Abstract
Background and Aim
Benchmarking has proven beneficial in improving the quality of surgery. Mortality rate is an objective indicator, of which the 30‐day mortality rate is the most widely used. However, as a result of recent advances in medical care, the 30‐day mortality rate may not cover overall surgery‐related mortalities. We examined the significance and validity of the 30‐day mortality rate as a quality indicator.
Methods
The present study was conducted on cancer surgeries of esophagectomy, total gastrectomy, distal gastrectomy, right hemicolectomy, low anterior resection, hepatectomy, and pancreaticoduodenectomy that were registered in the first halves of 2012, 2013 and 2014 in a Japanese nationwide large‐scale database. This study examined the mortality curve for each surgical procedure, “sensitivity of surgery‐related death” (capture ratio) at each time point between days 30‐180, and the association between mortality within 30 days, mortality after 31 days, and preoperative, perioperative, and postoperative factors.
Results
Surgery‐related mortality rates of each surgical procedure were 0.6%‐3.0%. Regarding 30‐day mortality rates, only 38.7% (esophagectomy) to 53.3% (right hemicolectomy) of surgery‐related mortalities were captured. The capture ratio of surgery‐related deaths reached 90% or higher for 120‐day to 150‐day mortality rates. Factors associated with mortality rate within 30 days/after the 31st day were different, depending on the type of surgical procedure.
Conclusion
Thirty‐day mortality rate is useful as a quality indicator, but is not necessarily sufficient for all surgical procedures. Quality of surgery may require evaluation by combining 30‐day mortality rates with other indicators, depending on the surgical procedure.
Keywords: 30‐day mortality rate, gastrointestinal cancer, hepatobiliary cancer, pancreatic cancer, quality indicator
1. INTRODUCTION
Benchmarking and evaluations of surgical quality have proven beneficial and indispensable for improving surgical quality.1, 2 A technique that focuses on the “structure,” “process,” “results,” and “outcomes” of medical care has been proposed as a method for evaluating the quality of medical care, and a variety of other parameters are also being used as indicators in the assessment of surgical quality.3 For example, number of surgeries has been used as a “structural” indicator, rate of laparoscopic surgeries and length of hospital stay have been used as “process” indicators, and surgery‐related mortality rate, as well as the rate of complications, has been used as “outcome” indicators. In recent years, interest has been increasingly shown in, as well as expectations from, outcome‐oriented evaluations and measurements of medical care quality.
Mortality is an objective indicator, and assessment methods using overall mortality, surgery‐related mortality, in‐hospital mortality, 30‐day mortality, and 90‐day mortality are commonly used. Historically, the 30‐day mortality rate has been used to measure performance across a wide range of surgical disciplines, and the American College of Surgeons National Surgical Quality Improvement Program (ACSNSQIP) database, which is widely considered as the gold standard database for surgical quality improvements and future pay‐for‐performance programs, records 30‐day complication and mortality outcomes after surgery.4, 5, 6 The 30‐day mortality rate, as an objective indicator, may not entirely encompass the overall surgery‐related mortality rate.7, 8 As a result of recent progress in medical care, especially advances in anaesthesia9 and intensive care,10 early postoperative mortality rates have decreased and survival rates have improved among patients who could have died early after surgery if treated using previous methods. However, this does not necessarily mean that all patients who avoid death during the early postoperative period will continue to survive. Medical circumstances, such as length of hospital stay after surgery, are different in each country, and patients particularly in Europe and the USA are discharged from hospitals during the early postoperative period. As a result, surgery‐related complications that occur after discharge might be impossible to determine on the basis of 30‐day mortality rates or in‐hospital mortality rates. There is an absence of available data pertaining to how much of the surgery‐related mortality rate can be determined based on the 30‐day mortality rate, although this observation varies depending on situations in each country.
Herein, our study examines the significance and validity of the 30‐day mortality rate as a surgery‐related quality indicator (QI).
2. METHODS
2.1. Data collection
The National Clinical Database (NCD) was established in 2010 as a nationwide database that registers all surgical cases in cooperation with the surgical board certification system. Registrations started in 2011 and, currently, approximately 4300 facilities all over Japan are participating in the registry, and approximately 97% of surgical operations carried out by surgeons have been registered.11 The NCD, a Web‐based data management system, continuously involves individuals who approve data, those in charge of annual case reports from various departments, and data entry personnel, thereby assuring data traceability. Among gastrointestinal surgeries registered in the NCD, data on items similar to those of the ACSNSQIP have been collected in regard to esophagectomy, total gastrectomy, distal gastrectomy, right hemicolectomy, low anterior resection, hepatectomy, pancreaticoduodenectomy, and acute diffuse peritonitis. Hepatectomy included only extended lobectomy, lobectomy and segmentectomy other than lateral segmentectomy for primary liver cancer or gallbladder cancer.
NCD records between January 1, 2012 and December 31, 2014 were analyzed for this study. The study was conducted on cases of gastrointestinal cancers, which were treated with esophagectomy, total gastrectomy, distal gastrectomy, right hemicolectomy, low anterior resection, hepatectomy, and pancreaticoduodenectomy. To examine the significance and validity of the 30‐day mortality rate as a surgical QI, the study was carried out without including acute diffuse peritonitis, in which the patient's condition before surgery may have a great influence on the mortality rate. In addition, cases of non‐curative resection were excluded to exclude cancer‐related deaths. In order to increase the quality of the endpoints, a longer duration was given to the postoperative observation period, and data on surgical cases operated between January and June, which allowed for acquisition of follow‐up data from 30 days to 180 days after surgery, were analyzed. Records from patients who refused use of their data were excluded from this analysis. Records with missing data for age, gender, or status at postoperative day 30 were also excluded. Because non‐curative resection cases had been excluded, postoperative mortality was equivalent to surgery‐related mortality and, therefore, was considered as an endpoint.
2.2. Mortality curve for each operative procedure
Mortality curves were drawn using death within 30 days or postoperative death as events to visualize when mortality events occurred in the postoperative course.
2.3. Sensitivity of surgery‐related mortality (capture ratio)
In order to assess the mortality at various time points, ranging from 30 days to 180 days, which covers the surgery‐related mortality rate, we calculated the sensitivity of mortality at each point (capture ratio) on the basis of the 210‐day mortality, and we also calculated respective 95% confidence intervals.
2.4. Association between mortality within 30 days, mortality after 31 days, and preoperative, perioperative, and postoperative factors
Preoperative, perioperative, and postoperative factors, extracted during the creation of surgery‐related mortality models, were used for examining association of mortality within 30 days and after 31 days. We investigated the distribution of patients who died within 30 days as well as that of patients who died after the 31st day, and the difference between the two was tested using Fisher's exact test. In both categories, a two‐sided P‐value of <.05 was considered statistically significant. Statistical analyses were carried out using STATA14 (Stata Corp., College Station, TX, USA).
The present study followed the ethical guidelines of human subjects based on the Helsinki Declaration. Review and approval by the ethics committee was not carried out because existing unlinkable, anonymized data were used in the present study.
