Abstract
Aims
Argon has been shown to prevent ischaemic injuries in several scenarios of regional ischaemia. We determined whether it could provide a systemic effect in a model of multiorgan failure (MOF) induced by aortic cross‐clamping.
Methods
Anaesthetized rabbits were submitted to aortic cross‐clamping (30 min) and subsequent reperfusion (300 min). They were either ventilated with oxygen‐enriched air throughout the protocol [fraction of inspired oxygen (FiO2) = 30%; control group) or with a mixture of 30% oxygen and 70% argon (argon groups). In a first group treated with argon (‘Argon‐Total’), its administration was started 30 min before ischaemia and maintained throughout the protocol. In the two other groups, the administration was started either 30 min before ischaemia (‘Argon‐Pre’) or at the onset of reperfusion (‘Argon‐Post’), for a total duration of 2 h. Cardiovascular, renal and inflammatory endpoints were assessed throughout protocol.
Results
Compared with control, shock was significantly attenuated in Argon‐Total and Argon‐Pre but not Argon‐Post groups (e.g. cardiac output = 62±5 vs. 29 ± 5 ml min−1 kg−1 in Argon‐Total and control groups at the end of the follow‐up). Shock and renal failure were reduced in all argon vs. control groups. Histopathological examination of the gut showed attenuation of ischaemic lesions in all argon vs. control groups. Blood transcription levels of interleukin (IL) 1β, IL‐8, IL‐10 and hypoxia‐inducible factor 1α were not significantly different between groups.
Conclusion
Argon attenuated clinical and biological modifications of cardiovascular, renal and intestinal systems, but not the inflammatory response, after aortic cross‐clamping. The window of administration was crucial to optimize organ protection.
Keywords: acute kidney injury (nephrology), animal models (drug development), critical care, intensive care (critical care)
Introduction
Ischaemia–reperfusion contributes to the physiopathology of many diseases and syndromes in anaesthesia and critical care. It can lead to multiorgan failure (MOF) in several pathological conditions, such as cardiac arrest, major surgery or solid organ transplantation 1. Besides the need for organ replacement therapies, the research for an alternative therapeutic strategy is crucial to reduce the ultimate morbidity and mortality after MOF 2.
Inhalation of noble gas, such as xenon and argon, has recently been shown to protect against ischaemia–reperfusion injuries. Although xenon has demonstrated beneficial effects in various models of neurological injury 3, its high cost precludes routine use. Conversely, argon is easy to produce, harmless and has no ecological impact. To date, argon has shown neuroprotective effects in models of stroke 4, subarachnoid haemorrhage 5, cardiac arrest 6, 7, 8 and hypoxic encephalopathy 9, 10, 11. It has also been shown to reduce infarct size 12 and prevent renal graft dysfunction in animal studies 13. Despite protective effects in such situations, the consequences of argon administration need to be investigated during ischaemia–reperfusion‐induced MOF.
Accordingly, we hypothesized that argon could attenuate MOF after aortic cross‐clamping. We used a well‐established model of MOF eliciting major haemodynamic dysfunction and shock, as well as renal, gut and liver alterations, in anaesthetized rabbits 14. We report the effect of argon by testing its effect both throughout ischaemia–reperfusion and also during only the acute phase of ischaemia or reperfusion.
Methods
The animal instrumentation and the ensuing experiments were approved by the institutional review board for animal research (ComEth Anses/EnvA/UPEC; Approval 10/11/15–9).
Animal preparation, aortic occlusion and reperfusion
As previously described 14, 15, male New Zealand rabbits (2.5–3.5 kg) were anaesthetized using zolazepam, tiletamine and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5480 [all 20–30 mg kg−1 intravenously (i.v.)]. After intubation, they were artificially ventilated with an fraction of inspired oxygen (FiO2) of 30% (SAR‐830P, CWE Inc., Ardmore, OK, USA). Tidal volume was set to 10 ml kg−1 and respiratory rate to 28–30 cycles min−1. Anaesthesia was maintained with additional administration of pentobarbitone (5 mg kg−1 h−1, i.v.).
Arterial blood pressure in the right carotid artery, and the electrocardiogram were recorded continuously (HEM version 4.2, Notocord, Croissy‐sur‐Seine, France). A temperature probe was inserted into the rectum and body temperature was maintained at 38.5 ± 0.5°C throughout experiments with heating pads. After left thoracotomy, a flow probe was placed around the ascending aorta in order to measure cardiac output (PS‐Series Probes, Transonic, New York, NY, USA). The chest was closed in layers and a median laparotomy was performed. All animals received heparin (250 IU kg−1 i.v.), and the supracoeliac aorta was occluded with a vascular clamp. Occlusion was released after 30 min of aortic cross‐clamping, as previously described 14. Animals were then monitored for 300 min. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=505 administration was permitted after reperfusion, with a target mean arterial pressure of 70 mmHg. Saline was administered throughout experiment, with the following regimen in all animals: 5 ml kg−1 i.v. after intubation, 10 ml kg−1 i.v. immediately after unclamping and 10 ml kg−1 h−1 throughout follow‐up. After 300 min of reperfusion, all animals were euthanized (pentobarbitone 60 ml kg−1 i.v.) and the liver, gut and kidney were fixed in formaldehyde (4%) for blind histological analysis by a pathologist 14, 15. Intestinal lesions were quantified using Chiu's score 16. Briefly, intestinal damage was graded from 0 (normal) to 5 (severely damaged) as follows: grade 0 = normal mucosal villi; grade 1 = mild extension of subepithelial space; grade 2 = extension of the subepithelial space with moderate lifting of the epithelial layer; grade 3 = massive epithelial lifting down the sides of villi; grade 4 = denuded villi with lamina propria and dilated capillaries exposed; grade 5 = digestion and disintegration of the lamina propria, haemorrhage and ulceration.
