Abstract
Vitamin D is a particularly important sterol hormone, with evidence emerging of its beneficial effects well beyond bone. In consequence of this and increased global recognition of vitamin D deficiency in the general population, there has been a resurgence in treatment with vitamin D preparations. However, the increasing use of vitamin D treatments has also seen a substantial increase in the number of reports of vitamin D intoxication, with the majority (75%) of reports published since 2010. Many of these cases are a consequence of inappropriate prescribing, and the use of high‐dose over‐the‐counter preparations or unlicensed preparations. This review highlights that the majority of cases were preventable and discusses the inappropriate use of poorly formulated, and unlicensed vitamin D preparations.
Keywords: hypervitaminosis D, iatrogenic, toxic, toxicity, vitamin D
Introduction
Vitamin D is somewhat of a misnomer as it is, in fact, a potent sterol hormone 1. In consequence, there has been considerable research interest focused on this molecule over the past decade, particularly when compared with the relatively stable research output associated with other vitamins (Figures 1 and 2). The high prevalence of vitamin D deficiency is well recognized in Europe but is in fact a global problem, with female adolescents in the Middle East at particular risk 2, 3, 4, 5. This, and the beneficial effects of treatment on areas beyond bone 1, may have prompted the marked increase in the use of vitamin D therapies 6. Similarly, population‐based guidelines 7 and advice from chief medical officers 8 have further supported the widespread use of vitamin D supplementation, with a recommended intake of 400 IU per day for those aged 4 years and above in the UK. However, vitamin D treatment is not without risk, as vitamin D toxicity is a potentially serious adverse effect of treatment. Vitamin D deficiency may also rise with increasing obesity rates as obesity is a key risk factor for vitamin D deficiency 9.
Figure 1.
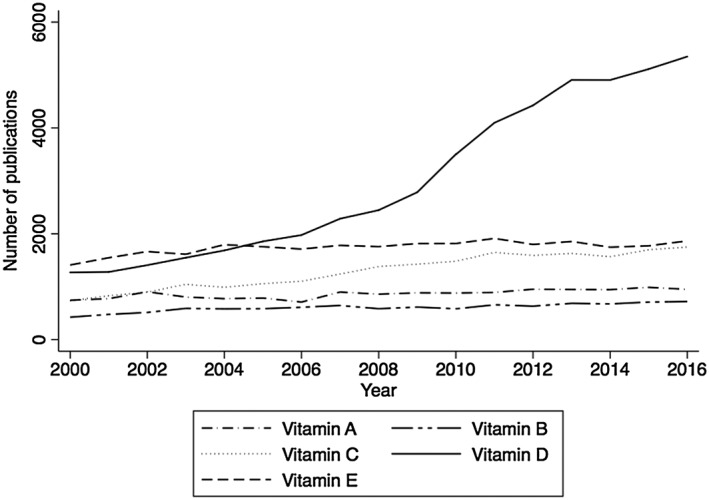
Comparison of number of published scientific articles retrieved by Web of Knowledge for vitamins A–E between 2000 and 2016
Figure 2.
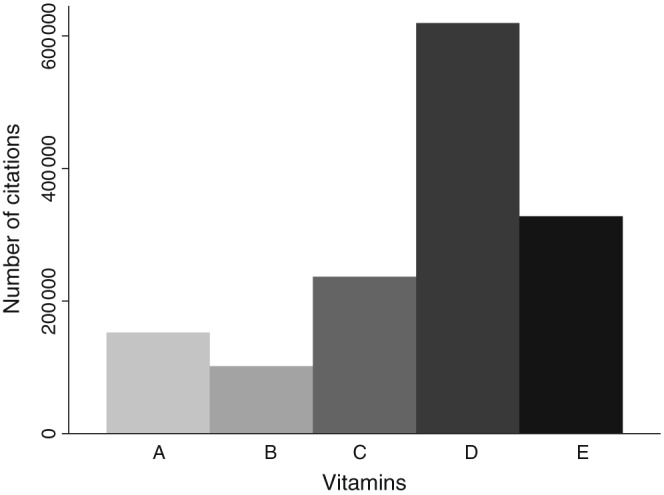
Number of citations for vitamins A–E between 2000 and 2016
Vitamin D is synthesized mainly in the skin 1; only 10% is derived from dietary sources. Cholecalciferol is synthesized from 7‐dehydrocholesterol via an enzymatic photosynthetic process involving ultraviolet radiation. This photosynthetic process is carefully regulated, such that balanced enzymatic degradation of cholecalciferol occurs within the skin, effectively preventing the accumulation of excess cholecalciferol 10. Following the photosynthesis of cholecalciferol, the molecule undergoes 25‐hydroxylation in the liver to form 25‐hydroxy‐cholecalciferol (25OHD) and then undergoes a further 1‐hydroxylation to form the active hormone, calcitriol, 1,25‐dihydroxycholecalciferol (1,25OHD) 1. It is this molecule that is the active component of vitamin D, acting upon the widespread vitamin D receptors (VDRs) distributed ubiquitously throughout the body's organs. Through interaction with the VDRs, calcitriol exerts its main effects on calcium homeostasis, causing increased absorption of both calcium and phosphorus from the gut. Vitamin D deficiency is therefore associated with reduced calcium absorption and hence secondary hyperparathyroidism, resulting in recruitment of calcium from the bone in order to maintain adequate plasma calcium concentrations. Similarly, vitamin D toxicity is associated with increased absorption of calcium from the gut, and hypercalcaemia. Furthermore, vitamin D excess may increase bone resorption, further increasing calcium levels 11.
