Abstract
Aims
The objective of this study was to determine the effectiveness and safety of cefazolin vs. antistaphylococcal penicillin (ASP) in the treatment of methicillin‐sensitive Staphylococcus aureus (MSSA) bacteraemia.
Methods
The databases of PubMed, Embase and Cochrane Central were used to identify comparative trials of cefazolin vs. ASP in MSSA bacteraemia. Meta‐analysis of included trials was performed to assess any differences regarding mortality, clinical cure, recurrence and withdrawal from adverse effects between groups. Data were analysed using fixed effect model. Studies were weighted using Mantel–Haenszel methodology. Heterogeneity was calculated using the I2 statistic.
Results
Nine retrospective and one prospective trials were identified involving 4728 patients, 2954 with ASP and 1774 with cefazolin. Meta‐analysis showed a lower mortality rate with cefazolin vs. ASP using fixed effect model [risk ratio (RR) 0.78, 95% confidence interval (CI) 0.69–0.88, P < 0.0001] with borderline high heterogeneity (I2 = 51%). Clinical cure was noted more often with cefazolin (RR 1.09, 95% CI 1.02–1.17, P = 0.02), although no difference was noted with relapse (RR 1.29, 95% CI 0.96–1.74 P = 0.09). Analysis also showed more withdrawals from adverse events with ASP vs. cefazolin (RR 0.27, 95% CI 0.16–0.47, P < 0.00001). A minority of patients enrolled in these trials were admitted to the intensive care unit or had endocarditis (11.4% with ASP and 9% with cefazolin).
Conclusion
Our meta‐analysis of retrospective data demonstrate that cefazolin is more effective and safer ASP in patients with MSSA bacteraemia from various causes. Low quality of trials, borderline high heterogeneity, and possible publication bias may limit the validity of our findings. Randomized trials are needed to confirm these findings.
Keywords: bacteraemia, cefazolin, nafcillin, oxacillin, Staphylococcus aureus
What is Already Known about this Subject
Numerous trials have suggested that cefazolin is more effective and safer than ASP in patients with MSSA, although the data are not consistent.
What this Study Adds
Our meta‐analysis show that cefazolin is safer and more effective than ASP in MSSA bacteremia associated with a variety of infection sources.
Introduction
Treatment of methicillin‐sensitive Staphylococcus aureus (MSSA) bacteraemia continues to be a common clinical issue despite being eclipsed by the more publicized methicillin‐resistant strain 1. Various sources recommend antistaphylococcal penicillins (ASP) as the drugs of choice for MSSA bacteraemias, especially in endocarditis 2, 3, 4. Advantages of ASP are good tissue penetration, lack of an inoculum effect, a narrow spectrum of action and decades of successful clinical experience. However, these drugs can present problems for patients and hospitals, especially when used in high doses for prolonged periods, which may be required for these infections. Such problems can include a relative high incidence of adverse effects (nephritis, bone marrow suppression, hepatotoxicity etc.), frequent dosing or constant infusion and high acquisition costs 5. Cefazolin can be an attractive alternative considering its favorable side effect profile, less frequent dosing, and low costs. However, concerns regarding the cefazolin inoculum effect leading to treatment failure, especially for deep seated infections, has dampened the enthusiasm for using cefazolin in MSSA bacteraemias. Emerging data now suggest that cefazolin may be equally or actually more effective than ASP in improving mortality for MSSA bacteraemias with fewer adverse effects, although the data are not uniform 6, 7, 8, 9, 10, 11, 12, 13, 14, 15. Therefore, we decided to identify these comparative trials, pool the data and perform a meta‐analysis to assess the efficacy and safety of cefazolin when compared to ASP in the treatment of MSSA bacteraemias.
Methods
Data sources and searches
The databases of PubMed, EMBASE and Cochrane Central Register of Controlled Trials were used to identify trials comparing the efficacy and toxicity of cefazolin vs. ASP in patients with MSSA bacteraemia. The PubMed database was searched using text and MeSH terms cefazolin nafcillin, cefazolin oxacillin, cefazolin cloxacillin, cefazolin bacteraemia. All fields were utilized in the MeSH search. A similar search was performed using EMBASE, but narrowed by adding MSSA to the search due to the high number of citations. In addition, the bibliographies of current trials and review articles were reviewed to capture those trials missed in the database searches.
