Abstract
Chronic pain is a highly prevalent debilitating condition for which treatment options remain limited for many patients. Ionotropic ATP signalling through excitatory and calcium‐permeable P2X receptor channels is now rightfully considered as a critical player in pathological pain generation and maintenance; therefore, their selective targeting represents a therapeutic opportunity with promising yet untapped potential. Recent advances in the structural, functional and pharmacological characterization of rodent and human ATP‐gated P2X receptor channels have shed brighter light on the role of specific subtypes in the pathophysiology of chronic inflammatory, neuropathic or cancer pain. Here, we will review the contribution of P2X3, P2X4 and P2X7 receptors to chronic pain and discuss the opportunities and challenges associated with the pharmacological manipulation of their function.
Linked Articles
This article is part of a themed section on Recent Advances in Targeting Ion Channels to Treat Chronic Pain. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.12/issuetoc
Abbreviations
- αβ‐meATP
αβ‐methylene ATP
- BBG
brilliant blue G
- BDNF
brain‐derived neurotrophic factor
- BNP
brain natriuretic peptide
- DRG
dorsal root ganglion
- HSV
herpes simplex virus
- IRF
IFN regulatory factor
- NPR
natriuretic peptide receptor
- PNI
peripheral nerve injury
- SNP
single‐nucleotide polymorphism
Introduction
Initially discovered as the main chemical energy currency in eukaryotic cells, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1713 is now fully recognized as an important extracellular signalling molecule (North, 2016). For around 50 years, this nucleotide has been known to play a critical role in nociception as well as in chronic pain mechanisms (Burnstock, 2016). Extracellular ATP elicits its downstream signalling via activation of two classes of surface P2 purinergic receptors: the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=77 and the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=52. P2X ATP‐gated channels can assemble from seven subunits (P2X1–7) as homotrimers or heterotrimers around a non‐selective cationic pore. For their role in transducing an ATP signal contributing to chronic pain generation and maintenance, they are regarded as highly interesting therapeutic targets for novel pharmacological approaches to analgesia. Here, we will review the latest advances in the understanding of the involvement of P2X receptor channels in pain pathways. We will mainly focus on P2X subtypes critically implicated in pathological pain: sensory http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=480&familyId=77&familyType=IC and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=479/3, microglial http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=481&familyId=77&familyType=IC and immune http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=484&familyId=77&familyType=IC receptors (see Table 1 and Figure 1).
Table 1.
Synopsis of functional properties and pharmacology of the P2X ATP‐gated channels involved in nociception and chronic pain. Calcium permeability of P2X receptor subtypes is indicated in fractional current (data from Egan and Khakh, 2004). The selective antagonists in bold typeface are displayed in Figure 3.
| Subtype | P2X3 | P2X2/3 | P2X4 | P2X7 |
|---|---|---|---|---|
| Current phenotype |

|
|

|

|
| Calcium permeability (%) | 2.7 (rat) | 3.5 (rat) | 15 (human) | 4.6 (rat) |
| Large pore | No | Yes | Yes | Yes |
| Link to pannexin | No | No | No | Yes |
| Agonists | ATP, αβ‐meATP, 2‐MeS‐ATP, BzATP and Ap(n)A | As for the P2X3 homomer | ATP > BzATP, 2‐MeS‐ATP, AP4, γ‐S‐ATP and αβ‐meATP | BzATP > ATP, 2‐MeS‐ATP and αβ‐meATP |
| Antagonists | Suramin, PPADS, TNP‐ATP, Ap4A analogs, A317491, RO‐3, AF‐219, RO‐51, AF‐353, AF‐906, NF279, MK‐3901, MRS 3357, Tricyclics, 5‐OH‐pyridines and 3,4‐dicarboxy‐pyridines | As for the P2X3 homomer | PPADS, paroxetine, duloxetine, 5‐BDBD, PSB‐12054, PSB‐12062, CORM‐2, carbamazepines, artemisinin, NP‐1815‐PX and BX430 | PPADS, BBG, oATP, decavanadate, KN62, NF279, A‐740003, A438079, A804598, A839977, AZ10606120, AZ11645373, GW791343, AZD9056, GSK1482160, GSK314181A, CE‐224535, JNJ‐479655, pyrimidine‐2,4‐diones, ZINC09315614, chloro‐purines, RO‐3, AF‐353, AF‐906, acetamides, 3,5‐DiCl‐pyridines, protoberberines, pyrazol‐acetamides, cyanoguanidines, teniposide, tanshinone IIAS, methanones, nanoAb 13A7 and nanoAb Dano1 |
| Selective for P2X3 homomers: IP5I, AZ004, RO‐85, mAb 12D4 and spinorphin | ||||
| Cellular distribution (pain‐related) | Primary sensory neurons (dorsal root, nodose and trigeminal ganglia) | As for the P2X3 homomer | CNS neurons, monocytes, macrophages and microglia | Lymphocytes, monocytes, macrophages, microglia, CNS neurons and astrocytes |
Figure 1.
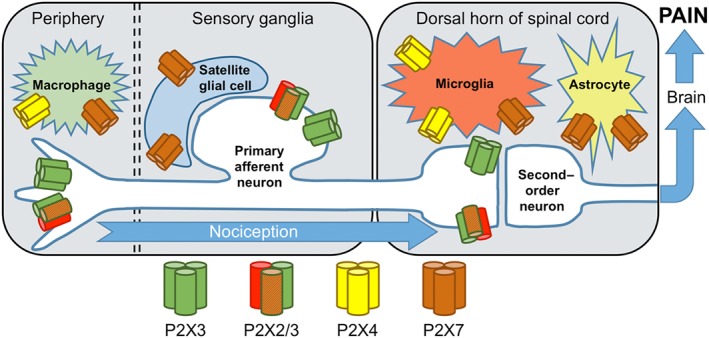
Schematic cellular distribution of the main P2X receptor subtypes expressed in mammalian peripheral pain pathways. The exact subunit stoichiometry of native heteromeric P2X2/3 receptors in primary sensory neurons remains unknown.
P2X3 and P2X2/3 receptors in primary sensory neurons
P2X3 homomers and P2X2/3 heteromers are predominantly localized on peripheral primary sensory afferents, in small‐diameter unmyelinated C‐fibres, while being mostly absent from medium‐diameter and large‐diameter NF200+ sensory neurons. Non‐peptidergic, P2X3‐expressing, C‐fibres, also isolectin B4+ and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=152&familyId=16&familyType=GPCR+, represent a large fraction of cutaneous and visceral nociceptors in rodents and humans. Located on nerve terminals in free nerve endings in peripheral tissues, P2X channels can drive the initial nerve impulse from the nociceptor's receptive field. Therefore, excitatory P2X3 and P2X2/3 ATP‐gated receptor channels are thought to exert their effect by directly sensitizing C‐fibres by membrane depolarization and calcium entry to facilitate pain transmission. Of particular importance for chronic pain diseases is the expression of P2X3 receptors in the central terminals of dorsal root ganglia (DRGs) (or trigeminal ganglia) terminating in the inner lamina II (substantia gelatinosa) of the dorsal horn of the spinal cord. These presynaptic P2X3 receptors are appropriately located to exert facilitation of glutamate release at the first synapse in the nociceptive pathway.