Experimental protocol
Rabbits were randomly assigned to one of the four experimental groups. In the control group, rabbits were mechanically ventilated with oxygen‐enriched air (FiO2 = 30%) throughout the protocol. In the three other groups, rabbits received a gas mixture with argon (70%) and oxygen (30%), with different timings of administration. The inhaled fraction of argon was chosen as the maximal fraction in the gas mixture that could be administered while maintaining 30% oxygen. This fraction has been shown to provide neuroprotective effects in other situations 6, 7, 10, 11, 12. Endpoints were haemodynamic parameters and biochemical markers of shock, renal function, hepatic lysis and inflammatory activation after cross‐clamping.
In the ‘Argon‐Total’ group, noble gas administration was started 30 min before aortic cross‐clamping and continued during the entire procedure. In the ‘Argon‐Pre’ group, gas administration was also started 30 min before cross‐clamping but continued for only 1 h after reperfusion, which represents a total exposure of 2 h to argon. In the ‘Argon‐Post’ group, argon administration was started at the onset of the reperfusion and continued for 2 h.
Biological assessment
Arterial blood samples were withdrawn at baseline and 15, 60 and 300 min after reperfusion for blood gases and biochemical analyses. As previously described 14, acute kidney injury was evaluated by urine output, creatinine urinary clearance and blood creatinine (Cobas bioanalyser; Roche‐Diagnostics, France). Hepatic injury was evaluated through determination of alanine aminotransferase (ALAT). We also measured blood mRNA levels after 300 min of reperfusion for markers of immunity, inflammation or hypoxia using real‐time quantitative reverse transcription polymerase chain reaction (RTq‐PCR), as previously described 14. We assessed the expression of interleukin (IL) 1β, IL‐8, IL‐10 and hypoxia‐inducible factor 1α (HIF‐1α). Expression levels were normalized to housekeeping gene ribosomal protein L5 (RPL5), and transcript levels were expressed as fold‐change from baseline levels.
Statistical analysis
Data are expressed as mean ± standard error of the mean, or median for continuous parameters or histological scores, respectively. Haemodynamic and biochemical parameters were compared between the groups using a two‐way analysis of variance for repeated measures at baseline, during aortic cross‐clamping and after reperfusion. A post hoc Holm–Sidak test was performed for comparisons with the control group when necessary. In order to reduce the number of comparisons, we did not compare values between other groups and/or between different time points. On the basis of a previous study, we estimated that eight rabbits per group were required to demonstrate an 80% increase in cardiac output in argon groups vs. control (α = 0.05 and β = 0.8) 14. Histological scores of intestinal lesions were compared between groups using a Kruskal–Wallis test, followed by a Mann–Whitney analysis. Significant differences were determined at P ≤ 0.05.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 17.
Results
Number of animals
A total of 32 rabbits were randomly included in the study and were divided into four experimental groups of eight rabbits.
Effects of argon on cardiovascular parameters
As shown in Figure 1, animals experienced a progressive decrease in cardiac output in the control group (87 ± 5 ml min−1 kg−1 at baseline vs. 29 ± 5 ml min−1 kg−1 at the end of the procedure). At the end of the procedure, cardiac output was significantly improved in Argon‐Total and Argon‐Pre, but not in Argon‐Post, compared with control (62 ± 5, 49 ± 5 and 42 ± 5 vs. 29 ± 5 ml min−1 kg−1, respectively).
Figure 1.
