Summary
Bioactive components of human milk, such as human lactoferrin (hLF), play an essential role in gut microbiome homeostasis and protection against neonatal inflammatory diseases. Neonatal intestinal macrophages display a proinflammatory profile that might contribute to inflammatory mucosal injury. Therefore, the aim of the study was to investigate the immunomodulatory effects of hLF on differentiation and activation of monocyte‐derived macrophages (moMϕ). Monocytes isolated from umbilical cord blood of term neonates and peripheral blood of healthy adults were differentiated in the absence or presence of hLF, and differentiation, apoptosis and phagocytosis were evaluated. Cytokine production, Toll‐like receptor (TLR) signalling and activation marker expression were investigated upon activation with lipopolysaccharide (LPS) and lipoteichoic acid (LTA) challenge. We demonstrate that hLF‐differentiated moMϕ exhibit decreased TLR‐4 expression, TLR signalling, proinflammatory cytokine secretion and intracellular tumour necrosis factor (TNF)‐α production. Investigation of differentiation markers, morphology and induction of apoptosis showed no alteration in lactoferrin‐differentiated moMϕ. Taken together, hLF promote anergic/anti‐inflammatory effects by TLR expression and pathway interference, resulting in a diminished proinflammatory moMϕ phenotype. The anergic/anti‐inflammatory properties of hLF might contribute to the prevention of harmful TLR‐mediated inflammatory disorders in the developing gut of premature infants.
Keywords: lactoferrin, monocyte‐derived macrophages, neonatal immunity, Toll‐like receptor
Introduction
Macrophages are essential for the elimination of invading pathogens, playing a pivotal role in inflammation as well as regulating tissue homeostasis 1. Phenotype and function of macrophages appear to adapt to the multifarious stimuli of the surrounding microenvironment. This functional heterogeneity can be reproduced depending on the activation and polarizing stimuli in vitro leading to M1‐activated (proinflammatory) or M2‐activated (anti‐inflammatory/tissue remodelling) macrophages 2, 3. Intestinal macrophages are engaged permanently with immunostimulatory bacteria and, therefore, appear to acquire an ‘inflammatory anergy’ to prevent sustained inflammation of the gut 4, 5.
In the premature gut, restitution of the epithelial border as well as mucin production are diminished leading towards a ‘leaky gut’ with increased translocation of bacteria into the lamina propria 6. Intestinal macrophages of premature neonates are in a proinflammatory state and acquire a gestational age‐dependent non‐inflammatory profile 5, 7. Thus, the ‘leaky gut’ as well as the higher proinflammatory state of macrophages might predispose premature infants to inflammatory mucosal injury resulting in complications such as necrotizing enterocolitis (NEC) 8.
In recent years, diverse immunoregulatory properties of lactoferrin have been described in vitro. Lactoferrin, a mammalian iron‐binding whey glycoprotein, exhibits direct effects against pathogens by iron depletion as well as binding and neutralizing pathogen‐associated molecular patterns and, furthermore, appears to attenuate the proinflammatory immune response via interaction with Toll‐like receptors (TLRs) 9, 10. Lactoferrin showed protective effects in a neonatal gut‐related sepsis model against Escherichia coli leading to reduced mortality 11. Randomized control trials revealed that oral administration of lactoferrin alone and combined with probiotics significantly reduced the incidence of NEC stage II or greater in premature neonates compared to placebo 12. Hence, lactoferrin seems to feature manifold immunoregulatory effects and showed promising results for NEC prevention in preterm infants in clinical studies.
To date, data concerning the functional properties of lactoferrin on neonatal monocytes and macrophages are scarce. Hence, the overarching aim of the current study was to provide deeper insights into the functional properties of neonatal and adult monocyte‐derived macrophages (moMϕ) and the effects of human lactoferrin (hLF) on functional response and differentiation after TLR‐dependent activation.
Materials and methods
Study population
Heparinized whole blood from the umbilical cord from term neonates (n = 18, 37–42 weeks of gestation) after caesarian section and peripheral blood from healthy adult volunteers (n = 10, age = 18–60 years) was collected aseptically and processed immediately. Exclusion criteria for newborns were known maternal autoimmune diseases or maternal immune deficiencies, regular intake of immunomodulatory medication, congenital malformations and congenital infections. The study was approved by the local ethics committee of the Medical University of Vienna (no. 1923/2012) and written informed consent was obtained from the parents before birth and healthy adults.
Cell isolation and cell culture
Peripheral blood mononuclear cells (PBMCs) were isolated sterilely from heparinized cord and peripheral blood through Ficoll density centrifugation immediately after drawing. CD14+ monocytes were extracted from PBMCs with anti‐CD14 MicroBeads (Miltenyi Biotec, Bergisch‐Gladbach, Germany), according to the manufacturer's protocol. Isolated cells (1·25 × 105/ml) were cultivated in RPMI‐1640 medium supplemented with 10% fetal calf serum (FCS; Gibco, Carlsbad, CA, USA) in a 24‐well flat‐bottomed plate (Greiner Bio‐One, Kremsmünster, Austria). To obtain moMϕ, monocytes were differentiated with macrophage colony‐stimulating factor (M‐CSF) (100 ng/ml; PeproTech, Rocky Hill, NJ, USA) in the absence or presence of hLF (50 or 500 µg/ml; Sigma Aldrich, St Louis, MO, USA) for 7 days in an incubator with 37°C, 5% CO2 and 95% humidity. M‐CSF and hLF were added on days 0, 3 and 5 of cultivation.
