Abstract
Aims
The full benefits of myocardial revascularization strategies applied to acute myocardial infarction patients might be reduced by myocardial ischaemia and reperfusion (I/R) injury. It is known that inflammation plays an important role in the pathogenesis of I/R injury and galectin‐3, a known inflammatory factor, is actively involved in ischaemia‐induced inflammation and fibrosis of various organs. Previous studies demonstrated that anti‐platelets therapy with ticagrelor, a new P2Y12 receptor antagonist, could effectively attenuate myocardial I/R injury and I/R injury‐related inflammatory responses. It remains unknown whether the cardioprotective effects of ticagrelor are also mediated by modulating myocardial galectin‐3 expression.
Methods
We determined the ratio of infarct area (IA)/area at risk (AAR), expression of galectin‐3, TNF‐α and IL‐6 in infarct area of rats treated with placebo (equal volume saline per gastric gavage immediately after LAD ligation, then once daily till study end) or ticagrelor (150 mg kg−1 dissolved in saline per gastric gavage immediately after LAD ligation, then once daily till study end) at 24 h, 3 and 7 days post I (45 min)/R injury. Sham‐operated rats served as control.
Results
Our results showed that ticagrelor treatment significantly reduced IA/AAR ratio at 3 and 7 days post I/R, downregulated mRNA and protein expression of galectin‐3, as well as mRNA expression of TNF‐α and IL‐6 in infarct area at 24 h, 3 and 7 days post I/R.
Conclusions
Our results suggest that the cardioprotective effects of ticagrelor might partly be mediated by downregulating galectin‐3 expression in infarct area in this rat model of myocardial I/R injury.
Keywords: galectin‐3, inflammation, ischaemia–reperfusion injury, ticagrelor
What is Already Known about this Subject
Ischaemia/reperfusion (I/R) injury is linked with increased inflammation.
The expression of galectin‐3 is upregulated in renal I/R injury.
Ticagrelor could reduce myocardial I/R injury.
What this study Adds
Galectin‐3 is upregulated in the infarct area post myocardial I/R injury.
Ticagrelor reduced I/R injury and myocardial galectin‐3 expression.
Downregulating galectin‐3 in the infarct area post I/R might be a novel working mechanism of ticagrelor.
Introduction
Although revascularization strategies have significantly reduced the acute mortality of patients with acute myocardial infarction (AMI) 1, revascularization‐related myocardial ischaemia and reperfusion (I/R) injury still remains an issue of concern in the modern interventional cardiology era 2, 3. It is known that inflammation plays an important role in the pathophysiology of I/R injury 4, 5, 6. Myocardial I/R injury‐induced inflammatory responses are typically associated with increased cytokines secretion, upregulated expression of cell adhesion molecules, and enhanced neutrophil infiltration and microvascular permeability 7. Previous study revealed that platelets are crucially involved in inflammatory response in the process of I/R injury 8, it was shown that platelets could enhance I/R injury by promoting inflammatory response in ischaemic myocardium 9, 10. Enhanced inflammatory response by platelets is usually mediated through two signalling pathways: (1) via P‐selectin expressed on the surface of activated platelets, which could accelerate the binding of white blood cells and platelet, and the activated white cell system could then mediate the subsequent endothelial inflammation responses; and (2) via platelet‐dependent CD40 and CD40L interaction, which could enhance the synthesis of adhesion molecules, chemokines and tissue factors, resulting in the activation of matrix metalloproteinases and further upgrading the inflammation responses 8. Recent research results indicate that anti‐inflammatory strategy may be one of the promising therapy options for cardiovascular diseases, including I/R injury 11, 12, 13. In fact, previous studies have demonstrated that anti‐platelet therapy with ticagrelor, a new P2Y12 receptor antagonist, could effectively attenuate I/R injury and I/R injury‐related inflammatory responses 14, 15.
Accumulating evidence suggests that galectins play an important role in regulating the physiological and pathological processes of I/R injury via modulating the inflammatory responses 16. Galectin‐3 is one of the most studied galectins and there is mounting evidence to suggest that it is actively involved in the pathogenesis of cardiovascular diseases 17. Galectin‐3 is a multi‐functional lectin with a broad range of actions, including promotion of neutrophil adhesion, induction of oxidative stress, mastocyte migration and degranulation. It is known that macrophages, as well as many other cells, such as neutrophils, eosinophils, mast cells and fibroblasts that all play important regulatory roles in the process of myocardial infarction, could produce galectin‐3 18, 19. Previous studies found that galectin‐3 was actively involved in the pathophysiology of inflammation and fibrosis in heart, kidney, lung, liver and other organs through activating fibroblasts and enhancing macrophage infiltration 20, 21. Li et al. 22 found that enhanced myocardial injury after I/R injury in mice deficient in Akt2 was associated with increased cardiac macrophage density and macrophage marker galectin‐3. Sanchez‐Mas et al. 23 reported that the mRNA expression of galectin‐3 in the infarcted area was the highest at 1 week after myocardial infarction and then gradually decreased in the next few weeks. Hashmi and Al‐Salam 24 also showed significantly upregulated mRNA and protein expressions of galectin‐3 at 60 min and 24 h after myocardial infarction in mice. Our group recently demonstrated that inflammation might participate in the worsening cardiorenal functions and remodelling processes post aortocaval fistula in unilateral nephrectomized rats 25, and significant upregulation of galectin‐3 expression in the hearts and kidney was evidenced in this model 26. These data collectively indicate that upregulated myocardial galectin‐3 expression might be an important determinant responsible for the initiation and progression of various myocardial diseases.