3. RESULTS
3.1. Subjects
Our study included 7448 cases of esophagectomy, 22 453 cases of total gastrectomy, 48 774 cases of distal gastrectomy, 24 260 cases of right hemicolectomy, 27 046 cases of low anterior resection, 7486 cases of hepatectomy, and 10 550 cases of pancreaticoduodenectomy. Among patients with esophagectomies, 39.5% presented a history of smoking tobacco within 1 year before surgery, and 62.0% had an alcohol‐consumption habit before surgery. Among patients with hepatectomies and those with pancreaticoduodenectomies, patients who were diagnosed with diabetes mellitus before surgery accounted for 28.1% and 30.2%, respectively, and these rates were higher than those found in patients undergoing other surgical procedures. Patients with intraoperative blood loss of 1000 mL or more accounted for 37.9% of those treated for hepatectomies, and 34.2% of those treated for pancreaticoduodenectomies, and patients with postoperative complications of Clavien‐Dindo classification grade III or greater accounted for 16.9% of those treated for esophagectomies and 16.0% of those treated for pancreaticoduodenectomies. Patients with postoperative anastomotic leakage accounted for 11.6% of those treated for esophagectomies, 8.5% of those treated for low anterior resections, and 10.6% of those treated for pancreaticoduodenectomies. Finally, 20.7% of patients treated for pancreaticoduodenectomies developed pancreatic fistulas (any grade), 2.3% developed grade C pancreatic fistulas, and bile leakage was present in 6.7% of patients treated for hepatectomies (Table 1).
Table 1.
Characteristics of analyzed subjects
| Esophagectomy (n = 7448) | Total gastrectomy (n = 22 453) | Distal gastrectomy (n = 48 774) | Right hemicolectomy (n = 24 260) | Low anterior resection (n = 27 046) | Hepatectomy (n = 7486) | Pancreaticoduodenectomy (n = 10 550) | Total (n = 148 017) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Age (y) | ‐59 | 1387 | 18.6 | 3340 | 14.9 | 7953 | 16.3 | 2569 | 10.6 | 6085 | 22.5 | 989 | 13.2 | 1372 | 13.0 | 23 695 | 16.0 |
| 60‐64 | 1477 | 19.8 | 3148 | 14.0 | 7005 | 14.4 | 2377 | 9.8 | 4587 | 17.0 | 1056 | 14.1 | 1474 | 14.0 | 21 124 | 14.3 | |
| 65‐69 | 1671 | 22.4 | 3652 | 16.3 | 7675 | 15.7 | 3247 | 13.4 | 4806 | 17.8 | 1305 | 17.4 | 1983 | 18.8 | 24 339 | 16.4 | |
| 70‐74 | 1586 | 21.3 | 4553 | 20.3 | 8877 | 18.2 | 4464 | 18.4 | 4698 | 17.4 | 1618 | 21.6 | 2361 | 22.4 | 28 157 | 19.0 | |
| 75‐79 | 947 | 12.7 | 4207 | 18.7 | 8339 | 17.1 | 4732 | 19.5 | 3607 | 13.3 | 1659 | 22.2 | 2122 | 20.1 | 25 613 | 17.3 | |
| 80‐ | 380 | 5.1 | 3553 | 15.8 | 8925 | 18.3 | 6871 | 28.3 | 3263 | 12.1 | 859 | 11.5 | 1238 | 11.7 | 25 089 | 17.0 | |
| Gender | Female | 1223 | 16.4 | 5701 | 25.4 | 16 204 | 33.2 | 12 450 | 51.3 | 9442 | 34.9 | 2042 | 27.3 | 4164 | 39.5 | 51 226 | 34.6 |
| Male | 6225 | 83.6 | 16 752 | 74.6 | 32 570 | 66.8 | 11 810 | 48.7 | 17 604 | 65.1 | 5444 | 72.7 | 6386 | 60.5 | 96 791 | 65.4 | |
| Smoking habits | (‐) | 4509 | 60.5 | 17 534 | 78.1 | 38 700 | 79.3 | 21 378 | 88.1 | 21 410 | 79.2 | 6077 | 81.2 | 8657 | 82.1 | 118 265 | 79.9 |
| (+) | 2939 | 39.5 | 4919 | 21.9 | 10 074 | 20.7 | 2882 | 11.9 | 5636 | 20.8 | 1409 | 18.8 | 1893 | 17.9 | 29 752 | 20.1 | |
| Drinking habits | (‐) | 2830 | 38.0 | 16 000 | 71.3 | 35 455 | 72.7 | 20 008 | 82.5 | 19 739 | 73.0 | 5477 | 73.2 | 7950 | 75.4 | 107 459 | 72.6 |
| (+) | 4618 | 62.0 | 6453 | 28.7 | 13 319 | 27.3 | 4252 | 17.5 | 7307 | 27.0 | 2009 | 26.8 | 2600 | 24.6 | 40 558 | 27.4 | |
| Hypertension | (‐) | 5033 | 67.6 | 14 128 | 62.9 | 30 376 | 62.3 | 14 715 | 60.7 | 17 790 | 65.8 | 4407 | 58.9 | 6595 | 62.5 | 93 044 | 62.9 |
| (+) | 2415 | 32.4 | 8325 | 37.1 | 18 398 | 37.7 | 9545 | 39.3 | 9256 | 34.2 | 3079 | 41.1 | 3955 | 37.5 | 54 973 | 37.1 | |
| Diabetes | (‐) | 6507 | 87.4 | 18 488 | 82.3 | 40 319 | 82.7 | 19 876 | 81.9 | 22 305 | 82.5 | 5385 | 71.9 | 7369 | 69.8 | 120 249 | 81.2 |
| (+) | 941 | 12.6 | 3965 | 17.7 | 8455 | 17.3 | 4384 | 18.1 | 4741 | 17.5 | 2101 | 28.1 | 3181 | 30.2 | 27 768 | 18.8 | |
| Chronic obstructive pulmonary disease | (‐) | 6945 | 93.2 | 21 405 | 95.3 | 46 734 | 95.8 | 23 568 | 97.1 | 26 221 | 96.9 | 7219 | 96.4 | 10 253 | 97.2 | 142 345 | 96.2 |
| (+) | 503 | 6.8 | 1048 | 4.7 | 2040 | 4.2 | 692 | 2.9 | 825 | 3.1 | 267 | 3.6 | 297 | 2.8 | 5672 | 3.8 | |