As vitamin D is mainly produced through photosynthesis, a multitude of factors influence levels, such as amount of sun exposure, time of day, season, latitude, altitude, cloud cover, air pollution, clothing and sunscreen use. Current opinion suggests that vitamin D concentrations are best reflected through the measurement of 25OHD, although the definition of sufficiency differs between authorities as either a 25OHD concentration >50 nmol l–1 12 or >75 nmol l–1 (Table 1) 13. Thus, with public health advice increasingly recommending against prolonged sun exposure, the only way to restore adequate vitamin D concentrations is through supplemental vitamin D 7. Either oral ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) is recommended, with the latter being more popular. Intramuscular preparations may be used, although this is rarely required, tending to be reserved for cases of malabsorption 12. A popular approach to treating deficiency is via a total dose of 300 000 IU of either cholecalciferol or ergocalciferol, delivered as split doses over a 6–12‐week period, then followed by maintenance therapy of around 800–1000 IU daily 12.
Table 1.
| Category | nmol l–1 | μg l–1 |
|---|---|---|
| Deficiency | <50 | <20 |
| Insufficiency | 51–74 | 21–29 |
| Sufficient | >75 | >30 |
| Excess | >250 | >100 |
| Intoxication | >375 | >150 |
Vitamin D toxicity
The features of vitamin D toxicity are mediated through hypercalcaemia, and symptoms range from mild, such as thirst and polyuria, to severe, including seizures, coma and death 14. Deterioration in clinical symptoms relate to the calcium concentration, which, in turn, is to a certain degree related to the 25OHD concentration 14. As per the debate relating to adequate 25OHD concentrations, there is also debate regarding optimal concentrations. Concentrations above 250 nmol l–1 are considered excessive, with concentrations above 375 nmol l–1 (150 μg l–1) being associated with toxicity (Table 1 ) 14. Vitamin D treatment appears to be safe at doses of up to 10 000 IU day–1, delivering 25OHD concentrations below levels associated with toxicity 14, 15. Indeed, others have shown that an average daily intake of 15 000 IU day–1 and plasma 25OHD concentrations up to 300 nmol l–1 are not associated with deranged calcium or phosphate metabolism or toxicity 16. However, with the increasing use of vitamin D treatments, there are increasing reports of vitamin D toxicity in the literature which appear to relate to manufacturing errors, prescribing errors and increasing use of supplemental high‐dose products 17.
It is therefore of substantial public health importance to highlight both the potential consequences of vitamin D toxicity and the common underlying causes. In the present review, we evaluate vitamin D toxicity and explore its causes for recurrent themes. We have classified doses higher than 4000 IU as ‘high dose’, and those higher than 100 000 as ‘mega dose’.
Methods
We searched Medline for relevant articles published between January 1945 and August 2017. We used various combinations of the following terms: ‘hypervitaminosis D’, ‘vitamin D’, ‘toxicity’, ‘toxic’ and ‘iatrogenic’. Additional publications were sourced from references in individual articles. Relevant articles were selected after reading through all titles and abstracts, and full texts were obtained if the information contained in the title or abstract was insufficient to exclude the study.
We selected studies for review if they indicated that a patient had received an excessive dose of vitamin D or had complications related to high vitamin D levels as a result of supplementation. The consequences of the vitamin D toxicity and the apparent causes behind its occurrence were also examined. As the nature of the research in this area has been predominantly case reports and series, we did not make a formal assessment of the quality of the research or undertake a formal systematic review with meta‐analysis and GRADE assessment of the quality of evidence.
Our search criteria, used by both authors, identified 437 publications, of which 109 were potentially relevant based on examining the title and abstract, and 62 were included in the final analysis. It is noteworthy that the earliest identified papers regarding the causes and complications of excess vitamin D supplementation were first published over 50 years ago 18, 19.
Results
Summary
All references used in the present work were identified in a Medline search; no additional references were found by searching the reference lists in the identified papers. In our review, three main themes emerged, with vitamin D excess and toxicity arising from errors in formulation or fortification (the most common), inappropriate prescribing or dispensing, and errors in administration. Of the 62 papers included in the final analysis, 44 were relevant (the rest being reviews or explanations of the physiology of vitamin D). Twenty papers related to fortification errors; these occurred globally, with events occurring in North America, South America, India and Australia. There were 17 reports of inappropriate prescribing or dispensing, with nine of these coming from India, the largest of which comprised a case series of 62 patients, and the others from the USA, Canada and the UK. Of the 17 reports of inappropriate prescribing, the majority 15 were due to errors in prescribing. There were seven reports of errors in administration (including self‐administration); the largest analysis, from the UK, comprised 372 cases of excess vitamin D levels (>220 nmol l–1), with 349 of these not under direct medical supervision. Other reports of errors in administration were from the USA, Netherlands, Japan and Brazil.
