Abstract
Aims
Evidence for drug use in newborns is sparse, which may cause large differences in drug prescriptions. We aimed to investigate the differences between neonatal intensive care units (NICUs) in the Netherlands in currently prescribed drugs.
Methods
This multicentre study included neonates admitted during 12 months to four different NICUs. Drugs were classified in accordance with the Anatomical Therapeutic Chemical (ATC) classification system and assessed for on/off‐label status in relation to neonatal age. The treatment protocols for four common indications for drug use were compared: pain, intubation, convulsions and hypotension.
Results
A total of 1491 neonates (GA range 23+6–42+2 weeks) were included with a total of 32 182 patient days, 181 different drugs and 10 895 prescriptions of which 23% was off‐label in relation to neonatal age. Overall, anti‐infective drugs were most frequently used with a total of 3161 prescriptions, of which 4% was off‐label in relation to neonatal age. Nervous system drugs included 2500 prescriptions of which 31% was off‐label in relation to neonatal age. Nervous system drugs, blood and blood forming organs, and cardiovascular drugs showed the largest differences between NICUs with ranges of 919–2278, 554–1465, and 238–952 total prescriptions per 1000 patients per ATC class, respectively.
Conclusions
We showed that drug use varies widely in neonatal clinical practice. The drug classes with the highest proportion of off‐label drugs in relation to neonatal age showed the largest differences between NICUs, i.e. cardiovascular and nervous system drugs. Drug research in neonates should receive high priority to guarantee safe and appropriate medicines and optimal treatment.
Keywords: ATC class, differences between NICUs, drug prescriptions, neonatal intensive care units, off‐label
What is Already Known about this Subject
Most drugs for neonates are prescribed off‐label and the evidence for use is limited due to a scarcity of clinical trials on efficacy, dosage and safety.
Considerable variation is observed between Dutch NICUs both regarding the number of antibiotics and the total dosage.
What this Study Adds
The prescription of nervous system and cardiovascular drugs is highly variable between NICUs. These differences become larger with decreasing postmenstrual age, although the proportion of off‐label prescriptions in relation to neonatal age decreased.
Despite the new FDA and EMA drug legislation, many drugs are still used off‐label and the variability in drug prescriptions reflects the lack of evidence on drug use, especially in the smallest newborns.
Consensus meetings on the treatment of common diseases and development of (inter)national guidelines should receive the highest priority.
Introduction
Infants in the neonatal intensive care unit (NICU) are exposed to a large number of drugs. Most drugs are off‐label for neonates and evidence for use in this population is sparse, due to a limited number of clinical trials on efficacy, dosage and safety 1, 2. These knowledge gaps are prone to large differences in interpretation of available evidence and will consequently be translated into different drug therapies described in local treatment protocols and neonatal practice.
Previous studies have described drug prescriptions during infancy, reporting a large proportion of off‐label drug use 2, 3, 4, 5, 6, 7. The proportion of off‐label prescribed drugs increases with decreasing age. Therefore, the most vulnerable paediatric group – preterm infants – has the highest exposure to drugs that are insufficiently documented 2. In neonatal care, almost all patients are exposed to at least one off‐label or non‐approved drug during admission. Off‐label use of drugs has been associated with the risk of adverse drug reactions 8. To improve paediatric drug therapy, new legislation was introduced more than a decade ago in the United States with the Pediatric Research Equity Act in 2003 9, the Food and Drug Administration Reauthorization Act of 2017 10, and in the European Union with the Paediatric Regulation in 2006 11 to encourage paediatric drug research in the pre‐ and post‐marketing phase. However, these have not yet led to increased licensing 12, 13.
We aimed to investigate the differences in currently prescribed drugs between neonatal intensive care units (NICUs) in the Netherlands, and to study the off‐label proportions, as well as drug‐class and age‐related differences.
Methods
Patients and setting
In this retrospective cohort, all patients with an admission date between 1 September 2014 and 31 August 2015 to one of the four participating Dutch level III NICUs (Radboud University Medical Center Nijmegen, Maastricht University Medical Center Maastricht, Máxima Medical Center Veldhoven and Sophia Children's Hospital Rotterdam) were eligible for inclusion. The study was conducted according to Good Clinical Practice and the Declaration of Helsinki.
Definitions and drug classification
A prescription was defined as a patient for whom a specific drug was prescribed during admission to the NICU, regardless of how often it was prescribed and of the route of administration. Patient days were defined as the sum of treatment days of each drug during admission to the NICU, which was calculated per patient and as a total. All drugs were classified in accordance with the Anatomical Therapeutic Chemical (ATC) classification system.
The definition by Neubert et al. for “off‐label use” was applied, meaning ‘all uses of a marketed drug not detailed in the summary of product characteristics (SmPC) including therapeutic indication, use in age‐subsets, appropriate strength (dosage), pharmaceutical form and route of administration’ 14. However, the on/off‐label status could be assessed only for the active substance in relation to age‐subsets, as information concerning dosage, route of administration, indication, drug preparation and formulation, could not be collected from all four hospitals. Therefore, on/off‐label status in relation to neonatal age (<1 month after birth) was assessed according to the SmPC, which was consulted via the Dutch Medicines Evaluation Board (http://www.cbg-meb.org, accessed on 12 October 2017). The status of a drug was considered on‐label if an SmPC for that active substance describes an indication that includes infants below 1 month of age, which is also the case if the drug is indicated for children in general.
Data collection
All four hospitals prescribed drugs using a computerized physician order entry system. Patient characteristics and drug prescriptions were retrieved from the electronical medical records of each hospital. Data were collected on date of admittance, birth date, gestational age, birthweight, gender, survival, drugs administered, and date and duration of drug administration until death or discharge from the NICU. We excluded ATC class ‘Q’ of veterinary drugs. We also excluded electrolytes, total parenteral nutrition, Dutch national health care system vaccines, supportive dermatological products (not containing a drug), and contrast media. We followed the guidelines in the Reporting of Studies Conducted using Observational Routinely Collected Data (RECORD) statement to report our study 15.
