only reducing the risk of transition for at least 1‐2 years, but also in improving functional outcomes18, 19, 20. There is increasing recognition in the field that transition to psychosis should in fact not be the sole focus of intervention, and that the variety of unfavourable trajectories, including poor functional outcome, should be critical targets21, 22, 23. Recent work identified as many as seventeen clinical trajectories in a UHR sample, with 43% of patients having favourable (recovery or remission from UHR state) and 57% unfavourable (recurrence, relapse, no‐remission, transition) outcomes over one year24.
In addition, it has been increasingly recognized that the “at risk mental state” should be regarded as a syndrome in its own right, in addition to being seen as connoting risk for disorder progression. It is a symptomatic state (albeit with psychotic symptoms below threshold for traditional diagnostic categories) associated with distress, functional impairment and diminished quality of life, closer in level to other coded psychiatric disorders and first episode psychosis than to the state of healthy controls25. Indeed, this was one of the reasons that its formulation in DSM‐5 was as “attenuated psychosis syndrome” rather than as a risk category26.
Another key learning which has opened a pathway for wider utility and progress is that, in addition to transition to psychosis and longer term psychotic disorder or persistent subthreshold psychotic symptoms, progression to persistent mood, anxiety, personality and/or substance use disorders is also a very common outcome27, 28. Hence, at this subthreshold or early stage of illness, extending the boundary beyond psychosis (both at the case identification point and as a preventive target) is likely to be essential. Cuijpers29 anticipated this in proposing a widening of the target syndromes based largely on power considerations and efficiency of prediction.
Complementary to this notion is the recognition that it is not uncommon for onset of mental disorders to follow a heterotypic course (i.e., symptoms of one type/category evolving into another type/category). This is illustrated by the fact that onset of first episode psychosis can emerge out of non‐psychotic precursor states. A review by Lee et al30 demonstrated that people at risk of non‐psychotic disorders (identified through the presence of subthreshold non‐psychotic symptoms) were at elevated risk of psychotic disorder (3.87% three‐year incidence rate) – not as high as people meeting UHR criteria (24.63% three‐year incidence rate), but substantially higher (77.4‐fold) than the general population.
On the one hand, the UHR criteria do have greater valence for psychosis outcomes31, but also have some valence for persistent or incident non‐psychotic disorders27, 32, 33. On the other, full‐threshold psychosis may emerge out of risk states not characterized by attenuated psychotic symptoms30, 34.
HOW DO MENTAL DISORDERS EMERGE AND EVOLVE?
When people are floridly psychotic, manic or deeply depressed, it is obvious that they are ill and in need of care. But how did they get there? How did the pathway to obvious and severe illness start? Some authors' choice of where to define the illness border has been driven by concerns of overdiagnosis, overtreatment and labelling. While these problems do exist in some pockets and jurisdictions, the serious treatment gap that exists in every single country in the world, with only a minority of those in need of quality care being able to access it, indicates that the problem of underdiagnosis and failure to deliver treatment is a dramatically more urgent issue.
Defining a boundary is nevertheless important, because it is linked to a categorical decision of whether treatment or at least some kind of help is indicated and should be offered. We argue that this should be a fuzzy boundary in which the patient has a major say, not only health professionals, funders and polemicists35, 36. There should be a soft entry policy but safeguards linked to proportional treatment, balancing benefits vs. risks, guided by the maxim primum non nocere.
Defining a boundary or border zone must be complemented by an understanding of the dynamics of how people move from being “well” to “ill”37. Eaton et al38 have described how this occurs in very clear terms. People develop symptoms either by intensification of existing traits or features within the normal range of experience, such as anxiety or sadness, or the acquisition of novel subjective experiences such as hallucinations or obsessional thoughts, or a combination of the two. Syndromes or constellations of symptoms develop through the concurrent or sequential accumulation of such experiences and behaviours, and when they manifest some coherence and stability.
The key characteristics for determining whether there is a disorder are severity and persistence39, though some argue that distress and/or functional impairment must also be present. In real life, these phenomena emerge in sporadic or gradual ways, often ebbing and flowing, sometimes following familiar trajectories and sequences, other times in a more fluid and reversible manner. How they stabilize or fade, how they attract other features and comorbid patterns and behaviours has not been systematically studied as yet.
In the early stages of mental ill health, diffuse and unstable subthreshold states of anxiety and depression are common, but often commingle with other features, including psychotic‐like disturbances of salience and perception, and emotional dysregulation, to produce a kaleidoscopic series of microphenotypes39, 40. We have not yet defined which set of variables to include in systematic studies of this stage of illness development, but they could include traditional symptom concepts, momentary emotional and perceptual states, self or corporeal disturbances, and sleep and motor activity changes. In this sea of emerging psychopathology, we already know that early psychotic symptoms, particularly if persistent in nature41, indicate enhanced risk, not only for traditional psychotic disorders, for which they do have a greater valence, but also for other syndromal and functional outcomes42, 43, 44.
In addition to the emergence and evolution of symptoms and syndromes, patienthood, help‐seeking and need for care are influenced and defined by sociological factors37, notably prejudice, stigma and illness behaviour45, 46, 47. Financial constraints can have a strong influence on where the bar is set by governments, social welfare agencies and health insurers for access to financial coverage for care. Ideological forces also seek to deny the reality of need for care, by asserting against all available evidence that mental ill health is actually part of the human condition (the “worried well”) and naturally heals through “resilience”. The same could be said about limb fractures, which are common, subject to a natural health process, and yet require professional intervention for optimal healing. These factors are arguably more potent in the mental health field in distorting the definition of need for care and the boundary between health and illness. More subtle variants of this invalidation involve the unhelpful distinction between high and low prevalence disorders.
