Abstract
The essay reviews John Kendrew's pioneering work on the structure of myoglobin for which he shared the Nobel Prize for Chemistry in 1962. It reconstructs the status of protein X‐ray crystallography at the time Kendrew entered the field in 1945, after distinctive service in operational research during the war. It reflects on the choice of sperm whale myoglobin as research material. In particular, it highlights Kendrew's early use of digital electronic computers for crystallographic computations and the marshaling of other tools and approaches that made it possible to solve the structure at increasing resolution. The essay further discusses the role of models in structure resolution and their broader reception. It ends by briefly reviewing Kendrew's other contributions in the formation and institutionalization of molecular biology.
Keywords: John Kendrew, myoglobin, protein structure, X‐ray crystallography; EDSAC; molecular biology
Quite exactly 60 years ago, an article in Nature presented the first low‐resolution model of a globular protein derived by direct structure determination. The work was part of an ongoing effort by John Kendrew and his colleagues at the Medical Research Council Unit for Molecular Biology in Cambridge (UK) that two years later led to the publication of the first atomic structure of the same protein, myoglobin.1, 2 In 1962, John Kendrew together with Max Perutz, working in the same laboratory, received the Nobel Prize for Chemistry for their studies of the structures of globular proteins. This essay retraces the work that led to this extraordinary achievement. It centers on the contribution of John Kendrew although, as is well known, every result in science relies on the work of many people that contribute to it in myriads of ways. It reconstructs how Kendrew took up the challenge of protein X‐ray crystallography, a field then still in its infancy. It recounts how Kendrew settled on sperm whale myoglobin as the best source for growing crystals, his early use of electronic computers and other resources he marshaled to achieve the atomic resolution of the protein structure. It discusses the role of models in reaching the result and their reception. The essay ends with a short outlook of Kendrew's career after his work on myoglobin and his other contributions to the establishment of the new science of molecular biology.
Protein X‐Ray Crystallography
According to Kendrew's own account, it was the crystallographer John Desmond Bernal who “in a jungle in Ceylon” (now Sri Lanka) in 1944, while both were working as scientific advisors to the allied air commander in chief Lord Mountbatton, convinced him that it should be possible to use X‐ray diffraction to solve the structure of proteins and that this was a most worthwhile problem to embark on.3 To fill in the necessary background here, Kendrew had a BA in Chemistry from Trinity College in Cambridge and had just started a PhD in physical chemistry on reaction kinetics, when war broke out. As many science graduates of his generation, he became engaged as scientist in the war effort. He first worked on radar research and then joined operational research, a field then in still in formation to which he remained engaged for the duration of the war, taking on increasing responsibilities. It was in this capacity that he had been called to serve in the South Pacific. With the end of the war in sight, Kendrew was looking for a meaningful project to go back to research rather than simply picking up where he had left before the war. A conversation with Linus Pauling, the distinguished physical chemist at Caltech, whom he had occasion to meet on a mission‐related stopover in California on his eastern route back to Europe in spring 1945, confirmed that protein crystallography would be a challenging and worthwhile field to enter.
Despite these undoubtedly decisive encounters, Kendrew seriously contemplated a career in the Scientific Civil Service. With others, he was convinced that science had an important role to play in postwar reconstruction and felt that scientists who, like him, had gained “experience of administration and of working alongside Government” had the “strongest moral obligation” to remain in government service. However, after some hesitation, he decided to return to academia, at least temporarily and for as long as the career chances in the Civil Service were not more clearly defined.4, 5 Helping this decision was his discovery that he could pick up his Trinity fellowship that he had interrupted for war service.
