Abstract
Aim
The aim of the present study was to characterize patterns of use of methylphenidate (MPH), a prescription stimulant medication recommended in the treatment of attention deficit hyperactivity disorder (ADHD) and of narcolepsy, in France, both in children and adults, over a 3‐year period.
Methods
Using the French General Health Insurance database, limited to two areas covering approximately 4 million individuals, we made up a cohort of incident MPH users between July 2010 and June 2013. Splitting them into distinct age groups (18–24, 25–49 and ≥50 years of age for adults and <6, 6–11 and 12–17 years of age for children), we established the characteristics of these populations at MPH initiation and during follow‐up according to the duration of treatment, quantities dispensed and coprescription with central nervous system (CNS) drugs.
Results
We included a cohort of 3534 incident users, involving 30 238 dispensings of MPH, leading to an annual rate of 29 incident users per 100 000 in 2013. Children (66% of new users) were characterized by long‐term use of MPH with few comedications. The group of 25–49‐year‐old patients were dispensed MPH more frequently than other groups, had the highest mean dose and were more often coprescribed other CNS drugs. The ≥50 year‐old group was more often coprescribed antidepressants and antiparkinsonian drugs.
Conclusions
Our pharmacoepidemiological study involving incident MPH users with a large number of characteristics showed different patterns of MPH use among children and adults. The results from the 25–49‐year‐old group suggested that MPH might be being used for medical conditions other than ADHD or narcolepsy in adults, and that it might be subject to misuse and/or abuse.
Keywords: ADHD, adults, children, drug utilization, methylphenidate, prescription database, retention
What is Already Known about this Subject
Methylphenidate (MPH) use in France and worldwide has increased significantly since 2000.
What this Study Adds
Thirty‐four per cent of new users of MPH are adults.
Patterns of drug use reveal distinct types of MPH use: long‐term use in children; associated with medical conditions other than attention deficit hyperactivity disorder and with high levels of coprescription of other central nervous system drugs; and also MPH misuse and potential abuse in the adult population.
Introduction
Since 2000, the dispensing of methylphenidate (MPH), a prescription stimulant medication commonly used in the treatment of attention deficit hyperactivity disorder (ADHD), has significantly increased worldwide in children and adults 1, 2, 3, 4, 5, 6, 7, 8, the concern actually are for efficacy and safety of the long term use for all the users (principally for young people where MPH is authorized in ADHD; and the use for young children (<6 years) and adults for whom MPH isn't authorized (only for narcolepsy in adults). To established and assess the patterns of this increase, numerous studies have been performed across a wide range of countries, with the aim of estimating the prevalence of MPH use and associated factors (European countries 2, 5, 6, 8, 10, 11, 12, 15, 16, 17, 18, United States 3, 13, 14, and Australia 14).
National guidelines for initiating drug therapy in children or adolescents to treat ADHD have been based on solid evidence from short‐term studies, without addressing the major concern about the long‐term use of, treatment adherence with, and the misuse or inappropriate use of MPH. Improved diagnosis of ADHD among children may explain this huge increase throughout the world. Studies in children have focused on establishing the increase in psychotropic drug use over time, and characterizing its patterns of use (regular, occasional or short‐term) 14, 18, 19, 20. Other studies have focused on patients with ADHD, irrespective of their age (adults and/or children) 3, 20, 21, 22, 23, 24, 25, with the aim of characterizing treatment initiation and persistence.
For adults, the increase in MPH use may be linked to improved diagnosis of ADHD in this population,for whom prevalence rates of ADHD have been established varying between 1% and 7% in a metaanalysis 26. Nevertheless, there remains uncertainty concerning the diagnosis of ADHD, and the long‐term efficacy and safety of MPH in this adult population. In relation to the duration of MPH use for the adult population, precedent studies that have been carried out specifically in Nordic countries, where the increase in, and amount of MPH use have been an important concern 12, 20, 27, found short treatment durations and/or early treatment discontinuations 20 but with discrepancies throughout distinct age groups 11, 27. Other important concerns are related to the possible use of MPH for medical conditions other than ADHD, such as Parkinson's disease 28 or bipolar depression 29, or in patients with cognitive slowing 30, 31, 32 or substance use disorder (SUD) 1, 29, 33, 34.
Despite a large amount of literature on the topic, our overall understanding of patterns of MPH use is sparse due to the variation in the populations analysed in the different studies. Indeed, some studies have focused only on children, others only on adults and others only on patients diagnosed with ADHD. Nevertheless, the concerns are from one study to another, even if the studied outcomes are not exactly the same, the overall concern converge to the description of different patterns of use like coprescription of other drugs, including psychotropic medications, and drug abuse. Taken alone, these studies are informative and give a clear comprehension of the different patterns which resumes profiles of MPH users considering the different patterns of use as a whole, at least in France.
To our knowledge, few pharmacoepidemiological studies have focused both on children and adults. A recent study in Denmark found an overuse of other psychotropic medications, an increase in dose and differences in the duration of treatment between children and adults 11. This study was carried out on prevalent users, thus preventing any characterization of MPH use at its initiation – in particular, the specialty of the prescribing physician – and of the concomitant use of other medications at the time of its initiation.
We sought to assess patterns of MPH use both in children and adult incident users over a 3‐year period according to distinct age groups, using a unique data source, and according to characteristics both at initiation and during follow‐up, such as: the quantities dispensed, time between refills, treatment duration, concomitant use of other drugs (6 months before initiation and during follow‐up), polypharmacy and multiple prescribers.
Methods
Database
This was a retrospective observational cohort study based on data from the French general health insurance drug database [General Health Insurance System (GHIS)] in two regions of the south of France (Provence‐Alpes–Côte‐d'Azur and Corsica) over a 4‐year‐period (2010–2013). The GHIS is the main national health insurance fund in France and covers employed and unemployed people and students, which corresponds to about 87% of the entire French population (self‐employed people and farmers are covered by other specific funds). In 2011, these two regions included 4 147 748 individuals covered by the GHIS. Drug prescription claims are automatically recorded in a database by the pharmacists and transmitted online to the GHIS. According the French legislation on privacy, patient, prescriber and pharmacy identifiers are unique anonymous numbers.
Population selected
From the GHIS database, we first included patients with GHIS coverage who had MPH dispensed (ATC code: N06BA04) at least once from 1 July 2010 to 30 June 2013. The starting date of inclusion was chosen so as to include only incident patients – i.e. patients with no dispensing of MPH over the 6‐month period before the screening period, which was took place from 1 January 2010 to 30 June 2010. The end of the inclusion period was chosen so as to ensure at least 6 months of follow‐up for people included in 2013. We performed a sensitivity analysis, using a threshold of 1 year to analyse the impact of a different definition of incident user to the characteristisation of the population.
The only ADHD drug marketed in France is MPH, which is marketed for patients aged ≥6 years who are affected by ADHD 35. Two MPH formulations are available: one immediate‐release (IR) (Ritalin ®) and several extended‐release (ER) (Ritalin®, Quasym®, Concerta® and Medikinet®) formulations. In adults, MPH is licensed as a second‐line treatment in narcolepsy when modafinil has been found to lack efficacy (only for Ritalin 10 mg ®), and only one (Concerta ®) of the extended‐release formulations has been licensed in adult ADHD in cases where it has been efficacious during childhood, but cannot be started in adults. According to narcotic substance legislation in France, MPH is subject to restrictive conditions of prescription and delivery: initiation in hospitals must be by neurologists, psychiatrists or paediatricians; prescription is limited to a 28‐day period and valid for 1 year; refill of the prescription can be authorized by any other physician for a maximum period of 1 year but it must be for the same dose and frequency as initially prescribed.
Collection of data
The following variables relevant to the study were extracted: age, gender, chronic disease, student system of healthcare reimbursement, prescriber and type of prescriber (general practitioner, practice specialist or hospital physician), date of dispensing, pharmacy identifier code, drug name, drug code and quantity delivered. Drugs were categorized according to the Anatomical Therapeutic Chemical (ATC) classification [antidepressants (ATC: N06A), benzodiazepines (ATC: N05BA, N05CD; N03AE01 for clonazepam), opiate maintenance treatments, including methadone and high‐dose buprenorphine (N07 BC), opioid analgesics (N02A), antipsychotic agents (N05A) and modafinil (N06BA07)]. The total quantities of drugs dispensed were expressed in defined daily doses (DDD) according to the World Health Organization Collaborating Centre for Drug Statistics Methodology 36; for MPH, one DDD corresponds to 30 mg.