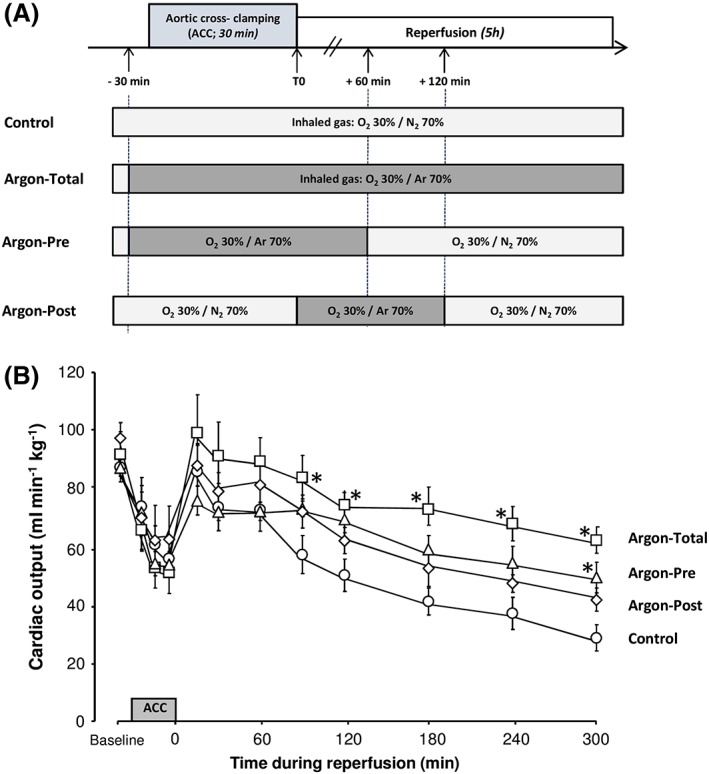
Schematic representation of the experimental protocol (A) and cardiac output throughout follow‐up (B). n = 8 in each group. ACC, aortic cross‐clamping; Argon‐Post, Argon administration was started at the onset of the reperfusion and continued during 2 hours; Argon‐Pre, gas administration was started 30 min before cross‐clamping but continued during only one hour after reperfusion; Argon‐Total, noble gas administration was started 30 min before aortic cross‐clamping and continued during the entire procedure; *P < 0.05 vs. corresponding control value. Statistical comparisons were only made between argon vs. control groups
As shown in Figure 2, heart rate and mean arterial pressure were not different among groups, except after 240 min of reperfusion, after which heart rate was significantly lower in control vs. argon groups. Conversely, stroke volume was significantly increased in Argon‐Total vs. control, meaning that improved cardiac output was the consequence of a true improvement of left ventricular function. Norepinephrine requirements were also significantly greater in control, compared with the three argon groups. For example, the norepinephrine requirement averaged 19.6 ± 3.5, 3.4 ± 1.5, 4.2 ± 1.9 and 10.9 ± 4.1 μg kg−1 min−1 at the end of follow‐up in control, Argon‐Total, Argon‐Pre and Argon‐Post groups, respectively (P < 0.05 between each argon group and control). Furthermore, two rabbits were completely weaned off norepinephrine at the end of the follow‐up in the Argon‐Total group.
Figure 2.
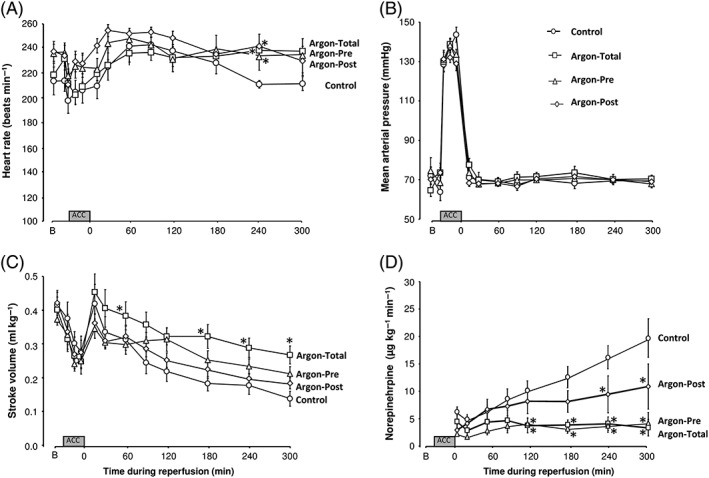
Heart rate (A), mean arterial pressure (B), cardiac stroke volume (C) and required doses of norepinephrine (D) throughout the protocol. The regimen for norepinephrine administration was adjusted in order to maintain mean arterial pressure at 70 mmHg after reperfusion. n = 8 in each group. Argon‐Post, Argon administration was started at the onset of the reperfusion and continued during 2 hours; Argon‐Pre, gas administration was started 30 min before cross‐clamping but continued during only one hour after reperfusion; Argon‐Total, noble gas administration was started 30 min before aortic cross‐clamping and continued during the entire procedure; *P < 0.05 vs. corresponding control value. Statistical comparisons were only made between argon vs. control groups
As shown in Table 1, argon administration also did not impair the oxygenation or decarboxylation of ventilated animals compared with control. The blood levels of HCO3 − were significantly improved after reperfusion in each argon group vs. control, suggesting attenuated metabolic acidosis, as also shown by a significantly higher arterial pH.
Table 1.