As P2X3 receptors can increase nociception via direct sensitization of pain fibres, there is much evidence for the idea that dysregulation of this purinergic pathway is involved in pathological pain. One key question in the field is whether the aberrant activation of P2X3 receptors in chronic pain stems from increased P2X3 expression and function or from increased availability of its endogenous agonist, namely, ATP. While up‐regulation of P2X3 receptors and ATP release will clearly affect the contribution of purinergic mechanisms to chronic pain, many reports have shown that other receptor signalling systems co‐expressed with P2X3 receptors in C‐fibres can directly regulate P2X3 and ATP‐induced neuronal sensitization. Furthermore, new insights into post‐translational modifications of P2X3 channels may lead to novel pharmacological approaches and might represent a way to alter P2X3 receptor function in specific chronic pain conditions. This new evidence will be reviewed here, with a focus on how targeting these regulatory pathways can affect pain responses.
It has been suggested that P2X3 receptors can form functional units with co‐expressed sensory transducers. For instance, P2X3 receptors interact with http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=507&familyId=78&familyType=IC channels, long known for their involvement in nociceptive thermal sensing. In a model of mechanical hyperalgesia of the masseter muscle induced by injection of the selective P2X agonist αβ‐methylene ATP (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4093), pretreatment with the TRPV1 antagonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6347 prevents the induction of pain (Saloman et al., 2013). Both P2X3 and TRPV1 receptors are co‐expressed in a subpopulation of trigeminal neurons, and capsaicin‐induced calcium transients were amplified by treatment with P2X3 receptor agonists, further indicating a co‐facilitatory interaction between the two sensory receptor channels. This facilitation is likely to depend on P2X3‐induced phosphorylation of serine residues on TRPV1 receptors. While no proof of direct physical proximity exists, several lines of evidence point towards their co‐localization within lipid rafts, as well as their differential regulation by cholesterol (Vacca et al., 2004; Liu et al., 2006; Szoke et al., 2010; Gnanasekaran et al., 2011). This raises the possibility of micro‐environmental changes affecting not only P2X3 channel activity but also P2X3–TRPV1 functional interaction.
The co‐expression of P2X3 and TRPV1 receptors in subpopulations of nociceptive neurons plays a key role in the severe pain induced by the snake venom toxin, BomoTx. The recent study by Zhang et al. (2017) demonstrated that BomoTx induced the release of ATP from sensory neurons, probably through the transient opening of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=121 hemichannels and that this ATP release further activates P2X3‐containing receptors on neighbouring neurons (Zhang et al., 2017). These findings uncover a novel mechanism by which ATP can diffuse from pannexin pores in a PLC‐dependent and calcium‐dependent way, a mechanism that might underlie excessive ATP release in other forms of pain pathologies. Furthermore, this indicates that stimulation of specific C‐fibre types, either expressing TRPV1 or P2X3 receptors, elicits distinct painful behaviours. Of note, the ATP release triggered by BomoTx activates P2X receptors on C‐fibres that mediate mechanical allodynia. Interestingly, ATP release through pannexin was also shown to be induced downstream of P2X3 activation in trigeminal sensory neurons. This calcium/calmodulin‐dependent ATP efflux pathway potentially drives feedforward potentiation of painful signals (Bele and Fabbretti, 2016).
Control of the surface localization of P2X3 receptors is directly involved in several multi‐receptor crosstalks. The depression of P2X3 receptor function induced by co‐activation of the natriuretic peptide receptor‐A (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=662) by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4890) relies on the redistribution of P2X3 receptors out of lipid rafts (Marchenkova et al., 2015). In a genetic mouse model of familial hemiplegic migraine, sustained inactivation of the BNP/NPR‐A pathway affected the phosphorylation state of P2X3 receptors and enhanced P2X3 receptor currents in trigeminal ganglion neurons (Marchenkova et al., 2016). Another peptide involved in nociception, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=681, was also shown to regulate the membrane localization of P2X3 receptors in trigeminal nociceptive neurons. CGRP is believed to induce a slow and sustained up‐regulation of P2X3 receptors and contribute to pain sensitization in migraine (Fabbretti et al., 2006; Wang et al., 2012). In inflammatory pain models, the agonists trypsin and tryptase sensitize sensory neurons via cleavage of their receptor protease‐activated receptor‐2 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=348&familyId=59&familyType=GPCR). PAR‐2 activation was shown to increase P2X3 channel activity in DRGs, via http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=284‐ and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=286&familyType=ENZYME‐dependent translocation of P2X3 receptors to the cell surface (Wang et al., 2012).
Another major signalling molecule involved in inflammatory pain, PGE2, modulates P2X3 receptor activity in sensory neurons. The increased excitability of DRG neurons observed following PGE2 treatment is attributed to the increased activity of P2X3 channels. The http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=342&familyId=58&familyType=GPCR receptors activated by PGE2 triggers a cAMP/PKA‐dependent pathway, ultimately enhancing P2X3 receptor responses, as demonstrated both in vitro and in vivo behaviourally in pain models through the use of the PKA blocker H89 (Wang et al., 2007). A similar regulatory mechanism was also suggested in a study investigating the increased involvement of P2X3 receptors in bone cancer pain, where an up‐regulation of these receptors could be triggered by PGE2 released from tumour cells (Wu et al., 2012).
While several lines of evidence clearly show that PKA is involved in linking EP receptor activation to up‐regulation of P2X3 receptors, recent data suggest that in inflamed neurons, an increase in PKC signalling is also involved in PGE2‐driven sensitization of P2X3 receptors. Exchange proteins directly activated by cAMP (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=259 can enhance the PKC‐dependent signalling under inflammatory pain conditions induced by complete Freund's adjuvant (Gu et al., 2016a). The same group demonstrated that this mechanism requires F‐actin as a link between PGE2 activation of PKC and subsequent enhancement of P2X3 receptor responses, in a process likely affecting membrane insertion of the receptor channels (Gu et al., 2016b). While more work is needed to further our understanding of the PKC‐P2X3 axis in sensory neurons, both http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1486 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1482 isoforms have been implicated in regulating P2X3 receptor function and may represent potent pharmacological targets for inflammatory pain (Prado et al., 2013).
Interestingly, metabotropic purinergic P2Y receptors were also shown to modulate activity of P2X3 and P2X2/3 receptors within nociceptive neurons. Both http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=323&familyId=52&familyType=GPCR and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=324&familyId=52&familyType=GPCR receptors are expressed along with P2X3 receptors in DRG nociceptors (Kobayashi et al., 2006). Activation of P2Y1 receptors with the agonists http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1755 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1710, as well as activation of P2Y2 receptors with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1734 inhibits responses of P2X3 receptors to αβ‐meATP (Gerevich et al., 2005). The mechanism suggested relies on Gq‐coupled activation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=274 and subsequent hydrolysis of the phospholipid http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2387, a known facilitator of P2X function (Mo et al., 2009; Mo et al., 2013). This inhibition provided by P2Y receptors might underlie a homeostatic mechanism to prevent excessive activation of P2X3 receptors and subsequent excess of intracellular calcium, as the metabotropic and ionotropic receptors are activated by purinergic signalling molecules, likely to come from the same sources following tissue damage. Most P2Y receptors show cross‐sensitivity to ATP‐ and UTP‐derived ligands; therefore, their activation under high ATP concentrations can dampen P2X3 receptor activity in pathological conditions. Interestingly, similar Gq‐coupled and phosphoinositide‐dependent modulation of channel activity was also observed with P2X4 and P2X7 receptors in immune cells, likely to represent a ubiquitous regulatory mechanism of purinergic transduction in pain circuits (Zhao et al., 2007; Bernier et al., 2008, 2013a,b).