Data analysis
We defined age categories according the following thresholds: children under 6 years of age, children (6–11 years of age), adolescents (12–17 years of age), young adults (18–24 years of age), adults (25–49 years of age) and adults over 50 years of age.
Firstly, we established the profiles of MPH incident users stratified by age group. We then focused on patients with more than one dispensing of MPH, and followed up these patients. We were particularly interested in collecting information on concomitant use of other medications, specifically central nervous system (CNS) drugs (opioid analgesics, benzodiazepines, antipsychotic agents); the time between two dispensings; and the dose dispensed. We defined concomitant use of medication as at least one dispensing of another drug between the first and last dispensing of MPH, irrespective of the prescribing physician. We defined the dose dispensed as the quantity of drug dispensed (in mg) divided by the time until the next dispensing. Use of other CNS drugs was assessed over two periods: during the 6 months preceding the initiation with MPH and during the entire follow‐up. This allowed us to get an indication of the proportion of patients stopping or continuing use of these other medications during MPH treatment. All of the dispensings between the inclusion of the patient until the end of their follow‐up (last MPH dispensing or 31 December 2013) were included. As our objective was to create a descriptive and exhaustive overview, all patients, including outliers (such as those with a high number of dispensings) were analysed.
Retention rates and Kaplan–Meier drug survival plots
For patients with at least two dispensings, we then computed retention rates of MPH and the median duration of treatment, with a threshold of 90 days as the maximum time allowed between two dispensings. For each patient, the duration of treatment was calculated as the time between the first and the last prescription occurring before a gap of at least 90 days until the next dispensing. For this sub‐analysis, treatment was considered as terminated when 90 days had passed without the individual filling a prescription for MPH, and the date of 31 December 2013 was the censoring date. The long interval (90 days) allowed between prescriptions was chosen to avoid assigning a false treatment termination date for patients who had long intervals between prescriptions. We illustrated this using Kaplan–Meier drug‐survival plots. Sensitivity analyses using two different time intervals (45 days and 365 days) between two dispensings were performed.
Results
Population included
Overall, we included 3534 incident users between 1 July 2010 and 30 June 2013 (see Figure 1). Considering the annual number of people affiliated to the GHIS, the estimated annual number of incident users increased from 27/100 000 people in 2011 to 29/100 000 people in 2013 (an increase of 7%).
Figure 1.
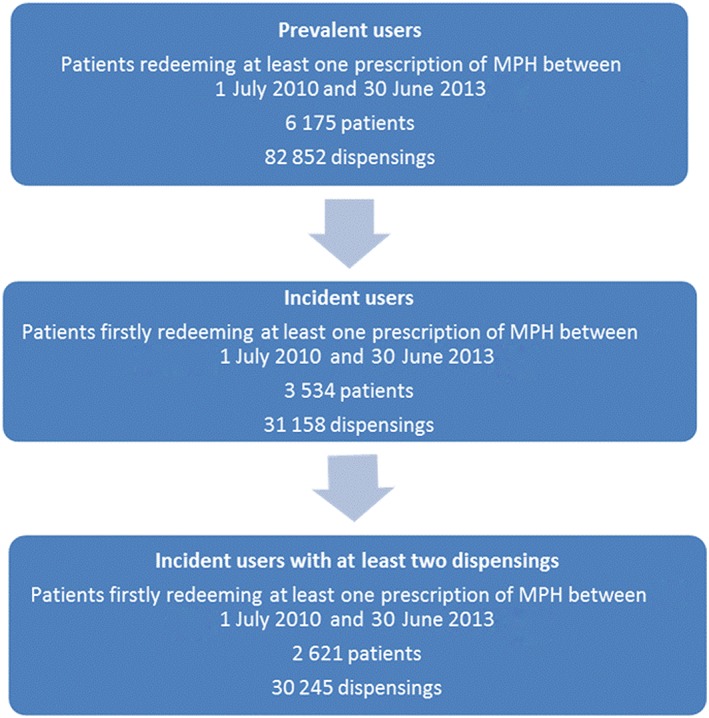
Flowchart of inclusion of methylphenidate (MPH) users
These incident MPH users were aged from 1 year to 91 years, with 2335 (66%) children and 1199 (34%) adults. Profiles of incident users according to age groups are presented in Table 1, dispensing characteristics in Table 2, and the Kaplan–Meier plots for drug survival are presented in Figure 2. Among the children, we observed a predominance of boys, with a gender ratio (boys/girls) of 2, while for adults the gender ratio was 1. The proportion of patients with chronic disease (available only for adults) was higher with increasing age (23% for 18–24‐year‐olds, 46% for 25–49‐year‐olds and 61% for ≥50 year‐olds). Among the incident patients, 2621 (75%) reported at least two dispensings of MPH but this proportion tended to decrease with increasing age, from 74% for the youngest children to 45% for the oldest adults. Among these incident users, there were 113 students, 88% of whom were aged between 18 and 24 years; the others were aged between 25 and 49 years.
Table 1.
Follow‐up of incident methylphenidate (MPH) users between 1 July 2010 and 30 June 2013: characteristics of users
| Children under 6 years old | Children 6–11 years old | Children 12–17 years old | Adults 18–24 years old | Adults 25–49 years old | Adults 25–49 years old | All | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 117 (3.3%) | N = 1560 (44.1%) | N = 658 (18.6%) | N = 198 (5.6%) | N = 831 (23.5%) | N = 170 (4.8%) | N = 3534 | |||||||||
| Number of dispensings | 1 dispensing | 31 | 27% | 227 | 15% | 125 | 19% | 52 | 26% | 384 | 46% | 94 | 55% | 913 | 26% |
| ≥2 dispensings | 86 | 74% | 1333 | 86% | 533 | 81% | 146 | 74% | 447 | 54% | 76 | 45% | 2621 | 74% | |
| Free universal healthcare | 30 | 26% | 398 | 26% | 145 | 22% | 38 | 19% | 374 | 45% | 33 | 19% | 1018 | 29% | |
| Patients with multiple dispensings of MPH (n = 2621) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 86 | N = 1333 | N = 533 | N = 146 | N = 447 | N = 76 | N = 2621 | |||||||||
| Gender | Men | 68 | 79% | 1034 | 78% | 432 | 81% | 96 | 66% | 259 | 58% | 37 | 49% | 1926 | 74% |
| Women | 18 | 21% | 299 | 22% | 101 | 19% | 50 | 34% | 188 | 42% | 39 | 51% | 695 | 26% | |
| Speciality of the 1st prescriber [n (%)] | Physician working in an hospital | 60 | 70% | 837 | 63% | 314 | 59% | 51 | 35% | 162 | 36% | 39 | 51% | 1463 | 56% |
| General practitioner | 5 | 6% | 136 | 10% | 75 | 14% | 43 | 30% | 208 | 47% | 20 | 26% | 487 | 19% | |
| Paediatrician | 4 | 5% | 152 | 11% | 34 | 6% | 1 | 1% | 4 | 1% | . | .0% | 291 | 11% | |
| Psychiatrist | 6 | 7% | 63 | 5% | 21 | 4% | 15 | 10% | 46 | 10% | 10 | 13% | 195 | 7% | |
| Neurologist | 8 | 9% | 133 | 10% | 81 | 15% | 9 | 6% | 13 | 3% | 6 | 8% | 250 | 10% | |
| Others/missing information | 3 | 4% | 12 | 1% | 8 | 2% | 27 | 19% | 14 | 3% | 1 | 1% | 65 | 3% | |
| Formulation of the 1st dispensing | IR and ER | 7 | 8% | 97 | 7% | 31 | 6% | 13 | 9% | 12 | 3% | 2 | 3% | 162 | 16% |
