Abstract
Objective
To provide detailed long-term outcome data of children and adolescents following pediatric anti-N-methyl-d-aspartate receptor (anti-NMDAR) encephalitis, to identify neuropsychological impairments, and to evaluate the influence of these factors on quality of life (QoL).
Methods
All Dutch children diagnosed with anti-NMDAR encephalitis were identified. Patients currently aged 4 years or older were included in the follow-up study, consisting of a visit to our clinic for a detailed interview and a standardized neuropsychological assessment. The following domains were included: attention, memory, language, executive functioning, QoL, and fatigue. Primary outcome measures were z scores on sustained attention, long-term verbal memory, QoL, fatigue, and working memory.
Results
Twenty-eight patients were included. Median Pediatric Cerebral Performance Category at last visit was 1 (interquartile range 1–2, range 1–4), and 64% (18/28) of patients returned consistently to their previous school level. Twenty-two patients were included in the cross-sectional part of the long-term follow-up study. Median follow-up time was 31 months (interquartile range 15–49, range 5–91). There were problems with sustained attention (z = −2.10, 95% confidence interval = −2.71 to −1.46, p < 0.0001) and fatigue (z = −0.96, 95% confidence interval = −1.64 to −0.28, p = 0.008). Cognitive deficits were not correlated with QoL, while fatigue was strongly correlated with QoL (r = 0.82, p < 0.0001).
Conclusions
Although follow-up is often reported as “good” following pediatric anti-NMDAR encephalitis, many patients have cognitive problems and fatigue, even up until adolescence, resulting in academic achievement problems and lower QoL. For physicians, it is essential to be aware of these problems, to provide valuable advice to patients and caregivers in the acute and follow-up phase, and to consider early neuropsychological counseling.
Anti-N-methyl-d-aspartate receptor (anti-NMDAR) encephalitis is an autoimmune disorder, initially described in 2007.1 Increased awareness has led to more frequent diagnoses, and currently more than 1,000 patients have been reported, of whom 35% are children.2 The disease course can be severe, with intensive care unit (ICU) admission in 75% of children. Nevertheless, if treated with adequate immunotherapy, outcome is considered favorable in 85% of children.2
However, there are signals that actual recovery might be less positive than initially reported. Small studies in both adults and children describe substantial deficits in multiple cognitive domains and also behavioral problems.3–9 Given these findings, it seems that despite apparent good outcome, full neuropsychological recovery is certainly not always achieved.
Functioning can be studied from different perspectives,10 including activities and participation. Outcome of anti-NMDAR encephalitis is currently measured in terms of activities with relatively crude measures, such as the modified Rankin Scale (mRS),11 while participation and quality of life (QoL) are also of major importance, especially in children and adolescents. Neuropsychological deficits can seriously affect participation and career choices as transition into adulthood might call for full cognitive abilities.
Therefore, the aim of this nationwide Dutch cohort study was to provide more insight into long-term outcome following pediatric anti-NMDAR encephalitis, with special emphasis on neuropsychological outcome, and to evaluate whether these neuropsychological factors influence QoL.
Methods
Patients
The Departments of Neurology and Pediatric Neurology of the Erasmus University Medical Center–Sophia Children's Hospital, Rotterdam, the Netherlands, are national referral sites for patients with suspected autoimmune encephalitis. In addition, the Department of Immunology is the national referral site for antineuronal antibody testing of samples from patients with suspected autoimmune encephalitis. Therefore, we had the opportunity to identify all Dutch children diagnosed with anti-NMDAR encephalitis, from January 2008 until March 2017, aged 0 to 18 years at disease onset. NMDAR antibodies were confirmed in serum and/or CSF by both commercial cell-based assay and immunohistochemistry.
Clinical information
Data about disease course were obtained from medical records and from detailed interviews with patients and caregivers during a visit to our clinic. Neurologic level of function was determined using the Pediatric Cerebral Performance Category (PCPC) scale (table e-1, links.lww.com/WNL/A495).12
Standard protocol approvals, registrations, and patient consents
The institutional review board of the Erasmus University Medical Center approved the study protocol. Informed consent was obtained from adult patients and for children from their parents, and if applicable, also from children aged 12 to 18 years.
Cross-sectional follow-up study
All patients currently aged 4 years or older were approached to participate in the follow-up study, as neuropsychological testing and the questionnaires required a minimal age for participation. Patients were invited for a visit to our clinic, in which current complaints and level of functioning were discussed. In addition, patients underwent a standardized neuropsychological assessment. If a visit was not possible, current problems were discussed by phone, and questionnaires were sent to us by mail and checked in additional calls if necessary.