It remains unknown if the previously reported cardioprotective effects of ticagrelor in I/R injury models might also be related to its role in modulating myocardial galectin‐3 expression. We therefore tested the hypothesis that ticagrelor might reduce myocardial I/R injury in rats at least partly via downregulating the myocardial galectin‐3 expression.
Methods
Animal groups
Sprague–Dawley (SD) rats, weighing 200–250 g, were purchased from the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology. The rats were divided into the following groups:
Sham group (n = 5 each): 24‐h sham‐operated group; 3‐day sham‐operated group; 7‐day sham‐operated group. Rats in sham‐operated groups underwent similar surgical procedures without ligating the left anterior descending artery (LAD).
Placebo group (n = 5 each): 24‐h placebo group, 3‐day placebo group and 7‐day placebo group. Rats in the placebo groups received equal volume saline per gastric gavage immediately after ligation of LAD, then once daily post LAD ligation till the study end.
Ticagrelor group (n = 5 each): 24‐h ticagrelor group; 3‐day ticagrelor group; 7‐day ticagrelor group. Rats in ticagrelor groups received ticagrelor [150 mg kg−1, 10 mg ticagrelor dissolved in 1 ml saline] per gastric gavage (3 to 3.75 ml) immediately after LAD ligation, then once daily till the study end. The dose of 150 mg kg−1 of ticagrelor was chosen based on a previous study showing that this dose (per oral gavage, once daily for 7 days) could significantly reduce the infarct size in male Sprague–Dawley rats that underwent 30‐minute coronary artery ligation and 24‐h reperfusion 27.
The experiment protocol was approved by the Ethics Committee of Puai Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology. All experiments were conducted in compliance with the ARRIVE guidelines and in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 85‐23, revised 1996).
Establishment of ischaemia–reperfusion model
Rats were weighed and anesthetized by intraperitoneal injection of 3% pentobarbital sodium (50 mg kg−1). Rats were then intubated and connected with a small animal ventilator (ALC‐V8S, Shanghai Orcote Biotech Co., Ltd). Ventilator parameters were adjusted to: tidal volume of 3 ml per 100 g, ventilation frequency of 70 beats min−1, and breathing ratio of 1:2. I/R injury was induced as previously described 28. Briefly, a 5‐0 ophthalmic suture was placed around the left anterior descending coronary (LAD) after pericardiotomy following an incision in the left fourth intercostal. The LAD was completely ligated to obtain regional ischaemia. The visualization of pale colour in the myocardium distal to the occlusion served as evidence of effective LAD occlusion. After 45 min of ischaemia, blood flow was restored by releasing the ligature and the 5‐0 ophthalmic suture remained in position. The reperfusion of the ischaemic region was confirmed by visual inspection of the return of a bright red colour. The sham rats were subjected to the same surgical procedures as performed on the myocardial I/R rats but without LAD occlusion.
Determination of myocardial infarct size
Area at risk (AAR) and infarct size were determined as previously described 29. Briefly, 24 h, 3 days or 7 days after reperfusion, the LAD was religated with the 5‐0 ophthalmic suture remaining in situ and 3 ml of 2% Evans blue dye (Fluka, Switzerland) was injected via the right jugular vein to stain non‐ischaemic myocardium and delineate the AAR. When the epicardial surface turned blue, the heart was harvested and the right atrium, the right ventricle and the left atrium were removed, the left ventricle was frozen and cut into 5–6 slices perpendicular to the base–apex axis. The mid‐LV slice (2‐mm thick) was weighed, the ischaemic and non‐ischaemic parts separated, frozen on dry ice and kept in the freezer (−80°C) until analysed. All other slices were weighed, scanned from both sides for the determination of the AAR and put in 1% triphenyltetrazolium chloride solution for 15 min at 37°C to distinguish viable myocardium from necrotic. After 24 h of incubation in 4% formaldehyde, slices were scanned again from both sides, and the extent of myocardial necrosis and the AAR were determined by planimetry of computer images (Image J, version 1.31, NIH, Bethesda, MD).