THE NEW DIAGNOSIS: WHY CATEGORIES STILL MATTER AND HOW TO DEFINE THEM TO GUIDE TREATMENT AND RESEARCH
Psychiatric diagnosis is once again experiencing a crisis of confidence, which has been created by a range of forces. Some derive from fundamental issues, including our failure to bridge the mind/body dichotomy of Descartes and the complications of what philosophers call the “explanatory gap” or the “hard problem of consciousness”48. Others involve the notion that psychiatry can be shoehorned into mainstream medical practice without thoughtful and serious redesign, and the related overreach of biological psychiatry49; the invalidity of reifying syndromal descriptions as disease entities, and the naïve and diluted phenomenological and psychological constructs partly associated with the “operational revolution” of DSM‐III onwards50; the polemics of antipsychiatry; and, most tellingly, the fact that diagnosis has rather low utility for treatment decisions. These forces have combined to fuel this crisis, which reached a peak during the launch period for DSM‐5. The question has been quite reasonably raised: why do we need diagnosis anyway?
The fact that in large transdiagnostic samples there is a general psychopathology factor (the “p” factor) which has good predictive validity51, and that most domains of psychopathology appear to conform to dimensional rather than categorical models, seem to favour a unitary or at least a non‐categorical approach. This thinking has helped to inspire the creation of the Research Domain Criteria (RDoC) project, which has embraced a transdiagnostic approach in research, attempting to base psychiatric nosology on neuroscience and behavioural science rather than DSM‐defined diagnostic categories52.
In our view, this approach overly downplays the role of clinical phenotype‐based classification and overamplifies the role of neuroscience and behavioural constructs, which, although no doubt contribute to the understanding of the aetiology of psychiatric disorder, should be regarded as complementary rather than central to the “object” of psychiatric research and clinical practice. As we have argued elsewhere50, part of the frustration with phenotype‐based classification, and the perceived roadblock that it has introduced to research progress, may be attributable not to that classification per se but rather to the oversimplified and broad nature of contemporary psychopathological descriptions present in DSM‐III onwards and in many of the instruments used to measure psychopathology in research studies48. To borrow geological terminology, focusing on plate tectonics (underlying neurobiology) should not replace or compensate for poor characterization of topography (phenomenology). In addition, the RDoC approach as yet confers no diagnostic benefit to clinical care, and its feasibility in many clinical settings is questionable.
Another related approach has been the Hierarchical Taxonomy of Psychopathology (HiTOP), which attempts to provide a hierarchical dimensional approach to psychiatric classification53. Although these approaches may contribute to mapping and describing nature (although, as noted above and elsewhere48, 50, there are reservations on this front and the jury is still out), they are of no help when it comes to making key decisions in patient care, which will always depend on binary or categorical 0/1 approaches.
It is all too easy to look at such issues and data sets from a population health or epidemiological perspective and critique concepts like “transition”7, but clinicians and patients who have to make decisions about treatment approaches and life goals need to be more pragmatic. How do we harness the reality of dimensional ebbs and flows of symptoms across a wide range to make decisions about which treatments and in which sequence and combination to offer to which patients23? This is where clinical staging provides a solution.
We have described clinical staging in several previous papers9, 54, 55. Its key goal is to provide a more accurate guide to treatment selection (and also to prognosis). It also serves to organize research into psychosocial risk factors, neurocognitive variables, and biomarkers (both of current stage and risk for stage development). The model attempts to determine the position of an individual along a continuum of illness, defined according to stages: Stage 0 = no current symptoms, Stage 1a = help‐seeking with distress, Stage 1b = attenuated (i.e., subthreshold) syndrome, Stages 2‐4 = full threshold disorder with varying degrees of recurrence and severity.
The best known application of clinical staging has been in oncology. There one could argue that the progression or resolution of cancer is also a dimensional issue, but we have imposed categories or stages in a successful effort to intervene proportionally and preventively to reduce the risk of extension of the disease and ultimately death. The risk/benefit ratio is a guide to how aggressively to intervene, with the balance in favour of slight overtreatment at each stage, rather than waiting for treatment failure and then stepping up the intensity, as with “stepped care” in mental health, which responds often very belatedly to treatment resistance.
It might still be an open question whether discrete traditional syndromes such as bipolar disorder, schizophrenia and severe depression have utility at any stage, given the ubiquitous comorbidity that manifests across all stages. Other key influences on the complexion of intervention strategies are developmental and personal goals, such as vocational pathways and individuation and identity formation, that people identify and struggle with, and which are equally transdiagnostic. These might also correlate more with stage of illness than with individual syndrome or classical diagnosis.
SOLVING THE PREVENTION PARADOX: UNLOCKING THE SECRET TO PRE‐EMPTIVE CLINICAL CARE
The prevention paradox
The prevention paradox refers to the fact that, with low incidence events such as suicide, transition to psychosis, or onset of anorexia nervosa, numerically more of the ultimately true positive cases will develop from lower risk than higher risk groups. van Os and Guloksuz7 applied this logic to transition to psychosis in quoting a recent study56 which found that only a very small proportion (4.1%) of patients who developed a first episode psychotic disorder attending local mental health services had been in previous contact with the local UHR service.
Our own data suggest that this may be a particularly low‐end case example reflecting local clinic service pathways. In the case of Orygen Youth Health Clinical Program in Melbourne, a public mental health specialist early intervention service, 12.5% of first episode psychosis patients over a three‐year period were referred from our UHR service (the PACE clinic) and 7% from other Orygen clinics.
