Abstract
Background
Small hepatocellular carcinomas (HCC ≤3 cm) are generally considered to have low malignant potential; however, some of them display pathological microvascular invasion (MVI).
Methods
Between 1991 and 2013, 414 patients with a single HCC ≤3 cm underwent curative hepatic resection (HR). Predictors for MVI were identified. Using another cohort (149 patients during 2000‐2014), our predictors for MVI in HCC ≤3 cm were validated. In 428 patients with a single HCC ≤3 cm who had predictors for MVI, survival was compared among anatomical HR (n = 149), partial HR (n = 227), and radiofrequency ablation (RFA) (n = 52).
Results
The positive rate of MVI reached 40.6% (168/414 patients). Independent predictors for MVI were as follows: tumor diameter ≥2 cm (odds ratio 1.84, P = .0052), alpha‐fetoprotein (AFP) ≥200 ng/mL (odds ratio 1.82, P = .0466), and des‐gamma‐carboxy prothrombin (DCP) ≥40 mAU/mL (odds ratio 1.79, P = .0126). Matching at least one predictor among these three could predict MVI in HCC ≤3 cm well (sensitivity 82.8%, positive predictive value [PPV] 48.7%). This criterion could also predict MVI in HCC ≤3 cm well in another cohort (sensitivity 82.8%, PPV 30.3%). In patients with single HCC ≤3 cm matching our criterion for predicting MVI, anatomical HR led to significantly better survival in both disease‐free (hazard ratio 0.689, P = .0231) and overall (hazard ratio 0.589, P = .0316) survivals.
Conclusion
Matching at least one factor among three (tumor diameter ≥2 cm, AFP ≥200 ng/mL, or DCP ≥40 mAU/mL) can predict MVI in HCC ≤3 cm. In such patients, anatomical HR would be recommended to improve survival.
Keywords: alpha‐fetoprotein, anatomical hepatic resection, des‐gamma‐carboxy prothrombin, hepatocellular carcinoma, microvascular invasion
Short abstract
Matching at least one factor among three (tumor diameter ≥2 cm, AFP ≥200 ng/mL, or DCP ≥40 mAU/mL) can predict MVI in HCC ≤3 cm. In such patients, anatomical HR should be recommended to improve survival.
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide. Its incidence has doubled in the past 20 years, making it the second leading cause of cancer death.1 It is estimated that by 2020 the number of HCC cases in Europe and the USA will reach 78 000 and 27 000, respectively.2 Management of HCC has significantly improved over the last decade as a result of better knowledge of HCC behavior, improvements in staging systems and treatment algorithms, and emerging therapeutic options.3 One of the most reliable and widely adopted methods for staging HCC is the Barcelona Clinic Liver Cancer (BCLC) system, which stratifies patients according to the characteristics of the tumor, underlying liver disease and performance status.4
HCC diameter value “3 cm or less (≤3 cm)” has a major impact for treatment choice in both the BCLC system and the 3rd Japan Society of Hepatology (JSH)‐HCC guidelines.5 Percutaneous ablation therapy such as radiofrequency ablation (RFA) and hepatic resection (HR) are equally recommended for HCC ≤3 cm. The reason why HCC ≤3 cm would be proposed for RFA was not clear, although two reasons can be considered. One is that HCC ≤3 cm is thought to have low malignant potential, and the other is the limitation of the safety margin for ablation.
Many reports have noted the heterogeneity of small HCC ≤3 cm, and some of these tumors display microvascular invasion (MVI) ranging from 18.1% to 37.0%, which indicated locally advanced HCC.6, 7, 8 We previously reported that HR with a wide margin (>0.5 cm) led to better survival in patients with solitary HCC ≤2 cm that displayed MVI. To realize personalized treatment for small HCC, the establishment of reliable predictions for MVI in HCC ≤3 cm is very important.
Here, we present a retrospective analysis of predictors of MVI in single HCC ≤3 cm, and of survival in patients with single HCC ≤3 cm matching predictors for MVI who underwent anatomical HR, partial HR, or RFA.
2. METHODS
2.1. Patients
Four hundred and fourteen patients with initial solitary HCC ≤3 cm underwent HR at the Department of Surgery and Science, Kyushu University Hospital, between 1991 and 2013. Maximum tumor diameter of ≤3 cm was confirmed by cutting the surface after HR. In all cases, sufficient non‐cancerous lesions were collected for analysis of MVI. Medical records of these 414 patients were followed up through March 2015. Median follow‐up period in this series was 71 months. Predictors for MVI in HCC ≤3 cm were identified in this cohort.