Neuropsychological assessment
The neuropsychological assessment consisted of a selection of the Cambridge Neuropsychological Test Automated Battery (CANTAB Research Suite 6.0, Cambridge Cognition Ltd., Cambridge, UK), additional neuropsychological tests, and questionnaires (table e-2, links.lww.com/WNL/A495). Tests and questionnaires were selected based on our own experiences and on disorders found in prior studies, were administrated in their Dutch versions, and are reliable and validated in the Netherlands. The tests and questionnaires were administered to assess skills in 6 domains:
Attention: Reaction Time (CANTAB), Dutch Dot Cancellation Test (Bourdon-Vos).13
Memory: Paired Associated Learning (CANTAB), Rey Auditory Verbal Learning Test (RAVLT).14
Executive functioning: Intra-Extra Dimensional Set Shift, Spatial Span, Stockings of Cambridge (all CANTAB), Word Generation (NEPSY-II [ A Developmental Neuropsychological Assessment, Second Edition]),17 Behavior Rating Inventory of Executive Function (BRIEF–Self-Report and BRIEF–Adult) questionnaire,18 Strength and Difficulties Questionnaire (self-report and parent-proxy report).19
QoL: Pediatric Quality of Life Inventory 4.0 (PedsQL Self-Report and PedsQL Parent Proxy-Report).20
Fatigue: PedsQL Multidimensional Fatigue Scale questionnaire (PedsQL-MFS Self-Report and PedsQL-MFS Parent Proxy-Report).21
Statistical analysis
For group comparisons, we used the Mann-Whitney U test (age), Fisher exact test (sex, immunotherapy), Fisher-Freeman-Halton extension (PCPC), and the Kruskal-Wallis one-way analysis of variance (character profiles). Results of neuropsychological assessments were compared with normative data of healthy individuals, corrected for age, sex, and educational level. Normative data for the CANTAB were obtained by CANTAB, Cambridge, UK. Scores were converted into standardized z scores for comparison. For statistics, z scores were set on minimum of −3 and maximum of +3 to prevent statistical differences by outliers (winsorization). In the graphs, the uncorrected z scores are shown, but corrected z scores were used for statistics. Displayed correlations were also calculated with corrected z scores. The z scores were analyzed using a one-sample t test (test value = 0). Primary outcome measures were sustained attention (Dutch Dot Cancellation Test–attention fluctuations), long-term verbal memory (RAVLT–Delayed Recall), fatigue (PedsQL-MFS Self-Report–Total Score), QoL (PedsQL Self-Report–Total Score), and working memory (BRIEF–Self-Report–Working Memory). Primary outcome measures were considered significant if p < 0.017 (Bonferroni). For the secondary outcome measures of the neuropsychological assessment, p values <0.005 were considered significant. Values between 0.005 and 0.05 should be interpreted carefully and considered exploratory. The relationship between our primary outcome measures and QoL were computed with a two-sided Pearson correlation coefficient. SPSS version 21.0 (IBM Corp., Armonk, NY) was used for statistical analyses, as well as GraphPad Prism 7 (GraphPad Software, La Jolla, CA) for Windows.