Study selection
Only full length published trials in humans were included in the analysis. Abstracts and non‐English studies were excluded. Both retrospective and prospective trials were included. All considered studies needed, as the primary objective, assessment of the incidence of mortality at some point during the study period when cefazolin was compared to ASP. Secondary endpoints included rates of clinical cure, relapse and withdrawal rates due to adverse effects. Secondary endpoints were not mandatory for inclusion in the meta‐analysis. Baseline characteristics between cefazolin and ASP groups (e.g. demographics, comorbidities, types of infections, doses) must be included in these trials.
Data extraction
The authors independently searched databases and discussed which trials were eligible for inclusion. Data extraction was also performed independently and entered into the statistical software and compared for accuracy.
Statistical analysis
Percent mortality, clinical cure, relapse and discontinuation due to adverse effects between cefazolin and ASP groups were the endpoints used in our analysis. Groups were compared using risk ratios (RR) and fixed effect model with 95% confidence intervals (CIs). Studies were weighed using the Mantel–Haenszel method. Publication bias was assessed using a funnel plot. Data were presented by generation of a forest plot with RR. Heterogeneity of the studies was assessed using the I2 statistic. An I2 value of 50% or more represented significant heterogeneity among trials. An α of <0.05 was considered statistically significant. Meta‐analyses were performed using Review Manager 5.3 software from the Cochrane Collaboration.
Results
In this study, 602 citations from PubMed and 249 citations from EMBASE (from 1973 to December 2017) were identified and reviewed. Of these, 10 trials were extracted and included for analysis. The breakdown of our systematic review is outlined in Figure 1.
Figure 1.
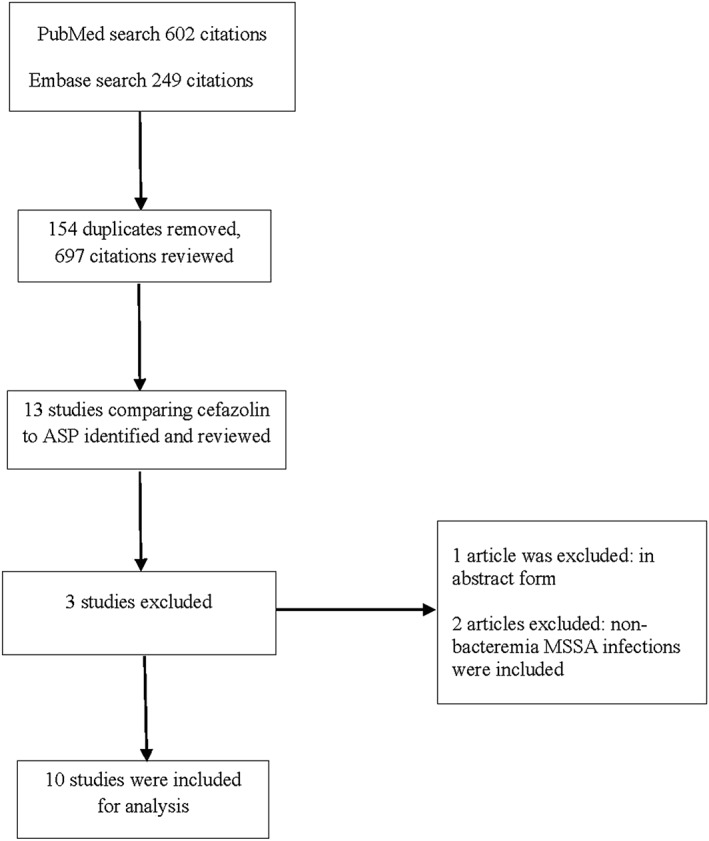
Results of the systematic review. ASP, antistaphylococcal penicillin; MSSA, methicillin‐sensitive Staphylococcus aureus
A total of 4728 patients were included in this analysis: 2954 patients receiving ASP and 1774 receiving cefazolin (Table 1). Five‐hundred thirty received cloxacillin, 328 received nafcillin and 92 received oxacillin. The largest of these trials combined both oxacillin and nafcillin as a single ASP group and did not differentiate absolute numbers. Nine trials were retrospective and one was prospective. Nine trials accounted for infection source (1 trial listed as “other infections”), six trials included percent admitted to the intensive care unit (ICU) and three trials listed a comorbidity index between groups. Dosing information of antibiotics was offered in four trials. The frequency of endocarditis among nine trials averaged 11.4% in the ASP group and 9% in the cefazolin group. One trial did not differentiate the percentage of endocarditis between ASP and cefazolin groups. Study quality was considered fair for all trials based on the Downs and Black checklist score.
