Abstract
Aims
It remains inconclusive whether the use of nonsteroidal anti‐inflammatory drugs (NSAIDs) increases the risk of atrial fibrillation (AF), especially in middle‐aged Asian populations. In this study, we evaluated the association between NSAID use and the risk of AF in a nationwide population‐based study of middle‐aged individuals in Taiwan.
Methods
A nested case–control study was conducted using the National Health Insurance Research Database (NHIRD) in Taiwan. We identified the cases with a diagnosis of AF (ICD‐9‐CM codes: 427.31) and the matched controls from three independent Longitudinal Health Insurance Databases (LHIDs) derived from the NHIRD from data collected from 2001 to 2013. Conditional logistic regression models with covariate adjustment were performed to evaluate the association between NSAID use and the risk of AF.
Results
A total of 57 058 participants (28 529 AF cases and 28 529 matched controls) were included. Participants with NSAID use had an elevated risk of AF compared to non‐users [adjusted odds ratio (AOR) = 1.18, 95% confidence interval (CI): 1.14–1.23]. When further assessing the effects of different classes of NSAIDs on the risk of AF, the results showed that participants who used non‐selective NSAIDs had a significantly elevated risk of AF (AOR = 1.18, 95% CI: 1.13–1.23), as did participants with a combined use of selective and non‐selective NSAIDs (AOR = 1.30, 95% CI: 1.21–1.39).
Conclusions
NSAID use was associated with an increased risk of AF occurrence among the participants included in our study cohort. Closely monitoring the adverse effects of NSAID treatment on the risk of AF will be important, particularly among individuals at high risk.
Keywords: atrial fibrillation, middle‐aged population, nationwide population‐based study, nonsteroidal anti‐inflammatory drugs
What is Already Known about this Subject
Atrial fibrillation (AF) affects approximately 0.5% of the general population, but more than 6% of the elderly population, and the prevalence of AF has been rising during the past decades.
It remains inconclusive whether the use of nonsteroidal anti‐inflammatory drugs (NSAIDs) increases risk of AF, especially in middle‐aged Asian populations.
What this Study Adds
We found that NSAID use was associated with an increased risk of atrial fibrillation occurrence among our study participants from an Asian population.
Based on these findings, it will be important to closely monitor the adverse effects of NSAID treatment on the risk of AF, particularly among individuals at high risk.
The underlying mechanisms associated with our findings deserve further investigation.
Introduction
Atrial fibrillation (AF), a common cardiac arrhythmia, affects approximately 0.5% of the general population, but more than 6% of the elderly population 1, 2. It is noteworthy that the prevalence of AF has been rising during the past decades 3. Several studies have reported that AF is associated with an elevated risk of cardiovascular or cerebrovascular diseases and death 4, 5, 6. The underlying mechanisms that lead to the development of AF remain unclear, but recent studies have suggested that inflammation may precede the pathogenesis of AF 7, 8.
Previous studies have documented a positive association between the use of non‐steroidal anti‐inflammatory drugs (NSAIDs) and the risk of AF, but limited studies have been conducted in Asian populations 9, 10, 11, 12, 13. NSAIDs are cyclooxygenase (COXs)‐mediated inhibitors, and NSAID mechanisms are related to the inhibition of the formation of prostaglandins, prostacyclins and thromboxanes 14, 15. For these reasons, at present, NSAIDs are widely prescribed as symptomatic treatment for various clinical conditions, e.g., acute pain, chronic inflammatory and degenerative joint diseases, etc. Looking more closely, in consideration of the clinical and public health implications and their widespread use, it is important to confirm the previously observed association between NSAID use and AF 16.
Although previous studies have investigated the role of NSAIDs on AF, most were conducted in general populations. Limited studies have evaluated the adverse effects of NSAIDs on AF in a middle‐aged population, especially Asian populations. To extend our understanding of this issue, this study presents the findings of a nested case–control study designed to elucidate the effects of NSAID use on AF occurrence in a middle‐aged population using a large nationwide population‐based cohort in Taiwan.
Methods
Data source
This study used data derived from three different Longitudinal Health Insurance Databases (LHIDs) comprised of medical claims data, which are part of the National Health Insurance Research Database (NHIRD) in Taiwan. In detail, the NHIRD includes demographic characteristics, outpatient visits and inpatient claims data, prescription records and disease diagnoses for approximately 99% of the entire population of 23 million people in Taiwan 17. The LHIDs were generated by randomly selecting one million enrolees from the National Health Insurance (NHI) programme in 2000 (LHID 2000), 2005 (LHID 2005) and 2010 (LHID 2010), separately. As a result, data from a total of three million enrolees were investigated in this study. All medical claims data in the LHIDs from January 1, 2001 to December 31, 2013 were included, accordingly. The study protocol was approved by the Institutional Review Board of the National Health Research Institutes, Taiwan.
Cases of atrial fibrillation and matched controls
The incident cases of AF were defined as individuals aged 45 years and older in 2001 with medical claims (either a one‐time hospitalization or at least two or more outpatient visits within one year) for AF (ICD‐9‐CM codes: 427.31) during the study period. The date of the first medical claim record for AF was identified as the index date. We applied three different matching algorithms: (1) matching by index year, age and gender; (2) matching by index year, age, gender and four related diseases; and (3) matching by disease risk score (DRS) at the fourth decimal point by accounting for potential confounding factors (Table S1). In detail, the DRS, which in this case was a patient's predicted probability of developing AF, was estimated using logistic regression 18 and calculated based on the individual covariate values multiplied by the regression coefficients. All of the considered covariates (as shown in Table S1) in the year prior to the index date were included in the logistic regression model without variable selection. We selected the second matching algorithm for subsequent analyses in this study. Specifically, for each case, we randomly selected one control who did not have any AF diagnosis at the time that the matched case was first diagnosed with AF. The controls were matched with the case by age (the same birth year), sex, type 2 diabetes mellitus (T2DM), hypertension, congestive heart failure (CHF), osteoarthritis, and index year. Matched controls were assigned the same index date as their matched cases. Of note, among cases, participants were excluded if they were ever diagnosed as having AF or valvular heart disease (VHD) prior to 2004. For both cases and controls, we excluded participants taking part in the NHI programme for less than one year before the index date.