Data processing and statistical analysis
Data from the four NICUs were combined for the overall analyses of neonatal prescriptions. The prescription frequency was ranked, together with an analysis of the proportion of prescriptions that were off‐label in relation to neonatal age. For comparison of the NICU prescriptions, patients were classified into five different postmenstrual age groups at start of drug use, because gestational age groups would be confounded by drug use at a later postnatal age: <26 weeks, 26–28 weeks, 28–32 weeks, 32–37 weeks and term neonates ≥37 weeks. Exposure to drugs was defined as either the absolute number of prescriptions or expressed per 1000 infants. Variability in prescribed drugs per ATC class between NICUs was quantified by calculating the range of total prescriptions per ATC class per 1000 patients between NICUs. This range was used to select the ATC classes for further investigation. All data were stored and analysed in SPSS Statistics version 21 (IBM, Armonk, NY, USA), using the non‐parametric Kruskal–Wallis test for continuous variables and Pearson's χ2 test for nominal variables, with a P‐value of <0.05 for significance.
Treatment protocol comparison
Four common indications for drug use in neonatal care were selected to compare the drugs and their suggested order as written in the treatment protocols of the four NICUs. This could give more insight into possible causes for differences in drug prescriptions. The selected indications were pain, intubation, convulsions and hypotension.
Results
During the one‐year period, 1491 neonates were included in the study with a total of 32 182 patient days, and a median gestational age of 32+5 weeks (IQR: 29+6–37+6 weeks). The median birth weight of all neonates was 1865 g (IQR: 1253–3000 g), of which 14.5% had an extremely low birth weight (ELBW; <1000 g). The median duration of admission to the NICU was 12 days (IQR: 5–32 days). Data on post menstrual age (PMA) at discharge was missing in six cases. Gestational age, birth weight, duration of admission to the NICU, and postmenstrual age at discharge were all significantly different between the four hospitals (Table 1).
Table 1.
Baseline characteristics of hospitalized neonates in four different NICUs in the Netherlands
| NICU 1 | NICU 2 | NICU 3 | NICU 4 | p value | Total/overall | |
|---|---|---|---|---|---|---|
| Number of beds | 18 | 15 | 13 | 31 | ||
| Number of patients given drugs | 314 | 353 | 223 | 601 | 1491 | |
| Male gender (%) | 60 | 59 | 55 | 58 | 0.615a | 58 |
| Gestational age (weeks +days ) | 31+5 (29+2–35+5) | 33+2 (30+2–38+0) | 34+6 (30+5–38+3) | 32+2 (29+4–37+5) | <0.001b | 32+5 (29+6–37+6) |
| <26 weeks (%) | 17 (5.4) | 15 (4.3) | 8 (3.6) | 39 (6.5) | 0.001c | 79 (5.3) |
| 26–28 weeks (%) | 33 (10.5) | 32 (9.1) | 15 (6.7) | 59 (9.8) | 139 (9.3) | |
| 28–32 weeks (%) | 112 (35.7) | 96 (27.3) | 51 (22.9) | 185 (30.8) | 444 (29.8) | |
| 32–37 weeks (%) | 82 (26.1) | 88 (25.0) | 53 (23.8) | 139 (23.2) | 362 (24.3) | |
| ≥ 37 weeks (%) | 70 (22.3) | 121 (34.4) | 96 (43.0) | 178 (29.6) | 465 (31.2) | |
| Birth weight (g) | 1695 (1228–2613) | 2012 (1350–3091) | 2100 (1370–3120) | 1800 (1200–2970) | 0.007b | 1865 (1253–3000) |
| ELBW (%) | 48 (15.3) | 51 (14.4) | 22 (9.9) | 95 (15.8) | 0.214c | 216 (14.5) |
| Number of days at NICU | 24 (8–47) | 12 (6–30) | 12 (5–25) | 7 (4–17) | <0.001b | 12 (5–32) |
| PMA at discharge | 37+2 (35+4–39+6) | 36+6 (33+0–40+3) | 38+1 (35+0–40+5) | 35+0 (32+1–39+3) | <0.001b | 36+5 (33+2–40+0) |
| Total patient days | 9789 | 7769 | 4716 | 9908 | 32 182 | |
| Total prescriptions | 2216 | 3371 | 1143 | 4165 | 10 895 | |
| Drugs per patient | 5 (3–10) | 7 (4–14) | 4 (2–6) | 5 (3–8) | <0.001b | 5 (3–10) |
| Patient days on drugs | 28 (12–80) | 36 (15–98) | 18 (7–52) | 13 (6–43) | <0.001b | 21 (8–71) |
| % OL in relation to neonatal age | 21 | 29 | 11 | 23 | <0.001b | 23 |
Data presented as median (IQR).
χ2 test
Kruskal–Wallis one‐way analysis
χ2 for distributions in all strata of gestational ages in four NICUs
Overall prescription of drugs and off‐label use in relation to neonatal age
In total, 181 different drugs were prescribed 10 895 times, of which 23% was off‐label in relation to neonatal age (see Supporting Information File S1 for on‐label age‐range in SmPC). The proportion of off‐label prescriptions in relation to neonatal age increased with PMA at start of drug therapy: 19% for infants with PMA at start below 32 weeks, 26% for infants with PMA between 32 and 37 weeks, and 29% above 37 weeks PMA. During admission, 54% of the neonates were exposed to at least one off‐label drug. The median number of prescribed drugs per patient was five (IQR: 3–10). This was significantly different between hospitals varying from a median of four to seven drugs per patient.
