Abstract
Background
The prognostic relevance of left atrial (LA) morphological and functional variables, including those derived from speckle tracking echocardiography (STE), has been little investigated in veterinary medicine.
Objectives
To assess the prognostic value of several echocardiographic variables, with a focus on LA morphological and functional variables in dogs with myxomatous mitral valve disease (MMVD).
Animals
One‐hundred and fifteen dogs of different breeds with MMVD.
Methods
Prospective cohort study. Conventional morphologic and echo‐Doppler variables, LA areas and volumes, and STE‐based LA strain analysis were performed in all dogs. A survival analysis was performed to test for the best echocardiographic predictors of cardiac‐related death.
Results
Most of the tested variables, including all LA STE‐derived variables were univariate predictors of cardiac death in Cox proportional hazard analysis. Because of strong correlation between many variables, only left atrium to aorta ratio (LA/Ao > 1.7), mitral valve E wave velocity (MV E vel > 1.3 m/s), LA maximal volume (LAVmax > 3.53 mL/kg), peak atrial longitudinal strain (PALS < 30%), and contraction strain index (CSI per 1% increase) were entered in the univariate analysis, and all were predictors of cardiac death. However, only the MV E vel (hazard ratio [HR], 4.45; confidence interval [CI], 1.76‐11.24; P < .001) and LAVmax (HR, 2.32; CI, 1.10‐4.89; P = .024) remained statistically significant in the multivariable analysis.
Conclusions and Clinical Importance
The assessment of LA dimension and function provides useful prognostic information in dogs with MMVD. Considering all the LA variables, LAVmax appears the strongest predictor of cardiac death, being superior to LA/Ao and STE‐derived variables.
Keywords: canine, cardiology, left atrium, strain
Abbreviations
- Ao
aorta
- BW
body weight
- CHF
congestive heart failure
- CSI
contraction strain index
- FAC
fractional area change
- LA
left atrium
- LAAmax
left atrial maximal area
- LAAmin
left atrial minimal area
- LA/Ao
left atrial diameter to aorta ratio
- LAVmax
left atrial maximal volume
- LAVmin
left atrial minimal volume
- LAEi
left atrial expansion index
- LV
left ventricle
- LVIDd
left ventricular internal dimension in diastole
- LVIDd/Ao
left ventricular internal dimension in diastole to aorta ratio
- LVIDs
left ventricular internal dimension in systole
- LVIDs/Ao
left ventricular internal dimension in systole to aorta ratio
- MMVD
myxomatous mitral valve disease
- MV E vel
mitral valve E wave velocity
- PACS
peak atrial contraction strain
- PALS
peak atrial longitudinal strain
- STE
speckle tracking echocardiography
- TDI
tissue Doppler imaging
- 2D
two‐dimensional
1. INTRODUCTION
Myxomatous mitral valve disease (MMVD) is the most common cardiac disease in dogs, with a prevalence of > 75% in individuals of specific canine breeds > 5 years of age.1, 2 In dogs weighing < 9 kg, the most important cause of death is cardiac‐related.3 Therefore, the study of prognostic indicators of mortality in this population of animals is of primary importance. Several studies have focused on evaluating clinical and echocardiographic variables that may predict cardiac mortality in dogs with MMVD.4, 5, 6, 7 Because echocardiography is the most commonly used diagnostic test in assessing dogs with cardiac disease, it is also the most studied technique for stratification of dogs with cardiac disease. Different Doppler variables, two‐dimensional (2D) echocardiographic indicators of mitral valve (MV) pathology, and degree of left atrial (LA) dilatation are strong predictors of cardiac‐related death in dogs with MMVD.4, 5, 8 In particular, LA size is easy to measure and strongly associated with negative prognosis in affected dogs.4, 5, 8
Left atrial dimension can be assessed using different echocardiographic methods,9, 10, 11 but a recent study has shown that bi‐planar‐derived LA volume is superior to traditional simple LA diameter in predicting LA dilatation in dogs with MMVD.12 Until now, survival studies have considered only LA diameter and its ratio to aortic root diameter (Ao; [LA/Ao]) as predictors of survival.4, 5, 6 Canine and feline LA function has been non‐invasively estimated with echocardiography using several approaches, such as measuring LA fractional shortening, LA fractional area change (FAC), and LA ejection fraction (EF).13, 14, 15, 16 Assessment of atrial deformation profiles obtained using tissue Doppler imaging (TDI) and its derived variables, strain and strain rate, recently has been proposed as an alternative method of exploring LA mechanics both in humans17, 18, 19 and dogs.20 Nevertheless, a number of potential drawbacks of this approach should be considered, including suboptimal reproducibility, angle dependence, and the confounding effect of noise artifacts.17, 18, 19, 20 Many of these limitations may be overcome by using 2D speckle tracking echocardiography (STE), the most recent and promising ultrasound technology for direct evaluation of LA function from standard gray‐scale echocardiographic images.17, 18, 19 Until now, only a few studies have used STE in evaluating LA function both in healthy21 and diseased dogs,15, 22 highlighting a progressive decrease in LA function according to the progression of MMVD class. Decreased LA function also has been recognized as a valuable prognostic indicator in dogs with MMVD.23 However, studies comparing different echocardiographic variables, particularly those of LA dimension and function, as predictors of cardiac mortality in dogs with MMVD are lacking.