Utilization of nonsteroidal anti‐inflammatory drugs (NSAIDs)
A list of NSAIDs was identified from prescription records in the LHIDs, including: the different medication components, time of prescription, and duration of drug supply and dosage, individually. Specifically, NSAIDs were classified as follows: (1) selective COX‐2 inhibitors: celecoxib, etoricoxib and rofecoxib; and (2) non‐selective COX‐1/COX‐2 inhibitors: aceclofenac, acemetacin, alclofenac, alminoprofen, diclofenac, diflunisal, etodolac, fenbufen, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, ketoprofen, ketorolac, mefenamic acid, meloxicam, nabumetone, naproxen, niflumic acid, nimesulide, piroxicam, sulindac, tenoxicam, and indomethacin. We also grouped NSAIDs into: high COX‐2 selectivity: celecoxib, etoricoxib, rofecoxib; intermediate COX‐2 selectivity: aceclofenac, etodolac, nabumetone, nimesulide, meloxicam, diclofenac, diflunisal, piroxicam, sulindac; and more selective for COX‐1: acemetacin, alclofenac, alminoprofen, fenbufen, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, mefenamic acid, naproxen, niflumic acid, tenoxicam. In addition, acetaminophen was also examined in this study.
NSAID exposure
We investigated the effect of long‐term NSAID use on the development of AF. The definition of exposure to NSAID use in this study was as follows. First, participants exposed to NSAIDs were defined as ‘users’ if they had at least one day of an NSAID supply within 365 days prior to the index date. Next, among the users, we defined participants as ‘current users’ if they were exposed to NSAIDs within 30 days prior to the index date, or as ‘past users’ if they were exposed to NSAIDs within 31–365 days (but not within 30 days) prior to the index date. Furthermore, current users were defined as ‘new users’ if they were first exposed to NSAIDs during 30 days prior to the index date, but not exposed to NSAIDs between 31 and 365 days prior to the index date, or ‘continuous users’ if NSAIDs were started between 31–365 days prior to the index date. Participants were defined as ‘non‐users’ if no NSAID was prescribed within 365 days prior to the index date 1, 11.
Adjustment of potential confounding factors
The list of potential confounders considered in this study included medical comorbid disorders (anaemia, coronary heart disease, myocardial infarction (MI), heart failure, cerebrovascular disease, coronary artery disease, chronic obstructive pulmonary disease (COPD), inflammatory bowel disease, malignant neoplasm, peripheral vascular disease, Parkinson's disease, rheumatoid arthritis and renal failure), concomitant medications (antidepressants, anxiolytics, anticonvulsants, antihypertensives, bisphosphonates, benzodiazepines, inhaled glucocorticosteroids, oral glucocorticosteroids, hormone replacement therapy), and healthcare utilization within one year prior to the index date (both outpatient visits and hospitalizations) 11, 19.
Data analysis
Descriptive statistics of the AF cases and matched controls (e.g., demographic and clinical characteristics, comorbid medical disorders, concomitant medication use, and healthcare utilization) were summarized as counts and percentages, or as mean and standard deviation (SD). To compare the distribution of demographic and clinical characteristics between the AF cases and matched controls, a chi‐square test was used for testing discrete variables and the Student's t‐test was used for testing continuous variables. Conditional logistic regression analyses (with and without covariate adjustment) were performed to determine the association of NSAID use (overall, selective and non‐selective NSAIDs, individually), defined daily dose (DDD) of NSAIDs, which was defined as ‘the assumed maintenance dose per day for a drug used for its main indication’, and number of prescriptions, respectively, with AF. We further examined the various exposure statuses, different classes, and each individual drug effect of the NSAIDs. If participants took two or more classes of NSAIDs at the same time, we defined those participants as combined users. The covariates that were adjusted for in the models are listed above.
We declared P‐values less than 0.05 as statistically significant. All analyses were carried out using SAS version 9.2 for Windows (SAS Institute, Cary, NC).
Results
A total of 57 058 participants (28 529 AF cases and 28 529 matched controls) were included in the study. Among them, there were 21 416 participants from LHID 2000 (10 708 AF cases and 10 708 matched controls), 22 014 participants from LHID 2005 (11 007 AF cases and 11 007 matched controls), and 17 808 participants from LHID 2010 (8904 AF cases and 8904 matched controls), respectively. Figure 1 depicts the detailed flow chart for identifying participants included in the study cohort. Of note, we excluded participants who were in two out of the three LHID datasets (n = 2090). The distributions of demographic and clinical characteristics, concomitant medication use, and healthcare utilization in LHID2000, LHID2005, LHID2010 and the three LHID combined datasets are presented in Table S2 and Table 1, respectively.
Figure 1.
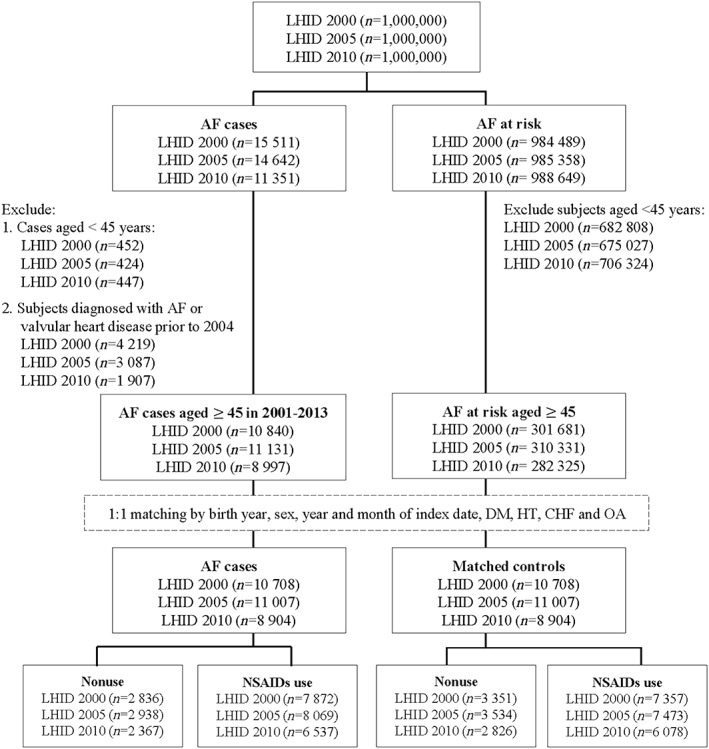
Flow chart of inclusion/exclusion criteria for study population
Note: AF, atrial fibrillation; DM, diabetes mellitus; HT, hypertension; CHF, congestive heart failure; OA, osteoarthritis
Table 1.