M‐CSF receptor expression and signalling
Monocytes were cultivated in the absence or presence of M‐CSF (100 ng/ml) and hLF (50 or 500 µg/ml) for 24 h at 37°C to evaluate the impact of hLF on M‐CSF receptor expression. After incubation, cells were stained with anti‐CD14‐fluorescein isothiocyanate (FITC) (clone 322A‐1; Beckman Coulter, Krefeld, Germany) and anti‐CD115‐phycoerythrin (PE) (clone 12‐3A3‐1B10; eBioscience, Vienna, Austria) for 20 min at room temperature and analysed by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
To investigate whether the M‐CSF signalling pathway was affected, monocytes were preincubated with hLF (50 or 500 µg/ml) for 10 min at 37°C and stimulated afterwards with M‐CSF (100 ng/ml) for 1 min. Immediately after stimulation, fixation of monocytes was performed with prewarmed (37°C) lyse/fix buffer (BD Biosciences) for 10 min at 37°C, followed by a 30‐min permeabilization on ice with precooled (–20°C) Perm Buffer III (BD Biosciences). Monocytes were washed three times, stained with the phosphospecific antibody phospholipase C (PLC)‐γ2‐Alexa Fluor 647 (clone K86–689.37; BD Biosciences) for 20 min at room temperature and then analysed on a LSRFortessaTM flow cytometer (BD Biosciences).
Apoptotic assay
Proportions of apoptotic cells were analysed from macrophages that were differentiated with M‐CSF and in the absence or presence of 50 or 500 µg/ml hLF. Macrophages were stained with annexin V‐V450 and 7‐aminoactinomycin D (7‐AAD) viability staining solution (both from BD Biosciences) and analysed subsequently by flow cytometry.
Surface receptor staining of macrophages
To analyse expression levels of surface receptors, monocyte‐differentiated macrophages were left untreated or stimulated with lipopolysaccharide (LPS) (1 ng/ml; E. coli O111:B4) or lipoteichoic acid (LTA) (1 µg/ml; Staphylococcus aureus ultrapure, both from InvivoGen, San Diego, CA, USA) for 24 h. Unstimulated macrophages were stained with (i) anti‐CD14‐FITC (Beckman Coulter), anti‐TLR‐2‐allophycocyanin (APC) (clone TL2.1) and anti‐TLR‐4‐phycoerythrin (PE) (clone HTA125, both from eBioscience), and (ii) anti‐CD16‐FITC (clone eBioCB16), anti‐CD64‐APC (clone 10.1) and anti‐CD206‐PE (clone 19.2, all from eBioscience) for 20 min at room temperature. After TLR‐specific activation, cells were stained with anti‐CD14‐FITC (Beckman Coulter), anti‐CD40‐PE (clone 5C3) and anti‐human leucocyte antigen D‐related (HLA‐DR)‐V500 (clone G46‐6, both from BD Biosciences) for 20 min at room temperature. After antibody incubation, cells were washed once with staining buffer and analysed immediately via flow cytometry.
Analysis of cytokine production
Intracellular tumour necrosis factor (TNF)‐α production was measured in unstimulated and stimulated macrophages with LPS (1 ng/ml) or LTA (1 µg/ml) for 4 h in the presence of brefeldin A (eBioscience). After stimulation, cells were stained with anti‐CD14‐FITC (Beckman Coulter) for 10 min at 4°C and afterwards fixed with intracellular (IC) fixation buffer for 20 min. Cells were then permeabilized using permeabilization buffer (eBioscience), stained subsequently with anti‐TNF‐α‐PE (clone Mab11, eBioscience) for 20 min and analysed by flow cytometer.
In addition, TNF‐α and TGF‐β were measured via enzyme‐linked immunosorbent assay (ELISA) from the cell culture supernatants after 4 h of stimulation. Additionally, cytokine levels of interleukin (IL)‐1β, IL‐6, IL‐8, IL‐10, TNF‐α and IL‐12p70 were analysed using the human inflammatory cytokine CBA kit (BD Biosciences) after 24 h of stimulation.
TLR signalling
Macrophages were either treated with cell culture medium or stimulated with LPS (100 ng/ml) or LTA (1 µg/ml) for 10 min at 37°C. After stimulation, cells were fixed and permeabilized under the same conditions as for M‐CSF signalling analysis. For intracellular evaluation of TLR signalling pathways, macrophages were stained with the phosphospecific antibodies nuclear factor kappa B (NF‐κB) p65 (pS529)‐PE (clone B33B4WP; eBioscience) and extracellular kinase (ERK)1/2 (pT202/pY204)‐Alexa Fluor 647 (clone 20A; BD Biosciences) for 20 min at room temperature and analysed afterwards by flow cytometric analysis.