Data availability
Any data not published within this article are available at Erasmus University Medical Center. Patient-related data will be shared on request from any qualified investigator, maintaining anonymization of the individual patients.
Results
Clinical characteristics
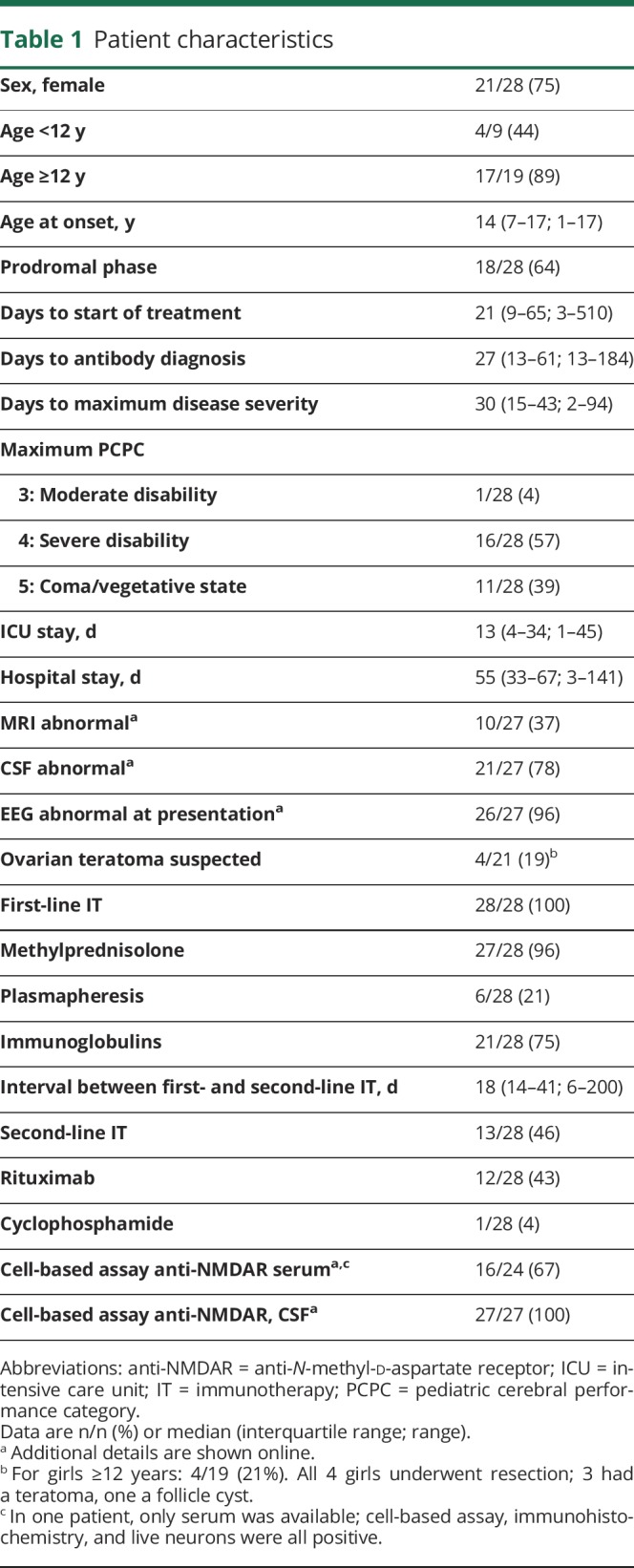
Thirty children were identified, of whom 28 were included (for patient selection, see figure 1). Twenty-one patients were female (75%), mainly in those aged 12 years or older (89%). Median age at onset was 14 years. Eighteen patients (64%) reported a prodromal phase, including headache, blurred vision, or upper respiratory infection. Three children (11%) developed anti-NMDAR encephalitis 3 to 7 weeks after a herpes simplex virus (HSV) type 1 encephalitis. In addition to those 3, one patient had a preexistent mild psychomotor developmental delay. The others were healthy before disease onset.
Figure 1. Flowchart of patient selection.
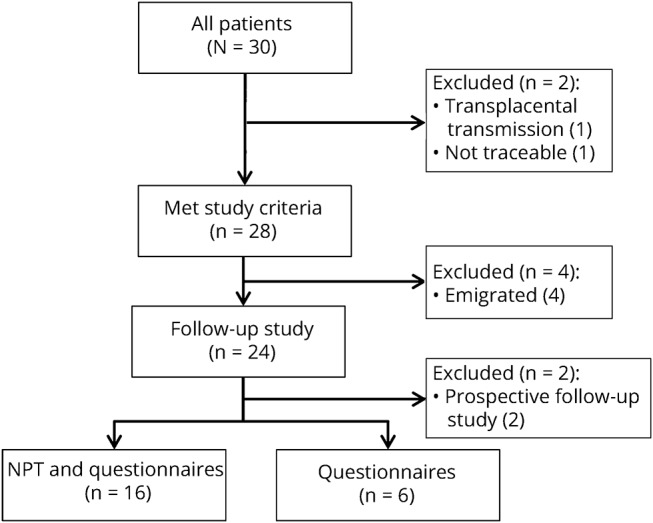
One patient was excluded because he was younger than 4 years (transplacental transmission of anti-N-methyl-d-aspartate receptor),31 and one patient was untraceable. Twenty-four patients participated in the follow-up study, of whom 2 are followed prospectively. Sixteen of the 22 participants completed the full neuropsychological assessment, 6 patients only completed the questionnaires, 3 visited our clinic, and 3 were contacted by phone because of geographical distance. NPT = neuropsychological testing.
Most children presented with behavioral disorders (36%) or seizures (36%), less frequently with speech disorders and movement disorders. In 2 of 28 patients (7%), hemiparesis was the presenting symptom, only occurring in children younger than 12 years (figure 2A). All patients presented to the initial physician with a maximum of 3 symptoms, while at maximum disease severity, 21 patients had developed more than 4 symptoms (figure 2, B and C). The numbers of symptoms between treatment and diagnosis were often comparable. Four patients developed one additional symptom after start of treatment, i.e., hypoventilation (n = 3) and bradycardia (n = 1). One patient developed seizures after diagnosis but before treatment, with a delay between diagnosis and treatment of 2 days (patient 16). One patient developed seizures 3 days after diagnosis and 9 days after initiation of treatment (patient 9).
Figure 2. Patient symptoms.
(A) Distribution of presenting symptoms of patients younger than 12 years and of patients aged 12–18 years. (B) Cumulative symptoms during disease course. (C) Number of core symptoms at presentation, treatment, and antibody diagnosis, and total number of symptoms.