The ATC class with the most frequently prescribed drugs was anti‐infective drugs with a total of 3161 prescriptions (29%), of which 4% was off‐label in relation to neonatal age (Figure 1, Table 2). The second largest ATC class was the nervous system drugs with 2500 prescriptions (23%) of which 31% was off‐label in relation to neonatal age. The drug class of blood and blood‐forming organs was the third largest with 1386 prescriptions (13%). However, this result was confounded since 54% of these prescriptions concerned phytomenadione prescribed as supplementary vitamin instead of the labelled indication as an antidote to anticoagulant drugs of the coumarin type. The large proportion of 28% off‐label prescriptions was caused by heparin for 86%, which was indicated for arterial catheter patency. Alimentary tract and metabolism drugs were fourth largest with 1327 prescriptions (12%), 17% of which was off‐label in relation to neonatal age. Cardiovascular drugs were the fifth largest class with 958 prescriptions (9%), of which 30% was off‐label in relation to neonatal age, for 84% due to dopamine and noradrenaline. The sixth largest ATC class was the respiratory drugs with 36% off‐label prescriptions, of which 76% was accounted for by xylometazoline and doxapram.
Figure 1.

Total number of prescriptions and proportion off‐label in each Anatomical Therapeutic Chemical (ATC) groupIn total, 10 895 prescriptions of 181 different drugs were retrieved, of which 23% was off‐label in relation to neonatal age. * Range of total prescriptions per ATC class per 1000 patients between NICUs.
Table 2.
Most frequently prescribed drugs per 1000 neonates
| No | All drugs | Prescriptions | No | Off‐label drugs in relation to neonatal age | Prescriptions |
|---|---|---|---|---|---|
| 1 | Phytomenadione | 668 | 1 | Heparin | 219 |
| 2 | Cholecalciferol | 521 | 2 | Fentanyl | 193 |
| 3 | Caffeine | 480 | 3 | Propofol | 117 |
| 4 | Amoxicillin | 375 | 4 | Dopamine | 109 |
| 5 | Gentamicin | 375 | 5 | Phenobarbital | 91 |
| 6 | Tobramycin | 302 | 6 | Hydrocortisone | 79 |
| 7 | Benzylpenicillin | 287 | 7 | Xylometazoline | 68 |
| 8 | Paracetamol | 273 | 8 | Miconazole | 66 |
| 9 | Surfactant | 251 | 9 | Phenylephrine + Tropicamide | 57 |
| 10 | Morphine | 247 | 10 | Norepinephrine | 53 |
| 11 | Heparin | 219 | 11 | Insulin | 50 |
| 12 | Fentanyl | 193 | 12 | Meropenem | 43 |
| 13 | Amoxicillin+ clavulanic acid | 165 | 13 | Dexamethasone | 42 |
| 14 | Midazolam | 148 | 14 | Doxapram | 42 |
| 15 | Atropine | 137 | 15 | Phenylephrine | 38 |
| 16 | Flucloxacillin | 133 | 16 | Chloralhydrate | 30 |
| 17 | Rocuronium | 133 | 17 | Ranitidine | 27 |
| 18 | Vancomycin | 132 | 18 | Levetiracetam | 25 |
| 19 | Furosemide | 130 | 19 | Cefazolin | 15 |
| 20 | Propofol | 117 | 20 | Cisatracurium | 15 |
| 21 | Dopamine | 109 | 21 | Ursodeoxycholic acid | 15 |
| 22 | Ceftazidime | 95 | 22 | Antitrombin | 14 |
| 23 | Phenobarbital | 91 | 23 | Esketamine | 14 |
| 24 | Ibuprofen | 91 | 24 | Tocopherol | 13 |
| 25 | Nystatin | 81 | 25 | Retinol | 11 |
| 26 | Hydrochlorothiazide | 80 | 26 | Trimethoprim | 11 |
| 27 | Spironolactone | 80 | 27 | Levomepromazine | 10 |
| 28 | Hydrocortisone | 79 | 28 | Sildenafil | 10 |
| 29 | Dobutamine | 70 | 29 | Dornase | 9 |
| 30 | Xylometazoline | 68 | 30 | Lidocaine | 9 |
Total of 10 985 prescriptions for 1491 patients. The number (No) indicates the ranking of prescribed drugs per 1000 patients
Table 2 provides the most prescribed drugs overall and off‐label in all NICUs, which overall were, in rank order, phytomenadione, cholecalciferol, caffeine, amoxicillin, gentamicin, tobramycin, benzylpenicillin, paracetamol, surfactant and morphine. Of these, none are off‐label in relation to neonatal age.
Differences in drug use between NICUs
The largest differences between NICUs were found for nervous system drugs, with total prescriptions between NICUs ranging 919–2278 per 1000 patients followed by 554–1465 for blood and blood‐forming organs, and 238–952 for cardiovascular system drugs, respectively (Figure 1). As 86% of the range of prescribed drugs from blood and blood‐forming organs is caused by heparin and phytomenadione, cardiovascular and nervous system drugs were considered most interesting for a more extensive comparison (Figure 2).
Figure 2.

Range of prescriptions per drug per 1000 patients between NICUs in descending order. The top 35 drugs are listed in descending order of the largest difference between minimum and maximum prescriptions.
Cardiovascular drug prescriptions differed between the four NICUs (Table 3), and with PMA (Figure 3A). Table 3 shows that the prescription of cardiovascular drugs varied from none to six different agents between the different NICUs in infants with PMA below 26 weeks. Dopamine exposure for those neonates was high in two NICUs, where another NICU showed larger variety of other haemodynamic agonists for these preterm infants, i.e. dobutamine and adrenaline. Furthermore, nervous system drugs showed large variety (Figure 3B, Table 4). Interesting differences included the variable use of propofol, levetiracetam and diuretics between NICUs for all PMAs. Prescriptions of paracetamol and phenobarbital were particularly different in the youngest infants.
Table 3.
