Abstract
Aims
Saliva, as a matrix, offers many benefits over blood in therapeutic drug monitoring (TDM), in particular for infantile TDM. However, the accuracy of salivary TDM in infants remains an area of debate. This review explored the accuracy, applicability and advantages of using saliva TDM in infants and neonates.
Methods
Databases were searched up to and including September 2016. Studies were included based on PICO as follows: P: infants and neonates being treated with any medication, I: salivary TDM vs. C: traditional methods and O: accuracy, advantages/disadvantages and applicability to practice. Compounds were assessed by their physicochemical and pharmacokinetic properties, as well as published quantitative saliva monitoring data.
Results
Twenty‐four studies and their respective 13 compounds were investigated. Four neutral and two acidic compounds, oxcarbazepine, primidone, fluconazole, busulfan, theophylline and phenytoin displayed excellent/very good correlation between blood plasma and saliva. Lamotrigine was the only basic compound to show excellent correlation with morphine exhibiting no correlation between saliva and blood plasma. Any compound with an acid dissociation constant (pKa) within physiological range (pH 6–8) gave a more varied response.
Conclusion
There is significant potential for infantile saliva testing and in particular for neutral and weakly acidic compounds. Of the properties investigated, pKa was the most influential with both logP and protein binding having little effect on this correlation. To conclude, any compound with a pKa within physiological range (pH 6–8) should be considered with extra care, with the extraction and analysis method examined and optimized on a case‐by‐case basis.
Keywords: infants, paediatric, pharmacokinetic parameters, physicochemical properties, saliva, systematic review, therapeutic drug monitoring
Introduction
Therapeutic drug monitoring (TDM) is defined as measuring serum concentrations of a drug in a single or multiple time points in a biological matrix after a dosage 1. It is especially useful when the relationship between drug plasma concentration and effect is stronger than between drug dosage and effect, and is vital when measuring drugs with a narrow therapeutic range 1, 2 or if concentrations resulting from a given dose are unpredictable due to high inter‐/intrapatient variability 3. Pharmacodynamics and pharmacokinetic factors such as absorption, distribution, metabolism and excretion lead to individual variability 2. This is especially true in young children, who, for example, have a higher rate of development of these processes, which is greatest from birth to 1 month but changes also occur during infancy and again during childhood 2, 4, 5, 6. They tend to have lower protein binding, which increases the free fraction of drugs and a less mature glomerular filtration rate 7, which means drugs may be retained for longer in those aged <6 months, while glucuronidation may not reach adult values until age 3–6 months 5. It is therefore not surprising that validated dosage regimens are limited in this population, with approximately 70% of drugs prescribed to children and >93% prescribed to critically ill neonates being off‐label 8, 9.
The use of serum/plasma in TDM, is well established and the most clinically used method 10, 11. Nevertheless, extensive blood sampling in infants is not practical, or ethical due to pain, anxiety and risk of infection 12. There are a significant number of validated matrices available for TDM in adults 11, 13, 14; however, these are not always appropriate for TDM in infants or neonates, for example tears, sweat and hair due to lack of availability. Table 1 reflects the available matrices for TDM in infants and outlines the advantages and disadvantages of each.
Table 1.
Biological matrices used in infants and neonates for therapeutic drug monitoring – advantages and disadvantages
| Matrix | Advantages | Disadvantages | References |
|---|---|---|---|
| Blood (Plasma/serum) |
• Traditional and well recognized method • Methods can be used to separate the free and bound drug components • Can be collected via indwelling catheters • Large volumes can be obtained • High concentration of drug in specimen |
• Invasive • Painful • Risk of infection • Poor patient compliance • Requires expertise training • Expensive • Excessive blood loss |
18, 26, 84 |
| Blood (dried blood spot) |
• Minimal sample preparation and storage • Minimal expense • Routine sampling done • High compliance • High accuracy in sample collection • Thought to be stable for most drugs at room temperature • Less invasive than venous puncture • Smaller blood volume required • Home monitoring potential |
• Invasive • Painful • Very small blood volume obtained • Restricting timing if kept in line with routine sampling • Training required • Lack of standardized process • Very sensitive analytical methods needed • Highly unstable drugs may degrade during drying process • Haematocrit can affect results |
13, 14 |
| Saliva |
• Noninvasive • Repeat samples easy to get • Represents the free nonprotein‐bound drug concentration in plasma • Inexpensive • Home monitoring potential • Reduced infection risk • Preferred by children and parents • Oral fluid assays are technically easier than plasma due to the lower content of proteins and lipids • Stimulated saliva aids collection and the pH is then very close to that of plasma, which is important when ionized drugs are measured. It also leaves samples less viscous |
• Low sample volume • Highly accurate analytical methods required • Interference from oral consumption of medication • Usefulness primarily determined by the variability of drug ionization over the variation in pH • Stimulation with citric acid may not be suitable in infants and care must be taken as too much can interfere with certain assays • Contraindicated in patients with inflammatory conditions of buccal mucosa or salivary glands • Does not correlate with blood for all drugs • No universal method • Large patient intervariability in ratios |
15, 18, 19, 26, 41, 85 |
| Faeces/meconium |
• Relies on excretion of drugs into bile, which then accumulates in meconium • Can detect substances which have a short half‐life in blood which can prove maternal drug use from 16 weeks' gestation. • Meconium offers a larger window of exposure than urine |
• Meconium only available during the first 2–3 days of life, with the first meconium being the most sensitive | 26 |
| Urine |
• Noninvasive • May be tested as a dried spot • Used to detect drugs or their metabolites • can be used for toxicology screening • 24‐h analysis useful for heavy metal detection • Useful to detect renal clearance of drugs and drugs in combination • High concentration of drug in specimen |
• Catheters pose infection risk • Not all drugs can be detected • Difficult to collect timed, quantitative samples from infants • Drugs can only be detected for 2–3 days after use |
1, 26 |
Technological advances in TDM in the past 4 decades have led to saliva testing becoming more popular in the diagnosis of diseases, disease progression and detection of drugs 15, 16. It has become a valuable clinical method due to its noninvasive nature, no need for specialist training or equipment, patient preference and the ability to compare easily with plasma concentrations 16, 17. This is because most compounds found in blood are also present in saliva. In infants, this method seems optimal to prevent distress and ensure patient safety 18. If there is a consistent comparison between saliva and plasma, and if the biological response to the drug is proportionate to its plasma concentration, then salivary concentrations may provide a valuable measurement of TDM.
Several studies have been conducted in adults in regard to saliva TDM, with it being a proven accurate method in relation to many drugs including artemisinin 19, digoxin 20, lamotrigine 21 and a number of other antiepileptic drugs 22. However, these reference ranges do not automatically transfer to infants, and the accuracy of salivary TDM in infants is still an area of debate 23, 24, with confusion between collection methods and simulation vs. no stimulation.
The number of studies looking at TDM involving infants is low, primarily due to poor consent rates, limited blood availability, low volume drug concentration assays and a lack of expertise in this area 25. Studies in paediatrics are usually disease orientated and expensive, as drug companies are unable to recover costs in this limited population 26. Consequently, there is a need for novel and accurate study designs that reduce these barriers: saliva TDM may meet these requirements.
This review aims to explore the accuracy, applicability and advantages of using saliva TDM in infants and neonates.
Methods
The review question was defined using PICO as follows: P: infants and neonates being treated with any medication. An infant is defined by Medline as a child aged between 1 and 23 months 27 and a neonate as an infant during the first 28 days after birth. For the purpose of this review, the term infant has been used to include all those aged <24 months. I: salivary TDM vs. C: traditional methods and O: accuracy, advantages/disadvantages and applicability to practice.
Search methods for identification of studies
A search strategy was defined with the help of the subject librarian and was adapted according to each database. Five databases: Medline, Embase, CINAHL, PubMed and Scopus were searched, along with The Cochrane Library and Google Scholar up to and including September 2016. There were 321 potential studies, following removal of duplicates and screening by two members of the research team by title and abstract, 24 were eligible for full paper review. In all cases the primary sources were used unless no subsequent peer reviewed article was published. Review articles of importance were reviewed. The search terms used in Medline are detailed in Figure 1. The search terms used included therapeutic drug monitoring, saliva*, infant and their numerous variations. We also scrutinized reference lists for those which met the criteria.
Figure 1.

Medline search strategy
The saliva/plasma concentration ratio (S/P) is a very useful parameter when assessing drugs contained in the saliva. This ratio is determined by a number of physiological factors that affect the passage of drug from blood to saliva. These include the pH of the oral fluid and plasma, the saliva flow rate and the pathophysiology of the oral cavity 28. With the exception of salivary pH, most of these remain constant. The primary drug properties that also play a key role in the passage of drugs into saliva include the acid dissociation constant (pKa) of the drug, its molecular weight, spatial configuration and lipid solubility of the analyte, and degree of protein binding in plasma and saliva 15. Once identified, compounds were assessed by their physicochemical and pharmacokinetic properties, as well as published quantitative saliva monitoring data.
Results
Thirteen different compounds were considered suitable for inclusion in this review. The compounds chosen were categorized as being acidic (n = 4), basic (n = 3) or neutral (n = 6). In the case of an amphoteric compound, such as morphine, it was included in the category to which it has the highest ionization potential, in this case basic. Acidic compounds were deemed to have a pKa ≤ 10, and basic compounds were considered as having a pKa ≥ 4.0. Any pKa value outside of this range (whether acidic or basic) would not be substantially affected by the physiological pH range of saliva (≤0.1% ionized) and was thereby categorized as neutral. Table 2 displays the compounds assessed in this review and includes the chemical structure, indications and both the physicochemical and pharmacokinetic parameters.
Table 2.