Therefore, the aims of our study were: (1) to investigate the prognostic value of LA STE variables on survival in dogs with MMVD; and, (2) to compare the usefulness of different echocardiographic variables, particularly those related to LA dimension and function, as predictors of cardiac mortality in dogs with MMVD.
2. MATERIALS AND METHODS
2.1. Animals
Dogs had been examined during a previous study evaluating LA deformation and function using STE in a larger group of dogs, and only those with MMVD and follow‐up available were enrolled in the present study.15 Dogs were presented to the cardiology unit of the Veterinary Teaching Hospital of the University of Bologna for cardiac evaluation on the basis of cardiac murmur clarification, clinical signs referable to heart disease (eg, dyspnea, cough, syncope), for breed screening, or for preanesthetic evaluation. Dogs were prospectively recruited from January 2011 to December 2014 and included in the study if they had clinical and echocardiographic signs typical for MMVD and body weight (BW) < 21 kg. Only dogs with follow‐up information available, as stated in the following sections, were included. Dogs with congenital cardiac malformations, dilated cardiomyopathy, or other acquired cardiac diseases were not included. Only dogs in sinus rhythm were included in the study. Dogs were allowed to receive medications for congestive heart failure (CHF) if considered necessary by the clinician. Thoracic radiography in 2 perpendicular projections was performed to exclude or confirm the presence of CHF (eg, pulmonary edema) to guide medical treatment.
2.2. Procedures and assessment of LA dimension and function
All dogs underwent complete clinical examination and cardiac auscultation. Complete transthoracic echocardiography was performed according to standard techniques24 by 2 investigators (MBT, MC) with several years of expertise in echocardiography, using ultrasound units (a iU22 ultrasound system, Philips Healthcare, Monza, Italy; iE33 ultrasound system, Philips Healthcare, Monza, Italy) equipped with phased array transducers of various frequencies and continuous ECG tracing. All of the examinations then were reviewed by a single experienced cardiologist (MBT). The diagnosis of MMVD was made on the basis of clinical examination and echocardiographic criteria including thickening or prolapse of the MV leaflets or both on 2D echocardiography and mitral regurgitation on color Doppler examination. Chamber diameters of the left ventricle (LV) were obtained from 2D‐guided M‐mode images acquired from a right parasternal short axis view at the level of the papillary muscles and then indexed to the Ao,25 measured from a right parasternal short axis view. The LA and Ao were measured on 2D images obtained from a right parasternal short axis view at the basilar level just after Ao valve closure, and the LA/Ao was calculated.9 The LV diastolic inflow was recorded from the left apical 4‐chamber view, placing the sample volume at the level of the tips of the MV on the ventricular side. Three consecutive measurements were averaged for each variable.
For the analysis of LA areas, volumes, and STE, the left apical 4‐chamber view was optimized to perfectly visualize the LA walls. Then, a 3‐consecutive cardiac beats cine‐loop was acquired and stored in Digital Images and Communications in Medicine format for subsequent analysis. Dedicated software (OsiriX MD Software, Pixeo SARL, Geneva, Switzerland) was used to review the video clips, the beat with the best image quality was chosen, and the endocardial border of the LA was traced during maximal (just before the MV opening) and minimal (just before the MV closure) LA expansion and LA areas were provided. Accordingly, end systolic and end diastolic LA lengths were measured, starting from the MV annulus to the atrial roof. The aortic cross sectional‐area (AoArea) was obtained by tracing the internal border of the aorta at the level of the cusps at end systole from a right parasternal short axis view. Left atrial maximal area (LAAmax) and minimal area (LAAmin) expressed in cm2 were automatically calculated by the software and then indexed to the AoArea. Left atrial fractional area change then was calculated using the formula: FAC = ([LAAmax – LAAmin]/LAAmax) × 100, and expressed as a percentage.15 Left atrial maximal (LAVmax) and minimal (LAVmin) volume was calculated using the monoplane area‐length method using the formula: LAV = (0.85 × A1 × A1)/L, where A1 is the LAA and L is LA length.11 Left atrial volumes then were indexed to BW and expressed in mL/kg. Left atrial expansion index (LAEi) was calculated using the formula: ([LAVmax – LAVmin]/LAVmin) and expressed as a percentage.13
The same cine‐loops and beats used to measure LA areas and volumes were successively employed for the STE analysis using dedicated software (QLAB quantification software version 9.1, Philips Healthcare, Monza, Italy.) as previously described.15 Briefly, the operator selected 3 points on the LA (2 on the MV annulus and 1 on the LA roof) and the software automatically drew a region of interest on the entire LA wall dividing it into 7 regions (from the basilar segments to the roof of the LA), and manual editing was done if necessary. Those segments that showed artifactual values (because of the lung interposition or echo drop‐out at the level of the region of the fossa ovalis or the pulmonary veins inlet) were excluded from the analysis. Time/intensity curves then were generated for each segment displacing strain values (as percentages) on the y‐axis and time (in seconds) on the x‐axis over an entire cardiac cycle. The software also generated a mean curve of all the segments that was used by the operator to measure the peak strain value during LV contraction (peak atrial longitudinal strain [PALS]), and just before LA active contraction, on the peak of the P wave of the ECG trace (peak atrial contraction strain [PACS]). Finally, the contraction strain index (CSI) was calculated using the formula: CSI = ([PALS/PACS] × 100; Figure 1). This last variable represents the contribution of LA contraction to LV filling.15 The entire image analysis was performed by a single operator (MBT). Representative images showing how region of interest of LA area, volumes, and STE were drawn are shown in Figure 1.