Table 1.
Characteristics of analysed studies
| Renaud et al. 6 | Paul et al. 7 | Lee et al. 8 | Li et al. 9 | Bai et al. 10 | Rao et al. 11 | Pollett et al. 12 | Flynt et al. 13 | McDanel et al. 14 | Lee et al. 15 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Year published | 2011 | 2011 | 2011 | 2014 | 2014 | 2015 | 2016 | 2017 | 2017 | 2017 |
| Methodology | Retrospective cohort | Retrospective cohort | Retrospective, propensity matched, case controlled | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Prospective cohort |
| No. of centres | 1 | 1 | 1 | 2 | 6 | 2 | 1 | 4 | 119 | 10 |
| No. of patients reviewed |
Naf – 13 Cef – 14 |
Clox – 281 Cef – 72 |
Naf – 41 Cef – 41 |
Ox – 34 Cef – 59 |
Clox – 249 Cef – 105 |
Ox– 58 Cef – 103 |
Naf – 30 Cef – 70 |
Naf – 81 Cef – 68 |
Naf/Ox – 2004 Cef – 1163 |
Naf – 163 Cef – 79 |
| Ages (years) |
Naf – 50 Cef – 50 (mean) |
69 (mean) entire cohort |
Naf – 54 Cef – 53 (mean) |
Ox – 51 Cef – 51 (mean) |
Clox – 67 Cef – 69 (median) |
Ox – 53 Cef – 53 (mean) |
Naf – 50 Cef – 53 (mean) |
Naf – 54 Cef – 65 (median) |
Naf/Ox – 76% >56 yo Cef – 75% >56 yo |
Naf – 55% ≥65 yo Cef – 41% ≥65 yo |
| Male (%) |
Naf – 53 Cef – 64 |
55 (total) |
Naf – 61 Cef – 56 |
Ox – 82 Cef – 77 |
Clox – 67 Cef – 55 |
Ox – 62 Cef – 58 |
Naf – 33 Cef – 31 |
Naf – 58 Cef – 33 |
Naf/Ox – 99 Cef – 97 |
Naf – 65 Cef – 60 |
| Charlson comorbidity index score (median) | NR | NR | NR | NR | NR | NR |
Naf – 3 Cef – 4 |
NR |
Naf/Ox – 2 Cef – 2 |
Naf – 71% ≥3 Cef – 65% ≥3 |
| Intensive care unit admission (%) | NR | 5.2 (total) | NR |
Ox −18 Cef – 7 |
Clox – 18 Cef – 10 |
Ox – 32.8 Cef – 41.8 |
Naf – 27 Cef – 13 |
NR |
Naf/Ox – 19 Cef – 15 |
NR |
| Disease severity |
Severe sepsis: Clox 15% Cef 0% |
Shock: 12.2% total |
High disease wburden: Naf 44% Cef 46% |
Pitt bacteraemia score (mean): Ox 0 Cef 0 |
Shock: Clox 24% Cef 30% |
APACHE score (mean) Ox 10.3 Cef 13 |
NR | NR |
APACHE III score > 34 Naf/Ox 52% Cef 56% |
SOFA score ≥ 2: Naf 68% Cef 45% |
| Doses of antibiotics |
Clox – 500 mg to 2 g 4×/day Cef – 2–3 g after dialysis |
NR | NR |
Ox – 10–12 g day–1
Cef – 4–8 g day–1 per renal fuction Cef – 2–3 g after dialysis (15 patients) |
Clox – 12 g day–1
Cef – 3 g day–1 |
Ox – 12 g day–1
Cef – 4 g day–1 |
NR | NR | NR | NR |
| Duration of antibiotic | Up to 6 weeks for both drugs | NR |
Days (median) Naf 15 Cef 17 |
Days (median) Ox 31 Cef 39 |
Days (median) Clox 19 Cef 17 |
NR |