One hundred and forty‐nine patients with initial solitary HCC ≤3 cm underwent HR at the Department of Gastroenterological Surgery, Kumamoto University Hospital, between 2000 and 2014. The medical records of these 149 patients were also followed up through March 2017. Median follow‐up period in this series was 62 months. Predictors for MVI in HCC ≤3 cm, identified in the former cohort, were validated in this next cohort.
2.2. Surgical techniques and follow‐up methods
Thorough intraoperative ultrasonography was carried out to determine the extent of disease and the line of parenchymal transection. A decision was then made regarding the type of liver resection to carry out, such as anatomical or partial HR, while considering the patient's liver function.9 Anatomical resection included hemi‐hepatectomy, segmentectomy, and subsegmentectomy or more, based on Couinaud's classification.10 In almost all HR, intermittent Pringle's maneuvers consisting of clamping the portal triad for 15 minutes and then releasing the clamp for 5‐minute intervals were applied. The CUSA system (Valley Lab, Boulder, CO, USA) was mainly used to transect the liver parenchyma. Our standard skin incision for HCC ≤3 cm was an upper midline and/or right subcostal incision. Forty‐one patients (9.9%) underwent laparoscopic hepatic resection.11 Any death that occurred in the hospital after treatment was recorded as a mortality. Complications were evaluated by Clavien's classification, and those with a score of Grade II or more were defined as positive.12
Gross classification of HCC ≤3 cm was made according to the general rules for clinical and pathological study of primary liver cancer established by the Liver Cancer Study Group of Japan,13 as non‐invasive gross type (vaguely nodular type, n = 7; and single nodular type, n = 315) and invasive gross type (single nodular type with extranodular growth, n = 42; and confluent multinodular type, n = 50). Pathological diagnoses were carried out by two or three certified experts in the individual institute according to the above general rules for pathological diagnosis.13
After discharge, all patients were examined for recurrence by ultrasonography, using tumor markers such as α‐fetoprotein (AFP) and des‐γ‐carboxy prothrombin (DCP) every month, and by computed tomography (CT) or magnetic resonance imaging (MRI) every 3 months. When recurrence was suspected, we treated the recurrent HCC by repeat HR,14 RFA, or lipiodolization.15
2.3. Statistics
Continuous variables are expressed as the means ± standard deviations (SD) and were compared using Student's t‐test. Categorical variables were compared using the χ2‐test. Survival curves were generated by the Kaplan‐Meier method and compared using the log‐rank test. Variables at P < .05 on univariate analysis were subjected to stepwise logistic regression analysis to identify independent predictors for MVI in HCC ≤3 cm. To identify better prognostic factors after treatments in patients with single HCC ≤3 cm matching our criterion for predicting MVI, 11 clinical, surgical, and tumor‐related variables were included in a Cox proportional hazard model in accordance with the findings of previous reports:5, 6, 9, 14 age (older vs younger than 65); preoperative total bilirubin (T‐bil) level (> vs ≤1 mg/dL); preoperative albumin level (> vs ≤3.5 mg/dL); indocyanine green retention rate at 15 minutes (ICGR15) (> vs ≤20%); liver damage13 (A vs B and C); preoperative AFP level (≥ vs <200 ng/mL); preoperative DCP level (≥ vs <40 mAU/mL); tumor diameter (≥ vs <2 cm); anatomical resection (yes vs no); surgical blood loss (> vs ≤1000 mL), and intraoperative blood cell transfusion (yes vs no). All analyses were carried out with JMP Pro 12.2.0 (SAS Institute, Cary, NC, USA). P‐values < .05 were considered significant.
3. RESULTS
3.1. Details of MVI in HCC ≤3 cm
Microvascular invasion was found in 168 patients (40.6%) among 414 patients with HCC ≤3 cm. Most of these patients (160/168 patients; 95.2%) had portal venous infiltration (vp), and two patients (0.5%) had hepatic venous infiltration (vv). Six patients (1.4%) had intrahepatic metastasis (im) without vp/vv, and one patient (0.2%) had bile duct infiltration (b).
3.2. Clinical characteristics of HCC ≤3 cm with and without MVI
Comparisons of background characteristics between the MVI (−) group (n = 246) and the MVI (+) group (n = 168) are summarized in Table 1. No variables showed significant differences between the two groups in background characteristics such as age, BMI, and liver function tests.