Compounds for infant and neonate therapeutic drug monitoring in saliva with chemical and biological data
| Compound | Therapeutic indication a | Structure | Physicochemical properties b | Pharmacokinetic properties b | Salt | ||||
|---|---|---|---|---|---|---|---|---|---|
| MW | LogP | Pkac | Half‐life (h) | % Protein bindingb | |||||
| Acidic |
Digoxin
C41H64O14 |
Arrhythmias Chronic heart failure |
|
780.9 | 2.37 | 7.15 | 84–120 | 25 | |
|
Phenobarbital
C12H12N2O3 |
Antiepileptic |
|
232.2 | 1.47 | 7.3 | 53–118 | 20–45 | ||
|
Phenytoin
C15H12N2O2 |
Antiepileptic |
|
252.3 | 2.1 | 8.3 | 22 | 90 | ✓ | |
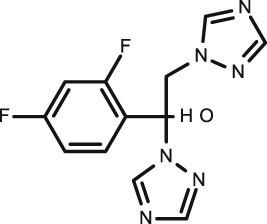
|
Theophylline
C7H8N4O2 |
Neonatal apnoea Asthma |
|
180.2 | –0.77 | 8.81 | 8 | 40 | ||
| Basic |
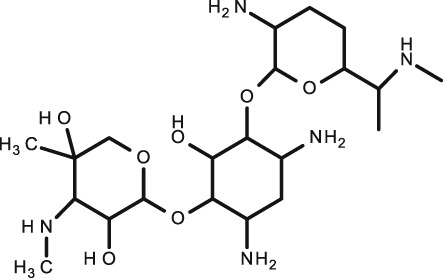
Gentamicin
C21H43N5O7 |
Bacterial infections |
|
477.6 | –3.1 |
10.18 (base) 12.55 (acid) |
3–5½ | 0–30 | ✓ |
|
Morphine
C17H19NO3 |
Analgesic |
|
285.3 | 0.89 |
9.12 (base) 10.26 (acid) |
2–4 | 30–40 | ✓ | |
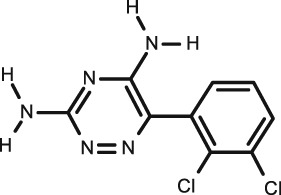
|
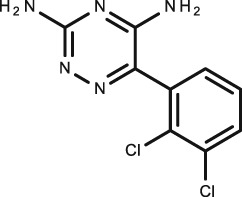
Lamotrigine
C9H7Cl2N5 |
Antiepileptic |
|
256.1 | 1.93 | 5.87 | 25 | 55 | ||
| Neutral |
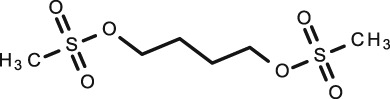
Busulfan
C6H14O6S2 |
Conditioning treatment before haematopoietic progenitor cell transplantation |
|
246.3 | –0.76 | N/A | 2.6 | 32 | |
|
Caffeine
C8H10N4O2 |
Neonatal apnoea |
|
194.2 | –0.55 | –0.92 (base) | 65–130 | 25–36 | ✓ | |
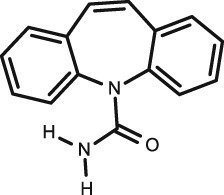
|
Carbamazepine
C15H12N2O |
Antiepileptic |
|
236.3 | 2.8 | 15.9 (acid) | 25–65 | 76 | ||
|
Fluconazole
C13H12F2N6O |
Fungal infections |
|
306.3 | 0.56 |
12.71 (acid) 2.56 (base) |
30 | 11–12 | ||
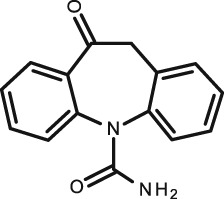
| oxcarbazepine C 15 H 12 N 2 O 2 | Antiepileptic |
|
252.3 | 1.82 | 12.92 (acid) | Active metabolite 9 h | 40 | ||
|
Primidone
C12H14N2O2 |
Antiepileptic |
|
218.3 | 1.12 | 11.5 (acid) | 3–23 | 70 | ||
British National Formulary for children (online version)
Drug bank
British Pharmacopeia (on‐line version)
Nature of dissociation given in parenthesis, with base referring to the pKa of the conjugate acid.
As the various authors have described the correlation between the experimental blood plasma levels of a compound and that of saliva levels in different manners, for the purpose of this review, the correlation R value has been categorized as either excellent, very good, good, fair or poor. Tables 3, 4, 5 summarize the information on each of the 13 compounds.
Table 3.
Acidic drugs assessed for salivary therapeutic drug monitoring in infants and neonates
| compound | Correlation a | Patients | Storage conditions | Collection details | Analysis method | Stimulated | R | Saliva/plasma correlation ratio | Reference | Saliva/plasma correlation ratio range in adults (nonconclusive) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Theophylline
IV or oral |
Excellent | 31 children 2–17.5 years | Samples stored at 4°C until analysis | Samples collected 7 h after dose Saliva: spitting into container Blood: venous | EMIT |
Y 1 N 2 |
P < 0.001 1–0.98 2.0.97 |
N/A | 30 | 0.46–0.69 | 34, 86, 87, 88, 89, 90 |
| Excellent | 8 premature neonates | Stored at 4°C for up to 48 h before analysis | Saliva: suctioning with syringe Blood: heel prick | HPLC | N | 0.98 P < 0.01 | 0.98 | 29 | |||
| Excellent | 13 neonates <38 weeks | Frozen at –20°C for 48–72 h before analysis | Samples collected 2–4 h after dose Saliva: aspiration Blood: venous | MS | Y | 0.973 P < 0.01 | 0.65 | 32 | |||
| Excellent | 13 infants <24 months | N/A | Samples collected 2 and 8 h after dosing. Saliva: aspiration Blood: venous | GC–MS | Y | 0.97 | 0.69 | 31 | |||
| Fair | 10 premature neonates | Frozen until analysis | Samples collected 2–3 times 4–6 h after dosing Saliva: suctioning with a rubber ear syringe. Blood: venous | HPLC | N | 0.70 ± 2.8 | 0.80 | 23 | |||
|
Phenytoin
oral |
Excellent | 38 patients (8 months–62 years). Number of infants not defined | N/A | Saliva: spiting/capillary‐pipette‐dilution system. Blood: venous | RIA | N | 0.98 P < 0.001 | 0.101 ± 0.003 | 41 | 0.09–0.13 | 22, 28, 41, 47, 48, 71, 72, 91, 92, 93, 94, 95 |
| Excellent/ Good | 202 children (5 months–18 years). | Frozen until analysis | Saliva: mucous trap suction device Blood: venous |
1. GLC 2. EMIT |
N |
1. 0.94 2. 0.86 |
0.11 ± 0.03 | 42 | |||
| Excellent | 16 children (1.5–15 years) | Fridge for up to 24 h before analysis | Water rinsed around mouth before samples taken Samples taken 1–24 h after dosing Saliva: syringe Blood: venous | FPIA | Y | 0.99 P < 0.01 | 0.10 | 44 | |||
| Excellent | 17 infants (<24 months) | N/A | Samples taken at 3 weeks, 6 weeks and 3 month intervals for 1 year. 30 min–9 h after dosing Saliva: aspiration with mucous extractor. Blood: N/A | GC | Y | 0.98 | 0.13 ± 0.01 | 43 | |||
|
Phenobarbital
oral |
Excellent | 38 patients (8 months–62 years) | N/A | Saliva: spitting/capillary‐pipette‐dilution system Blood: N/A | RIA | N | 0.98 P < 0.001 | 0.3 | 41 | 0.27–0.41 | 28, 41, 47, 48, 53, 71, 72, 94, 96, 97 |
| Very Good |
15 infants <24 months (age groups: 5 months–2 years, 2‐6 years, 6–12 years and 12–18 years) |
frozen | Saliva: mucous trap suction device Blood: venous | GLC/EMIT | N |
GLC‐0.94 EMIT‐0.92 |
0.32 ± 0.06 | 42 | |||
| N/A | 19 infants <24 months | N/A | Samples collected 30 min–9 h after dosing Saliva: N/A Blood: N/A | GC | Y | N/A | 0.22 | 43 | |||
| Poor | 32 patients (0.3–17 years) | Frozen until analysis | Mouths rinsed before sampling Saliva: aspiration with a syringe Blood: venous | HPLC | Y | 0.65 P < 0.001 | N/A | 50 | |||
|
Digoxin
oral |
Good | 11 children (9–24 months) | N/A | Samples collected at trough Saliva: syringe‐ mouths rinsed Blood: N/A | FPIA | Y | 0.87 | N/A | 24 | 0.60–0.87 | 20, 98, 99, 100, 101, 102, 103 |
| Good | 18 children (9–24 months) | Refrigerated at 4°C for up to 24 h | Samples collected 10–12 h after dosing, mouths rinsed Saliva: mucous extractor Blood: venous | FPIA | Y | 0.83 P < 0.05 | 0.35 | 54 | |||
| Fair | 12 infants (1–12 months) | Plasma stored at –18°C, saliva stored at 4°C | Samples collected 6 h after previous dose Saliva: syringe Blood: N/A | RIA digoxin Kit | N | 0.71 (P < 0.001) | 0.66 ± 0.20 | 55 |
Correlation R value: excellent >0.95; very good >0.90; good >0.80; fair >0.70; poor <0.70
EMIT, enzyme multiplied immunoassay technique; FPIA, fluorescence polarization immunoassay; GC, gas chromatography; GLC, gas–liquid chromatography; HPLC, high‐pressure liquid chromatography; IV, intravenous; MS, mass spectrometry; N/A, not applicable; RIA, radioimmunoassay
Table 4.
Basic drugs assessed for salivary therapeutic drug monitoring in infants
| Compound | Correlation a | Patients | Storage Conditions | Collection details | Analysis method | Stimulated | R | Saliva/Plasma correlation ratio | Reference | Saliva/Plasma correlation ratio range in adults (Nonconclusive) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lamotrigine oral | Excellent | 20 children (1–16 years) | N/A | Saliva: N/A Blood: venous | HPLC | Y/N: stimulation by piece of polymer not possible in infant | R = 0.9841 P < 0.0001 | 0.49. | 60 | 0.40–0.56 | 59, 60, 104, 105, 106 |
| Good | 1 patient <24 months | frozen at –20°C | Samples taken 3 h after dosing Saliva: suction with a pipette Blood: venous | HPLC/HPLC‐UV | N | Two separate laboratories: 0.81/0.86 P < 0.05 | 0.62 | 59 | |||
| Gentamycin IV | Good | 10 children (5 < 24 months) | N/A | Samples taken before next dose Saliva: syringe Blood: N/A | FPIA | Y | 0.89 P < 0.0001 | N/A | 24 | No available data | |
| Morphine IV | Poor | 15 children (some <24 months) | Frozen at –20°C until analysis | Mouths rinsed if taking any oral medication Saliva: mucous extractor Blood: venous | solid phase serum morphine RIA | Y | 0.04 P = 0.89 | 2.28 ± 2.84. | 58 | No available data | |
Correlation R value: excellent >0.95; very good >0.90; good >0.80; fair >0.70; poor <0.70
FPIA, fluorescence polarization immunoassay; HPLC, high‐pressure liquid chromatography; IV, intravenous; RIA, radioimmunoassay
Table 5.