Publications for vitamin D had a 4.1 fold increase in annual frequency from 2001 to 2016, with the greatest increases after 2009 (Figure 1). Similarly, only five of the 20 reports of fortification errors, five of the 17 errors in prescribing or dispensing, and one of the seven reports of administration errors were published before 2010. Problems appeared to be most common in paediatric and elderly populations.
Causes of vitamin D toxicity
Formulation or fortification errors
With the early and widespread fortification of foodstuffs with vitamin D, there were numerous reports of vitamin D intoxication 20, suggesting errors in food manufacturing. Indeed, other studies of fortified foodstuffs revealed substantial manufacturing errors, confirming marked differences between the stated and actual doses 21. Overfortification of vitamin D in milk resulted in hypervitaminosis D in the local population, leading to at least 56 cases; 41 required hospitalization and there were two deaths 22, reinforcing the need for careful ongoing production monitoring 23.
The problem of accurate dosing of vitamin D is not just confined to food fortification, and is a particular issue when supplements are unlicensed. The manufacture of both ergocalciferol and cholecalciferol requires considerable pharmaceutical expertise if inaccurate dosing is to be avoided. Thus, many countries have agencies which oversee the production and safety of pharmaceutical products; in the UK, this role is performed by the Medicines and Healthcare Products Regulatory Authority (MHRA).
A New Zealand study of 14 (12 unlicensed and two licensed) vitamin D3 formulations revealed that only eight were within 10% of the stated dose 24. Similarly, a US study 25 demonstrated that, of the 15 vitamin D3 preparations analysed, there was substantial variation compared with the stated dose, both in pills from the same bottle (52–136% of expected dose) and between separate preparations (9–140% of stated dose). Only one‐third of the pills analysed were within 10% of the stated dose. Of these, the licensed products revealed the greatest accuracy and least variation with the stated dose. Similarly, an Indian study revealed that, of 14 commonly used preparations, only four were found to be within the accepted 90–125% of stated dose, defined by the Indian Pharmacopeia 26. Furthermore, US studies on the fortification of foods with vitamin D have also revealed wide variations from the stated dose as nutritional products are not as well regulated as medicines.
While the problematic manufacture of vitamin D products may at first appear trivial, such inaccuracies appear to be responsible for the majority of cases of vitamin D toxicity reported in the literature (Table 2). Koutkia et al. 27 reported severe hypercalcaemia and renal failure due to vitamin D toxicity in a subject taking vitamin D3 at a stated dose of 2000 IU day–1, yet analysis of the medication revealed actual doses of up to 2.6 million IU day–1. Another US study reported on a woman with vitamin D toxicity associated with the use of a vitamin D supplement containing 188 000 IU of cholecalciferol rather than the stated dose of 400 IU 28. Benemei et al. 29 reported three cases of vitamin D intoxication with severe hypercalcaemia, where the patients had been treated with a vitamin D formulation with a stated dose of 600 IU, whereas, in fact, the actual content was 52 800 IU. Similar manufacturing errors, producing serious toxicity and hospitalization, has also been reported in children 30, 31, 32, 33. Seven paediatric cases of hypercalcaemia due to vitamin D intoxication were reported in association with a fish oil supplement, where the stated dose was roughly 4000 times less than the actual dose 31; in one of these cases, an infant in whom the stated daily dose was 2000 IU day–1 was actually taking 6000 IU day–1 30. Toxicity associated with inaccurately manufactured and labelled vitamin D supplements is a globally reported problem 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43. To our knowledge, such errant labelling has not been reported in conjunction with licensed formulations.
Table 2.