Median time from symptom onset to maximum PCPC (maximum disease severity) was 30 days. Forty-six percent of patients (13/28) were treated in the ICU with a median stay of 13 days. Total hospital stay was more than a month in 78% of patients. All patients were treated with first-line immunotherapy. Forty-six percent of patients received either rituximab (n = 12) or cyclophosphamide (n = 1). In 14 of 28 patients (50%), treatment was started before diagnosis, in 6 of 28 patients (21%), treatment was initiated on the day of diagnosis, and in 8 of 28 patients (29%), treatment was started after diagnosis. For all clinical characteristics, see table 1 and supplemental material (links.lww.com/WNL/A493).
Table 1.
Patient characteristics
Outcome
Three patients had a relapse 3, 5, and 35 months after first symptoms. One patient had a higher PCPC during the relapse than during the initial disease episode, leading to the initiation of rituximab. At hospital discharge, the median PCPC was 3 (interquartile range [IQR] 2–3, range 1–4). Seventeen patients were discharged home, although 10 concurrently started with an outpatient rehabilitation program. Eleven patients (39%) were transferred directly to an inpatient rehabilitation center. Median rehabilitation time was 98 days (IQR 58–194, range 34–578). The median PCPC at last visit was 1 (IQR 1–2, range 1–4). Twenty-six patients (93%) resumed school after admission or rehabilitation. In 6 of the 26 patients (23%) who resumed school, the current educational level was lower, including 5 patients with special educational needs. During follow-up, 3 patients stopped school prematurely because of fatigue (n = 2) or anxiety (n = 1). Overall, 18 of 28 patients (64%) returned consistently to their previous school level.
Cross-sectional follow-up study
Twenty-two patients participated in the follow-up study, with a median follow-up time after symptom onset of 31 months (IQR 15–49, range 5–91). Nineteen were seen at our clinic, while 3 had an interview by phone. All 22 patients completed questionnaires, while 16 patients completed the full neuropsychological assessment (figure 1). Individual information is shown online in table e-3 (links.lww.com/WNL/A495). Median age at last visit was 17 years (IQR 12–19, range 4–25). Three patients had post-HSV encephalitis anti-NMDAR encephalitis, 2 with a follow-up PCPC of 3 (patients 14 and 18) and 1 with a PCPC of 4 (patient 19). One patient had a PCPC of 4 because of spasticity and vocal cord paralysis (patient 6).
Neuropsychological outcome
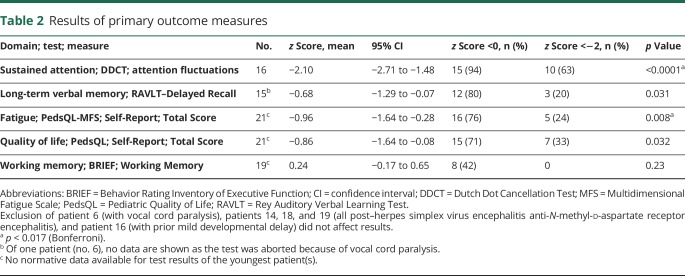
Characteristics of the 16 patients who underwent full neuropsychological assessment were similar to those of the other patients (n = 13; table e-4, links.lww.com/WNL/A495). Patients had lower sustained attention scores (z = −2.10, puncorrected < 0.0001; table 2), and these were consistent among almost all patients. The mean score on long-term verbal memory tended to be lower (z = −0.68, puncorrected = 0.031). Patients reported more fatigue (z = −0.96, puncorrected = 0.008), and QoL tended to be lower (z = −0.87, puncorrected = 0.032), while working memory was not different (z = 0.24, puncorrected = 0.23). Results were similar when the 3 patients with anti-NMDAR encephalitis post HSV encephalitis were excluded (1 full neuropsychological assessment, 2 only completed questionnaires; data not shown).
Table 2.
Results of primary outcome measures
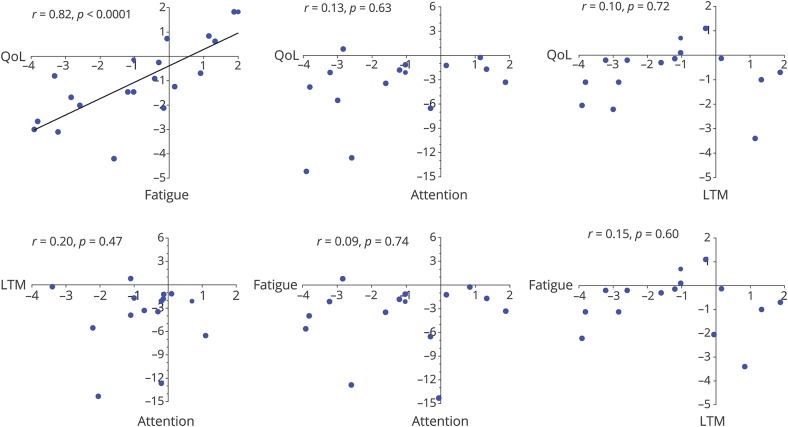
There was a strong correlation between self-reported fatigue and QoL (r = 0.82, p < 0.0001; figure 3), also as reported by parents (Parent Proxy-Report−Total Score; r = 0.70, p = 0.004). There were no significant correlations between QoL and fatigue and the cognitive domains sustained attention and long-term verbal memory (figure 3). Treatment delay, follow-up time, age at onset, ICU stay, maximum PCPC, and PCPC at follow-up were not correlated with sustained attention, long-term verbal memory, or fatigue (figure e-1, links.lww.com/WNL/A494). Sustained attention and long-term verbal memory were also not correlated with QoL scores as reported by parents (sustained attention: r = 0.20, p = 0.62; long-term verbal memory: r = 0.45, p = 0.27).