Neutral drugs assessed for salivary therapeutic drug monitoring in neonates and infants
| Compound | Correlation a | Patients | Storage Conditions | Collection details | Analysis method | Stimulated | R | Saliva/plasma correlation ratio | Reference | Saliva/plasma correlation ratio range in adults (nonconclusive) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Busulfan oral | Excellent | 10 children (1–24 months) | Frozen immediately –25°C | Samples collected 0–360 min after dose administration Saliva: modified medical pacifier Blood: venous | LC–MS/MS | N | 0.958 | 1.09 | 61 | No available data | |
| Fluconazole oral or IV | Excellent | 19 children (10–24 months) |
Frozen ‐20°C |
Samples collected at trough levels Saliva: suction device Blood: N/A | LC–MS–MS | N | 0.96 P < 0.1 | 1.0 | 62 | 0.76–1.16 | 107, 108 |
| Primidone oral | Excellent/Good | 202 children (5 months–18 years) | Frozen | Saliva: mucous trap suction device Blood: venous |
1.GLC 2.EMIT |
N |
1.0.94 2.0.98 |
1.1.04 2.0.95 |
42 | 0.70–1.15 | 22, 47, 48, 53, 94 |
| Carbamazepine oral | Excellent | 39 children (2–15 years) | Up to 1 month in a domestic freezer. | Samples collected 3 h after dosing Saliva: gauze wrapped in cotton wool Blood: venous | HPLC | N | 0.99 | N/A | 68 | 0.26–0.40 | 22, 53, 71, 72, 74, 94, 109, 110, 111, 112, 113 |
| Very good/good | 202 children (5 months‐18 years) | frozen | Saliva: mucous trap suction device Blood: venous | GLC/EMIT | N | GLC = 0.91, EMIT = 0.83 | 0.26 | 42 | |||
| Good | 85 children (1–16 years) | frozen | Mouths rinsed Saliva: aspiration/spitting into a container Blood: venous | HPLC | Y | 0.84 P < 0.001 | N/A | 50 | |||
| Oxcarbazepine oral | Very Good | 47 children (7 < 24 months) | Frozen –20°C until analysis | Samples collected in morning before dosing Saliva: plastic pipette Blood: venous | HPLC | N | 0.908 | 0.71 | 77 | 0.19–1.01 | 114, 115, 116, 117, 118 |
| Caffeine oral or IV | Good | 140 premature neonates (<34 weeks) | Refrigerated for max 24 h | Samples collected at least 6 h after dosing Saliva: gauze attached to a cotton swab Blood: heel prick/arterial lines | HPLC‐UV |
Y Y N |
2. 0.89 | 0.70 | 63 | 0.55–0.79 | 47, 119, 120 |
| Good | 29 premature neonates | Frozen at –20°C until analysis | Samples collected at trough saliva: salimetrics collection device Blood: N/A | HPLC | N | 0.87 P < 0.001 | N/A | 67 | |||
| Good | 7 premature neonates | Both frozen until analysis | Samples collected 4–6 h after dosing Saliva: rubber ear syringe Blood: heel prick | straight phase HPLC | N | 0.84 ± 2.8 μg ml–1 | 0.71 | 23 | |||
| Fair | 13 neonates (<32 weeks) | N/A | Samples collected at trough Saliva: N/A Blood: N/A | EMIT | N/A | 0.76 | N/A | 66 | |||
| N/A | 59 premature neonates (<32 weeks) | Both stored at –75°C until analysis | Samples collected before dosing Saliva: vacuum aspiration Blood: heel prick | HPLC | N | N/A | N/A | 65 | |||
Correlation R value: excellent >0.95; very good >0.90; good >0.80; fair >0.70; poor <0.70
EMIT, enzyme multiplied immunoassay technique; GLC, gas–liquid chromatography; HPLC, high‐pressure liquid chromatography; IV, intravenous; LC, liquid chromatography; MS, mass spectrometry; N/A, not applicable; RIA, radioimmunoassay
Acidic compounds (pKa ≤ 10)
Four acidic compounds have previously been studied for saliva content in infants (Table 2) with the pKa ranging from digoxin (7.15) to theophylline (8.81). The individual compounds are discussed below and detailed in Table 3.
Theophylline
Five studies were included in this review on TDM of theophylline in infants 23, 29, 30, 31, 32. Of these studies, four were deemed to have excellent correlation 29, 30, 31, 32 (R = 0.97, P < 0.01). Of these four studies, two used stimulation to enhance saliva collection, one did not use stimulation and the other collected both stimulated and unstimulated saliva for comparison. There was no difference in correlation in any of these studies by the introduction of stimulation to increase saliva production.
The earliest study to be included for the monitoring of theophylline was published in 1980 by Khanna et al. 23 assessing 10 premature neonates. This was the only study to conclude only a fair correlation between the blood and saliva monitoring. In contrast to the Toback et al. 29 study, just 3 years later Khanna et al. 23 reported a correlation value as low as 0.7 compared to Toback's 0.98. Both studies were similar in their use of high‐pressure liquid chromatography (HPLC) for analysis, no stimulation, a similar time period for sample collection following intravenous (IV) administration and assessed a similar number of premature neonates. The only difference appears to be storage method, with the Khanna et al. 23 study freezing samples until analysis (no further details given) whereas the Toback study either stored samples at 4°C for 48 h or analysed immediately.
The method chosen for analysis (Table 3) also varied between the five studies. In the most recent study (2007) by Chereches‐Panta et al. 32, assessing 13 infants, gas chromatography–mass spectroscopy (MS) was used. MS was used in the 2001 study by Culea et al. 31 with 13 neonates whereas the enzyme multiplied immunoassay technique (EMIT) was used in the 1990 study by Siegel et al. 30 with 31 children and HPLC by Toback et al. 29 in 1983 and Khanna et al. in 1980 23.
One further point of interest when comparing these five studies, is that only four describe the S/P ratio, which varies significantly between the studies, ranging from 0.69 32 to 1.53 31. When considering theophylline, overall stimulated saliva sampling is thought to be an accurate method of TDM, while treating levels >8 μg/ml with caution 30 and consideration needs to be given to the higher S/P theophylline concentration ratio in neonates as compared to children and adults. The S/P ratio of 1.24 in Khanna et al.'s study 23 was similar to the observations of others 33, 34, while Toback et al. 29 suggest the ratio to be closer to 1. Also, that found by Culea et al. 31 is low, although they found the ratio to be higher in the infants than children. The lower correlations with unstimulated saliva which were seen by Siegel et al. 30 were thought to be due to greater variability in the theophylline saliva concentration due to changes in flow rates and pH. However, it has been determined that the secretion of theophylline is not influenced by these factors 32, 33, 35, 36. The difference seen between the populations could be due to differences in protein binding in these groups, but this requires further exploration. It has, however, been suggested that neonates have minimal protein binding of theophylline 37 and that proteins in this group generally differ from those found in children and adults 38, 39. In addition, high plasma concentrations of free fatty acids, unconjugated bilirubin and steroids in neonates can compete with certain drugs for binding sites. It has also been suggested that pH changes in blood as little as 0.2 may affect the protein binding 40.
With the exception of the 1980 study 23, all were in agreement that theophylline shows excellent correlation between blood and saliva, regardless of whether stimulation is used and independent on analytical method indicating that saliva TDM is useful for this compound.
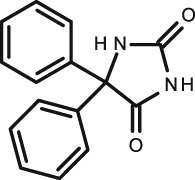
Phenytoin
There were four studies published assessing phenytoin in infant and children's saliva included in this article. The studies all took place in the last century; 1975 41, 1981 42, 43 and 1990 44. The correlation seen between the samples and indeed between the different studies was excellent–very good. Similar to the theophylline study a range of different analytical methods was employed to quantify the samples. In 1981 42, a large study was carried out using two different assay methods, gas–liquid chromatography (GLC) and EMIT, assessing 202 children between the ages of 5 months and 18 years. It reported that the GLC assay had very good correlation (R = 0.94) whereas EMIT was not as good (R = 0.86). They also describe better correlation with fresh compared to frozen samples.
In the most recent study on phenytoin (1990) by Lifschitz et al. 44, 16 children and infants taking oral phenytoin were studied. Stimulated saliva and blood were analysed by fluorescence polarization immunoassay (FPIA) and the correlation between blood and saliva described as excellent (R = 0.99). When comparing the S/P ratio for all four studies, they are in close agreement to one another, with an average value of 0.11 ± 0.013.
Phenytoin in adults and older children has shown wide individual and marked interpatient variability, including age‐dependent metabolism 45, although it has a well‐documented S/P ratio between 0.10–0.12 22, 41, 46, 47, 48, 49, which agrees with all of these studies 41, 42. All studies showed high correlations, meaning TDM using saliva is an accurate method for phenytoin; however, as there is a wide interpatient metabolism associated with it, lack of correlation between drug dose and concentration needs consideration. The use of stimulated/unstimulated saliva seems to have little effect on correlation and researchers should use EMIT with caution.