Table 1.
Comparisons of background characteristics between the MVI (−) group and the MVI (+) group
| Variable | MVI (−) (n = 246) | MVI (+) (n = 168) | P‐value |
|---|---|---|---|
| Age (y) | 66 ± 9 | 66 ± 10 | .6567 |
| Male/Female | 168/78 | 113/55 | .8255 |
| BMI (kg/m2) | 23.1 ± 3.0 | 23.2 ± 3.0 | .7779 |
| DM (+) (%) | 76 (31%) | 40 (24%) | .1071 |
| HBs‐Ag (+) (%) | 36 (14.6%) | 30 (17.9%) | .4108 |
| HCV‐Ab (+) (%) | 182 (74.0%) | 122 (72.6%) | .7578 |
| Plt (× 104/μL) | 19.3 ± 57.7 | 15.7 ± 17.8 | .4325 |
| T‐bil (mg/dL) | 0.8 ± 0.4 | 0.8 ± 0.4 | .7817 |
| Alb (g/dL) | 3.9 ± 0.4 | 3.9 ± 0.4 | .6978 |
| AST (IU/L) | 53 ± 45 | 48 ± 27 | .1456 |
| ALT (IU/L) | 51 ± 40 | 49 ± 34 | .3881 |
| PT (%) | 86 ± 15 | 87 ± 14 | .5402 |
| ICG15R (%) | 18.0 ± 9.7 | 18.9 ± 11.3 | .3865 |
| Child A (%) | 230 (93%) | 156 (93%) | .7999 |
| Liver damage A (%) | 167 (68%) | 126 (75%) | .1364 |
Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DM, diabetes mellitus; HBs‐Ag, hepatitis B virus surface antigen; HCV‐Ab, hepatitis C antibody; ICGR‐15, indocyanine green retention rate at 15 min; MVI, microvascular invasion; Plt, platelet count; PT, prothrombin time; T‐bil, total bilirubin.
Comparisons of surgical factors between the two groups are summarized in Table 2. Resected volume was significantly larger in the MVI (+) group (99 vs 73 g, P = .0071), and the rate of anatomical HR was higher in the MVI (+) group (36 vs 28%, P = .0993). Mean duration of hospital stay was significantly longer in the MVI (+) group (18 vs 16 days, P = .0414).
Table 2.
Comparisons of surgical factors between the MVI (−) group and the MVI (+) group
| Variable | MVI (−) (n = 246) | MVI (+) (n = 168) | P‐value |
|---|---|---|---|
| Intraoperative factors | |||
| Operation time (min) | 227 ± 93 | 219 ± 89 | .3539 |
| Bleeding (g) | 436 ± 449 | 469 ± 626 | .5233 |
| Resected volume (g) | 73 ± 75 | 99 ± 117 | .0071 |
| Transfusion (+) (%) | 24 (9.8%) | 15 (8.9%) | .7665 |
| Anatomical resection (%) | 69 (28%) | 60 (36%) | .0993 |
| Surgical margin (mm) | 4.9 ± 5.7 | 5.7 ± 6.8 | .2036 |
| Postoperative factors | |||
| Mortality (%) | 1 (0.4%) | 2 (1.2%) | .3606 |
| Morbidity (%) | 51 (21%) | 45 (27%) | .1601 |
| Hospital stay (days) | 16 ± 9 | 18 ± 12 | .0414 |
MVI, microvascular invasion.
Comparisons of tumor‐related factors between the two groups are summarized in Table 3. Variables of the MVI (+) group more often showed features of advanced tumor stage, such as tumor diameter (2.2 vs 2.0 cm; P < .0001), invasive gross type (46 vs 6%; P < .0001), poorly differentiated (33 vs 13%; P < .0001), fc‐inf (+) (63 vs 31%; P < .0001), and higher DCP level (124 vs 48 mAU/mL; P = .0031).
Table 3.