Kendrew came to Cambridge in late 1945 and—again on Bernal's advice—joined the Cavendish where there was a small group, mainly consisting of Max Perutz, working under the tutelage of Lawrence H. Bragg, the Director of the Cavendish Laboratory, that was engaged in applying X‐ray crystallography to determine the structure of proteins. Kendrew's official supervisor was William Taylor, head of Crystallography at the Cavendish. Taylor was working on the structure of silicates. Like most professional crystallographers, he regarded protein crystallography as a hopeless undertaking and a “waste of time” but accepted the formal agreement regarding Kendrew.6
Mad Pursuit
What was the status of protein X‐ray crystallography when Kendrew joined the field? Despite Oswald Avery's experiments in 1944 that proved that DNA carried the genetic information, proteins were considered to be the most important molecules to understand life. Protein crystallography was just one of many approaches employed to study proteins. It was carried by the idea that knowing the structure was a way to understand function. The question was how to interpret the complex diffraction images. In the mid‐1940s, the hope to make some headway was very much based on the expectation that there was a general plan of protein structure and that the solution of one protein would give clues to the structure of proteins more generally. As became increasingly clear, this was a wrong expectation. Yet without this expectation, the problem would have seemed so hopeless that nobody would have taken up the challenge. As Bragg saw it, it was a “false star” but a helpful star nevertheless.7
Yet this disillusionment came later. Kendrew first collaborated with Perutz on the structure analysis of hemoglobin, embarking on a comparison of fetal and adult hemoglobin. This work gained him his PhD in 1949. From the very beginning, Kendrew also attempted the crystal analysis of myoglobin, the protein responsible for oxygen storage in muscle. Myoglobin was only a quarter of the size of hemoglobin and for this reason a better molecule for a first attempt to establish the molecular structure of a protein. Nevertheless, the project was long hampered by the difficulty of growing crystals of a size suitable for X‐ray analysis. Kendrew first tried to work with myglobin from the heart muscle of the horse but eventually found that diving animals had more myoglobin. He tried to grow crystals from porpoise, seal, dolphin, penguin, tortoise, and carp before finally discovering that myoglobin from sperm whale meat, a stock of which was stored in the Low Temperature Research Station at Cambridge, grew beautiful crystals.1 A letter Kendrew wrote to Hugh Huxley, his former graduate student, captured the excitement about the achievement after a seven‐year trial: “… 3 weeks ago we got the most marvellous myoglobin crystals, from sperm whale of all places. They are gigantic crystals which give excellent precession pictures in 12 h, and, best of all, they are P21 with 2 molecules in the cell. The first Pattersons are very promising indeed. So, we are going hell for leather for the three‐dimensional.”8
Soon thereafter, Perutz proved that the isomorphic replacement method could be applied to the structural analysis of crystalline proteins.9 The method was already well known but it was not clear if the changes in intensity produced by the heavy atoms in the big molecule were strong enough that they could be used to calculate the phases. After much toil, Kendrew and his collaborators managed to attach heavy atoms at five distinct sites of the molecule. With this, the way was open to pursue the 3D structure (Figs. 1 and 2).
Figure 1.

Crystals of native and heavy metal derivates of myoglobin used for X‐ray analysis. Each of the 100 or so crystals shown in the figure is mounted in a thin‐walled glass tube about 1 mm in diameter. The tubes are sealed at each end to preserve humidity and one end is enclosed in a “lump” of modeling clay that was used to align the crystal prior to X‐ray data collection. Photograph by Bror Strandberg. Courtesy of Bror Strandberg and Medical Research Council Laboratory of Molecular Biology.
Figure 2.
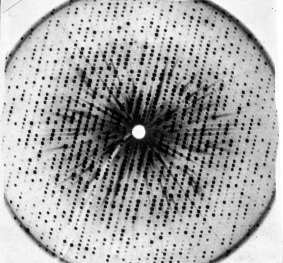
X‐ray photograph of a myoglobin crystal (late 1950s). Photograph by John Kendrew. Courtesy of Medical Research Council Laboratory of Molecular Biology.
Managing Data
Solving the structure remained a daunting enterprise, especially because of the many measurements and calculations this involved. Kendrew seemed to have prepared for this, having acquainted himself with the experimental electronic digital computer, the EDSAC (Electronic Delay Storage Automatic Calculator) that was being built at the Mathematical Laboratory at Cambridge.