Myocardial mRNA expression of galectin‐3, IL‐6 and TNF‐α in infarct area detected by real‐time PCR
The total RNA was extracted from the frozen tissue using the TRIzol reagent (Ambion, Cat. No. 15596‐026, Carlsbad, CA, USA) according to the instruction provided by the manufacturers, and the mRNA was reverse transcribed using the reverse transcription kit PrimeScript RT Master Mix Perfect Real Time (Takara, Cat. No. RR036A, Otsu, Shiga, Japan) (1 μg). Real‐time polymerase chain reaction was performed to detect the expression of various cytokines by QuantiFast SYBR Green PCR Kit (Qiagen, Cat. No. 208054, Germantown, Maryland, USA). Quantitative RT‐PCR analysis was performed using a T100‐Thermal Cycler (BIO‐RAD, Hercules, CA, USA) and a real‐time system (BIO‐RAD, Hercules, CA, USA). The PCR was set up to maintain 39 cycles at 95°C for 3 min, 95°C for 5 s, 56°C for 10 s, and 72°C for 25 s, 65°C for 5 s, 95°C for 50 s. GAPDH was used as internal control; primers are shown in Table 1.
Table 1.
RT‐PCR primers
| Genes | Sequence 5′‐3′ |
|---|---|
| Il‐6 forward | GCCAGAGTCATTCAGAGCAAT |
| Il‐6 reverse | CTTGGTCCTTAGCCACTCCT |
| TNF‐α forward | CACCACGCTCTTCTGTCTACTG |
| TNF‐α reverse | GCTACGGGCTTGTCACTCG |
| Galectin‐3 forward | CAACTGGCCCTAGTGCTTATC |
| Galectin‐3 reverse | CAGAGTGATACTGTTTGCGTTG |
| GAPDH forward | CGCTAACATCAAATGGGGTG |
| GAPDH reverse | TTGCTGACAATCTTGAGGGAG |
Myocardial galectin‐3 protein expression determined by Western blot
The protein labelling was performed by chemiluminescence ECL colorimetric technique. The protein was first extracted from the tissue sample with lysate and then the extracted protein (10 μg) was added to the 12% SDS‐PAGE gel wells (concentrated glue 80 V 40 min, separation glue 120 V 50 min). After electrophoresis, the membrane was blocked in TBS solution containing 5% nonfat dry milk at room temperature for 2 h and then incubated overnight at 4°C. This was followed by 1:10 000 dilution of HRP‐labelled secondary antibody, incubated with the membrane at room temperature for 1 h. Finally, the protein expression was measured using a fully automated chemiluminescence analyser (Tanon‐5200, Shanghai Tianneng) according to the manufacturer's instructions. Rabbit anti‐galectin‐3 (113486, 1:10 000; GENETEX), anti‐GAPDH (1039, 1:10 000; ASPEN) and Goat Anti‐Rabbit IgG (PAB160011, 1:10 000; BIOSWAMP) were used to determine the myocardial protein expression of galectin‐3.
Statistics
All data were presented as mean ± standard deviation. The data were analysed using a homogeneity of variances test. Data with P < 0.05 by the test of homogeneity of variances were analysed by the Games–Howell test. Data with P > 0.05 by the test of homogeneity of variances were analysed by the Tukey HSD test. All data were analysed by SPSS22.0 statistical analysis software, and P < 0.05 was considered statistically significant.
Results
Ticagrelor limits myocardial infarct size
Compared to the placebo group, the IA/AAR ratio in the ticagrelor group was reduced 12.62% at 24 h (34.51 ± 12.19% vs. 47.13 ± 12.42%, P = 0.144), 38.41% at 3 days (17.74 ± 9.9% vs. 56.15 ± 9.44%, P < 0.01) and 34.29% at 7 days (14.02 ± 5.08% vs. 48.31 ± 19.01%, P < 0.01) (Figure 1).
Figure 1.
