Abstract
Objective
We tested whether in vivo neuroinflammation relates to the distinctive distributions of pathology in Alzheimer disease (AD) and progressive supranuclear palsy (PSP).
Methods
Sixteen patients with symptomatic AD (including amnestic mild cognitive impairment with amyloid-positive PET scan), 16 patients with PSP–Richardson syndrome, and 13 age-, sex-, and education-matched healthy controls were included in this case-control study. Participants underwent [11C]PK11195 PET scanning, which was used as an in vivo index of neuroinflammation.
Results
[11C]PK11195 binding in the medial temporal lobe and occipital, temporal, and parietal cortices was increased in patients with AD, relative both to patients with PSP and to controls. Compared to controls, patients with PSP showed elevated [11C]PK11195 binding in the thalamus, putamen, and pallidum. [11C]PK11195 binding in the cuneus/precuneus correlated with episodic memory impairment in AD, while [11C]PK11195 binding in the pallidum, midbrain, and pons correlated with disease severity in PSP.
Conclusions
Together, our results suggest that neuroinflammation has an important pathogenic role in the 2 very different human neurodegenerative disorders of AD and PSP. The increase and distribution of microglial activation suggest that immunotherapeutic strategies may be useful in slowing the progression of both diseases.
There is evidence that microglia show increased activation in Alzheimer disease (AD), Parkinson disease, Huntington disease, and progressive supranuclear palsy (PSP).1–12 Furthermore, genetic association studies in AD reveal variations in genes that contribute to immune signaling as risk factors.13 This raises the possibility of immune-therapeutic strategies for prevention and disease modification.
However, key issues need to be addressed before such strategies can be applied, including the confirmation of clinicopathologic correlations of neuroinflammation and the establishment of the potential utility of biomarkers for measuring and tracking neuroinflammation in vivo. Despite the importance of neuroinflammation, there is still insufficient information regarding the extent and regional distribution of microglial activation in patients with neurodegenerative conditions, and their association with clinical markers of disease severity or their relationship to systemic inflammatory markers.
[11C]PK11195 is a well-established PET marker of in vivo microglial activation,1,2,6–8,10,14 although microglial activation represents only part of the complex cascade of events in neuroinflammation.15 Here, we assessed the magnitude and patterns of [11C]PK11195 binding in 2 very different neurodegenerative entities, AD and PSP, characterized by distinct anatomical distributions of pathology. The value of this comparison does not lie in the differential diagnosis between these clinically diverse entities but rather in establishing the distribution of neuroinflammation in 2 distinct tauopathies. We tested whether [11C]PK11195 binding relates to the distinctive distributions of pathology in typical amnestic AD and PSP–Richardson syndrome, and whether [11C]PK11195 binding relates to different measures of clinical severity in AD and PSP.
Methods
Participants
The current study was conducted within the context of the NIMROD (Neuroimaging of Inflammation in Memory and Related Other Disorders) Study.16 We recruited 16 PSP patients with probable PSP by the 1996 Movement Disorder Society criteria (representing a “classic phenotype,” which is sometimes referred to as Richardson syndrome), but all patients also met 2017 revised criteria for probable PSP–Richardson syndrome17,18; 9 patients met diagnostic criteria for probable AD19 (typical amnestic phenotype, without biomarkers) and 7 patients had amnestic mild cognitive impairment (MCI). The patients with amnestic MCI had (1) a Mini-Mental State Examination (MMSE) score of >24/30, (2) memory impairment at least 1.5 SDs below that expected for age and education,20 and (3) biomarker evidence of amyloid pathology (positive Pittsburgh compound B [PiB]-PET scan) (MCI+). Thirteen age-, sex-, and education-matched healthy controls with no history of major psychiatric or neurologic illnesses, head injury, or any other significant medical comorbidity were also recruited. All participants were older than 50 years, had sufficient proficiency in English for cognitive testing, did not have any acute infectious or symptomatic systemic inflammatory disorder (e.g., lupus, rheumatoid arthritis, Crohn disease, polymyalgia rheumatica), and had no contraindications to MRI. Patients were identified from the specialist clinics at the Cambridge University Hospitals NHS Trust and the Dementias and Neurodegenerative Diseases Research Network, while healthy controls were recruited via the Dementias and Neurodegenerative Diseases Research Network, which is part of the National Institute for Health Research (NIHR) Clinical Research Network (nihr.ac.uk/nihr-in-your-area/dementias-and-neurodegeneration/).