Comparisons of tumor‐related factors between the MVI (−) group and the MVI (+) group
| Variable | MVI (−) (n = 246) | MVI (+) (n = 168) | P‐value |
|---|---|---|---|
| Tumor diameter (cm) | 2.0 ± 0.6 | 2.2 ± 0.5 | <.0001 |
| Tumor diameter ≥2 cm (%) | 129 (52%) | 115 (68%) | .0011 |
| Invasive gross type (%) | 14 (6%) | 78 (46%) | <.0001 |
| Poorly differentiated (%) | 32 (13%) | 56 (33%) | <.0001 |
| fc (+) (%) | 113 (46%) | 115 (68%) | <.0001 |
| fc‐inf (+) (%) | 78 (32%) | 106 (63%) | <.0001 |
| AFP (ng/mL) | 201 ± 1039 | 199 ± 515 | .9756 |
| AFP ≥200 ng/mL (%) | 27 (11%) | 30 (18%) | .0479 |
| DCP (mAU/mL) | 48 ± 86 | 124 ± 367 | .0031 |
| DCP ≥40 mAU/mL (%) | 51 (21%) | 60 (36%) | .0029 |
| lc (+) (%) | 135 (55%) | 92 (55%) | .8894 |
AFP, alfa‐fetoprotein; DCP, des‐gamma‐carboxy prothrombin; fc, fibrous capsule; fc‐inf, fibrous capsule infiltration; lc, histological liver cirrhosis; MVI, microvascular invasion.
3.3. Independent risk factors or predictors for MVI in HCC ≤3 cm
Results of multivariate analysis with stepwise logistic regression analysis are summarized in Table 4. Independent risk factors for MVI were invasive gross type (odds ratio 13.68), fc‐inf (+) (odds ratio 4.11), and tumor diameter ≥2 cm (odds ratio 1.96). As for predictive factors for MVI which can be evaluated preoperatively, multivariate analysis (Table 5) showed that all three factors were independently significant predictors for MVI: tumor diameter ≥2 cm (odds ratio 1.84), AFP ≥200 ng/mL (odds ratio 1.82), and DCP ≥40 ng/mL (odds ratio 1.79).
Table 4.
Independent risk factors for MVI in HCC ≤3 cm
| Variable | Odds ratio | 95% CI | P‐value |
|---|---|---|---|
| Invasive gross type | 13.68 | 6.47‐31.76 | <.0001 |
| fc‐inf (+) | 4.11 | 1.77‐10.51 | .0008 |
| Tumor diameter ≥2 cm | 1.96 | 1.16‐3.33 | .0113 |
| Poorly differentiated | 1.43 | 0.91‐3.22 | .1985 |
| DCP ≥40 mAU/mL | 1.39 | 0.80‐2.42 | .2424 |
| fc (+) | 1.65 | 0.69‐4.32 | .2674 |
| AFP ≥200 ng/mL | 1.08 | 0.50‐2.36 | .8533 |
AFP, alpha‐fetoprotein; CI, confidence interval; DCP, des‐gamma‐carboxy prothrombin; fc, fibrous capsule; fc‐inf, fibrous capsule infiltration; HCC, hepatocellular carcinoma; MVI, microvascular invasion.
Table 5.
Independent predictors for MVI in HCC ≤3 cm
| Variable | Odds ratio | 95% CI | P‐value |
|---|---|---|---|
| Tumor diameter ≥2 cm | 1.84 | 1.20‐2.85 | .0052 |
| AFP ≥200 ng/mL | 1.82 | 1.01‐3.32 | .0466 |
| DCP ≥40 mAU/mL | 1.79 | 1.13‐2.83 | .0126 |
AFP, alpha‐fetoprotein; CI, confidence interval; DCP, des‐gamma‐ carboxy prothrombin; HCC, hepatocellular carcinoma; MVI, microvascular invasion.
3.4. Criterion for predicting MVI in HCC ≤3 cm
Sensitivity and positive predictive value (PPV) of our predictors are summarized in Table 6. Matching at least one predictor among the three predictors could predict MVI well (sensitivity 82.8%, PPV 48.7%). Matching two predictors led to a higher PPV (55.6%); however, sensitivity decreased (36.2%). Matching all three predictors also led to a higher PPV (69.2%); however, sensitivity decreased drastically (5.8%). According to these results, our criterion for predicting MVI in HCC ≤3 cm was defined as follows: matching at least one predictor among tumor diameter ≥2 cm, AFP ≥200 ng/mL, and DCP ≥40 ng/mL.
Table 6.
Diagnostic value of our predictors for MVI in HCC ≤3 cm
| Variable | Sensitivity (%) | Positive predictive value (%) |
|---|---|---|
| Matching one predictor | 82.8 | 48.7 |
| Matching two predictors | 36.2 | 55.6 |
| Matching three predictors | 5.8 | 69.2 |
HCC, hepatocellular carcinoma; MVI, microvascular invasion.