According to van Os and Guloksuz7, the above low percentage indicates that “the impact of prodromal services in public health terms may be negligible in relation to their costs”. While the authors fail to note that the UHR service may well have prevented onset for a number of first episode psychosis cases (i.e., the “false false positive” cases57), there are likely to be better clinical outcomes for first episode psychosis cases who have previously been seen at a UHR clinic compared to those who have not58, and these services have been shown to be cost‐effective59, 60. The fact remains, however, that UHR services see only a minority proportion of those who develop first episode psychosis.
If we aim to address the falling transition rate in UHR samples57, we should seek to increase efficiency of risk detection by enhancing methods of predicting psychosis within the UHR group. There are a number of ways in which this might be achieved. One approach is to improve screening and enrichment strategies. Screening tools such as the Prodromal Questionnaire61 have been found to identify UHR cases who transition with high sensitivity (87%) and specificity (87%) and have also been found to detect a more enriched sample for psychosis risk62. Another approach is to apply new analytic strategies to data collected at study entry. There are currently several consortia‐based efforts underway (e.g., PSYSCAN63 and PRONIA64) applying machine learning approaches to develop clinical translation tools for enhanced prediction of psychosis onset in the UHR population.
Another important advance has been to use iterative probabilistic multimodal models to combine assessment domains, such as patient history, clinical assessments and biomarkers. This approach incorporates data from different modalities to increase predictive strength. For example, a probabilistic multimodal model in a UHR cohort using a combination of patient history, clinical assessment and fatty‐acid biomarkers was able to identify over 70% of UHR cases who transitioned within one year65. However, it is unlikely that this approach will widen the entry channel, such that a higher percentage of first episode psychosis cases will pass through the UHR service portal.
Another response is to accept that a UHR service with a focus on psychosis risk and early warning signs of psychosis may be too narrow a channel to attract many of the young people experiencing and manifesting this phenotype. Such clinics struggle to detect and engage more than a small percentage of those expected within a given population in this stage of illness. On the other hand, with broad spectrum youth mental health care primary care platforms, such as headspace66, 67, we now know that a much higher number of such young people can be engaged. In a recent study, we found that 38% of young people accessing these services reported attenuated psychotic symptoms likely to be in the UHR range68.
Also, a recent retrospective study by Shah et al34 reported that 32% of their first episode psychosis sample did not undergo a period of subthreshold psychotic experiences prior to the onset of frank psychosis, and that the most prevalent early symptomatology was depression, anxiety and low functioning. Together, these findings suggest that a broader identification approach could overcome the prevention paradox by also identifying lower risk cases with possibly different phenotypic pathways to first episode psychosis34, 69, and also at risk of other full threshold or Stage 2 disorders. This would pave the way to a truly transdiagnostic approach.
Transdiagnostic risk: the Clinical High At Risk Mental State (CHARMS) approach
The high prevalence and impact of mental disorders in young people, the limitations of current diagnostic systems and risk identification approaches, the diffuse symptom patterns in early stages, and their pluripotent, transdiagnostic trajectories all indicate the need to develop a new diagnostic and predictive strategy. The approach we have recently adopted, guided by the clinical staging model and consistent with broad spectrum youth clinical service structures such as headspace, is an adaptation of the original “at risk mental state” approach to encompass a broader range of inputs and output target syndromes.
This Clinical High At Risk Mental State (CHARMS), as it has been named, is a broad composite definition of a syndrome warranting treatment in its own right due to help‐seeking and distress associated with presenting symptoms, albeit below DSM/ICD‐defined threshold for diagnosis. Figures 1 and 2 show the shift in approach from the traditional UHR to the CHARMS paradigm in the context of clinical staging.
Figure 1.
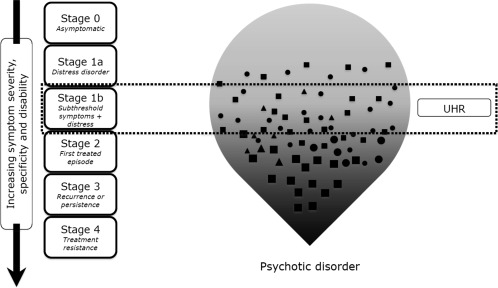
Traditional ultra high risk (UHR) paradigm in the context of clinical staging. The shapes represent different types of symptoms
Figure 2.
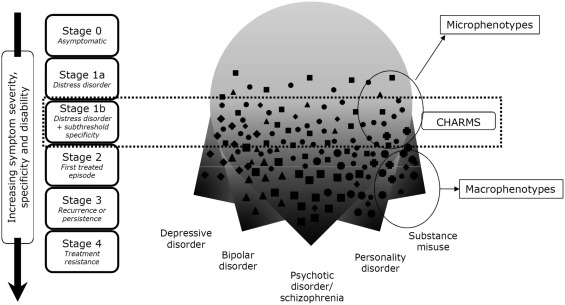
New transdiagnostic Clinical High At Risk Mental State (CHARMS) paradigm in the context of clinical staging. The shapes represent different types of symptoms
The subthreshold (Stage 1b) states covered in the criteria at present include attenuated psychotic symptoms, subthreshold bipolar states, mild‐moderate depression, and borderline personality features of reduced range and shorter duration than full diagnostic threshold70. The trait vulnerability of the UHR criteria is expanded to include history of serious mental disorder in a first degree relative, in addition to functional decline or chronic low functioning in the young person. Early data indicate a ∼30% transition rate to Stage 2 disorder over a 6‐12 month period in young people meeting these criteria and receiving treatment in our headspace clinical services, as opposed to <5% transition rate in help‐seeking young people below this threshold (Stage 1a).