Standard protocol approvals, registrations, and patient consents
All participants had mental capacity, and we obtained informed written consent from patients (as principal participants) and patients' designated informants (for providing informant information) in accordance with the Declaration of Helsinki. The study was approved by the local ethics committee.
Clinical, cognitive, and blood assessment
Participants' assessment included clinical indices of disease severity, such as Rey Auditory Verbal Learning Test (RAVLT) in AD/MCI+ patients and Progressive Supranuclear Palsy Rating Scale (PSPRS) in patients with PSP.21 Demographic measures and neuropsychological tests (i.e., MMSE and Addenbrooke's Cognitive Examination–Revised) as well as a blood sample to assess the levels of 3 basic peripheral markers of inflammation (i.e., C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], and white blood cell count) were also obtained from all participants. The CRP, ESR, and white blood cell biomarkers were included on the basis that peripheral inflammation may facilitate the development of neuroinflammation and neurodegeneration,22–24 and such peripheral markers might augment the monitoring of immunotherapeutic trials if related to central inflammation.
Neuroimaging assessment
All participants underwent MRI on a 3T Siemens Magnetom Tim Trio or Verio scanner (medical.siemens.com) using a magnetization-prepared rapid-acquisition gradient echo T1-weighted sequence. The T1-weighted sequence (repetition time = 2,300 milliseconds, echo time = 2.98 milliseconds, field of view = 240 × 256 mm2, 176 slices of 1-mm thickness, flip angle = 9°) was used to facilitate tissue class segmentation (gray and white matter, together with CSF) and to allow nonrigid registration of standard space regions of interest (ROIs) to subject MRI space (using a modified version of the Hammers atlas, which included the midbrain, pons, cerebellar gray matter, and dentate nucleus of the cerebellum ROIs). Each T1 image was nonrigidly registered to the ICBM2009a template brain using ANTS (picsl.upenn.edu/ANTS/), and the inverse transform was applied to the modified Hammers atlas (resliced from MNI152 to ICBM2009a space) to bring the ROIs to subject MRI space.
All participants underwent [11C]PK11195 PET imaging for assessment of the extent and distribution of brain inflammation. [11C]PK11195 and [11C]PiB were produced with high radiochemical purity (>95%), with [11C]PiB having a specific activity >150 GBq/μmol at the end of synthesis, while [11C]PK11195 specific activity was around 85 GBq/μmol at the end of synthesis. PET scanning was performed with a GE Advance PET scanner (GE Healthcare, Waukesha, WI) and a GE Discovery 690 PET/CT, with attenuation correction provided by a 15-minute 68Ge/68Ga transmission scan and a low-dose CT scan, respectively. The emission protocols were 550 MBq [11C]PiB injection followed by imaging from 40 to 70 minutes post injection, and 75 minutes of dynamic imaging (55 frames) starting concurrently with a 500-MBq [11C]PK11195 injection. Each emission frame was reconstructed using the PROMIS 3-dimensional filtered back projection algorithm into a 128 × 128 matrix 30-cm transaxial field of view, with a transaxial Hann filter cutoff at the Nyquist frequency.25 Corrections were applied for randoms, dead time, normalization, scatter, attenuation, and sensitivity. Each emission image series was aligned using SPM8 to reduce the effect of patient motion during data acquisition (fil.ion.ucl.ac.uk).
The mean aligned PET image (and hence the corresponding aligned PET image series) was rigidly registered to the T1-weighted MRI. For [11C]PiB, we used reference tissue ROI defined by ≥90% on the SPM8 gray matter probability map (smoothed to PET resolution) in the superior cerebellar cortex.26 For [11C]PK11195, supervised cluster analysis was used to determine the reference tissue time-activity curve.27 All ROI data were corrected for CSF contamination through division with the mean ROI probability (normalized to 1) of gray + white matter, using SPM8 probability maps smoothed to PET resolution. To test whether correction for CSF affected the main results, we repeated all the [11C]PK11195 ROI PET analyses using data not corrected for CSF contamination (see PET statistical analyses and results sections).