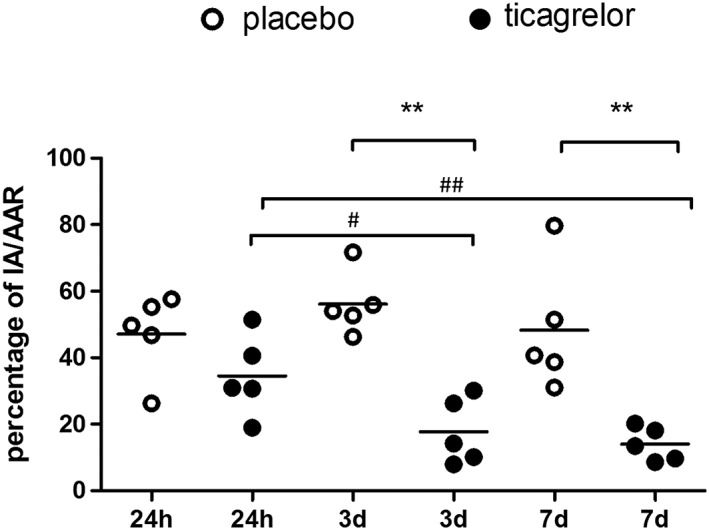
Ratio of infarct area (IA)/area at risk (AAR). Ticagrelor (black circle) significantly reduced IA/AAR ratio at 3 days and 7 days post I/R injury compared to saline‐treated rats (open circle). IA/AAR ratio was also significantly lower at 3 days and 7 days compared to 24 h in ticagrelor group. ** P < 0.01 vs. placebo group. # P < 0.05 vs. 24 h, ## P < 0.01 vs. 24 h
Myocardial mRNA expression of galectin‐3, TNF‐α and IL‐6 in sham‐operated rats and in infarct area post I/R injury
Myocardial galcectin‐3 mRNA expression was low in sham‐operated rats, and was significantly upregulated at 24 h, 3 days and 7 days post I/R injury in the infarct area of placebo groups, which was significantly higher at 3‐day and 7‐day groups compared to the 24‐h group post I/R injury in placebo‐treated rats. Ticagrelor treatment significantly downregulated galcectin‐3 mRNA expression in the infarct area at 24 h, 3 days and 7 days post I/R injury (Figure 2A) as compared to placebo‐treated rats. Compared to the sham group, myocardial galcectin‐3 mRNA expression increased 3.12‐fold in the placebo group and 1.95‐fold in the ticagrelor group at 24 h; increased 10.80‐fold in the placebo group and 6.45‐fold in the ticagrelor group at 3 days; increased 12.26‐fold in the placebo group and 7.45‐fold in the ticagrelor group at 7 days. As shown in Figure 2A, that relative mRNA expression of galectin‐3 is higher at 3 days and 7 days compared to 24 h in the placebo group, which is line with previous findings showing that galectin‐3 mRNA expression peaked at 7 days in the infarct area 23.
Figure 2.
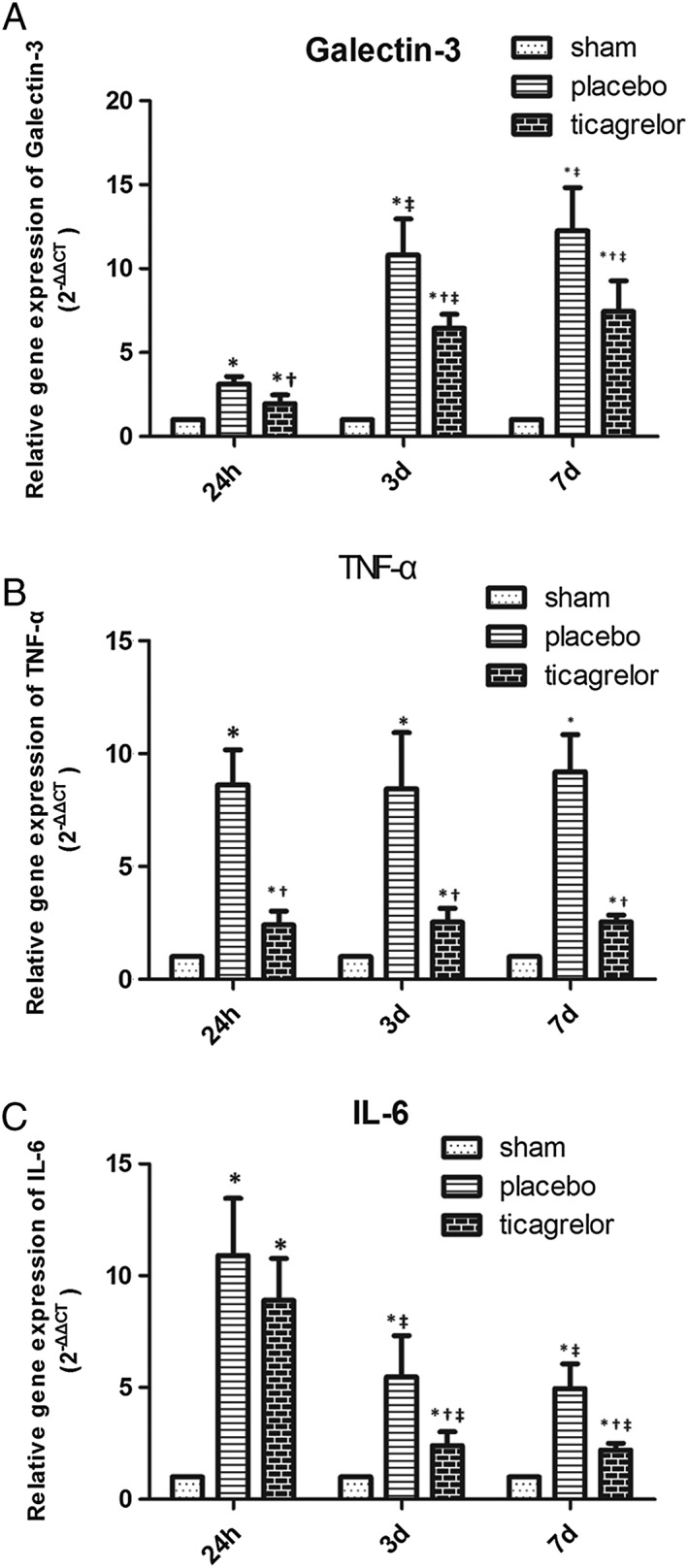
mRNA expression of galectin‐3 (A), TNF‐ α (B) and IL‐6 (C) in the myocardial tissue of sham group (white bar), placebo group (gray bar) and ticagrelor group (black bar) in infarct area at 24 h, 3 days and 7 days post I/R. * P < 0.05 vs. sham‐operated group; † P < 0.05 vs. placebo group at the same time point; ‡ P < 0.05 vs. 24‐h group within the same treatment group
As shown in Figure 2B, the mRNA expression of TNF‐α was also significantly and equally upregulated post I/R in the infarct area of placebo groups from 24 h to 7 days compared to sham hearts for the corresponding area. Ticagrelor treatment significantly downregulated TNF‐α mRNA expression in the infarct area at 24 h, 3 days and 7 days post I/R injury compared to placebo‐treated rats.