Biochemical markers of hepatic and renal function
| Baseline | Reperfusion 60 min | Reperfusion 300 min | |
|---|---|---|---|
| Arterial pH | |||
| Control | 7.42 ± 0.02 | 7.14 ± 0.01 | 6.81 ± 0.04 |
| Argon‐Total | 7.38 ± 0.03 | 7.26 ± 0.03 | 7.17 ± 0.02* |
| Argon‐Pre | 7.39 ± 0.03 | 7.24 ± 0.02 | 7.23 ± 0.06* |
| Argon‐Post | 7.41 ± 0.02 | 7.20 ± 0.03 | 7.10 ± 0.06* |
| Arterial pCO 2 (mmHg) | |||
| Control | 38 ± 4 | 41 ± 3 | 31 ± 4 |
| Argon‐Total | 40 ± 5 | 43 ± 3 | 39 ± 1 |
| Argon‐Pre | 43 ± 3 | 44 ± 4 | 36 ± 3 |
| Argon‐Post | 43 ± 2 | 48 ± 4 | 38 ± 4 |
| Arterial pO 2 (mmHg) | |||
| Control | 196 ± 16 | 159 ± 13 | 195 ± 8 |
| Argon‐Total | 178 ± 20 | 123 ± 10 | 150 ± 17 |
| Argon‐Pre | 173 ± 10 | 147 ± 12 | 165 ± 8 |
| Argon‐Post | 171 ± 13 | 127 ± 11 | 185 ± 16 |
| Arterial HCO 3 − (mmol l −1 ) | |||
| Control | 25.5 ± 1.9 | 14.4 ± 0.8 | 4.9 ± 0.8 |
| Argon‐Total | 25.2 ± 1.7 | 17.5 ± 1.3 | 14.2 ± 1.1* |
| Argon‐Pre | 27.8 ± 1.0 | 19.8 ± 2.1 * | 15.2 ± 2.0* |
| Argon‐Post | 27.6 ± 0.8 | 19.4 ± 1.2 * | 12.3 ± 2.1* |
| ALAT (IU l −1 ) | |||
| Control | 27 ± 6 | 63 ± 14 | |
| Argon‐Total | 23 ± 3 | 63 ± 10 | |
| Argon‐Pre | 29 ± 3 | 66 ± 13 | |
| Argon‐Post | 26 ± 7 | 52 ± 11 | |
n = 8 in each group. ALAT, alanine aminotransferase; Argon‐Post, Argon administration was started at the onset of the reperfusion and continued during 2 hours; Argon‐Pre, gas administration was also started 30 min before cross‐clamping but continued during only one hour after reperfusion; Argon‐Total, noble gas administration was started 30 min before aortic cross‐clamping and continued during the entire procedure; pCO2, partial pressure of CO2; pO2; partial pressure of O2
P < 0.05 vs. corresponding control. Statistical comparisons were only made between argon vs. control groups
Effects of argon on acute kidney and hepatic injuries
As shown in Figure 3, acute kidney injury was attenuated in each argon group vs. control. The protection was maximal in Argon‐Total vs. control, as shown by decreased creatinine blood levels (107 ± 19 vs. 164 ± 19 μmol l−1, respectively; P < 0.05), as well as improved diuresis (30 ± 6 vs. 6 ± 3 ml, respectively; P < 0.05) and creatinine clearance after reperfusion (5.9 ± 1.6 vs. 0.5 ± 0.2 ml min−1, respectively; P < 0.05). A similar trend was observed in Argon‐Pre and Argon‐Post groups but the difference only achieved statistical significance for renal clearance of creatinine. Conversely, ALAT blood levels were no different between the argon groups vs. control at the end of the follow‐up (Table 1).
Figure 3.
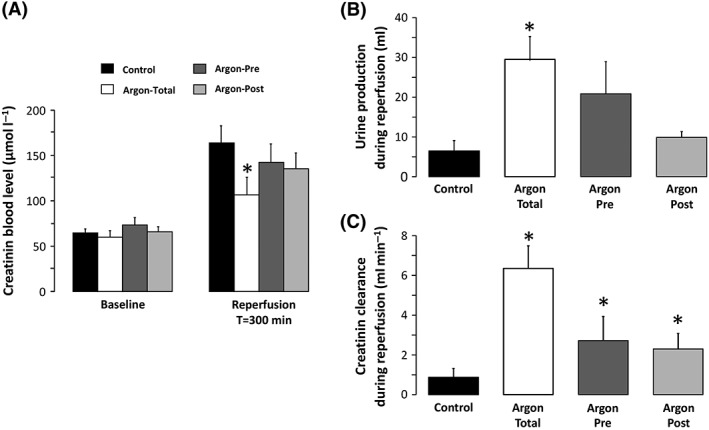
Creatinine blood levels (A), urine production (B) and creatinine clearance (C) throughout the protocol. n = 8 in each group. Argon‐Post, Argon administration was started at the onset of the reperfusion and continued during 2 hours; Argon‐Pre, gas administration was started 30 min before cross‐clamping but continued during only one hour after reperfusion; Argon‐Total, noble gas administration was started 30 min before aortic cross‐clamping and continued during the entire procedure; *P < 0.05 vs. control. Statistical comparisons were only made between argon vs. control groups
Effect of argon on organ damage
In order to investigate the level of kidney and liver damage at the end of the procedure, histopathological evaluation was blindly performed in three rabbits from each of the Argon‐Total and control groups. In the control group, kidney samples showed tubular necrosis (Figure 4A) and microangiopathy (Figure 4B), but this was not observed in Argon‐Total animals (Figure 4C). Liver samples from the control group presented ischaemic lesions and necrosis (Figure 4D‐E), which were not observed in Argon‐Total (Figure 4G).
Figure 4.