[11C]PiB data were quantified using standardized uptake value ratio by dividing the mean CSF-corrected radioactivity concentration in each Hammers atlas ROI by the corresponding mean CSF-corrected radioactivity concentration in the reference tissue ROI. For [11C]PK11195, nondisplaceable binding potential (BPND), a measure of specific binding, was determined for each ROI using a basis function implementation of the simplified reference tissue model, both with and without CSF contamination correction.28 [11C]PK11195 BPND maps were also generated using this basis function simplified reference tissue model approach. [11C]PiB data were treated as dichotomous measures (i.e., positive or negative) and considered positive if the average standardized uptake value ratio across the cortical ROIs was >1.5.29
To compare [11C]PK11195 binding across groups (AD/MCI PiB+, PSP, and controls), individual ROI BPND values for [11C]PK11195 were used in a repeated-measures general linear model to test for the main effect of ROI, main effect of group, and group × ROI interaction. Age and sex were included as covariates of no interest. For the AD/MCI+ and PSP groups, we also tested Pearson correlations between regional [11C]PK11195 BPND and disease severity using the RAVLT scores for AD/MCI+ patients and the PSPRS for patients with PSP. Finally, we tested for associations between neuroinflammation and peripheral markers of inflammation using Pearson correlations.
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
Clinical, cognitive, and blood findings
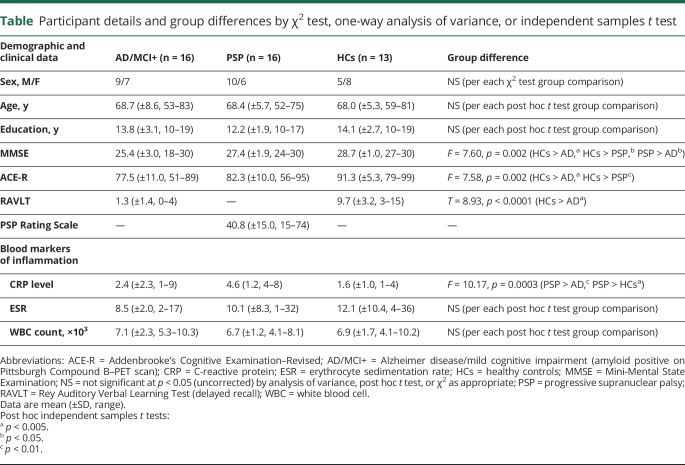
The patient and control groups were matched for age, sex, and education (table). Nevertheless, to account for any possible residual confounding effect associated with variability in demographic measures, age and sex were included as covariates of no interest in the general linear models of the main effect of ROI, the main effect of group, and the group × ROI interaction. As expected, there was a significant main effect of group for cognitive measures, driven by reduced MMSE and Addenbrooke's Cognitive Examination–Revised scores in AD/MCI+ and PSP patients relative to healthy controls (table). Episodic memory, as assessed via the RAVLT (delayed recall), was significantly impaired in AD/MCI+ patients relative to controls (table). Although none of the participants included had acute inflammatory conditions (see exclusion criteria), patients with PSP displayed higher CRP levels than AD/MCI+ patients and controls, despite normal leukocyte count and ESR (table).
Table.
Participant details and group differences by χ2 test, one-way analysis of variance, or independent samples t test
Neuroimaging findings
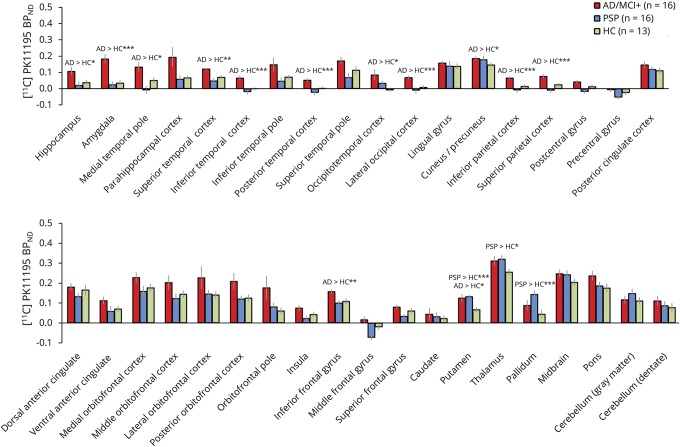
In the repeated-measures analysis of regional binding, we found a significant main effect of ROI (F2,36 = 3.8, p < 0.001), main effect of group (F2,36 = 5.7, p < 0.006), and a group × ROI interaction (F2,70 = 2.6, p < 0.001) (figure 1). The group and interaction effects were driven in part by higher [11C]PK11195 BPND values in the AD/MCI+ group relative to both the PSP and control groups, in cortical and subcortical ROIs, including occipital, parietal, and temporal cortices, as well as in the hippocampus, amygdala, and other medial temporal lobe ROIs (figure 1). The PSP group, relative to controls, showed increased [11C]PK11195 BPND in the thalamus, putamen, and pallidum (figure 1).