3.5. Validation of our criterion for predicting MVI in HCC ≤3 cm using another cohort
Our criterion for predicting MVI in HCC ≤3 cm was validated using another cohort consisting of 149 patients with an initial single HCC ≤3 cm. MVI was found in 30 patients (20.1%). Among those with HCC ≤3 cm with MVI, most patients (22/30; 73.3%) had vp, and five (16.7%) had vv. Three patients (10.0%) had im without vp/vv, and three patients (10%) had b.
Sensitivity and PPV of our criterion for predicting MVI in this next cohort are summarized in Table 7. Matching at least one predictor among three predictors could also predict MVI well (sensitivity 82.8%, PPV 30.3%). Matching two predictors led to a higher PPV (38.9%); however, sensitivity decreased (72.4%). Matching all three predictors also led to a higher PPV (41.2%); however, sensitivity dropped markedly (24.1%). Thus, in a separate cohort, our criterion for predicting MVI in HCC ≤3 cm also worked well.
Table 7.
Diagnostic value of our predictors for MVI in HCC ≤3 cm in another cohort
| Variable | Sensitivity (%) | Positive predictive value (%) |
|---|---|---|
| Matching one predictor | 82.8 | 30.3 |
| Matching two predictors | 72.4 | 38.9 |
| Matching three predictors | 24.1 | 41.2 |
HCC, hepatocellular carcinoma; MVI, microvascular invasion.
3.6. Survival of patients matching the criterion for predicting MVI in HCC ≤3 cm
In 563 patients, comprising both cohorts with initial single HCC ≤3 cm, 376 patients (67%) matched our criterion for predicting MVI. Anatomical HR was carried out in 149 patients, partial HR in 227 patients, and survivals were compared to 52 patients matched our criterion who underwent RFA. Disease‐free survival (DFS) and overall survival (OS) curves of these three groups are shown in Figure 1A and 1B, respectively. There were significant differences in both DFS and OS curves (P = .0478 and P = .0358, respectively). The 5‐year DFS of the anatomical HR group reached 55%; however, the 5‐year DFS of the RFA group was 28%. The 5‐year OS of the anatomical HR group reached 61%, whereas that of the RFA group was 36%.
Figure 1.
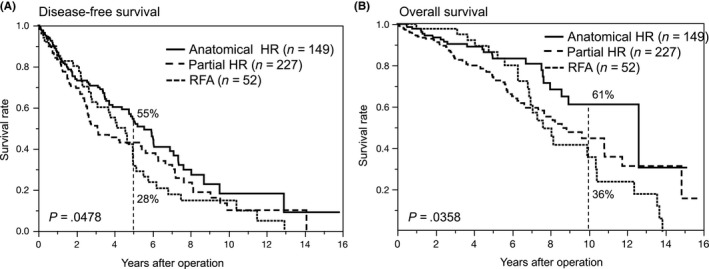
(A) Disease‐free and (B) overall survival curves after anatomical hepatic resection (HR), partial HR, and radiofrequency ablation (RFA) in patients with hepatocellular carcinoma ≤3 cm matching our criterion for predicting microvascular invasion
Multivariate analysis using Cox proportional hazard models identified five better prognostic factors (absence of transfusion, T‐bil ≤1.0 mg/dL, age ≤65 years, ICGR15 ≤20%, and anatomical HR) influencing DFS, and three better prognostic factors (ICGR15 ≤20%, anatomical HR, and age ≤65 years) influencing OS (Table 8). Anatomical HR led to significantly better survival in both disease‐free (hazard ratio 0.689, P = .0231) and overall (hazard ratio 0.589, P = .0316) survivals.
Table 8.
Multivariate analysis for better prognostic factors in DFS and OS
| Variables | Hazard ratio | 95% CI | P‐value |
|---|---|---|---|
| DFS | |||
| Transfusion (−) | 0.422 | 0.168‐0.922 | .0294 |
| T‐bil ≤1.0 mg/dL | 0.615 | 0.447‐0.858 | .0046 |
| Age ≤65 y | 0.661 | 0.495‐0.858 | .0046 |
| ICGR15 ≤20% | 0.664 | 0.471‐0.946 | .0238 |
| Anatomical resection | 0.689 | 0.501‐0.950 | .0231 |
| OS | |||
| ICGR15 ≤20% | 0.532 | 0.351‐0.812 | .0036 |
| Anatomical resection | 0.589 | 0.349‐0.956 | .0316 |
| Age ≤65 y | 0.594 | 0.405‐0.864 | .0064 |
CI, confidence interval; DFS, disease‐free survival; ICGR‐15, indocyanine green retention rate at 15 min; OS, overall survival; T‐bil, total bilirubin.