The data also indicate that evolution of symptoms may not necessarily follow a homotypic course (e.g., subthreshold psychosis evolving into threshold psychosis), but may be heterotypic in nature (e.g., moderate depression without attenuated psychotic symptoms at entry evolving into first episode psychosis), consistent with the pluripotent model. While this heterotypic course has been regarded as a shortcoming in the UHR approach (i.e., indicating lack of specificity of the criteria), it is welcomed within the CHARMS approach, because the target is any Stage 2 “exit syndrome” rather than a specific disorder outcome.
Importantly, this broad input‐output approach can still support research into “narrowing” down on predictors and mechanisms at play in specific disorders or symptom clusters: the UHR subgroup, for example, can be identified within the broad Stage 1b cohort, and specific predictors of outcome within this subgroup or specific Stage 2 outcomes, such as psychosis, can be studied, and predictors of this specific outcome within the broad Stage 1b at risk group can be researched.
This pluripotent risk paradigm tackles many of the shortcomings associated with the UHR approach. It addresses the low transition to psychosis rates observed in recent years, allowing for capturing a broad range of outcomes and therefore a higher “transition rate” to serious mental disorder generally. It also places attenuated psychotic symptoms within the context of a range of multidimensional psychopathology, deemphasising these symptoms as a form of “schizophrenia light”71.
It also provides a clinical identification approach for transdiagnostic preventive intervention trials. Such trials, which may consist of psychosocial or biological interventions or combinations and/or sequences of the two, would target the range of presenting symptomatology, rather than focus on a particular set of symptoms. In reality, this is what has occurred in UHR intervention trials anyway, particularly cognitive‐behavioural therapy trials, where it is counterproductive to separate attenuated psychotic symptoms from the rest of the clinical picture (which is often more clinically distressing72) and focus treatment exclusively on those symptoms.
A suitable trial design for such studies are Sequential Multiple Assignment Randomized Trials (“SMART”), used in several recent large‐scale studies in psychiatry to develop an evidence base to support adaptive clinical care73. This trial design methodology is a good fit with the clinical staging model, as it involves multiple intervention stages that correspond to the critical decisions involved in adaptive interventions. These are interventions in which the type or dosage is individualized on the basis of patient characteristics, such as psychological features, clinical presentation or mechanism linked biomarkers, and then is repeatedly adjusted over time in response to patient progress73. Interventions can also be tailored at critical decision points according to response or other patient characteristics, such as specific biomarker changes or comorbidity, and also patient preference.
Our group is currently conducting a SMART trial in a UHR sample23, and plans to follow this with a further trial involving a combination of psychosocial and biological approaches targeting disorder progression, in the broader pluripotent at risk group (Stage 1b, identified using CHARMS criteria). Timing, personalization through biological and psychological markers, sequencing and admixture, and proportionality to stage are the key guiding principles.
New approaches to model and predict evolution of mental disorder
The model of the onset of mental disorder involving symptoms that ebb and flow, and consolidate or recede across stages, as described above, suggests the utility of approaching psychopathology as an evolving, complex system implying a combination of intra‐individual and contextual factors interacting over time74. While it is useful to impose categories on this system for clinical decision‐making, modelling change in psychopathology and predicting its evolution might more effectively be achieved using dynamic, time‐dependent approaches.
Although searching for particular static factors that signal risk for future disorder (as with the Huntingtin gene mutation in Huntington's disease) may play a role, modelling risk for mental disorder may also require capturing factors (and their possible interaction) over time, i.e., must be dynamic in nature and able to incorporate fluctuations in key variables16, 40, 50.
The traditional approach in psychiatric prediction studies, notably psychosis prediction, is to assess a range of variables (clinical, neurocognitive, neurobiological, genetic, etc.) upon entry to a mental health service and to determine whether these variables predict disorder onset (in the case of UHR research, first episode psychosis) or an increase/remission in symptom severity. This methodology rests on the notion that a single sampling of cross‐sectional data can accurately predict the outcome of interest. The highly dynamic and changeable nature of psychopathology and the heterogeneous nature of early symptoms and symptom trajectories (see above) indicates the need for more dynamic models of prediction24, 74. Such models of dynamic change have predominantly emerged from disciplines outside of psychiatry and therefore cross‐disciplinary fertilization is important for progress in the field.
An example is dynamical systems theory, with origins in mathematics and physics, which seeks to describe the behaviour of complex dynamical systems such as the climate, ecosystems and financial markets75. Increasingly, mental health has been conceptualized in these terms, i.e., as a system with many elements which interact with each other over time (as in network theory76, see below). The architecture of such a system reflects how it will change over time77: in a system with loosely connected, heterogeneous elements, change occurs gradually in response to changing conditions, whereas a system with highly interconnected, homogenous elements may initially resist change but then reach a critical threshold or “tipping point” towards another state.
In the context of psychopathology, these two “system states” may correspond to “healthy” and “disordered”/“ill” states78, 79. Tipping points tend to be preceded by early warning signs, such as the phenomenon of “critical slowing down”, which refers to the system taking increasingly longer to return to its previous state after a perturbation/stressor80, 81. There is emerging evidence, using simulation data and fine‐grained longitudinal time series data collected using ecological momentary assessment, that transitions in mental health (at this stage, depression and bipolar disorder) are preceded by critical slowing down78, 79.
A conceptually related approach is the “network perspective” of psychopathology, which has gained traction in recent years. This approach conceptualizes mental disorder not as the consequence of an underlying latent variable (a “common cause”), but as a result of a dynamic interplay of symptoms82, 83, 84, with symptoms actively influencing/causing each other, rather than being the passive expression of an underlying disease process. Within the context of pluripotency during early psychopathology, it has been proposed that the way in which networked symptoms influence each other during early stages of mental ill health may be less concentrated and stable than in later stages76.