A recent study also uncovered a potential interaction between P2X3 receptors and the lysophosphatidic acid receptor (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=272&familyId=36&familyType=GPCR). In a rat model of bone cancer pain‐induced mechanical pain, activation of LPA1, co‐expressed with P2X3 receptors in DRG neurons, potentiated ATP‐mediated currents (Wu et al., 2016). Activation of the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=289 signalling pathway downstream from LPA1 receptors is suggested as the functional link, as either LPA1 receptor or Rho/ROCK antagonists inhibited αβ‐meATP‐evoked pain responses.
A microarray‐based gene expression study investigating 25 inbred mouse strains identified the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=467 subunit as a gene playing a central role in mechanical allodynia and in nicotine‐induced analgesia (Wieskopf et al., 2015). Gain‐of‐function and loss‐of‐function mutants correlate with the level of analgesia induced by activation of α6 subunits, in both neuropathic and inflammatory pain models. It has been suggested that the analgesic role of α6 subunits is mediated through cross‐inhibition of P2X2/3 heteromeric channels in mouse DRG neurons. While the study also hints at a similar role of α6 subunits in humans, it is likely that a different cross‐regulation exists with the P2X component of pain sensitization, as the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=479&familyId=77&familyType=IC receptor subunit has not been found in human and primate DRGs (Serrano et al., 2012).
Direct pharmacological blockade of P2X3 receptors by selective antagonists has shown clinical promise when used on various preclinical models of chronic pain, including inflammatory, neuropathic and cancer‐induced bone pain (Hansen et al., 2012; Prado et al., 2013; Schiavuzzo et al., 2015). Moreover, recent high‐resolution structural information on crystallized human P2X3 receptors, with the competitive antagonists TNP‐ATP and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4115 bound to the orthosteric site (see Figure 2) and in different stages of its activation cycle, has the potential to boost the development of novel potent analgesics (Mansoor et al., 2016). In parallel, as more and more data become available on the various regulatory mechanisms governing sensitization of P2X3 receptors in chronic pain pathologies, it is likely that some of the sensory receptors known to interact with P2X3 receptors in the complex process of C‐fibre sensitization will also provide effective therapeutic targets.
Figure 2.
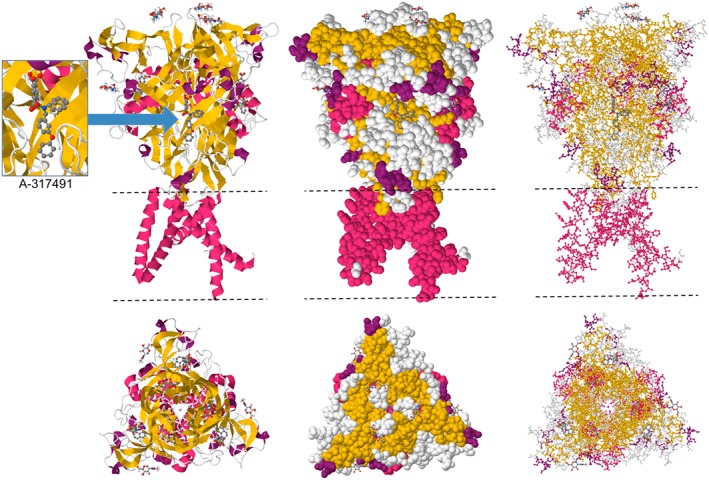
Crystal structure of the trimeric human P2X3 receptor channel showing interactions with the competitive antagonist A‐317491 bound to the orthosteric ATP site. Cartoon (left), space fill (centre) and ball‐and‐stick (right) representations are displayed in two orientations. The dashed lines indicate the position of the lipid bilayer. Coordinates from PDB #5SVR (Mansoor et al., 2016) viewed in JSmol.
Microglial P2X4 receptors
In the last 15 years, a lot of attention has been paid to the role of P2X4 receptors in the generation and maintenance of chronic pain. While earlier investigations were traditionally aimed at neurocentric mechanisms by which intrinsic changes in primary or second‐order nociceptive neurons would induce hyperexcitability, more and more studies demonstrated that modifications in the glial micro‐environment surrounding sensory neurons could trigger aberrant excitability and nociceptive responses. The contribution of P2X4 receptors to such indirect effects on nociceptive pathways was first documented via its up‐regulation in activated spinal cord microglia. The mechanism by which activation of microglial P2X4 receptors leads to neuronal hyperexcitability within the dorsal horn of the spinal cord has been reviewed previously (Trang and Salter, 2012; Tsuda et al., 2013) and will be briefly described here. Additional aspects of the regulation of P2X4 receptor activity in painful pathologies will be discussed.
Following peripheral nerve injury, microglia in the superficial laminae of the spinal cord dorsal horn, where the first somatosensory relays take place, undergo activation, a complex process characterized by changes in morphology, gene expression and cell number. Activated microglia increase their expression of P2X4 receptors, hinting at the importance of purinergic transduction in this process. The timing of microglial activation correlates with mechanical pain hypersensitivity and is necessary and sufficient to trigger neuronal hyperexcitability. A critical molecular event in this pathway was the release of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4872 by activated microglia (Tsuda et al., 2003; Ulmann et al., 2008; Trang et al., 2009). Interestingly, the release of BDNF is dependent on activation of microglial P2X4 receptors by ATP. In turn, BDNF activates postsynaptic http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1818&familyId=326&familyType=CATALYTICRECEPTOR receptors on second‐order spinal neurons in lamina I, inducing down‐regulation of the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=184#972 (Coull et al., 2003, 2005). The dysregulation of neuronal anionic gradients brought by this cascade ultimately weakens GABAergic synapses, resulting in a dis‐inhibition of the spinal circuits, so critical in pain pathologies.
As the abnormal expression of P2X4 receptors in microglia contributes to peripheral nerve injury (PNI)‐induced mechanical pain, several studies have focused on the mechanisms underlying up‐regulation of P2X4 receptors. Masuda et al. (2014) identified IFN regulatory factor‐5 (IRF‐5) as the major transcription factor involved in the transcriptional control of P2X4 receptors. Mice lacking IRF‐5 did not up‐regulate spinal P2X4 receptors after PNI and exhibited substantial resistance to pain hypersensitivity. Furthermore, IRF‐8, which had previously been identified as a critical regulator of reactive‐state microglia and the neuropathic pain responses after PNI (Masuda et al., 2012), was shown to regulate IRF‐5 activation through binding to its promoter site. Another key player in the up‐regulation of microglial P2X4 receptors is http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2060&familyId=619&familyType=ENZYME, a member of the Src family of tyrosine kinases involved in microglial activation. In Lyn−/− mice, the up‐regulation of P2X4 receptors following PNI is absent, as is tactile allodynia. The upstream up‐regulation of Lyn is likely to reflect IFN‐γ activation of microglia following PNI (Tsuda et al., 2008, 2009b). The involvement of the chemokine CCL21 as a mediator of increased expression of P2X4 receptors was also suggested. CCL21 is rapidly expressed in nociceptive C‐fibres following injury and transported to their central terminals in the dorsal horn (Biber et al., 2011). CCL21 deficiency prevents subsequent overexpression of P2X4 receptors in spinal cord microglia, while in vitro and in vivo application of CCL21 results in up‐regulation of microglial P2X4 receptors. An additional key step in this now well‐characterized pathway is the link between P2X4 receptor activation and BDNF release from microglia. P2X4 receptor channels are highly permeable to calcium ions (Egan and Khakh, 2004), and P2X4 channel‐dependent calcium entry triggers a biphasic BDNF release, initially from a pre‐existing pool of BDNF followed by de novo synthesis (Trang et al., 2009). This process requires activation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=519, while the release of BDNF is mediated by SNARE‐mediated exocytosis. Activation of p38 MAPK driven by microglial P2X4 receptors was also observed in bone cancer pain models, where BDNF release is accompanied by activation of toll‐like receptor 4 and TNF‐α release from microglia (Jin et al., 2014).