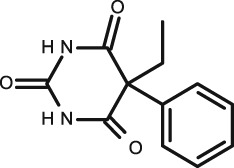
Phenobarbital
Four published studies are included in this review 41, 42, 43, 50. Each of these studies assessed phenobarbital in saliva in infants combined with that of other anticonvulsants such as phenytoin, as described above. The correlation of saliva to blood varied significantly between studies, ranging from excellent as described by Cook et al. 41, where unstimulated saliva and plasma were analysed by radioimmunoassay (R = 0.98, P < 0.001) in a study assessing 38 patients aged between 8 months and 62 years. In stark contrast to this, the 1981 study described by Mucklow et al. 43 assessing 19 infants, was completely unable to identify a linear relationship between dosage and saliva concentration for phenobarbital. Goldsmith and Ouvrier 42 found a very good correlation in 15 infants with a S/P ratio = 0.32 ± 0.06 and no significant difference was seen between the different age groups studied. For the GLC assay, the correlation was very good (R = 0.94) and the EMIT was similar (R = 0.92). A later study by Gorodischer et al. in 1997 50 studied stimulated saliva and plasma, which were analysed by HPLC, and found the correlation coefficient to be poor (R = 0.65). However, those older than 8 years had a higher correlation (R = 0.93). It has been suggested that salivary excretion of phenobarbital is regulated differently in older and younger children. A study by Knotts reported an excellent correlation (R = 0.95) in older children 51.
The S/P ratio was given in three of the four studies, and found to be in good agreement with one another, with an average ratio of 0.28 ± 0.06. The S/P ratio described by Cook et al. 41 and Goldsmith et al. 42 is similar to that found in adults, which varied between 0.30 and 0.37 41, 46, 47, 48, 49, 52, 53.
Therefore, salivary TDM of phenobarbital has the potential to be an accurate method in infants. However, the age of the patient must be considered, as well as the use of stimulation. In the two studies which displayed the best correlation, neither used any method of saliva stimulation.
Digoxin
In the three studies including digoxin, the correlation varies from good 24, 54 to fair 55. As seen in the 1998 study by Berkovitch et al. 24 good correlation (R = 0.87) in 11 children (nine of whom were infants) receiving oral digoxin was presented. Plasma and saliva was analysed using FPIA. A further study in 2003 by Zalzstein et al. 54 also showed a good correlation (R = 0.83, P < 0.01) between stimulated saliva and serum in 18 children (again nine of whom were infants) receiving oral digoxin. The samples were also analysed using FPIA; however, variability existed in individual S/P concentration ratios. Low plasma concentrations correlated with negligible or undetectable saliva concentrations. No plasma levels were above the agreed toxic limit of 2 ng ml–1; however, one saliva sample was deemed toxic, without the plasma following suit. In three saliva samples, the digoxin concentration was not detectable even though the plasma levels were within therapeutic range. An earlier study by Krivoy et al. conducted in 1981 55 compared plasma with unstimulated saliva using the radioimmunoassay digoxin kit. An S/P ratio of 0.66 ± 0.20 and a fair correlation coefficient (R = 0.71, P < 0.001) was noted, however, it showed a wide range in the ratios. The S/P ratios quoted are vastly different in the two studies that present them.
Digoxin is an acidic drug with a pKa of 7.15, meaning that % ionization will vary significantly with small changes in physiological pH. An increase in pH from 6 to 7 will see the % of digoxin ionized increase from 6.6 to 41.45%. Krivoy et al. 55 stated that saliva was an accurate method of TDM; however, the poor correlation coefficient does not reflect this. In contrast, Berkovitch et al. 24 reported a much higher correlation and stated that it was not a reliable method. They thought it was possible that digoxin did not enter saliva by diffusion alone, but also by active secretion and that there was a possibility of endogenous digoxin‐like substances present in the saliva 6. Krivoy et al. 55 found similar correlations to Berkovitch et al. 24, showing that the stimulation of saliva yields better correlation, yet further studies will need conducted to determine if, as in adults, TDM using saliva is an accurate method in infants when no stimulation is used. There is also a need for a standardized method of sample collection, storage and analysis before a ratio can be accurately determined as high variability is demonstrated in these studies.
Basic compounds (pKa ≥ 4)
Three basic compounds have previously been studied for saliva content in infants (Table 2) with the pKa ranging from gentamicin (10.18) to lamotrigine (5.87). The individual compounds are discussed below. Unlike with the acidic compounds where each compound had more than two studies to allow comparison, the number of infant studies for TDM in basic drugs is much more limited with only four studies in total for the three compounds (Table 4).
Gentamicin
Only one study satisfied conditions for inclusion in this review. In the 2000 study by Berkovitch et al. 56, serum and stimulated saliva in 10 infants were analysed using FPIA. A good correlation was noticed in once daily dosing patients (R = 0.89, P < 0.0001) but no S/P ratio was determined. Gentamicin is a hydrophilic drug and with a pKa of 10.18 and will therefore be positively charged at physiological pH 56, hence, although it is only weakly protein bound, it would be expected that it does not appear in saliva as Mahmod et al. 57 states. Berkovitch et al. 24 did find levels in saliva, although, as they found no correlation when the dose was three times a day, it was thought that it must take longer for the ionized molecules to enter and equilibrate with saliva and hence once‐daily dosing allows such a time lag as the measurements are taken 24 h after dosing. This study suggests that it is a valid and accurate method to monitor trough levels in infants on a once daily dosing regimen; however, the lack of studies on this drug leave the results unreliable and more studies need to be conducted before an accurate representation of the S/P ratio can be determined.
Morphine
In one 1997 study by Kopecky et al. 58, 15 children receiving IV infusions of morphine were analysed using a solid phase serum morphine radioimmunoassay. The S/P ratio was 2.28 ± 2.84. There was no correlation between saliva and plasma (R = 0.04, P = 0.89). As citric acid decreases the salivary pH, the authors examined the analytical effect of different pH values on the morphine concentrations in saliva across a pH range of 3.96–8.06 and reported no difference in assay performance. This study would suggest that determination of morphine levels from saliva cannot be used as a quantitative tool to predict serum concentrations, although further studies are required to confirm these findings.
Lamotrigine
Two studies for lamotrigine were found and included in this review. In 2003 Ryan et al. 59 studied unstimulated saliva and serum using HPLC‐UV analysis. The correlations were tested over two laboratories (R = 0.81 and R = 0.86). The S/P ratio was 0.62 ± 0.19. They found the correlation to be excellent in children (R = 0.94) and that the S/P ratio was less (0.56) than in adults; however, these values was based on only one infant.
In a later study (2005) conducted by Malone et al. 60 stimulated and unstimulated saliva along with serum were studied in an adult group and in 20 children (1–16 years) using HPLC analysis. The data in adults showed that within the first 2 h following oral administration, the results had a wide scatter, after exclusion of these the results they showed an excellent correlation (R = 0.9841, P < 0.0001; n = 98). The S/P ratio was lower in the adults than children (41.7 ± 7.07% for unstimulated saliva vs. 42.1 ± 6.52% for stimulated). In children the ratio was 47.6 ± 7.2% and 46.7 ± 6.2%. There was a close correlation between the concentrations of lamotrigine in stimulated and unstimulated saliva, with the mean S/P ratio = 0.49. As plasma lamotrigine concentrations increased from 1 mg l–1 to 10 mg l–1, the mean S/P ratio changed from 41.8% to 48.8% indicating that lamotrigine ratios are concentration dependent. They believe this to be due to plasma protein binding reaching saturation. Saliva TDM of lamotrigine can be an accurate and reliable method, although it must be ensured that no oral contamination occurs 59.
Neutral compounds
Six neutral compounds have been included in this review. They have been classified as neutral if they have no ionizable functionality or if their pKa value is such that a unit change in physiological pH would have less than a 0.1 variation in % ionization, Table 2. This is further classified as a basic pKa <4 and an acidic pKa >10. The individual compounds are discussed below with detailed criteria presented in Table 5.
Busulfan
The one study included in this article suggests that TDM of busulfan in unstimulated saliva is a valid alternative to plasma sampling in infants with excellent correlation (R = 0.958) 61. Rauh et al. 61 demonstrated that busulfan in saliva was stable for up to 48 h at 4°C (concentration decrease <5%). Analysis was conducted using LC–MS/MS also allowing for high output sample analysis. The S/P ratio was 1.09. Their saliva collection method, however, would not be suitable for all infants as some do not actually take a pacifier. The excellent linear regression makes this drug look ideal for TDM using salivary samples in this population; however, only one infant was involved in the study conducted with 10 children and therefore further work is required to support the findings.
Fluconazole
One study has been conducted on fluconazole TDM using unstimulated saliva compared with serum in paediatric patients 62. The study involved 19 children, 10 of which were infants, and analysis was again by LC–MS/MS. The correlation was excellent (R = 0.96, P < 0.1) and the S/P ratio was 1.0. Van der Elst et al. 62 describes how at high concentrations of fluconazole, the amount in saliva was less than in serum, suggesting saturation in oral fluid. The S/P drug concentration ratio did not significantly differ in patients receiving oral treatment vs. IV (P = 0.791).
Salivary fluconazole was shown to be stable for 17 days at room temperature giving more evidence to the potential for home monitoring 62.
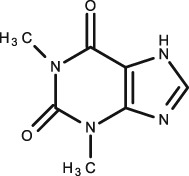
Caffeine
Together with theophylline, caffeine had the highest number of studies included in this systematic review, with each compound having five studies on TDM using saliva in infants. The largest of these studies was carried out in 2001 by De Wildt et al. 63; 140 premature neonates were given IV caffeine. Their saliva was stimulated immediately before collection, 5–10 min before collection and unstimulated saliva was also collected. Plasma and saliva were analysed using HPLC with UV detection. The unstimulated and stimulated immediately before the collection groups were not well correlated. The citric acid stimulated 5–10 min before collection had the strongest correlation (R = 0.89) with little variability. In conclusion, they determined that the S/P ratio was 0.7 and that this method was reproducible and feasible when saliva was stimulated 5–10 min before collection. An earlier study was conducted by Khanna et al. 23 on seven premature infants who received caffeine via an orogastric tube. Serum and unstimulated saliva was analysed using straight phase HPLC. A good correlation (R = 0.84 ± 2.8 μg ml–1) was seen. The P/S ratio was determined at 1.40, however if we consider the S/P ratio, which has been quoted in the majority of other articles, then it is identical to that given by De Wildt at 0.7. In this study, total methylxantine concentration was also measured as caffeine can convert to theophylline and vice versa 64. In this case, R = 0.81 ± 3.3 μg ml–1. With the exception of one patient, when the total concentration in saliva was <8 μg ml–1, the total in serum did not exceed 15 μg ml–1.