Preliminary empirical work is consistent with this proposal, positioning network dynamics theory within the clinical staging framework and suggesting that, with increased clinical stage severity, symptomatology becomes more specialized and differentiated, giving rise to diagnostic specificity associated with greater inter‐ and intra‐mental state connection strength, and greater inter‐ and intra‐mental state connection variability85. Empirical investigations into the predictive potential of dynamic symptom networks for the onset and progression of psychosis are currently underway86.
Another dynamic prediction approach, more agnostic with regard to theoretical principles, is joint modelling. This is a statistical method that combines multilevel modelling (using repeated clinical assessments) with survival analysis (allowing for the time‐to‐event nature of determining outcome in prediction studies)87, 88, 89. The approach can be used to identify symptom trajectories (e.g., persistence of negative symptoms, intensification of general psychopathology) that predict outcome, taking into account censored data and time to follow‐up (as in survival analysis).
Importantly, it allows for the generation of a risk calculator that can be updated over time based on repeated assessments (using clinical or other information), a more refined method of predicting outcome than the existing risk calculators90, 91. Initial work using this approach with data from our recent UHR intervention trial19 shows that dynamic prediction using joint modelling produces much stronger predictive models, particularly positive predictive values, than using baseline data alone89. This approach could equally be applied to transdiagnostic outcomes within a broader risk group, such as a CHARMS cohort.
We have recently argued that such concepts and analytic approaches may be useful for predicting onset of more severe stages of disorder transdiagnostically, as they take the evolving clinical picture into account74. They offer a means of modelling and predicting how mental disorder may evolve across clinical stages, capturing how and why microphenotypes disperse, cohere, sustain, expand or entrench. They may also guide the identification of “dynamic signatures” for risk of particular disorders74 (e.g., critical slowing down may prove to be a more reliable indicator of imminent onset of depression than of psychotic disorder). As indicated above, “broadening” and “narrowing” the approach to risk identification and predictive factors are not mutually exclusive.
Importantly, these new prediction approaches link well with the process that occurs in real‐world clinical decision making92. Clinical decision making regarding possible treatment changes and prognostic judgements is generally “adaptive” in nature – it reacts to and is updated based on gathering further clinical information and the unfolding symptomatology of the patient, rather than relying solely on the profile of the patient's first clinical presentation23. Using the conceptual and analytic approaches outlined here may provide an empirically‐based and rigorous guide for making decisions regarding treatment modification in response to the evolution of a patient's clinical profile over time. In this way, they may help refine treatment decision making and possibly be incorporated into adaptive clinical trial designs, described above, which are currently generally based purely on a category of response/non‐response at the end of a pre‐specified time period93.
CONCLUSIONS
The “at risk mental state” for psychosis approach has been a highly productive research paradigm over the last 25 years. However, the limitations of current risk identification approaches, the diffuse and unstable symptom patterns in early stages, and their pluripotent, transdiagnostic trajectories all indicate the need to develop a new strategy. The approach we have recently adopted has been guided by the clinical staging model and adapts the original “at risk mental state” model to encompass a broader range of inputs and output target syndromes. This approach is supported by a number of novel modelling and prediction strategies, such as dynamical systems theory, network theory, and joint modelling.
A holistic strategy can be developed that applies these new prediction approaches, as well as machine learning and iterative probabilistic multimodal models, to a blend of subjective psychological data, physical disturbances and biomarkers acquired through fine‐grained sequential or longitudinal assessments. This strategy will ultimately enhance our understanding and ability to predict the onset, early course and evolution of mental ill health, further opening pathways for preventive interventions.
REFERENCES
- 1. McGorry PD, Edwards J, Mihalopoulos C et al. EPPIC: an evolving system of early detection and optimal management. Schizophr Bull 1996;22:305‐26. [DOI] [PubMed] [Google Scholar]
- 2. McGorry PD, Copolov DL, Singh BS. Current concepts in functional psychosis. The case for a loosening of associations. Schizophr Res 1990;3:221‐34. [DOI] [PubMed] [Google Scholar]
- 3. Henry LP, Harris MG, Amminger GP et al. Early Psychosis Prevention and Intervention Centre long‐term follow‐up study of first‐episode psychosis: methodology and baseline characteristics. Early Interv Psychiatry 2007;1:49‐60. [DOI] [PubMed] [Google Scholar]
- 4. Fusar‐Poli P, Bonoldi I, Yung AR et al. Predicting psychosis: meta‐analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry 2012;69:220‐9. [DOI] [PubMed] [Google Scholar]