Demographic and clinical characteristics among study participants across three different datasets
| Database | Three LHID combined | |||||
|---|---|---|---|---|---|---|
| Characteristic | Controls | AF | ORa | 95% CI | ||
| (n = 28 529) | (n = 28 529) | |||||
| Demographic characteristics | ||||||
| Age (years; n, %) | ||||||
| 45–54 | 2142 | (7.51) | 2142 | (7.51) | N/A | N/A |
| 55–64 | 4631 | (16.23) | 4631 | (16.23) | ||
| 65–74 | 7362 | (25.81) | 7362 | (25.81) | ||
| ≥75 | 14 394 | (50.45) | 14 394 | (50.45) | ||
| Gender (n, %) | ||||||
| Male | 15 595 | (54.66) | 15 595 | (54.66) | N/A | N/A |
| Female | 12 934 | (45.34) | 12 934 | (45.34) | ||
| Clinical characteristics: comorbidities | ||||||
| T2DM | 6815 | (23.89) | 6815 | (23.89) | N/A | N/A |
| CKD | 2528 | (8.86) | 3669 | (12.86) | 1.56 | 1.47–1.65 |
| Hypertension | 17 183 | (60.23) | 17 183 | (60.23) | N/A | N/A |
| MI | 500 | (1.75) | 981 | (3.44) | 2.02 | 1.81–2.25 |
| CHF | 9353 | (32.78) | 9353 | (32.78) | N/A | N/A |
| Sleep apnoea | 4331 | (15.18) | 4704 | (16.49) | 1.11 | 1.06–1.16 |
| Hyperthyroidism | 146 | (0.51) | 277 | (0.97) | 1.90 | 1.56–2.33 |
| AS | 67 | (0.23) | 71 | (0.25) | 1.06 | 0.76–1.48 |
| SLE | 29 | (0.10) | 30 | (0.11) | 1.03 | 0.62–1.72 |
| RA | 273 | (0.96) | 341 | (1.20) | 1.26 | 1.07–1.47 |
| Osteoarthritis | 7012 | (24.58) | 7012 | (24.58) | N/A | N/A |
| Gout | 2784 | (9.76) | 3311 | (11.61) | 1.22 | 1.16–1.29 |
| CAD | 5676 | (19.90) | 9147 | (32.06) | 2.05 | 1.96–2.13 |
| VHD | 890 | (3.12) | 2081 | (7.29) | 2.53 | 2.32–2.74 |
| Concomitant medication use | ||||||
| Antidepressants | 2811 | (9.85) | 3391 | (11.89) | 1.24 | 1.18–1.31 |
| Anticonvulsants | 2562 | (8.98) | 3287 | (11.52) | 1.32 | 1.25–1.40 |
| Anxiolytics | 10 506 | (36.83) | 12 735 | (44.64) | 1.41 | 1.36–1.46 |
| Benzodiazepines | 4822 | (16.90) | 6020 | (21.10) | 1.33 | 1.27–1.39 |
| Bisphosphonates | 67 | (0.23) | 72 | (0.25) | 1.08 | 0.77–1.50 |
| Glucocorticosteroids | 4142 | (14.52) | 5106 | (17.90) | 1.29 | 1.23–1.35 |
| HRT | 606 | (2.12) | 700 | (2.45) | 1.17 | 1.04–1.31 |
Conditional logistic regression was used to compute odds ratio and 95% confidence interval.
AF, atrial fibrillation; AS, ankylosing spondylitis; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; HRT, hormone replacement therapy; LHID, Longitudinal Health Insurance Database; MI, myocardial infarction; OR, odds ratio; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; T2DM, type 2 diabetes mellitus; VHD, valvular heart disease.
In addition, we applied three matching algorithms as presented in Table S1. The results were comparable across the different matching algorithms. Given this finding, we used the dataset that was created from individual disease matching; that is, the controls were matched with each AF case by age (the same birth year), sex, T2DM, hypertension, CHF, osteoarthritis, and index year, to perform the subsequent analyses, and reported the results.
Table 2 presents the association between NSAID use on the risk of AF. Compared to non‐users, participants with NSAID use had a positive association with AF in the LHID combined dataset [adjusted odds ratio (AOR) = 1.18, 95% confidence interval (CI): 1.14–1.23]. When further classifying NSAID use into new users, continuous users and past users, similar associations between NSAID use and increased risk of AF were found (AOR = 2.18, 95% CI: 1.95–2.43 for new users; AOR = 1.44, 95% CI: 1.36–1.52 for continuous users; AOR = 1.05, 95% CI: 1.01–1.10 for past users). Additionally, similar results were observed in each LHID dataset (LHID 2000, LHID 2005 and LHID 2010) (Table 2). In addition, when we treated 3 months prior to the index date as the latent period in this study and repeated the analyses accordingly, similar results were observed (Table S3).
Table 2.