Reports of vitamin D toxicity due to errors in formulation
| Author, year (reference) | Country | Stated dose | Actual dose received | Number of patients affected |
|---|---|---|---|---|
| Koutkia et al., 2001 27 | US | 2000 IU | 156 000–2.6 M IU day–1 | 1 adult |
| Klontz et al., 2007 28 | US | 400 IU | 188 000 | 1 adult +3 children |
| Kaptein et al., 2010 34 | Netherlands | 150 IU | 15 000–150 000 IU | 2 adults |
| Lowe et al., 2011 35 | Dominican Republic | 600 000 IU/5 ml | 900 000–1 M IU | 9 adults |
| Araki et al., 2011 21 | US | 1600 IU | 186 000 IU | 2 adults |
| Granado‐Lorencio et al., 2012 41 | Ecuador |
Unstated Vial day–1 for 4 weeks |
600 000 IU/vial | 1 adult |
| Kara et al, 2014 31 | Turkey | 2000 IU/5 ml | 800 000 IU | 7 children |
| Bell et al., 2013 42 | Australia | 300 IU day–1 | 300 000 IU | 1 adult |
| Anik et al., 2013 32 | Turkey | 10 IU ml–1 | Unknown | 3 children |
| Benemei et al., 2013 29 | Italy | 600 IU | 52 800 IU | 3 adults |
| Marins et al., 2014 43 | Brazil | 2000 day–1 | 4 M IU day–1 | 1 adult |
| Ketha et al., 2015 30 | US | 2000 IU/drop | 6000 IU/drop | 1 child |
| Zigenhorn et al., 2016 37 | Netherlands | 78× stated dose | 1 adult | |
| Guerra et al., 2016 36 | Brazil | 2000 IU | Unknown | 1 adult |
Inappropriate prescribing or dispensing
High‐dose formulations are a particular cause for concern. Stoss therapy for vitamin D deficiency, with high doses of vitamin D over relatively short periods, progressing to much lower maintenance doses, has encouraged the development of high‐dose vitamin D therapies (60 000 IU and above). With evidence indicating that doses of 10 000 IU day–1 or below do not cause vitamin D toxicity 15, and that such doses are effective in resolving vitamin D deficiency 38, it may seem that having super‐strength doses might expose subjects to unnecessary risk through either errant prescribing or inappropriate self‐medication. Indeed, vitamin D toxicity is described in association both with high‐dose over‐the‐counter supplements 39 being taken too frequently and prescribed in error by the physician 40, 44, 45, and being filled incorrectly by the pharmacist – for example, a 50 000 IU prescription provided daily instead of weekly 46. With regard to the former, a case report of a patient using 60 000 IU of supplemental vitamin D two to three times a week, Chatterjee et al. 39 noted vitamin D toxicity to be associated with reversible parkinsonism. Similarly, doses of 88 000 IU day–1 were reported to be associated with toxicity in a patient self‐medicating for multiple sclerosis 16. Worryingly, there have been a number of case reports on the mistaken administration of high doses of vitamin D to children by parents, resulting in hospitalization with severe toxicity 47, 48. Vitamin D toxicity following excessive replacement by the medical community with prolonged high doses of vitamin D has also been reported 44, 45, 49, 50, 51, 52, 53, 54. All of these patients had been treated injudiciously with high doses of parenteral vitamin D, and all had been receiving vitamin D treatment under medical supervision. These reports of toxicity associated with injudicious use of high doses of parenteral vitamin D preparations seem to be a particular, although not unique, problem of the Indian subcontinent 55. Furthermore, in some cases these treatments were initiated for inappropriate reasons, such as failure to thrive 56. Ziaie et al. 57 reported the case of an Iranian male who had been treated with 300 000 IU of parenteral vitamin D weekly presenting with severe and prolonged vitamin D toxicity.
In children with rickets, for whom a single parenteral megadose of vitamin D is considered effective and economical, substantially elevated 25OHD concentrations have been noted, exposing patients to the risk of toxicity 52, 58. Indeed, toxicity has been reported in an infant treated with high‐dose vitamin D while under medical supervision 59, and also in an infant treated with supplemental high‐dose vitamin D drops 60. There is a clear risk of vitamin D toxicity arising in these patients as there are several different treatment regimens and monitoring schedules, compounded by a lack of age‐specific guidance 1. A recent French case series of nine children who presented with severe hypercalcaemia following a cumulative dose of 600 000 units also indicated the need to adhere to current recommended vitamin D doses 61. There is also a case of megadoses of vitamin D being used inappropriately by an alternative health practitioner, resulting in patient harm 62.
While it might appear that prescribing errors account for a worryingly high number of cases of vitamin D toxicity, population‐based studies suggest that these represent a minority of such cases 63. In a UK study of requests for commercially available direct blood spot analysis of 25OHD concentrations, 372 (2.5% of total) requests had concentrations above 220 nmol l–1, with 28 of these having concentrations above 500 nmol l–1 63. Of the entire group with elevated 25OHD concentrations (>220 nmol l–1), only 6% were under direct medical supervision, and the majority (74%) obtained their vitamin D supplement directly over the internet 63. As this was a blood spot analysis, the authors could not determine toxicity through measurement of calcium concentrations, although this, again, suggests that the public is being put at risk through the availability of high‐dose preparations. In fact, an earlier analysis of these data revealed that, of the respondents with 25OHD concentrations >220 nmol l–1 who were contacted, 55% indicated that they were taking doses of 10 000 IU day–1 or less. With studies revealing that toxicity does not occur with daily vitamin D doses up to 10 000 IU day–1 15, these data suggest that, again, errant manufacturing may be exposing the public to risk 64. Of the six subjects who had 25OHD concentrations above 550 nmol l–1, one was under medical supervision, taking high doses against medical advice, with the remaining five independently using high doses (11 000–100 000 IU day–1) of vitamin D 63. In a Spanish study, a similar figure of 1.86% of patients for whom requests for 25OHD concentrations were made were found to have hypervitaminosis D, as defined by 25OHD levels above 160 nmol l–1, of whom, 51 (0.002%) had hypercalcaemia 65. An Irish study 66 revealed a prevalence of 4.8% of raised vitamin D 25OHD levels, although an elevated result was considered as 25OHDlevels >125 nmol l–1, as per Institute of Medicine (IOM) criteria. All of these patients appeared to be under medical supervision.