| Brain disorder | (‐) | 7273 | 97.7 | 21 610 | 96.2 | 46 946 | 96.3 | 23 295 | 96.0 | 26 181 | 96.8 | 7273 | 97.2 | 10 214 | 96.8 | 142 792 | 96.5 |
| (+) | 175 | 2.3 | 843 | 3.8 | 1828 | 3.7 | 965 | 4.0 | 865 | 3.2 | 213 | 2.8 | 336 | 3.2 | 5225 | 3.5 | |
| ≥ASA2 | (‐) | 2418 | 32.5 | 6986 | 31.1 | 16 262 | 33.3 | 6649 | 27.4 | 9829 | 36.3 | 1732 | 23.1 | 2678 | 25.4 | 46 554 | 31.5 |
| (+) | 5030 | 67.5 | 15 467 | 68.9 | 32 512 | 66.7 | 17 611 | 72.6 | 17 217 | 63.7 | 5754 | 76.9 | 7872 | 74.6 | 101 463 | 68.5 | |
| Intraoperative cardiac complications | (‐) | 7438 | 99.9 | 22 443 | 100.0 | 48 755 | 100.0 | 24 251 | 100.0 | 27 037 | 100.0 | 7481 | 99.9 | 10 547 | 100.0 | 14 7952 | 100.0 |
| (+) | 10 | 0.1 | 10 | 0.0 | 19 | 0.0 | 9 | 0.0 | 9 | 0.0 | 5 | 0.1 | 3 | 0.0 | 65 | 0.0 | |
| Intraoperative blood loss ≥1000 mL | (‐) | 6709 | 90.1 | 20 261 | 90.2 | 47 436 | 97.3 | 23 718 | 97.8 | 25 721 | 95.1 | 4649 | 62.1 | 6938 | 65.8 | 135 432 | 91.5 |
| (+) | 739 | 9.9 | 2192 | 9.8 | 1338 | 2.7 | 542 | 2.2 | 1325 | 4.9 | 2837 | 37.9 | 3612 | 34.2 | 12 585 | 8.5 | |
|
Postoperative complications Clavien‐Dindo classification ≥grade III |
(‐) | 6188 | 83.1 | 20 642 | 91.9 | 46 507 | 95.4 | 23 195 | 95.6 | 24 612 | 91.0 | 6595 | 88.1 | 8867 | 84.0 | 136 606 | 92.3 |
| (+) | 1260 | 16.9 | 1811 | 8.1 | 2267 | 4.6 | 1065 | 4.4 | 2434 | 9.0 | 891 | 11.9 | 1683 | 16.0 | 11 411 | 7.7 | |
|
Postoperative complications Clavien‐Dindo classification ≥grade IV |
(‐) | 7199 | 96.7 | 22 118 | 98.5 | 48 322 | 99.1 | 24 044 | 99.1 | 26 782 | 99.0 | 7268 | 97.1 | 10 251 | 97.2 | 145 984 | 98.6 |
| (+) | 249 | 3.3 | 335 | 1.5 | 452 | 0.9 | 216 | 0.9 | 264 | 1.0 | 218 | 2.9 | 299 | 2.8 | 2033 | 1.4 | |
| Reoperation within 30 days after operation | (‐) | 6768 | 90.9 | 21 332 | 95.0 | 47 301 | 97.0 | 23 414 | 96.5 | 24 654 | 91.2 | 7218 | 96.4 | 9882 | 93.7 | 140 569 | 95.0 |
| (+) | 680 | 9.1 | 1121 | 5.0 | 1473 | 3.0 | 846 | 3.5 | 2392 | 8.8 | 268 | 3.6 | 668 | 6.3 | 7448 | 5.0 | |
|
Surgical site infection other than anastomotic leakage |
(‐) | 6828 | 91.7 | 21 304 | 94.9 | 47 406 | 97.2 | 23 016 | 94.9 | 25 835 | 95.5 | 7042 | 94.1 | 9458 | 89.6 | 140 889 | 95.2 |
| (+) | 620 | 8.3 | 1149 | 5.1 | 1368 | 2.8 | 1244 | 5.1 | 1211 | 4.5 | 444 | 5.9 | 1092 | 10.4 | 7128 | 4.8 | |
| Anastomotic leakage | (‐) | 6584 | 88.4 | 21 524 | 95.9 | 47 749 | 97.9 | 23 929 | 98.6 | 24 741 | 91.5 | 7373 | 98.5 | 9432 | 89.4 | 141 332 | 95.5 |
| (+) | 864 | 11.6 | 929 | 4.1 | 1025 | 2.1 | 331 | 1.4 | 2305 | 8.5 | 113 | 1.5 | 1118 | 10.6 | 6685 | 4.5 | |
| Transfusion ≥5 U | (‐) | 7279 | 97.7 | 22 234 | 99.0 | 48 502 | 99.4 | 24 144 | 99.5 | 26 914 | 99.5 | 7224 | 96.5 | 10 220 | 96.9 | 146 517 | 99.0 |
| (+) | 169 | 2.3 | 219 | 1.0 | 272 | 0.6 | 116 | 0.5 | 132 | 0.5 | 262 | 3.5 | 330 | 3.1 | 1500 | 1.0 | |
| Postoperative unexpected intubation | (‐) | 7012 | 94.1 | 22 165 | 98.7 | 48 414 | 99.3 | 24 150 | 99.5 | 26 909 | 99.5 | 7353 | 98.2 | 10 334 | 98.0 | 146 337 | 98.9 |
| (+) | 436 | 5.9 | 288 | 1.3 | 360 | 0.7 | 110 | 0.5 | 137 | 0.5 | 133 | 1.8 | 216 | 2.0 | 1680 | 1.1 | |
| Postoperative mechanical ventilation ≥48 h | (‐) | 6881 | 92.4 | 22 157 | 98.7 | 48 418 | 99.3 | 24 099 | 99.3 | 26 878 | 99.4 | 7332 | 97.9 | 10 313 | 97.8 | 146 078 | 98.7 |
| (+) | 567 | 7.6 | 296 | 1.3 | 356 | 0.7 | 161 | 0.7 | 168 | 0.6 | 154 | 2.1 | 237 | 2.2 | 1939 | 1.3 | |
| Postoperative renal dysfunction | (‐) | 7318 | 98.3 | 22 264 | 99.2 | 48 493 | 99.4 | 24 121 | 99.4 | 26 852 | 99.3 | 7331 | 97.9 | 10 378 | 98.4 | 146 757 | 99.1 |
| (+) | 130 | 1.7 | 189 | 0.8 | 281 | 0.6 | 139 | 0.6 | 194 | 0.7 | 155 | 2.1 | 172 | 1.6 | 1260 | 0.9 | |
| Postoperative central nervous system disorder | (‐) | 7430 | 99.8 | 22 400 | 99.8 | 48 689 | 99.8 | 24 215 | 99.8 | 26 998 | 99.8 | 7476 | 99.9 | 10 528 | 99.8 | 147 736 | 99.8 |
| (+) | 18 | 0.2 | 53 | 0.2 | 85 | 0.2 | 45 | 0.2 | 48 | 0.2 | 10 | 0.1 | 22 | 0.2 | 281 | 0.2 | |
| Postoperative cardiac complications | (‐) | 7389 | 99.2 | 22 360 | 99.6 | 48 653 | 99.8 | 24 203 | 99.8 | 26 981 | 99.8 | 7445 | 99.5 | 10 478 | 99.3 | 147 509 | 99.7 |
| (+) | 59 | 0.8 | 93 | 0.4 | 121 | 0.2 | 57 | 0.2 | 65 | 0.2 | 41 | 0.5 | 72 | 0.7 | 508 | 0.3 | |
| Postoperative sepsis | (‐) | 7066 | 94.9 | 21 954 | 97.8 | 48 198 | 98.8 | 23 978 | 98.8 | 26 509 | 98.0 | 7299 | 97.5 | 10 096 | 95.7 | 145 100 | 98.0 |
| (+) | 382 | 5.1 | 499 | 2.2 | 576 | 1.2 | 282 | 1.2 | 537 | 2.0 | 187 | 2.5 | 454 | 4.3 | 2917 | 2.0 | |