The first contact to the new machine was created by Huxley, Kendrew's first research student. Shortly after his arrival in 1949, Bragg, who closely followed the protein crystallography work in the Cavendish, suggested to him that it would be “good for [his] soul” to calculate a two‐dimensional Patterson projection of one of the hemoglobin forms. Huxley remembered: “This was a terrible chore with Beevers‐Lipson strips and an adding machine cranked by hand. It took me about two weeks of solid work with much loud complaining.”10 One of the people Huxley complained to was John Bennett, his friend and fellow research student at Christ's College. Bennett, an engineer‐mathematician from Australia, was part of Maurice Wilkes’ team at the Mathematical Laboratory working on the pioneering electronic computer with a stored memory program that had just started operating. He “immediately” realized that the calculations Huxley was complaining about were programmable and managed to produce a program which could do Huxley's two weeks’ work in about half an hour. Another half an hour was needed to print out the results (Fig. 3).10 Already determined to switch from crystallography to electron microscopy to study muscle fibers, Huxley handed the results over to Kendrew. With Bennett's help, Kendrew learnt programming himself and, together, they perfected the program. In 1952, they published a joint paper on their work. It was the first paper written on crystallographic computations on electronic digital computers.11 From then on, Kendrew became a devoted EDSAC user.
Figure 3.

Patterson projection of electron density for whale myoglobin printed on EDSAC 1 (c. 1951). From J. M. Wheeler, IEEE Annals of the History of Computing 14. n. 4 (1992), 31, figure 1. Copyright 1992 by the Institute of Electrical and Electronic Engineers, Inc. Reproduced with permission.
Kendrew's move to using EDSAC was not an obvious step as can be gauged by the fact that Perutz, who was working on the much more complex molecule, did not “trust” the electronic computer and continued doing his calculations on the Hollerith machine, when Kendrew was already routinely using EDSAC.6 Also Bragg was not at once convinced of the necessity to move to electronic computing.12 EDSAC was faster. Yet there were drawbacks. EDSAC was an experimental machine and constant tests had to be run to achieve reliable results. Working with it required considerable technical skills. The machine tended to break down and users needed to know how to test and replace tubes, replace chassis and lift and test diodes. Another serious limitation of EDSAC was its storage capacity. Even the planned doubling of the EDSAC storage positions from 512 (little over 1 Kbyte) to 1024 was not sufficient to calculate a three‐dimensional synthesis without breaking it into parts.13
What then inclined Kendrew to spend so much of his time on a new calculating device that still had to prove its viability, at a point where his research did not really require it? Kendrew, I would like to propose, from the beginning viewed protein structure analysis as a huge data‐handling problem and approached it in operational terms by assessing the efficiency of the single steps and devices involved as well as of the operation as a whole. This general attitude came from his wartime experience with Operational Research and also determined his approach to the new electronic computer. In her informed guide to Kendrew's archival papers, Jeannine Alton confirms: “From the first, and long before the choice of a suitable material had been achieved, Kendrew realized how important a factor would be the rapid handling of very large data and information. It is interesting to see the fascination with note‐keeping, filing and organization present in the schoolboy and fostered by operational research in war finding a kind of bureaucratic apotheosis in the sustained effort of accuracy required for the long haul to the final successful three‐dimensional picture.”14
The storage of data and their retrieval and display most occupied Kendrew in his work with EDSAC.
Again, EDSAC may not have been indispensable for the crystallographic calculations Kendrew was performing around 1950, when he first got interested in the new device, but increasingly it became so. Kendrew decided to start with a resolution of 6 Å, which he expected to be sufficient to show the general layout of the molecule. From there the analysis jumped to a resolution of 2 and then 1.4 Å. This meant moving from an analysis of around 400 reflections to one including 10,000 and then 25,000 reflections per heavy atom derivative. By 1962, Kendrew and his collaborators had examined 110 myoglobin derivatives and measured the intensities of about 250,000 X‐ray reflections.15 This expansion of the work could only be contemplated using the computer. As Kendrew made clear: “Without that computer we would certainly have been simply unable, I mean, the amount of calculation by hand, it would have been impossible, even if you had the money to hire 20, 50 people” (Fig. 4).16
Figure 4.

Hand‐sorted data of the myoglobin calculations on EDSAC 2 are carried over by postdoctoral researchers Bror Strandberg (rear) and Richard Dickerson (front) from the Mathematical Laboratory to the MRC unit. Photograph by Bror Strandberg. Courtesy of Bror Strandberg and Medical Research Council Laboratory of Molecular Biology.