- 5. Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci 2015;19:744‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wood SJ, Yung AR, McGorry PD et al. Neuroimaging and treatment evidence for clinical staging in psychotic disorders: from the at‐risk mental state to chronic schizophrenia. Biol Psychiatry 2011;70:619‐25. [DOI] [PubMed] [Google Scholar]
- 7. van Os J, Guloksuz S. A critique of the “ultra‐high risk” and “transition” paradigm. World Psychiatry 2017;16:200‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cannon TD, Chung Y, He G et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry 2015;77:147‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGorry P, Keshavan M, Goldstone S et al. Biomarkers and clinical staging in psychiatry. World Psychiatry 2014;13:211‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pantelis C, Velakoulis D, McGorry PD et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross‐sectional and longitudinal MRI comparison. Lancet 2003;361:281‐8. [DOI] [PubMed] [Google Scholar]
- 11. Fusar‐Poli P, McGuire P, Borgwardt S. Mapping prodromal psychosis: a critical review of neuroimaging studies. Eur Psychiatry 2012;27:181‐91. [DOI] [PubMed] [Google Scholar]
- 12. Cotter J, Drake RJ, Bucci S et al. What drives poor functioning in the at‐risk mental state? A systematic review. Schizophr Res 2014;159:267‐77. [DOI] [PubMed] [Google Scholar]
- 13. Yung AR, Cotter J, Wood SJ et al. Childhood maltreatment and transition to psychotic disorder independently predict long‐term functioning in young people at ultra‐high risk for psychosis. Psychol Med 2015;45:3453‐65. [DOI] [PubMed] [Google Scholar]
- 14. Yung AR, Nelson B. The ultra‐high risk concept – a review. Can J Psychiatry 2013;58:5‐12. [DOI] [PubMed] [Google Scholar]
- 15. Ruhrmann S, Schultze‐Lutter F, Salokangas RK et al. Prediction of psychosis in adolescents and young adults at high risk: results from the Prospective European Prediction of Psychosis Study. Arch Gen Psychiatry 2010;67:241‐51. [DOI] [PubMed] [Google Scholar]
- 16. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry 2016;73:191‐2. [DOI] [PubMed] [Google Scholar]
- 17. Fusar‐Poli P, Carpenter WT, Woods SW et al. Attenuated psychosis syndrome: ready for DSM‐5.1? Annu Rev Clin Psychol 2014;10:155‐92. [DOI] [PubMed] [Google Scholar]
- 18. van der Gaag M, Smit F, Bechdolf A et al. Preventing a first episode of psychosis: meta‐analysis of randomized controlled prevention trials of 12 month and longer‐term follow‐ups. Schizophr Res 2013;149:56‐62. [DOI] [PubMed] [Google Scholar]
- 19. McGorry PD, Nelson B, Markulev C et al. Effect of omega‐3 polyunsaturated fatty acids in young people at ultrahigh risk for psychotic disorders: the NEURAPRO randomized clinical trial. JAMA Psychiatry 2017;74:19‐27. [DOI] [PubMed] [Google Scholar]
- 20. Stafford MR, Jackson H, Mayo‐Wilson E et al. Early interventions to prevent psychosis: systematic review and meta‐analysis. BMJ 2013;346:f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yung AR. Treatment of people at ultra‐high risk for psychosis. World Psychiatry 2017;16:207‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yung AR, Nelson B, Thompson A et al. The psychosis threshold in ultra high risk (prodromal) research: is it valid? Schizophr Res 2010;120:1‐6. [DOI] [PubMed] [Google Scholar]
- 23. Nelson B, Amminger GP, Yuen HP et al. Staged Treatment in Early Psychosis: a sequential multiple assignment randomised trial of interventions for ultra high risk of psychosis patients. Early Interv Psychiatry (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Polari A, Lavoie S, Yuen HP et al. Clinical trajectories in the ultra‐high risk for psychosis population. Schizophr Res (in press). [DOI] [PubMed] [Google Scholar]
- 25. Fusar‐Poli P, Rocchetti M, Sardella A et al. Disorder, not just state of risk: meta‐analysis of functioning and quality of life in people at high risk of psychosis. Br J Psychiatry 2015;207:198‐206. [DOI] [PubMed] [Google Scholar]
- 26. Carpenter WT, Regier D, Tandon R. Misunderstandings about attenuated psychosis syndrome in the DSM‐5. Schizophr Res 2014;152:303. [DOI] [PubMed] [Google Scholar]
- 27. Lin A, Wood SJ, Nelson B et al. Outcomes of nontransitioned cases in a sample at ultra‐high risk for psychosis. Am J Psychiatry 2015;172:249‐58. [DOI] [PubMed] [Google Scholar]
- 28. Rutigliano G, Valmaggia L, Landi P et al. Persistence or recurrence of non‐psychotic comorbid mental disorders associated with 6‐year poor functional outcomes in patients at ultra high risk for psychosis. J Affect Disord 2016;203:101‐10. [DOI] [PubMed] [Google Scholar]
- 29. Cuijpers P. Examining the effects of prevention programs on the incidence of new cases of mental disorders: the lack of statistical power. Am J Psychiatry 2003;160:1385‐91. [DOI] [PubMed] [Google Scholar]
- 30. Lee TY, Lee J, Kim M et al. Can we predict psychosis outside the clinical high‐risk state? A systematic review of non‐psychotic risk syndromes for mental disorders. Schizophr Bull 2018;44:276‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nelson B, Yuen HP, Wood SJ et al. Long‐term follow‐up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiatry 2013;70:793‐802. [DOI] [PubMed] [Google Scholar]
- 32. Wigman JT, van Nierop M, Vollebergh WA et al. Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity – implications for diagnosis and ultra‐high risk research. Schizophr Bull 2012;38:247‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lim J, Rekhi G, Rapisarda A et al. Impact of psychiatric comorbidity in individuals at ultra high risk of psychosis – findings from the Longitudinal Youth at Risk Study (LYRIKS). Schizophr Res 2015;164:8‐14. [DOI] [PubMed] [Google Scholar]