Figure 1. [11C]PK11195 binding in AD, PSP, and HCs.
The bar plots represent the mean values (± SE) of the [11C]PK11195 BPND in each region of interest for the participant groups: AD and MCI+, PSP, and HCs. The [11C]PK11195 BPND data reported here are corrected for CSF contamination. See the results section for statistics related to CSF-corrected and uncorrected data. Post hoc t tests: *p < 0.05, **p < 0.01, ***p < 0.005. AD = Alzheimer disease; BPND = nondisplaceable binding potential; HC = healthy control; MCI+ = amyloid-positive mild cognitive impairment; PSP = progressive supranuclear palsy.
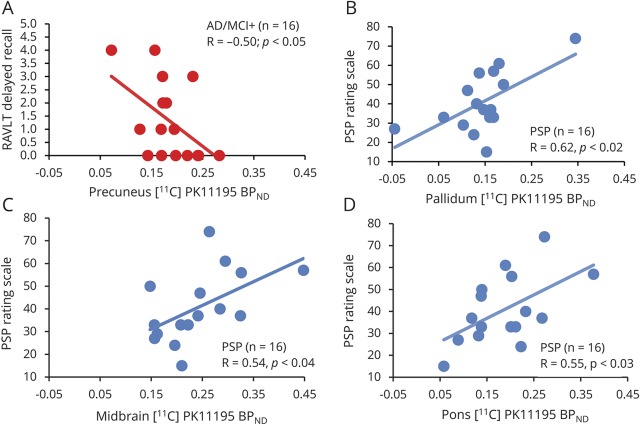
Repeating these analyses using ROI [11C]PK11195 BPND values that were not corrected for CSF partial volume effects yielded similar results (F2,36 = 2.2, p < 0.0001 for the main effect of ROIs; F2,36 = 6.1, p < 0.006 for the main effect of group; and F2,70 = 2.0, p < 0.0001 for the group × ROI interaction). We then tested whether regional [11C]PK11195 BPND related to disease severity in each clinical group. In the AD/MCI+ group, there was a significant negative correlation between the RAVLT scores (delayed recall at 30 minutes) and [11C]PK11195 BPND in the precuneus (figure 2A). In the PSP group, we found a significant positive correlation for [11C]PK11195 BPND in the pallidum, midbrain, and pons and disease severity, as assessed via the PSPRS (figure 2, B–D).
Figure 2. [11C]PK11195 binding correlates with clinical severity in AD and PSP.
(A) Correlation between [11C]PK11195 BPND values in the precuneus (x-axis) and RAVLT scores (y-axis) in patients with AD and MCI+ (red dots). (B–D) Correlation between [11C]PK11195 BPND values in the pallidum, midbrain, and pons (x-axes) and PSP Rating Scale (y-axes) in patients with PSP. AD = Alzheimer disease; BPND = nondisplaceable binding potential; MCI+ = amyloid-positive mild cognitive impairment; PSP = progressive supranuclear palsy; RAVLT = Rey Auditory Verbal Learning Test.
Discussion
The brain regions with the most marked abnormalities of [11C]PK11195 binding in AD/MCI+ and PSP groups were those predicted from the established distribution of neurodegeneration of each disease. Specifically, patients with amnestic AD/MCI+ had evidence of increased neuroinflammation in the medial temporal lobe as well as parietal and lateral temporal cortices.30–33 Conversely, patients with PSP had evidence of enhanced neuroinflammation in the thalamus, pallidum, and putamen, a group of subcortical regions that have been implicated in the pathophysiology of PSP.34,35 The increased [11C]PK11195 binding in the basal ganglia in PSP is also consistent with preliminary findings reported in a study with 4 patients with PSP.10
Our data demonstrate that the density and distribution of activated microglia in living patients with AD and PSP mirror the typical neuropathologic changes characteristic of each disorder. This could result from a causal link between neuroinflammation and neurodegeneration, although the association might also derive from the process of neurodegeneration itself. A cross-sectional and noninterventional study such as this one cannot alone provide the direction of causality. Nevertheless, the disease-specific anatomical distributions of activated microglia in AD and PSP suggest a regional association rather than a side effect of a global increased [11C]PK11195 binding in response to a general inflammatory insult.