Cardiovascular drug prescriptions according to PMA (per 1000 neonates per PMA group)
| NICU 1 | NICU 2 | NICU 3 | NICU 4 | |||||
|---|---|---|---|---|---|---|---|---|
| PMA | Drug | No | Drug | No | Drug | No | Drug | No |
| <26 | Dopamine | 353 | Dopamine | 467 | Dobutamine | 231 | ||
| Norepinephrine | 59 | Dopamine | 154 | |||||
| Epinephrine | 77 | |||||||
| Hydrochlorothiazide | 77 | |||||||
| Spironolactone | 77 | |||||||
| Furosemide | 26 | |||||||
| 26 < 28 | Dopamine | 273 | Dopamine | 156 | Furosemide | 67 | Furosemide | 339 |
| Furosemide | 242 | Furosemide | 125 | Dobutamine | 254 | |||
| Hydrochlorothiazide | 91 | Norepinephrine | 63 | Hydrochlorothiazide | 237 | |||
| Spironolactone | 91 | Epinephrine | 31 | Spironolactone | 237 | |||
| Dobutamine | 61 | Dobutamine | 31 | Dopamine | 153 | |||
| Epinephrine | 30 | Milrinone | 31 | Epinephrine | 85 | |||
| 28 < 32 | Furosemide | 107 | Furosemide | 156 | Furosemide | 157 | Furosemide | 205 |
| Dopamine | 63 | Dopamine | 146 | Hydrochlorothiazide | 78 | Hydrochlorothiazide | 184 | |
| Epinephrine | 27 | Norepinephrine | 94 | Spironolactone | 78 | Spironolactone | 184 | |
| Norepinephrine | 27 | Hydrochlorothiazide | 42 | Dobutamine | 114 | |||
| Hydrochlorothiazide | 18 | Spironolactone | 42 | Dopamine | 97 | |||
| Spironolactone | 18 | Dobutamine | 31 | Epinephrine | 92 | |||
| 32 < 37 | Furosemide | 98 | Dopamine | 170 | Hydrochlorothiazide | 113 | Furosemide | 151 |
| Dopamine | 85 | Norepinephrine | 148 | Spironolactone | 113 | Hydrochlorothiazide | 122 | |
| Hydrochlorothiazide | 85 | Furosemide | 125 | Furosemide | 75 | Spironolactone | 122 | |
| Spironolactone | 85 | Hydrochlorothiazide | 34 | Metoprolol | 19 | Dobutamine | 94 | |
| Dobutamine | 24 | Spironolactone | 34 | Epinephrine | 58 | |||
| Norepinephrine | 24 | Epinephrine | 23 | Dopamine | 58 | |||
| ≥37 | Dopamine | 100 | Dopamine | 289 | Furosemide | 63 | Dobutamine | 129 |
| Hydrochlorothiazide | 71 | Norepinephrine | 248 | Hydrochlorothiazide | 52 | Epinephrine | 84 | |
| Spironolactone | 71 | Furosemide | 215 | Spironolactone | 52 | Dopamine | 56 | |
| Norepinephrine | 57 | Milrinone | 149 | Digoxine | 21 | Furosemide | 51 | |
| Epinephrine | 43 | Alprostadil | 116 | Propranolol | 10 | Alprostadil | 45 | |
| Alprostadil | 29 | Dobutamine | 99 | Hydrochlorothiazide | 39 | |||
The top five prescribed cardiovascular drugs are shown for every PMA group.
NICU, neonatal intensive care unit; PMA, postmenstrual age
Figure 3.
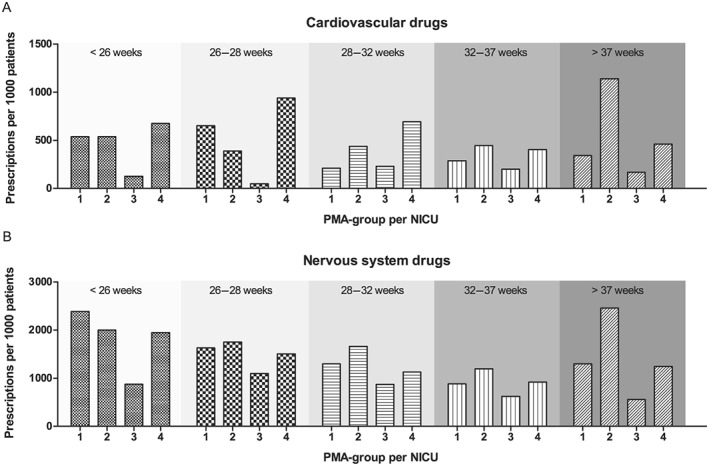
Exposure of preterm neonates in four NICUs to (A) cardiovascular and (B) nervous system drugs at different PMAs. The number of cardiovascular drug prescriptions is expressed per 1000 patients in each PMA group. NICU, neonatal intensive care unit; PMA, postmenstrual age
Table 4.