| Postoperative septic shock | (‐) | 7364 | 98.9 | 22 324 | 99.4 | 48 618 | 99.7 | 24 156 | 99.6 | 26 921 | 99.5 | 7438 | 99.4 | 10 447 | 99.0 | 147 268 | 99.5 |
| (+) | 84 | 1.1 | 129 | 0.6 | 156 | 0.3 | 104 | 0.4 | 125 | 0.5 | 48 | 0.6 | 103 | 1.0 | 749 | 0.5 | |
| Postoperative pneumonia | (‐) | 6543 | 87.8 | 21 783 | 97.0 | 47 884 | 98.2 | 23 991 | 98.9 | 26 844 | 99.3 | 7364 | 98.4 | 10 316 | 97.8 | 144 725 | 97.8 |
| (+) | 905 | 12.2 | 670 | 3.0 | 890 | 1.8 | 269 | 1.1 | 202 | 0.7 | 122 | 1.6 | 234 | 2.2 | 3292 | 2.2 | |
| Postoperative deep vein thrombosis | (‐) | 7413 | 99.5 | 22 415 | 99.8 | 48 714 | 99.9 | 24 212 | 99.8 | 26 983 | 99.8 | 7460 | 99.7 | 10 511 | 99.6 | 147 708 | 99.8 |
| (+) | 35 | 0.5 | 38 | 0.2 | 60 | 0.1 | 48 | 0.2 | 63 | 0.2 | 26 | 0.3 | 39 | 0.4 | 309 | 0.2 | |
| Postoperative pulmonary embolism | (‐) | 7428 | 99.7 | 22 429 | 99.9 | 48 727 | 99.9 | 24 244 | 99.9 | 27 011 | 99.9 | 7465 | 99.7 | 10 521 | 99.7 | 147 825 | 99.9 |
| (+) | 20 | 0.3 | 24 | 0.1 | 47 | 0.1 | 16 | 0.1 | 35 | 0.1 | 21 | 0.3 | 29 | 0.3 | 192 | 0.1 | |
| Postoperative urinary tract infection | (‐) | 7406 | 99.4 | 22 353 | 99.6 | 48 589 | 99.6 | 24 134 | 99.5 | 26 783 | 99.0 | 7440 | 99.4 | 10 471 | 99.3 | 147 176 | 99.4 |
| (+) | 42 | 0.6 | 100 | 0.4 | 185 | 0.4 | 126 | 0.5 | 263 | 1.0 | 46 | 0.6 | 79 | 0.7 | 841 | 0.6 | |
| Postoperative pancreatic fistula | (‐) | 21 394 | 95.3 | 47 929 | 98.3 | 8363 | 79.3 | 77 686 | 95.0 | ||||||||
| (+) | 1059 | 4.7 | 845 | 1.7 | 2187 | 20.7 | 4091 | 5.0 | |||||||||
|
Postoperative pancreatic fistula ≥grade C |
(‐) | 22 382 | 99.7 | 48 706 | 99.9 | 10 304 | 97.7 | 81 392 | 99.5 | ||||||||
| (+) | 71 | 0.3 | 68 | 0.1 | 246 | 2.3 | 385 | 0.5 | |||||||||
| Postoperative biliary fistula | (‐) | 6981 | 93.3 | 6981 | 93.3 | ||||||||||||
| (+) | 505 | 6.7 | 505 | 6.7 | |||||||||||||
ASA2, American Society of Anesthesiologists Classification 2.
3.2. Mortality curves for each surgical procedure
When survival or death at day 210 was used as an endpoint, the surgery‐related mortality rate was 3.0% for patients with hepatectomies, 2.5% for patients with pancreaticoduodenectomies, 2.3% for patients with esophagectomies, 1.4% for patients with total gastrectomies, 1.1% for patients with right hemicolectomies, 0.8% for patients with distal gastrectomies, and 0.6% for patients with low anterior resections, respectively, in descending order. All types of surgical procedures showed mortality rates that increased over time.
For right hemicolectomies, low anterior resections, and hepatectomies, the mortality rate nearly reached a plateau between days 90 and 150. For esophagectomies, total gastrectomies, gastrectomies, and pancreaticoduodenectomies, the mortality rate continuously increased until day 210 (Figure 1).
Figure 1.
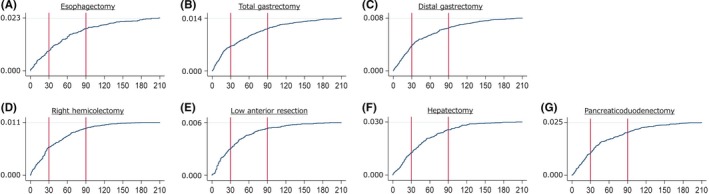
Mortality curve for each type of surgical procedure. A, Esophagectomy; B, Total gastrectomy; C, Distal gastrectomy; D, Right hemicolectomy; E, Low anterior resection; F, Hepatectomy; G, Pancreaticoduodenectomy
3.3. Sensitivity of the 30‐to‐180‐day mortality rate (capture ratio for surgery‐related mortalities) in comparison with the 210‐day mortality rate (surgery‐related mortalities)
The 30‐day mortality rate captured only 38.7% (esophagectomies) to 53.3% (right hemicolectomies) of surgery‐related mortalities. The capture ratio for surgery‐related mortalities reached 90% or greater when the 120‐day mortality rate was taken into consideration for right hemicolectomy, low anterior resection, hepatectomy, and pancreaticoduodenectomy, as well as when the 150‐day mortality rate was taken into account for esophagectomy, total gastrectomy, and distal gastrectomy. The capture ratio for surgery‐related mortalities reached 90% or greater when the 120‐day mortality rate was taken into consideration for right hemicolectomy, low anterior resection, hepatectomy, and pancreaticoduodenectomy, and when the 150‐day mortality rate was taken into consideration for esophagectomy, total gastrectomy and distal gastrectomy. The capture ratio of 90‐day mortality rate was 80.3% in esophagectomies, 80.3% in total gastrectomies, 81.6% in distal gastrectomies, 89.0% in right hemicolectomies, 88.5% in low anterior resections, 85.2% in hepatectomies and 81.2% in pancreaticoduodenectomies (Table 2).
Table 2.