Inappropriate administration of vitamin D
A single dose of 2 000 000 IU of vitamin D was given in error to two nursing home residents 67, leading to a call to replace multiple use bottles with smaller single‐unit dose formulations. At the other extreme of age, most premature infant formulas have high vitamin D levels, which, while safe for short durations, if prolonged feeding is undertaken can result in excessive levels of vitamin D (>100 nmol l–1) 68. Careful monitoring in these patients is therefore essential. Errors in maternal vitamin D replacement can also result in substantial hypercalcaemia in the offspring. In one study, an exclusively breastfed infant required emergency admission 69. The mother was on a vitamin D prescription of 1 ml (400 IU per 1 ml daily); however, the concentration of the vitamin D supplement she had purchased online was 400 IU per drop, resulting in a 30‐fold higher dose than intended (12 000 IU day–1), with excess vitamin D being present in her breast milk 69.
Toxic vitamin D levels can also arise from misuse and inappropriate administration. A 19‐year‐old male was admitted with acute kidney injury and hypercalcaemia, with a vitamin D level of 150 ng ml–1. He was using a parenteral formulation of vitamins A, D and E that was restricted for veterinary use, containing 20 000 000 IU of vitamin A, 5 000 000 IU of vitamin D3 and 6800 IU of vitamin E per 100 ml. He was using the preparation as a ‘filler’ to enhance his muscle definition, rather than for any nutritional benefit 70. Other lifestyle causes of excess vitamin D should also be considered, including excess use of tanning beds 71.
Discussion
Vitamin D sufficiency is a key determinant of health, and supplementation is commonly required. The substantial public health benefits of ensuring vitamin D sufficiency may, however, be partially offset by a minority of individuals who suffer from adverse outcomes owing to vitamin D toxicity. The fact that it is a vitamin and a frequently used nutritional supplement, may have led to considerable complacency regarding its potential for toxic effects. This, combined with the dramatic expansion in vitamin D interest arising, in part, from popular books extolling the virtues of high‐dose vitamin D 72, it is perhaps not surprising there has been such an increase in the number of cases of vitamin D toxicity.
The causes of vitamin D toxicity appear to be multiple, and include the use of unlicensed and poorly manufactured products. In addition, there is widespread availability and inappropriate use of high‐dose over‐the‐counter supplements, and prescribing errors arising from the injudicious use of high‐dose supplements. Prescribers and dispensers need to appreciate the potential dangers to their patients and, wherever possible, mitigate against causing them harm. Standardizing replacement and monitoring regimes, and growing awareness among physicians of the potential risks of vitamin D toxicity, with acceptance of a therapeutic window, are clearly essential 73. It is, however, surprising that vitamin D toxicity still occurs, through lack of clear guidance and regulation, despite awareness of this problem for over 50 years.
To our knowledge, this is the first comprehensive review of vitamin D toxicity. It highlights the pressing need for substantial improvements in the delivery of vitamin D products. However, there are key limitations; the nature of this topic has limited the literature predominantly to case reports and case series, and the evidence base is therefore of only moderate quality. Nevertheless, we observed clear themes in the causes of vitamin D toxicity; furthermore, the majority of cases appeared to have been easily preventable.
Conclusion
In summary, vitamin D toxicity remains an ongoing issue and its incidence is likely to rise, owing to both increasing interest and the widespread prescribing of vitamin D. The patients at greatest risk are likely to be at the extremes of age. Simple legislation to ensure the quality of all vitamin D products, together with limited and restricted use of very high‐dose formulations, may substantially reduce future public harm, as the majority of cases appear, in retrospect, to have been easily preventable.
Competing Interests
There are no competing interests to declare.
Contributors
J.S.D. conceived the study. Both authors undertook literature searching and reviewed the papers, and both drafted and refined the manuscript.
Taylor, P. N. , and Davies, J. S. (2018) A review of the growing risk of vitamin D toxicity from inappropriate practice. Br J Clin Pharmacol, 84: 1121–1127. doi: 10.1111/bcp.13573.
References
- 1. Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr 2008; 88: 491s–499s. [DOI] [PubMed] [Google Scholar]
- 2. Zgaga L, Theodoratou E, Farrington SM, Agakov F, Tenesa A, Walker M, et al Diet, environmental factors, and lifestyle underlie the high prevalence of vitamin D deficiency in healthy adults in Scotland, and supplementation reduces the proportion that are severely deficient. J Nutr 2011; 141: 1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grace C, Vincent R, Aylwin SJ. High prevalence of vitamin D insufficiency in a United Kingdom urban morbidly obese population: implications for testing and treatment. Surg Obes Relat Dis 2014; 10: 355–360. [DOI] [PubMed] [Google Scholar]
- 4. Janner M, Ballinari P, Mullis PE, Fluck CE. High prevalence of vitamin D deficiency in children and adolescents with type 1 diabetes. Swiss Med Wkly 2010; 140: w13091. [DOI] [PubMed] [Google Scholar]
- 5. Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol 2014; 144 (Pt A): 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davies JS, Poole CD. Vitamin D: too much of a good thing? Br J Gen Pract 2014; 64: 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scientific Advisory Committee on Nutrition . Vitamin D and Health. London, 2016. Available at https://www.gov.uk/government/groups/scientific-advisory-committee-on-nutrition (last accessed 1 October 2017). [Google Scholar]
- 8. Davies SC, Jewell T, McBride M, Burns H. Vitamin D – Advice on supplements for at risk groups. London: Department of Health, 2012. [Google Scholar]
- 9. Pereira‐Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta‐analysis. Obes Rev 2015; 16: 341–349. [DOI] [PubMed] [Google Scholar]
- 10. Holick MF. Photosynthesis of vitamin D in the skin: effect of environmental and life‐style variables. Fed Proc 1987; 46: 1876–1882. [PubMed] [Google Scholar]
- 11. Selby PL, Davies M, Marks JS, Mawer EB. Vitamin D intoxication causes hypercalcaemia by increased bone resorption which responds to pamidronate. Clin Endocrinol 1995; 43: 531–536. [DOI] [PubMed] [Google Scholar]
- 12. National Osteoporosis Society . Vitamin D and bone health: a practical clinical guideline for patient management [online]. Available at https://nos.org.uk/media/2073/vitamin-d-and-bone-health-adults.pdf (last accessed 2 July 2017).