Nervous system drug prescriptions according to PMA (per 1000 neonates per PMA group)
| NICU1 | NICU2 | NICU3 | NICU4 | |||||
|---|---|---|---|---|---|---|---|---|
| PMA | Drug | No | Drug | No | Drug | No | Drug | No |
| <26 | Caffeine | 647 | Caffeine | 667 | Caffeine | 750 | Caffeine | 923 |
| Fentanyl | 529 | Morphine | 400 | Paracetamol | 125 | Propofol | 462 | |
| Morphine | 176 | Fentanyl | 333 | Morphine | 231 | |||
| Paracetamol | 114 | Midazolam | 133 | Fentanyl | 103 | |||
| Paracetamol | 25 | Midazolam | 77 | |||||
| Phenobarbital | 51 | |||||||
| 26 < 28 | Caffeine | 970 | Caffeine | 844 | Caffeine | 800 | Caffeine | 864 |
| Fentanyl | 394 | Fentanyl | 469 | Paracetamol | 267 | Propofol | 492 | |
| Paracetamol | 333 | Morphine | 281 | Fentanyl | 200 | Morphine | 305 | |
| Morphine | 273 | Midazolam | 156 | Morphine | 67 | Fentanyl | 186 | |
| Phenobarbital | 91 | Paracetamol | 125 | Phenobarbital | 67 | Midazolam | 119 | |
| Midazolam | 61 | Phenobarbital | 94 | Midazolam | 67 | Phenobarbital | 102 | |
| 28 < 32 | Caffeine | 857 | Caffeine | 927 | Caffeine | 902 | Caffeine | 724 |
| Fentanyl | 304 | Fentanyl | 317 | Paracetamol | 78 | Propofol | 314 | |
| Paracetamol | 259 | Morphine | 238 | Fentanyl | 59 | Morphine | 195 | |
| Morphine | 170 | Paracetamol | 222 | Methadone | 59 | Fentanyl | 108 | |
| Propofol | 27 | Midazolam | 95 | Phenobarbital | 39 | Midazolam | 81 | |
| Midazolam | 18 | Phenobarbital | 63 | Midazolam | 39 | Phenobarbital | 49 | |
| 32 < 37 | Paracetamol | 427 | Morphine | 364 | Caffeine | 566 | Caffeine | 403 |
| Caffeine | 378 | Paracetamol | 352 | Paracetamol | 226 | Morphine | 266 | |
| Morphine | 134 | Caffeine | 341 | Etomidate | 57 | Propofol | 252 | |
| Fentanyl | 122 | Fentanyl | 307 | Fentanyl | 57 | Paracetamol | 209 | |
| Phenobarbital | 98 | Midazolam | 182 | Midazolam | 38 | Fentanyl | 173 | |
| Propofol | 98 | Chloralhydrate | 80 | Dexmedetomidine | 19 | Midazolam | 165 | |
| >37 | Paracetamol | 557 | Morphine | 686 | Paracetamol | 396 | Paracetamol | 309 |
| Morphine | 257 | Paracetamol | 512 | Phenobarbital | 73 | Midazolam | 281 | |
| Phenobarbital | 157 | Midazolam | 504 | Caffeine | 63 | Morphine | 253 | |
| Midazolam | 157 | Fentanyl | 388 | Fentanyl | 31 | Phenobarbital | 185 | |
| Propofol | 114 | Phenobarbital | 231 | Midazolam | 31 | Levetiracetam | 129 | |
| Chloralhydrate | 100 | Chloralhydrate | 149 | Morphine | 21 | Propofol | 79 | |
The top five prescribed nervous system drugs are shown for every PMA group.
NICU, neonatal intensive care unit; PMA, postmenstrual age
Treatment protocol comparison
Table 5 gives an overview of the drugs and the order in which they should be prescribed according to the local treatment protocols in the different NICUs for the four selected indications per gestational age groups.
Table 5.
Order of drugs in treatment protocols concerning four major care indications in the four NICUs
| No | NICU1 | No | NICU2 | No | NICU3 | No | NICU4 | |
|---|---|---|---|---|---|---|---|---|
| Pain | Paracetamol | EMLA | Paracetamol | Fentanyl | ||||
| EMLA | Lidocaine | Lidocaine | Morphine | |||||
| Morphine | Morphine | Fentanyl | Midazolam | |||||
| Lidocaine | Fentanyl | Methadone | Paracetamol | |||||
| Fentanyl | Paracetamol | Morphine | ||||||
| Midazolam | Lorazepam | |||||||
| Levomepromazine | ||||||||
| Esketamine | ||||||||
| Hypotension | 1 | Dopamine | 1 | Dopamine | 1 | Dopamine | ||
| 2 | Dobutamine/Norepinephrine | 2 | Dobutamine/Norepinephrine | 1 | Dobutamine | |||
| 3 | Hydrocortisone | 3 | Dexamethasone | 1 | Norepinephrine | |||
| 3 | Epinephrine | 1 | Epinephrine | |||||
| 3 | Milrinone | 2 | Hydrocortisone | |||||
| 3 | Hydrocortisone | 2 | Methylene blueb | |||||
| 2 | Naloxoneb | |||||||
| Intubation | 1 | Atropine (<32 weeks) | 1 | Atropine | 1 | Atropine | 1 | Propofol |
| 1 | Fentanyl (<32 weeks) | 1 | Fentanyl/morphine | 1 | Fentanyl/morphine | |||
| 1 | Rocuronium (<32 weeks) | 1 | Rocuronium/vecuronium | 1 | Rocuronium/etomidate | |||
| 2 | Propofol (>32 weeks) | |||||||
| Convulsions | 1 | Phenobarbital | 1 | Phenobarbital | 1 | Phenobarbital | 1 | Phenobarbital |
| 2 | Levetiracetam | 2 | Midazolam (+ pyridoxine) | 2 | Midazolam | 2 | Midazolam (+ pyridoxine) | |
| 3 | Lidocaine | 3 | Lidocaine | 3 | Lidocaine | 3 | Lidocaine | |
| 4 | Midazolama (+ pyridoxine) | 4 | Levetiracetam | 4 | Pyridoxine | 4 | Levetiracetam | |
| 5 | Clonazepam | 5 | Pyridoxine | 5 | Thiopental | 5 | Pyridoxine | |
| 6 | Thiopental | 5 | Clonazepam |
The number (No) indicates the order in which drugs should be prescribed for treatment of each indication. If the same number has been used multiple times for one indication in one NICU, this means that their preference is equal, meaning that the attending physician is free to select one of the suggestions. One drug may be prescribed or a combination of drugs simultaneously. The absence of a number concerning pain treatment indicates none of the NICUs suggest a certain order in the drugs to be prescribed for pain treatment.