Figure 1.
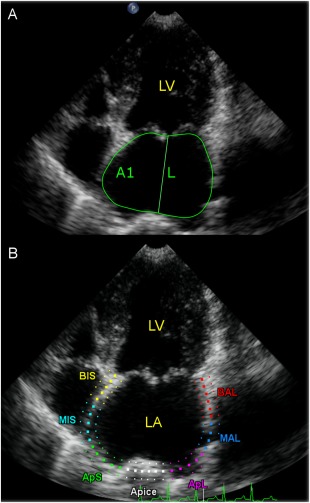
A, Representative images from a dog included in the study, showing how region of interest of LA area, volumes, and STE were drawn. Endocardial border tracing and length measurement of LA in left apical 4‐chamber view at end systole (maximal dimensions). The software automatically gives the LA area. The same procedure was then repeated at end diastole (minimal dimensions). Left atrial volume was then calculated using the monoplane area‐length formula, by measuring the LA area (A1) and length (L). B, LA STE analysis obtained from left apical 4‐chamber view. A region of interest is manually drawn to include the LA wall, which is automatically divided into seven different segments with different colors. LA, left atrium; LV, left ventricle; STE, speckle tracking echocardiography
2.3. Survival analysis
Survival data were obtained from an internal database or by telephone questionnaires. Dogs were classified as still alive, dead for cardiac‐unrelated causes, or dead for cardiac‐related causes (defined as sudden death, CHF refractory to medical treatment, euthanasia because of worsening cardiac condition, or death within 2 hours from the onset of pulmonary edema).4 Time in days from the echocardiographic examination to the phone call for dogs still alive (follow‐up time) or to death (survival time) was recorded.
2.4. Statistical analysis
Descriptive statistics were used for age, sex, and BW. Normal distribution of the echocardiographic, echo‐Doppler, and STE‐derived variables was assessed using a Shapiro‐Wilks test. Data were reported as mean ± standard deviation (SD) or as median (min‐max) if variables were normally or not normally distributed, respectively.
The approach used to analyze days of survival after diagnosis was that previously reported.8 Dogs still alive at the end of the study and dogs that died for noncardiac related events were right‐censored. The effect of each predictive variable on survival time was evaluated using Kaplan‐Meier survival analysis. Predictive continuous variables were divided into quartiles. Pairwise post‐hoc log‐rank analyses were performed across the quartiles of variables to identify significant differences among them. Cut‐off values were based on significantly different sequential quartiles and were used to dichotomize each variable. Variables showing an ordinal increase or decrease in the hazard within each quartile were taken forward to the multivariable analysis as continuous data, whereas variables not significant on Kaplan‐Meier analysis were not further considered. Hazard ratios (HR) and 95% confidence intervals (CI) for the Cox univariate factors were calculated using the Cox proportional hazards model. The association between predictors was evaluated using the Spearman's rank correlation index (r). For any pair of variables with a correlation coefficient (r) > .8, only 1 variable (that with the greatest Wald chi‐square in the Cox univariate analysis) was kept for further analyses. Variables with P < .2 in univariate analysis were entered into a Cox regression multivariable analysis using a manual forward selection method to identify independent predictors of survival. The variable with the most significant association with cardiac death (ie, lowest P value) was entered into the model at each iteration. The order in which variables were entered into the model was based on the strength of their association, and those with the lowest P values from the previous step entered first. Goodness‐of‐fit of the model was evaluated using Akaike criteria. The assumptions of the model were visually inspected using graphical representation of the residuals. All statistical analyses were performed using 2 commercial dedicated software programs (SAS version 9.3, SAS Institute Inc, Cary, North Carolina; MedCalc version 12.6.1.0, MedCalc Software, Ostend, Belgium).
Measurements of all STE variables (PALS, PACS, CSI, LAAmax, LAAmin, and FAC) previously had shown good intraobserver and interobserver repeatability in our laboratory.15 Because LA volumes were directly derived from LA areas measurements, variability analysis of these variables was not performed.
3. RESULTS
One‐hundred fifteen dogs met the inclusion criteria of the study. Descriptive statistics and echocardiographic variables are summarized in Table 1. At the time of inclusion, 37 dogs were in CHF confirmed by thoracic radiography, and they were clinically stable under medical treatment. None of the other animals experienced CHF before or at inclusion. Drugs used to control clinical signs of CHF were: 37 dogs received benazepril (mean dosage, 0.38 mg/kg q12–24h); 35 dogs received pimobendan (mean dosage, 0.31 mg/kg q12h); 5 dogs received amlodipine (mean dosage, 0.3 mg/kg q24h); 3 dogs received spironolactone (mean dosage, 2 mg/kg q24h); and 37 dogs received furosemide (mean dosage, 2 mg/kg q12h; Table 1).