- 34. Shah JL, Crawford A, Mustafa SS et al. Is the clinical high‐risk state a valid concept? Retrospective examination in a first‐episode psychosis sample. Psychiatr Serv 2017;68:1046‐52. [DOI] [PubMed] [Google Scholar]
- 35. Frances A. Saving normal: an insider's revolt against out‐of‐control psychiatric diagnosis, DSM‐5, big pharma, and the medicalization of ordinary life. New York: Morrow, 2013. [Google Scholar]
- 36. Greenberg G. The book of woe: the DSM and the unmaking of psychiatry. New York: Blue Rider, 2013. [Google Scholar]
- 37. Scott RD. The treatment barrier: part 2. The patient as an unrecognized agent. Br J Med Psychol 1973;46:57‐67. [DOI] [PubMed] [Google Scholar]
- 38. Eaton WW. The sociology of mental disorders, 3rd ed Westport: Praeger, 2001. [Google Scholar]
- 39. McGorry P, van Os J. Redeeming diagnosis in psychiatry: timing versus specificity. Lancet 2013;381:343‐5. [DOI] [PubMed] [Google Scholar]
- 40. van Os J. The dynamics of subthreshold psychopathology: implications for diagnosis and treatment. Am J Psychiatry 2013;170:695‐98. [DOI] [PubMed] [Google Scholar]
- 41. van Os J, Linscott RJ, Myin‐Germeys I et al. A systematic review and meta‐analysis of the psychosis continuum: evidence for a psychosis proneness‐persistence‐impairment model of psychotic disorder. Psychol Med 2009;39:179‐95. [DOI] [PubMed] [Google Scholar]
- 42. Kelleher I, Corcoran P, Keeley H et al. Psychotic symptoms and population risk for suicide attempt: a prospective cohort study. JAMA Psychiatry 2013;70:940‐8. [DOI] [PubMed] [Google Scholar]
- 43. Kelleher I, Devlin N, Wigman JT et al. Psychotic experiences in a mental health clinic sample: implications for suicidality, multimorbidity and functioning. Psychol Med 2013;44:1615‐24. [DOI] [PubMed] [Google Scholar]
- 44. Kelleher I, Keeley H, Corcoran P et al. Clinicopathological significance of psychotic experiences in non‐psychotic young people: evidence from four population‐based studies. Br J Psychiatry 2012;201:26‐32. [DOI] [PubMed] [Google Scholar]
- 45. Kirmayer L, Lemelson R, Cummings C. (eds). Revisioning psychiatry: integrating biological, clinical and cultural perspectives. Cambridge: Cambridge University Press, 2015. [Google Scholar]
- 46. Pilowsky I. Abnormal illness behaviour. Br J Med Psychol 1969;42:347‐51. [DOI] [PubMed] [Google Scholar]
- 47. Pilowsky I. A general classification of abnormal illness behaviours. Br J Med Psychol 1978;51:131‐7. [DOI] [PubMed] [Google Scholar]
- 48. Parnas J. The RDoC program: psychiatry without psyche? World Psychiatry 2014;13:46‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Borsboom D, Cramer A, Kalis A. Brain disorders? Not really… Why network structures block reductionism in psychopathology research. Behav Brain Sci (in press). [DOI] [PubMed] [Google Scholar]
- 50. Nelson B, Hartmann JA, Parnas J. Detail, dynamics and depth: useful correctives for some current research trends. Br J Psychiatry (in press). [DOI] [PubMed] [Google Scholar]
- 51. Lahey BB, Krueger RF, Rathouz PJ et al. Validity and utility of the general factor of psychopathology. World Psychiatry 2017;16:142‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanislow CA. Updating the Research Domain Criteria. World Psychiatry 2016;15:222‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kotov R, Krueger RF, Watson D. A paradigm shift in psychiatric classification: the Hierarchical Taxonomy Of Psychopathology (HiTOP). World Psychiatry 2018;17:24‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McGorry PD. Issues for DSM‐V: clinical staging: a heuristic pathway to valid nosology and safer, more effective treatment in psychiatry. Am J Psychiatry 2007;164:859‐60. [DOI] [PubMed] [Google Scholar]
- 55. McGorry PD. Early clinical phenotypes, clinical staging, and strategic biomarker research: building blocks for personalized psychiatry. Biol Psychiatry 2013;74:394‐5. [DOI] [PubMed] [Google Scholar]
- 56. Ajnakina O. First episode psychosis: looking backwards and forwards. London: King's College London, 2017. [Google Scholar]
- 57. Yung AR, Yuen HP, Berger G et al. Declining transition rate in ultra high risk (prodromal) services: dilution or reduction of risk? Schizophr Bull 2007;33:673‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Valmaggia LR, Byrne M, Day F et al. Duration of untreated psychosis and need for admission in patients who engage with mental health services in the prodromal phase. Br J Psychiatry 2015;207:130‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Valmaggia LR, McCrone P, Knapp M et al. Economic impact of early intervention in people at high risk of psychosis. Psychol Med 2009;39:1617‐26. [DOI] [PubMed] [Google Scholar]
- 60. Phillips LJ, Cotton S, Mihalopoulos C et al. Cost implications of specific and non‐specific treatment for young persons at ultra high risk of developing a first episode of psychosis. Early Interv Psychiatry 2009;3:28‐34. [DOI] [PubMed] [Google Scholar]
- 61. Savill M, D'Ambrosio J, Cannon TD et al. Psychosis risk screening in different populations using the Prodromal Questionnaire: a systematic review. Early Interv Psychiatry 2018;12:3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rietdijk J, Klaassen R, Ising H et al. Detection of people at risk of developing a first psychosis: comparison of two recruitment strategies. Acta Psychiatr Scand 2012;126:21‐30. [DOI] [PubMed] [Google Scholar]
- 63. PSYSCAN . Translating neuroimaging findings from research into clinical practice. http://psyscan.eu.
- 64. PRONIA . Personalised prognostic tools for early psychosis management. https://www.pronia.eu.