Figure 3. Overview of correlations between primary outcome measures.
Outcome measures: sustained attention (Dutch Dot Cancellation Test–attention fluctuations), long-term verbal memory (RAVLT–Delayed Recall), fatigue (PedsQL-MFS Self-Report–Total Score), QoL (PedsQL Self-Report–Total Score), and working memory (BRIEF–Working Memory). In all graphs, results of uncorrected z scores are shown, but the correlations are calculated with corrected z scores (maximum 3, minimum −3). Anti-NMDAR = anti-N-methyl-d-aspartate receptor; BRIEF = Behavior Rating Inventory of Executive Function; HSV = herpes simplex virus; LTM = long-term verbal memory; MFS = Multidimensional Fatigue Scale; PedsQL = Pediatric Quality of Life; QoL = quality of life; RAVLT = Rey Auditory Verbal Learning Test.
Among the secondary outcome measures (tables e-5 and e-6, links.lww.com/WNL/A495), the mean z score on domain speed was lower (Dutch Dot Cancellation Test−reaction time; z = −1.53, puncorrected = 0.002). Scores on the domains visual memory (Paired Associated Learning−total errors; z = −0.90, puncorrected = 0.016), short-term verbal memory (RAVLT Trials 1–5); z = −0.76, puncorrected = 0.023), and naming (Boston Naming Test−total score; z = −0.78, puncorrected = 0.019) were low, but between 0.05 and 0.005.
Results of the questionnaires completed by parents were comparable to those of children (table e-7, links.lww.com/WNL/A495).
Patients and parents mentioned similar difficulties in the detailed interview (17/22). Regarding school or work performance, the most notable problems were word finding difficulties (24%), dyslexia (12%), and attention and concentration deficits (18%). Other problems were impulsiveness (18%), anxiety (18%), and indecisiveness (12%). Concerning the disease period, 21 of 22 patients (95%) had a persistent (fragmented or complete) amnesia.
Based on our own observations during the visits to our clinics, we could differentiate 3 frontal lobe syndrome profiles using the character descriptions by parents and the main complaints of the patients themselves. This way, we allocated the patients visiting our clinic into 3 groups: (1) passive (apathy, n = 5), (2) moderate (no signs of a frontal lobe syndrome, n = 6), and (3) active (impulsive, n = 7). The median scores on QoL and fatigue were compared between these groups (visualized in figure e-2, links.lww.com/WNL/A494). Among the passive patients, the school dropout rate was 80% (4/5), while for the active patients, school resumption was achieved in all 7, of whom 2 did not retain previous school level.
Discussion
We have demonstrated that, despite good functional recovery (according to the mRS or PCPC), persistent cognitive deficits are common in young children and adolescents following pediatric anti-NMDAR encephalitis, and that important parameters for good outcome, such as treatment delay or age at onset, do not specifically affect neuropsychological outcome. Other interesting and important findings are that patients reported more fatigue, and that patients with fatigue also reported a poorer QoL, while poorer cognitive outcome did not affect QoL.
Fatigue has not been evaluated before in patients with anti-NMDAR encephalitis. However, it is known to be a common disabling symptom in pediatric acquired brain injury,22,23 making our results that fatigue was associated with poorer QoL plausible. This finding is supported further by the frequent reporting of fatigue by patients as the most disabling symptom often hampering normal participation.
Remarkably, poorer cognitive outcome did not influence QoL, possibly because QoL questionnaires comprise general topics, while patients often reported specific task-related problems, which might be underestimated in current questionnaires. In addition, patients becoming accustomed to a new “stable” situation and reduced awareness might be other explanations. The latter is less likely because parents' QoL scores were comparable.