Table 1.
Descriptive statistics of 115 dogs with MMVD
| Variable | Observed values | N | Reference range | Reference # |
|---|---|---|---|---|
| Age (years) | 10.9 ± 3.3 | |||
| BW (kg) | 9.2 ± 4.3 | |||
| Breed | ||||
| Mixed breed | 54 | |||
| CKCS | 8 | |||
| Yorkshire terrier | 7 | |||
| Miniature poodle | 7 | |||
| Shih‐Tzu | 5 | |||
| Maltese | 4 | |||
| Jack Russel Terrier | 3 | |||
| Bolognese | 2 | |||
| Beagle | 2 | |||
| Dachshund | 2 | |||
| Other breeds | 21 | |||
| Sex | ||||
| Male | 67 | |||
| Female | 48 | |||
| LVIDd/Ao | 2.2 ± 0.5 | 1.6 ± 0.2 | 25 | |
| LVIDs/Ao | 1.2 ± 0.3 | 1.1 ± 0.2 | 25 | |
| MV E vel (cm/s) | 105.5 ± 39.8 | 71.3 ± 5.64 | 15 | |
| LA/Ao | 1.8 ± 0.4 | <1.5 | 12 | |
| LAAmax/Ao | 4.3 ± 2.5 | 2.2 (1.9‐2.4) | 15 | |
| LAAmin/Ao | 2.6 ± 1.8 | 0.9 (0.8‐1.1) | 15 | |
| LA FAC (%) | 42.6 ± 9.7 | 55.1 ± 2.09 | 15 | |
| LAVmax (mL/kg) | 2.8 ± 2.0 | 0.58 (0.92) a | 13 | |
| LAVmin (mL/kg) | 1.4 ± 1.3 | 0.21 (0.38) a | 13 | |
| LAEi (%) | 95.3 ± 70.5 | 173 (76–394) | 13 | |
| PALS (%) | 40.9 ± 13.8 | 60.4 ± 2.36 | 15 | |
| PACS (%) | 18.1 ± 10.1 | 26.3 ± 1.77 | 15 | |
| CSI (%) | 42.2 ± 17.2 | 42.8 ± 3.24 | 15 | |
| Drugs for CHF | N | Mean dose (mg/kg) | ||
| Furosemide | 37 | 2 q12h | ||
| Benazepril | 37 | 0.38 q12–24h | ||
| Pimobendan | 35 | 0.31 q12h | ||
| Spironolactone | 3 | 2 q24h | ||
| Amlodipine | 5 | 0.30 q24h |
Data are expressed as mean ± SD or median (min‐max).
Abbreviations: CHF, congestive heart failure; CKCS, Cavalier king Charles spaniel; CSI, contraction strain index; LA/Ao, left atrial diameter to aorta ratio; LAEi, left atrial expansion index; LA FAC, left atrial fractional area change; LAAmax/Ao, left atrial maximal area to aortic area ratio; LAAmin/Ao, left atrial minimal area to aortic area ratio; LAVmax, left atrial maximal volume indexed to body weight; LAVmin, left atrial minimal volume indexed to body weight; LVIDd/Ao, left ventricular internal dimension in diastole to aorta ratio; LVIDs/Ao, left ventricular internal dimension in systole to aorta ratio; MV E vel, mitral valve E wave peak velocity; N, number of dogs; PALS, peak atrial longitudinal strain; PACS, peak atrial contraction strain
aReported as mean (95th percentile).
At the end of the follow‐up period, 35 dogs had died because of cardiac‐related causes (median survival, 212 days; range, 16–1,179 days), 28 dogs had died of non cardiac‐related causes (median survival, 318 days; range, 81–1,067 days), and 52 dogs were still alive (median follow‐up, 539 days; range, 151–1,259 days).
Each Kaplan‐Meier survival curve was calculated (the curves of the variables that were not significant in the multivariable analysis are presented as on‐line supporting information). The following variables were dichotomized: LA/Ao, MV E vel, LAVmax, PALS, LAAmax/Ao, LAAmin/Ao, LAVmin, and PACS, whereas CSI, LVIDs/Ao, and LVIDd/Ao were considered in the following analyses as continuous predictive variables (Table 2). The LAEi and LA FAC were not significantly associated with survival time and were not considered in further analyses. Of the above considered variables, only LVIDs/Ao was not a significant predictor of survival in the Cox univariate analysis. The LAAmax/Ao, LAAmin/Ao, and LAVmin were not further considered in the following evaluations because of their high correlation with LAVmax (r = .87, r = .84, and r = .94, respectively). In the same way, PACS was excluded from the multivariable analysis because it was highly correlated with CSI (r = .80) and similarly LVIDd/Ao was highly correlated with LA/Ao (r = .85). The LA/Ao, MV E vel, LAVmax, PALS, and CSI were retained as significant predictors after multivariable analysis. The risk of event increased with higher values of LA/Ao, MV E vel, and LAVmax, and with lower values of PALS, whereas it decreased with higher values of CSI (Table 2). When these variables were entered into the multivariable model, only MV E vel > 1.3 m/s and LAVmax > 3.53 mL/kg were retained with HRs of 4.45 (CI, 1.76‐11.24; P < .001) and 2.32 (CI, 1–4.89; P = .024), respectively (Table 3). The Kaplan‐Meier curves for the variables that were significant in the multivariable analysis are presented in Figure 2.