Association between NSAID use and atrial fibrillation, based on various NSAID exposure statuses
| Controls | AF | Total | COR | 95% CI | AORa | 95% CI | |
|---|---|---|---|---|---|---|---|
| LHID2000 | |||||||
| Non‐users | 3351 (31.29) | 2836 (26.48) | 6187 | ||||
| Users | 7357 (68.71) | 7872 (73.52) | 15 229 | 1.29 | 1.22–1.38 | 1.15 | 1.08–1.23 |
| New users | 198 (1.85) | 399 (3.73) | 597 | 2.40 | 2.01–2.87 | 2.28 | 1.89–2.74 |
| Continuous users | 2045 (19.10) | 2718 (25.38) | 4763 | 1.64 | 1.52–1.78 | 1.42 | 1.30–1.55 |
| Past users | 5114 (47.76) | 4755 (44.41) | 9869 | 1.12 | 1.05–1.20 | 1.02 | 0.95–1.09 |
| Total | 10 708 | 10 708 | 21 416 | ||||
| LHID2005 | |||||||
| Non‐users | 3534 (32.11) | 2938 (26.69) | 6472 | ||||
| Users | 7473 (67.89) | 8069 (73.31) | 15 542 | 1.34 | 1.26–1.42 | 1.20 | 1.13–1.29 |
| New users | 244 (2.22) | 407 (3.70) | 651 | 2.04 | 1.72–2.41 | 1.99 | 1.67–2.36 |
| Continuous users | 2071 (18.82) | 2728 (24.78) | 4799 | 1.66 | 1.53–1.80 | 1.45 | 1.33–1.58 |
| Past users | 5158 (46.86) | 4934 (44.83) | 10 092 | 1.19 | 1.11–1.27 | 1.08 | 1.01–1.16 |
| Total | 11 007 | 11 007 | 22 014 | ||||
| LHID2010 | |||||||
| Non‐users | 2826 (31.74) | 2367 (26.58) | 5193 | ||||
| Users | 6078 (68.26) | 6537 (73.42) | 12 615 | 1.31 | 1.23–1.40 | 1.18 | 1.10–1.27 |
| New users | 175 (1.97) | 313 (3.52) | 488 | 2.15 | 1.77–2.61 | 2.14 | 1.75–2.61 |
| Continuous users | 1721 (19.33) | 2303 (25.86) | 4024 | 1.66 | 1.52–1.82 | 1.46 | 1.32–1.60 |
| Past users | 4182 (46.97) | 3921 (44.04) | 8103 | 1.14 | 1.06–1.23 | 1.04 | 0.96–1.12 |
| Total | 8904 | 8904 | 17 808 | ||||
| Three LHID combined | |||||||
| Non‐users | 9065 (31.77) | 7581 (26.57) | 16 646 | ||||
| Users | 19 464 (68.23) | 20 948 (73.43) | 40 412 | 1.32 | 1.27–1.37 | 1.18 | 1.14–1.23 |
| New users | 573 (2.01) | 1058 (3.71) | 1631 | 2.24 | 2.01–2.49 | 2.18 | 1.95–2.43 |
| Continuous users | 5441 (19.07) | 7188 (25.20) | 12 629 | 1.65 | 1.57–1.74 | 1.44 | 1.36–1.52 |
| Past users | 13 450 (47.15) | 12 702 (44.52) | 26 152 | 1.16 | 1.11–1.21 | 1.05 | 1.01–1.10 |
| Total | 28 529 | 28 529 | 57 058 | ||||
Adjusted variables included: medical comorbid disorders (CKD, MI, sleep apnoea, hyperthyroidism, ankylosing spondylitis, SLE, RA, gout, CAD and VHD), and concomitant medication use (antidepressants, anticonvulsants, anxiolytics, benzodiazepines, bisphosphonates, glucocorticosteroids and HRT).
AF, atrial fibrillation; AOR, adjusted odds ratio; CAD, coronary artery disease; CKD, chronic kidney disease; COR, crude odds ratio; HRT, hormone replacement therapy; LHID, Longitudinal Health Insurance Database; MI, myocardial infarction; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; VHD, valvular heart disease.
When further assessing the effects of different classes of NSAIDs on the risk of AF (Table 3), we found that participants taking non‐selective NSAIDs had a significantly elevated risk of AF (AOR = 1.18, 95% CI: 1.13–1.23) as did participants with a combined use of selective and non‐selective NSAIDs (AOR = 1.30, 95% CI: 1.21–1.39) in the LHID combined dataset, but this was not true for participants taking selective NSAIDs only. We also examined the effects of each individual NSAID on AF among participants in LHID 2000, LHID 2005 and LHID 2010, separately. The results suggest that various individual NSAIDs might have a different degree of adverse effect on the risk of AF (Tables S2–S4). Instead of comparing non‐selective NSAIDs and selective COX‐2 inhibitors, we further grouped the drugs by more selective for COX‐1, intermediate COX‐2 selectivity, and high COX‐2 selectivity, separately. Similar results were found as shown in Table 3. When investigating defined daily dose (DDD) and number of prescriptions, respectively, no dose–response effects were found for the use of NSAIDs (Table 4).
Table 3.