Phagocytosis assay
Phagocytosis was assessed by incubating macrophages for 1 h at 37°C with non‐opsonized E. coli–FITC (Orpegen, Heidelberg, Germany), according to the manufacturer's protocol. Additionally, the pHrodoTM Green E. coli BioParticles® kit for flow cytometry (Life Technologies, Carlsbad, CA, USA) was used to measure the potential of macrophages to process E. coli particles. Therefore, cells were incubated with non‐opsonized E. coli particles for 1 h at 37°C. In addition, moMϕ were stained with anti‐CD14‐V450 (clone M5E2; BD Biosciences).
RNA extraction and reverse transcription–quantitative polymerase chain reaction (RT–qPCR)
For VentX mRNA quantification, 2 × 105 monocytes were left untreated or were differentiated with M‐CSF in the absence or presence of hLF. Cells were harvested on day 6 and total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen, Venlo, the Netherlands). RNA quantity and quality were assessed using NanoDrop8000 (Thermo Fisher Scientific, Waltham, MA, USA). The ABI PRISM 7500HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) was used for RT‐qPCR analysis. Primer‐probes sets for VentX FAM and 18S rRNA VIC were obtained predesigned from Applied Biosystems and tested for primer efficacy (gene expression assays: Hs99999901_s1 18S VIC, Hs00797729_s1 VentX FAM). Multiplex amplification was carried out in a total volume of 20 µl for 40 cycles of 3 s at 95°C, 30 s at 60°C. Initial denaturation was performed for 3 min at 95°C. Target gene expression was normalized to 18s rRNA housekeeping gene expression. Normalized target gene expression was analysed by the comparative ΔΔCT method and calculated as x‐fold expression.
Statistical analysis
Statistical analysis was performed using spss version 24.0. The Shapiro–Wilk test was performed to prove normal distribution and the Levene test was applied to verify the homogeneity of variance. Data were then analysed using one‐way analysis of variance (anova) in accordance with Tukey. Data that were not normally distributed were analysed using the Kruskal–Wallis test. Flow cytometry data were analysed using FlowJo X (FlowJo LLC, TreeStar Inc., Ashland, OR, USA). A P‐value of ≤ 0·05 was considered statistically significant.
Results
Presence of hLF does not interfere with macrophage differentiation
In the first step, we examined the influence of hLF on M‐CSF receptor expression and M‐CSF signalling of monocytes. M‐CSF receptor expression was down‐regulated significantly after engagement with M‐CSF or 500 μg/ml hLF alone and M‐CSF + 50 or 500 μg/ml hLF combined in comparison to monocytes that were cultivated in medium alone (Fig. 1a). M‐CSF signalling was increased in monocytes that were stimulated with 500 μg/ml hLF compared to medium‐only controls. Activated monocytes with M‐CSF or M‐CSF + 500 μg/ml hLF showed identical signalling up‐regulation (Fig. 1a). Investigations on morphology and induction of apoptosis did not differ in lactoferrin‐differentiated macrophages or in controls (Fig. 1b,c).
Figure 1.
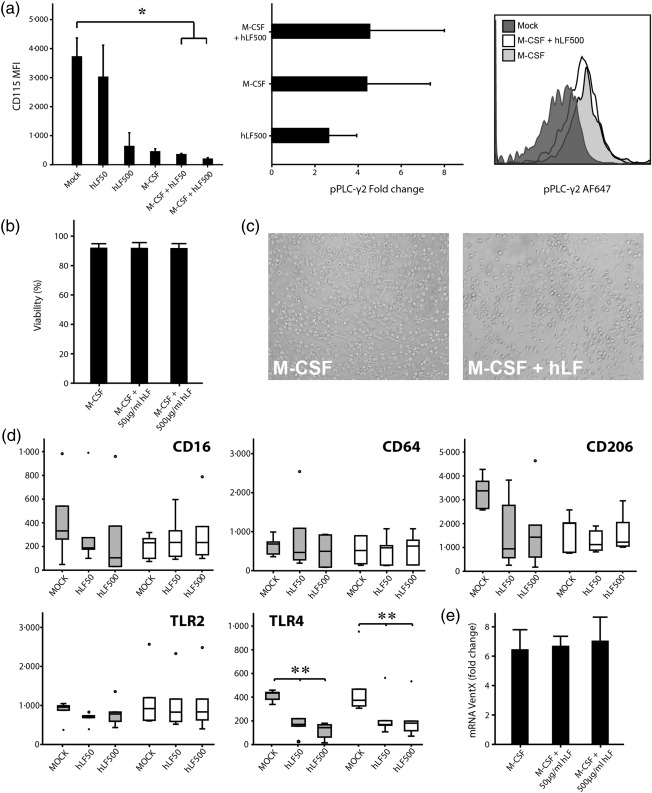
Influence of human lactoferrin (hLF) on monocyte‐to‐macrophage differentiation. (a) CD115 expression [mean fluorescence intensity (MFI)] as well as phosphoinositide phospholipase C (pPLC)‐γ2 signalling (fold change compared to unstimulated control) was evaluated after stimulation with macrophage colony‐stimulating factor (M‐CSF) or hLF alone or in combination with different concentrations of hLF by flow cytometry in adult monocytes. One representative histogram from three independent experiments is shown (n = 3). (b) Macrophage viability was evaluated by flow cytometry using annexin V and 7‐aminoactinomycin D (7‐AAD) staining and (c) light microscopy after 7 days of culture. One representative image from four independent experiments is shown. Bars show mean ± standard deviation from four healthy adult probands. (d) Expression of CD16, CD64, CD206, Toll‐like receptor (TLR)‐2 and TLR‐4 of macrophages differentiated in the presence or absence of hLF from healthy adults and term neonates (each group n = 6) was evaluated by flow cytometry. Boxes indicate the 25th and 75th percentiles, error caps indicate the 5th and 95th percentiles and the middle line represents the median. Dots represent outliers. (e) Monocyte‐derived macrophages differentiated in the presence or absence of hLF from healthy adults (n = 4) were harvested at day 6 and VentX mRNA expression was determined by reverse transcription–polymerase chain reaction (RT–PCR). VentX mRNA was normalized to 18sRNA and compared between unstimulated macrophages without M‐CSF and hLF at day 5. Values are shown as fold change in relation to unstimulated control. Normal distribution was determined with the Shapiro–Wilk test. Normally distributed data were analysed using one‐way analysis of variance in accordance with Tukey and non‐normally distributed data were analysed using the Kruskal–Wallis test. *P < 0·05; **P < 0·01.