| IR only | 40 | 47% | 349 | 26% | 121 | 23% | 34 | 23% | 146 | 33% | 35 | 46% | 725 | 19% | |
| ER only | 39 | 45% | 887 | 67% | 381 | 71% | 99 | 68% | 289 | 64% | 39 | 51% | 1734 | 65% | |
| Formulations dispensed during follow‐up (n %) | IR and ER | 44 | 51% | 471 | 35% | 154 | 29% | 34 | 23% | 80 | 18% | 12 | 16% | 795 | 30% |
| IR only | 11 | 13% | 114 | 9% | 49 | 9% | 21 | 14% | 104 | 23% | 29 | 38% | 328 | 13% | |
| ER only | 31 | 36% | 748 | 56% | 330 | 62% | 91 | 62% | 263 | 59% | 35 | 46% | 1498 | 57% | |
| Time between first and last dispensing (days) | Mean±std | 588.2±337.1 | 528.4±330.9 | 395.0±306.6 | 321.1±259.9 | 393.7±319.4 | 261.4±255.6 | 461.0±328.9 | |||||||
| Median [Q1;Q3] | 578.5 [344.0;824.0] | 478.0 [245.0;779.0] | 303.0 [145.0;604.0] | 273.5 [116.0;428.0] | 313.0 [120.0;618.0] | 157.0 [44.0;406.0] | 395.0 [190.0;699.0] | ||||||||
| Number of different physicians | Mean±std | 3.5±1.6 | 3.3±1.8 | 2.7±1.6 | 2.6±2.3 | 4.2±5.4 | 2.3±1.5 | 3.3±2.8 | |||||||
| Median [Q1;Q3] | 3.0 [2.0;4.0] | 3.0 [2.0;4.0] | 2.0 [2.0;3.0] | 2.00 [1.0;3.0] | 3.0 [2.0;4.0] | 2.00 [1.0;3.0] | 3.0 [2.0;4.0] | ||||||||
| Number of different pharmacists | Mean±std | 2.4±1.3 | 2.1±1.2 | 1.8±1.0 | 2.2±2.2 | 4.0±5.6 | 1.8±0.9 | 2.4±2.7 | |||||||
| Median [Q1;Q3] | 2.0 [1.0;3.0] | 2.0 [1.0;2.0] | 2.0 [1.0;2.0] | 2.0 [1.0;2.0] | 2.0 [1.0;4.0] | 2.0 [1.0;2.0] | 2.0 [1.0;3.0] | ||||||||
| Median [95% CI] of drug survival (months) with 90 days allowed between two dispensings | 26.8 [18.7; −] | 23.1 [21.1; 25.4] | 9.9 [7.9; 11.7] | 5.5 [4.0; 7.3] | 7.5 [5.9; 9.3] | 3.4 [1.5; 6.6] | 14.8 [13.8; 16.0] | ||||||||
| Retention rates with 90 days allowed between two dispensings | |||||||||||||||
| ‐ Retention rate at 1 month | 80 | 93% | 1271 | 95% | 484 | 91% | 124 | 85% | 354 | 79% | 55 | 72% | 2368 | 90% | |
| ‐ Retention rate at 3 months | 77 | 90% | 1165 | 87% | 402 | 75% | 96 | 66% | 297 | 66% | 39 | 51% | 2076 | 79% | |
| ‐ Retention rate at 6 months’ | 67 | 78% | 1033 | 78% | 317 | 59% | 66 | 45% | 236 | 53% | 32 | 42% | 1751 | 67% | |
CI, confidence interval; ER, extended release; IR, immediate release; std, standard deviation; Q1, 1st quartile; Q3, 3rd quartile
Table 2.
Follow‐up of incident methylphenidate (MPH) users between 1 July 2010 and 30 June 2013: characteristics of use
| Dispensing characteristics of MPH for patients with multiple dispensings of MPH: | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Children under 6 years old | Children 6–11 years old | Children 12–17 years old | Adults 18–24 years old | Adults 25–49 years old | Adults > =50 years olds | All | |||||||||
| N = 1350 | N = 16 779 | N = 4531 | N = 1152 | N = 5851 | N = 575 | N = 30 238 | |||||||||
| Formulations dispensed (n %) | IR | 412 | 31% | 2396 | 14% | 602 | 13% | 194 | 17% | 1917 | 32% | 199 | 35% | 5720 | 19% |
| ER | 775 | 57% | 13 270 | 79% | 3653 | 80% | 882 | 77% | 3773 | 65% | 310 | 54% | 22 663 | 75% | |
| IR + ER | 163 | 12% | 1113 | 6% | 276 | 6% | 76 | 6% | 161 | 3% | 66 | 11% | 1855 | 6% | |
| Quantities per dispensing in DDD | Mean±std | 19.7±8.8 | 24.9±13.7 | 36.0±21.7 | 46.9±28.1 | 59.0±28.1 | 39.0±24.0 | 34.0±26.9 | |||||||
| Median[Q1;Q3] | 18.7 [16.8;20.0] | 20.0 [18.7;30.0] | 33.6 [28.0;46.7] | 40.0 [30.0;56.0] | 56.0 [30.0;74.6] | 33.6 [20.0;50.4] | 28.0 [18.7;37.3] | ||||||||
| Time between 2 dispensings (days) | Mean±std | 40.0±38.6 | 45.6±44.88 | 52.7±55.99 | 46.6±61.6 | 32.6±72,1 | 39.8±62.8 | 43.8±54.0 | |||||||
| Median [Q1;Q3] | 30.0 [27.0;40.0] | 32.0 [28.0;49.0] | 35.0 [28.0;56.0] | 30.0 [21.0;46.0] | 16.0 [6.0;31.0] | 29.0 [23.0;35.0] | 31.0 [26.0;46.0] | ||||||||
| Dosage (mg) per day (Quantity divided by time) | Mean±std | 21.7±32.3 | 26.1±51.5 | 34.3±63.0 | 93.9±218.5 | 269.5±545.3 | 59.3±173.9 | 77.4±266.9 | |||||||
| Median [Q1;Q3] | 17.5 [10.7;23.3] | 19.4 [11.7;30.0] | 26.1 [14.7;40.0] | 39.6 [20.6;72.0] | 89.6 [41.5; 228.0] | 35.3 [;20.0; 57.54] | 24.0 [14.3;42.9] | ||||||||
DDD, defined daily doses; ER, extended release; IR, immediate release; std, standard deviation; Q1, 1st quartile; Q3, 3rd quartile
Figure 2.
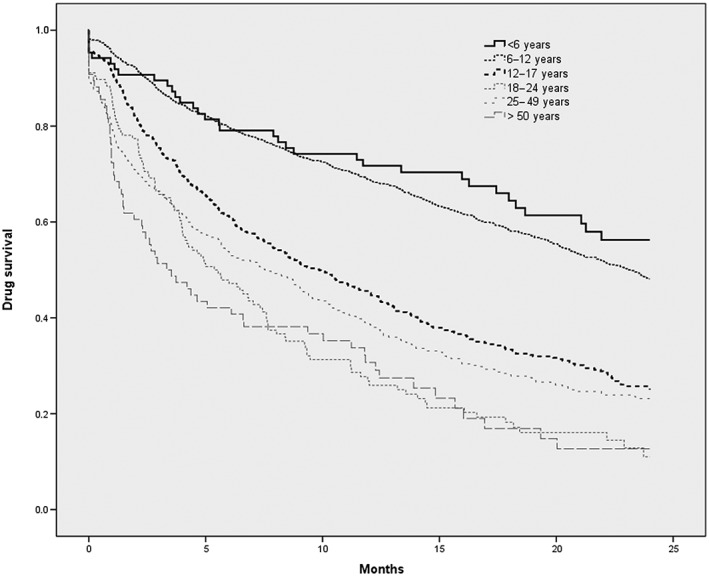
Kaplan–Meier plot for drug survival for methylphenidate (for patients with at least two dispensings; n = 2621), with treatment cessation being the ‘event’. The vertical axis represents the probability of stopping MPH use (between 0 and 1)
We then established patterns of MPH use for the 2621 patients dispensed MPH at least twice. For children, the treatment was mostly initiated in a hospital by a specialist (neurologist, paediatrician or psychiatrist), while for adults initiation was mostly shared between the general practitioner and the hospital physician. Overall, the formulation of the first dispensing of MPH differed according to age: the IR form at initiation was more often used for the 1‐5 year old children (47%) and for ≥50 ‐year‐old adults (46%) than for the four other age groups for whom the percentage of IR form at initiation varies between 23% to 33%.
The median number of different physicians visited by patients varied between the different age groups, between two (for 12‐17 ; 18‐24 and ≥50 year‐old patients) and three (for ≤6; 6‐11 and 25‐49 year‐olds patients). The duration of follow‐up (time between the first and last dispensing), and median drug survival and retention rates (with 90 days allowed between two dispensings) decreased with age: 6‐month retention rates ranged from 78% for 1–5‐year‐old children to 42% for ≥50‐year‐old adults. The Kaplan–Meier plot for drug survival (Figure 2) shows that children initiating treatment before the age of 12 years had a higher treatment retention rate, which persisted over time.
Dose computing (quantity dispensed divided by the duration between two dispensings) showed that the mean and median dose increased with age, with the highest values for 25–49‐year‐old adults, for whom the median dose was 89.6 (41.5; 228.0) [median (Q1;Q3)] mg day–1, compared with 39.6 (20.6; 72.0) mg day–1 for 18–24‐year‐old adults.