Kendrew's focus on efficiency and accuracy manifested itself in other parts of the project. When Kendrew entered the field, diffraction patterns were recorded on photographic films and the intensities of the diffraction spots were estimated by comparison with a reference scale. Seeing a colleague in a cell biology laboratory in London using a self‐built densitometer to measure the density of cell sections, Kendrew at once realized that the same device could be used to measure diffraction spots. Although the peaks of the trace the instruments recorded still had to be measured by hand, it nevertheless represented a substantial improvement, both in terms of speed and accuracy, in comparison to the eye estimation method used before. Kendrew arranged to use the instrument after hours, traveling to London to measure his pictures, until a commercial model became available and the Cambridge group acquired its own densitometer. It became the standard equipment to collect data until automated diffractometers came into use in the 1960s.
When the myoglobin work was gearing up and “computers” (mostly young women hired straight out of local high schools, also known as “computer girls”) were hired to do the measurements, Kendrew introduced a routine to secure that the work was done accurately. At weekends, he took the pack of new measurements home and checked their accuracy according to symmetry considerations displayed in the pictures. If there was a 5% error rate, he discarded the whole data set. If the error occurred again, the “computer” was replaced.6, 16
There was another aspect of the work that Kendrew addressed early on. In the mid‐1950s, as early as Fred Sanger published the first complete sequence of insulin, Kendrew started searching intensively for someone who would undertake a sequence analysis of myoglobin. Advances in protein sequencing threatened to render protein crystallography obsolete as it was believed that knowledge of the sequence alone would make it possible to predict the 3D‐structure of a protein.15 In characteristic way, Kendrew confronted the problem straight on and set out to compare the efficiency of the two approaches. Eventually, Allen Edmundson, a research student of William Stein and Stanford Moore at the Rockefeller Institute of New York, responded to his call and later moved to Cambridge with his automatic amino acid analyzer in tow. As it turned out, the sequence analysis of myoglobin posed unexpected problems, while the high‐resolution map of myoglobin yielded more information than expected. Nevertheless, the sequence data provided crucial confirmation for the atomic structure of myoglobin.17 Protein crystallographers became the biggest clients for protein sequences and the early atomic structure analyses all relied on sequence information to help with the interpretation of the electron density maps.18
It was Kendrew's constant aim for utmost efficiency regarding the tools, especially those for data handling, and the organization of the work that contributed decisively to the successful completion of the pioneering work. The extraordinary effort this included by Kendrew and his team can be measured by the fact that it took five years for the determination of the second high‐resolution protein structure (lysozyme) to be presented.19 David Phillips who led the team at the Royal Institution that achieved the result had contributed to the data collection of the myoglobin structure.
“Sausage” and “Forest of Rods” Model
The first model of myoglobin was not beautiful (Fig. 5). It was hard for Kendrew to hide his disappointment. In the first publication on the structure, he expressed his surprise at the “unexpected twists” the protein chain was performing.1 It certainly did not compare well with the double helical model of DNA, built a few years earlier in the same laboratory in Cambridge, of which James Watson had quipped that it was so “pretty” it just had to exist.20 A fellow crystallographer, seeing the photograph of the model published in Nature, put it succinctly: “It's a horrible object, but beautiful work” (Fig. 6).21 Kendrew agreed that the structure could “not be recommended on aesthetic grounds.”22 The most common association of the dark plaster model was with “abdominal viscera”.23 Others viewed it as “worm‐like”.24 One more kindly inclined correspondent described it as “a dog quietly huddled up”. Kendrew replied, remarking that the model “reminded many people of many things” but nobody had yet thought of a huddled‐up dog.25
Figure 5.

“Sausage model” of myoglobin (1957). Source: Science Museum/Science & Society Picture Library, slide no. SCM/PHY/C100369A. Kendrew presented the black plasticine model to the Science Museum describing it as “the first model of a protein”; J. Kendrew to F. Greenaway, 14 October 1975, Science Museum, file T/6762. How literally this needs to be understood remains open to interpretation. The model differs from the one which first appeared in print in a series of photographs and which later returned to the Laboratory of Molecular Biology from Kendrew's private collection. This (much better preserved) model is covered with a glossy white paint except for the oxygen binding structure that is represented by a red disc. The structure is not sitting on pegs but is supported by a few metal rods inserted between the bends. At the laboratory, priority is given to this model. The available record gives no definite answer. The “white” model also exists in a few large‐scale reproductions used for demonstrations and exhibitions and certainly found a wider circulation at the time.