Next, we examined the effect of hLF on the expression of the differentiation markers CD16, CD64 and CD206, as well as TLR‐2/4. TLR‐4 expression was down‐regulated significantly on adult and term moMϕ under the influence of hLF. Interestingly, adult moMϕ showed lower, although not significant, levels of TLR‐2 and CD206 after concomitant differentiation with hLF, although this was not statistically significant. Expression levels of CD14, CD16 and CD64 in both groups and TLR‐2/CD206 in term neonates were not modified in the presence of hLF when compared to M‐CSF conditions (Fig. 1d). Furthermore, we investigated the expression of the transcription factor VentX in moMϕ after 6 days of differentiation. The expression of VentX was not influenced by the presence of hLF during differentiation (Fig. 1e).
Diminished TNF‐α production and TLR signalling after LPS and LTA challenge in hLF‐differentiated moMϕ
In the next step, we determined the immune response of differentiated moMϕ after TLR‐2 (LTA)‐ and TLR‐4 (LPS)‐specific activation. First, we investigated the capability of moMϕ to produce TNF‐α after TLR engagement. Upon 4 h of stimulation, hLF‐treated adult and term moMϕ showed a dose‐dependent decrease of TNF‐α secretion in cell culture supernatants compared to M‐CSF controls. hLF alone had no effect on TNF‐α secretion (Fig. 2a,b). In accordance with these results, intracellular TNF‐α production was significantly lower in hLF‐differentiated moMϕ stimulated with LPS or LTA compared to M‐CSF controls in both study groups (Fig. 2c,d). To deepen our insight into the TLR‐dependent pathways, we examined the phosphorylation of the signalling molecules ERK1/2 and NF‐κB p65. Both signalling proteins were significantly lower phosphorylated upon LPS and LTA stimulation in adult hLF‐differentiated moMϕ compared to M‐CSF‐differentiated moMϕ (Fig. 3a).
Figure 2.
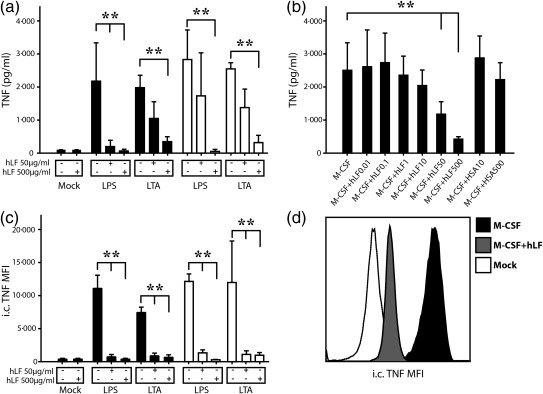
Influence of human lactoferrin (hLF) on the proinflammatory response of monocyte‐differentiated macrophages (moMϕ). (a) MoMϕ were stimulated with lipopolysaccharide (LPS) or lipoteichoic acid (LTA) for 4 h and tumour necrosis factor (TNF)‐α was measured with enzyme‐linked immunosorbent assay in the cell culture supernatant (n = 5 per group: black bars adults, white bars neonates). (b) Influence of increasing dosage of hLF on adult monocyte‐derived macrophages on the secretion of TNF‐α after 4 h LPS stimulation. Human serum albumin (HSA) was used as protein control (n = 5). (c) In parallel, intracellular (i.c.) TNF‐α was assessed on a single‐cell level (n = 5 per group: black bars adults, white bars neonates) using flow cytometry, and (d) one representative histogram from 10 independent experiments is shown. Bars show mean ± standard deviation. Normal distribution was determined with the Shapiro–Wilk test. Normally distributed data were analysed using one‐way one‐way analysis of variance in accordance with Tukey and non‐normally distributed data were analysed using the Kruskal–Wallis test. *P < 0·05; **P < 0·01.
Figure 3.