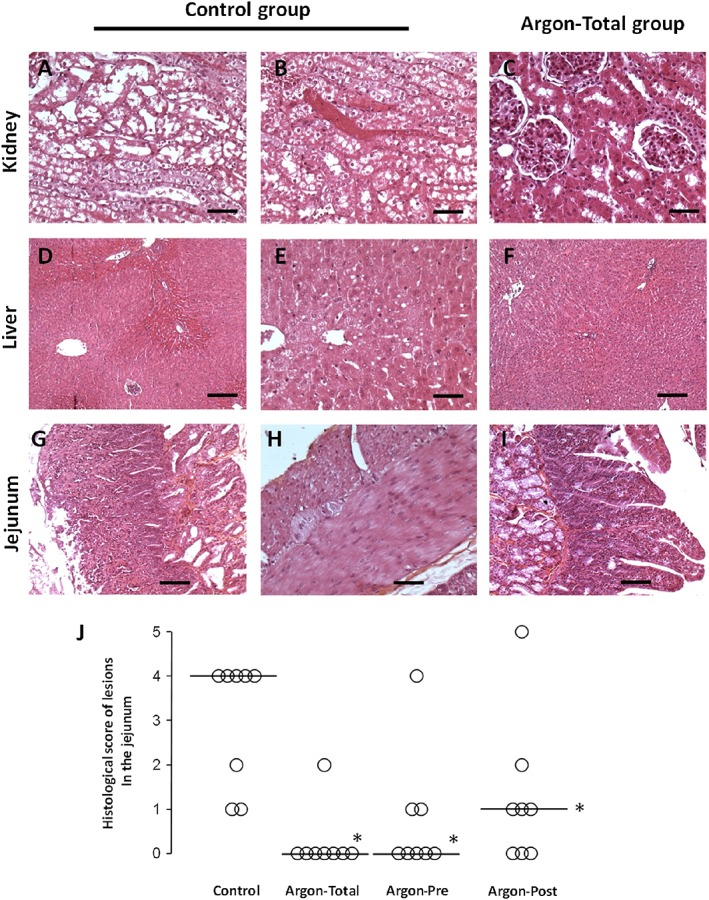
Histological appearance of the kidney (A–C), liver (D–F) and jejunum (G–I) in the control group compared with the Argon‐Total group, that is to mean with Argon administration throughout the protocol. (A) Renal cortex from a rabbit in the control group with tubular necrosis (bar = 50 μm; haematoxylin and eosin (H&E) stain). (B) Renal cortex from a rabbit in the control group with microangiopathy (bar = 50 μm; H&E stain). (C) Renal cortex from a rabbit in the Argon‐Total group with a normal histological appearance (bar = 50 μm; H&E stain). (D) Liver from a rabbit in the control group showing centrilobular congestion. (E) Liver from a rabbit in the control group with macrovacuolar steatosis (bar = 50 μm; H&E stain). (F) Liver from a rabbit in the Argon‐Total group with a normal histological appearance (bar = 200 μm; H&E stain). (G) Jejunum from a rabbit in the control group with villous atrophy and extensive necrosis in the mucosal layer (bar = 200 μm; H&E stain). (H) Jejunum from a rabbit in the control group, showing necrosis of the muscularis externa (bar = 100 μm; H&E stain). (I) Jejunum from a rabbit in the Argon‐Total group with a normal histological appearance (bar = 200 μm; H&E stain). (J) Histological scores of intestinal ischaemic lesions in the animals from the different groups. The open circles represent individual values for each animal. The bold line represents the median value for each group. *, P < 0.05 vs. control group
For intestinal tissues, we evaluated all samples from all animals. In the control group, the jejunum showed villous atrophy (Figure 4G), diffuse inflammation and extended necrosis (Figure 4H). By comparison, intestinal villi structure was well preserved in the argon groups, as shown in Figure 4I for the Argon‐Total animals. As shown in Figure 4J, the semi‐quantitative analysis of intestinal lesions showed a significant reduction in histological scores in all argon groups vs. control.
Effect of argon on inflammation markers
As shown in Figure 5, transcription levels of IL‐1β, IL‐8 and IL‐10 in the blood strongly increased at the end of the procedure compared with baseline in the control group (fold‐change >3 for all these markers). This was not significantly different in the argon groups vs. control. The increases in the blood transcripts of HIF‐1α were smaller but no difference was observed among groups.
Figure 5.
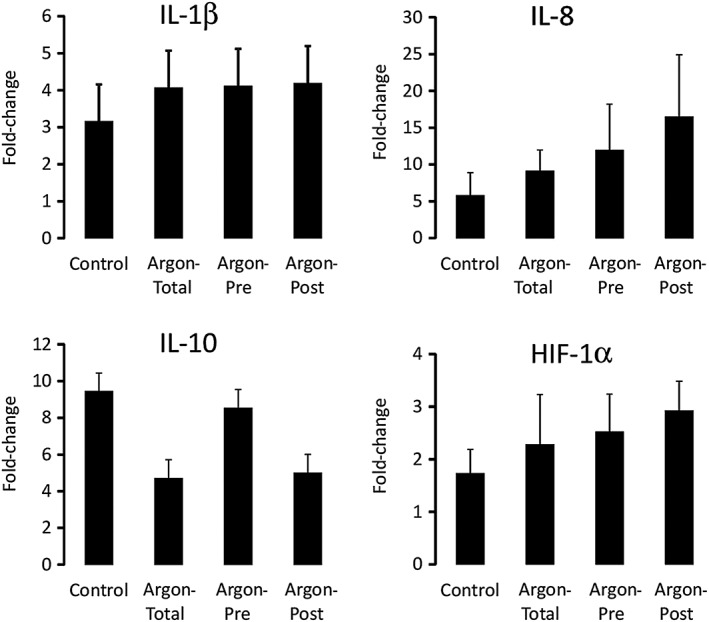
Transcriptomic evaluation by real‐time quantitative polymerase chain reaction of innate immunity and hypoxic markers. Transcripts levels were expressed as fold‐change from baseline levels. n = 8 in each group. HIF‐1α, hypoxia‐inducible factor 1α; IL, interleukin
Discussion
In the present study, inhalation of argon attenuated MOF induced by aortic cross‐clamping. The protection against cardiovascular, renal and intestinal damages was evidenced by physiological, biological and histological parameters. Administration of argon during the entire procedure (i.e. prior to ischaemia and throughout reperfusion) conferred the maximal organ protection, while transient administration provided smaller benefits.