mRNA expression of IL‐6 was significantly upregulated at 24 h, then reduced at 3 days and 7 days post I/R, which were significantly higher than in the respective sham groups at all three measured time points in the placebo groups. Ticagrelor treatment significantly downregulated IL‐6 mRNA expression in infarct area at 3 days and 7 days post I/R as compared to placebo‐treated rats (Figure 2C). As shown in Figure 2C, mRNA expression of IL‐6 was lower at 3 days and 7 days than in 24 h in the placebo group. This result is in line with a previous finding showing that IL‐6 peaked at 24 h in 93 patients receiving thrombolytic treatment for their first AMI 30.
Protein expression of galcectin‐3 in myocardial tissue of sham rats and in infarct area of rats post I/R injury
As shown in Figure 3, protein expression of galcectin‐3 was significantly upregulated in the infarct area from 24 h to 7 days post I/R in placebo groups, which was significantly higher at 3 days and 7 days post I/R compared to 24 h post I/R in placebo‐treated rats. Ticagrelor treatment significantly downregulated protein expression of galcectin‐3 in the infarct area at 24 h, 3 days and 7 days post I/R as compared to placebo‐treated rats (Figure 3A, 3B).
Figure 3.
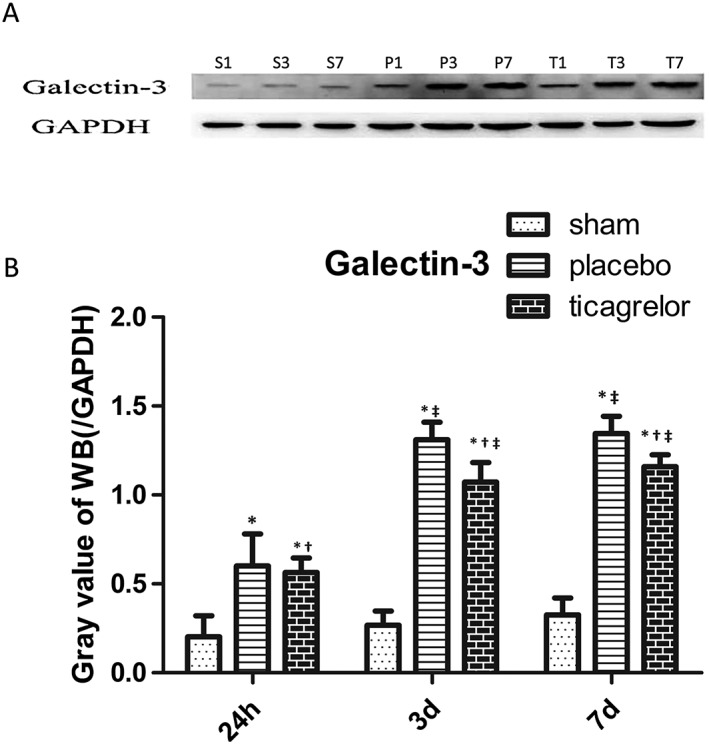
(A) S1, S3 and S7 represents sham group at 24 h, 3 days and 7 days; P1, P3, P7 represents placebo group at 24 h, 3 days and 7 days; T1, T3, T7 represents ticagrelor group at 24 h, 3 days and 7 days. Representative blot for galectin‐3 of various groups at 24 h, 3 days and 7 days post sham operation or I/R injury. Protein expression of galectin‐3 (B) in the myocardial tissue of sham group (white bar) and infarct area of placebo group (gray bar) and ticagrelor group (black bar) at 24 h, 3 days and 7 days post I/R injury. * P < 0.05 vs. sham‐operated group; † P < 0.05 vs. placebo group at the same time point; ‡ P < 0.05 vs. 24‐h group within the same treatment group
Discussion
The major findings of the present study are as follows: (1) ticagrelor application per gavage immediately after LAD ligation significantly reduced the ratio of infarct area (IA)/area at risk (AAR); (2) ticagrelor use immediately after LAD ligation significantly downregulated galectin‐3, TNF‐α and IL‐6 expression in the infarct area in this rat model of I/R injury.