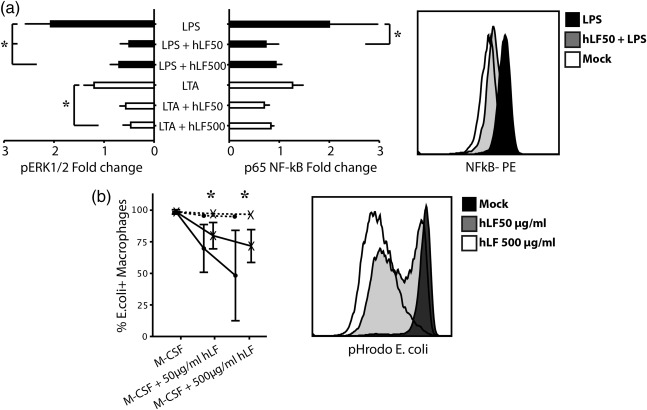
Influence of human lactoferrin (hLF) on the phagocytic activity and Toll‐like receptor (TLR)‐2/4 signalling in monocyte‐differentiated macrophages (moMϕ) (a) Phosphorylation levels of extracellular‐regulated kinase (ERK) 1/2 and nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB) in macrophages from term neonates (n = 5) upon stimulation with lipopolysaccharide (LPS) (black bars) or lipoteichoic acid (LTA) (white bars). A representative histogram from 10 independent experiments is shown. (b) Phagocytic capacity of moMϕ under increasing concentrations of hLF using fluorescein isothiocyanate (FITC)‐marked Echerichia coli (dotted lines) and pHrodo‐E. coli (solid line) in adults (black dots) and neonates (black cross, n = 5 per group) was determined by flow cytometry. A representative histogram from 10 independent experiments is shown. Bars show mean ± standard deviation. Normal distribution was determined with the Shapiro–Wilk test. Normally distributed data were analysed using one‐way analysis of variance in accordance with Tukey and non‐normally distributed data were analysed using the Kruskal–Wallis test. *P < 0·05.
Human lactoferrin attenuates phagocytic activity, activation marker expression and cytokine response
To investigate how hLF influences phagocytic activity, moMϕ were incubated with non‐opsonized E. coli particles. In neonatal and adult moMϕ, the presence of high‐dosage hLF did not influence the uptake of FITC‐marked E. coli. In the next step, we used pHrodo‐E. coli to assess the acidification of the phagosome after uptake of E. coli. The presence of hLF during differentiation led to a significantly lower potential of acidification of the phagosome to process E. coli in both study groups (Fig. 3b). We further investigated the regulation of surface activation markers after TLR‐2 or TLR‐4 engagement in hLF‐treated or ‐untreated moMϕ after 24 h. hLF down‐regulated the expression of CD40 and HLA‐DR independently in untreated moMϕ. After LPS or LTA challenge, the presence of hLF during macrophage differentiation led to a significantly lower expression of CD40 (Fig. 4a) and HLA‐DR (Fig. 4b) on the surface of moMϕ compared to LPS‐ or LTA‐activated moMϕ.
Figure 4.
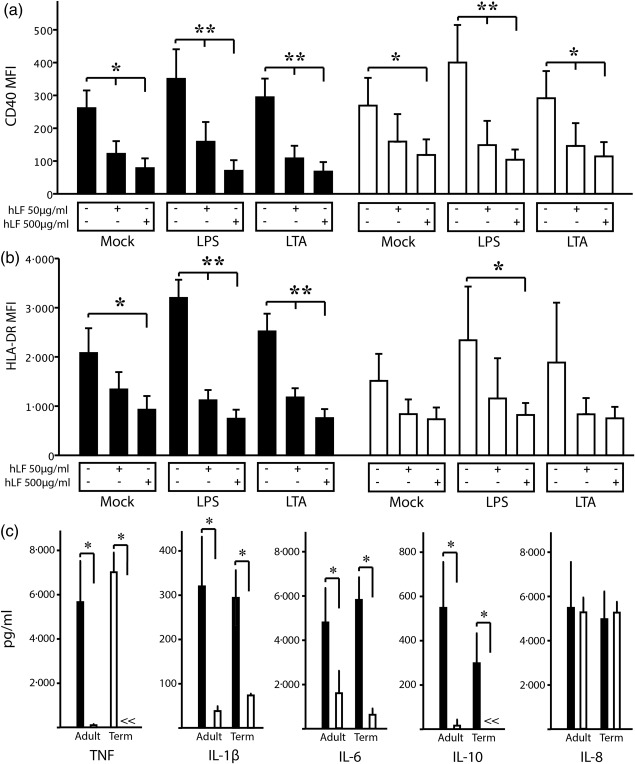
Effects of human lactoferrin (hLF) on activation markers, cytokine secretion and phagocytosis. (a) CD 40 and (b) human leucocyte antigen D‐related (HLA‐DR) expression on adult (black bars) and neonatal (white bars, n = 5 per group) macrophages after stimulation with lipopolysaccharide (LPS) and lipoteichoic acid (LTA) in the presence or absence of different doses of hLF as determined by flow cytometry. (c) Cell culture supernatants from LPS‐stimulated monocyte‐differentiated macrophages (moMϕ) in the presence (500 µg/ml) or absence of hLF were assessed for cytokine levels of tumour necrosis factor (TNF)‐α, interleukin (IL)‐1, IL‐6, IL‐10 and IL‐8 (n = 8 per group) using a flow cytometry‐based bead array. Bars show mean ± standard deviation. Normal distribution was determined with the Shapiro–Wilk test. Normally distributed data were analysed using one‐way analysis of variance in accordance with Tukey and non‐normally distributed data were analysed using the Kruskal–Wallis test. *P < 0·05; **P < 0·01.