In contrast to this and in a much later study, Lee et al. 65 administered IV caffeine over 7 days to 59 premature neonates. Unstimulated saliva was compared with serum using HPLC analysis. They used the assessment of precision and bias between two methods and determined that there was no significant difference in precision between the serum and salivary data. They concluded that saliva can be used instead of serum to monitor caffeine at practically any concentration even in high maintenance doses of 30 mg kg–1day –1. A study by Chioukh et al. 66, demonstrated that saliva caffeine levels were comparable to plasma in 13 infants receiving IV caffeine. The trough caffeine levels were determined from saliva and serum and analysed by EMIT with a fair correlation (R = 0.76). The most recent study for TDM of caffeine in infants was published in 2016 by Dobson et al. 67. This study included 29 premature neonates. Plasma and unstimulated saliva were analysed by HPLC. They showed good correlation between the salivary and plasma caffeine concentrations over different doses (R = 0.87, P < 0.001).
When considering caffeine, the use of saliva for TDM has been shown to have conflicting results and with major differences in collection methods, use of stimulation and different statistical analysis, it is difficult to determine the usefulness of saliva as a method of TDM for caffeine. In theory this should be an accurate method and if a more robust collection and analysis method is determined, could prove to be a very useful in this patient group. Lee et al. 65 suggested that the transport of caffeine from blood to saliva is independent of concentration, meaning that it is likely that free caffeine enters salivary ducts by passive diffusion and not via capacity‐limited active transport processes that other drugs may use. De Wilt et al. 63 found the unstimulated and stimulated immediately before the collection groups were not well correlated, and it was noted that in more than half of the samples, <50 μl of saliva was collected, which may have been a contributing factor to the weak correlation and wide variability. Also, applying the citric acid 5 min before collection prevented dilution of the sample by citric acid.
Carbamazepine
Three studies on carbamazepine are included in this review. The earliest of these was published in 1981 by Goldsmith et al. 42, who collected unstimulated saliva and plasma from 202 children (15 of which were infants) and analysed it using GLC and EMIT. They found that there was better correlation with fresh samples compared to frozen ones. A good correlation was found in the 15 infants with an S/P ratio of 0.26 and no significant difference was seen between the age groups. For the GLC assay the correlation was excellent (R = 0.91) and the EMIT was not as significant (R = 0.83).
In a later study by Gorodischer et al. (1997) 50, 85 children were studied. They collected stimulated saliva and plasma and analysed them by HPLC. Good correlation was seen (R = 0.84, P < 0.001), which was similar to that seen in the EMIT assay by Goldsmith.
Chee et al. published a study in 1993 68 where they compared carbamazepine and its active metabolite carbamazepine‐10,11‐epoxide levels in plasma and unstimulated saliva samples in 39 children. Analysis was conducted using HPLC. Both carbamazepine and carbamazepine‐10,11‐epoxide concentrations had excellent correlation (R = 0.99 and 0.98 respectively) and storage in a domestic freezer for 1 month had no significant effect on correlation. They believed that no oral contamination occurred despite not rinsing the participants' mouths with water. The S/P ratio was only provided by Goldsmith et al. 42 as 0.26; it was not quoted in the other studies.
Salivary concentrations of carbamazepine have been shown to correlate significantly with plasma total and unbound concentrations in children 22, 53, 69, 70 and in adults 71, 72, 73, 74; however, ratios have been seen to vary widely 0.24–0.37 22, 46, 49, 74, 75. The ratio from Goldsmith et al. 42 agrees well with three of these studies, two of which 70, 76 were conducted in children. Rylance et al. 76 found that even though a patient is in so called steady state, carbamazepine levels in saliva may change by 100% during the course of the day, probably due to its short half‐life in children, which also helps explain the wide variety of ratios seen in studies. Furthermore, EMIT analysis may have a cross reaction with carbamazepine‐10,11 epoxide as mentioned by Bartels et al. 70, which may explain the lower correlation seen in Goldsmith et al.'s study 42. Overall, unstimulated saliva in infants was shown to yield better correlations and a standardization of techniques and assay methods is needed to confirm these findings.
Oxcarbazepine
Oxcarbazepine (OXC) is rapidly reduced to its pharmacologically active metabolite, monohydroxy derivative (MHD). In a recent study (2016) conducted by Rui‐Rui et al. 77, the unstimulated saliva and plasma MHD concentrations in 47 children (seven of whom were infants) were compared using HPLC analysis. There was a very good correlation between saliva and plasma concentrations (R = 0.908) with the S/P ratio being 0.71. There was no significant correlation with patient age or sex and there was no significant difference with age, OXC daily dose, plasma and saliva MHD concentrations and S/P ratio when OXC was given in conjunction with other AEDs. No other studies on oxcarbazepine are included in this review.
Primidone
In the 1981 study by Goldsmith et al. 42 unstimulated saliva and plasma were collected from 202 children and analysed using GLC and EMIT. The S/P ratio was 1.04 for gas chromatography and 0.95 for EMIT. No significant difference was seen between the age groups studied. For the GLC assay the correlation was very good (R = 0.94) and the EMIT was excellent (R = 0.98), this same study analysed the concentrations of carbamazepine, and found the opposite trend with analysis methods, in that the GLC was considered to be the better method for carbamazepine. This corresponds well to adult studies describing the S/P ratios between 0.75–1.08 28, 46, 47, 78.The authors felt the very short half‐life of primidone may have contributed to the greater variation in S/P ratio. However, this single study is too limited to give an accurate representation of the accuracy of saliva TDM for primidone in infants, further studies looking into the use of stimulation and collection methods are required.
Discussion
There is a significant amount of evidence available to suggest that saliva is indeed a useful matrix for TDM in infants (Tables 4–6). Figure 2 displays the average R value achieved from each of the 13 compounds analysed. Morphine was the only compound to show negligible correlation between blood and saliva samples, with all twelve remaining compounds displaying at least good correlation between saliva and plasma (R > 0.8). In general, the neutral compounds displayed the best correlation between saliva and plasma concentrations with three neutral compounds (busulfan, fluconazole and primidone) displaying an R value of >0.95. Acidic drugs also displayed very good correlation, in particular weakly acidic drugs with a pKa > 8.3. It is perhaps not surprising that the neutral and very weakly acidic compounds perform better for saliva TDM as derivations in saliva pH would have no effect on the % ionization of the compounds. There is less evidence available regarding basic compounds for this method of analysis. However, of the three compounds included in this review, they display a lower correlation value when compared to either the acidic or neutral compounds. Of those compounds studied, only one basic compound, lamotrigine, had a pKa less affected by physiological pH (pKa 5.87), which would be predominately unionised at physiological pH. Lamotrigine gave excellent potential for saliva TDM (Figure 2). Analysis of the physicochemical parameters for the compounds suggests that both protein binding and logP had little effect on the correlation. Some highly protein bound compounds such as phenytoin (90% protein bound) displayed a reduced S/P ratio (0.1) and displayed excellent correlation; likewise, primidone, which is 70% protein bound did not have a reduced S/P ratio (1.1) and still recorded excellent correlation. Additionally, fluconazole with a relatively low protein binding (30%) has an S/P ratio of 1. Compounds with a pKa within physiological range, i.e. acidic drugs with a pKa < 7.2 or basic drugs with a pKa > 6 had less correlation, probably due to variation in % ionization with variation in saliva pH. The most variation in correlation between different studies of the same compound was seen from those compounds with a pKa close to 7 (digoxin pKa 7.15 and phenobarbital pKa 7.3) where correlation ranged from excellent to poor (Tables 4–6), suggesting that the pH of saliva is significantly affecting the reliability of these compounds for analysis via this method. When considering a compound for this type of TDM, the physicochemical properties of the drug must be considered and, where possible, any compound with a pKa within physiological range should be considered with extra care.
Figure 2.
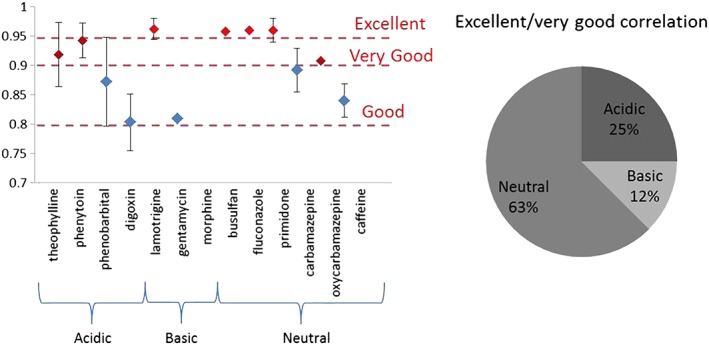
Comparison of mean R values for the 13 compounds assessed
When considering the method for analysis, a wide range of techniques is available. Some of the assay methods have been validated, but many are out dated and more accurate options now available. Method of analysis significantly affects the correlation and, in particular, compounds with limited absorbance extinction coefficients. LC–MS/MS displayed excellent correlation on a wide range of compounds with impressive limits of detection.
With regard to the method used for the extraction of saliva, there have been many collection methods tried, and, although spitting into a container seems to be the easiest method in adults and children, this is not practical for infants. When considering infants, it is thought that wiping the mouth with sterile gauze and rinsing with 5 ml of sterile water and waiting for 5 min helps to reduce the risk of contamination 79 from either oral medication or mother's milk. Some research has shown that turning the infant onto their side to let the saliva pool in the cheek was the easiest method of collection 79. Other authors have extracted the saliva from under the tongue and have collected it using various devices such as pipettes 77, mucous extractors 18, adapted pacifiers 61, suction devices 23, 62 and more recently Salivettes/salimetrics collection devices 67.
There are advantages and disadvantages associated with all these collection devices: pipettes have limitations due to saliva viscosity; the adapted pacifier is not applicable to all infants; and suction devices are more troublesome than the other methods. The Salivette collection device has been specifically designed for the purpose and consists of a cotton swab, which is placed in the mouth until saturated, and is then placed into its container where the fluid can be collected. The only disadvantage recorded for this collection method is that some drugs may adsorb onto the cotton swab 80. Despite this, it remains suitable for most drugs.