Association between NSAID use and atrial fibrillation, based on various classes of NSAIDs
| Controls | AF | Total | COR | 95% CI | AORa | 95% CI | |
|---|---|---|---|---|---|---|---|
| LHID2000 | |||||||
| Non‐user | 3351 (31.29) | 2836 (26.48) | 6187 | ||||
| Non‐selective NSAIDs | 6178 (57.70) | 6464 (60.37) | 12 642 | 1.27 | 1.19–1.35 | 1.14 | 1.07–1.22 |
| Selective NSAIDs | 214 (2.00) | 198 (1.85) | 412 | 1.17 | 0.95–1.44 | 1.10 | 0.88–1.36 |
| Combination | 965 (9.01) | 1210 (11.30) | 2175 | 1.60 | 1.44–1.78 | 1.32 | 1.18–1.48 |
| Total | 10 708 | 10 708 | 21 416 | ||||
| LHID2005 | |||||||
| Non‐user | 3534 (32.11) | 2938 (26.69) | 6472 | ||||
| Non‐selective NSAIDs | 6229 (56.59) | 6684 (60.72) | 12 913 | 1.33 | 1.25–1.42 | 1.20 | 1.13–1.29 |
| Selective NSAIDs | 216 (1.96) | 176 (1.60) | 392 | 1.03 | 0.84–1.27 | 0.97 | 0.78–1.20 |
| Combination | 1028 (9.34) | 1209 (10.98) | 2237 | 1.53 | 1.37–1.70 | 1.31 | 1.16–1.46 |
| Total | 11 007 | 11 007 | 22 014 | ||||
| LHID2010 | |||||||
| Non‐user | 2826 (31.74) | 2367 (26.58) | 5193 | ||||
| Non‐selective NSAIDs | 5088 (57.14) | 5424 (60.92) | 10 512 | 1.30 | 1.21–1.39 | 1.18 | 1.10–1.27 |
| Selective NSAIDs | 180 (2.02) | 177 (1.99) | 357 | 1.22 | 0.98–1.51 | 1.13 | 0.90–1.42 |
| Combination | 810 (9.10) | 936 (10.51) | 1746 | 1.47 | 1.30–1.65 | 1.19 | 1.04–1.35 |
| Total | 8904 | 8904 | |||||
| Three LHID combined | |||||||
| Non‐user | 9065 (31.77) | 7581 (26.57) | 16 646 | ||||
| Non‐selective NSAIDs | 16 308 (57.16) | 17 312 (60.68) | 33 620 | 1.30 | 1.25–1.36 | 1.18 | 1.13–1.23 |
| Selective NSAIDs | 568 (1.99) | 509 (1.78) | 1077 | 1.13 | 0.99–1.28 | 1.06 | 0.93–1.20 |
| Combination | 2588 (9.07) | 3127 (10.96) | 5715 | 1.55 | 1.45–1.66 | 1.30 | 1.21–1.39 |
| Total | 28 529 | 28 529 | 57 058 | ||||
| LHID2000 | |||||||
| Non‐user | 3351 (31.29) | 2836 (26.48) | 6187 | ||||
| Non‐selective NSAIDs | 1800 (16.81) | 1799 (16.80) | 3599 | 1.19 | 1.10–1.30 | 1.11 | 1.02–1.21 |
| Intermediate COX‐2 NSAIDs | 1604 (14.98) | 1581 (14.76) | 3185 | 1.20 | 1.10–1.31 | 1.12 | 1.02–1.22 |
| High COX‐2 NSAIDs | 215 (2.01) | 201 (1.88) | 416 | 1.17 | 0.95–1.43 | 1.09 | 0.88–1.35 |
| Combination | 3738 (34.91) | 4291 (40.07) | 8029 | 1.42 | 1.32–1.52 | 1.21 | 1.12–1.31 |
| Total | 10 708 | 10 708 | 21 416 | ||||
| LHID2005 | |||||||
| Non‐user | 3534 (32.11) | 2938 (26.69) | 6472 | ||||
| Non‐selective NSAIDs | 1753 (15.93) | 1921 (17.45) | 3674 | 1.34 | 1.23–1.45 | 1.27 | 1.17–1.38 |
| Intermediate COX‐2 NSAIDs | 1688 (15.34) | 1555 (14.13) | 3243 | 1.14 | 1.05–1.25 | 1.06 | 0.97–1.16 |
| High COX‐2 NSAIDs | 217 (1.97) | 177 (1.61) | 394 | 1.03 | 0.84–1.26 | 0.96 | 0.78–1.19 |
| Combination | 3815 (34.66) | 4416 (40.12) | 8231 | 1.47 | 1.37–1.58 | 1.27 | 1.17–1.37 |
| Total | 11 007 | 11 007 | 22 014 | ||||
| LHID2010 | |||||||
| Non‐user | 2826 (31.74) | 2367 (26.58) | 5193 | ||||
| Non‐selective NSAIDs | 1474 (16.55) | 1471 (16.52) | 2945 | 1.21 | 1.10–1.32 | 1.15 | 1.05–1.26 |
| Intermediate COX‐2 NSAIDs | 1339 (15.04) | 1347 (15.13) | 2686 | 1.23 | 1.12–1.36 | 1.14 | 1.03–1.26 |
| High COX‐2 NSAIDs | 183 (2.06) | 181 (2.03) | 364 | 1.23 | 0.99–1.53 | 1.13 | 0.90–1.42 |
| Combination | 3082 (34.61) | 3538 (39.73) | 6620 | 1.43 | 1.32–1.55 | 1.23 | 1.13–1.34 |
| Total | 8904 | 8904 | 17 808 | ||||
| Three LHID combined | |||||||
| Non‐user | 9065 (31.77) | 7581 (26.57) | 16 646 | ||||
| Non‐selective NSAIDs | 4715 (16.53) | 4842 (16.97) | 9557 | 1.24 | 1.18–1.31 | 1.17 | 1.11–1.24 |
| Intermediate COX‐2 NSAIDs | 4284 (15.02) | 4191 (14.69) | 8475 | 1.20 | 1.14–1.27 | 1.12 | 1.06–1.18 |
| High COX‐2 NSAIDs | 572 (2.00) | 516 (1.81) | 1088 | 1.13 | 1.00–1.28 | 1.05 | 0.93–1.20 |
| Combination | 9893 (34.68) | 11 399 (39.96) | 21 292 | 1.45 | 1.38–1.51 | 1.24 | 1.18–1.30 |
| Total | 28 529 | 28 529 | 57 058 | ||||
Adjusted variables included: medical comorbid disorders (CKD, MI, sleep apnea, hyperthyroidism, ankylosing spondylitis, SLE, RA, gout, CAD and VHD), and concomitant medication use (antidepressants, anticonvulsants, anxiolytics, benzodiazepines, bisphosphonates, glucocorticosteroids and HRT).
AF, atrial fibrillation; AOR, adjusted odds ratio; CAD, coronary artery disease; CKD, chronic kidney disease; COR, crude odds ratio; HRT, hormone replacement therapy; LHID, Longitudinal Health Insurance Database; MI, myocardial infarction; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; VHD, valvular heart disease.
Table 4.