Interestingly, in a model of peripheral inflammatory pain, it was shown that macrophages participate in pain hypersensitivity via a similar yet distinctive mechanism. Up‐regulation of P2X4 receptors is also observed in macrophages under inflammatory conditions, and activation of these receptors by ATP is necessary and sufficient to induce pain hypersensitivity, as the phenotype was absent in P2X4‐null mice. Also, ATP‐primed macrophages injected in the paw could directly lead to hypersensitivity (Ulmann et al., 2010). In this model however, the downstream effector is likely to be the release of the PGE2, driven by P2X4 receptors, via activation of p38 MAPK, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=269 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=275.
An intriguing and novel aspect of the control of P2X4 receptor up‐regulation was brought by a study by Sorge et al. (2012), who demonstrated striking sex differences in the pathogenesis of mechanical allodynia after PNI and during inflammatory pain in mice. While the previously described mechanism of up‐regulation of microglial P2X4 receptors followed by p38 MAPK activation and BDNF release was observed in males, blocking any step of this pathway in females was found ineffective at treating pain. These results indicate that microglial up‐regulation of the P2X4 receptor via an upstream modulator is absent in females. As the transcription factors IRF‐5 and IRF‐8 showed no sex dimorphism, it remains unclear which driver of P2X4 receptor expression is silent in females. While the authors observed no up‐regulation of P2X4 receptors in females following PNI, they did observe microglial activation in the dorsal horn at the same level as in the male counterparts, indicating that the microglial phenotype alone is not a valid marker of pain. This further confirms the specific importance of P2X4 receptors in driving the pain phenotype and the dissociation between expression of P2X4 receptors and microglial activation. Accordingly, in P2X4‐null mice, PNI does not induce allodynia as it does in wild‐type mice; however, the same level of microglial proliferation and activation is observed in both genotypes (Tsuda et al., 2009a).
The numerous investigations performed on the BDNF‐KCC2‐driven neuronal sensitization have uncovered the importance of this pathway in other pain pathologies. Notably, it was shown to be critical in the development of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1627 hyperalgesia, a phenomenon where chronic morphine use induces a long term tolerance to the opioid accompanied by paradoxical pain hypersensitivity (Ferrini et al., 2013). This represents a widespread therapeutic problem since opioids remain the gold standard in chronic pain management. Chronic morphine use induces microglial BDNF release‐dependent down‐regulation of KCC2 and subsequent hyperexcitability of rat lamina 1 neurons. Chronic morphine treatment increases expression of microglial P2X4 receptors, whereas knocking out P2X4 or blocking its function with the antagonist TNP‐ATP prevents the appearance of hyperalgesia. Overexpression of microglial P2X4 receptors depends on http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=319 activation and is a necessary step for morphine‐induced hyperalgesia.
While most pharmacological efforts at controlling pain through its purinergic aspects have been aimed at the P2X3 receptor, several P2X4 receptor antagonists (see Figure 3) also showed analgesic potential. For example, the compound NP‐1815‐PX, a potent and selective P2X4 receptor blocker, shows significant efficacy in the treatment of pain induced by herpes simplex virus (HSV)‐1 inoculation, a model of herpetic pain (Matsumura et al., 2016). Whereas it inhibited the allodynia caused by traumatic nerve damage following HSV‐1 infection, the compound had no effect on acute nociception or motor function. However, it was found ineffective at treating spinal nerve injury‐induced mechanical allodynia. Antidepressants, in particular, the selective 5‐HT reuptake inhibitor http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4790, have also been suggested to possess significant indirect P2X4 receptor antagonism and were effective at treating certain forms of neuropathic pain (Nagata et al., 2009). We know now that researchers using preclinical animal models in the evaluation of the potential of P2X4 receptor blockers as analgesics will have to consider the dimorphism between the P2X4‐dependent pathogenesis observed in males and P2X4‐independent mechanisms at play in females.
Figure 3.
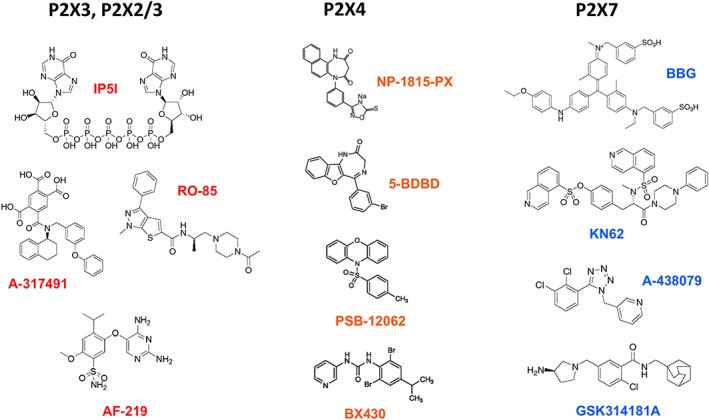
Structures of subtype‐selective P2X receptor antagonists. The compounds IP5I and RO‐85 can discriminate between P2X3 homomers and P2X2/3 heteromers. The orally bioavailable compound AF‐219 is currently tested in Phase III clinical trials for hypersensitized C‐fibre afferents and refractory chronic cough.
P2X7 receptors in immune cells
A more recent player in the field of purinergic signalling in chronic pain is the P2X7 receptor channel, which shows the lowest sensitivity to ATP among the P2X family. This high‐threshold activation gives P2X7 a role in damage‐sensing, only triggering downstream effects when ATP concentration is pathologically elevated. P2X7 ATP receptors in immune cells have long been known for their involvement in peripheral inflammation, where their activation leads to inflammasome recruitment, IL‐1β processing and release, as well as multiple inflammatory cascades. In the CNS, they are mainly expressed in microglia and are thought to perform similar functions by inducing the release of inflammatory cytokines.