The type of collecting tube also needs to be taken into account when beginning a saliva collection. Studies have shown that tubes containing serum separator gels can significantly affect the determination of some drugs such as phenytoin 81, 82 or ribavirin 83. Therefore, it is strongly recommended to evaluate the matrix effect of collecting tubes 3. Once collected, the majority of the samples that we considered were frozen at –20°C before analysis with no detrimental effect. One study 42 reported better correlation with fresh samples compared with frozen; however, there was insufficient evidence to conclude that this is the case for the majority of compounds. Finally, the question of whether to stimulate saliva production when collecting the samples also needs addressed prior to creating a reliable reproducible method. From the compounds studied, stimulation had limited effect on those drugs with excellent correlation. This is probably due to the method of stimulation using critic acid, and thereby lowering saliva pH further and not affecting % ionization of the weakly acidic or neutral drugs. Stimulation displayed a varied effect overall on the compounds, with no apparent correlation with chemical properties, and therefore should only be considered if necessary.
In conclusion, there is significant potential for infantile saliva testing, and in particular for neutral and weakly acidic compounds.
Competing Interests
The views expressed in this manuscript are those of the authors and do not reflect that of the Department for the Economy. The authors have no conflicts of interest.
The authors acknowledge the support of the Department for the Economy, Northern Ireland.
Hutchinson, L. , Sinclair, M. , Reid, B. , Burnett, K. , and Callan, B. (2018) A descriptive systematic review of salivary therapeutic drug monitoring in neonates and infants. Br J Clin Pharmacol, 84: 1089–1108. doi: 10.1111/bcp.13553.
Contributor Information
Marlene Sinclair, Email: m.sinclair1@ulster.ac.uk.
Bridgeen Callan, Email: b.callan@ulster.ac.uk.
References
- 1. Dasgupta A. Handbook of Drug Monitoring Methods. New Jersey: Humana Press Inc, 2008. [Google Scholar]
- 2. Buchanan N. Therapeutic drug monitoring. Indian J Pediatr 1986; 53: 149–162. [DOI] [PubMed] [Google Scholar]
- 3. Zhao W, Jacqz‐Aigrain E. Principles of therapeutic drug monitoring In: Pediatric Clinical Pharmacology, Vol. 205 Berlin: Springer, 2011; 77–90. [DOI] [PubMed] [Google Scholar]
- 4. Kearns GL, Sbdel‐Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med 2003; 18: 1157–1167. [DOI] [PubMed] [Google Scholar]
- 5. Routledge PA. Pharmacokinetics in children. J Antimicrob Chemother 1994; 34 (Suppl A): 19–24. [DOI] [PubMed] [Google Scholar]
- 6. Koren G, Parker R. Interpretation of excessive serum concentrations of digoxin in children. Am J Cardiol 1985; 55: 1210–1214. [DOI] [PubMed] [Google Scholar]
- 7. Koren G. Therapeutic drug monitoring principles in the neonate. Clin Chem 1997; 43: 222–227. [PubMed] [Google Scholar]
- 8. Jong GWT, Vulto AG, de Hoog M, Schimmel KJ, Tibboel D, Van den Anker JN. Unapproved and off‐label use of drugs in a children's hospital. N Engl J Med 2000; 343: 1125. [DOI] [PubMed] [Google Scholar]
- 9. Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, et al Survey of unlicensed and off label drug use in paediatric wards in European countries. BMJ 2000; 320: 79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gross AS. Best practice in therapeutica drug monitoring. Br J Clin Pharmacol 1998; 46: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pichini S, Alteri I, Zuccaro P, Pacifici R. Drug monitoring in non conventional biological fluids and matrices. Clin Pharm 1996; 30: 211–228. [DOI] [PubMed] [Google Scholar]
- 12. Hawcutt DB, Rose AC, Fuerst‐Recktenwald S, Nunn T, Turner MA. Points to consider when planning the collection of blood or tissue samples in clinical trials of investigational medicinal products in children, infants and neonates In: Guide to Paediatric Drug Development and Clinical Research, eds Van den Anker JN, Rose K. Washington D.C: Karger Publishers, 2010. [Google Scholar]
- 13. Edelbroek PM, Van Der Hejjden J, Stolk LM. Dried blood spot methods in therapeutic drug monitoring: methods, assays, and pitfalls. Ther Drug Monit 2009; 31: 327–336. [DOI] [PubMed] [Google Scholar]
- 14. Li W, Tse FL. Dried blood spot sampling in combination with LC‐MS/MS for quantitative analysis of small molecules. Biomed Chromat 2010; 24: 49–65. [DOI] [PubMed] [Google Scholar]
- 15. Choo RE, Huestis MA. Oral fluid as a diagnostic tool. Clin Chem Lab Med 2004; 42: 1273–1287. [DOI] [PubMed] [Google Scholar]
- 16. Patsalos PN, Berry DJ. Therapeutic drug monitoring of antiepileptic drugs by use of saliva. Ther Drug Monit 2013; 35: 4–29. [DOI] [PubMed] [Google Scholar]
- 17. Gorodischer R, Burtin P, Hwang P, Levine M, Koren G. Saliva versus blood sampling for therapeutic drug monitoring in children: patient and parental preferences and an economic analysis. Ther Drug Monit 1994; 16: 437–443. [DOI] [PubMed] [Google Scholar]
- 18. Gorodischer R, Koren G. Salivary excretion of drugs in children: theoretical and practical issues in therapeutic drug monitoring. Dev Pharmacol Ther 1992; 19: 161–177. [DOI] [PubMed] [Google Scholar]
- 19. Gordi T, Hai TN, Hoai NM. Use of saliva and capillary blood samples as substitutes for venous blood sampling in pharmacokinetic investigations of artemisinin. Eur J Clin Pharmacol 2000; 56: 561–566. [DOI] [PubMed] [Google Scholar]
- 20. Huffman DH. Relationship between digoxin concentrations in serum and saliva. Clin Pharmacol Ther 1975; 17: 310–312. [DOI] [PubMed] [Google Scholar]
- 21. Tsiropoulos I, Kristensen O, Klitgaard NA. Saliva and serum concentration of lamotrigine in patients with epilepsy. Ther Drug Monit 2000; 22: 517–521. [DOI] [PubMed] [Google Scholar]
- 22. McAuliffe JJ, Sherwin AL, Leppik IE, Fayle SA, Pippenger CE. Salivary levels of anticonvulsants a practical approach to drug monitoring. Neurology 1977; 27: 409–413. [DOI] [PubMed] [Google Scholar]
- 23. Khanna NN, Bada HS, Somani SM. Use of salivary concentrations in the prediction of serum caffeine and theophylline concentrations in premature infants. J Pediatr 1980; 96: 494–499. [DOI] [PubMed] [Google Scholar]
- 24. Berkovitch M, Bistritzer T, Aladjem M, Burtin P, Dagan T, Chen‐Levi Z, et al Clinical relevance of therapeutic drug monitoring of digoxin and gentamicin in the saliva of children. Ther Drug Monit 1998; 20: 253–256. [DOI] [PubMed] [Google Scholar]
- 25. Autmizguine J, Benjamin DK Jr, Smith PB, Sampson M, Ovetchkine P, Cohen‐Wolkowiez M, et al Pharmacokinetic studies in infants using minimal‐risk study designs. Curr Clin Pharmacol 2014; 9: 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bailey B, Klein J, Koren G. Noninvasive methods for drug measurement in pediatrics. Pediatr Clin North Am 1997; 44: 15–26. [DOI] [PubMed] [Google Scholar]
- 27. Ovid Technologies , 2017. [Online]. Available at http://ovidsp.uk.ovid.com/sp-3.26.1a/ovidweb.cgi (last accessed 14 August 2017).