Association with other CNS medications
Medication dispensed during the 6‐month period prior to MPH initiation and during MPH treatment is shown in Table 3, and details of the top three medications codispensed during follow‐up are presented in Table 4.
Table 3.
Associated dispensing during the follow‐up and 6 months before methylphenidate (MPH) initiation (patients with >1 dispensing of methylphenidate by pharmaceutical class)
| Children under 6 years old | Children 6–11 years olds | Children 12–17 years olds | Adults 18–24 years olds | Adults 25–49 years olds | Adults ≥50 years olds | All | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 86 | N = 1333 | N = 533 | N = 146 | N = 447 | N = 76 | N = 2621 | |||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| Benzodiazepines (BZDs) | 6 months before | 2 | 2% | 14 | 1% | 12 | 2% | 22 | 15% | 266 | 60% | 38 | 50% | 354 | 14% |
| ‐ n who persist | 1 | 4 | 6 | 16 | 245 | 34 | 306 | ||||||||
| ‐ n who stop | 1 | 10 | 6 | 6 | 21 | 4 | 48 | ||||||||
| During follow‐up | 2 | 2% | 27 | 2% | 18 | 3% | 31 | 21% | 298 | 67% | 47 | 62% | 423 | 16% | |
| Opiate maintenance treatments | 6 months before | . | . | . | . | . | . | 5 | 3% | 133 | 30% | 4 | 5% | 142 | 5% |
| – n who persist | 4 | 119 | 4 | 127 | |||||||||||
| – n who stop | 1 | 14 | 0 | 15 | |||||||||||
| During follow‐up | . | . | . | . | . | . | 5 | 3% | 143 | 32% | 5 | 7% | 153 | 6% | |
| Antipsychotic agents | 6 months before | 11 | 13% | 48 | 4% | 34 | 6% | 18 | 12% | 141 | 32% | 16 | 21% | 268 | 10% |
| – n who persist | 8 | 30 | 27 | 14 | 121 | 15 | 215 | ||||||||
| – n who stop | 3 | 18 | 7 | 4 | 20 | 1 | 53 | ||||||||
| During follow‐up | 12 | 14% | 106 | 8% | 40 | 8% | 21 | 14% | 158 | 35% | 18 | 24% | 355 | 14% | |
| Modafinil | 6 months before | . | . | 1 | 0.1% | 1 | 0.2% | 5 | 3% | 11 | 2% | 9 | 12% | 27 | 1% |
| – n who persist | 0 | 0 | 1 | 5 | 2 | 8 | |||||||||
| – n who stop | 1 | 1 | 4 | 6 | 7 | 19 | |||||||||
| During follow‐up | . | . | 0 | . | 0 | . | 3 | 2% | 18 | 4% | 2 | 3% | 23 | 1% | |
| Antidepressants | 6 months before | 1 | 1% | 4 | 0.3% | 13 | 2% | 25 | 17% | 152 | 34% | 44 | 58% | 239 | 9% |
| – n who persist | 0 | 0 | 9 | 19 | 121 | 39 | 188 | ||||||||
| – n who stop | 1 | 4 | 4 | 6 | 31 | 5 | 51 | ||||||||
| During follow‐up | 1 | 1% | 10 | 1% | 16 | 3% | 28 | 19% | 179 | 40% | 44 | 58% | 278 | 11% | |
| Opioid analgesics | 6 months before | 2 | 2% | 18 | 1% | 5 | 1% | 11 | 8% | 128 | 30% | 16 | 21% | 180 | 7% |
| – n who persist | 0 | 1 | 1 | 5 | 97 | 10 | 114 | ||||||||
| – n who stop | 2 | 17 | 4 | 6 | 31 | 6 | 66 | ||||||||
| During follow‐up | 4 | 5% | 43 | 3% | 30 | 6% | 18 | 12% | 173 | 39% | 20 | 26% | 288 | 11% | |
| Antiparkinsonian agents | 6 months before | 1 | 1% | 1 | 0.1% | 1 | 0.2% | 3 | 2% | 26 | 6% | 21 | 28% | 53 | 2% |
| – n who persist | 0 | 0 | 1 | 1 | 17 | 19 | 38 | ||||||||
| – n who stop | 1 | 1 | 0 | 2 | 9 | 2 | 15 | ||||||||
| During follow‐up | . | . | 1 | 0.1% | 3 | 1% | 5 | 3% | 29 | 7% | 20 | 26% | 58 | 2% | |
| Anxiolytic and hypnotic agents other than BZDs | 6 months before | 2 | 2% | 43 | 3% | 21 | 4% | 8 | 6% | 63 | 14% | 9 | 12% | 146 | 6% |
| – n who persist | 1 | 15 | 10 | 3 | 35 | 5 | 79 | ||||||||
| – n who stop | 1 | 28 | 11 | 5 | 28 | 4 | 77 | ||||||||
| During follow‐up | 3 | 4% | 75 | 6% | 34 | 6% | 12 | 8% | 72 | 16% | 10 | 13% | 206 | 8% | |
Table 4.
Top three concomitant drugs dispensed for each pharmaceutical class and age group (incident users, n = 2621)
| Children under 6 years old (n = 86) | Children 6–11 years old (n = 13 33) | Children 12–17 years old (n = 533) | Adults 18–24 years old (n = 146) | Adults 25–49 years old (n = 447) | Adults ≥50 years old (n = 76) | |
|---|---|---|---|---|---|---|
| Antipsychotic agents | 12 (14%) | 106 (8%) | 40 (8%) | 21 (14%) | 158 (35%) | 18 (24%) |
| Risperidone, n = 11, 13% | Risperidone, n = 90, 7% | Risperidone, n = 28, 5% | Lithium, n = 9, 6% | Olanzapine, n = 54, 12% | Lithium, n = 9, 12% | |
| Cyamemazine, n = 3, 4% | Cyamemazine, n = 15, 1% | Cyamemazine, n = 8, 2% | Cyamemazine, n = 6, 4% | Lithium, n = 46, 10% | Cyamemazine, Olanzapine, Quetiapine, n = 5, 7% | |
| Tiapride, n = 1, 1% | Pimozide, n = 1, 0.2% | Risperidone, n = 5, 3% | Aripiprazole, n = 44, 10% | |||
| Opioids analgesics | 4 (5%) | 43 (3%) | 30 (6%) | 18 (12%) | 173 (39%) | 20 (26%) |
| Codeine, n = 4, 5% | Codeine, n = 36, 3% | Tramadol, n = 19, 4% | Morphine sulphate, n = 10, 7% | Morphine sulphate, n = 98, 22% | Tramadol, n = 12, 16% | |
| Tramadol n = 9, 1% | Codeine, n = 11, 2% | Tramadol, n = 6, 4% | Tramadol, n = 89, 20% | Morphine sulphate, n = 7, 9% | ||
| Codeine, n = 5, 3% | Codeine, n = 41, 9% | Codeine, n = 5, 7% | ||||
| Anxiolytic and hypnotic agents other than BZDs | 3 (4%) | 75 (6%) | 34 (6%) | 12 (8%) | 72 (16%) | 10 (13%) |
| Hydroxyzine, n = 3, 4% | Hydroxyzine, n = 71, 5% | Hydroxyzine, n = 32,6% | Hydroxyzine, n = 8, 6% | Hydroxyzine, n = 52, 12% | Hydroxyzine, n = 5, 7% | |
| Etifoxine, n = 3, 0.3% | Etifoxine, n = 3, 1% | Etifoxine, n = 5, 3% | Etifoxine, n = 14, 3% | Etifoxine, n = 2, 3% | ||
| Meprobamate with aceprometazine, n = 1, 0.2% | Meprobamate with aceprometazine, n = 1, 0.7% | Meprobamate only or in association with aceprometazine, n = 12, 3% | Meprobamate only or in association with aceprometazine, n = 1, 1% | |||
| BZDs | 2 (2%) | 27 (2%) | 18 (3%) | 31 (21%) | 298 (67%) | 47 (62%) |
| Diazepam, n = 2, 2% | Clonazepam, n = 7, 1% | Prazepam, n = 4, 0.8% | Zolpidem, n = 13, 9% | Bromazepam, n = 108, 24% | Bromazepam, n = 11, 15% | |
| Clobazam, n = 1, 1% | Diazepam, n = 5, 04% | Alprazolam, n = 4, 0.8% | Oxazepam, n = 9, 6% | Flunitrazepam, n = 107, 24% | Zopiclone, n = 11, 15% | |
| Prazepam, n = 4, 0.3% | Diazepam, zolpidem, n = 3, 0.6% | Alprazolam, n = 9, 6% | Zolpidem, n = 101, 23% | Zolpidem, n = 10, 13% | ||
| Antidepressants | 1 (1%) | 10 (1%) | 16 (3%) | 28 (19%) | 179 (40%) | 44 (58%) |
| Amitriptyline n = 1, 1% | Clomipramine, n = 3, 0.2% | Escitalopram, n = 5, 1% | Duloxetine, fluoxetine n = 7, 5% | Escitalopram, n = 58, 13% | Escitalopram, n = 19, 25% | |
| Amitriptyline n = 2, 0.2% | Sertraline, n = 4, 0.8% | Mirtazapine, n = 6, 4% | Duloxetine, n = 54, 12% | Duloxetine, n = 10, 13% | ||
| Imipramine, n = 2, 0.2% | Fluoxetine, n = 3, 0.6% | Mirtazapine, n = 41, 9% | Fluoxetine, Mirtazapine, n = 7, 9% | |||
| Antiparkinsonian agents | 1 (0.1%) | 3 (1%) | 5 (3%) | 29 (7%) | 20 (26%) | |
| Pramipexole, n = 1, 0.1% | Pramipexole, n = 1, 0.2% | Tropatepine, n = 3, 2% | Trihexyphenidyl, n = 15, 3% | L‐dopa, n = 19, 25% | ||