In order to mimic the complex situation of MOF, we used an original model of ischaemia–reperfusion injury induced by supracoeliac aortic cross‐clamping. MOF severity was reflected by a major drop in cardiac output, high norepinephrine requirements, profound metabolic acidosis, and acute intestinal and kidney injury in control conditions. The latter injury was evidenced by oliguria and increased creatinine blood levels. By contrast, hepatic failure was less prominent. Beyond haemodynamic and visceral dysfunction, MOF was associated with a high burden of inflammatory and ischaemic stress, with a major activation of inflammatory markers, such as IL‐1 β, IL‐8 and IL‐10. These alterations are the hallmarks of MOF. Therefore, the experimental model can be considered as an original model mimicking shock in critically ill patients 14.
The present study could have important clinical implications. Up until now, xenon has been the main noble gas studied for medical applications but its routine clinical use is hampered by its high cost. By contrast, argon is easy to produce and is one‐hundredth of the cost of xenon 18. The protective effects of argon have mostly been shown after cardiac arrest or regional ischaemia. In rodents, Brücken et al. showed that the administration of argon 1 h after cardiac arrest improved functional neurological recovery after experimental cardiac arrest 6, 19. The same result was observed when argon was administered 3 h after cardiac arrest 20. Pigs receiving post‐resuscitation treatment with argon also had complete neurological recovery 7. In a piglet model of perinatal asphyxia, argon administration was shown to be safe 21, and reduced neuronal cell death 9. It also reduced cerebral infarct size after transient cerebral artery occlusion in rats 4, and the risk of mortality after subarachnoid haemorrhage 5. However, a recent study in a rodent model of cardiac arrest provided conflicting results, with argon showing no neuroprotective effect 8. The smaller proportion of argon in the gas mixture in the latter study (i.e. 50%) might explain this absence of effect. Importantly, in addition to its well‐established neuroprotective effect, argon has also previously demonstrated nephroprotective 13 and cardioprotective 22 effects. In a rat model of myocardial ischaemia, argon administration after reperfusion prevented the decrease in left ventricular ejection fraction 22. A previous study also demonstrated a reduction in myocardial infarct size when argon was given as a preconditioning agent in a rabbit model of coronary obstruction 12.
However, none of these previous experimental models studied shock or MOF. To our knowledge, our study is the first to show the protective effect of argon in a model of MOF. As it is a safe product, it would be highly relevant to test its effect further in different clinical presentations of MOF, such as scheduled surgical ischaemia–reperfusion injuries. Argon could then become a part of the therapeutic armamentarium during cardiovascular and hepatic surgery, solid organ transplantation or resuscitated shock. Importantly, it should be administered throughout injury, to provide maximum benefit.
Importantly, in the present study, argon administration attenuated circulatory failure and the decrease in cardiac output. The protective effect was greatest with the longest administration of argon, including the pre‐, intra‐ and post‐ischaemic phase. The cardioprotective effect of argon was also evidenced by the lack of decrease in heart rate at the end of the follow‐up, compared with the control group. However, this improvement did not fully explain the increase in cardiac output as argon also enhanced stroke volume after ischaemia–reperfusion. The reduction in the demand for norepinephrine for the treatment of hypotension was a positive effect of argon, as high doses of norepinephrine are associated with several drawbacks 23.
Along with attenuated cardiovascular failure, intestinal and acute kidney injury was gradually reduced by early and prolonged administration of argon. If these results were partially due to an improvement in haemodynamic parameters, direct intestinal or renal cytoprotective effects would be likely to occur. In the jejunum, we observed a potent attenuation of ischaemic epithelial lesions. In the kidney, we observed both reduced tubular necrosis and improved creatinine clearance. This is in agreement with a previous report showing a nephroprotective effect of a cold storage solution enriched by argon in a model of kidney transplantation in mice. Grafts stored in argon‐enriched solution showed decreased expression of caspase 3 after ischaemia–reperfusion, and preserved tubular architecture without a loss in CD‐10 expression 13.
Conversely, the beneficial effect of argon was not related to a modulation in inflammation as argon did not influence the levels of inflammatory biomarkers. This suggests that inflammation activation is not strictly correlated with the short‐term severity of shock in this model. It also suggests that the effect of argon is mediated by a direct anti‐ischaemic effect to prevent the occurrence of MOF, as supported by its maximal effect when administered before ischaemia.