Ticagrelor is a reversibly binding and selective oral P2Y12 antagonist, the disposition and metabolism of which has been investigated in mice, rats and marmosets. The results of those studies showed that the metabolic profiles were similar between these animal species and humans, and the in vivo metabolite profiles were also qualitatively similar across all species 31. The plasma concentration of oral ticagrelor peaked at 2 h and the half‐life ranged from 10.9 to 14.9 h. The average absolute bioavailability of ticagrelor was approximately 36% (range 25.4–64.0%) in humans 32.
The observed cardioprotective results represented by reduced infarct area and myocardial proinflammatory cytokine levels from our study are in line with previous finding showing that a single acute dose of ticagrelor via intraperitoneal injection (30 mg kg−1) 5 min before reperfusion significantly reduced infarct size and reduced the proinflammatory cytokine levels in a rat ischaemia/reperfusion model 15. It is noted that IA/AAR ratio tended to be lower at 24 h as compared to 3 days and 7 days in the placebo‐treated rats (P > 0.05). Although not statistically significant, this deviation should be kept in mind when interpreting the data for potential bias in the analysis process. IA/AAR ratio was significantly lower at 3 days and 7 days as compared to 24 h in the ticagrelor group (both P < 0.05), which suggests that the drug accumulation in rats after 3 days and 7 days might contribute to the stronger cardioprotective effects of ticagrelor, which has a half‐life around of 10.9–14.9 h 32.
Beyond above findings, we showed for the first time that ticagrelor application post LAD also significantly downregulated the galectin‐3 expression in infarct area; thus, the cardioprotective effects of ticagrelor might also be associated with its modulating effects on galectin‐3 expression in the ischaemic area in this rat model of I/R injury.
Multiple mechanisms have been indicated for the beneficial effects of ticagrelor in attenuating ischaemic insult, including increasing myocardial adenosine levels, augmenting the phosphorylation of the pro‐survival kinases Akt and ERK 1/2 and endothelial NO synthase 15 as well as inhibiting glandular transporter (ENT‐1) on erythrocytes glycoside reuptake, strengthening of the local adenosine response, and vasodilation 14, 27, 33. Previous studies also suggested that the cardioprotective effects of ticagrelor was independent of its role in inhibition of platelet aggregation, as the cardioprotective effects were not observed post clopidogrel application 14, 15. Beyond the above knowledge on the potential therapeutic mechanisms of ticagrelor in I/R injury models, the present study results suggest that the observed cardioprotective effects of ticagrelor might at least partly be mediated through downregulating the expression of galectin‐3 in the infarct area in this rat model of I/R injury. Supportive data are reported by Fernandes Bertocchi et al., who demonstrated that galectin‐3 knockout animals presented less acute tubular necrosis and a more prominent tubular regeneration when compared with wild‐type controls, concurrently with lower expression of MCP‐1, IL‐6, IL‐1beta, less macrophage infiltration and lower ROS production in mice models of renal I/R 34. Accordingly, Cohen and colleagues revealed that the reno‐protective effects of a reno‐protective cocktail was accompanied by the reduced tissue galectin‐3 level in a rat model of renal ischaemia 35. The above evidence, including ours, thus collectively indicate a crucial role of galectin‐3 in ischaemia injuries of various organs, and targeted downregulating of galectin‐3 might be a promising anti‐ischaemic strategy.
Although we did not observe the impact of ticagrelor on inflammatory cell infiltration in the infarct area, including macrophages, neutrophils, eosinophils, mast cells and fibroblasts, which are all capable of producing galectin‐3, our results indirectly suggest that the reduced expression of galectin‐3 in the infarct area might be the result of reduced inflammatory cell infiltration in this area post ticagrelor use. We have no data to show whether or not the downregulation of galectin‐3 expression in the infarct area of rats by ticagrelor is dose‐dependent, but future studies are planned to address this issue.
Future studies using galectin‐3 antagonist are warranted to show the direct therapeutic evidence in both in vivo and in vitro I/R models and prove the hypothesis that targeting galectin‐3 is a novel anti‐inflammatory and anti‐ischaemic strategy in heart, liver, kidney and cerebral diseases.