Figure 6.
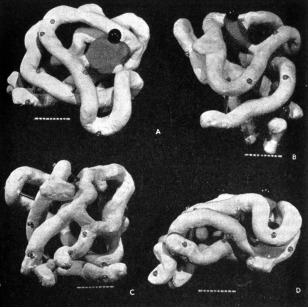
Myoglobin model as it first appeared in print. From Kendrew JC, Bodo G, Dintzis HM, Parrish RG, Wyckoff H, Phillips DC (1958). Nature 181:665, Figure 2. Reprinted by permission of Springer Nature.
The irregular structure of the molecule also made it difficult to describe it. Kendrew's attempts read: “… embracing [the haem group], so to speak, are two segments of polypeptide chain arranged like the letter V.” Or: “The polypeptide chain is shown as an irregular rod winding its way around the molecule.”26 Most importantly, the myoglobin model brought home the realization that there was no regularity in the structure of proteins and that the structure of every single protein would need to be established from scratch.
The ambivalent reactions to the first model were swept aside when Kendrew and his team presented their atomic model of myoglobin. Perutz described the structure built of a forest of 6‐foot tall steel rods, colored Meccano clips and custom‐made skeletal model parts as the “Eighth Wonder of the World” (Fig. 7).27 Irving Geis's drawing of the model, stripped of its mechanical parts, for Scientific American, established a new aesthetics for molecular structures (Fig. 8).18 Although the journal editor reportedly stamped the drawing a “mass of crumpled chicken wire,” it nevertheless launched Geis's career as a “molecular artist”.28 With his many drawings, he deeply influenced the conventions for depicting and viewing intricate protein structures.29, 30, 31 Geis also managed to give the original myoglobin model a better appearance (Fig. 9). By this time, he had shifted from an atom‐to‐atom representation to an increasingly abstract and “artistic”, though always stereochemically correct rendering of molecular structures or to what a critic called the production of “understandable metaphor(s) for molecules.”28 This shift was an expression of his attempt to illustrate the function (besides the structure) of the molecules in response to the development of molecular computer graphics that borrowed his ribbon‐and‐arrow style of representation.
Figure 7.

John Kendrew with his “forest of rods” model of myoglobin (1959). Courtesy of Medical Research Council Laboratory of Molecular Biology.
Figure 8.
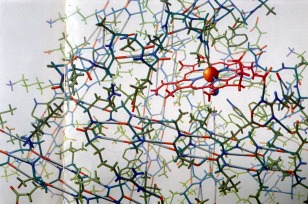
Drawing of the atomic model of myoglobin by Irving Geis. From the Geis Archives (presentation booklet and undated). Copyright Scientific American (1961). Image from the Irving Geis Collection. Rights owned and administered by the Howard Hughes Medical Institute. Reproduction by permission only.
Figure 9.
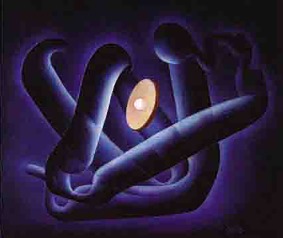
Myoglobin in a textbook drawing by Irving Geis. From the Geis Archives (presentation booklet and undated). Image from the Irving Geis Collection. Rights owned and administered by the Howard Hughes Medical Institute. Reproduction by permission only.