It has also been demonstrated previously that argon activates protective pathways in the neuronal and cardiac tissue during regional ischaemia. For example, argon increased heme oxygenase 1 expression and Akt phosphorylation in hypoxic neurones 11. Its effect was found to be blunted by the pharmacological inhibition of phosphoinositide 3‐kinase 11 or extracellular regulated kinase 10, 11. The same findings were observed in an in vitro model of ischaemia–reperfusion in human atrial appendages 22. The protective effect of argon on functional recovery was, again, blunted by pharmacological inhibitors of phosphoinositide 3‐kinase, Akt and extracellular regulated kinase, and also by activators of mitochondrial permeability transition pore opening 22. Höllig et al. also demonstrated that argon increased the hippocampal expression of HIF‐1α and heme oxygenase 1 after experimental subarachnoid haemorrhage 5. In isolated cardiomyocytes, preconditioning with argon was found to increase cell viability and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1250, superoxide dismutase 2 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=784 27 levels 24. In retinal ganglion cells, Ulbrich et al. showed that argon reduced retinal apoptosis and inhibited Bax and Bcl‐2 expression, as well as caspase 3 cleavage 25. The same authors found that argon‐induced neuroprotection could be mediated by inhibition of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1752 and 4 26. However, all the above‐mentioned studies focused on regional ischaemia and mostly isolated cells. Further mechanistic studies are therefore required to investigate the mechanism of action of argon after global ischaemia.
Our study had several limitations. First, we did not investigate the dose‐effect of argon on organ injury, and we only assessed the effect of one ‘dose’ of argon with an inspired fraction of 70%; indeed, this was the maximal value allowing an oxygen fraction of 30%. In previous studies, argon was also administered at an inspired fraction ranging from 50% to 70% 27. Secondly, we investigated only a few inflammatory markers. One could argue that blood transcripts might not be truly relevant, compared with tissue transcripts or blood proteins. Therefore, we could not rule out a possible effect of argon on other markers. We could also speculate that a longer follow‐up might have shown an effect of argon on inflammation. However, it would be hard to follow animals for longer durations in the control group because of the severity of shock. A longer follow‐up would therefore not permit a proper comparison between ‘protected’ and control groups. Finally, argon did not completely blunt the multiple patterns of MOF in this model, while hypothermic total liquid ventilation was more powerfull but also more difficult to translate into surgical practice in the very short term 14.
In conclusion, argon attenuated ischaemia–reperfusion‐induced MOF in anaesthetized rabbits after aortic cross‐clamping. This protection was maximal when argon was administered throughout ischaemia and reperfusion. The clinical perspectives of this cost‐effective noble gas should be viewed in the light of potential ease of administration as an adjunct to the resuscitation of the critically ill.
Competing Interests
There are no competing interests to declare.
The authors gratefully acknowledge Natacha Martin for her excellent technical support. This study was supported by a grant from the “Fondation pour la Recherche Médicale” (DBS20140930781) and by Région Ile‐de‐France (CORDDIM). Guillaume Savary was supported by the Agence Régionale de Santé d'Ile‐De‐France.
Contributors
G.S., F.L., M.K., N.M. and R.T. designed the study. G.S., F.L., N.M. and P.B. were responsible for the conduct of the study. G.S., F.L., J.R., M.K., T.H., P.B., B.C., A.C., B.G., N.M. and R.T. analysed the data. R.T. carried out the statistical analysis. G.S., B.G., N.M. and R.T. wrote the manuscript.
Savary, G. , Lidouren, F. , Rambaud, J. , Kohlhauer, M. , Hauet, T. , Bruneval, P. , Costes, B. , Cariou, A. , Ghaleh, B. , Mongardon, N. , and Tissier, R. (2018) Argon attenuates multiorgan failure following experimental aortic cross‐clamping. Br J Clin Pharmacol, 84: 1170–1179. doi: 10.1111/bcp.13535.
References
- 1. Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury 2009; 40: 912–918. [DOI] [PubMed] [Google Scholar]
- 2. Mongardon N, Dyson A, Singer M. Is MOF an outcome parameter or a transient, adaptive state in critical illness? Curr Opin Crit Care 2009; 15: 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Deken J, Rex S, Monbaliu D, Pirenne J, Jochmans I. The efficacy of noble gases in the attenuation of ischemia reperfusion injury: a systematic review and meta‐analyses. Crit Care Med 2016; 44: e886–e896. [DOI] [PubMed] [Google Scholar]
- 4. Ryang Y‐M, Fahlenkamp AV, Rossaint R, Wesp D, Loetscher PD, Beyer C, et al Neuroprotective effects of argon in an in vivo model of transient middle cerebral artery occlusion in rats. Crit Care Med 2011; 39: 1448–1453. [DOI] [PubMed] [Google Scholar]
- 5. Höllig A, Weinandy A, Liu J, Clusmann H, Rossaint R, Coburn M. Beneficial properties of argon after experimental subarachnoid hemorrhage: early treatment reduces mortality and influences hippocampal protein expression. Crit Care Med 2016; 44: e520–e529. [DOI] [PubMed] [Google Scholar]