Association between NSAID use and atrial fibrillation in the three LHID combined dataset, grouped by defined daily dose (DDD) and cumulative prescriptions
| Controls | AF | Total | COR | 95% CI | AOR a | 95% CI | |
|---|---|---|---|---|---|---|---|
| Defined daily dose (DDD) | |||||||
| Non‐user | 9065 (31.77) | 7581 (26.57) | 16 646 | ||||
| DDD ≤ 0.5 | 9383 (32.89) | 10 980 (38.49) | 20 363 | 1.43 | 1.37–1.49 | 1.26 | 1.21–1.32 |
| 0.5 < DDD ≤ 1 | 7780 (27.27) | 7791 (27.31) | 15 571 | 1.23 | 1.17–1.29 | 1.11 | 1.06–1.17 |
| 1 < DDD | 2301 (8.07) | 2177 (7.63) | 4478 | 1.15 | 1.07–1.23 | 1.08 | 1.01–1.16 |
| Total | 28 529 | 28 529 | 57 058 | ||||
| Cumulative prescriptions | |||||||
| Non‐user | 9065 (31.77) | 7581 (26.57) | 16 646 | ||||
| 0 < prescriptions ≤ 2 | 7012 (24.58) | 7362 (25.81) | 14 374 | 1.28 | 1.22–1.33 | 1.19 | 1.14–1.25 |
| 2 < prescriptions ≤ 6 | 5925 (20.77) | 6236 (21.86) | 12 161 | 1.30 | 1.24–1.37 | 1.17 | 1.11–1.23 |
| 6 < prescriptions | 6527 (22.88) | 7350 (25.76) | 13 877 | 1.41 | 1.35–1.48 | 1.19 | 1.13–1.26 |
| Total | 28 529 | 28 529 | 57 058 | ||||
Adjusted variables included: medical comorbid disorders (CKD, MI, sleep apnoea, hyperthyroidism, ankylosing spondylitis, SLE, RA, gout, CAD and VHD), and concomitant medication use (antidepressants, anticonvulsants, anxiolytics, benzodiazepines, bisphosphonates, glucocorticosteroids and HRT).
AF, atrial fibrillation; AOR, adjusted odds ratio; CAD, coronary artery disease; CKD, chronic kidney disease; COR, crude odds ratio; HRT, hormone replacement therapy; LHID, Longitudinal Health Insurance Database; MI, myocardial infarction; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; VHD, valvular heart disease.
Figures 2 and [Link], [Link], [Link] present the results of the subgroup analyses, stratified by age, sex, index year and various comorbid conditions [including T2DM, chronic kidney disease (CKD), hypertension, MI, CHF, sleep apnoea, osteoarthritis, gout and Charlson comorbidity index (CCI), separately]. No interactive effect among those examined factors was observed (all p‐values > 0.05).
Figure 2.
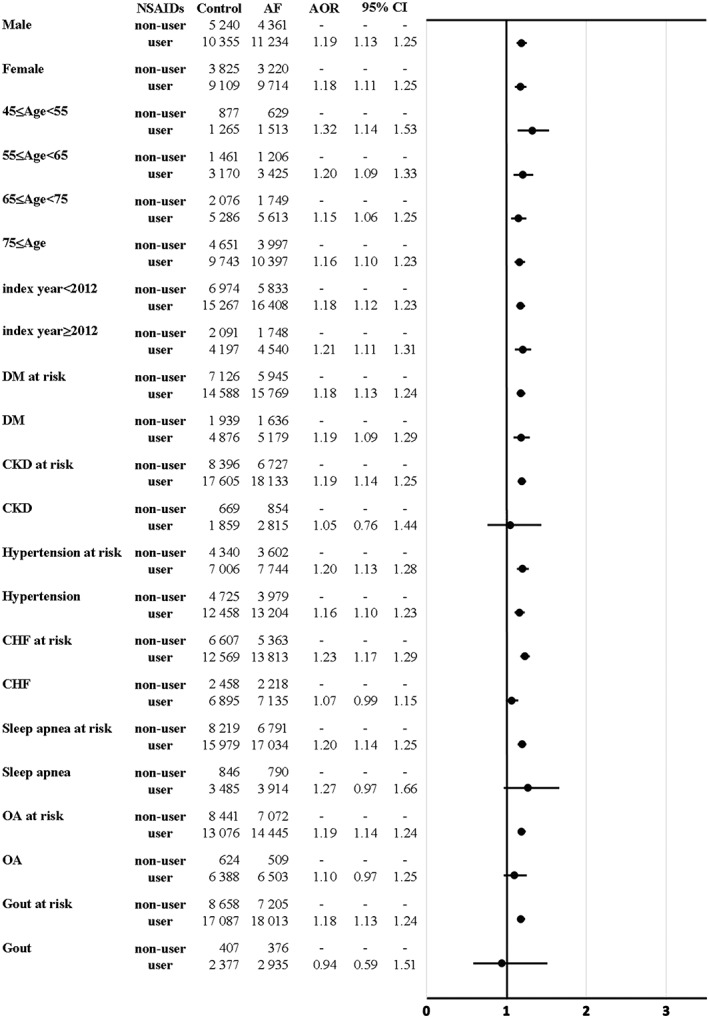
Risk of atrial fibrillation in relation to NSAID use among participants in the LHID combined dataset, stratified by age, gender and various clinical characteristics. a Adjusted variables included: medical comorbid disorders (chronic kidney disease, myocardial infarction, sleep apnoea, hyperthyroidism, ankylosing spondylitis, systemic lupus erythematosus, rheumatoid arthritis, gout, coronary artery disease and valvular heart disease), and concomitant medication use (antidepressants, anticonvulsants, anxiolytics, benzodiazepines, bisphosphonates, glucocorticosteroids and hormone replacement therapy)
Discussion
In this nationwide population‐based case–control study, our results provide supportive evidence that NSAID use is associated with increasing incidence of AF in a middle‐aged Taiwanese population. Importantly, positive associations between NSAID use and AF were found in each of the three independent LHID datasets and in the combined LHID dataset. When classifying NSAIDs as non‐selective NSAIDs, selective NSAIDs and combined NSAIDs, similar adverse effects of NSAID use on AF were observed among non‐selective NSAID and combined NSAID users.