| Ropinirole, n = 1, 0.2% | Trihexyphenidyl, n = 2, 1% | Tropatepine, n = 11, 2% | Pramipexole, n = 4, 5% Apomorphine, ropinirole, rasagiline, amantadine, n = 3, 4% | |||
| Tropatepine, n = 1, 0.2% | Piribedil, n = 1, 0.7% | Ropinirole, pramipexole, L‐dopa, n = 1, 0.2% | ||||
| Opiate maintenance treatments | 5 (3%) | 143 (32%) | 5 (7%) | |||
| Methadone, n = 3, (2%) | High‐dose buprenorphine, n = 104, 23% | High‐dose buprenorphine, n = 3, 4% | ||||
| High‐dose buprenorphine, n = 2, 1% | Methadone, n = 58, 13% | Methadone, n = 2, 3% |
BZD, benzodiazepines
For children less than 6 years of age, 14% were prescribed an antipsychotic drug concomitantly with their MPH treatment. The majority of these treatments were introduced at least 6 months before MPH initiation. Four children (4.7%) had codeine prescribed concomitantly.
For children aged 6–11 and 12–17 years, 8% were prescribed an antipsychotic drug (mostly risperidone) concomitantly with their MPH treatment, and this was mainly introduced at least 6 months before MPH treatment. About 6% were prescribed an anxiolytic agent (mostly hydroxyzine) other than a benzodiazepine (BZD). For young adults (18–24 years of age), about 21% were prescribed BZDs concomitantly, and 19% antidepressants. These treatments were mainly prescribed at least 6 months before MPH initiation.
Patients aged 25–49 years were characterized by higher concomitant use of other CNS drugs. Indeed, 67% were coprescribed BZDs, 40% antidepressants, 39% opioid analgesics, 35% antipsychotic agents and 32% opiate maintenance treatments (high‐dosage buprenorphine for 23% and methadone for 13%).
Patients ≥50 years of age were also characterized by concomitant use of CNS drugs such as BZDs (62%), but more specifically presented the highest concomitant use of antidepressants (58%) and antiparkinsonian medications (26%), which were already in use before the MPH initiation.
For adults, even though MPH is indicated for the second‐line treatment of narcolepsy in cases where modafinil has been found to lack efficacy, only a minority were prescribed modafinil 6 months before MPH initiation: between 2% and 3% for adults under 50 years of age, and 12% for those ≥50 years of age.
Discussion
The present study provides a detailed overview of MPH initiation and use both in children and adults over a 3‐year period in a geographical area in the south of France covering more than 4 million individuals (those covered by the GHIS). The use of MPH increased during the study period (an increase of 7% in the number of incident users between 2010 and 2013), although remained at a lower number in 2013 (29/100 000 inhabitants) than in other European countries 2, 12 (0.3 defined daily dose /1000 inhab/day in France in 2012 vs. 4.53 ddd/1000 inhab/day in the Netherlands as an example). Yet, the prevalence of ADHD has been estimated (using self‐reports) as being higher in France (1.7%) than in other European countries (1.6% in the Netherlands, for example 29). Moreover, a recent study carried out in the same geographical area as used in the present study 37 found that the use of MPH has increased in the past few years by 135% in children and 343% in adult populations, in a context where the use of MPH is already higher in children than in adults 7.
The current pharmacoepidemiological study is rare because it established the characteristics of new users of MPH, irrespective of age, over a 3‐year period, and included dose, duration, concomitant use of other medication, visits to multiple prescribers and the number of dispensings in multiple pharmacies. Thus, evaluation of different age groups revealed that the huge increase in MPH use these over the past few years can have multiple causes. The present analysis demonstrated that the pattern of use for children is consistent with guidelines, with regular visits to physicians, adequate doses and a long duration of treatment. Patterns of use are different for adults, as multiple profiles for adults seem to emerge, and one of them the 25‐49 year olds suggest abusive behaviors.
Children had characteristically higher retention rates and less concomitant use of other psychotropic medication than adults. For children, the mean duration of treatment was longer for those initiating MPH therapy at a young age (6–11 years), while the highest dropout rate after one dispensing was observed for 1–5‐year‐old children (27%). Use of MPH for these preschool children has not been approved and is controversial due to the lack of pertinent data about its safety on the developing brain of these children, and on its efficacy in this population 38. One‐quarter of these preschool children dropped out after one prescription. This may have been the consequence of adverse events 39, 40 or of uncertainty about the diagnosis, but as such data were not available in our database, we could not state this definitively.
Adults represented 34% of incident MPH users, although this agent is not recommended for this population (only one of the ER formulations, Concerta®, is authorized for adult patients for continuation of an effective treatment of ADHD during childhood, and Ritalin® 10 mg for narcolepsy in cases where modafinil has lacked efficacy). Modafinil is rarely prescribed before MPH initiation, leading us to hypothesize that a small proportion of these patients are prescribed MPH for narcolepsy. Compared with children, among whom boys were predominant in terms of MPH use, this gender ratio tended towards 1 after young adulthood, in accordance with studies demonstrating that although boys are more commonly diagnosed with ADHD than girls, when diagnosed for ADHD in young adulthood, women and men are equally likely to be diagnosed 26, 33.
Patterns of MPH use for adults seem to differ according to their age group. The characteristics of 18–24‐year‐old patients were found to be similar to those for 12–17‐year‐old patients, with regular visits to their physician, which may prove the hypothesis that some ADHD patients are diagnosed later, in young adulthood 26. However, they took more comedications (in particular BZDs) and had a lower median drug survival (5.5 months), and the fact that 42% of them were students led us hypothesize that this might reflect misuse by non‐ADHD students, to boost their academic performance and/or induce euphoria (party, social enhancement) 41, 42. However, we cannot disentangle these hypotheses, and it would be particularly interesting to compare patterns of MPH use between students and others in future research.