- 6. Brücken A, Cizen A, Fera C, Meinhardt A, Weis J, Nolte K, et al Argon reduces neurohistopathological damage and preserves functional recovery after cardiac arrest in rats. Br J Anaesth 2013; 110 (Suppl. 1): i106–i112. [DOI] [PubMed] [Google Scholar]
- 7. Ristagno G, Fumagalli F, Russo I, Tantillo S, Zani DD, Locatelli V, et al Postresuscitation treatment with argon improves early neurological recovery in a porcine model of cardiac arrest. Shock 2014; 41: 72–78. [DOI] [PubMed] [Google Scholar]
- 8. Zuercher P, Springe D, Grandgirard D, Leib SL, Grossholz M, Jakob S, et al A randomized trial of the effects of the noble gases helium and argon on neuroprotection in a rodent cardiac arrest model. BMC Neurol 2016; 16: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Broad KD, Fierens I, Fleiss B, Rocha‐Ferreira E, Ezzati M, Hassell J, et al Inhaled 45–50% argon augments hypothermic brain protection in a piglet model of perinatal asphyxia. Neurobiol Dis 2016; 87: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao H, Mitchell S, Ciechanowicz S, Savage S, Wang T, Ji X, et al Argon protects against hypoxic–ischemic brain injury in neonatal rats through activation of nuclear factor (erythroid‐derived 2)‐like 2. Oncotarget 2016; 7: 25640–25651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao H, Mitchell S, Koumpa S, Cui YT, Lian Q, Hagberg H, et al Heme oxygenase‐1 mediates neuroprotection conferred by argon in combination with hypothermia in neonatal hypoxia–ischemia brain injury. Anesthesiology 2016; 125: 180–192. [DOI] [PubMed] [Google Scholar]
- 12. Pagel PS, Krolikowski JG, Shim YH, Venkatapuram S, Kersten JR, Weihrauch D, et al Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo . Anesth Analg 2007; 105: 562–569. [DOI] [PubMed] [Google Scholar]
- 13. Irani Y, Pype JL, Martin AR, Chong CF, Daniel L, Gaudart J, et al Noble gas (argon and xenon)‐saturated cold storage solutions reduce ischemia–reperfusion injury in a rat model of renal transplantation. Nephron Extra 2011; 1: 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mongardon N, Kohlhauer M, Lidouren F, Hauet T, Giraud S, Hutin A, et al A brief period of hypothermia induced by total liquid ventilation decreases end‐organ damage and multiorgan failure induced by aortic cross‐clamping. Anesth Analg 2016; 123: 659–669. [DOI] [PubMed] [Google Scholar]
- 15. Darbera L, Chenoune M, Lidouren F, Kohlhauer M, Adam C, Bruneval P, et al Hypothermic liquid ventilation prevents early hemodynamic dysfunction and cardiovascular mortality after coronary artery occlusion complicated by cardiac arrest in rabbits. Crit Care Med 2013; 41: e457–e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low‐flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 1970; 101: 478–483. [DOI] [PubMed] [Google Scholar]
- 17. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acid Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coburn M, Rossaint R. Argon in the fast lane: noble gases and their neuroprotective effects. Crit Care Med 2012; 40: 1965–1966. [DOI] [PubMed] [Google Scholar]
- 19. Brücken A, Kurnaz P, Bleilevens C, Derwall M, Weis J, Nolte K, et al Dose dependent neuroprotection of the noble gas argon after cardiac arrest in rats is not mediated by K(ATP)‐channel opening. Resuscitation 2014; 85: 826–832. [DOI] [PubMed] [Google Scholar]
- 20. Brücken A, Kurnaz P, Bleilevens C, Derwall M, Weis J, Nolte K, et al Delayed argon administration provides robust protection against cardiac arrest‐induced neurological damage. Neurocrit Care 2015; 22: 112–120. [DOI] [PubMed] [Google Scholar]
- 21. Alderliesten T, Favie LMA, Neijzen RW, Auwärter V, Nijboer CHA, Marges REJ, et al Neuroprotection by argon ventilation after perinatal asphyxia: a safety study in newborn piglets. PloS One 2014; 9: e113575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lemoine S, Blanchart K, Souplis M, Lemaitre A, Legallois D, Coulbault L, et al Argon exposure induces postconditioning in myocardial ischemia–reperfusion. J Cardiovasc Pharmacol Ther 2017; 22: 564–573. [DOI] [PubMed] [Google Scholar]
- 23. Mongardon N, Dyson A, Singer M. Pharmacological optimization of tissue perfusion. Br J Anaesth 2009; 103: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayer B, Soppert J, Kraemer S, Schemmel S, Beckers C, Bleilevens C, et al Argon induces protective effects in cardiomyocytes during the second window of preconditioning. Int J Mol Sci 2016; 17 pii: E1159. https://doi.org/10.3390/ijms17071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ulbrich F, Schallner N, Coburn M, Loop T, Lagrèze WA, Biermann J, et al Argon inhalation attenuates retinal apoptosis after ischemia/reperfusion injury in a time‐ and dose‐dependent manner in rats. PLoS One 2014; 9: e115984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ulbrich F, Kaufmann K, Roesslein M, Wellner F, Auwärter V, Kempf J, et al Argon mediates anti‐apoptotic signaling and neuroprotection via inhibition of toll‐like receptor 2 and 4. PLoS One 2015; 10: e0143887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Höllig A, Schug A, Fahlenkamp AV, Rossaint R, Coburn M, Argon Organo‐Protective Network (AON) . Argon: systematic review on neuro‐ and organoprotective properties of an ‘inert’ gas. Int J Mol Sci 2014; 15: 18175–18196. [DOI] [PMC free article] [PubMed] [Google Scholar]