Early reports using antagonists (Figure 3) and knockout mice have implicated microglial P2X7 receptors in chronic neuropathic and inflammatory pain through the release of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974 (Dell'Antonio et al., 2002a,b; Chessell et al., 2005), a process that has been reviewed previously (Donnelly‐Roberts and Jarvis, 2007; Giuliani et al., 2017). Notably, the mechanical hyperalgesia observed in rats in inflammatory conditions can be abrogated by oxidized ATP, a known P2X7 receptor antagonist. Using P2X7‐null mice, Chessell et al. (2005) showed that the tactile or thermal hypersensitivity induced by inflammatory adjuvant injection or partial nerve ligation requires P2X7 receptor expression. Specifically, the release of IL‐1β and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4975 was disrupted in P2X7‐null mice. It was later reported that LPS priming of microglia in the spinal cord is necessary for the P2X7/IL‐1β release pathway to take place (Clark et al., 2010). Using antagonists and P2X7 receptor KO mice, they showed that LPS induction of painful symptoms requires P2X7 receptors by engaging a p38 MAPK‐dependent pathway. Furthermore, there is increasing evidence that enhanced release of IL‐1β after activation of P2X7 receptors antagonizes morphine analgesia and accounts for the development of morphine tolerance, which could explain the lack of efficacy of opioids in the treatment of pain in neuropathic patients (Shavit et al., 2005). IL‐1β is known to be a key mediator in neurodegeneration, chronic inflammation and chronic pain (Allan et al., 2005; Dinarello, 2011). It also induces the transcription of COX‐2 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1250, which both play a central role in the generation and maintenance of inflammatory pain (Samad et al., 2001). More recently, the expression of P2X7 receptors was measured in peripheral blood lymphocytes and monocytes, as well as the levels of IL‐1β in patients suffering from chronic nociceptive and neuropathic pain (Luchting et al., 2016). P2X7 receptor mRNA and protein levels were found to be increased on monocytes and lymphocytes of patients with neuropathic pain, but not in patients with nociceptive low back pain. Similarly, IL‐1β concentrations in serum were significantly elevated only in patients with neuropathic pain. While direct release of mature soluble IL‐1β is generally thought to be the main pathway for microglia‐to‐neuron communication, recent reports show that IL‐1β could also be packaged within microvesicles budding from microglia (Li et al., 2017). Following spinal nerve ligation in rats, microglial microvesicles were detected in the CSF, and the associated pain symptoms could be alleviated with shRNA targeted against IL‐1β. These microvesicles, the release of which is dependent on activation of P2X7 receptors and p38 MAPK, can directly sensitize neuronal activity. A strong body of evidence therefore suggests that activation of P2X7 receptors and the subsequent release of IL‐1β represent highly critical steps in chronic pain maintenance.
Up‐regulation of P2X7 receptor expression in microglia in chronic pain conditions, showing many similarities to the involvement of P2X4 receptors, has also been suggested. While the underlying transcriptional control of P2X7 receptors remains to be investigated, evidence shows that these receptors are up‐regulated at both the mRNA and protein level in spinal microglia after peripheral nerve injury (Kobayashi et al., 2011). Up‐regulation of P2X7 receptors was critical for mechanical hypersensitivity as the selective antagonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4118 blocked its development. A similar increase in microglial P2X7 receptors was observed in a model of postsurgical pain, where pre‐surgery i.t. injection of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4147 (BBG), a potent P2X7 receptor blocker, prevented mechanical allodynia (Ying et al., 2014). Interestingly, up‐regulation of microglial P2X7 receptors is also present in models of morphine tolerance, a prevalent problem in pain management (Zhou et al., 2010). Loss of morphine potency, microglial activation and increased P2X7 receptors were all significantly reduced by intrathecal BBG treatment, but only if given before the induction of tolerance.
Two singular characteristics of the P2X7 receptors and their contribution to pain mechanisms were highlighted by the work of Sorge et al. (2012). The first is that P2X7 receptors have two distinct modes of operation. The first mode is to function as a non‐selective ATP‐gated cation channel and as an opener of pannexin hemichannels to form a large pore, permeable to molecules with a mass of up to 900 Da, under prolonged application of ATP (Surprenant et al., 1996; Locovei et al., 2007). The second is that the P2X7 receptor gene shows a high frequency of single‐nucleotide polymorphisms (SNPs). Using genome‐wide linkage analysis, Sorge et al. (2012) showed that variations within the coding sequence of the P2X7 gene affects chronic pain sensitivity in both mice and humans. These genetic variations partly account for the high degree of variability observed in chronic pain symptoms and, importantly for therapeutic purposes, in the response to analgesics. Specifically, they report an association between mechanical allodynia and the P451L SNP, a mutation known to specifically affect pore formation in cell membranes (Adriouch et al., 2002). Mice carrying the P451L mutation display impaired pore formation of the P2X7 channel, with normal non‐selective cation permeability, and less allodynia than mice expressing the pore‐forming P2X7 wild‐type allele. Moreover, in two independent human chronic pain cohorts, one with mastectomy‐induced pain and another with osteoarthritis, a genetic association was observed between lower pain intensity and the hypofunctional His270 allele of P2X7. The findings suggest a specific requirement for large pore formation by P2X7 receptors in the pathophysiology of chronic inflammatory and neuropathic pain.
While the mechanisms underlying the relevance of the large pore formation in P2X7 receptors to pain remain unknown, P2X7 gene polymorphisms appear to be linked to numerous diseases (Caseley et al., 2014) and studies have followed on their importance in chronic pain. In patients with diabetic neuropathy, the gain‐of‐function His155Tyr and Ala348Thr SNPs are associated with an increased pain score (Ursu et al., 2014). Interestingly, this was shown in female, but not in male patients, suggesting a sex‐specific mechanism for this involvement of P2X7 receptors in pain. Whether this gender effect relies on the recruitment of different immune cells as the main effector cell types, as it has been suggested for P2X4 receptors in microglia, as distinct from T‐cells, will remain to be demonstrated. In another report on P2X7 receptor polymorphisms, specific P2X7 haplotypes in humans were linked to different responses to cold pain as well as to differing analgesic effects of the opioid fentanyl (Ide et al., 2014).
There is a growing focus on the involvement of P2X7 receptors in bone cancer pain. About 90% of advanced bone cancer patients must cope with chronic pain syndromes related to tumour growth (Mantyh, 2006; Colvin and Fallon, 2008). In a rodent model of bone cancer pain, microglia activation seems to arise later than in inflammation or neuropathic injury, suggesting a difference in pain induction mechanisms (Yang et al., 2015). Furthermore, minocycline blockade of microglial activation is effective at reducing pain at later disease stages, but ineffective at preventing its development. In this paradigm, spinal microglial P2X7 receptors, phosphorylated p38 MAPK and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4983 levels were all increased, while spinal inhibition of the P2X7/p38/IL‐18 pathway reduced pain at advanced stages of bone cancer. Direct antagonism of P2X7 receptors with A839977 was also successfully used in another study investigating bone cancer pain models (Falk et al., 2015). However, another study demonstrated that P2X7 receptor‐deficient mice were more susceptible to bone cancer pain (Hansen et al., 2011). The discrepancy is likely to be due to the use of a mouse line that still expresses the P2X7(k) splice variant (Nicke et al., 2009).