- 13. Holick MF, Binkley NC, Bischoff‐Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 1911–1930. [DOI] [PubMed] [Google Scholar]
- 14. Alshahrani F, Aljohani N. Vitamin D: deficiency, sufficiency and toxicity. Forum Nutr 2013; 5: 3605–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vieth R. Vitamin D supplementation, 25‐hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999; 69: 842–856. [DOI] [PubMed] [Google Scholar]
- 16. Kimball S, Vieth R. Self‐prescribed high‐dose vitamin D3: effects on biochemical parameters in two men. Ann Clin Biochem 2008; 45: 106–110. [DOI] [PubMed] [Google Scholar]
- 17. Davies JS, Poole CD, Feldschreiber P. The medico‐legal aspects of prescribing vitamin D. Br J Clin Pharmacol 2014; 78: 1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ross SG. Vitamin D intoxication in infancy: a report of four cases. J Pediatr 1952; 41: 815–822. [DOI] [PubMed] [Google Scholar]
- 19. Howard JE, Meyer RJ. Intoxication with vitamin D. J Clin Endocrinol 1948; 8: 895–910. [DOI] [PubMed] [Google Scholar]
- 20. Holick MF. Vitamin D deficiency: what a pain it is. Mayo Clin Proc 2003; 78: 1457–1459. [DOI] [PubMed] [Google Scholar]
- 21. Araki T, Holick MF, Alfonso BD, Charlap E, Romero CM, Rizk D, et al Vitamin D intoxication with severe hypercalcemia due to manufacturing and labeling errors of two dietary supplements made in the United States. J Clin Endocrinol Metab 2011; 96: 3603–3608. [DOI] [PubMed] [Google Scholar]
- 22. Blank S, Scanlon KS, Sinks TH, Lett S, Falk H. An outbreak of hypervitaminosis D associated with the overfortification of milk from a home‐delivery dairy. Am J Public Health 1995; 85: 656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jacobus CH, Holick MF, Shao Q, Chen TC, Holm IA, Kolodny JM, et al Hypervitaminosis D associated with drinking milk. N Engl J Med 1992; 326: 1173–1177. [DOI] [PubMed] [Google Scholar]
- 24. Garg S, Sabri D, Kanji J, Rakkar PS, Lee Y, Naidoo N, et al Evaluation of vitamin D medicines and dietary supplements and the physicochemical analysis of selected formulations. J Nutr Health Aging 2013; 17: 158–161. [DOI] [PubMed] [Google Scholar]
- 25. LeBlanc ES, Perrin N, Johnson JD Jr, Ballatore A, Hillier T. Over‐the‐counter and compounded vitamin D: is potency what we expect? JAMA Intern Med 2013; 173: 585–586. [DOI] [PubMed] [Google Scholar]
- 26. Khadgawat R, Ramot R, Chacko KM, Marwaha RK. Disparity in cholecalciferol content of commercial preparations available in India. Indian J Endocrinol Metab 2013; 17: 1100–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koutkia P, Chen TC, Holick MF. Vitamin D intoxication associated with an over‐the‐counter supplement. N Engl J Med 2001; 345: 66–67. [DOI] [PubMed] [Google Scholar]
- 28. Klontz KC, Acheson DW. Dietary supplement‐induced vitamin D intoxication. N Engl J Med 2007; 357: 308–309. [DOI] [PubMed] [Google Scholar]
- 29. Benemei S, Gallo E, Giocaliere E, Bartolucci G, Menniti‐Ippolito F, Firenzuoli F, et al It's time for new rules on vitamin D food supplements. Br J Clin Pharmacol 2013; 76: 825–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ketha H, Wadams H, Lteif A, Singh RJ. Iatrogenic vitamin D toxicity in an infant – a case report and review of literature. J Steroid Biochem Mol Biol 2015; 148: 14–18. [DOI] [PubMed] [Google Scholar]
- 31. Kara C, Gunindi F, Ustyol A, Aydin M. Vitamin D intoxication due to an erroneously manufactured dietary supplement in seven children. Pediatrics 2014; 133: e240–e244. [DOI] [PubMed] [Google Scholar]
- 32. Anik A, Catli G, Abaci A, Dizdarer C, Bober E. Acute vitamin D intoxication possibly due to faulty production of a multivitamin preparation. J Clin Res Pediatr Endocrinol 2013; 5: 136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Conti G, Chirico V, Lacquaniti A, Silipigni L, Fede C, Vitale A, et al Vitamin D intoxication in two brothers: be careful with dietary supplements. J Pediatr Endocrinol Metabol 2014; 27: 763–767. [DOI] [PubMed] [Google Scholar]