Table 2.
Cox proportional hazard univariate analysis in 115 dogs with MMVD
| Variable | HR | 95% CI | P |
|---|---|---|---|
| LA/Ao > 1.7 | 5.10 | 2.41‐10.8 | <.001 |
| MV E vel > 1.3 m/s | 6.49 | 2.76‐15.25 | <.001 |
| LAAmax/Ao > 3.6 | 4.69 | 2.35‐9.36 | <.001 |
| LAAmin/Ao > 2 | 5.02 | 2.51‐10.03 | <.001 |
| LAVmin > 1.5 mL/kg | 5.46 | 2.75‐10.83 | <.001 |
| LAVmax > 3.53 mL/kg | 4.53 | 2.28‐8.99 | <.001 |
| PALS < 30% | 3.11 | 1.58‐6.12 | .001 |
| PACS < 11% | 3.05 | 1.55‐6.03 | .001 |
| LVIDd/Ao per 0.1 increase | 3.87 | 1.98‐7.52 | <.001 |
| LVIDs/Ao per 0.1 increase | 1.67 | 0.47‐5.83 | .420 |
| CSI per 1% increase | 0.97 | 0.95‐0.99 | .007 |
Abbreviations: CI, confidence interval; CSI, contraction strain index; HR, hazard ratio; LA/Ao, left atrium to aorta ratio; LAAmax/Ao, left atrial maximal area to aortic area; LAAmin/Ao, left atrial minimal area to aortic area; LAVmin, left atrial maximal volume indexed to body weight; LAVmax, left atrial minimal volume indexed to body weight; LVIDd/Ao, left ventricular internal dimension in diastole to aorta ratio; LVIDs/Ao, left ventricular internal dimension in systole to aorta ratio; MV E vel, mitral valve E wave peak velocity; PALS, peak atrial longitudinal strain; PACS, peak atrial contraction strain
Table 3.
Final model results in the multivariable cox proportional hazard analysis in 115 dogs with MMVD
| Variable | HR | 95% CI | P |
|---|---|---|---|
| MV E vel > 1.3 m/s | 4.45 | 1.76‐11.24 | <.001 |
| LAVmax > 3.53 mL/kg | 2.32 | 1.10‐4.89 | .024 |
Abbreviations: CI, confidence interval; HR, hazard ratio; LAVmax, left atrial maximal volume indexed to body weight; MV E vel, mitral valve E wave peak velocity.
Figure 2.
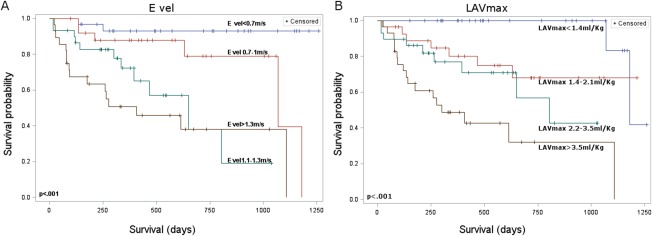
Kaplan‐Meier survival curves of the two variables that remained significant in the multivariable analysis for 115 dogs with myxomatous MV disease. E vel, E wave velocity; LAVmax, left atrial maximal volume
4. DISCUSSION
We evaluated the prognostic value of several echocardiographic variables on survival in dogs with MMVD. In particular, we focused on LA morphological and functional variables, also including for the first time in veterinary medicine some STE‐derived variables.
The survival analysis showed that STE‐derived LA strain and the related variables PALS, PACS, and CSI are univariate predictors of cardiac‐related death in dogs with MMVD, but these parameters lost prognostic significance in multivariable analysis. Only MV E vel > 1.3 m/s and LAVmax > 3.53 mL/kg were independent predictors of high mortality risk. The association between increased MV E vel and poor prognosis in dogs with MMVD already has been demonstrated, with cut‐off values of 1.2 and 1.4 m/s, depending on the study.4, 5, 8, 26 Moreover, increasing MV E vel values over serial examinations also have been associated with a poorer outcome.27 Mitral valve E vel is determined mainly by LV filling pressures and relaxation.28 In cardiac disease, such as MMVD, where the preload is disproportionally increased, the filling pressure component is dominant on relaxation. Therefore, an increase in MV E vel is an indirect measure of mitral regurgitation severity, reflecting worsening pressure differences between the LA and LV with increasing regurgitant volumes.8, 28 Not surprisingly, the 2 variables that better reflect increased LA preload, namely MV E vel and LAVmax, were both predictors of cardiac death in the multivariable analysis.