Our PET data are also in keeping with previous postmortem findings,3 which demonstrated that microglia burden (as assessed via LN3 immunostaining) and interleukin 1β and transforming growth factor β expression showed a disease-specific topological relationship with the pathologic hallmarks of AD and PSP.3 More specifically, the previous postmortem study3 found that patients with AD had significantly higher microglia density and interleukin 1β expression in the parietal cortices compared to patients with PSP and controls, while the microglia density and cytokine expression was greater in the substantia nigra of patients with PSP relative to patients with AD and controls.3 The expression of transforming growth factor β was also increased in frontal and parietal cortices in patients with AD relative to patients with PSP or controls.3 Together with our findings, these data suggest that microglia activation and cytokine expression coexist with the pathogenic processes underlying AD and PSP and could contribute to the process of ongoing neurodegeneration.3 If so, this would warrant further investigation of immune-therapeutic strategies to modulate neuroinflammation in AD and PSP, although evidence from earlier anti-inflammatory trials in AD remains controversial,36,37 and no such clinical trials have been conducted in PSP.
Our data also confirmed the hypothesis that [11C]PK11195 binding correlates with disease severity in both AD/MCI+ and PSP; more specifically, with severity of episodic memory impairment as assessed via the RAVLT in AD/MCI+, and with PSP severity as measured via the PSPRS in PSP. Again, these effects were not global correlations but adhered to the functional anatomy of cognitive and motor symptoms in AD and PSP (i.e., cuneus/precuneus in relation to episodic memory deficits in AD as well as the pallidum, midbrain, and pons in relation to PSPRS in PSP).
Despite that a symptomatic acute infection and chronic extraneural inflammatory condition were exclusion criteria of our study, the patients with PSP showed higher levels of CRP in the blood, a common peripheral marker of inflammation. We acknowledge that the dysphagia and bladder dysfunction frequently experienced by patients with PSP can put them at risk of respiratory and urinary tract infections. However, the average CRP level in our PSP group was 4.6 (±1.2), which is well below the values expected in acute inflammatory states. In addition, the leucocyte count and ESR were both normal. The increased CRP levels in PSP might therefore reflect underlying chronic inflammatory states that sustain or even accelerate the neuroinflammation associated with PSP, in the absence of acute infection. It will be useful to replicate these findings in larger clinical cohorts and use more detailed peripheral markers of inflammation (e.g., immune-phenotyping) for characterization and classification of the immune cells in circulation in AD and PSP.
Overall, the use of [11C]PK11195 PET could provide helpful information to stratify patients in future clinical trials or to track the effects of treatments targeting neuroinflammation in neurodegenerative disorders such as AD and PSP. However, to fully meet its potential toward these directions, additional properties are necessary to show for this biomarker of neuroinflammation. Specifically, although recent longitudinal studies in AD have demonstrated that changes in [11C]PK11195 binding may be associated with disease progression,14,38 such a correlation has not been established in PSP. Neuroinflammation might be stable in symptomatic stages of PSP, as suggested by a pilot study of 2 patients with PSP.10 Furthermore, increased levels of serum neurofilament light protein have been found in both AD and PSP, and correlated with disease severity39–41; hence, future studies may test whether markers of neuroinflammation are associated with neurofilament light protein levels in the serum. Perhaps more importantly, it remains to be determined whether the putative effects of anti-inflammatory therapies can reduce the elevated [11C]PK11195 binding in AD and PSP and, consequently, could help slow the progression of these disorders. This would also enable mediation analysis to test the causality between immune-reactivity and disease progression in dementia and related disorders. Furthermore, we suggest that multitracer PET studies will be useful to formally assess how neuroinflammation relates to other important molecular aspects in dementia and related disorders including, for example, studying how neuroinflammation is associated with amyloid load in AD14 as well as with tau burden in AD and PSP. A cross-sectional and single-tracer study like the present one is not able to address such interesting and open questions, although it represents the necessary first step toward achieving this goal.