NICU, neonatal intensive care unit
Preferably avoid midazolam use for premature born infants
Experimental drugs
Discussion
We evaluated drug prescriptions between NICUs for a period of one year and found that a considerable percentage of the drugs are still used off‐label and that large differences exist in drug prescriptions between the four NICUs. The largest variability was found for drug classes with the highest proportion of off‐label drugs in relation to neonatal age, i.e. cardiovascular and nervous system drugs. These differences became larger with decreasing PMA, although the proportion of off‐label prescriptions became smaller. Despite the new FDA and EMA drug legislations, many drugs are still used off‐label and the variability in drugs prescriptions reflects the lack of evidence on drug use, especially in the smallest newborns.
Prescribed drugs
Of almost 11 000 drug prescriptions for neonates, 23% was off‐label in relation to neonatal age. Comparable proportions of off‐label prescriptions in relation to neonatal age were found in the last decade by Neubert et al. with 38% in Germany 16, Hsieh et al. with 35% in the USA 1, and Cuzzolin et al. with 34% in Italy 17. A comforting finding was that the proportion of off‐label prescriptions in relation to neonatal age increased with PMA at start of drug therapy. Therefore, the most vulnerable infants with the lowest PMA were exposed to fewer off‐label drugs than infants at higher PMA. This might reflect the cautiousness of clinicians in treating the most vulnerable patients. Dell'Aera et al. and Avenel et al. also found a higher prevalence of off‐label prescriptions within the full‐term neonates compared to the preterms 5, 18.
Also comforting was the small proportion of off‐label drug prescriptions in relation to neonatal age (4%) in the largest drug class of anti‐infective drugs. On the other hand, the second largest class concerned the nervous system drugs, of which 31% was off‐label in relation to neonatal age. These findings are in agreement with those of Cuzzolin et al. and Neubert et al. who also found that anti‐infective drugs were the largest ATC class prescribed with a proportion off‐label in relation to neonatal age of 24% and 11%, respectively 16, 17. For nervous system drugs, these studies found a proportion of 67% and 56% of off‐label prescriptions in relation to neonatal age, which is comparable with our results.
Nevertheless, off‐label drug use does not necessarily imply inadequate drug use, although this is generally suggested 19. Instead of referring to the label, adequate drug use should be based on the level of evidence, with an expert interpretation. Consequently, several sources have been developed which are periodically updated and released, such as the British National Formulary, Pediatric Dosages by Lexicomp, Pediatric Injectable Drugs, and Micromedex. Ceelie et al. reported on large differences between four commonly used paediatric drug formularies, which indicates the challenges in the availability and reliability of paediatric drug dosing guidelines in present drug formularies 20. Recently, in the Netherlands a continuously updated online paediatric formulary has been released – the Dutch Paediatric Formulary 21. Despite the valuable interpretation regarding dosages and safe drug use, the sources mentioned above do not suggest which drug to choose for certain indications and therefore do not help to reduce the differences in prescriptions between physicians and hospitals.
Comparing NICUs
Large differences between NICUs were found in neonatal drug use. Drug classes with a high proportion of off‐label drug prescriptions in relation to neonatal age showed the largest differences between NICUs, i.e. cardiovascular and nervous system drugs. Also, these ATC classes, together with ATC class blood and blood‐forming organs, showed the largest range of total prescriptions per ATC class per 1000 patients between NICUs. As the high rank of blood and blood‐forming organs was driven by heparin and phytomenadione alone, this class was of limited interest for further comparison.
The large differences among cardiovascular drugs strengthen the alarming message of a severe lack in paediatric documentation, which has been reported by Bajcetic et al. 22 and Pasquali et al. 23. Nervous system drugs also showed large variability, which may be a reflection of the variation in drugs mentioned in pain treatment protocols of these four NICUs. This may be explained by the worldwide discussion on the neurodevelopmental safety of nervous system drugs such as opioids, paracetamol and benzodiazepines in the preterm brain 24. A comparable discussion accounts for the choice of premedication for intubation 25, 26, 27. This can be recognized in treatment protocols in our study, choosing either an opioid with a muscle relaxant, or propofol. Mehler et al. studied analgesic and sedative drug use in very low birth weight infants in German NICUs and reported large differences, as well as many changes over time in analgesic and sedative treatment 28. On the other hand, the treatment protocol of neonatal convulsions showed less differences between NICUs, which seems to be the result of an existing national guideline 29. Even though all mentioned drugs in the guideline were off‐label for treatment of convulsions in neonates, this publicly accessible expert opinion appears to reduce different interpretations of sparse evidence.
Liem et al. reported a comparable approach for antibiotic drugs alone and found considerable variation between Dutch NICUs in the number of different antibiotics used and in the total dosage of antibiotics 30. This heterogeneity indicates that empirical antibiotic treatment varies among NICUs and there are currently no consensus guidelines regarding the choice of empirical antibiotics.
Although all four participating NICUs were level 3, considerable differences were found in the general descriptives between the NICUs; i.e. duration of admission, gestational ages and specific treatments (surgery, extracorporeal membrane oxygenation). These may partly explain the large variability in prescribed drugs between NICUs. Another cause for differences in drug use concerns the steps by which new evidence is adapted to clinical care, which may depend on local expert opinions.