Regarding LA measurement, LA/Ao was an independent predictor of a poor prognosis in the first study on clinical and echocardiographic predictors of mortality in dogs with MMVD,4 but this finding was not confirmed in a more recent study.8 Our findings about the negative prognostic value of LAVmax, but not of LA/Ao, might suggest that the use of linear measurements to predict LA enlargement may underestimate the risk stratification of dogs with MMVD. Although LA/Ao has been proven to predict cardiac‐related death in dogs with MMVD,4 the volumetric approach of measuring the LA seems more effective in predicting mortality. In humans, presence of LA dilatation correlates with increased risk of developing stroke, atrial fibrillation, and cardiac‐related death.29 Therefore, detecting cardiac chamber dilatation is crucial for stratifying patients with cardiac disease, and the recommended way to assess LA dilatation in people now is the use of volumetric methods.30 In cats with cardiac disease, LA volume and LA EF, obtained from volume measurement over the cardiac cycle, have been reported to be useful diagnostic and prognostic echocardiographic variables, showing better performance when compared to simple diameter measurements.31 One study comparing LA/Ao with LA volume assessment in a population of dogs with MMVD showed that mild LA dilatation could be detected in some dogs only by measuring LA volume, but not LA diameter.12 Results of our study confirm that the use of a volumetric method also has prognostic relevance in assessing LA dimension in dogs compared to standard linear measurement. Whether this kind of measurement is reproducible among different cardiologists at different centers still must be clearly elucidated. At present, studies from 2 different research groups have shown acceptable reproducibility in assessing LA volumes both in healthy dogs10, 13 and dogs with MMVD.11
Among the variables of LA function, only STE‐derived variables were predictors of cardiac related death in our population of dogs, whereas LA FAC and LAEi were not (Figure 2). This finding is in contrast with a previous study where LA FAC appeared to be a prognostic factor within 1 year in dogs with MMVD.23 In that study, a different type of statistical analysis was carried out, with a direct comparison of LA FAC values between dogs that were dead or alive at 1 year after inclusion. In our study, STE‐derived PALS, PACS, and CSI were all univariate predictors of survival. Left atrial strain recently has been studied to validate the technique in clinically healthy dogs21 and to evaluate strain changes in dogs under experimental conditions,32, 33 or in dogs with naturally occurring MMVD.15, 22 With progression of disease, STE‐derived variables tend to decrease as a consequence of progressive impairment in LA contraction and relaxation.15, 22 This might be secondary to changes in loading conditions, LA preload and afterload, and progressive LA fibrosis that occur during MMVD.15, 34 In people, LA strain analysis is useful for stratifying patients with valvular disease, atrial fibrillation, or acute coronary disease.35, 36, 37 Moreover, LA strain has been shown to be an independent predictor of death or the need for cardiac transplantation in people with symptomatic systolic dysfunction.38 Our study is the first in veterinary medicine to show that, similarly to people, STE‐derived strain can be used as a prognostic indicator also in dogs with cardiac disease, namely MMVD.
Our study had some limitations. We focused mainly on echocardiographic indicators of LA morphology and function, without introducing echocardiographic variables of MV pathology into the analysis, the radiographically derived vertebral heart score, or clinical variables, such as syncope, presence of heart murmur, presence of CHF, and dyspnea, that are known to be independent prognostic indicators in dogs with MMVD.4, 7, 8, 39 Moreover, for the assessment of LA volume we used the monoplane area‐length method obtained from the 4‐chamber view. In people, the bi‐plane method from the 4‐ and 2‐chamber views is preferred to derive LA volumes.30 However, studies in dogs have shown that use of a monoplane method overestimates the volume by only 5.8% when compared with the bi‐plane method.11 Moreover, a strong correlation exists among different methods of calculation of LA volumes in dogs when using 2D echocardiographic images.10, 11 Therefore, different echocardiographic approaches in measuring LA volumes in dogs appear interchangeable in the clinical setting, and it appears unlikely that the method we chose negatively affected our results. Another possible limitation to our study is the use of a single cardiac cycle to perform the STE analysis. Whether the beat‐to‐beat variation and sinus arrhythmia could significantly affect LA STE‐derived variables in dogs remains to be elucidated in further studies. Finally, the effect of cardiac medication and diuretics on LA function and STE‐derived variables has not been investigated. In clinically healthy dogs, experimentally induced volume overload enhances LA phasic function because of the increased preload, according to the Frank‐Starling mechanism.32 However, in naturally occurring disease, LA phasic function decreases with the progression of the disease, likely in response to the presence of other concurrent mechanisms, such as increased LA afterload and LA fibrosis.15 It might be speculated that using some medications that decrease LA pressure (eg, furosemide, angiotensin converting enzyme inhibitors, amlodipine, pimobendan)39, 40, 41, 42, 43 could have an effect on LA function, leading to some bias when comparing untreated dogs with LA dilatation and dogs under medical treatment. To prove whether or not this assumption is correct requires further studies.
In conclusion, we evaluated the prognostic significance of several echocardiographic variables of LA morphology and function in dogs with MMVD. Although many variables, including all STE variables, were able to predict mortality in this population of animals, only MV E vel > 1.3 m/s and LAVmax > 3.53 mL/kg remained significant predictors of cardiac death in the multivariable analysis.
CONFLICT OF INTEREST DECLARATION
The authors declare that they have no conflict of interest with the contents of this article.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
This work was done at the Department of Veterinary Medical Sciences, Alma Mater Studiorum‐University of Bologna. This study was presented in part as an abstract at the 25th Congress of the European Society of Veterinary Internal Medicine—Companion Animals, Lisbon, Portugal, 10th‐12th September 2015.