Further, the production of TNF‐α, IL‐1β, IL‐6, IL‐8, IL‐10 and IL‐12p70 was analysed after 24 h of stimulation with LPS and LTA. TNF‐α, IL‐1β, IL‐6 and IL‐10 were decreased significantly in LPS‐stimulated moMϕ of adults when compared to M‐CSF conditions. In LTA‐stimulated cells, a reduction of the cytokine production was also visible in hLF‐differentiated moMϕ, but did not reach statistical significance. In neonates, TNF‐α, IL‐1β, IL‐6 and IL‐10 levels were decreased significantly in both LPS‐ and LTA‐stimulated hLF‐differentiated moMϕ. Production of IL‐12p70 and TGF‐β could not be detected in both groups (data not shown). IL‐8 levels remained unchanged in the presence of hLF after LPS or LTA challenge in both study groups (Fig. 4c and Supporting information, Fig. S1).
Discussion
We show that hLF attenuates the proinflammatory response of moMϕ and, therefore, mediates a decreased immune response via interaction of the TLR‐4‐specific pathway. hLF‐differentiated moMϕ displayed decreased potential of phagosome acidification as well as down‐regulated antigen‐presenting and co‐stimulatory surface markers. Although proinflammatory cytokines such as TNF‐α or IL‐6 were down‐regulated significantly in hLF‐treated moMϕ, anti‐inflammatory counterparts such as IL‐10 or TGF‐β were missing upon activation of hLF‐differentiated moMϕ. Thus, our data might suggest that hLF polarizes macrophages into a more ‘anergic’ instead of an ‘anti‐inflammatory’ state. The lactoferrin‐induced anergic/anti‐inflammatory state probably contributes to the protective effects to proinflammatory disorders such as NEC in the intestine of premature neonates.
NEC is a life‐threating gastrointestinal complication in extremely premature infants of mainly unknown origin. Besides an imbalance of intestinal microperfusion, reduced mucus production and diminished epithelial gut barrier, it is assumed that a dysregulated excessive inflammatory immune response against luminal bacteria might further damage the vulnerable gut of premature infants leading to a systemic inflammatory immune response 8, 13. Thus, the premature ‘leaky gut’ might result in a higher probability of translocation of harmful bacteria from the intestinal lumen into the tissue, causing inflammation by tissue‐resident macrophages. Additionally, intestinal macrophages of premature infants exhibit an elevated proinflammatory profile which might aggravate gut inflammation, predisposing to the development of NEC 5, 7, 8. This is supported by the finding that premature infants who developed NEC display a gut microbial dysbiosis with lower diversity and higher amounts of Gram‐negative bacteria prior to disease onset 14, 15. Furthermore, the bacterial cell wall component LPS was found to be a top transcriptional upstream regulator in NEC 16. Interestingly, eradication of Gram‐negative bacteria with orally given aminoglycosides appears to be protective against NEC in premature infants 17.
NEC often occurs within the first weeks of life. Therefore, we have chosen to investigate the monocyte‐to‐macrophage differentiation, as premature infants receive human breast milk containing lactoferrin and/or supplemental lactoferrin after birth. Additionally, as shown by Maheshwari et al. 5, the intestinal macrophage pool in the developing gut is replenished by blood monocytes migrating into the intestinal lamina propria and differentiating into macrophages. M‐CSF seems to play a crucial role in local homeostasis of monocytes and macrophages – especially in the gut – and was therefore chosen as differentiation stimulus in our experiments 18, 19.
We sought first to investigate the potential interference of hLF on the differentiation process of monocytes to macrophages. M‐CSF receptor expression as well as signalling was not influenced by hLF. Interestingly, high dosages of hLF weakly induced phospholipase C gamma 2 (PLC‐γ2) without M‐CSF stimulation. hLF incubation did not cause apoptosis of macrophages and furthermore, using light microscopy, hLF‐differentiated moMϕ were normally shaped. Macrophage surface markers showed comparable levels regardless of different hLF concentrations. Additionally, the expression level of VentX, a homeobox transcription factor for terminal differentiation and proinflammatory activation of macrophages, was unaffected by hLF treatment 20. Interestingly, TLR‐4 surface expression – as opposed to TLR‐2 – was down‐regulated selectively on hLF‐treated moMϕ. Stimulation with the TLR‐2‐specific agonist LTA and the TLR‐4‐specific agonist LPS resulted in diminished TLR‐dependent signalling and proinflammatory cytokine secretion in a dose‐dependent manner. Although the TLR‐2 expression was not affected by hLF exposure, we observed a significant reduction of the proinflammatory response after LTA stimulation in hLF‐treated moMϕ. The reduced cytokine response can be explained partly by reduced downstream signalling of the TLR‐2‐pathway in hLF‐treated moMϕ. Previous studies have revealed that lactoferrin activates TLR‐4‐dependent and ‐independent pathways, resulting in macrophage activation 21, 22. Those studies were conducted after completion of macrophage differentiation or using macrophage cell lines and did not investigate the impact of hLF on the differentiation process 21, 22. Using recombinant hLF and GM‐CSF as differentiation stimulus, van der Does et al. 23 described a moMϕ subset with increased responsiveness to microbial structures. This might be explained by the use of GM‐CSF, which seems to be an important growth and homeostasis factor during acute inflammation 24. Interestingly, the presence of bovine lactoferrin during differentiation of monocyte‐derived dendritic cells leads to an attenuated immune response after TLR engagement 25. These data suggest a pivotal role of lactoferrin during macrophage differentiation, depending on the used differentiation stimulus. Hence, data on the effect of hLF on neonatal moMϕ differentiated in the presence of GM‐CSF are missing. In recent years, several functional deficiencies in the neonatal immune system regarding the monocyte function have been suggested, showing limited immune responses of neonatal monocytes compared to healthy adults 26, 27. Interestingly, we found no significant difference in moMϕ phenotype or function between term neonates and adults.