Technical considerations regarding the [11C]PK11195 BPND PET methods should also be considered. In particular, our main regional PET analyses used partial volume correction for CSF, which controlled for differences in CSF signal contamination within each region and across the different diagnostic groups (i.e., AD/MCI+, PSP, and control groups). Although this approach is important to reduce the potential influence of brain volume loss seen in AD/MCI+ and PSP, this MRI-guided method is subject to error because of imperfect registration of PET and MRIs, together with errors in segmentation and point spread function modeling. However, we note that using uncorrected PET data yielded similar results in terms of the main effect of ROI, main effect of group, and group × ROI interaction, which provides substantiation of the CSF-corrected results. The supervised cluster method for estimating [11C]PK11195 BPND could also have introduced an underestimation bias, as the reference tissue may have still included specific binding of the radioligand. In any case, this may have only reduced the effect sizes without altering the risk of reporting false-positive results.
We also highlight that our data are specific to [11C]PK11195 and do not inevitably generalize to second-generation translocator protein (TSPO) ligands (e.g., PBR28) or alternative tracers of neuroinflammation over and above those that bind to TSPO (e.g., COX-1, MPO, macrophage infiltration).42–44 Further studies should assess the utility of such novel markers for in vivo imaging of neuroinflammation, bearing in mind that the binding of second-generation TSPO tracers like PBR28 can be affected by genetic variations (i.e., the rs6971 TSPO polymorphism).45
In conclusion, we have provided clear evidence that [11C]PK11195 is a sensitive PET ligand for in vivo studies of neuroinflammation in clinical populations with AD and its prodromal stage of amnestic MCI, as well as in a non-AD tauopathy, PSP–Richardson syndrome. The brain regions that showed increased [11C]PK11195 binding were those predicted from the well-established pattern of regional cortical and subcortical neurodegeneration in each disease. Our data support the further use of [11C]PK11195 PET to study microglia activation in neurodegenerative disorders and in clinical trials that aim to modulate neuroinflammation in neurodegenerative disease.
Glossary
- AD
Alzheimer disease
- BPND
nondisplaceable binding potential
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- NIHR
National Institute for Health Research
- PiB
Pittsburgh compound B
- PSP
progressive supranuclear palsy
- PSPRS
Progressive Supranuclear Palsy Rating Scale
- RAVLT
Rey Auditory Verbal Learning Test
- ROI
region of interest
- TSPO
translocator protein
Author contributions
L.P.: conception and design of the study, acquisition and analysis of data, drafting the manuscript and figures. P.V.R.: conception and design of the study, acquisition of data. Y.T.H.: acquisition and analysis of data, drafting of the manuscript. K.S.J.A.: acquisition and analysis of data, drafting of the manuscript. W.R.B.-J.: acquisition of data, drafting the manuscript. D.W.: analysis of data. P.S.J.: analysis of data, drafting the manuscript and figures. R.A.: acquisition of data, drafting the manuscript. R.J.B.: analysis of data, drafting the manuscript. A.S.: drafting the manuscript. E.M.: analysis of data. L.S.: analysis of data. T.D.F.: acquisition and analysis of data, drafting the manuscript. F.I.A.: conception and design of the study, obtaining funding. J.T.O.: conception and design of the study, obtaining funding, drafting the manuscript. J.B.R.: conception and design of the study, obtaining funding, drafting the manuscript and figures.
Study funding
This study was funded by the National Institute for Health Research Cambridge Biomedical Research Centre and Biomedical Research Unit in Dementia (NIHR, RG64473), the PSP Association, the Wellcome Trust (JBR 103838), and the Medical Research Council (MC-A060-5PQ30 and MR/P01271X/1). Dr. Li Su is supported by Alzheimer's Research UK.
Disclosure
L. Passamonti, P. Vázquez Rodríguez, Y. Hong, K. Allinson, R. Bevan-Jones, D. Williamson, P. Jones, R. Arnold, R. Borchert, A. Surendranathan, E. Mak, L. Su, and T. Fryer report no disclosures relevant to the manuscript. F. Aigbirhio has served as review editor for Journal of Labelled Compounds and Radiopharmaceuticals, received academic grant support from GE Healthcare, and served as a consultant for Avid and Cantabio, all for matters not related to the current study. J. O'Brien has served as deputy editor of International Psychogeriatrics, received grant support from Avid (Lilly), and served as a consultant for Avid and GE Healthcare, all for matters not related to the current study. J. Rowe serves as editor to Brain, has been a consultant for Asceneuron and Syncona, and has received academic grant funding from AZ-MedImmune, Janssen, and Lilly, unrelated to this study. Go to Neurology.org/N for full disclosures.