Given the numerous similarities between P2X4 and P2X7 receptors with regard to their role in neuropathic pain, it is interesting to evaluate the possible interactions between these two purinergic subtypes. First reported to form functional P2X4/7 heteromers in heterologous expression systems (Guo et al., 2007), the two proteins were also shown to co‐precipitate in macrophages (Perez‐Flores et al., 2015). As both receptors are up‐regulated in microglia and involved in chronic pain conditions, the presence of a functional P2X4/7 heteromer, or a reciprocal regulation between P2X4 and P2X7 homomers would greatly affect how purinergic stimulation drives nociceptive hyperexcitability. For instance, P2X4 and P2X7 units can interact via the long intracellular C‐terminal domain of the P2X7 unit and, further, knocking out the P2X4 protein disrupts P2X7‐induced cell death and IL‐1β release from macrophages (Perez‐Flores et al., 2015). Whether heteromeric P2X4/7 ATP receptors play a critical role in pathological pain, for example in activated microglia‐dependent BDNF or IL‐1β release, remains to be assessed.
While most studies have looked at the involvement of P2X7 receptors in pain through their expression on microglia and other immune cells, roles of P2X7 receptors in pain, independent of immune cells, have also been suggested. In satellite glial cells surrounding sensory neurons in DRGs, an analgesic role for P2X7 receptors was postulated based on evidence that activity of these receptors in satellite glial cell induces ATP release through pannexin‐1 hemichannels. Subsequent activation of neuronal P2Y1 receptors then drives P2X3 down‐regulation via p38 MAPK activation (Chen et al., 2008). This could be particularly important in aged animals, where levels of P2X7 receptors are relatively high compared with the neuronal P2X3 receptors. The functional link between P2X7 receptors and pannexin hemichannels has been shown to be critical in the induction of gliogenic LTP in pain circuits in pathological conditions (Kronschlager et al., 2016). Following high‐frequency stimulation and ATP release from C‐fibres, the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75 agonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4171 is released from glia through P2X7 receptor‐coupled pannexin‐1 hemichannels. d‐serine then diffuses in the dorsal horn of spinal cord where it induces homosynaptic and heterosynaptic LTP at nociceptive synapses. Interestingly, this pathological neuron–glia crosstalk requires the combined activation of microglial and astrocytic P2X7 receptors.
Conclusions and challenges
A large body of evidence has now fully established that ionotropic ATP signalling contributes to pain mechanisms in physiological and pathological contexts through the engagement of P2X3, P2X4 and P2X7 ATP receptors. These P2X receptor subtypes have the appropriate biophysical properties and the appropriate cellular expression to justify their prioritization as potential targets for novel pharmacological approaches to analgesia. Like other ion channels, P2X receptor channels are not easy targets for drug development, and we have identified a number of key challenges. These include species differences in pharmacology that hinders preclinical validation, unclear distribution and subunit composition of P2X receptors in human tissues in health and disease, poor selectivity of antagonists for homomeric against heteromeric P2X receptor complexes and its physiological consequences, genetic polymorphisms affecting channel function and gender differences in P2X receptor involvement in pain pathways. Nevertheless, several P2X receptor ligands have successfully reached Phase II or Phase III clinical trials, including a P2X3 receptor antagonist (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9540, Figure 3) that shows a promising therapeutic potential in the treatment of hypersensitized sensory afferents in chronic cough and possibly chronic visceral pain (Abdulqawi et al., 2015).
Recent details on the protein structure of human P2X3 receptors (Mansoor et al., 2016) and P2X4 receptor orthologues (Kawate et al., 2009; Hattori and Gouaux, 2012) at the atomic level are a boon for drug designers, and we can predict with confidence that more P2X receptor structures, including that for the P2X7 receptor, will be available in the near future. This will improve our capacity to identify potent subtype‐selective antagonists for preclinical validation in rodent and primate models. It is only a matter of time before some compounds display the right properties for good oral bioavailability and translation to first‐in‐class P2X receptor analgesics, in the treatment of chronic pain without the side effects of opiates.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c,d,e).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
L.‐P.B. is funded through a CIHR Banting Fellowship and the Michael Smith Foundation for Health Research. A.R.A. and P.S. acknowledge the support of CIHR and NSERC.
Bernier, L.‐P. , Ase, A. R. , and Séguéla, P. (2018) P2X receptor channels in chronic pain pathways. British Journal of Pharmacology, 175: 2219–2230. doi: 10.1111/bph.13957.
References
- Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP et al (2015). P2X3 receptor antagonist (AF‐219) in refractory chronic cough: a randomised, double‐blind, placebo‐controlled phase 2 study. Lancet 385: 1198–1205. [DOI] [PubMed] [Google Scholar]
- Adriouch S, Dox C, Welge V, Seman M, Koch‐Nolte F, Haag F (2002). Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol 169: 4108–4112. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ (2005). Interleukin‐1 and neuronal injury. Nat Rev Immunol 5: 629–640. [DOI] [PubMed] [Google Scholar]
- Bele T, Fabbretti E (2016). The scaffold protein calcium/calmodulin‐dependent serine protein kinase controls ATP release in sensory ganglia upon P2X3 receptor activation and is part of an ATP keeper complex. J Neurochem 138: 587–597. [DOI] [PubMed] [Google Scholar]
- Bernier LP, Ase AR, Boué‐Grabot E, Seguela P (2013a). Inhibition of P2X4 function by P2Y6 UDP receptors in microglia. Glia 61: 2038–2049. [DOI] [PubMed] [Google Scholar]
- Bernier LP, Ase AR, Chevallier S, Blais D, Zhao Q, Boue‐Grabot E et al (2008). Phosphoinositides regulate P2X4 ATP‐gated channels through direct interactions. J Neurosci 28: 12938–12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier LP, Ase AR, Séguéla P (2013b). Post‐translational regulation of P2X receptor channels: modulation by phospholipids. Front Cell Neurosci 7: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K, Tsuda M, Tozaki‐Saitoh H, Tsukamoto K, Toyomitsu E, Masuda T et al (2011). Neuronal CCL21 up‐regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J 30: 1864–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G (2016). Purinergic mechanisms and pain. Adv Pharmacol 75: 91–137. [DOI] [PubMed] [Google Scholar]
- Caseley EA, Muench SP, Roger S, Mao HJ, Baldwin SA, Jiang LH (2014). Non‐synonymous single nucleotide polymorphisms in the P2X receptor genes: association with diseases, impact on receptor functions and potential use as diagnosis biomarkers. Int J Mol Sci 15: 13344–13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang X, Wang C, Li G, Gu Y, Huang LY (2008). Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci U S A 105: 16773–16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P et al (2005). Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114: 386–396. [DOI] [PubMed] [Google Scholar]