4. DISCUSSION
In the BCLC system, HCC ≤3 cm is considered to be “early stage”, which denotes an early clinical entity with a high rate of cure. In both the BCLS system and the JSH‐HCC guidelines, HR and RFA are equally recommended for HCC ≤3 cm. This is presumably based on the concept that HCC ≤3 cm is homogeneous and has low malignant potential. In our series, MVI was found in 168 patients (40.6%) among 414 patients with single HCC ≤3 cm, suggesting that the population of HCC ≤3 cm is not homogeneous, and that there may be a subgroup of an advanced biological nature with high‐grade malignancy. We previously reported that MVI was also found in HCC ≤2 cm (very early stage in the BCLC system); however, its rate is low at 28.9%.16 Among 244 patients with 2 cm ≤HCC ≤3 cm in our series, MVI was found in 115 patients (47.1%). This high rate would mean that 2 cm ≤HCC ≤3 cm has high‐grade malignancy and, from our data, HR would be preferable to RFA for treating this subgroup.
We have reported that the elevation of DCP is a strong predictor for MVI of HCC.16, 17, 18 DCP may have the ability to enhance cell proliferation by Met receptor and angiogenesis by vascular endothelial growth factor.19, 20 Koike et al21 carried out a prospective study to clarify the significance of DCP and concluded that DCP positivity was the strongest predictive factor for portal vein invasion. We know that DCP has not been measured worldwide; however, the significance of DCP elevation for predicting MVI in early HCC has been recognized in Western countries.22 To establish personalized treatment for early HCC, measurement of DCP should be strongly recommended.
Although many reports refer to the superiority of DCP to AFP in predicting MVI in HCC, AFP is nevertheless another tool for evaluating the malignant potential of HCC.23 In our series, the value of AFP itself had no correlation with MVI in HCC ≤3 cm; however, AFP ≥200 ng/mL had a significant correlation with MVI (+). The cut‐off value of 200 ng/mL was from the appropriate value of 196 ng/mL identified by the ROC curve for predicting MVI in our first cohort. Mild to moderate elevation of AFP is sometimes found in patients with hepatitis or cirrhosis, but severe elevation of AFP to levels of 200 ng/mL or more is likely to be caused by HCC with high malignant potential.
We previously reported that invasive gross type was the strongest predictor for MVI in HCC ≤2 cm.16 In the present study, invasive gross type was also the strongest predictor for MVI in HCC ≤3 cm (odds ratio 13.68). Therefore, the preoperative diagnosis for HCC gross type should be another potent tool. We previously found that preoperative diagnosis for gross type of HCC ≤2 cm is highly challenging, because distinguishing between single nodular type with extranodular growth from single nodular type and confluent multinodular type from vaguely nodular type is difficult.16 Hui et al24 tried to classify the gross type of HCC by reviewing CT images, but the rate of correct diagnosis was only 46%. Nakayama et al25 also reported an objective morphological classification system using multiphase CT. These systems should be useful for diagnosis for multinodular type, but are unlikely to be effective for single nodular type with extranodular growth type, especially for small HCC. Diffusion‐weighted imaging of MRI26, 27 or standardized uptake value (SUV)max in positron emission tomography (PET)26, 27 are other possible tools for predicting MVI in HCC ≤3 cm; for these tools, however, the small tumor size itself presents an obstacle for accurate evaluation.