References
- 1.Edison P, Archer HA, Gerhard A, et al. Microglia, amyloid, and cognition in Alzheimer's disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis 2008;32:412–419. [DOI] [PubMed] [Google Scholar]
- 2.Fan Z, Aman Y, Ahmed I, et al. Influence of microglial activation on neuronal function in Alzheimer's and Parkinson's disease dementia. Alzheimers Dement 2015;11:608–621.e7. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Botran R, Ahmed Z, Crespo FA, et al. Cytokine expression and microglial activation in progressive supranuclear palsy. Parkinsonism Relat Disord 2011;17:683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuitemaker A, Kropholler MA, Boellaard R, et al. Microglial activation in Alzheimer's disease: an (R)-[(1)(1)C]PK11195 positron emission tomography study. Neurobiol Aging 2013;34:128–136. [DOI] [PubMed] [Google Scholar]
- 5.Stefaniak J, O'Brien J. Imaging of neuroinflammation in dementia: a review. J Neurol Neurosurg Psychiatry 2016;87:21–28. [DOI] [PubMed] [Google Scholar]
- 6.Gerhard A, Pavese N, Hotton G, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis 2006;21:404–412. [DOI] [PubMed] [Google Scholar]
- 7.Edison P, Ahmed I, Fan Z, et al. Microglia, amyloid, and glucose metabolism in Parkinson's disease with and without dementia. Neuropsychopharmacology 2013;38:938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cagnin A, Brooks DJ, Kennedy AM, et al. In-vivo measurement of activated microglia in dementia. Lancet 2001;358:461–467. [DOI] [PubMed] [Google Scholar]
- 9.Anderson AN, Pavese N, Edison P, et al. A systematic comparison of kinetic modelling methods generating parametric maps for [(11)C]-(R)-PK11195. Neuroimage 2007;36:28–37. [DOI] [PubMed] [Google Scholar]
- 10.Gerhard A, Trender-Gerhard I, Turkheimer F, Quinn NP, Bhatia KP, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in progressive supranuclear palsy. Mov Disord 2006;21:89–93. [DOI] [PubMed] [Google Scholar]
- 11.Wiley CA, Lopresti BJ, Venneti S, et al. Carbon 11-labeled Pittsburgh compound B and carbon 11-labeled (R)-PK11195 positron emission tomographic imaging in Alzheimer disease. Arch Neurol 2009;66:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kropholler MA, Boellaard R, van Berckel BN, et al. Evaluation of reference regions for (R)-[(11)C]PK11195 studies in Alzheimer's disease and mild cognitive impairment. J Cereb Blood Flow Metab 2007;27:1965–1974. [DOI] [PubMed] [Google Scholar]
- 13.Villegas-Llerena C, Phillips A, Garcia-Reitboeck P, Hardy J, Pocock JM. Microglial genes regulating neuroinflammation in the progression of Alzheimer's disease. Curr Opin Neurobiol 2016;36:74–81. [DOI] [PubMed] [Google Scholar]
- 14.Fan Z, Okello AA, Brooks DJ, Edison P. Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer's disease. Brain 2015;138:3685–3698. [DOI] [PubMed] [Google Scholar]
- 15.Agostinho P, Cunha RA, Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer's disease. Curr Pharm Des 2010;16:2766–2778. [DOI] [PubMed] [Google Scholar]
- 16.Bevan-Jones WR, Surendranathan A, Passamonti L, et al. Neuroimaging of Inflammation in Memory and Related Other Disorders (NIMROD) study protocol: a deep phenotyping cohort study of the role of brain inflammation in dementia, depression and other neurological illnesses. BMJ Open 2017;7:e013187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996;47:1–9. [DOI] [PubMed] [Google Scholar]
- 18.Hoglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the Movement Disorder Society criteria. Mov Disord 2017;32:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 21.Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain 2007;130:1552–1565. [DOI] [PubMed] [Google Scholar]
- 22.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun 2004;18:407–413. [DOI] [PubMed] [Google Scholar]
- 23.Bonotis K, Krikki E, Holeva V, Aggouridaki C, Costa V, Baloyannis S. Systemic immune aberrations in Alzheimer's disease patients. J Neuroimmunol 2008;193:183–187. [DOI] [PubMed] [Google Scholar]