Lactoferrin also exerts distinct immunomodulatory properties on intestinal epithelial cells. LF promotes enterocyte growth and proliferation as well as stimulating the secretion of TGF‐β 28, 29. Maheshwari et al. showed that TGF‐β plays a crucial role in the developing premature intestine by suppression of the proinflammatory immune response of macrophages. In early gestational age TGF‐β production of stromal cells is diminished, leading to an inflammatory response in intestinal macrophages. This observation is underlined by the finding that enteral supplementation with TGF‐β protected mice from experimentally induced NEC‐like injuries 5. Thus, the induction of TGF‐β in intestinal epithelial cells by LF might be a further protective mechanism against excessive gut inflammation. Further in‐vivo studies are needed to validate this hypothesis. Based on clinical trials and data, enteral administration of lactoferrin leads to a reduced incidence of NEC and late‐onset sepsis in premature infants 12. As LF concentrations are highest in the colostrum, reaching from 5 to 7 g/dl 10, neonatologists aim at the feeding of colostrum and breast milk as well as supplementation of LF as a dietary supplement for the prevention of NEC in very low‐birthweight neonates.
Taken together, LF seems to have an impact upon various immunological players in the gut with direct and indirect effects on intestinal macrophages. The results of the present study shed some light on the proposed mechanism of the underlying ‘anergic’/anti‐inflammatory properties of hLF, highlighting the importance of monocyte/macrophages as potential prophylactic and therapeutic targets in inflammatory diseases.
Disclosure
The authors declare no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. Cell culture supernatants from lipopolysaccharide (LPS)‐ and lipoteichoic acid (LTA)‐stimulated monocyte‐differentiated macrophages from adult (black bars) and neonates (white bars) in the presence or absence of human lactoferrin (hLF) were assessed for cytokine levels of (a) tumour necrosis factor (TNF)‐α, (b) interleukin (IL)‐1, (c) IL‐6, (d) IL‐8 and (e) IL‐10 (n = 8 per group). Bars show mean ± standard deviation. Normal distribution was determined using the Shapiro–Wilk test. Normally distributed data were analysed using one‐way analysis of variance in accordance with Tukey and non‐normally distributed data were analysed using the Kruskal‐Wallis test. *P < 0·05; **P < 0·01. Neonatal intestinal macrophages display a proinflammatory profile that might contribute to inflammatory mucosal injury. The human bioactive milk component lactoferrin attenuates the proinflammatory immune response of neonatal monocyte‐derived macrophages by interference with Toll‐like receptor (TLR) pathways. Thus, the anergic/anti‐inflammatory properties of human lactoferrin might contribute to the prevention of harmful TLR‐mediated inflammatory disorders in the developing gut of premature infants.
Acknowledgement
We thank Günther Hofbauer for technical assistance with flow cytometry experiments and Rudolf Öhler for critically reviewing the manuscript. This study was founded by in‐house funding of the Medical University of Vienna.