To eradicate the MVI of HCC, anatomical HR28 or partial HR with a wide tumor margin15 should be recommended. Anatomical HR, in theory, can ideally eradicate MVI confined to tumor‐bearing portal tributaries. In our series involving 428 patients with HCC ≤3 cm matching our criterion of predicting MVI, a positive survival impact of anatomical HR was found. The 5‐year DFS of the anatomical HR group was 55%, compared to 46% for the partial HR group and 28% for the RFA group. This result meant high curability for HCC ≤3 cm with MVI by anatomical HR, and anatomical HR would therefore be recommended for HCC ≤3 cm, matching our criterion for predicting MVI. In contrast, liver function reserve may be one of the most important factors for patients’ survival after treatment for HCC.9 Actually, in our own study, there were significant differences in the ICGR15 values among anatomical HR (15.6 ± 6.8%), partial HR (21.3 ± 8.6%), and RFA groups (27.9 ± 7.9%). Recent meta‐regression analysis concerning patients’ survival after anatomical HR versus partial HR for HCC showed this critical concern.29
Limitations of the present study are its retrospective design; in addition, our results may be biased as a result of the physicians’ varying therapeutic policies. Furthermore, patients’ backgrounds, such as liver function reserves, differ among the anatomical HR, partial HR, and RFA groups. These differences may substantially affect survival after treatment. Propensity score matching is one of the potent methods to compare treatment modalities under homogeneous conditions; however, in our series, matching was very difficult because of the definite difference of liver functional reserve among the three groups. Therefore, randomized control study with the same therapeutic policies and the same patients’ backgrounds will be necessary to confirm our results. Second, the specificity of our criterion for predicting MVI in HCC ≤3 cm was relatively low. Actual positive rate of MVI in this cohort was 35% (198 in 563 patients); therefore, there is a possibility of carrying out unnecessary anatomical HR in patients without MVI. We consider that “sensitivity” has more priority than “specificity” in this situation; however, a better criterion with high specificity should be discussed. Finally, in this study, the positive rates of MVI in HCC ≤3 cm considerably differed between the two institutions (40.6% vs 20.1%). This difference may be related to the characteristics of patients; however, there is no significant difference in the positive rates of poorly differentiated HCC (21.3 vs 20.8%, P = .9079). The same pathologist or pathological team did not examine MVI; however, pathologists of these two institutions are skilled experts because both institutions are high‐volume centers of HR for HCC of over 100 cases per year. In addition, pathologists examined MVI according to the same published general rules.13 We cannot show the reason there was a big difference in the positive rate of MVI in HCC ≤3 cm between the two institutions, but this difference itself would be a problem to be resolved by additional concerns of the pathological definitions of MVI for small‐sized HCC.
In conclusion, matching at least one factor among three factors (tumor diameter ≥2 cm, AFP ≥200 ng/mL, or DCP ≥40 mAU/mL) can predict MVI in HCC ≤3 cm. In such patients, we recommend anatomical HR for better survival.
DISCLOSURE
Conflicts of Interest: Authors declare no conflicts of interest for this article.
Yamashita Y‐I, Imai K, Yusa T, et al. Microvascular invasion of single small hepatocellular carcinoma ≤3 cm: Predictors and optimal treatments. Ann Gastroenterol Surg. 2018;2:197–203. https://doi.org/10.1002/ags3.12057
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24:1–17. [DOI] [PubMed] [Google Scholar]
- 3. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. European Association for the Study of the Liver ; European Organisation for Research and Treatment of Cancer . EASL‐EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. [DOI] [PubMed] [Google Scholar]
- 5. Kokudo N, Hasegawa K, Akahane M, et al. Evidence‐based clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2013 update (3rd JSH‐HCC Guidelines). Hepatol Res. 2015;45:126 https://doi.org/10.1111/hepr.12464. [DOI] [PubMed] [Google Scholar]
- 6. Giuliante F, Ardito F, Pinna AD, et al. Liver resection for hepatocellular carcinoma ≤3 cm: results of an Italian multicenter study on 588 patients. J Am Coll Surg. 2012;215:244–54. [DOI] [PubMed] [Google Scholar]