The 25–49‐year‐old group differed from the other adults. Firstly, this group was characterized by lower retention rates, with almost half having used MPH only once. Moreover, for patients with multiple dispensings, we observed higher quantities dispensed, a higher number of different physicians and/or pharmacies visited, less time between two dispensings and higher rates of concomitant use of other psychoactive drugs, with BZDs being the most frequently used. These observed characteristics may illustrate distinct behaviours of MPH misuse related to comorbid psychiatric disorders and SUD. Our first hypothesis is that MPH is used by adults with ADHD who have SUD or another psychiatric disorder concomitantly. Indeed, this 25–49‐year‐old group had a higher prevalence of use of antidepressants, antipsychotic agents and opioid maintenance treatment (OMT) prior to MPH initiation, which has already been observed in other studies 14, 17, and these other medications may be used to treat these comorbidities. The prevalence of comorbid psychiatric disorders in this age group, particularly depression, anxiety, mood and SUD 34, 43, 44, 45 is high, which also makes diagnosis and treatment challenging. Moreover, this raises concerns about the safety and impact of MPH on comorbid mental health problems associated with ADHD in adults, and the literature on this topic is controversial 20, 44, 46, 47. It is possible that the higher dropout rate (46%) after one dispensing in 25–49‐year‐old patients is due to side effects related to these other comorbidities after the first administration of MPH. In particular, MPH is contraindicated for some psychiatric comorbidities, such as severe depression, psychotic symptoms, severe mood disorders, mania, schizophrenia and psychopathic/borderline personality disorders. It would have been interesting to analyse medications introduced after the discontinuation of MPH treatment. Concerning patients with SUD in particular, it has been demonstrated that ADHD is highly prevalent in treatment‐seeking patients with an SUD (23.1% in a recent meta‐analysis 48) and that SUD patients required higher doses of MPH 49. In our 25–49‐year‐old group, we observed higher doses of MPH and also a higher percentage of OMT. The literature concerning MPH treatment for OMT patients is scarce, and conclusions concerning evidence for the benefits of treatment with ADHD drugs in patients with SUD have been divergent 46, 47, 50, 51, 52. It is also possible that a high proportion of patients drop out after one dispensing because of a lack of efficacy of MPH. Yet, even if MPH medications could be effective in treating ADHD in patients with SUD, close monitoring would be necessary to ensure that these stimulants are used in a therapeutic manner and to see how MPH ultimately affects, or not, the patient's SUD 46.
These considerations on MPH use for patients suffering from SUD are related to the potential for abuse associated with this drug. The second hypothesis concerning these particular characteristics of MPH use in the 25–49‐year‐old group is related to the possible use of MPH by patients who did not have ADHD but suffered from SUD and may have been abusing MPH 53. The potential for abuse and addiction to this drug, which is increasingly misused, has now been clearly established, and some of the characteristics observed in the population studied here may have been related to particular behaviours linked to drug abuse. Indeed, in this subpopulation, a non‐negligible proportion of adults resorted to the IR formulation, which has already been linked to diversion 1. They also used flunitrazepam (24%) or morphine sulphate (22%), which are both potential drugs of abuse 54, 55, and visited a larger number of different physicians, reinforcing the hypothesis of a possible diversion of MPH by ‘doctor shopping’, which consists of obtaining overlapping prescriptions by two or more prescribers 56. This behaviour has, in particular, already been characterized in the PACA‐Corse region (the same geographical region as investigated in our study), with an estimated increase of 6% in the overall quantities of MPH obtained by diversion through doctor shopping in 2011 1. The abuse of MPH has also been reported in other countries 57, 58, 59, with people using it as a cognitive enhancer and/or for recreational purposes 1, 42, 58, 59, 60, 61, 62, 63, particularly in association with the intravenous route of administration 1, 59, 64. In a recent study carried out in France in subjects abusing MPH 1, a few patients reported a history of ADHD and some of them reported using other CNS drugs concomitantly with MPH, to manage symptoms during ‘comedown’, such as anxiety, tiredness, paranoia, feeling sick or depression. The growing availability of the drug due to its increasing use for medical purposes may be a factor influencing its abuse by non‐ADHD patients. Indeed, the availability of prescription drugs has been demonstrated to be linked to the nonmedical use and abuse of these medications with abuse potential 65, 66. There have been serious concerns about the relationship between the increase in the consumption of stimulants (including MPH) in the USA and a possible diversion 67. Increased clinician awareness is essential in helping to reduce prescription drug abuse, while continuing to provide effective treatment. The results observed in the older adult group (>50 years) suggested that they consumed MPH for a shorter period of time (median drug survival 3.4 months) and had a poorer health status (61% were affected by a chronic disease, the nature of which was unknown to us). The fact that they had high rates of comedication with other CNS drugs (mostly BZDs and antidepressants), large increases in BZD use after MPH initiation, and the highest rate of use of antiparkinsonian medications (26%) raises questions about the medical context of MPH use. Indeed, this may be related to experimental MPH use for a variety of diseases (depressive symptoms, fatigue, apathy), as an adjuvant to narcotic analgesics and for cognitive slowing in populations such as medically ill older adults and patients receiving palliative care, as previously described in the literature 30, 32, 61, 62.
The present study had some limitations. As we used a medico‐administrative database, clinical data were not available to cross‐refer to information on the indication for MPH, the severity of the illness, or socioeconomic factors (such as educational levels, with the exception of the student status, depending on the student reimbursement plan). Yet, all of these factors have been proved to be linked to the persistence of MPH use for ADHD, contributing to the effectiveness of this drug for this condition 68. In our study, in which the persistence of MPH use was measured throughout the duration of treatment, until its first interruption (with 90 days allowed between two dispensings), the results were consistent with those from another study from northern Europe 20, involving young users (and a longer duration of treatment). Sensitivity analyses extending the interval allowed between prescription fillings to 365 days, or reducing it to 45 days, provided the same conclusions, but with different values for median drug survival (see Table S1). For this analysis of treatment retention, only the first treatment episode for each person was included – i.e. a person refilling a prescription after more than 90 days of interruption was not reintroduced into the analysis. Another perspective would be to analyse multiple sequences of MPH treatment per patient, involving different doses and comedications. In our study, we could not determine the reasons why patients interrupted their treatment, as we did not have access to a hospitalization database to investigate acute adverse events, and did not know if any patients died. Such studies will be done, using the exhaustive database linking hospitalizations, deaths and the dispensing of medications throughout the whole of France.
Another limitation was that the database we used did not include the entire French population, but only inhabitants of a French region in the south of France who were covered by the GHIS, so we could not consider the results to be fully representative for the whole of France. Nevertheless, we estimated that there were 27 new users per 100 000 people in 2010, and an analysis on another French sample estimated 21 incident users per 100 000 people 69, which was reasonably similar. Moreover, self‐employed people were not included into the present study, and we do not know if these patients tends to consume more or less MPH. We defined incident patients as those with no dispensings of MPH during a previous six‐month period. A sensitivity analysis using a threshold of 1 year with no previous dispensings of MPH provided the same results (Table S2). Nevertheless, further investigation is needed to quantify the proportion of adults considered as incident MPH users who had already been prescribed MPH during childhood.
In conclusion, MPH use in France has increased both in young and adult patients, although the prevalence of MPH use seems to be lower in France compared with that in other European countries and in north America. We have demonstrated different patterns of use across age groups, highlighting long‐term use for children, use in medical conditions other than ADHD for adults and possible drug abuse/misuse behaviours, in particular for 25–49‐year‐old patients. These different patterns imply that various strategies are needed, in order to minimize the risk of adverse events, in particular in older people, and also the risk of abuse.
Competing Interests
There are no competing interests to declare.
We thank Mme Eleonore Ronfle, Mr François Natali, Mr Reggio and Mme Banide from the French GHIS medical department, for their technical help with data extraction, and Mme Giocanti Adeline for data management.
Contributors
V.P., E.F., L.M. and MM conceived the study. All authors critically reviewed the study protocol. V.P., E.F. and Q.B. analysed the data. V.P. performed the literature review. J.M./M.M. and L.M. had an invaluable role in the interpretation of the data. All coauthors provided critical revisions of the manuscript. V.P. was responsible for drafting the manuscript and incorporating the suggestions of the coauthors, and is the guarantor of the manuscript. The lead author certifies that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any deviation from the study as planned (and, if relevant, registered) has been explained.
Supporting information
Table S1 Sensitivity analyses for retention rates with various definitions of time allowed between two dispensings
Table S2 Follow‐up of incident methylphenidate users between 1 January 2011 and 30 June 2013: characteristics of users
Pauly, V. , Frauger, E. , Lepelley, M. , Mallaret, M. , Boucherie, Q. , and Micallef, J. (2018) Patterns and profiles of methylphenidate use both in children and adults. Br J Clin Pharmacol, 84: 1215–1227. doi: 10.1111/bcp.13544.