Baron Toaldo M, Romito G, Guglielmini C, et al. Prognostic value of echocardiographic indices of left atrial morphology and function in dogs with myxomatous mitral valve disease. J Vet Intern Med. 2018;32:914–921. https://doi.org/10.1111/jvim.15093
REFERENCES
- 1. Borgarelli M, Häggström J. Canine degenerative myxomatous mitral valve disease: natural history, clinical presentation and therapy. Vet Clin North Am Small Anim Pract. 2010;40:651–663. [DOI] [PubMed] [Google Scholar]
- 2. Borgarelli M, Buchanan JW. History review, epidemiology and natural history of degenerative mitral valve disease. J Vet Cardiol. 2012;14:93–101. [DOI] [PubMed] [Google Scholar]
- 3. Parker HG, Kilroy‐Glynn P. Myxomatous mitral valve disease in dogs: does size matter? J Vet Cardiol. 2012;14:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med. 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 5. Borgarelli M, Crosara S, Lamb K, et al. Survival characteristics and prognostic variables of dogs with preclinical chronic degenerative mitral valve disease attributable to myxomatous degeneration. J Vet Intern Med. 2012;26:69–75. [DOI] [PubMed] [Google Scholar]
- 6. Borgarelli M, Abbott J, Braz‐Ruivo L, et al. Prevalence and prognostic importance of pulmonary hypertension in dogs with myxomatous mitral valve disease. J Vet Intern Med. 2015;29:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. López‐Alvarez J, Elliott J, Pfeiffer D, et al. Clinical severity score system in dogs with degenerative mitral valve disease. J Vet Intern Med. 2015;29:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sargent J, Muzzi R, Mukherjee R, et al. Echocardiographic predictors of survival in dogs with myxomatous mitral valve disease. J Vet Cardiol. 2015;17:1–12. [DOI] [PubMed] [Google Scholar]
- 9. Rishniw M, Erb HN. Evaluation of four 2‐dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med. 2000;14:429–435. [DOI] [PubMed] [Google Scholar]
- 10. LeBlanc N, Scollan K, Sisson D. Quantitative evaluation of left atrial volume and function by one‐dimensional, two‐dimensional, and three‐dimensional echocardiography in a population of normal dogs. J Vet Cardiol. 2016;18:336–349. [DOI] [PubMed] [Google Scholar]
- 11. Höllmer M, Willesen JL, Tolver A, Koch J. Comparison of four echocardiographic methods to determine left atrial size in dogs. J Vet Cardiol. 2016;18:137–145. [DOI] [PubMed] [Google Scholar]
- 12. Wesselowski S, Borgarelli M, Bello NM, Abbott J. Discrepancies in identification of left atrial enlargement using left atrial volume versus left atrial‐to‐aortic root ration in dogs. J Vet Intern Med. 2014;28:1527–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Höllmer M, Willesen JL, Tolver A, Koch J. Left atrial volume and phasic function in clinically healthy dogs of 12 different breeds. Vet J. 2013;197:639–645. [DOI] [PubMed] [Google Scholar]
- 14. Tidholm A, Höglund K, Häggström J, Bodegård‐Westling A, Ljungvall I. Left atrial ejection fraction assessed by real‐time 3‐dimensional echocardiography in normal dogs and dogs with myxomatous mitral valve disease. J Vet Intern Med. 2013;27:884–889. [DOI] [PubMed] [Google Scholar]
- 15. Baron Toaldo M, Romito G, Guglielmini C, et al. Assessment of left atrial deformation and function using two‐dimensional speckle tracking echocardiography in healthy dogs and dogs with myxomatous mitral valve disease. J Vet Intern Med. 2017;31:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johns SM, Nelson OL, Gay JM. Left atrial function in cats with left‐sided cardiac disease and pleural effusion or pulmonary edema. J Vet Intern Med. 2012;26:1134–1139. [DOI] [PubMed] [Google Scholar]
- 17. To AC, Flamm SD, Marwick TH, Klein AL. Clinical utility of multimodality LA imaging. Assessment of size, function, and structure. JACC Cardiovasc Imaging. 2011;4:788–798. [DOI] [PubMed] [Google Scholar]
- 18. Vizzardi E, D'Aloia A, Rocco E, et al. How should we measure left atrial size and function? J Clin Ultrasound. 2012;40:155–166. [DOI] [PubMed] [Google Scholar]
- 19. Rosca M, Lancellotti P, Popescu BA, Piérard LA. Left atrial function: pathophysiology, echocardiographic assessment, and clinical applications. Heart. 2011;97:1982–1989. [DOI] [PubMed] [Google Scholar]
- 20. Baron Toaldo M, Guglielmini C, Diana A, Sarcinella F, Cipone M. Feasibility and reproducibility of echocardiographic assessment of regional left atrial deformation and synchrony by tissue Doppler ultrasonographic imaging in healthy dogs. Am J Vet Res. 2014;75:59–66. [DOI] [PubMed] [Google Scholar]
- 21. Caivano D, Rishniw M, Patata V, Giorgi ME, Birettoni F, Porciello F. Left atrial deformation and phasic function determined by 2‐dimensional speckle tracking echocardiography in healthy dogs. J Vet Cardiol. 2016;18:146–155. [DOI] [PubMed] [Google Scholar]