References
- 1. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11:723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci 2008; 13:453–61. [DOI] [PubMed] [Google Scholar]
- 3. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smythies LE, Maheshwari A, Clements R et al Mucosal IL‐8 and TGF‐beta recruit blood monocytes: evidence for cross‐talk between the lamina propria stroma and myeloid cells. J Leukoc Biol 2006; 80:492–9. [DOI] [PubMed] [Google Scholar]
- 5. Maheshwari A, Kelly DR, Nicola T et al TGF‐beta2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 2011; 140:242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siggers RH, Hackam DJ. The role of innate immune‐stimulated epithelial apoptosis during gastrointestinal inflammatory diseases. Cell Mol Life Sci 2011; 68:3623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sherman MP. Lactoferrin and necrotizing enterocolitis. Clin Perinatol 2013; 40:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011; 364:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haversen L, Ohlsson BG, Hahn‐Zoric M, Hanson LA, Mattsby‐Baltzer I. Lactoferrin down‐regulates the LPS‐induced cytokine production in monocytic cells via NF‐kappa B. Cell Immunol 2002; 220:83–95. [DOI] [PubMed] [Google Scholar]
- 10. Chatterton DE, Nguyen DN, Bering SB, Sangild PT. Anti‐inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell Biol 2013; 45:1730–47. [DOI] [PubMed] [Google Scholar]
- 11. Edde L, Hipolito RB, Hwang FF, Headon DR, Shalwitz RA, Sherman MP. Lactoferrin protects neonatal rats from gut‐related systemic infection. Am J Physiol Gastrointest Liver Physiol 2001; 281:G1140–50. [DOI] [PubMed] [Google Scholar]
- 12. Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2015;CD007137. [DOI] [PubMed] [Google Scholar]
- 13. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid Based Child Health 2014; 9:584–671. [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Hoenig JD, Malin KJ et al 16S rRNA gene‐based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 2009; 3:944–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Warner BB, Deych E, Zhou Y et al Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case‐control study. Lancet 2016; 387:1928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. MohanKumar K, Namachivayam K, Cheng F et al Trinitrobenzene sulfonic acid‐induced intestinal injury in neonatal mice activates transcriptional networks similar to those seen in human necrotizing enterocolitis. Pediatr Res 2017; 81:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bury RG, Tudehope D. Enteral antibiotics for preventing necrotizing enterocolitis in low birthweight or preterm infants. Cochrane Database Syst Rev 2001;CD000405. [DOI] [PubMed] [Google Scholar]
- 18. Jenkins SJ, Hume DA. Homeostasis in the mononuclear phagocyte system. Trends Immunol 2014; 35:358–67. [DOI] [PubMed] [Google Scholar]
- 19. Hashimoto D, Chow A, Noizat C et al Tissue‐resident macrophages self‐maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013; 38:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu X, Gao H, Ke W, Giese RW, Zhu Z. The homeobox transcription factor VentX controls human macrophage terminal differentiation and proinflammatory activation. J Clin Invest 2011; 121:2599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ando K, Hasegawa K, Shindo K et al Human lactoferrin activates NF‐kappaB through the Toll‐like receptor 4 pathway while it interferes with the lipopolysaccharide‐stimulated TLR4 signaling. FEBS J 2010; 277:2051–66. [DOI] [PubMed] [Google Scholar]
- 22. Curran CS, Demick KP, Mansfield JM. Lactoferrin activates macrophages via TLR4‐dependent and ‐independent signaling pathways. Cell Immunol 2006; 242:23–30. [DOI] [PubMed] [Google Scholar]
- 23. van der Does AM, Bogaards SJ, Ravensbergen B, Beekhuizen H, van Dissel JT, Nibbering PH. Antimicrobial peptide hLF1–11 directs granulocyte–macrophage colony‐stimulating factor‐driven monocyte differentiation toward macrophages with enhanced recognition and clearance of pathogens. Antimicrob Agents Chemother 2010; 54:811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fleetwood AJ, Dinh H, Cook AD, Hertzog PJ, Hamilton JA. GM‐CSF‐ and M‐CSF‐dependent macrophage phenotypes display differential dependence on type I interferon signaling. J Leukoc Biol 2009; 86:411–21. [DOI] [PubMed] [Google Scholar]
- 25. Puddu P, Latorre D, Carollo M et al Bovine lactoferrin counteracts Toll‐like receptor mediated activation signals in antigen presenting cells. PLOS ONE 2011; 6:e22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sadeghi K, Berger A, Langgartner M et al Immaturity of infection control in preterm and term newborns is associated with impaired Toll‐like receptor signaling. J Infect Dis 2007; 195:296–302. [DOI] [PubMed] [Google Scholar]
- 27. Wisgrill L, Groschopf A, Herndl E et al Reduced TNF‐alpha response in preterm neonates is associated with impaired nonclassic monocyte function. J Leukoc Biol 2016; 100:607–12. [DOI] [PubMed] [Google Scholar]
- 28. Lonnerdal B, Jiang R, Du X. Bovine lactoferrin can be taken up by the human intestinal lactoferrin receptor and exert bioactivities. J Pediatr Gastroenterol Nutr 2011; 53:606–14. [DOI] [PubMed] [Google Scholar]
- 29. Buccigrossi V, de Marco G, Bruzzese E et al Lactoferrin induces concentration‐dependent functional modulation of intestinal proliferation and differentiation. Pediatr Res 2007; 61:410–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. Cell culture supernatants from lipopolysaccharide (LPS)‐ and lipoteichoic acid (LTA)‐stimulated monocyte‐differentiated macrophages from adult (black bars) and neonates (white bars) in the presence or absence of human lactoferrin (hLF) were assessed for cytokine levels of (a) tumour necrosis factor (TNF)‐α, (b) interleukin (IL)‐1, (c) IL‐6, (d) IL‐8 and (e) IL‐10 (n = 8 per group). Bars show mean ± standard deviation. Normal distribution was determined using the Shapiro–Wilk test. Normally distributed data were analysed using one‐way analysis of variance in accordance with Tukey and non‐normally distributed data were analysed using the Kruskal‐Wallis test. *P < 0·05; **P < 0·01. Neonatal intestinal macrophages display a proinflammatory profile that might contribute to inflammatory mucosal injury. The human bioactive milk component lactoferrin attenuates the proinflammatory immune response of neonatal monocyte‐derived macrophages by interference with Toll‐like receptor (TLR) pathways. Thus, the anergic/anti‐inflammatory properties of human lactoferrin might contribute to the prevention of harmful TLR‐mediated inflammatory disorders in the developing gut of premature infants.