Our multicentre comparison of drug use in NICUs provides a unique view of neonatal pharmacology in practice but is limited by some assumptions. First, our data did not allow comparison of NICUs with respect to drug dosages, routes of administration, specific products or preparation of drugs for administration. Apart from judging whether a drug is registered for use in neonatal age, each of these items could also have been related to the label if the data were available. Second, since practically all drugs were first labelled for an adult indication, their ATC code was often incorrect with respect to their use in current neonatal practice. Even for drugs where the neonatal indication has been added to the label, their ATC code remains as primarily marketed. This concerns, for example, sildenafil, ibuprofen, caffeine and phytomenadione. Third, differences in local decision‐making practice determines treatments and drug use. In a smaller NICU it may be easier to reach consensus than in a larger NICU. Fourth, data was retrospectively collected from different prospective electronic health record systems, which may have caused some differences in definitions used for data output. Fifth, our findings from a single country cohort cannot easily be compared to other countries or reports, as the content of the SmPCs may be different between countries, and various definitions for off‐label status have been used, which has also been shown by Aronson et al. 31. Sixth, if the SmPC mentioned an indication for infants in general without mentioning an age range, this was considered to also include neonates and therefore on‐label in relation to neonatal age (see Supporting Information File S1). Nevertheless, physicians would not feel safe to prescribe these drugs in clinical practice based on this information, knowing that a general dosage for infants is not optimal and safe for (preterm) neonates. However, if these drugs, with an on‐label status for infants without mentioning an age range, were to be considered off‐label in relation to neonatal age instead, the overall proportion of off‐label prescriptions in our cohort increases from 23% to 41%. This is mainly due to changes in the ATC groups; cardiovascular drugs (from 30% to 94% off‐label), anti‐infectives (from 4% to 24% off‐label), and nervous system drugs (from 31% to 46% off‐label). In addition, an indication and dosage for neonates in the SmPC rarely differentiates for gestational age. As the definition of a ‘neonate’ is limited to a newborn infant during its first 30 days of life, without referring to a certain gestational age, we considered neonates to be term as well preterm newborn infants. Nevertheless, on‐label in relation to neonatal age should not necessarily mean on‐label for all gestational ages.
Future suggestions
Our study shows that there is great variability in the drug prescriptions for neonates in NICUs. Little consensus has been reached on these drugs, and therefore expert interpretation of current evidence and future research should be prioritized. New investigator‐initiated research is urgently required as there is little benefit to pharmaceutical companies in incorporating new findings in paediatrics, which has led to few drug‐labelling changes made under paediatric legislation, including neonates 12. Nevertheless, pharmacological trials involving neonates deal with multiple challenges. Appropriate dosing is hampered by the rapid physiological changes occurring at this stage of development. The selection of proper end‐points and biomarkers is complicated by the limited knowledge of the pathophysiology of the specific diseases of infancy. Coppini et al. have addressed possible perspectives to stimulate research in neonates and infants 32. Furthermore, as evidence on pharmacological treatment of neonates remains thin, more (inter)national guidelines on treatment of common indications should be published, following the successful example of the guideline for neonatal convulsions.
Conclusion
We showed that drugs used for neonatal care differed importantly between four Dutch level 3 NICUs. Our findings form a valuable contribution to the several pooled prescription data analyses of multiple NICUs that have been reported. The drug classes with the highest proportion of off‐label drugs in relation to neonatal age showed the largest differences between NICUs, i.e. cardiovascular and nervous system drugs. We believe that drug research in neonates should have high priority to ensure the use of safe and appropriate drug therapy in newborns.
Competing Interests
There are no competing interests to declare.
The authors thank the DINO‐trial staff of the participating NICUs for their contribution. This study was enabled by funding from the Netherlands Organisation for Health Research and Development ZonMw (Grant number: 80‐83600‐98‐10190).
Supporting information
File S1 Age range in SmPC per active substance and off‐label interpretation in relation to neonatal age
Flint, R. B. , van Beek, F. , Andriessen, P. , Zimmermann, L. J. , Liem, K. D. , Reiss, I. K. M. , de Groot, R. , Tibboel, D. , Burger, D. M. , Simons, S. H. P. , and DINO Research group (2018) Large differences in neonatal drug use between NICUs are common practice: time for consensus?. Br J Clin Pharmacol, 84: 1313–1323. doi: 10.1111/bcp.13563.
References
- 1. Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK Jr, Smith PB, et al Medication use in the neonatal intensive care unit. Am J Perinatol 2014; 31: 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kimland E, Odlind V. Off‐label drug use in pediatric patients. Clin Pharmacol Ther 2012; 91: 796–801. [DOI] [PubMed] [Google Scholar]
- 3. Barr J, Brenner‐Zada G, Heiman E, Pareth G, Bulkowstein M, Greenberg R, et al Unlicensed and off‐label medication use in a neonatal intensive care unit: a prospective study. Am J Perinatol 2002; 19: 67–72. [DOI] [PubMed] [Google Scholar]
- 4. Conroy S, McIntyre J, Choonara I. Unlicensed and off label drug use in neonates. Arch Dis Child Fetal Neonatal Ed 1999; 80: F142–F144 discussion F44–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dell'Aera M, Gasbarro AR, Padovano M, Laforgia N, Capodiferro D, Solarino B, et al Unlicensed and off‐label use of medicines at a neonatology clinic in Italy. Pharm World Sci 2007; 29: 361–367. [DOI] [PubMed] [Google Scholar]
- 6. Lass J, Kaar R, Jogi K, Varendi H, Metsvaht T, Lutsar I. Drug utilisation pattern and off‐label use of medicines in Estonian neonatal units. Eur J Clin Pharmacol 2011; 67: 1263–1271. [DOI] [PubMed] [Google Scholar]
- 7. O'Donnell CP, Stone RJ, Morley CJ. Unlicensed and off‐label drug use in an Australian neonatal intensive care unit. Pediatrics 2002; 110: e52. [DOI] [PubMed] [Google Scholar]
- 8. Turner S, Nunn AJ, Fielding K, Choonara I. Adverse drug reactions to unlicensed and off‐label drugs on paediatric wards: a prospective study. Acta Paediatr 1999; 88: 965–968. [DOI] [PubMed] [Google Scholar]
- 9. Food and Drug Administration . Pediatric Research Equity Act of 2003. Washington, DC: US Government, 2003. [Google Scholar]
- 10. Food and Drug Administration . FDA Reauthorization Act of 2017. Washington, DC: US Government, 2017.