- Clark AK, Staniland AA, Marchand F, Kaan TK, McMahon SB, Malcangio M (2010). P2X7‐dependent release of interleukin‐1beta and nociception in the spinal cord following lipopolysaccharide. J Neurosci 30: 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin L, Fallon M (2008). Challenges in cancer pain management – bone pain. Eur J Cancer 44: 1083–1090. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K et al (2005). BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438: 1017–1021. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A et al (2003). Trans‐synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424: 938–942. [DOI] [PubMed] [Google Scholar]
- Dell'Antonio G, Quattrini A, Cin ED, Fulgenzi A, Ferrero ME (2002a). Relief of inflammatory pain in rats by local use of the selective P2X7 ATP receptor inhibitor, oxidized ATP. Arthritis Rheum 46: 3378–3385. [DOI] [PubMed] [Google Scholar]
- Dell'Antonio G, Quattrini A, Dal Cin E, Fulgenzi A, Ferrero ME (2002b). Antinociceptive effect of a new P(2Z)/P2X7 antagonist, oxidized ATP, in arthritic rats. Neurosci Lett 327: 87–90. [DOI] [PubMed] [Google Scholar]
- Dinarello CA (2011). Interleukin‐1 in the pathogenesis and treatment of inflammatory diseases. Blood 117: 3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly‐Roberts DL, Jarvis MF (2007). Discovery of P2X7 receptor‐selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol 151: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan TM, Khakh BS (2004). Contribution of calcium ions to P2X channel responses. J Neurosci 24: 3413–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbretti E, D'arco M, Fabbro A, Simonetti M, Nistri A, Giniatullin R (2006). Delayed upregulation of ATP P2X3 receptors of trigeminal sensory neurons by calcitonin gene‐related peptide. J Neurosci 26: 6163–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk S, Schwab SD, Frosig‐Jorgensen M, Clausen RP, Dickenson AH, Heegaard AM (2015). P2X7 receptor‐mediated analgesia in cancer‐induced bone pain. Neuroscience 291: 93–105. [DOI] [PubMed] [Google Scholar]
- Ferrini F, Trang T, Mattioli TA, Laffray S, Del'guidice T, Lorenzo LE et al (2013). Morphine hyperalgesia gated through microglia‐mediated disruption of neuronal Cl(−) homeostasis. Nat Neurosci 16: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerevich Z, Muller C, Illes P (2005). Metabotropic P2Y1 receptors inhibit P2X3 receptor‐channels in rat dorsal root ganglion neurons. Eur J Pharmacol 521: 34–38. [DOI] [PubMed] [Google Scholar]
- Giuliani AL, Sarti AC, Falzoni S, Di Virgilio F (2017). The P2X7 receptor‐interleukin‐1 liaison. Front Pharmacol 8: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanasekaran A, Sundukova M, Van Den Maagdenberg AM, Fabbretti E, Nistri A (2011). Lipid rafts control P2X3 receptor distribution and function in trigeminal sensory neurons of a transgenic migraine mouse model. Mol Pain 7: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Li G, Chen Y, Huang LY (2016a). Epac‐protein kinase C alpha signaling in purinergic P2X3R‐mediated hyperalgesia after inflammation. Pain 157: 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Wang C, Li G, Huang LY (2016b). EXPRESS: F‐actin links Epac‐PKC signaling to purinergic P2X3 receptors sensitization in dorsal root ganglia following inflammation. Mol Pain 12 https://doi.org/10.1177/1744806916660557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Masin M, Qureshi OS, Murrell‐Lagnado RD (2007). Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol 72: 1447–1456. [DOI] [PubMed] [Google Scholar]
- Hansen RR, Nasser A, Falk S, Baldvinsson SB, Ohlsson PH, Bahl JM et al (2012). Chronic administration of the selective P2X3, P2X2/3 receptor antagonist, A‐317491, transiently attenuates cancer‐induced bone pain in mice. Eur J Pharmacol 688: 27–34. [DOI] [PubMed] [Google Scholar]
- Hansen RR, Nielsen CK, Nasser A, Thomsen SI, Eghorn LF, Pham Y et al (2011). P2X7 receptor‐deficient mice are susceptible to bone cancer pain. Pain 152: 1766–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Gouaux E (2012). Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 485: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide S, Nishizawa D, Fukuda K, Kasai S, Hasegawa J, Hayashida M et al (2014). Haplotypes of P2RX7 gene polymorphisms are associated with both cold pain sensitivity and analgesic effect of fentanyl. Mol Pain 10: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin XH, Wang LN, Zuo JL, Yang JP, Liu SL (2014). P2X4 receptor in the dorsal horn partially contributes to brain‐derived neurotrophic factor oversecretion and toll‐like receptor‐4 receptor activation associated with bone cancer pain. J Neurosci Res 92: 1690–1702. [DOI] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E (2009). Crystal structure of the ATP‐gated P2X(4) ion channel in the closed state. Nature 460: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A et al (2006). Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol 498: 443–454. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi E, Miyagawa Y, Yamanaka H, Noguchi K (2011). Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model. Neurosci Lett 504: 57–61. [DOI] [PubMed] [Google Scholar]
- Kronschlager MT, Drdla‐Schutting R, Gassner M, Honsek SD, Teuchmann HL, Sandkuhler J (2016). Gliogenic LTP spreads widely in nociceptive pathways. Science 354: 1144–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li X, Jiang X, Yang M, Yang R, Burnstock G et al (2017). Microvesicles shed from microglia activated by the P2X7‐p38 pathway are involved in neuropathic pain induced by spinal nerve ligation in rats. Purinergic Signal 13: 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Huang W, Wu D, Priestley JV (2006). TRPV1, but not P2X, requires cholesterol for its function and membrane expression in rat nociceptors. Eur J Neurosci 24: 1–6. [DOI] [PubMed] [Google Scholar]
- Locovei S, Scemes E, Qiu F, Spray DC, Dahl G (2007). Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett 581: 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchting B, Heyn J, Woehrle T, Rachinger‐Adam B, Kreth S, Hinske LC et al (2016). Differential expression of P2X7 receptor and IL‐1beta in nociceptive and neuropathic pain. J Neuroinflammation 13: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor SE, Lu W, Oosterheert W, Shekhar M, Tajkhorshid E, Gouaux E (2016). X‐ray structures define human P2X(3) receptor gating cycle and antagonist action. Nature 538: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW (2006). Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci 7: 797–809. [DOI] [PubMed] [Google Scholar]
- Marchenkova A, Vilotti S, Fabbretti E, Nistri A (2015). Brain natriuretic peptide constitutively downregulates P2X3 receptors by controlling their phosphorylation state and membrane localization. Mol Pain 11: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenkova A, Vilotti S, Ntamati N, Van Den Maagdenberg AM, Nistri A (2016). Inefficient constitutive inhibition of P2X3 receptors by brain natriuretic peptide system contributes to sensitization of trigeminal sensory neurons in a genetic mouse model of familial hemiplegic migraine. Mol Pain 12 https://doi.org/10.1177/1744806916646110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Iwamoto S, Yoshinaga R, Tozaki‐Saitoh H, Nishiyama A, Mak TW et al (2014). Transcription factor IRF5 drives P2X4R+‐reactive microglia gating neuropathic pain. Nat Commun 5: 3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Tsuda M, Yoshinaga R, Tozaki‐Saitoh H, Ozato K, Tamura T et al (2012). IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep 1: 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y, Yamashita T, Sasaki A, Nakata E, Kohno K, Masuda T et al (2016). A novel P2X4 receptor‐selective antagonist produces anti‐allodynic effect in a mouse model of herpetic pain. Sci Rep 6: 32461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo G, Bernier LP, Zhao Q, Chabot‐Dore AJ, Ase AR, Logothetis D et al (2009). Subtype‐specific regulation of P2X3 and P2X2/3 receptors by phosphoinositides in peripheral nociceptors. Mol Pain 5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo G, Peleshok JC, Cao CQ, Ribeiro‐DA‐Silva A, Séguéla P (2013). Control of P2X3 channel function by metabotropic P2Y2 utp receptors in primary sensory neurons. Mol Pharmacol 83: 640–647. [DOI] [PubMed] [Google Scholar]