- 34. Kaptein S, Risselada AJ, Boerma EC, Egbers PH, Nieboer P. Life‐threatening complications of vitamin D intoxication due to over‐the‐counter supplements. Clin Toxicol 2010; 48: 460–462. [DOI] [PubMed] [Google Scholar]
- 35. Lowe H, Cusano NE, Binkley N, Blaner WS, Bilezikian JP. Vitamin D toxicity due to a commonly available ‘over the counter’ remedy from the Dominican Republic. J Clin Endocrinol Metab 2011; 96: 291–295. [DOI] [PubMed] [Google Scholar]
- 36. Guerra V, Vieira Neto OM, Laurindo AF, Paula FJ, Moyses NM. Hypercalcemia and renal function impairment associated with vitamin D toxicity: case report. J Bras Nefrol 2016; 38: 466–469. [DOI] [PubMed] [Google Scholar]
- 37. Zigenhorn M, Westerman EM, Rietveld AP. Hypervitaminosis D due to a dietary supplement. Ned Tijdschr Geneeskd 2016; 160: A9360. [PubMed] [Google Scholar]
- 38. Ish‐Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab 2008; 93: 3430–3435. [DOI] [PubMed] [Google Scholar]
- 39. Chatterjee R, Chatterjee K, Sen C. Reversible parkinsonism due to vitamin D toxicity. J Neurosci Rural Pract 2017; 8: 305–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jacobsen RB, Hronek BW, Schmidt GA, Schilling ML. Hypervitaminosis D associated with a vitamin D dispensing error. Ann Pharmacother 2011; 45: e52. [DOI] [PubMed] [Google Scholar]
- 41. Granado‐Lorencio F, Rubio E, Blanco‐Navarro I, Perez‐Sacristan B, Rodriguez‐Pena R, Garcia Lopez FJ. Hypercalcemia, hypervitaminosis A and 3‐epi‐25‐OH‐D3 levels after consumption of an ‘over the counter’ vitamin D remedy. A case report. Food Chem Toxicol 2012; 50: 2106–2108. [DOI] [PubMed] [Google Scholar]
- 42. Bell DA, Crooke MJ, Hay N, Glendenning P. Prolonged vitamin D intoxication: presentation, pathogenesis and progress. Intern Med J 2013; 43: 1148–1150. [DOI] [PubMed] [Google Scholar]
- 43. Marins TA, Galvao Tde F, Korkes F, Malerbi DA, Ganc AJ, Korn D, et al Vitamin D intoxication: case report. Einstein 2014; 12: 242–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaur P, Mishra SK, Mithal A. Vitamin D toxicity resulting from overzealous correction of vitamin D deficiency. Clin Endocrinol 2015; 83: 327–331. [DOI] [PubMed] [Google Scholar]
- 45. Wani M, Wani I, Banday K, Ashraf M. The other side of vitamin D therapy: a case series of acute kidney injury due to malpractice‐related vitamin D intoxication. Clin Nephrol 2016; 86: 236–241. [DOI] [PubMed] [Google Scholar]
- 46. Carlton S, Clopton D, Cappuzzo KA. Vitamin D deficiency: appropriate replenishment therapies and the effects of vitamin D toxicity. Consult Pharm 2010; 25: 171–177. [DOI] [PubMed] [Google Scholar]
- 47. Barrueto F Jr, Wang‐Flores HH, Howland MA, Hoffman RS, Nelson LS. Acute vitamin D intoxication in a child. Pediatrics 2005; 116: e453–e456. [DOI] [PubMed] [Google Scholar]
- 48. Rajakumar K, Reis EC, Holick MF. Dosing error with over‐the‐counter vitamin D supplement: a risk for vitamin D toxicity in infants. Clin Pediatr 2013; 52: 82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mansuri ZH, Kaji BC, Dumra S, Buch HN. Hypervitaminosis‐D, an uncommon reality! J Assoc Physicians India 2014; 62: 58–60. [PubMed] [Google Scholar]
- 50. Bansal RK, Tyagi P, Sharma P, Singla V, Arora V, Bansal N, et al Iatrogenic hypervitaminosis D as an unusual cause of persistent vomiting: a case report. J Med Case Reports 2014; 8: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koul PA, Ahmad SH, Ahmad F, Jan RA, Shah SU, Khan UH. Vitamin D toxicity in adults: a case series from an area with endemic hypovitaminosis D. Oman Med J 2011; 26: 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sharawat IK. Hypervitaminosis D with dyslipidemia: an unusual scenario. Indian Pediatr 2016; 53: 174–175. [PubMed] [Google Scholar]
- 53. Cirillo M, Bilancio G, Cirillo C. Reversible vascular calcifications associated with hypervitaminosis D. J Nephrol 2016; 29: 129–131. [DOI] [PubMed] [Google Scholar]
- 54. Pandita KK, Razdan S, Kudyar RP, Beigh A, Kuchay S, Banday T. ‘Excess gooD can be dangerous’. A case series of iatrogenic symptomatic hypercalcemia due to hypervitaminosis D. Clin Cases Miner Bone Metab 2012; 9: 118–120. [PMC free article] [PubMed] [Google Scholar]