References
- 1. Frauger E, Amaslidou D, Spadari M, Allaria‐Lapierre V, Braunstein D, Sciortino V, et al Patterns of methylphenidate use and assessment of its abuse among the general population and individuals with drug dependence. Eur Addict Res 2015; 22: 119–126. [DOI] [PubMed] [Google Scholar]
- 2. Micallef J, Frauger E, Palmaro A, Boucherie Q, Lapeyre Mestre M. Example of an investigation of an ‘emergent’ phenomenon in addiction vigilance: the case of methylphenidate. Therapie 2015; 70: 191–202. [DOI] [PubMed] [Google Scholar]
- 3. Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, et al Trends in the parent‐report of health care provider‐diagnosed and medicated attention‐deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry 2014; 53: 34–46.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karanges EA, Stephenson CP, McGregor IS. Longitudinal trends in the dispensing of psychotropic medications in Australia from 2009–2012: focus on children, adolescents and prescriber specialty. Aust N Z J Psychiatry 2014; 48: 917–931. [DOI] [PubMed] [Google Scholar]
- 5. Renoux C, Shin J‐Y, Dell'Aniello S, Fergusson E, Suissa S. Prescribing trends of attention‐deficit hyperactivity disorder (ADHD) medications in UK primary care, 1995–2015. Br J Clin Pharmacol 2016; 82: 858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trip A‐M, Visser ST, Kalverdijk LJ, de Jong‐van den Berg LTW. Large increase of the use of psycho‐stimulants among youth in the Netherlands between 1996 and 2006. Br J Clin Pharmacol 2009; 67: 466–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ANSM . Agence nationale de sécurité du médicament et des produits de santé. Méthylphénidate : données d'utilisation et de sécurité d'emploi en France. 2017. [French National Safety Agency on drugs and health products]. Available at http://ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Methylphenidate-donnees-d-utilisation-et-de-securite-d-emploi-en-France-Point-d-Information (last accessed 14 March 2018).
- 8. Boland F, Galvin R, Reulbach U, Motterlini N, Kelly D, Bennett K, et al Psychostimulant prescribing trends in a paediatric population in Ireland: a national cohort study. BMC Pediatr 2015; 15: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inglis SK, Carucci S, Garas P, Häge A, Banaschewski T, Buitelaar JK, et al Prospective observational study protocol to investigate long‐term adverse effects of methylphenidate in children and adolescents with ADHD: the Attention Deficit Hyperactivity Disorder Drugs Use Chronic Effects (ADDUCE) study. BMJ Open 2016; 6: e010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pottegård A, Bjerregaard BK, Glintborg D, Hallas J, Moreno SI. The use of medication against attention deficit hyperactivity disorder in Denmark: a drug use study from a national perspective. Eur J Clin Pharmacol 2012; 68: 1443–1450. [DOI] [PubMed] [Google Scholar]
- 11. Pottegård A, Bjerregaard BK, Glintborg D, Kortegaard LS, Hallas J, Moreno SI. The use of medication against attention deficit/hyperactivity disorder in Denmark: a drug use study from a patient perspective. Eur J Clin Pharmacol 2013; 69: 589–598. [DOI] [PubMed] [Google Scholar]
- 12. Zoëga H, Furu K, Halldórsson M, Thomsen PH, Sourander A, Martikainen JE. Use of ADHD drugs in the Nordic countries: a population‐based comparison study. Acta Psychiatr Scand 2011; 123: 360–367. [DOI] [PubMed] [Google Scholar]
- 13. Safer DJ, Malever M. Stimulant treatment in Maryland public schools. Pediatrics 2000; 106: 533–539. [DOI] [PubMed] [Google Scholar]
- 14. Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. JAMA 2000; 283: 1025–1030. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt‐Troschke SO, Ostermann T, Melcher D, Schuster R, Erben CM, Matthiessen PF. The use of methylphenidate in children: analysis of prescription usage based in routine data of the statutory health insurance bodies concerning drug prescriptions. Gesundheitswesen Bundesverb Arzte Offentlichen Gesundheitsdienstes Ger 2004; 66: 387–392. [DOI] [PubMed] [Google Scholar]
- 16. Faber A, Kalverdijk LJ, de Jong‐van den Berg LTW, Hugtenburg JG, Minderaa RB, Tobi H. Co‐morbidity and patterns of care in stimulant‐treated children with ADHD in the Netherlands. Eur Child Adolesc Psychiatry 2010; 19: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karlstad Ø, Zoëga H, Furu K, Bahmanyar S, Martikainen JE, Kieler H, et al Use of drugs for ADHD among adults‐a multinational study among 15.8 million adults in the Nordic countries. Eur J Clin Pharmacol 2016; 72: 1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hartz I, Skurtveit S, Steffenak AKM, Karlstad Ø, Handal M. Psychotropic drug use among 0–17 year olds during 2004–2014: a nationwide prescription database study. BMC Psychiatry 2016; 16: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knellwolf A‐L, Deligne J, Chiarotti F, Auleley G‐R, Palmieri S, Boisgard CB, et al Prevalence and patterns of methylphenidate use in French children and adolescents. Eur J Clin Pharmacol 2008; 64: 311–317. [DOI] [PubMed] [Google Scholar]
- 20. Pottegård A, Bjerregaard BK, Kortegaard LS, Zoëga H. Early discontinuation of attention‐deficit/hyperactivity disorder drug treatment: a Danish nationwide drug utilization study. Basic Clin Pharmacol Toxicol 2015; 116: 349–353. [DOI] [PubMed] [Google Scholar]
- 21. Preen DB, Calver J, Sanfilippo FM, Bulsara M, Holman CDJ. Patterns of psychostimulant prescribing to children with ADHD in Western Australia: variations in age, gender, medication type and dose prescribed. Aust N Z J Public Health 2007; 31: 120–126. [DOI] [PubMed] [Google Scholar]
- 22. Giacobini M, Medin E, Ahnemark E, Russo LJ, Carlqvist P. Prevalence, patient characteristics, and pharmacological treatment of children, adolescents, and adults diagnosed with ADHD in Sweden. J Atten Disord 2018; 22: 3–13. [DOI] [PubMed] [Google Scholar]
- 23. McCarthy S, Asherson P, Coghill D, Hollis C, Murray M, Potts L, et al Attention‐deficit hyperactivity disorder: treatment discontinuation in adolescents and young adults. Br J Psychiatry 2009; 194: 273–277. [DOI] [PubMed] [Google Scholar]
- 24. Adis Medical Writers . Pharmacological treatment of attention‐deficit hyperactivity disorder in preschool‐age children requires care. Drugs Ther Perspect 2013; 29: 241–244. [Google Scholar]
- 25. Raman SR, Marshall SW, Gaynes BN, Haynes K, Naftel AJ, Stürmer T. An observational study of pharmacological treatment in primary care of children with ADHD in the United Kingdom. Psychiatr Serv 2015; 66: 617–624. [DOI] [PubMed] [Google Scholar]
- 26. Faraone SV, Biederman J, Mick E. The age‐dependent decline of attention deficit hyperactivity disorder: a meta‐analysis of follow‐up studies. Psychol Med 2006; 36: 159–165. [DOI] [PubMed] [Google Scholar]
- 27. Geirs DP, Pottegård A, Halldórsson M, Zoëga H. A nationwide study of attention‐deficit/hyperactivity disorder drug use among adults in Iceland 2003‐2012. Basic Clin Pharmacol Toxicol 2014; 115: 417–422. [DOI] [PubMed] [Google Scholar]
- 28. Devos D, Moreau C, Delval A, Dujardin K, Defebvre L, Bordet R. Methylphenidate: a treatment for Parkinson's disease? CNS Drugs 2013; 27: 1–14. [DOI] [PubMed] [Google Scholar]
- 29. Fayyad J, De Graaf R, Kessler R, Alonso J, Angermeyer M, Demyttenaere K, et al Cross‐national prevalence and correlates of adult attention‐deficit hyperactivity disorder. Br J Psychiatry 2007; 190: 402–409. [DOI] [PubMed] [Google Scholar]
- 30. Prommer E. Methylphenidate: established and expanding roles in symptom management. Am J Hosp Palliat Care 2012; 29: 483–490. [DOI] [PubMed] [Google Scholar]
- 31. Hardy SE. Methylphenidate for the treatment of depressive symptoms, including fatigue and apathy, in medically ill older adults and terminally ill adults. Am J Geriatr Pharmacother 2009; 7: 34–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sinita E, Coghill D. The use of stimulant medications for non‐core aspects of ADHD and in other disorders. Neuropharmacology 2014; 87: 161–172. [DOI] [PubMed] [Google Scholar]
- 33. Kooij SJJ, Bejerot S, Blackwell A, Caci H, Casas‐Brugué M, Carpentier PJ, et al European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry 2010; 10: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Emmerik‐van Oortmerssen K, van de Glind G, Koeter MWJ, Allsop S, Auriacombe M, Barta C, et al Psychiatric comorbidity in treatment‐seeking substance use disorder patients with and without attention deficit hyperactivity disorder: results of the IASP study. Addiction 2014; 109: 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frances C, Hoizey G, Millart H, Trenque T. Paediatric methylphenidate (Ritalin) restrictive conditions of prescription in France. Br J Clin Pharmacol 2004; 57: 115–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2017 [online]. Available at https://www.whocc.no/atc_ddd_index/ (last accessed 13 January 2017).