- 28. Mucklow JC, Bending MR, Kahn GC, Dollery CT. Drug concentration in saliva. Clini Pharmacol Ther 1978; 24: 563–570. [DOI] [PubMed] [Google Scholar]
- 29. Toback JW, Gal P, Erkan NV, Roop C, Robinson H. Usefulness of theophylline saliva levels in neonates. Ther Drug Monit 1983; 5: 185–189. [DOI] [PubMed] [Google Scholar]
- 30. Siegel IA, Ben‐Aryeh H, Gozal D, Colin AA, Szargel R, Laufer D. Comparison of unbound and total serum theophylline concentrations with those of stimulated and unstimulated saliva in asthmatic children. Ther Drug Monitor 1990; 12: 460–464. [DOI] [PubMed] [Google Scholar]
- 31. Culea M, Palibroda N, Chereches‐Panta P, Nanulescu M. Comparison of isotopic dilution methods for determination of theophylline in the plasma and saliva of infants and children. Chromatographia 2001; 53 (S1): 387–389. [Google Scholar]
- 32. Chereches‐Panta P, Nanukescu MV, Culea M, Palibroda N. Reliability of salivary theophylline in monitoring the treatment for apnoea of prematurity. J Perinatol 2007; 27: 709–712. [DOI] [PubMed] [Google Scholar]
- 33. Aranda JV, Sitar DS, Parsons WD, Loughnan PM, Neims AH. Pharmacokinetic aspects of theophylline in premature newborns. N Engl J Med 1976; 295: 413–416. [DOI] [PubMed] [Google Scholar]
- 34. Hendeles L, Burkey S, Bighley L, Richardson R. Unpredictability of theophylline saliva measurements in chronic obstructive pulmonary disease. J Allergy Clin Immunol 1977; 60: 335–338. [DOI] [PubMed] [Google Scholar]
- 35. Aranda JV, Turmen T. Methylxanthines in apnea of prematurity. Clin Perinatol 1979; 6: 87–108. [PubMed] [Google Scholar]
- 36. Levy G, Ellis EF, Koysooko R. Indirect plasma‐theophylline monitoring in asthmatic children by determination of theophylline concentration in saliva. Pediatrics 1974; 53: 873–876. [PubMed] [Google Scholar]
- 37. Koup JR, Hart BA. Relationship between plasma and whole blood theophylline concentration in neonates. J Pediatr 1979; 94: 320–321. [DOI] [PubMed] [Google Scholar]
- 38. Behrmann RE, Battaglia FC. Protein binding of human fetal and maternal plasmas to salicylate. J Appl Physiol 1967; 22: 125–130. [DOI] [PubMed] [Google Scholar]
- 39. Wallace S. Altered plasma albumin in the newborn infant. Br J Clin Pharmacol 1977; 4: 82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morselli PL, Rovei V. Placental transfer of pethidine and norpethidine and their pharmacokinetics in the newborn. Eur J Clin Pharmacol 1980; 18: 25–30. [DOI] [PubMed] [Google Scholar]
- 41. Cook CE, Amerson E, Poole WK, Lesser P, O'Tuama L. Phenytoin and phenobarbital concentrations in saliva and plasma measured by radiommunoassay. Clin Pharmacol Ther 1975; 18: 742–747. [DOI] [PubMed] [Google Scholar]
- 42. Goldsmith RF, Ouvrier RA. Salivary anticonvulsant levels in children: a comparison of methods. Ther Drug Monit 1981; 3: 151–158. [DOI] [PubMed] [Google Scholar]
- 43. Mucklow JC, Bacon CJ, Hierons AM, Webb JK, Rawlins MD. Monitoring of phenobarbitone and phenytoin therapy in small children by salivary samples. Ther Drug Monit 1981; 3: 275–277. [DOI] [PubMed] [Google Scholar]
- 44. Lifshitz M, Ben‐Zvi Z, Gorodischer R. Monitoring phenytoin therapy using citric acid‐stimulated saliva in infants and children. Ther Drug Monit 1990; 12: 334–338. [DOI] [PubMed] [Google Scholar]
- 45. Zysset T, Rudeberg A, Vassella F, Kupfer A, Bircher J. Phenytoin therapy for epileptic children: evaluation of salivary and plasma concentrations and of methods of assessing compliance. Dev Med Child Neurol 1981; 23: 66–75. [DOI] [PubMed] [Google Scholar]
- 46. Troupin AS, Friel P. Anticonvulsant level in saliva, serum, and cerebrospinal fluid. Epilepsia 1975; 16: 223–227. [DOI] [PubMed] [Google Scholar]
- 47. Horning MG, Brown L, Nowlin J, Letratanangkoon K, Kellaway P, Zion TE. Use of saliva in therapeutic drug monitoring. Clin Chem 1977; 23: 157–164. [PubMed] [Google Scholar]
- 48. Schmidt D, Kupferberg HJ. Diphenylhydantoin, phenobarbital, and primidone in saliva, plasma, and cerebrospinal fluid. Epilepsia 1975; 16: 735–741. [DOI] [PubMed] [Google Scholar]
- 49. Blom GF, Guelen PJM. The distribution of antiepileptic drugs between serum, saliva and cerebrospinal fluid In: Antiepileptic Drug Monitoring, eds Gardner‐Thorpe GW, Janz D, Meinardi H, Pippenger CE. London: Pitman Medical, 1977; 287–297. [Google Scholar]
- 50. Gorodischer R, Burtin P, Verjee Z, Hwang P, Koren G. Is saliva suitable for therapeutic monitoring of anticonvulsants in children: an evaluation in the routine clinical setting. Ther Drug Monit 1997; 19: 637–642. [DOI] [PubMed] [Google Scholar]
- 51. Knott C, Reynolds F. The place of saliva in antiepileptic drug monitoring. Ther Drug Monit 1984; 6: 35–42. [DOI] [PubMed] [Google Scholar]
- 52. Friedman IM, Litt IF, Henson R, Holtzman D, Halverson D. Saliva phenobarbital and phenytoin concentrations in epileptic adolescents. J Pediatr 1981; 98: 645–647. [DOI] [PubMed] [Google Scholar]
- 53. Tokugawa K, Ueda K, Fujito H, Kurokawa T. Correlation between the saliva and free serum concentration of phenobarbital in epileptic children. Eu J Clin Pharmacol 1986; 145: 401–402. [DOI] [PubMed] [Google Scholar]
- 54. Zalzstein E, Zucker N, Lifshitz M. Digoxin concentration in saliva and plasma in infants, children, and adolescents with heart disease. Current Therapeutic Research 2003; 64: 743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Krivoy N, Rogin N, Greif Z, Ben‐Aryeh H, Gutman D, Alroy G. Relationship between digoxin concentration in serum and saliva in infants. J Pediatr 1981; 99: 810–811. [DOI] [PubMed] [Google Scholar]
- 56. Berkovitch M, Goldman M, Silverman R, Chen‐Levi Z, Greenberg R, Marcus O, et al Therapeutic drug monitoring of oce daily gentamycin in serum and saliva in children. Eu J Pediatr 2000; 159: 697–698. [DOI] [PubMed] [Google Scholar]
- 57. Mahmod S, Al‐Hakiem MH, Landon J, Smith DS, Shaw EJ. Aminoglycoside antibiotics do not appear in saliva. Clin Chem 1983; 29: 988–989. [PubMed] [Google Scholar]
- 58. Kopecky EA, Jacobson S, Klein J, Kapur B, Koren G. Correlation of morphine sulfate in blood plasma and saliva in pediatric patients. Ther Drug Monit 1997; 19: 530–534. [DOI] [PubMed] [Google Scholar]
- 59. Ryan M, Grim SA, Miles MV, Tang PH, Fakhoury TA, Strawsbury RH, et al Correlation of lamotrigine concentrations between serum and saliva. Pharmacotherapy 2003; 23: 1550–1557. [DOI] [PubMed] [Google Scholar]
- 60. Malone SA, Eadie MJ, Addison RS, Wright AWE, Dickson RG. Monitoring salivary lamotrigine concentrations. Clin Neurosci 2006; 13: 902–907. [DOI] [PubMed] [Google Scholar]
- 61. Rauh M, Stachel D, Kuhlen M, Groschl M, Holter W, Rascher W. Quantification of busulfan in saliva and plasma in haematopoietic stem cell transplantation in children. Clin Pharmacokin 2006; 45: 305–316. [DOI] [PubMed] [Google Scholar]
- 62. van der Elst KCM, van Alst M, Lub‐de Hooge MN, van Hateren K, Kosterink JG, Alffennaar JW, et al Clinical validation of the analysis of fluconazole in oral fluid in hospitalised children. Antimicro agents Chemother 2014; 58: 6742–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. De Wilt SN, Kerkvliet KTM, Wezenberg MGA, Ottink S, Hop WC, Vulto AG, et al Use of saliva in therapeutic drug monitoring of caffeine in preterm infants. Ther Drug Monit 2001; 23: 250–254. [DOI] [PubMed] [Google Scholar]
- 64. Bada HS, Khanna NN, Somani SM, Tin AA. Interconversion of theophylline and caffeine in newborn infants. J Pediatr 1979; 94: 993–995. [DOI] [PubMed] [Google Scholar]
- 65. Lee TC, Charles BG, Steer PA, Flenady VJ. Saliva as a valid alternative to serum in monitoring intravenous caffeine treatment for apnea of prematurity. Ther Drug Monit 1996; 18: 288–293. [DOI] [PubMed] [Google Scholar]
- 66. Chioukh FZ, Chaabane A, Hamida HB, Ben Ameur K, Aouam K, Monastiri K. Saliva as an alternative to plasma in therapeutic drug monitoring of caffeine in preterm infants. J Matern Fetal Med 2014; 27 (S1): 398. [Google Scholar]
- 67. Dobson NR, Liu X, Rhein LM, Darnall RA, Corwin MJ, McEntire BL, et al Salivary caffeine concentrations are comparable to plasma concentrations in preterm infants receiving extended caffeine therapy. Br J Clin Pharmacol 2016; 82: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chee KY, Lee D, Byron D, Naidoo D, Bye A. A simple collection method for saliva in children: potential for home monitoring of carbamazepine therapy. Br J Clin Pharmacol 1993; 35: 311–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moreland TA, Priestman DA, Rylance GW. Saliva carbamazepine levels in children before and during multiple dosing. Br J Clin Pharmacol 1982; 13: 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bartels H, Gunther E, Wallis S. Flow‐dependent salivary primidone levels in epileptic children. Epilepsia 1979; 20: 431–436. [DOI] [PubMed] [Google Scholar]
- 71. Miles MV, Tennison MB, Greenwood RS, Benoit SE, Thorn MD, Messenheimer JA, et al Evaluation of the Ames seralyzer for the determination of carbamazepine, phenobarbital, and phenytoin concentrations in saliva. Ther Drug Monit 1990; 12: 501–510. [DOI] [PubMed] [Google Scholar]
- 72. Miles MV, Tennison MB, Greenwood RS. Intraindividual variability of carbamazepine, phenobarbital, and phenytoin concentrations in saliva. Ther Drug Monit 1991; 13: 166–171. [DOI] [PubMed] [Google Scholar]
- 73. Schramm W, Annesley TM, Siegel GJ, Sakellares JC, Smith RH. Measurement of phenytoin and carbamazepine in an ultrafiltrate of saliva. Ther Drug Monitor 1991; 13: 452–460. [DOI] [PubMed] [Google Scholar]