- 65. Clark SR, Baune BT, Schubert KO et al. Prediction of transition from ultra‐high risk to first‐episode psychosis using a probabilistic model combining history, clinical assessment and fatty‐acid biomarkers. Transl Psychiatry 2016;6:e897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rickwood DJ, Telford NR, Mazzer KR et al. The services provided to young people through the headspace centres across Australia. Med J Aust 2015;202:533‐6. [DOI] [PubMed] [Google Scholar]
- 67. Rickwood DJ, Telford NR, Parker AG et al. headspace ‐ Australia's innovation in youth mental health: who are the clients and why are they presenting? Med J Aust 2014;200:108‐11. [DOI] [PubMed] [Google Scholar]
- 68. Purcell R, Jorm AF, Hickie IB et al. Demographic and clinical characteristics of young people seeking help at youth mental health services: baseline findings of the Transitions Study. Early Interv Psychiatry 2015;9:487‐97. [DOI] [PubMed] [Google Scholar]
- 69. Shah JL. Sub‐threshold mental illness in adolescents: within and beyond DSM's boundaries. Soc Psychiatry Psychiatr Epidemiol 2015;50:675‐7. [DOI] [PubMed] [Google Scholar]
- 70. Hartmann JA, Nelson B, Spooner R et al. Broad clinical high‐risk mental state (CHARMS): methodology of a cohort study validating criteria for pluripotent risk. Early Interv Psychiatry (in press). [DOI] [PubMed] [Google Scholar]
- 71. van Os J, Murray RM. Can we identify and treat “schizophrenia light” to prevent true psychotic illness? BMJ 2013;346:f304. [DOI] [PubMed] [Google Scholar]
- 72. Power L, Polari AR, Yung AR et al. Distress in relation to attenuated psychotic symptoms in the ultra‐high‐risk population is not associated with increased risk of psychotic disorder. Early Interv Psychiatry 2015;10:258‐62. [DOI] [PubMed] [Google Scholar]
- 73. Lei H, Nahum‐Shani I, Lynch K et al. A “SMART” design for building individualized treatment sequences. Annu Rev Clin Psychol 2012;8:21‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nelson B, McGorry PD, Wichers M et al. Moving from static to dynamic models of the onset of mental disorder. JAMA Psychiatry 2017;74:528‐34. [DOI] [PubMed] [Google Scholar]
- 75. Scheffer M. Critical transitions in nature and society. Princeton: Princeton University Press, 2009. [Google Scholar]
- 76. Borsboom D. A network theory of mental disorders. World Psychiatry 2017;16:5‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Scheffer M, Carpenter SR, Lenton TM et al. Anticipating critical transitions. Science 2012;338:344‐8. [DOI] [PubMed] [Google Scholar]
- 78. van de Leemput IA, Wichers M, Cramer AO et al. Critical slowing down as early warning for the onset and termination of depression. Proc Natl Acad Sci USA 2014;111:87‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bayani A, Hadaeghi F, Jafari S et al. Critical slowing down as an early warning of transitions in episodes of bipolar disorder: a simulation study based on a computational model of circadian activity rhythms. Chronobiol Int 2017;34:235‐45. [DOI] [PubMed] [Google Scholar]
- 80. Scheffer M. Complex systems: foreseeing tipping points. Nature 2010;467:411‐2. [DOI] [PubMed] [Google Scholar]
- 81. Scheffer M, Bascompte J, Brock WA et al. Early‐warning signals for critical transitions. Nature 2009;461:53‐9. [DOI] [PubMed] [Google Scholar]
- 82. Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol 2013;9:91‐121. [DOI] [PubMed] [Google Scholar]
- 83. van Borkulo C, Boschloo L, Borsboom D et al. Association of symptom network structure with the course of longitudinal depression. JAMA Psychiatry 2015;72:1219‐26. [DOI] [PubMed] [Google Scholar]
- 84. Isvoranu AM, Borsboom D, van Os J et al. A network approach to environmental impact in psychotic disorder: brief theoretical framework. Schizophr Bull 2016;42:870‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wigman JTW, van Os J, Thiery E et al. Psychiatric diagnosis revisited: towards a system of staging and profiling combining nomothetic and idiographic parameters of momentary mental states. PLoS One 2013;8:e59559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Booij SH, Wichers M, de Jonge P et al. Study protocol for a prospective cohort study examining the predictive potential of dynamic symptom networks for the onset and progression of psychosis: the Mapping Individual Routes of Risk and Resilience (Mirorr) study. BMJ Open 2018;8:e019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yuen HP, Mackinnon A. Performance of joint modelling of time‐to‐event data with time‐dependent predictors: an assessment based on transition to psychosis data. PeerJ 2016;4:e2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yuen HP, Mackinnon A, Nelson B. A new method for analysing transition to psychosis: joint modelling of time‐to‐event outcome with time‐dependent predictors. Int J Methods Psychiatry Res (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yuen HP, Mackinnon A, Hartmann J et al. A novel approach for developing prediction models of transition to psychosis: dynamic prediction using joint modelling. Submitted for publication.
- 90. Cannon TD, Yu C, Addington J et al. An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry 2016;173:980‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fusar‐Poli P, Rutigliano G, Stahl D et al. Development and validation of a clinically based risk calculator for the transdiagnostic prediction of psychosis. JAMA Psychiatry 2017;74:493‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chitty RN. Why clinicians are natural bayesians: is there a bayesian doctor in the house? BMJ 2005;330:1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bhatt DL, Mehta C. Adaptive designs for clinical trials. N Engl J Med 2016;375:65‐74. [DOI] [PubMed] [Google Scholar]