Sensitivity of the 30‐to‐180‐day mortality rate (capture ratio for surgery‐related mortalities)
| 30 d | 60 d | 90 d | 120 d | 150 d | 180 d | |
|---|---|---|---|---|---|---|
| Esophagectomy | 38.7 (31.4‐46.4) | 64.7 (57.1‐71.8) | 80.3 (73.6‐85.9) | 87.2 (81.3‐91.8) | 93.0 (88.1‐96.3) | 96.5 (92.6‐98.7) |
| Total gastrectomy | 47.3 (41.7‐52.9) | 66.3 (60.8‐71.5) | 80.3 (75.6‐84.5) | 88.7 (84.8‐92.0) | 93.4 (90.1‐95.9) | 97.8 (95.6‐99.1) |
| Distal gastrectomy | 48.3 (43.4‐53.2) | 68.1 (63.3‐72.5) | 81.6 (77.5‐85.2) | 89.3 (85.9‐92.1) | 95.1 (92.6‐97.0) | 97.6 (95.6‐98.8) |
| Right hemicolectomy | 53.3 (47.1‐59.4) | 74.4 (68.7‐79.5) | 89.0 (84.7‐92.5) | 95.4 (92.2‐97.6) | 98.4 (96.1‐99.5) | 100.0 (98.6‐100.0) |
| Low anterior resection | 50.9 (42.8‐59.0) | 76.4 (69.0‐82.8) | 88.5 (82.4‐93.0) | 94.2 (89.3‐97.3) | 97.4 (93.6‐99.3) | 98.7 (95.5‐99.8) |
| Hepatectomy | 42.6 (36.0‐49.3) | 69.9 (63.4‐75.8) | 85.2 (79.8‐89.5) | 94.6 (90.7‐97.1) | 97.7 (94.8‐99.2) | 99.6 (97.5‐100.0) |
| Pancreaticoduodenectomy | 42.9 (36.9‐49.0) | 68.2 (62.3‐73.8) | 81.3 (76.1‐85.8) | 91.0 (86.9‐94.1) | 95.1 (91.8‐97.3) | 98.5 (96.2‐99.6) |
3.4. Association between mortality within 30 days, mortality after 31 days, and preoperative, perioperative and postoperative factors
Among factors that likely affect surgery‐related mortality rates, the following showed a significantly higher percentage of deaths within 30 days, irrespective of the type of surgical procedure: postoperative complications of Clavien‐Dindo classification grade III or higher (except esophagectomy), postoperative complications of Clavien‐Dindo classification grade IV or higher (all surgical procedures), unplanned postoperative intubation (except esophagectomy and hepatectomy), and cardiac complications (all surgical procedures). In addition, the following factors were associated with individual surgical procedures: renal dysfunction (distal gastrectomy, right hemicolectomy), central nervous system disorder (right hemicolectomy), sepsis (right hemicolectomy), and septic shock (total gastrectomy, low anterior resection). Conversely, the following factors displayed significantly lower percentages in terms of mortality within 30 days: reoperation within 30 days (esophagectomy, total gastrectomy, right hemicolectomy, pancreaticoduodenectomy), anastomotic leakage (total gastrectomy, distal gastrectomy), pulmonary embolism (low anterior resection), pneumonia (hepatectomy), and urinary tract infection (total gastrectomy, right hemicolectomy) (Table 3).
Table 3.
Association between mortality within 30 d, mortality after 31 d, and preoperative, perioperative and postoperative factors
| Esophagectomy | Total gastrectomy | Distal gastrectomy | Right colectomy | Low anterior resection | Hepatectomy | Pancreaticoduodenectomy | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Postoperative death within 30 days (n = 67) | Postoperative death after 31st day (n = 106) | P‐value | Postoperative death within 30 days (n = 152) | Postoperative death after 31st day (n = 169) | P‐value | Postoperative death within 30 days (n = 152) | Postoperative death after 31st day (n = 169) | P‐value | Postoperative death within 30 days (n = 152) | Postoperative death after 31st day (n = 169) | P‐value | Postoperative death within 30 days (n = 152) | Postoperative death after 31st day (n = 169) | P‐value | Postoperative death within 30 days (n = 152) | Postoperative death after 31st day (n = 169) | P‐value | Postoperative death within 30 days (n = 152) | Postoperative death after 31st day (n = 169) | P‐value | |
| Age (‐59/60‐64/65‐69/70‐74/75‐79/80‐) | 4/14/8/17/18/6 | 8/17/21/25/24/11 | .763 | 4/11/13/21/48/55 | 6/10/21/20/47/65 | .811 | 11/10/18/88/38/90 | 5/6/15/30/52/106 | .251 | 7/6/10/16/25/78 | 2/3/5/9/19/86 | .203 | 7/9/11/13/12/28 | 1/9/7/7/16/37 | .113 | 3/14/14/29/24/11 | 8/21/19/24/36/20 | .419 | 6/13/22/25/23/26 | 8/10/23/37/39/36 | .635 |
| Gender (F/M) | 6/61 | 7/99 | .568 | 26/126 | 31/138 | .884 | 52/148 | 56/158 | 1.000 | 63/79 | 60/64 | .539 | 17/63 | 15/62 | .844 | 21/74 | 30/98 | .873 | 27/88 | 36/117 | 1.000 |
| Smoking habits (+) | 36 (53.7%) | 48 (45.3%) | .349 | 19 (12.5%) | 36 (21.3%) | .039 | 31 (15.5%) | 29 (13.6%) | .580 | 9 (6.3%) | 14 (11.3%) | .190 | 19 (23.8%) | 13 (16.9%) | .325 | 20 (21.1%) | 32 (25.0%) | .525 | 25 (21.7%) | 22 (14.4%) | .144 |
| Drinking habits (+) | 39 (58.2%) | 67 (63.2%) | .526 | 30 (19.7%) | 42 (24.9%) | .287 | 31 (15.5%) | 47 (22.0%) | .103 | 17 (12.0%) | 16 (12.9%) | .854 | 17 (21.3%) | 14 (18.2%) | .691 | 22 (23.2%) | 39 (30.5%) | .288 | 27 (23.5%) | 36 (23.5%) | 1.000 |