Competing Interests
There are no competing interests to declare.
This work was supported by the Health and Family Planning Commission of Wuhan municipality (WX16B12), Wuhan, China; “Yellow crane talents program” sponsored by the Wuhan Government in 2015; and the “Shibaiqian talents program” sponsored by the Wuhan Government in 2015.
Liu, X. , Gu, Y. , Liu, Y. , Zhang, M. , Wang, Y. , and Hu, L. (2018) Ticagrelor attenuates myocardial ischaemia–reperfusion injury possibly through downregulating galectin‐3 expression in the infarct area of rats. Br J Clin Pharmacol, 84: 1180–1186. doi: 10.1111/bcp.13536.
References
- 1. Berger PB, Ellis SG, Holmes DR Jr, Granger CB, Criger DA, Betriu A, et al Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction: results from the global use of strategies to open occluded arteries in Acute Coronary Syndromes (GUSTO‐IIb) trial. Circulation 1999; 100: 14–20. [DOI] [PubMed] [Google Scholar]
- 2. Benhabbouche S, Crola da Silva C, Abrial M, Ferrera R. Ann Fr Anesth Reanim 2011). [The basis of ischemia‐reperfusion and myocardial protection]; 30 (Suppl 1): S2–S16. [DOI] [PubMed] [Google Scholar]
- 3. Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol 2011; 301: H1723–H1741. [DOI] [PubMed] [Google Scholar]
- 4. Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 2002; 53: 31–47. [DOI] [PubMed] [Google Scholar]
- 5. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007; 357: 1121–1135. [DOI] [PubMed] [Google Scholar]
- 6. Marchant DJ, Boyd JH, Lin DC, Granville DJ, Garmaroudi FS, McManus BM. Inflammation in myocardial diseases. Circ Res 2012; 110: 126–144. [DOI] [PubMed] [Google Scholar]
- 7. Pilipenko VV, Iabluchanskii NI. [Alteration of the microcirculation of the bulbar conjunctiva in patients with myocardial infarction during treatment with non‐steroidal anti‐inflammatory agents]. Sov Med 1983. (5); 105–107. [PubMed] [Google Scholar]
- 8. Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res 2002; 22: 913–922. [DOI] [PubMed] [Google Scholar]
- 9. Choudhri TF, Hoh BL, Zerwes HG, Prestigiacomo CJ, Kim SC, Connolly ES Jr, et al Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor‐mediated platelet aggregation. J Clin Invest 1998; 102: 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lefer AM, Campbell B, Scalia R, Lefer DJ. Synergism between platelets and neutrophils in provoking cardiac dysfunction after ischemia and reperfusion: role of selectins. Circulation 1998; 98: 1322–1328. [DOI] [PubMed] [Google Scholar]
- 11. Patel MB, Kilgore KS, Ortolano GA, Gryboski CL, Qureshi MA, Marcovitz P, et al Conditioned blood reperfusion during angioplasty (CoBRA) treatment of acute myocardial infarction. Perfusion 2001; 16 (Suppl): 39–49. [DOI] [PubMed] [Google Scholar]
- 12. Shernan SK, Collard CD. Role of the complement system in ischaemic heart disease: potential for pharmacological intervention. BioDrugs 2001; 15: 595–607. [DOI] [PubMed] [Google Scholar]
- 13. Kohtani T, Abe Y, Sato M, Miyauchi K, Kawachi K. Protective effects of anti‐neutrophil antibody against myocardial ischemia/reperfusion injury in rats. Eur Surg Res 2002; 34: 313–320. [DOI] [PubMed] [Google Scholar]
- 14. Wang K, Zhou X, Huang Y, Khalil M, Wiktor D, van Giezen JJ, et al Adjunctive treatment with ticagrelor, but not clopidogrel, added to tPA enables sustained coronary artery recanalisation with recovery of myocardium perfusion in a canine coronary thrombosis model. Thromb Haemost 2010; 104: 609–617. [DOI] [PubMed] [Google Scholar]
- 15. Ye Y, Birnbaum GD, Perez‐Polo JR, Nanhwan MK, Nylander S, Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol 2015; 35: 1805–1814. [DOI] [PubMed] [Google Scholar]
- 16. Nguyen JT, Evans DP, Galvan M, Pace KE, Leitenberg D, Bui TN, et al CD45 modulates galectin‐1‐induced T cell death: regulation by expression of core 2 O‐glycans. J Immunol 2001; 167: 5697–5707. [DOI] [PubMed] [Google Scholar]
- 17. Krześlak A, Lipińska A. Galectin‐3 as a multifunctional protein. Cell Mol Biol Lett 2004; 9: 305–328. [PubMed] [Google Scholar]