- 24.Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer's disease. J Neurol Neurosurg Psychiatry 2003;74:788–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinahan PE, Rogers JG. Analytic 3D image reconstruction using all detected events. IEEE Trans Nucl Sci 1989;36:964–968. [Google Scholar]
- 26.Schuitemaker A, van Berckel BN, Kropholler MA, et al. SPM analysis of parametric (R)-[11C]PK11195 binding images: plasma input versus reference tissue parametric methods. Neuroimage 2007;35:1473–1479. [DOI] [PubMed] [Google Scholar]
- 27.Turkheimer FE, Edison P, Pavese N, et al. Reference and target region modeling of [11C]-(R)-PK11195 brain studies. J Nucl Med 2007;48:158–167. [PubMed] [Google Scholar]
- 28.Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 1997;6:279–287. [DOI] [PubMed] [Google Scholar]
- 29.Hatashita S, Yamasaki H. Clinically different stages of Alzheimer's disease associated by amyloid deposition with [11C]-PIB PET imaging. J Alzheimers Dis 2010;21:995–1003. [DOI] [PubMed] [Google Scholar]
- 30.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarz AJ, Yu P, Miller BB, et al. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain 2016;139:1539–1550. [DOI] [PubMed] [Google Scholar]
- 32.Schöll M, Lockhart SN, Schonhaut DR, et al. PET imaging of tau deposition in the aging human brain. Neuron 2016;89:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ossenkoppele R, Schonhaut DR, Baker SL, et al. Tau, amyloid, and hypometabolism in a patient with posterior cortical atrophy. Ann Neurol 2015;77:338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 1994;44:2015–2019. [DOI] [PubMed] [Google Scholar]
- 35.Litvan I, Hauw JJ, Bartko JJ, et al. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 1996;55:97–105. [DOI] [PubMed] [Google Scholar]
- 36.Group AR, Martin BK, Szekely C, et al. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol 2008;65:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ADAPT Research Group, Lyketsos CG, Breitner JC, et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology 2007;68:1800–1808. [DOI] [PubMed] [Google Scholar]
- 38.Kreisl WC, Lyoo CH, Liow JS, et al. (11)C-PBR28 binding to translocator protein increases with progression of Alzheimer's disease. Neurobiol Aging 2016;44:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rojas JC, Bang J, Lobach IV, et al. CSF neurofilament light chain and phosphorylated tau 181 predict disease progression in PSP. Neurology 2018;90:e273–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jabbari E, Zetterberg H, Morris HR. Tracking and predicting disease progression in progressive supranuclear palsy: CSF and blood biomarkers. J Neurol Neurosurg Psychiatry 2017;88:883–888. [DOI] [PubMed] [Google Scholar]
- 41.Hampel H, Toschi N, Baldacci F, et al. Alzheimer's disease biomarker-guided diagnostic workflow using the added value of six combined cerebrospinal fluid candidates: Aβ1–42, total-tau, phosphorylated-tau, NFL, neurogranin, and YKL-40. Alzheimers Dement Epub 2018 Jan 9. [DOI] [PubMed]
- 42.Hamelin L, Lagarde J, Dorothee G, et al. Early and protective microglial activation in Alzheimer's disease: a prospective study using 18F-DPA-714 PET imaging. Brain 2016;139:1252–1264. [DOI] [PubMed] [Google Scholar]
- 43.Yokokura M, Terada T, Bunai T, et al. Depiction of microglial activation in aging and dementia: positron emission tomography with [11C]DPA713 versus [11C](R)PK11195. J Cereb Blood Flow Metab 2017;37:877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suridjan I, Pollock BG, Verhoeff NP, et al. In-vivo imaging of grey and white matter neuroinflammation in Alzheimer's disease: a positron emission tomography study with a novel radioligand, [18F]-FEPPA. Mol Psychiatry 2015;20:1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owen DR, Yeo AJ, Gunn RN, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 2012;32:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.