Predictors of good functional outcome such as treatment delay, maximum PCPC, and ICU stay were not correlated with QoL, fatigue, or sustained attention. This supports our statement that “good” outcome certainly not always means “good” total recovery. NMDAR antibodies are considered to compromise signal transmission, leading to problems in multiple functional networks, corresponding to the extent of symptoms. Finke et al.24 showed that a reduced connectivity of the anterior hippocampus and the anterior default mode network was associated with poorer memory in anti-NMDAR encephalitis.24 In addition, this reduced connectivity is also described in a broad spectrum of other neurologic conditions.25–28 These connections seem most vulnerable, which may explain the discrepancy between good outcome and poor memory recovery. A follow-up study testing patients by serial neuropsychological tests combined with fMRI will be essential to examine the correlation between cognitive functioning and this reduced connectivity over time, and to examine whether this process is reversible.
Most anti-NMDAR encephalitis follow-up studies concentrate on the neurobehavioral problems of disinhibition. However, frontal lobe syndromes are more widespread, and little is known about passive patients during rehabilitation and follow-up. Our data suggest that these “passive” patients might be more at risk to develop problems with normal participation because these patients showed more school dropout rates and reported more fatigue. This observation needs confirmation in future research, but may have important consequences for rehabilitation programs.
For cognitive outcome, we particularly observed lower scores in the domain sustained attention and speed. Possibly these cognitive deficits are most prominent and should be considered during cognitive rehabilitation. However, there was no correlation between the different cognitive test results, which underlines that the occurring cognitive deficits are diverse and probably different parts of the brain are affected. Short-term verbal memory and language scores were also lower. Apparently these domains are more vulnerable to dysfunction of the NMDAR. These findings are partially in concordance with earlier findings,3–9 although these previous published studies describe more diverse cognitive deficits, with additional deficits in executive functioning. However, these studies are difficult to interpret and to compare properly to our results because of limited patient numbers and unstandardized methods and because some patients were assessed in the acute disease phase. By using standardized performance-based measures, such as CANTAB, we found no prominent problems in executive functions. Nevertheless, by using rating measures (questionnaires, interviews), patients reported substantial difficulties in performing activities of daily living. An explanation for this disconnection is that performance-based measures and rating measures do not assess the same aspects in cognitive and behavioral functioning. Rating measures assess whether goals in activities of daily living are reached and have higher ecological validity.29 Next to the BRIEF (and other rating measures we performed), the BADS-C (Behavioral Assessment of the Dysexecutive Syndrome in Children) might be a useful addition.
The present study, with national coverage, detailed description of clinical data, and the use of a systematic neuropsychological assessment, provides broad, valuable results, likely to be externally valid. This study exclusively pertains to pediatric anti-NMDAR encephalitis, also a valuable aspect, because in comparison to adults, there are differences in disease onset, treatment decisions, and social functioning. First, children present more often with seizures or behavioral changes,30 whereas adults mostly present with psychiatric symptoms or memory dysfunction,2 which may lead to different intervals to diagnosis and treatment. Second, treatment decisions can be age-dependent and may affect outcome; i.e., physicians tend to be more aggressive in children, starting immunotherapy early while simultaneously being more careful with cyclophosphamide. Third, neuropsychological problems can seriously affect participation as successful transition into adulthood calls for full cognitive, emotional, and behavioral abilities.
We had the unique opportunity to include all Dutch children with anti-NMDAR encephalitis. Nevertheless, despite national coverage and increasing incidence, anti-NMDAR encephalitis is a rare disease. Therefore, to include a sufficient number of patients with a reasonable follow-up time, a retrospective study design was inevitable but with the associated problems. The first issue is missing data. The amount of missing data was minimized by contacting treating physicians, parents, and patients. Regarding selection bias (between patients participating and nonparticipating in the follow-up study), we found no difference in clinical characteristics. Furthermore, clinical characteristics are in line with previous studies.2,30 The results of the participants thus seem to be a good representation for the total group and results are probably generalizable.
Overall, our findings highlight the importance of awareness of persisting neuropsychological deficits and excessive fatigue following pediatric anti-NMDAR encephalitis. With a considerable median follow-up time (almost 3 years), our results clearly indicate that neuropsychological deficits can be prolonged. Currently, disease outcome is assessed with parameters measuring impairment and disabilities (mRS,11 PCPC), and treatment decisions are based on these parameters. Our results show that neuropsychological parameters measuring participation and QoL are also important and should be considered when assessing outcome, because these factors can substantially affect participation and well-being. Therefore, physicians should inform patients and parents correctly about the occurrence of prolonged neuropsychological problems. In addition, they should provide good accessibility to neuropsychological counseling in rehabilitation centers immediately following the acute disease course and during follow-up.
Acknowledgment
The authors gratefully thank all patients and their parents for their participation. They thank all referring physicians, with special thanks to Dr. F.M.C. Berkesteijn, Dr. J.M. de Bont, Prof. Dr. K.P.J. Braun, Prof. Dr. O.F. Brouwer, Dr. J.F.H.M. Claes, Dr. K.G.J. van Dijk, Dr. C. Erasmus, Dr. J.G.J. Hoeijmakers, Dr. W. Peper, Dr. J.P.A. Samijn, Dr. R.D. Thijs, Prof. Dr. R.J. Vermeulen. The authors thank Esther Hulsenboom and Mariska Nagtzaam for technical assistance.