Stereo slides of the 2 Å myoglobin model also much impressed various audiences. A young researcher witnessing one such presentation later recalled: “We had all been given stereo glasses and watched with great anticipation as the two stereo images were being adjusted on the large screen. Suddenly, they were adjusted and the picture of the skeletal model of myoglobin jumped into three‐dimensions for everyone in the room at the same time. The whole audience simultaneously let out a loud ‘Oh’.”32
After the Nobel Prize
In 1962, shortly before the announcement of the Nobel Prizes for Kendrew, Perutz, Watson, Francis Crick, and Maurice Wilkens, the protein crystallographers together with other groups from Cambridge and London moved to the newly founded MRC Laboratory of Molecular Biology at the outskirts of Cambridge. The many researchers who visited the laboratory in the following years found Perutz, who chaired the laboratory, still actively engaged at the laboratory bench while Kendrew, who was head of the Structural Studies Division, only came to the laboratory irregularly and was winding up his research group. Nevertheless, Kendrew during this time made other important contributions to the development of the new science of molecular biology. He founded the Journal of Molecular Biology and was its editor in chief from 1959 to 1987, giving the new science an important publication outlet. As member and later deputy chairman of the UK Council of Scientific Policy (CSP) (1964–1972) and its Standing Committee on International Scientific Relations (of which he was a member and later a chairman), Kendrew worked indefatigably for the promotion, better funding and institutionalization of molecular biology on a national and international level and for raising its public profile. Moreover, he was a leading force in the foundation of the European Molecular Biology Organization (EMBO) of which he was a founding member (1963–1971) and later Secretary General (1969–1974) and in the long negotiations that finally led to the foundation of the European Molecular Biology Laboratory (EMBL) in Heidelberg. Kendrew became EMBL's founding Director (1975–1982) and during his tenure decisively shaped the laboratory. Among the people he encouraged to join the laboratory was Jacques Dubochet. Kendrew was specifically interested in the method of vitrification Dubouchet was developing and its potential for cryo‐electron microscopy, the method that is currently stirring such excitement in the field of structural biology and that was recognized with last year's Nobel Prize. Together with his pioneering work on myoglobin, these different initiatives decisively supported and shaped the new field of molecular biology.
Acknowledgments
The essay is based on a talk presented at the conference ‘Revolutions in Structural Biology’, organized at EMBL in November 2017 to celebrate the 100th anniversary of the birth of Sir John Kendrew, the founding director of the laboratory. I thank the organizers of the conference for the invitation to present the paper as well as David Eisenberg and Brian Matthews for their encouragement to revise the paper for publication.
Footnotes
During the war, whale meat was investigated as supplement to butcher's meat to meet the shortage of supply. Later batches of sperm whale meat were sent to Kendrew by air from Lima, where the meat could be bought on the market.16
References
- 1. Kendrew JC, Bodo G, Dintzis HM, Parrish RG, Wyckoff H, Phillips DC (1958) A three‐dimensional model of the myoglobin molecule obtained by X‐ray analysis. Nature 181:662–666. [DOI] [PubMed] [Google Scholar]
- 2. Kendrew JC, Dickerson RE, Strandberg BE, Hart RG, Davies DR, Phillips DC, Shore VC (1960) Structure of myoglobin: a three‐dimensional Fourier synthesis at 2 Å resolution.” Nature 185:422–427. [DOI] [PubMed] [Google Scholar]
- 3.Video interview with Kendrew JC by Holmes K, 18 June 1997, LMB Archive.
- 4. Kendrew JC to Gordon C, 15 August 1945; Ms.Eng.c.2394, B.51, The correspondence and papers of Sir John Cowdery Kendrew, Bodleian Libraries, University of Oxford.
- 5. Kendrew JC to Snow CP, 8 October 1945. [draft version]; Ms.Eng.c.2606, R.7, The correspondence and papers of Sir John Cowdery Kendrew, Bodleian Libraries, University of Oxford.
- 6.Interview with Kendrew JC by the author, Cambridge, 14 July 1992.
- 7. Bragg WL (1965) First stages in the X‐ray analysis of proteins. Rep Prog Phys 28:1–16. [Google Scholar]
- 8. Kendrew JC to Huxley H, 23 Dec 1952; Ms.Eng.c.2397, C. 17, The correspondence and papers of Sir John Cowdery Kendrew, Bodleian Libraries, University of Oxford.
- 9. Green DW, Ingram VC, Perutz MF (1954) The structure of haemoglobin‐IV: sign determination by the isomorphous replacement method. Proc Roy Soc A 225:287–307. [Google Scholar]
- 10. Huxley HE, An early adventure in crystallographic computing In: Thomas JM, Phillips D, Ed. (1990) Selections and reflections: the legacy of Sir L. Bragg, Science Reviews: Northwood, pp 133–134. [Google Scholar]
- 11. Bennett JM, Kendrew JC (1952) The computation of Fourier synthesis with a digital electronic calculating machine. Acta Cryst 5:109–116. [Google Scholar]
- 12. Crowther JG (1974) The Cavendish Laboratory 1874–1974. London: Macmillan, p. 306. [Google Scholar]
- 13. Wilson AJC (1951) Summarized proceedings of a conference on the development and application of Fourier methods in crystal‐structure analysis−London, November 1950. Brit J Appl Phys 2:61–70. on p. 67. [Google Scholar]
- 14. Alton J (1989) Catalogue of the papers and correspondence of Sir John C. Kendrew. Oxford: National Cataloguing Unit for the Archives of Contemporary Scientists, p. 65.