- 55. Sharma LK, Dutta D, Sharma N, Gadpayle AK. The increasing problem of subclinical and overt hypervitaminosis D in India: an institutional experience and review. Nutrition 2017; 34: 76–81. [DOI] [PubMed] [Google Scholar]
- 56. Joshi R. Hypercalcemia due to hypervitaminosis D: report of seven patients. J Trop Pediatr 2009; 55: 396–398. [DOI] [PubMed] [Google Scholar]
- 57. Ziaie H, Razmjou S, Jomhouri R, Jenabi A. Vitamin D toxicity: stored and released from adipose tissue? Arch Iran Med 2016; 19: 597–600. [PubMed] [Google Scholar]
- 58. Bothra M, Gupta N, Jain V. Effect of intramuscular cholecalciferol megadose in children with nutritional rickets. J Pediatr Endocrinol Metabol 2016; 29: 687–692. [DOI] [PubMed] [Google Scholar]
- 59. Nimesh M, Singh P, Jhamb U, Dubey AP. An unsuspected pharmacological vitamin D toxicity in a child and its brief review of literature. Toxicol Int 2015; 22: 167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cantrell L, Hogen E. Vitamin D overdosage in an infant from nonprescription vitamin D drops. Am J Health‐Syst Pharm 2015; 72: 1262–1263. [DOI] [PubMed] [Google Scholar]
- 61. Hmami F, Oulmaati A, Amarti A, Kottler ML, Bouharrou A. Overdose or hypersensitivity to vitamin D? Arch Pediatr 2014; 21: 1115–1119. [DOI] [PubMed] [Google Scholar]
- 62. Kerstens PJ, van Ditzhuijsen TJ, van Tongeren JH. Mega‐dosages vitamin D: progressive medicine? Ned Tijdschr Geneeskd 1990; 134: 1959–1961. [PubMed] [Google Scholar]
- 63. Shea RL, Berg JD. Self‐administration of vitamin D supplements in the general public may be associated with high 25‐hydroxyvitamin D concentrations. Ann Clin Biochem 2017; 54: 355–361. [DOI] [PubMed] [Google Scholar]
- 64. Shea RL, Berg JD. Incidences of high to toxic 25‐hydroxy vitamin D levels amongst users of a direct‐to‐thepublic blood spot vitamin D testing service. Forum Nutr 2014; 108. [Google Scholar]
- 65. Perez‐Barrios C, Hernandez‐Alvarez E, Blanco‐Navarro I, Perez‐Sacristan B, Granado‐Lorencio F. Prevalence of hypercalcemia related to hypervitaminosis D in clinical practice. Clin Nutr 2016; 35: 1354–1358. [DOI] [PubMed] [Google Scholar]
- 66. Kilbane MT, O'Keane M, Morrin M, Flynn M, McKenna MJ. The double‐edged sword of vitamin D in Ireland: the need for public health awareness about too much as well as too little. Ir J Med Sci 2014; 183: 485–487. [DOI] [PubMed] [Google Scholar]
- 67. van den Ouweland J, Fleuren H, Drabbe M, Vollaard H. Pharmacokinetics and safety issues of an accidental overdose of 2,000,000 IU of vitamin D3 in two nursing home patients: a case report. BMC Pharmacol Toxicol 2014; 15: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nako Y, Tomomasa T, Morikawa A. Risk of hypervitaminosis D from prolonged feeding of high vitamin D premature infant formula. Pediatr Int 2004; 46: 439–443. [DOI] [PubMed] [Google Scholar]
- 69. Smollin C, Srisansanee W. Vitamin D toxicity in an infant: case files of the University of California, San Francisco medical toxicology fellowship. J Med Toxicol 2014; 10: 190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rocha PN, Santos CS, Avila MO, Neves CL, Bahiense‐Oliveira M. Hypercalcemia and acute kidney injury caused by abuse of a parenteral veterinary compound containing vitamins A, D, and E. J Bras Nefrol 2011; 33: 467–471. [PubMed] [Google Scholar]
- 71. Singh P, Trivedi N. Tanning beds and hypervitaminosis D: A case report. Ann Intern Med 2014; 160: 810–811. [DOI] [PubMed] [Google Scholar]
- 72. Bowles TJ. The Miraculous Results of Extremely High Doses of the Sunshine Hormone Vitamin D3 My Experiment with Huge Doses of D3 from 25,000 to 50,000 to 100,000 IU a Day over a 1 Year Period. CreateSpace Independent Publishing Platform.
- 73. Romagnoli E, Pepe J, Piemonte S, Cipriani C, Minisola S. Management of endocrine disease: value and limitations of assessing vitamin D nutritional status and advised levels of vitamin D supplementation. Eur J Endocrinol 2013; 169: R59–R69. [DOI] [PubMed] [Google Scholar]