- 7. Du M, Chen L, Zhao J, et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer. 2014;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Masuda T, Beppu T, Okabe H, et al. Predictive factors of pathological vascular invasion in hepatocellular carcinoma within 3 cm and three nodules without radiological vascular invasion. Hepatol Res. 2016;46:985–91. [DOI] [PubMed] [Google Scholar]
- 9. Yamashita Y, Taketomi A, Itoh S, et al. Longterm favorable results of limited hepatic resections for patients with hepatocellular carcinoma: 20 years of experience. J Am Coll Surg. 2007;205:19–26. [DOI] [PubMed] [Google Scholar]
- 10. Couinaud C. Lobes et segments hepatiques [in French]. Press Med. 1954;62:709–12. [PubMed] [Google Scholar]
- 11. Yamashita Y, Ikeda T, Kurihara T, et al. Long‐term favorable surgical results of laparoscopic hepatic resection for hepatocellular carcinoma in patients with cirrhosis: a single‐center experience over a 10‐year period. J Am Coll Surg. 2014;219:1117–23. [DOI] [PubMed] [Google Scholar]
- 12. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Ann Surg. 2009;250:187–96. [DOI] [PubMed] [Google Scholar]
- 13. Liver Cancer Study Group of Japan . The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 6th ed revised version. Tokyo: Kanehara; 2015. [Google Scholar]
- 14. Yamashita Y, Shirabe K, Tsuijita E, et al. Third or more repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery. 2013;154:1038–45. [DOI] [PubMed] [Google Scholar]
- 15. Kanematsu T, Furuta T, Takenaka K, et al. A 5‐year experience of lipiodolization: selective regional chemotherapy for 200 patients with hepatocellular carcinoma. Hepatology. 1989;10:98–102. [DOI] [PubMed] [Google Scholar]
- 16. Yamashita Y, Tsuijita E, Takeishi K, et al. Predictors for microinvasion of small hepatocellular carcinoma ≤ 2 cm. Ann Surg Oncol. 2012;19:2027–34. [DOI] [PubMed] [Google Scholar]
- 17. Shirabe K, Toshima T, Kimura K, et al. New scoring system for prediction of microvascular invasion in patients with hepatocellular carcinoma. Liver Int. 2014;34:937–41. [DOI] [PubMed] [Google Scholar]
- 18. Yamashita Y, Shirabe K, Aishima S, Maehara Y. Predictors of microvascular invasion in hepatocellular carcinoma. Dig Dis. 2015;33:655–60. [DOI] [PubMed] [Google Scholar]
- 19. Murata K, Suzuki H, Okano H, Oyamada T, Yasuda Y, Sakamoto A. Cytoskeletal changes during epithelial‐to‐fibroblastoid conversion as a crucial mechanism of des‐gamma‐carboxy prothrombin production in hepatocellular carcinoma. Int J Oncol. 2009;35:1005–14. [DOI] [PubMed] [Google Scholar]
- 20. Suzuki M, Shiraha H, Fujikawa T, et al. Des‐gamma‐carboxy prothrombin is a potential autologous growth factor for hepatocellular carcinoma. J Biol Chem. 2005;280:6409–15. [DOI] [PubMed] [Google Scholar]
- 21. Koike Y, Shiratori Y, Sato S, et al. Des‐gamma‐carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer. 2001;91:561–9. [DOI] [PubMed] [Google Scholar]
- 22. Poté N, Cauchy F, Albuquerque M, et al. Performance of PIVKA‐II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848–54. [DOI] [PubMed] [Google Scholar]
- 23. Marrero JA, Feng Z, Wang Y, et al. Alpha‐fetoprotein, des‐gamma carboxyprothrombin, and lectin‐bound alpha‐fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hui AM, Takayama T, Sano K, et al. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J Hepatol. 2000;33:975–9. [DOI] [PubMed] [Google Scholar]
- 25. Nakayama H, Takayama T, Okubo T, et al. Proposal of objective morphological classification system for hepatocellular carcinoma using preoperative multiphase computed tomography. J Gastroenterol. 2014;49:1430–7. [DOI] [PubMed] [Google Scholar]
- 26. Kim MJ, Lee M, Choi JY, Park YN. Imaging features of small hepatocellular carcinomas with microvascular invasion on gadoxetic acid‐enhanced MR imaging. Eur J Radiol. 2012;81:2507–12. [DOI] [PubMed] [Google Scholar]
- 27. Xu P, Zeng M, Liu K, Shan Y, Xu C, Lin J. Microvascular invasion in small hepatocellular carcinoma: is it predictable with preoperative diffusion‐weighted imaging? J Gastroenterol Hepatol. 2014;29:330–6. [DOI] [PubMed] [Google Scholar]
- 28. Ueno S, Kubo F, Sakoda M, et al. Efficacy of anatomic resection vs nonanatomic resection for small nodular hepatocellular carcinoma based on gross classification. J Hepatobiliary Pancreat Surg. 2008;15:493–500. [DOI] [PubMed] [Google Scholar]
- 29. Cucchetti A, Cescon M, Ercolani G, Bigonzi E, Torzilli G, Pinna AD. A comprehensive meta‐regression analysis on outcome of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Ann Surg Oncol. 2012;19:3697–705. [DOI] [PubMed] [Google Scholar]