- Nagata K, Imai T, Yamashita T, Tsuda M, Tozaki‐Saitoh H, Inoue K (2009). Antidepressants inhibit P2X4 receptor function: a possible involvement in neuropathic pain relief. Mol Pain 5: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicke A, Kuan YH, Masin M, Rettinger J, Marquez‐Klaka B, Bender O et al (2009). A functional P2X7 splice variant with an alternative transmembrane domain 1 escapes gene inactivation in P2X7 knock‐out mice. J Biol Chem 284: 25813–25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA (2016). P2X receptors. Philos Trans R Soc Lond B Biol Sci 371 https://doi.org/10.1098/rstb.2015.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Flores G, Levesque SA, Pacheco J, Vaca L, Lacroix S, Perez‐Cornejo P et al (2015). The P2X7/P2X4 interaction shapes the purinergic response in murine macrophages. Biochem Biophys Res Commun 467: 484–490. [DOI] [PubMed] [Google Scholar]
- Prado FC, Araldi D, Vieira AS, Oliveira‐Fusaro MC, Tambeli CH, Parada CA (2013). Neuronal P2X3 receptor activation is essential to the hyperalgesia induced by prostaglandins and sympathomimetic amines released during inflammation. Neuropharmacology 67: 252–258. [DOI] [PubMed] [Google Scholar]
- Saloman JL, Chung MK, Ro JY (2013). P2X(3) and TRPV1 functionally interact and mediate sensitization of trigeminal sensory neurons. Neuroscience 232: 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S et al (2001). Interleukin‐1beta‐mediated induction of Cox‐2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 410: 471–475. [DOI] [PubMed] [Google Scholar]
- Schiavuzzo JG, Teixeira JM, Melo B, Da Silva Dos Santos DF, Jorge CO, Oliveira‐Fusaro MC et al (2015). Muscle hyperalgesia induced by peripheral P2X3 receptors is modulated by inflammatory mediators. Neuroscience 285: 24–33. [DOI] [PubMed] [Google Scholar]
- Serrano A, Mo G, Grant R, Pare M, O'donnell D, Yu XH et al (2012). Differential expression and pharmacology of native P2X receptors in rat and primate sensory neurons. J Neurosci 32: 11890–11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavit Y, Wolf G, Goshen I, Livshits D, Yirmiya R (2005). Interleukin‐1 antagonizes morphine analgesia and underlies morphine tolerance. Pain 115: 50–59. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J et al (2012). Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med 18: 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G (1996). The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272: 735–738. [DOI] [PubMed] [Google Scholar]
- Szoke E, Borzsei R, Toth DM, Lengl O, Helyes Z, Sandor Z et al (2010). Effect of lipid raft disruption on TRPV1 receptor activation of trigeminal sensory neurons and transfected cell line. Eur J Pharmacol 628: 67–74. [DOI] [PubMed] [Google Scholar]
- Trang T, Beggs S, Wan X, Salter MW (2009). P2X4‐receptor‐mediated synthesis and release of brain‐derived neurotrophic factor in microglia is dependent on calcium and p38‐mitogen‐activated protein kinase activation. J Neurosci 29: 3518–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Salter MW (2012). P2X4 purinoceptor signaling in chronic pain. Purinergic Signal 8: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki‐Saitoh H, Inoue K (2009a). Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain 5: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki‐Saitoh H, Inoue K (2009b). IFN‐gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci U S A 106: 8032–8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Masuda T, Tozaki‐Saitoh H, Inoue K (2013). P2X4 receptors and neuropathic pain. Front Cell Neurosci 7: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto‐Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW et al (2003). P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424: 778–783. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Tozaki‐Saitoh H, Masuda T, Toyomitsu E, Tezuka T, Yamamoto T et al (2008). Lyn tyrosine kinase is required for P2X(4) receptor upregulation and neuropathic pain after peripheral nerve injury. Glia 56: 50–58. [DOI] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F et al (2008). Up‐regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 28: 11263–11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann L, Hirbec H, Rassendren F (2010). P2X4 receptors mediate PGE2 release by tissue‐resident macrophages and initiate inflammatory pain. EMBO J 29: 2290–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu D, Ebert P, Langron E, Ruble C, Munsie L, Zou W et al (2014). Gain and loss of function of P2X7 receptors: mechanisms, pharmacology and relevance to diabetic neuropathic pain. Mol Pain 10: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca F, Amadio S, Sancesario G, Bernardi G, Volonte C (2004). P2X3 receptor localizes into lipid rafts in neuronal cells. J Neurosci Res 76: 653–661. [DOI] [PubMed] [Google Scholar]
- Wang C, Li GW, Huang LY (2007). Prostaglandin E2 potentiation of P2X3 receptor mediated currents in dorsal root ganglion neurons. Mol Pain 3: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Dai Y, Kobayashi K, Zhu W, Kogure Y, Yamanaka H et al (2012). Potentiation of the P2X3 ATP receptor by PAR‐2 in rat dorsal root ganglia neurons, through protein kinase‐dependent mechanisms, contributes to inflammatory pain. Eur J Neurosci 36: 2293–2301. [DOI] [PubMed] [Google Scholar]
- Wieskopf JS, Mathur J, Limapichat W, Post MR, Al‐Qazzaz M, Sorge RE et al (2015). The nicotinic alpha6 subunit gene determines variability in chronic pain sensitivity via cross‐inhibition of P2X2/3 receptors. Sci Transl Med 7: 287ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JX, Xu MY, Miao XR, Lu ZJ, Yuan XM, Li XQ et al (2012). Functional up‐regulation of P2X3 receptors in dorsal root ganglion in a rat model of bone cancer pain. Eur J Pain 16: 1378–1388. [DOI] [PubMed] [Google Scholar]
- Wu JX, Yuan XM, Wang Q, Wei W, Xu MY (2016). Rho/ROCK acts downstream of lysophosphatidic acid receptor 1 in modulating P2X3 receptor‐mediated bone cancer pain in rats. Mol Pain 12 https://doi.org/10.1177/1744806916644929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li H, Li TT, Luo H, Gu XY, Lu N et al (2015). Delayed activation of spinal microglia contributes to the maintenance of bone cancer pain in female Wistar rats via P2X7 receptor and IL‐18. J Neurosci 35: 7950–7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying YL, Wei XH, Xu XB, She SZ, Zhou LJ, Lv J et al (2014). Over‐expression of P2X7 receptors in spinal glial cells contributes to the development of chronic postsurgical pain induced by skin/muscle incision and retraction (SMIR) in rats. Exp Neurol 261: 836–843. [DOI] [PubMed] [Google Scholar]
- Zhang C, Medzihradszky KF, Sanchez EE, Basbaum AI, Julius D (2017). Lys49 myotoxin from the Brazilian lancehead pit viper elicits pain through regulated ATP release. Proc Natl Acad Sci U S A 114: E2524–E2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Yang M, Ting AT, Logothetis DE (2007). PIP(2) regulates the ionic current of P2X receptors and P2X(7) receptor‐mediated cell death. Channels (Austin) 1: 46–55. [PubMed] [Google Scholar]
- Zhou D, Chen ML, Zhang YQ, Zhao ZQ (2010). Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats. J Neurosci 30: 8042–8047. [DOI] [PMC free article] [PubMed] [Google Scholar]