- 11. Borrell Fontelles J, Pekkarinen M. Regulation (EC) No. 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medicinal products for paediatric use and amending Regulation (EEC) No. 1768/92, Directive 2001/20/EC, Doirective 2001/83/EC and Regulation (EC) No. 726/2004. Official Journal of the European Union 2006; L378/1–L78/19. [Google Scholar]
- 12. Laughon MM, Avant D, Tripathi N, Hornik CP, Cohen‐Wolkowiez M, Clark RH, et al Drug labeling and exposure in neonates. JAMA Pediatr 2014; 168: 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ward RM, Sherwin CM. Newborns still lack drug data to guide therapy. Br J Clin Pharmacol 2016; 82: 1410–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neubert A, Wong IC, Bonifazi A, Catapano M, Felisi M, Baiardi P, et al Defining off‐label and unlicensed use of medicines for children: results of a Delphi survey. Pharmacol Res 2008; 58: 316–322. [DOI] [PubMed] [Google Scholar]
- 15. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement. PLoS Med 2015; 12: e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neubert A, Lukas K, Leis T, Dormann H, Brune K, Rascher W. Drug utilisation on a preterm and neonatal intensive care unit in Germany: a prospective, cohort‐based analysis. Eur J Clin Pharmacol 2010; 66: 87–95. [DOI] [PubMed] [Google Scholar]
- 17. Cuzzolin L, Agostino R. Off‐label and unlicensed drug treatments in Neonatal Intensive Care Units: an Italian multicentre study. Eur J Clin Pharmacol 2016; 72: 117–123. [DOI] [PubMed] [Google Scholar]
- 18. Avenel S, Bomkratz A, Dassieu G, Janaud JC, Danan C. Incidence des prescriptions hors autorisation de mise sur le marche en reanimation neonatale [The incidence of prescriptions without marketing product license in a neonatal intensive care unit]. Arch Pediatr 2000; 7: 143–147. [DOI] [PubMed] [Google Scholar]
- 19. Balan S, Hassali MA, Mak VS. Awareness, knowledge and views of off‐label prescribing in children: a systematic review. Br J Clin Pharmacol 2015; 80: 1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ceelie I, van der Starre C, Tibboel D, Stol K, Koren G, de Wildt SN. Evaluation of drug formularies for pediatric intensive care. Pediatr Crit Care Med 2011; 12: e14–e19. [DOI] [PubMed] [Google Scholar]
- 21. van der Zanden TM, de Wildt SN, Liem Y, Offringa M, de Hoog M, Dutch Paediatric Pharmacotherapy Expertise Network NKFK . Developing a paediatric drug formulary for the Netherlands. Arch Dis Child 2017; 102: 357–361. [DOI] [PubMed] [Google Scholar]
- 22. Bajcetic M, Jelisavcic M, Mitrovic J, Divac N, Simeunovic S, Samardzic R, et al Off label and unlicensed drugs use in paediatric cardiology. Eur J Clin Pharmacol 2005; 61: 775–779. [DOI] [PubMed] [Google Scholar]
- 23. Pasquali SK, Hall M, Slonim AD, Jenkins KJ, Marino BS, Cohen MS, et al Off‐label use of cardiovascular medications in children hospitalized with congenital and acquired heart disease. Circ Cardiovasc Qual Outcomes 2008; 1: 74–83. [DOI] [PubMed] [Google Scholar]
- 24. Smits A, van den Anker JN, Allegaert K. Clinical pharmacology of analgosedatives in neonates: ways to improve their safe and effective use. J Pharm Pharmacol 2017; 69: 350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borenstein‐Levin L, Synnes A, Grunau RE, Miller SP, Yoon EW, Shah PS, et al Narcotics and sedative use in preterm neonates. J Pediatr 2017; 180: 92–98 e1. [DOI] [PubMed] [Google Scholar]
- 26. Carbajal R, Eble B, Anand KJ. Premedication for tracheal intubation in neonates: confusion or controversy? Semin Perinatol 2007; 31: 309–317. [DOI] [PubMed] [Google Scholar]
- 27. Durrmeyer X, Daoud P, Decobert F, Boileau P, Renolleau S, Zana‐Taieb E, et al Premedication for neonatal endotracheal intubation: results from the epidemiology of procedural pain in neonates study. Pediatr Crit Care Med 2013; 14: e169–e175. [DOI] [PubMed] [Google Scholar]
- 28. Mehler K, Oberthuer A, Haertel C, Herting E, Roth B, Goepel W, et al Use of analgesic and sedative drugs in VLBW infants in German NICUs from 2003–2010. Eur J Pediatr 2013; 172: 1633–1639. [DOI] [PubMed] [Google Scholar]
- 29. Smit LS, Peeters‐Scholte CMPCD, van Rooij, L.G.M. Richtlijn voor behandeling van neonatale epileptische aanvallen. In Nederlands Vlaamse Werkgroep Neonatale Neurol ogie van de Sectie Neonatologie van de NVK en van de Nederlandse Vereniging voor Kinder neurologie [online]. Available at https://www.erasmusmc.nl/47445/5464293/5931241/674532/2253329/2261613.pdf, 2012: 1–10.
- 30. Liem TB, Krediet TG, Fleer A, Egberts TC, Rademaker CM. Variation in antibiotic use in neonatal intensive care units in the Netherlands. J Antimicrob Chemother 2010; 65: 1270–1275. [DOI] [PubMed] [Google Scholar]
- 31. Aronson JK, Ferner RE. Unlicensed and off‐label uses of medicines: definitions and clarification of terminology. Br J Clin Pharmacol 2017; 83: 2615–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coppini R, Simons SH, Mugelli A, Allegaert K. Clinical research in neonates and infants: Challenges and perspectives. Pharmacol Res 2016; 108: 80–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1 Age range in SmPC per active substance and off‐label interpretation in relation to neonatal age