| Hypertension (+) | 32 (47.8%) | 41 (38.7%) | .270 | 63 (41.4%) | 73 (43.2%) | .821 | 97 (48.5%) | 88 (41.1%) | .139 | 60 (42.3%) | 61 (49.2%) | .269 | 31 (38.8%) | 30 (39.0%) | 1.000 | 42 (44.2%) | 61 (47.7%) | .684 | 63 (54.8%) | 80 (52.3%) | .712 |
| Diabetes (+) | 16 (23.9%) | 20 (18.9%) | .447 | 24 (15.8%) | 14 (8.3%) | .053 | 49 (24.5%) | 52 (24.3%) | 1.000 | 26 (18.3%) | 28 (22.6%) | .446 | 20 (25.0%) | 21.0 (27.3%) | .856 | 26 (27.4%) | 43 (33.6%) | .380 | 43 (37.4%) | 60 (39.2%) | .800 |
| Chronic obstructive pulmonary disease (+) | 8 (11.9%) | 13 (12.3%) | 1.000 | 15 (9.9%) | 14 (8.3 %) | .698 | 21 (10.5%) | 21 (9.8%) | .871 | 9 (6.3%) | 10 (8.1%) | .638 | 5 (6.3%) | 3 (3.9%) | .720 | 4 (4.2%) | 7 (5.5%) | .762 | 10 (8.7%) | 8 (5.2%) | .326 |
| Brain disorder (+) | 3 (4.5%) | 4 (3.8%) | 1.000 | 24 (15.8%) | 16 (9.5%) | .093 | 21 (10.5%) | 19 (8.9%) | .620 | 14 (9.9%) | 9 (7.3%) | .516 | 12 (15.0%) | 9 (11.7%) | .641 | 5 (5.3%) | 7 (5.5%) | 1.000 | 8 (7.0%) | 14 (9.2%) | .654 |
| ≥ASA2 | 57 (85.1%) | 89 (84.0%) | 1.000 | 130 (85.5%) | 138 (81.7%) | .370 | 184 (92.0%) | 194 (90.7%) | .728 | 131 (92.3%) | 119 (96.0%) | .301 | 68 (85.0%) | 65 (84.4%) | 1.000 | 84 (88.4%) | 109 (85.2%) | .554 | 102 (88.7%) | 134 (87.6%) | .850 |
| Intraoperative cardiac complications (+) | 2 (3.0%) | 0 (0.0%) | .149 | 2 (1.3%) | 0 (0.0 %) | .223 | 2 (1.0%) | 0 (0.0%) | .233 | 3 (2.1%) | 0 (0.0%) | .251 | 1 (1.3%) | 0 (0.0%) | 1.000 | 1 (1.1%) | 0 (0.0%) | .426 | 0 (0.0%) | 0 (0.0%) | |
| Intraoperative blood loss ≥1000 mL | 18 (26.9%) | 27 (25.5%) | .860 | 32 (21.1%) | 46 (27.2%) | .241 | 18 (9.0%) | 32 (15.0%) | .071 | 11 (7.7%) | 17 (13.7%) | .160 | 12 (15.0%) | 12 (15.6%) | 1.000 | 65 (68.4%) | 88 (68.8%) | 1.000 | 70 (60.9%) | 81 (52.9%) | .215 |
|
Postoperative complications Clavien‐Dindo classification ≥grade III (+) |
55 (82.1%) | 79 (74.5%) | .268 | 131 (86.2%) | 103 (60.9%) | <.001 | 159 (79.5%) | 118 (55.1%) | <.001 | 113 (79.6%) | 57 (46.0%) | <.001 | 66 (82.5%) | 44 (57.1%) | <.001 | 88 (92.6%) | 90 (70.3%) | <.001 | 104 (90.4%) | 107 (69.9%) | <.001 |
|
Postoperative complications Clavien‐Dindo classification ≥grade IV (+) |
55 (82.1%) | 51 (48.1%) | <.001 | 127 (83.6%) | 64 (37.9%) | <.001 | 148 (74.0%) | 78 (36.4%) | <.001 | 108 (76.1%) | 29 (23.4%) | <.001 | 63 (78.8%) | 26 (33.8%) | <.001 | 87 (91.6%) | 63 (49.2%) | <.001 | 100 (87.0%) | 67 (43.8%) | <.001 |
| Reoperation within 30 days after operation (+) | 19 (28.4%) | 48 (45.3%) | .037 | 46 (30.3%) | 74 (43.8%) | .015 | 54 (27.0%) | 70 (32.7%) | .238 | 20 (14.1%) | 35 (28.2%) | .006 | 34 (42.5%) | 32 (41.6%) | 1.000 | 21 (22.1%) | 32 (25.0%) | .637 | 40 (34.8%) | 72 (47.1%) | .046 |
| Surgical site infection other than anastomotic leakage (+) | 6 (9.0 %) | 31 (29.2%) | .001 | 12 (7.9%) | 30 (17.8%) | .012 | 15 (7.5%) | 44 (20.6%) | <.001 | 19 (13.4%) | 31 (25.0%) | .018 | 14 (17.5%) | 18 (23.4%) | .430 | 4 (4.2%) | 34 (26.6%) | <.001 | 19 (16.5%) | 52 (34.0%) | .001 |
| Anastomotic leakage (+) | 15 (22.4%) | 38 (35.8%) | .065 | 22 (14.5%) | 53 (31.4%) | <.001 | 28 (14.0%) | 49 (22.9%) | .023 | 19 (13.4%) | 20 (16.1%) | .603 | 30 (37.5%) | 27 (35.1%) | .868 | 4 (4.2%) | 15 (11.7%) | .054 | 40 (34.8%) | 67 (43.8%) | .166 |
| Transfusion ≥5 U (+) | 17 (25.4%) | 29 (27.4%) | .860 | 36 (23.7%) | 30 (17.8%) | .214 | 37 (18.5%) | 44 (20.6%) | .622 | 18 (12.7%) | 13 (10.5%) | .702 | 15 (18.8%) | 16 (20.8%) | .842 | 47 (49.5%) | 55 (43.0%) | .345 | 53 (46.1%) | 61 (39.9%) | .321 |
| Postoperative unexpected intubation (+) | 33 (49.3%) | 52 (49.1%) | 1.000 | 77 (50.7%) | 52 (30.8%) | <.001 | 98 (49.0%) | 58 (27.1%) | <.001 | 32 (22.5%) | 14 (11.3%) | .022 | 34 (42.5%) | 20 (26.0%) | .043 | 42 (44.2%) | 41 (32.0%) | .070 | 56 (48.7%) | 54 (35.3%) | .033 |
| Postoperative mechanical ventilation ≥48 h (+) | 35 (52.2%) | 62 (58.5%) | .436 | 59 (38.8%) | 55 (32.5%) | .246 | 75 (37.5%) | 68 (31.8%) | .255 | 39 (27.5%) | 28 (22.6%) | .397 | 29 (36.3%) | 21 (27.3%) | .237 | 41 (43.2%) | 47 (36.7%) | .336 | 48 41.7%) | 61 (39.9%) | .802 |
| Postoperative renal dysfunction (+) | 22 (32.8%) | 28 (26.4%) | .392 | 44 (28.9%) | 38 (22.5%) | .201 | 61 (30.5%) | 44 (20.6%) | .024 | 35 (24.6%) | 17 (13.7%) | .030 | 27 (33.8%) | 16 (20.8%) | .076 | 41 (43.2%) | 42 (32.8%) | .125 | 38 (33.0%) | 43 (28.1%) | .421 |
| Postoperative central nervous system disorder (+) | 0 (0.0%) | 1 (0.9%) | 1.000 | 10 (6.6%) | 7 ( 4.1%) | .455 | 8 (4.0%) | 3 (1.4%) | .130 | 9 (6.3%) | 1 (0.8%) | .022 | 7 (8.8%) | 2 (2.6%) | .168 | 1 (1.1%) | 0 (0.0%) | .426 | 3 (2.6%) | 5 (3.3%) | 1.000 |
| Postoperative cardiac complications (+) | 33 (49.3%) | 11 (10.4%) | <.001 | 63 (41.4%) | 12 (7.1%) | <.001 | 76 (38.0%) | 12 (5.6%) | <.001 | 41 (28.9%) | 2 (1.6%) | <.001 | 38 (47.5%) | 11 (14.3%) | <.001 | 29 (30.5%) | 7 (5.5%) | <.001 | 46 (40.0%) | 9 (5.9%) | <.001 |