- 15. Kendrew JC (1964) Myoglobin and the structure of proteins. Nobel lecture, December 11, 1962 In: Nobel lectures, including presentation speeches and laureates’ biographies. Chemistry, 1942–1962. Amsterdam: Elsevier, pp. 676–698, on p. 651, 696. [Google Scholar]
- 16.Interview with Kendrew JC by the author, Linton, 18 March 1993.
- 17. Kendrew JC, Watson HC (1961) The amino‐acid sequence of sperm whale myoglobin: a partial determination by X‐ray methods, and its correlation with chemical data. Nature 190:670–670. 13783432 [Google Scholar]
- 18. Kendrew JC (1961) The three‐dimensional structure of a protein molecule. Sci Am 205:96–110. [DOI] [PubMed] [Google Scholar]
- 19. Blake CCF, Koenig DF, Mair GA, North ACT, Phillips DC, Sarma VR (1965) Structure of hen egg‐white lysozyme: a three‐dimensional Fourier synthesis at 2 Å resolution. Nature 206:757–761. [DOI] [PubMed] [Google Scholar]
- 20. Watson JD (1968) The double helix: a personal account of the discovery of the structure of DNA. New York: Weidenfeld and Nicolson, p 120. [Google Scholar]
- 21. Liquori AM to Kendrew JC, 20 March 1958; Ms.Eng.c.2408, C.278, The correspondence and papers of Sir John Cowdery Kendrew, Bodleian Libraries, University of Oxford.
- 22. Kendrew JC to Bloom G, 12 January 1958; Ms.Eng.c.2408, C.279, The correspondence and papers of Sir John Cowdery Kendrew, Bodleian Libraries, University of Oxford.
- 23.Dr. Norton, Visit to the Molecular Biology Research Unit, Cambridge, 2nd December 1957, unpublished internal report; FD12/291 (E243/29), National Archives, UK.
- 24. Astbury W to Kendrew JC, 19 June 1960; Ms.Eng.c.2408, C.281, The correspondence and papers of Sir John Cowdery Kendrew, Bodleian Libraries, University of Oxford.
- 25. Inoue S to Kendrew JC, 7 April 1958 and Kendrew JC to Inoue S, 17 April 1958; Ms.Eng.c.2408, C.278, The correspondence and papers of Sir John Cowdery Kendrew, Bodleian Libraries, University of Oxford. [Google Scholar]
- 26. Kendrew JC (1966) The thread of life: an introduction to molecular biology based on the series of B.B.C. Television lectures of the same title. London: G. Bell and Sons, captions to figures 18 and 19. [Google Scholar]
- 27. Perutz M, John Kendrew memorial meeting address, 1997. (typescript); Max Ferdinand Perutz Papers, PRTZ 4/2/11, Churchill Archives Centre, Cambridge, UK.
- 28. Gaber BP, Goodsell DS (1997) The art of molecular graphics. Irving Geis: dean of molecular illustration. J Mol Graph Model 15:57–59. [Google Scholar]
- 29. Dickerson RE (2008) Irving Geis, molecular artist, 1908–1997. Protein Sci 6:2483. [Google Scholar]
- 30. Dickerson RE (1997) Irving Geis, 1908–1997. Structure 5:1247–1249. [DOI] [PubMed] [Google Scholar]
- 31. Dickerson RE (1997) Molecular artistry. Curr Biol 7:R740–R741. [Google Scholar]
- 32. Steitz T in a letter to Henderson R on the occasion of Max Perutz's death, 8 Feb 2002; reproduced in Finch J (2008). A nobel fellow on every floor: a history of LMB. Cambridge: MRC Laboratory of Molecular Biology, p 332. [Google Scholar]