Glossary
- anti-NMDAR
anti-N-methyl-d-aspartate receptor
- BRIEF
Behavior Rating Inventory of Executive Function
- CANTAB
Cambridge Neuropsychological Test Automated Battery
- HSV
herpes simplex virus
- ICU
intensive care unit
- IQR
interquartile range
- mRS
modified Rankin Scale
- PedsQL
Pediatric Quality of Life
- PedsQL-MFS
Pediatric Quality of Life Multidimensional Fatigue Scale
- PCPC
Pediatric Cerebral Performance Category
- QoL
quality of life
- RAVLT
Rey Auditory Verbal Learning Test
Footnotes
Podcast: NPub.org/enhbn2
CME Course: NPub.org/cmelist
Contributor Information
Collaborators: CHANCE Study Group, P. B. Augustijn, D. P. Bakker, Maartje Boon, E.A. Cats, M.J. Eikelenboom, M. Engelen, C.P.W. Geleijns, C. Haaxma, J. Nicolai, J.M.F. Niermeijer, E.H. Niks, E.A. Peeters, R.P. Portier, A.B. Rietman, H.M. Schippers, A. Verrips, and M.C.Y. de Wit
Author contributions
M.A.A.M. de Bruijn: study design, acquisition of data, data analysis, medical writing. F.K. Aarsen: study design, acquisition of data, revising the manuscript for content. M.P. van Oosterhout: acquisition of data, revising the manuscript for content. M.M. van der Knoop: acquisition of data, revising the manuscript for content. C.E. Catsman-Berrevoets: revising the manuscript for content. M.W.J. Schreurs: revising the manuscript for content. D.E.M. Bastiaansen: acquisition of data, revising the manuscript for content. P.A.E. Sillevis Smitt: revising the manuscript for content. R.F. Neuteboom: study design, acquisition of data, revising the manuscript for content. M.J. Titulaer: study design, acquisition of data, data analysis, medical writing.
Study funding
M.T. was supported by an Erasmus MC fellowship, has received funding from the Netherlands Organization for Scientific Research (NWO, Veni incentive), from the Dutch Epilepsy Foundation (NEF, project 14-19), and from ZonMw (Memorabel program).
Disclosure
M. de Bruijn, F. Aarsen, M. van Oosterhout, M. van der Knoop, C. Catsman-Berrevoets, M. Schreurs, and D. Bastiaansen report no disclosures relevant to the manuscript. P. Sillevis Smitt holds a patent for the detection of anti-DNER and received research support from EUROIMMUN. R. Neuteboom reports no disclosures relevant to the manuscript. M. Titulaer has filed a patent for methods for typing neurologic disorders and cancer, and devices for use therein, and has received research funds for serving on a scientific advisory board of MedImmune LLC, for consultation at Guidepoint Global LLC, and an unrestricted research grant from EUROIMMUN AG. Go to Neurology.org/N for full disclosures.
References
- 1.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finke C, Kopp UA, Pruss H, Dalmau J, Wandinger KP, Ploner CJ. Cognitive deficits following anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry 2012;83:195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKeon GL, Scott JG, Spooner DM, et al. Cognitive and social functioning deficits after anti-N-methyl-D-aspartate receptor encephalitis: an exploratory case series. J Int Neuropsychol Soc 2016;22:828–838. [DOI] [PubMed] [Google Scholar]
- 5.Loughan AR, Allen A, Perna R, Malkin MG. Anti-N-methyl-D-aspartate receptor encephalitis: a review and neuropsychological case study. Clin Neuropsychol 2016;30:150–163. [DOI] [PubMed] [Google Scholar]
- 6.Hinkle CD, Porter JN, Waldron EJ, Klein H, Tranel D, Heffelfinger A. Neuropsychological characterization of three adolescent females with anti-NMDA receptor encephalitis in the acute, post-acute, and chronic phases: an inter-institutional case series. Clin Neuropsychol 2017;31:268–288. [DOI] [PubMed] [Google Scholar]
- 7.Matricardi S, Patrini M, Freri E, et al. Cognitive and neuropsychological evolution in children with anti-NMDAR encephalitis. J Neurol 2016;263:765–771. [DOI] [PubMed] [Google Scholar]
- 8.Iadisernia E, Battaglia FM, Vanadia E, Trapolino E, Vincent A, Biancheri R. Anti-N-methyl-D-aspartate-receptor encephalitis: cognitive profile in two children. Eur J Paediatr Neurol 2012;16:79–82. [DOI] [PubMed] [Google Scholar]
- 9.McKeon GL, Robinson GA, Ryan AE, et al. Cognitive outcomes following anti-N-methyl-D-aspartate receptor encephalitis: a systematic review. J Clin Exp Neuropsychol 2018;40:234–252. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. International Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization; 2001. [Google Scholar]