- 22. Nakamura K, Kawamoto S, Osuga T, et al. Left atrial strain at different stages of myxomatous mitral valve disease in dogs. J Vet Intern Med. 2017;31:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakamura K, Osuga T, Morishita K, et al. Prognostic values of left atrial function in dogs with chronic mitral valvular heart disease. J Vet Intern Med. 2014;28:1746–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med. 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 25. Brown DJ, Rush JE, MacGregor J, Ross JN Jr, Brewer B, Rand WM. M‐mode echocardiographic ratio indices in normal dogs, cats and horses: a novel quantitative method. J Vet Intern Med. 2003;17:653–662. [DOI] [PubMed] [Google Scholar]
- 26. Moonarmart W, Boswood A, Luis Fuentes V, Brodbelt D, Souttar K, Elliott J. N‐terminal pro B‐type natriuretic peptide and left ventricular diameter independently predict mortality in dogs with mitral valve disease. J Small Anim Pract. 2010;51:84–96. [DOI] [PubMed] [Google Scholar]
- 27. Hezzell MJ, Boswood A, Moonarmart W, Elliott J. Selected echocardiographic variables change more rapidly in dogs that die from myxomatous mitral valve disease. J Vet Cardiol. 2012;14:269–279. [DOI] [PubMed] [Google Scholar]
- 28. Schober KE, Hart TM, Stern JA, et al. Detection of congestive heart failure in dogs by Doppler echocardiography. J Vet Intern Med. 2010;24:1358–1368. [DOI] [PubMed] [Google Scholar]
- 29. Leung DY, Boyd A, Ng AA, Chi C, Thomas L. Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J. 2008;156:1056–1064. [DOI] [PubMed] [Google Scholar]
- 30. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 31. Linney CJ, Dukes‐McEwan J, Stephenson HM, López‐Alvarez J, Fonfara S. Left atrial size, atrial function and left ventricular diastolic function in cats with hypertrophic cardiomyopathy. J Small Anim Pract. 2014;55:198–206. [DOI] [PubMed] [Google Scholar]
- 32. Osuga T, Nakamura K, Morita T, et al. Effects of experimental cardiac volume loading on left atrial phasic function in healthy dogs. Am J Vet Res. 2016;77:952–960. [DOI] [PubMed] [Google Scholar]
- 33. Goldberg A, Kusunose K, Qamruddin S, et al. Left atrial size and function in a canine model of chronic atrial fibrillation and heart failure. PLoS One. 2016;11:e0147015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Janus I, Noszczyk‐Nowak A, Nowak M, Ciaputa R, Kandefer‐Gola M, Pasławska U. A comparison of the histologic pattern of the left atrium in canine dilated cardiomyopathy and chronic mitral valve disease. BMC Vet Res. 2016;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vieira MJ, Teixeira R, Gonçalves L, Gersh BJ. Left atrial mechanics: echocardiographic assessment and clinical implications. J Am Soc Echocardiogr. 2014;27:463–478. [DOI] [PubMed] [Google Scholar]
- 36. Di Salvo G, Caso P, Lo Piccolo R, et al. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent‐onset lone atrial fibrillation. Circulation. 2005;112:387–395. [DOI] [PubMed] [Google Scholar]
- 37. Cameli M, Lisi M, Giacomin E, et al. Chronic mitral regurgitation: left atrial deformation analysis by two‐dimensional speckle tracking echocardiography. Echocardiography 2011;28:327–334. [DOI] [PubMed] [Google Scholar]
- 38. Helle‐Valle T, Opdahl A, Broch K, et al. LA strain by STE in patients with heart failure – an independent and incremental predictor of cardiac death or need of heart transplantation. Circulation 2011;124:A8551 [Google Scholar]
- 39. Kim HT, Han SM, Song WJ, et al. A retrospective study of degenerative mitral valve disease in small‐breed dogs: Survival and prognostic variables. J Vet Sci. 2017;18:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suzuki S, Fukushima R, Ishikawa T, et al. Comparative effects of amlodipine and benazepril on left atrial pressure in dogs with experimentally‐induced mitral valve regurgitation. BMC Vet Res. 2012;8:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suzuki S, Fukushima R, Ishikawa T, et al. The effect of pimobendan on left atrial pressure in dogs with mitral valve regurgitation. J Vet Intern Med. 2011;25:1328–1333. [DOI] [PubMed] [Google Scholar]
- 42. Ishikawa T, Tanaka R, Suzuki S, et al. The effect of angiotensin‐converting enzyme inhibitors of left atrial pressure in dogs with mitral valve regurgitation. J Vet Intern Med. 2010;24:342–347. [DOI] [PubMed] [Google Scholar]
- 43. Suzuki S, Ishikawa T, Hamabe L, et al. The effect of furosemide on left atrial pressure in dogs with mitral valve regurgitation. J Vet Intern Med. 2011;25:244–250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