- 37. Ehrhardt C, Boucherie Q, Pauly V, Braunstein D, Ronflé E, Thirion X, et al Methylphenidate: gender trends in adult and pediatric populations over a 7 year period. Therapie 2017; 72: 635–641. [DOI] [PubMed] [Google Scholar]
- 38. Ghuman JK, Ghuman HS. Pharmacologic intervention for attention‐deficit hyperactivity disorder in preschoolers: is it justified? Pediatr Drugs 2013; 15: 1–8. [DOI] [PubMed] [Google Scholar]
- 39. Hawcutt DB, Mainie P, Riordan A, Smyth RL, Pirmohamed M. Reported paediatric adverse drug reactions in the UK 2000–2009. Br J Clin Pharmacol 2012; 73: 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wigal T, Greenhill L, Chuang S, McGOUGH J, Vitiello B, Skrobala A, et al Safety and tolerability of methylphenidate in preschool children with ADHD. J Am Acad Child Adolesc Psychiatry 2006; 45: 1294–1303. [DOI] [PubMed] [Google Scholar]
- 41. Bagot KS, Kaminer Y. Efficacy of stimulants for cognitive enhancement in non‐attention deficit hyperactivity disorder youth: a systematic review. Addiction 2014; 109: 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Teter CJ, McCabe SE, Cranford JA, Boyd CJ, Guthrie SK. Prevalence and motives for illicit use of prescription stimulants in an undergraduate student sample. J Am Coll Health 2005; 53: 253–262. [DOI] [PubMed] [Google Scholar]
- 43. Retz W, Retz‐Junginger P. Prediction of methylphenidate treatment outcome in adults with attention‐deficit/hyperactivity disorder (ADHD). Eur Arch Psychiatry Clin Neurosci 2014; 264 (Suppl. 1): 35–43. [DOI] [PubMed] [Google Scholar]
- 44. Cunill R, Castells X, Tobias A, Capellà D. Pharmacological treatment of attention deficit hyperactivity disorder with co‐morbid drug dependence. J Psychopharmacol 2015; 29: 15–23. [DOI] [PubMed] [Google Scholar]
- 45. Wilens TE, Morrison NR, Prince J. An update on the pharmacotherapy of attention‐deficit/hyperactivity disorder in adults. Expert Rev Neurother 2011; 11: 1443–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pérez de los Cobos J, Siñol N, Pérez V, Trujols J. Pharmacological and clinical dilemmas of prescribing in co‐morbid adult attention‐deficit/hyperactivity disorder and addiction. Br J Clin Pharmacol 2014; 77: 337–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Karlstad Ø, Furu K, Skurtveit S, Selmer R. Prescribing of drugs for attention‐deficit hyperactivity disorder in opioid maintenance treatment patients in Norway. Eur Addict Res 2014; 20: 59–65. [DOI] [PubMed] [Google Scholar]
- 48. Oortmerssen K van E, van de Glind G, van den Brink W, Smit F, Crunelle CL, Swets M, et al Prevalence of attention‐deficit hyperactivity disorder in substance use disorder patients: a meta‐analysis and meta‐regression analysis. Drug Alcohol Depend 2012; 122: 11–19. [DOI] [PubMed] [Google Scholar]
- 49. Skoglund C, Brandt L, D'Onofrio B, Larsson H, Franck J. Methylphenidate doses in attention deficit/hyperactivity disorder and comorbid substance use disorders. Eur Neuropsychopharmacol 2017; 27: 1144–1152. [DOI] [PubMed] [Google Scholar]
- 50. Mariani JJ, Levin FR. Treatment strategies for co‐occurring ADHD and substance use disorders. Am J Addict 2007; 16 (Suppl. 1): 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Levin FR, Evans SM, Brooks DJ, Kalbag AS, Garawi F, Nunes EV. Treatment of methadone‐maintained patients with adult ADHD: double‐blind comparison of methylphenidate, bupropion and placebo. Drug Alcohol Depend 2006; 81: 137–148. [DOI] [PubMed] [Google Scholar]
- 52. Abel KF, Bramness JG, Martinsen EW. Stimulant medication for ADHD in opioid maintenance treatment. J Dual Diagn 2014; 10: 32–38. [DOI] [PubMed] [Google Scholar]
- 53. National Institute on Drug Abuse (NIDA). The science of drug abuse and addiction: the basics [online]. Available at https://www.drugabuse.gov/publications/media-guide/science-drug-abuse-addiction-basics (last accessed 12 November 2017).
- 54. Pauly V, Pradel V, Pourcel L, Nordmann S, Frauger E, Lapeyre‐Mestre M, et al Estimated magnitude of diversion and abuse of opioids relative to benzodiazepines in France. Drug Alcohol Depend 2012; 126: 13–20. [DOI] [PubMed] [Google Scholar]
- 55. Peyriere H, Nogue E, Eiden C, Frauger E, Charra M, Picot M‐C, et al Evidence of slow‐release morphine sulfate abuse and diversion: epidemiological approaches in a French administrative area. Fundam Clin Pharmacol 2016; 30: 466–475. [DOI] [PubMed] [Google Scholar]
- 56. Rasmussen L, Zoëga H, Hallas J, Pottegård A. Deviant patterns of methylphenidate use in adults: a Danish nationwide registry‐based drug utilization study. Pharmacoepidemiol Drug Saf 2015; 24: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 57. Bruggisser M, Ceschi A, Bodmer M, Wilks MF, Kupferschmidt H, Liechti ME. Retrospective analysis of stimulant abuse cases reported to the Swiss Toxicological Information Centre during 1997–2009. Swiss Med Wkly 2010; 140: w13115. [DOI] [PubMed] [Google Scholar]
- 58. Gahr M, Freudenmann RW, Hiemke C, Kölle MA, Schönfeldt‐Lecuona C. Abuse of methylphenidate in Germany: data from spontaneous reports of adverse drug reactions. Psychiatry Res 2014; 215: 252–254. [DOI] [PubMed] [Google Scholar]
- 59. Bjarnadottir GD, Haraldsson HM, Rafnar BO, Sigurdsson E, Steingrimsson S, Johannsson M, et al Prevalent intravenous abuse of methylphenidate among treatment‐seeking patients with substance abuse disorders: a descriptive population‐based study. J Addict Med 2015; 9: 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Novak SP, Kroutil LA, Williams RL, Van Brunt DL. The nonmedical use of prescription ADHD medications: results from a national Internet panel. Subst Abuse Treat Prev Policy 2007; 2: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav 2001; 68: 611–627. [DOI] [PubMed] [Google Scholar]
- 62. Greely H, Sahakian B, Harris J, Kessler RC, Gazzaniga M, Campbell P, et al Towards responsible use of cognitive‐enhancing drugs by the healthy. Nature 2008; 456: 702–705. [DOI] [PubMed] [Google Scholar]
- 63. Cepeda MS, Fife D, Berwaerts J, Yuan Y, Mastrogiovanni G. Shopping behavior for ADHD drugs: results of a cohort study in a pharmacy database. Drugs R D 2014; 14: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vérité F, Micallef J. Acute psychiatric symptoms during methylphenidate intravenous injections: A case report. Therapie 2017; 72: 367–372. [DOI] [PubMed] [Google Scholar]
- 65. Gerada C, Ashworth M. ABC of mental health. Addiction and dependence–I: illicit drugs. BMJ 1997; 315: 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use, and diversion of abusable prescription drugs. J Am Coll Health 2006; 54: 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Swanson JM, Volkow ND. Increasing use of stimulants warns of potential abuse. Nature 2008; 453: 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. World Helath Organization . Adherence to long‐term therapies. Evidence for action [online]. Available at http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf (last accessed 3 February 2017).
- 69. Agence nationale de sécurité du médicament et des produits de santé . Méthylphénidate: données d'utilisation et de sécurité d'emploi en France [French national Agency on Drugs and Health Products. Methyphenidate: about use and safety data in France in 2013]. Available at http://ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Donnees-d-utilisation-et-mesures-visant-a-securiser-l-emploi-du-methylphenidate-en-France-publication-par-l-ANSM-d-un-rapport-d-analyse-et-d-une-brochure-d-information-a-destination-des-patients-et-de-leur-entourage-Point-d-information (last accessed 7 March 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Sensitivity analyses for retention rates with various definitions of time allowed between two dispensings
Table S2 Follow‐up of incident methylphenidate users between 1 January 2011 and 30 June 2013: characteristics of users