| Postoperative sepsis (+) | 34 (50.7%) | 47 (44.3%) | .437 | 55 (36.2%) | 50 (29.6%) | .234 | 62 (31.0%) | 70 (32.7%) | .752 | 46 (32.4%) | 26 (21.0%) | .039 | 33 (41.3%) | 22 (28.6%) | .132 | 25 (26.3%) | 45 (35.2%) | .190 | 40 (34.8%) | 66 (43.1%) | .207 |
| Postoperative septic shock (+) | 24 (35.8%) | 26 (24.5%) | .124 | 36 (23.7%) | 24 (14.2%) | .032 | 39 (19.5%) | 39 (18.2%) | .802 | 34 (23.9%) | 19 (15.3%) | .091 | 29 (36.3%) | 9 (11.7%) | <.001 | 11 (11.6%) | 24 (18.8%) | .192 | 27 (23.5%) | 34 (22.2%) | .883 |
| Postoperative pneumonia (+) | 34 (50.7%) | 55 (51.9%) | 1.000 | 46 (30.3%) | 67 (39.6%) | .081 | 63 (31.5%) | 70 (32.7%) | .833 | 25 (17.6%) | 32 (25.8%) | .134 | 16 (20.0%) | 22 (28.6%) | .264 | 9 (9.5%) | 37 (28.9%) | <.001 | 27 (23.5%) | 42 (27.5%) | .484 |
| Postoperative deep vein thrombosis (+) | 0 (0.0%) | 0 (0.0%) | 3 (2.0%) | 5 (3.0%) | .726 | 3 (1.5%) | 2 (0.9%) | .676 | 5 (3.5%) | 0 (0.0%) | .063 | 4 (5.0%) | 1 (1.3%) | .367 | 3 (3.2%) | 1 (0.8%) | .315 | 2 (1.7%) | 2 (1.3%) | 1.000 | |
| Postoperative pulmonary embolism (+) | 0 (0.0%) | 0 (0.0%) | 2 (1.3%) | 3 (1.8%) | 1.000 | 4 (2.0%) | 2 (0.9%) | .163 | 2 (1.4%) | 0 (0.0%) | .500 | 6 (7.5%) | 0 (0.0%) | .028 | 0 (0.0%) | 1 (0.8%) | 1.000 | 5 (4.3%) | 1 (0.7%) | .087 | |
| Postoperative urinary tract infection (+) | 1 (1.5%) | 8 (7.5%) | .156 | 0 (0.0%) | 8 (4.7%) | .008 | 4 (2.0%) | 9 (4.2%) | .263 | 4 (2.8%) | 12 (9.7%) | .021 | 2 (2.5%) | 8 (10.4%) | .053 | 5 (5.3%) | 6 (4.7%) | 1.000 | 3 (2.6%) | 10 (6.5%) | .162 |
| Postoperative pancreatic fistula (+) | 11 (7.2%) | 21 (12.4%) | .138 | 10 (5.0%) | 13 (6.1%) | .673 | 48 (41.7%) | 72 (47.1%) | .457 | ||||||||||||
|
Postoperative pancreatic fistula ≥grade C (+) |
4 (2.6%) | 10 (5.9%) | .179 | 6 (3.0%) | 8 (3.7%) | .789 | 29 (25.2%) | 39 (25.5%) | 1.000 | ||||||||||||
| Postoperative biliary fistula (+) | 8 (8.4%) | 34 (26.6%) | .001 | ||||||||||||||||||
ASA2, American Society of Anesthesiologists Classification 2.
The significance of bolded terms was “P < 0.05”.
4. DISCUSSION
We found that the 30‐day mortality, which was a standard QI for international comparisons, was not sufficient as a QI for all gastrointestinal cancer surgeries. Surgical stress, risk of complications, surgery‐related mortality rate, and the 30‐day mortality differ depending on the type of surgical procedure. Even when the type of surgical procedure was identical, the risk of surgery‐related deaths and complications varied depending on patient‐related risks, such as age and comorbidities.12, 13, 14 However, from an overall perspective, the risk roughly reflects the difference associated with the type of surgical procedure. Also, in our data, surgery‐related mortality rates varied depending on the type of surgical procedure, where highest values were found in hepatectomies and lowest in low anterior resections. Previous reports, based on a comparison of 30‐day mortality rates, have shown that surgical outcomes were more favorable in Japan than in Europe and the USA.15
The number of surgeries carried out, rate of laparoscopic surgeries, length of hospital stay, surgery‐related mortality rates, and rate of complications have been used as indicators for the evaluation of surgical outcomes, or qualities, in individual facilities, and have been used for comparisons between facilities in terms of outcomes (qualities). However, the number of surgeries carried out by each facility, as well as the length of hospital stay, varies depending on each country's health‐care system. In addition, for the rate of complications, such as postoperative surgical site infections and anastomotic leakage, the capture ratio may differ depending on diagnostic criteria and postoperative care systems, which makes it difficult to carry out a comparison of true incidences. Safety is the most basic requirement for carrying out surgery, and treatment outcomes should be evaluated upon securing a certain safety level. From our perspective, surgery‐related mortality rates are believed to be of utmost importance as indicators for the evaluation of surgical quality.
In Japan, the length of postoperative hospital stays are relatively long and, as a result, the capture ratio is high for postoperative complications, including those that are minor and delayed. Cases of hospital transfer or hospital discharge without alleviation of complications are few, and this could be the reason why surgery‐related mortalities nearly match in‐hospital mortalities. Meanwhile, in Europe and the USA, duration of postoperative hospital stay is often short, and surgery‐related deaths among in‐hospital deaths are difficult to determine. Similarly, 30‐day mortality rates do not reflect all aspects of surgical outcomes or qualities.