- 18. Dumic J, Dabelic S, Flogel M. Galectin‐3: an open‐ended story. Biochim Biophys Acta 2006; 1760: 616–635. [DOI] [PubMed] [Google Scholar]
- 19. Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B, et al Genetic and pharmacological inhibition of galectin‐3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail 2013; 6: 107–117. [DOI] [PubMed] [Google Scholar]
- 20. Filer A, Bik M, Parsonage GN, Fitton J, Trebilcock E, Howlett K, et al Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways. Arthritis Rheum 2009; 60: 1604–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao P, Simpson JL, Zhang J, Gibson PG. Galectin‐3: its role in asthma and potential as an anti‐inflammatory target. Respir Res 2013; 14: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li X, Mikhalkova D, Gao E, Zhang J, Myers V, Zincarelli C, et al Myocardial injury after ischemia‐reperfusion in mice deficient in Akt2 is associated with increased cardiac macrophage density. Am J Physiol Heart Circ Physiol 2011; 301: H1932–H1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanchez‐Mas J, Lax A, Asensio‐Lopez MC, Fernandez‐Del Palacio MJ, Caballero L, Garrido IP, et al Galectin‐3 expression in cardiac remodeling after myocardial infarction. Int J Cardiol 2014; 172: e98–e101. [DOI] [PubMed] [Google Scholar]
- 24. Hashmi S, Al‐Salam S. Galectin‐3 is expressed in the myocardium very early post‐myocardial infarction. Cardiovasc Pathol 2015; 24: 213–223. [DOI] [PubMed] [Google Scholar]
- 25. Wu J, Cheng Z, Zhang M, Zhu P, Gu Y. Impact of aortocaval shunt flow on cardiac and renal function in unilateral nephrectomized rats. Sci Rep 2016; 6: 27493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang MJ, Gu Y, Wang H, Zhu PF, Liu XY, Wu J. Valsartan attenuates cardiac and renal hypertrophy in rats with experimental cardiorenal syndrome possibly through down‐regulating galectin‐3 signaling. Eur Rev Med Pharmacol Sci 2016; 20: 345–354. [PubMed] [Google Scholar]
- 27. Nanhwan MK, Ling S, Kodakandla M, Nylander S, Ye Y, Birnbaum Y. Chronic treatment with ticagrelor limits myocardial infarct size: an adenosine and cyclooxygenase‐2‐dependent effect. Arterioscler Thromb Vasc Biol 2014; 34: 2078–2085. [DOI] [PubMed] [Google Scholar]
- 28. Lin C, Sui H, Gu J, Yang X, Deng L, Li W, et al Effect and mechanism of propofol on myocardial ischemia reperfusion injury in type 2 diabetic rats. Microvasc Res 2013; 90: 162–168. [DOI] [PubMed] [Google Scholar]
- 29. Xu B, Dong GH, Liu H, Wang YQ, Wu HW, Jing H. Recombinant human erythropoietin pretreatment attenuates myocardial infarct size: a possible mechanism involves heat shock Protein 70 and attenuation of nuclear factor‐kappaB. Ann Clin Lab Sci 2005; 35: 161–168. [PubMed] [Google Scholar]
- 30. Puhakka M, Magga J, Hietakorpi S, Penttilä I, Uusimaa P, Risteli J, et al Interleukin‐6 and tumor necrosis factor alpha in relation to myocardial infarct size and collagen formation. J Card Fail 2003; 9: 325–332. [DOI] [PubMed] [Google Scholar]
- 31. Li Y, Landqvist C, Grimm SW. Disposition and metabolism of ticagrelor, a novel P2Y12 receptor antagonist, in mice, rats, and marmosets. Drug Metab Dispos 2011; 39: 1555–1567. [DOI] [PubMed] [Google Scholar]
- 32. Teng R, Maya J. Absolute bioavailability and regional absorption of ticagrelor in healthy volunteers. J Drug Assess 2014; 3: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nylander S, Femia EA, Scavone M, Berntsson P, Asztely AK, Nelander K, et al Ticagrelor inhibits human platelet aggregation via adenosine in addition to P2Y12 antagonism. J Thromb Haemost 2013; 11: 1867–1876. [DOI] [PubMed] [Google Scholar]
- 34. Fernandes Bertocchi AP, Campanhole G, Wang PH, Goncalves GM, Damiao MJ, Cenedeze MA, et al A role for galectin‐3 in renal tissue damage triggered by ischemia and reperfusion injury. Transpl Int 2008; 21: 999–1007. [DOI] [PubMed] [Google Scholar]
- 35. Cohen J, Dorai T, Ding C, Batinic‐Haberle I, Grasso M. The administration of renoprotective agents extends warm ischemia in a rat model. J Endourol 2013; 27: 343–348. [DOI] [PubMed] [Google Scholar]