- 11.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 12.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr 1992;121:68–74. [DOI] [PubMed] [Google Scholar]
- 13.Vos PG. Bourdon-Vos Test Manual, 3rd ed. Amsterdam: Pearson; 1998. [Google Scholar]
- 14.van den Burg W, Kingma A. Performance of 225 Dutch school children on Rey's Auditory Verbal Learning Test (AVLT): parallel test-retest reliabilities with an interval of 3 months and normative data. Arch Clin Neuropsychol 1999;14:545–559. [DOI] [PubMed] [Google Scholar]
- 15.Storms G, Saerens J, De Deyn PP. Normative data for the Boston Naming Test in native Dutch-speaking Belgian children and the relation with intelligence. Brain Lang 2004;91:274–281. [DOI] [PubMed] [Google Scholar]
- 16.Paquier PF, van Mourik M, van Dongen HR, Catsman-Berrevoets C, Creten WL, Van Borsel J. Normative data of 300 Dutch-speaking children on the Token Test. Aphasiology 2009;23:427–437. [Google Scholar]
- 17.Korkman MKU, Kemp S. Dutch Technical Manual of NEPSY II-NL. Amsterdam: Pearson; 2010. [Google Scholar]
- 18.Smidts DP, Huizinga M. Manual of BRIEF Executive Functions Behavior Questionnaire. Amsterdam: Hogrefe; 2009. [Google Scholar]
- 19.van Widenfelt BM, Goedhart AW, Treffers PD, Goodman R. Dutch version of the Strengths and Difficulties Questionnaire (SDQ). Eur Child Adolesc Psychiatry 2003;12:281–289. [DOI] [PubMed] [Google Scholar]
- 20.Engelen V, Haentjens MM, Detmar SB, Koopman HM, Grootenhuis MA. Health related quality of life of Dutch children: psychometric properties of the PedsQL in the Netherlands. BMC Pediatr 2009;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordijn M, Cremers EM, Kaspers GJ, Gemke RJ. Fatigue in children: reliability and validity of the Dutch PedsQL Multidimensional Fatigue Scale. Qual Life Res 2011;20:1103–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chevignard M, Francillette L, Toure H, et al. Academic outcome, participation and health-related quality of life following childhood severe traumatic brain injury: results of a prospective longitudinal study: the seven-year follow-up of the TGE cohort. Ann Phys Rehabil Med 2016;59S:e133. [Google Scholar]
- 23.Toussaint-Duyster LC, Wong YYM, Van der Cammen-van Zijp MH, et al. Fatigue and physical functioning in children with multiple sclerosis and acute disseminated encephalomyelitis. Mult Scler Epub 2017 April 1. [DOI] [PMC free article] [PubMed]
- 24.Finke C, Kopp UA, Scheel M, et al. Functional and structural brain changes in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2013;74:284–296. [DOI] [PubMed] [Google Scholar]
- 25.Allen G, Barnard H, McColl R, et al. Reduced hippocampal functional connectivity in Alzheimer disease. Arch Neurol 2007;64:1482–1487. [DOI] [PubMed] [Google Scholar]
- 26.Grydeland H, Walhovd KB, Westlye LT, et al. Amnesia following herpes simplex encephalitis: diffusion-tensor imaging uncovers reduced integrity of normal-appearing white matter. Radiology 2010;257:774–781. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Liang M, Jiang T, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett 2007;417:297–302. [DOI] [PubMed] [Google Scholar]
- 28.Shapira-Lichter I, Weinstein M, Lustgarten N, et al. Impaired diffusion tensor imaging findings in the corpus callosum and cingulum may underlie impaired learning and memory abilities in systemic lupus erythematosus. Lupus 2016;25:1200–1208. [DOI] [PubMed] [Google Scholar]
- 29.Toplak ME, West RF, Stanovich KE. Practitioner review: do performance-based measures and ratings of executive function assess the same construct? J Child Psychol Psychiatry 2013;54:131–143. [DOI] [PubMed] [Google Scholar]
- 30.Armangue T, Titulaer MJ, Malaga I, et al. Pediatric anti-N-methyl-D-aspartate receptor encephalitis: clinical analysis and novel findings in a series of 20 patients. J Pediatr 2013;162:850–856.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilderink M, Titulaer MJ, Schreurs MW, Keizer K, Bunt JE. Transient anti-NMDAR encephalitis in a newborn infant due to transplacental transmission. Neurol Neuroimmunol Neuroinflamm 2015;2:e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any data not published within this article are available at Erasmus University Medical Center. Patient-related data will be shared on request from any qualified investigator, maintaining anonymization of the individual patients.