The findings of the present study show that postoperative complications categorized as Clavien‐Dindo classification grade III or greater, postoperative complications categorized as Clavien‐Dindo classification grade IV or greater, unplanned postoperative intubations, and cardiac complications are factors with significantly high percentages of mortality within 30 days, irrespective of the type of surgical procedure, and factors with low association with surgical procedures, such as renal dysfunction (distal gastrectomy, right hemicolectomy), central nervous system disorder (right hemicolectomy), sepsis (right hemicolectomy), and septic shock (total gastrectomy, low anterior resection) were also extracted. In contrast, our results also indicated that factors highly associated with the type of surgical procedure, such as reoperation within 30 days and anastomotic leakage, had significantly lower percentages of deaths occurring within 30 days after surgery. Our data show that factors linked to surgery‐related deaths differ depending on the type of surgical procedure, and that when the 30‐day mortality rate was used as a QI for an evaluation of surgery outcome or quality, the capture ratio for the determination of deaths associated with surgical technique‐related complications may be low or otherwise useless, depending on the respective surgical procedure. Furthermore, our data suggest that when the rate of surgical site infections (except those as a result of anastomotic leakage) is high in surgeries other than low anterior resection, the surgery‐related mortality rate is likely to be high even when the 30‐day mortality rate is low. Also, when the rate of anastomotic leakage is high in esophagectomy, total gastrectomy, and distal gastrectomy, the surgery‐related mortality rate is likely to be high, even when the 30‐day mortality rate is low. Similarly, in esophagectomy, right hemicolectomy, pancreaticoduodenectomy, the surgery‐related mortality rate is likely to be high when the rate of reoperation within 30 days is high, even when the 30‐day mortality rate is low, and in hepatectomies with large amounts of bile leakage, surgery‐related mortality rate is likely to be high, even when the 30‐day mortality rate is low and, as a result, surgery‐related mortality may be difficult to evaluate properly.
Meanwhile, in low anterior resections, surgery‐related mortalities can be evaluated properly through evaluation of the 30‐day mortality rate. Thus, in order to evaluate the outcomes or qualities of surgery, the 30‐day mortality rate and other indicators, such as complications, will need to be assessed in combination with outcomes or qualities of surgery. For example, the incidence of anastomotic leakage in total gastrectomy and distal gastrectomy or bile leakage in hepatectomy may be useful. And to use these indicators could fit the actual clinical feelings of surgeons. A comparison with worldwide outcomes or qualities of surgery, or benchmarking, will be necessary to improve the outcomes or qualities of surgery in Japan.16 However, data regarding Japan, in which surgery‐related mortality is well determined, were based on the Japanese health‐care system, and it remains unknown whether these observations are unique to Japan or are universal and shared worldwide. Our results, which were derived from data collected in Japan, could be used to guide an evaluation of their association with medical circumstances in all countries worldwide, through an international endeavor.
5. CONCLUSION
The 30‐day mortality rate is definitely useful as a QI for the evaluation of the outcomes or qualities of gastrointestinal cancer surgeries, but it is not necessarily sufficient to cover all types of surgical procedures. Depending on the type of surgical procedure, evaluations of surgical outcome (or qualities) may need to be carried out in combination with the use of the 30‐day mortality rate and other indicators, such as complications.
DISCLOSURE
Funding: Sources of funding for research and/or publication: The Japanese Society of Gastroenterological Surgery.
Conflicts of Interest: Hiroaki Miyata and Hiroyuki Yamamoto are affiliated with the Department of Healthcare Quality Assessment at the University of Tokyo, and the department is endowed by Johnson & Johnson K.K., Nipro Co., Teijin Pharma Ltd, Kaketsuken K.K., St. Jude Medical Japan Co., Ltd, Novartis Pharma K.K., Taiho Pharmaceutical Co., Ltd, W. L. Gore & Associates, Co., Ltd, Olympus Corporation, and Chugai Pharmaceutical Co., Ltd. None of the organizations had any role in design and conduct of the study, data collection, data analysis, data management, data interpretation, or the preparation, review, approval of this manuscript. The other authors declare that they have no conflicts of interest.
Author Contribution: Substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; T.M., H.Y., S.M., K.K., G.W., H.M., Y.D., M.M. Drafting the article or revising it critically for important intellectual content; T.M., H.Y., S.M., K.K., G.W., H.M. Final approval of the version to be published; Y.S., Y.D., M.M.
ACKNOWLEDGEMENTS
We would like to thank the participating surgeons and institutions contributing the data acquisition in NCD. We also would like to thank Mr Koji Sasaki, Secretariat of the Japanese Society of Gastroenterological Surgery, and Mr Naoki Saito, Secretariat of NCD, for their secretarial assistance.
Mizushima T, Yamamoto H, Marubashi S, et al. Validity and significance of 30‐day mortality rate as a quality indicator for gastrointestinal cancer surgeries. Ann Gastroenterol Surg. 2018;2:231–240. https://doi.org/10.1002/ags3.12070
REFERENCES
- 1. Kiefe CI, Allison JJ, Williams OD, Person SD, Weaver MT, Weissman NW. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285:2871–9. [DOI] [PubMed] [Google Scholar]
- 2. Gotoh M, Miyata H, Hashimoto H, et al. National Clinical Database feedback implementation for quality improvement of cancer treatment in Japan: from good to great through transparency. Surg Today. 2016;46:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966;44(Suppl):166–206. [PubMed] [Google Scholar]
- 4. Russell EM, Bruce J, Krukowski ZH. Systematic review of the quality of surgical mortality monitoring. Br J Surg. 2003;90:527–32. [DOI] [PubMed] [Google Scholar]
- 5. Johnson ML, Gordon HS, Petersen NJ, et al. Effect of definition of mortality on hospital profiles. Med Care. 2002;40:7–16. [DOI] [PubMed] [Google Scholar]
- 6. Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high‐risk surgery. N Engl J Med. 2011;364:2128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sacks GD, Lawson EH, Dawes AJ, et al. Relationship between hospital performance on a patient satisfaction survey and surgical quality. JAMA Surg. 2015;150:858–64. [DOI] [PubMed] [Google Scholar]
- 8. Damhuis RA, Wijnhoven BP, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30‐day, 90‐day and in‐hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99:1149–54. [DOI] [PubMed] [Google Scholar]
- 9. Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321:1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bellomo R, Goldsmith D, Uchino S, et al. Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med. 2004;32:916–21. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki H, Gotoh M, Sugihara K, et al. Nationwide survey and establishment of a clinical database for gastrointestinal surgery in Japan: targeting integration of a cancer registration system and improving the outcome of cancer treatment. Cancer Sci. 2011;102:226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kenjo A, Miyata H, Gotoh M, et al. Risk stratification of 7,732 hepatectomy cases in 2011 from the National Clinical Database for Japan. J Am Coll Surg. 2014;218:412–22. [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi H, Miyata H, Gotoh M, et al. Risk model for right hemicolectomy based on 19,070 Japanese patients in the National Clinical Database. J Gastroenterol. 2014;49:1047–55. [DOI] [PubMed] [Google Scholar]
- 14. Matsubara N, Miyata H, Gotoh M, et al. Mortality after common rectal surgery in Japan: a study on low anterior resection from a newly established nationwide large‐scale clinical database. Dis Colon Rectum. 2014;57:1075–81. [DOI] [PubMed] [Google Scholar]
- 15. Anazawa T, Paruch JL, Miyata H, et al. Comparison of national operative mortality in gastroenterological surgery using web‐based prospective data entry systems. Medicine (Baltimore). 2015;94:e2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colvin H, Mizushima T, Eguchi H, Takiguchi S, Doki Y, Mori M. Gastroenterological surgery in Japan: the past, the present and the future. Ann Gastroenterol Surg. 2017;1:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