Findings from this study are in line with results reported in previous studies 1, 20, 21. For instance, Schmidt et al. have suggested that NSAID use is positively associated with the risk of AF in a Danish population‐based study 11. Their study showed that increased risk of AF occurs during the initial period of NSAID use but may decrease over time. In addition, Krijthe et al., using data from the Rotterdam Study, have demonstrated that NSAID use is associated with an elevated risk of AF, especially among current users of NSAIDs 2. Consistently, Chao et al. have reported significant adverse effects of new NSAID use on AF in a Taiwanese population, particularly among patients at high risk, such as those with CKD or COPD 12.
It is known that NSAIDs inhibit cyclooxygenase isozymes (e.g., COX‐1 and COX‐2) and block biosynthesis of prostanoid (PG). As such, NSAIDs decrease total renal perfusion and redistribute renal blood flow, which may increase the risk of AF through adverse renal effects via fluid retention, electrolyte disturbances, and blood pressure destabilization. Furthermore, these changes may induce blood pressure elevation due to expansion of plasma volume, then increased left atrial (LA) pressure, LA stretch, consequently increased peripheral resistance, 22, 23 and fluctuation of serum potassium as a result of decreased potassium excretion in the distal nephron, which then triggers AF 24.
The strengths of this study include, first, the use of three independent datasets, specifically, LHID 2000, LHID 2005 and LHID 2010, to investigate the association between NSAID use and AF, in each of which we found similar results. These findings indicate the robustness of the observed adverse effects of NSAIDs on AF. Second, most studies have examined the associations between NSAID use and AF in general populations. While the majority of these previous studies have investigated the adverse effects of NSAID use on AF in European general populations, limited studies have reported its adverse effects on AF in Asian populations. This study is one of only a limited number of studies to evaluate the adverse effect on AF in Asian populations, especially in a middle‐aged Asian population. Third, we applied three different matching algorithms and repeated the analyses using LHID 2000, which could serve as a sensitivity analysis. Of note, similar results were observed no matter which matching algorithms were employed, which provided supportive confirmatory evidence in a single study.
Several limitations should be noted. First, we were not able to estimate the effect of over‐the‐counter NSAID use. However, this misclassification is likely to be non‐differential and lead to bias towards the null and an underestimation of risk. Second, we only examined the adverse effects of NSAID use on AF in Taiwanese study participants. It would be interesting to confirm these results in other ethnic populations, particularly in other Asian populations. Third, information on potential confounding factors such as smoking, alcohol and obesity are not available in the NHIRD. Therefore, we employed clinical proxy surrogates, for example, T2DM and hypertension for obesity, to adjust for those unmeasured confounding risk factors. However, it was likely that residual confounding effects could still exist due to those unmeasured variables. Fourth, the underlying pathophysiology of adverse effects of NSAID use on AF is still not well understood and merits further investigation. Fifth, the definition of NSAID use was somewhat limited, even though we defined NSAID use based on previous publications 1, 11. For instance, subjects with only one day of use were classified as users. However, the influence of this on our results should be limited as the percentage of subjects with only one day of NSAID use was small in this study.
In summary, the findings from this study indicate that NSAID use increases the risk of AF among middle‐aged adults in an Asian population. Based on these findings, it will certainly be important for physicians who prescribe NSAIDs to do so cautiously, especially among those at high risk for AF. Benefits and risks of NSAID use should be carefully evaluated when delivered in clinical practice. The underlying mechanisms associated with our findings deserve further investigation.
Competing Interests
There are no competing interests to declare.
We thank Tami R. Bartell at the Stanley Manne Children's Research Institute, Ann and Robert H. Lurie Children's Hospital of Chicago for English editing. This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes (Registered numbers: 99081, 99136, 99287, 101014, NHRID‐101‐548). The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health or National Health Research Institutes.
S.‐Y.C. and H.‐J.T. are supported in part by grants from the Ministry of Science and Technology and National Health Research Institutes (S.‐Y.C., MOST105‐2628‐B‐038‐001‐MY4; H.‐J.T., PH‐105‐SP‐05, PH‐105‐SP‐04 and PH‐106‐PP‐08).
Supporting information
Figure S1 Risk of atrial fibrillation in relation to NSAID use among participants in the LHID 2000 dataset, stratified by age, gender and various clinical characteristics
Figure S2 Risk of atrial fibrillation in relation to NSAID use among participants in the LHID 2005 dataset, stratified by age, gender and various clinical characteristics
Figure S3 Risk of atrial fibrillation in relation to NSAID use among participants in the LHID 2010 dataset, stratified by age, gender and various clinical characteristics
Table S1 Association between NSAID use and atrial fibrillation among participants in the LHID 2000 dataset, based on three different matching algorithms
Table S2 Demographic and clinical characteristics among study participants in three individual LHID datasets
Table S3 xAssociation between NSAID use and atrial fibrillation, based on various NSAID exposure statuses under consideration of 3‐month latency period
Table S4 Association between use of individual NSAIDs and atrial fibrillation among participants in the LHID 2000 dataset
Table S5 Association between use of individual NSAIDs and atrial fibrillation among participants in the LHID 2005 dataset
Table S6 Association between use of individual NSAIDs and atrial fibrillation among participants in the LHID 2010 dataset
Chuang, S.‐Y. , Hsu, P.‐F. , Lin, F.‐J. , Huang, Y.‐W. , Wang, G.‐Z. , Chang, W.‐C. , and Tsai, H.‐J. (2018) Association between nonsteroidal anti‐inflammatory drugs and atrial fibrillation among a middle‐aged population: a nationwide population‐based cohort. Br J Clin Pharmacol, 84: 1290–1300. doi: 10.1111/bcp.13558.