- 74. Westenberg HG, Van Der Kleijn E, Oei TT, De Zeeuw RA. Kinetics of carbamazepine and carbamazepine‐epoxide, determined by use of plasma and saliva. Clin Pharmacol and Ther 1978; 23: 320–328. [DOI] [PubMed] [Google Scholar]
- 75. Chambers R, Homeida M, Hunter KR, Teague RH. Salivary carbamazepine concentrations. The Lancet 1977; 309: 656–657. [DOI] [PubMed] [Google Scholar]
- 76. Rylance GW, Moreland TA, Butcher GM. Carbamazepine dose‐frequency requirement in children. Arch Dis Child 1979; 54: 454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rui‐Rui L, Sheng XY, Ma LY, Yao HX, Cai LX, Chen CY, et al Saliva and plasma Monohydroxcarbazepine concentrations in pediatric patients with epilepsy. Ther Drug Monit 2016; 38: 365–370. [DOI] [PubMed] [Google Scholar]
- 78. Gallagher BB, Baumel IP, Primidone . Absorption, distribution, and excretion. Antiepileptic Drugs 1972; 357–359. [Google Scholar]
- 79. Chang HP, Anderson GC, Wood CE. Feasible and valid saliva collection for cortisol in transitional newborn infants. Nurs Res 1995; 44: 117–119. [PubMed] [Google Scholar]
- 80. Bermejo AM, Lucas ACS, Tabernero MJ. Saliva/plasma ratio of methadone and EDDP. J Anal Toxicol 2000; 24: 70–72. [DOI] [PubMed] [Google Scholar]
- 81. Quattrocchi F, Karnes HT, Robinson JD, Hendeles L. Effect of serum separator blood collection tubes on drug concentrations. Ther Drug Monit 1983; 5: 359–362. [DOI] [PubMed] [Google Scholar]
- 82. Dasgupta A, Dean R, Saldana S, Kinnaman G, McLawhon RW. Absorption of therapeutic drugs by barrier gels in serum separator blood collection tubes: volume‐and time‐dependent reduction in total and free drug concentrationsa. Am J Clin Pathol 1994; 101: 456–461. [DOI] [PubMed] [Google Scholar]
- 83. Marquet P, Sauvage FL, Loustaud‐Ratti V, Babany G, Rousseau A, Lachatre G. Stability of ribavirin concentrations depending on the type of blood collection tube and preanalytical conditions. Ther Drug Monit 2010; 32: 237–241. [DOI] [PubMed] [Google Scholar]
- 84. Tennison M, Ali I, Miles MV. Feasibility and acceptance of salivary monitoring of antiepileptic drugs via the US postal service. Ther Drug Monit 2004; 26: 295–299. [DOI] [PubMed] [Google Scholar]
- 85. Tal A, Aviram M, Gorodischer R. Variations in theophylline concentrations detected by 24 hour saliva concentration profiles in ambulatory children with asthma. J Allergy Clin Immunol 1990; 86: 238–243. [PubMed] [Google Scholar]
- 86. Koysooko R, Ellis EF, Levy G. Relationship between theophylline concentration in plasma and saliva of man. Clin Pharmacol and Ther 1974; 15: 454–460. [DOI] [PubMed] [Google Scholar]
- 87. Plavsic F, Culig J, Bakran I, Vrhovac B. Theophylline concentration in saliva as a guide for individualization of its therapeutic use. Brit J Clin Pharmaco 1981; 1: 533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Culig J, Johnston A, Turner P. Saliva theophylline concentrations after a single oral dose. Br J Clin Pharmacol 1982; 13: 243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Knott CH, Bateman M, Reynolds F. Do saliva concentrations predict plasma unbound theophylline concentrations? A problem re‐examined. Brit J Clin Pharmaco 1984; 1: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ebden P, Leopold D, Buss D, Smith AP, Routledge PA. Relationship between saliva and free and total plasma theophylline concentrations in patients with chronic airflow obstruction. Thorax 1985; 1: 526–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Paxton JW, Whiting B, Stephen KW. Phenytoin concentrations in mixed, parotid and submandibular saliva and serum measured by radioimmunoassay. Brit J Clin Pharmaco 1977; 1: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Knott C, Hamshaw‐Thomas A, Reynolds F. Phenytoin‐valproate interaction: importance of saliva monitoring in epilepsy. Br Med J 1982; 2: 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tsanaclis LM, Allen J, Perucca E, et al Effect of valproate on free plasma phenytoin concentrations. Br J Clin Pharmacol 1984; 18: 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Van HG. Comparative study of the levels of anticonvulsants and their free fractions in venous blood, saliva and capillary blood in man. J Pharmacol 1984; 15: 27–35. [PubMed] [Google Scholar]
- 95. Knott C, Williams CP, Reynolds F. Phenytoin kinetics during pregnancy and the puerperium. BJOG 1986; 1: 1030–1037. [DOI] [PubMed] [Google Scholar]
- 96. Luoma PV, Heikkinen JE, Ylostalo PR. Phenobarbital pharmacokinetics and salivary and serum concentrations in pregnancy. Ther Drug Monit 1982; 4: 65–68. [DOI] [PubMed] [Google Scholar]
- 97. Shen D. Saliva phenobarbital concentration in epileptics. Chung Hua Shen Ching Ching Shen Ko Tsa Chih 1989; 22: 369–370. [PubMed] [Google Scholar]
- 98. Jusko WJ, Gerbracht L, Golden LH, Koup JR. Digoxin concentrations in serum and saliva. Commun Chem Pathol Pharmacol 1975; 10: 189–192. [PubMed] [Google Scholar]
- 99. Van der Vijgh WJF. Comparison of salivary digoxin concentration with plasma levels in man. Neth J Med 1975; 18: 269–272. [Google Scholar]
- 100. Jourbert PH, Muller FO, Aucamp BM. Salivary digoxin concentration in saliva and serum. Brit J Clin Pharmaco 1976; 3: 673–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Allonen H, Iisalo E, Kangas L, Lammintausta R, Salonen M. Estimation of pharmacokinetic parameters of digoxin from serum, saliva and urine. Int J Clin Pharmacol Biopharm 1978; 16: 420–423. [PubMed] [Google Scholar]
- 102. Lazowski J, Lypka A, Borkowski P. The relationship between digoxin concentration in saliva and serum. Pol Tyg Lek 1978; 30: 1709–1711. [PubMed] [Google Scholar]
- 103. Mahmod S, Smith DS, Landon J. Radioimmunoassay of salivary digoxin by simple adaptation of a kit method for serum digoxin: saliva/serum ratio and correlation. Ther Drug Monit 1987; 9: 91–96. [DOI] [PubMed] [Google Scholar]
- 104. Cohen AF, Ashby L, Crowley D, et al Lamotrigine (BW430C), a potential anticonvulsant. Effects on the central nervous system in comparison with phenytoin and diazepam. Br J Clin Pharmacol 1985; 20: 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Trnavska Z, Krejcova H, Ykaczykovam Z, et al Pharmacokinetics of lamotrigine (Lamictal) in plasma and saliva. Eur J Drug Metab Pharmacokinet 1991; 3: 211–215. [PubMed] [Google Scholar]
- 106. Incecayir T, Agabeyoglu I, Gucuyener K. Comparison of plasma and saliva concentrations of lamotrigine in healthy volunteers. Arzneimittelforschung 2007; 57: 517–521. [DOI] [PubMed] [Google Scholar]
- 107. Koks CH, Crommentuyn KM, Hoetelmans RM, Mathôt RA, Beijnen JH. Can fluconazole concentrations in saliva be used for therapeutic drug monitoring? Ther Drug Monit 2001; 1: 449–453. [DOI] [PubMed] [Google Scholar]
- 108. Koks CH, Rosing H, Meenhorst PL, Bult A, Beijnen JH. High‐performance liquid chromatographic determination of the antifungal drug fluconazole in plasma and saliva of human immunodeficiency virus‐infected patients. J Chromatogr 1995; 20: 345–351. [DOI] [PubMed] [Google Scholar]
- 109. Paxton JW, Donald RA. Concentrations and kinetics of carbamazepine in whole saliva, parotid saliva, serum ultrafiltrate, and serum. Clin Pharmacol Ther 1980; 28: 695–702. [DOI] [PubMed] [Google Scholar]
- 110. Kristensen O, Larsen HF. Value of saliva samples in monitoring carbamazepine concentrations in epileptic patients. Acta Neurol Scand 1980; 1: 344–350. [DOI] [PubMed] [Google Scholar]
- 111. MacKichan JJ, Duffner PK, Cohen ME. Salivary concentrations and plasma protein binding of carbamazepine and carbamazepine‐10, 11‐epoxide in epileptic patients. Br J Clin Pharmacol 1981; 12: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vasudev A, Tripathi KD, Puri V. Correlation of serum and salivary carbamazepine concentration in epileptic patients: implications for therapeutic drug monitoring. Neurol India 2002; 1: 60. [PubMed] [Google Scholar]
- 113. Đorđević S, Kilibarda V, Stojanović T. Determination of carbamazepine in serum and saliva samples by high performance liquid chromatography with ultraviolet detection. Vojnosanit Pregl 2009; 66: 347–352. [DOI] [PubMed] [Google Scholar]
- 114. Thiesohn M, Heimann G. Disposition of the anti‐epileptic oxcarbazepine and its metabolites in healthy volunteers. Eur J Clin Pharmacol 1982; 1: 545–551. [DOI] [PubMed] [Google Scholar]
- 115. Kristensen O, Klitgaard NA, Jonsson B, et al Pharmacokinetics of 10‐OH‐carbazepine, the main metabolite of the antiepileptic oxcarbazepine from serum and saliva concentrations. Acta Neurol Scand 1983; 1: 145–150. [DOI] [PubMed] [Google Scholar]
- 116. Klitgaard NA, Kristensen O. Use of saliva for monitoring oxcarbazepine therapy in epileptic patients. Eu J Clin Pharmacol 1986; 31: 91–94. [DOI] [PubMed] [Google Scholar]
- 117. Cardot JM, Degen P, Flesch G, Menge P, Dieterle W. Comparison of plasma and saliva concentrations of the active monohydroxy metabolite of oxcarbazepine in patients at steady state. Biopharm Drug Dispos 1995; 16: 603–614. [DOI] [PubMed] [Google Scholar]
- 118. Miles MV, Tang PH, Ryan MA. Feasibility and limitations of oxcarbazepine monitoring using salivary monohydroxycarbamazepine (MHD). Ther Drug Monit 2004; 1: 300–304. [DOI] [PubMed] [Google Scholar]
- 119. Zylber‐Katz E, Granit L, Levy M. Relationship between caffeine concentrations in plasma and saliva. Clin Pharmacol and Ther 1984; 36: 133–137. [DOI] [PubMed] [Google Scholar]
- 120. Newton R, Broughton LJ, Lind MJ, Morrison PJ, Rogers HJ, Bradbrook ID. Plasma and salivary pharmacokinetics of caffeine in man. Eu J Clin Pharmaco 1981; 21: 45–52. [DOI] [PubMed] [Google Scholar]