References
- 1. De Caterina R, Ruigomez A, Rodriguez LA. Long‐term use of anti‐inflammatory drugs and risk of atrial fibrillation. Arch Intern Med 2010; 170: 1450–1455. [DOI] [PubMed] [Google Scholar]
- 2. Krijthe BP, Heeringa J, Hofman A, Franco OH, Stricker BH. Non‐steroidal anti‐inflammatory drugs and the risk of atrial fibrillation: a population‐based follow‐up study. BMJ Open 2014; 4: e004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsang TS, Petty GW, Barnes ME, O'Fallon WM, Bailey KR, Wiebers DO, et al The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol 2003; 42: 93–100. [DOI] [PubMed] [Google Scholar]
- 4. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22: 983–988. [DOI] [PubMed] [Google Scholar]
- 5. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998; 98: 946–952. [DOI] [PubMed] [Google Scholar]
- 6. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003; 107: 2920–2925. [DOI] [PubMed] [Google Scholar]
- 7. Pokharel S, van Geel PP, Sharma UC, Cleutjens JP, Bohnemeier H, Tian XL, et al Increased myocardial collagen content in transgenic rats overexpressing cardiac angiotensin‐converting enzyme is related to enhanced breakdown of N‐acetyl‐Ser‐Asp‐Lys‐Pro and increased phosphorylation of Smad2/3. Circulation 2004; 110: 3129–3135. [DOI] [PubMed] [Google Scholar]
- 8. Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J 2006; 27: 136–149. [DOI] [PubMed] [Google Scholar]
- 9. Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005; 352: 1092–1102. [DOI] [PubMed] [Google Scholar]
- 10. Haag MD, Bos MJ, Hofman A, Koudstaal PJ, Breteler MM, Stricker BH. Cyclooxygenase selectivity of nonsteroidal anti‐inflammatory drugs and risk of stroke. Arch Intern Med 2008; 168: 1219–1224. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt M, Christiansen CF, Mehnert F, Rothman KJ, Sorensen HT. Non‐steroidal anti‐inflammatory drug use and risk of atrial fibrillation or flutter: population based case‐control study. BMJ 2011; 343: d3450. [DOI] [PubMed] [Google Scholar]
- 12. Chao TF, Liu CJ, Chen SJ, Wang KL, Lin YJ, Chang SL, et al The association between the use of non‐steroidal anti‐inflammatory drugs and atrial fibrillation: a nationwide case‐control study. Int J Cardiol 2013; 168: 312–316. [DOI] [PubMed] [Google Scholar]
- 13. Schmidt M, Lamberts M, Olsen AM, Fosboll E, Niessner A, Tamargo J, et al Cardiovascular safety of non‐aspirin non‐steroidal anti‐inflammatory drugs: review and position paper by the working group for Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J 2016; 37: 1015–1023. [DOI] [PubMed] [Google Scholar]
- 14. FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase‐2. N Engl J Med 2001; 345: 433–442. [DOI] [PubMed] [Google Scholar]
- 15. Habib I, Mazulis A, Roginsky G, Ehrenpreis ED. Nonsteroidal anti‐inflammatory drugs and inflammatory bowel disease: pathophysiology and clinical associations. Inflamm Bowel Dis 2014; 20: 2493–2502. [DOI] [PubMed] [Google Scholar]
- 16. Castellsague J, Riera‐Guardia N, Calingaert B, Varas‐Lorenzo C, Fourrier‐Reglat A, Nicotra F, et al Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta‐analysis of observational studies (the SOS project). Drug Saf 2012; 35: 1127–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu CS, Wang SC, Gau SS, Tsai HJ, Cheng YC. Association of stroke with the receptor‐binding profiles of antipsychotics – a case‐crossover study. Biol Psychiatry 2013; 73: 414–421. [DOI] [PubMed] [Google Scholar]
- 18. Arbogast PG, Ray WA. Use of disease risk scores in pharmacoepidemiologic studies. Stat Methods Med Res 2009; 18: 67–80. [DOI] [PubMed] [Google Scholar]
- 19. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64: e1–e76. [DOI] [PubMed] [Google Scholar]
- 20. Liu G, Yan YP, Zheng XX, Xu YL, Lu J, Hui RT, et al Meta‐analysis of nonsteroidal anti‐inflammatory drug use and risk of atrial fibrillation. Am J Cardiol 2014; 114: 1523–1529. [DOI] [PubMed] [Google Scholar]
- 21. Danelich IM, Wright SS, Lose JM, Tefft BJ, Cicci JD, Reed BN. Safety of nonsteroidal antiinflammatory drugs in patients with cardiovascular disease. Pharmacotherapy 2015; 35: 520–535. [DOI] [PubMed] [Google Scholar]
- 22. Whelton A. Renal aspects of treatment with conventional nonsteroidal anti‐inflammatory drugs versus cyclooxygenase‐2‐specific inhibitors. Am J Med 2001; 110 (Suppl 3A): 33S–42S. [DOI] [PubMed] [Google Scholar]
- 23. Aw TJ, Haas SJ, Liew D, Krum H. Meta‐analysis of cyclooxygenase‐2 inhibitors and their effects on blood pressure. Arch Intern Med 2005; 165: 490–496. [DOI] [PubMed] [Google Scholar]
- 24. van der Hooft CS, Heeringa J, van Herpen G, Kors JA, Kingma JH, Stricker BH. Drug‐induced atrial fibrillation. J Am Coll Cardiol 2004; 44: 2117–2124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Risk of atrial fibrillation in relation to NSAID use among participants in the LHID 2000 dataset, stratified by age, gender and various clinical characteristics
Figure S2 Risk of atrial fibrillation in relation to NSAID use among participants in the LHID 2005 dataset, stratified by age, gender and various clinical characteristics
Figure S3 Risk of atrial fibrillation in relation to NSAID use among participants in the LHID 2010 dataset, stratified by age, gender and various clinical characteristics
Table S1 Association between NSAID use and atrial fibrillation among participants in the LHID 2000 dataset, based on three different matching algorithms
Table S2 Demographic and clinical characteristics among study participants in three individual LHID datasets
Table S3 xAssociation between NSAID use and atrial fibrillation, based on various NSAID exposure statuses under consideration of 3‐month latency period
Table S4 Association between use of individual NSAIDs and atrial fibrillation among participants in the LHID 2000 dataset
Table S5 Association between use of individual NSAIDs and atrial fibrillation among participants in the LHID 2005 dataset
Table S6 Association between use of individual NSAIDs and atrial fibrillation among participants in the LHID 2010 dataset