Days (median) Naf 20 Cef 12 |
NR | NR | NR |
| Infection site (%): | ||||||||||
| Skin, soft tissue |
Naf – 7 Cef – 14 |
15 (total) |
Naf – 20 Cef – 22 |
Ox – 3 Cef – 14 |
Clox – 17 Cef – 25 |
Ox – 22 Cef – 14 |
Naf – 0 Cef – 4 |
Naf – 19 Cef – 23 |
Naf/Ox – 23 Cef – 25 |
Naf – 23 Cef – 34 |
| Bone & joint | NR | 9 (total) |
Naf – 17 Cef – 24 |
Ox – 59 Cef – 31 |
Clox – 11 Cef – 14 |
Ox – 13 Cef – 20 |
Naf – 10 Cef – 7 |
Naf – 24 Cef – 19 |
Naf/Ox – 13 Cef – 12 |
Naf – 37 Cef – 35 |
| Respiratory | NR | NR |
Naf – 10 Cef – 7 |
Ox – 6 Cef – 4 |
Clox – 18 Cef – 16 |
Ox – 1.7 Cef – 1.9 |
Naf – 13 Cef – 1 |
NR | NR |
Naf – 9 Cef – 3 |
| Catheter related |
Naf – 38 Cef – 35 |
11 (total) |
Naf – 17 Cef – 20 |
Ox – 3 Cef – 10 |
Clox – 14 Cef – 12 |
Ox – 24 Cef – 45 |
Naf – 10 Cef – 19 |
NR | NR |
Naf – 5 Cef – 14 |
| Presence of endocarditis (%) |
Naf – 0 Cef – 1 |
6 (total) |
Naf – 2 Cef – 2 |
Ox – 12 Cef – 25 |
Clox – 12 Cef – 2 |
Ox – 20 Cef – 16 |
Naf – 17 Cef – 14 |
Naf – 27 Cef – 16 |
Naf/Ox – 7 Cef – 4 |
Naf – 6 Cef – 1 |
| Mortality assessment | 30 days | 90 days | Not specified | 90 days | 90 days | In‐hospital | 90 days | 30 days | 90 days | Within 90 days |
| Mortality % |
Naf – 15.3 Cef – 7.1 |
Clox – 32 Cef – 40 |
Naf – 12 Cef – 2 |
Ox – 2.9 Cef – 0 |
Clox – 30 Cef – 20 |
Ox – 5.1 Cef – 0.9 |
Naf – 16.6 Cef – 7.1 |
Naf – 4.9 Cef – 5.8 |
Naf/Ox – 25 Cef – 19.8 |
Naf – 14.7 Cef – 2.5 |
| Downs and Black checklist score a | 15 | 12 | 14 | 17 | 16 | 16 | 15 | 16 | 15 | 16 |
Naf, nafcillin; Clox, cloxacillin; Ox, oxacillin; Cef, cefazolin; NR, not reported; yo, years old
maximum score = 28
The result of our analysis showed a mortality rate of 16.6% with cefazolin and 24% with ASP among the entire cohort. Meta‐analysis showed these differences to be statistically significant using fixed‐effect model, (RR 0.78, 95% CI 0.69–0.88, P < 0.0001) with borderline high heterogeneity (I2 = 51%, Figure 2). We performed a subgroup analysis omitting the largest trial by McDanel et al. 14 and continued to see a statistical mortality benefit in favor of cefazolin (RR 0.74, 95% CI 0.58–0.94, P = 0.01, Figure 3). There was statistical difference in clinical cure in favour of cefazolin (five trials, RR 1.09 95% CI 1.02–1.17, P = 0.02, Figure 4); however, no difference was noted with relapse rate between groups (7 trials, RR 1.29 95% CI 0.96–1.74, P = 0.09, Figure 5). Four trials evaluated discontinuation rate from adverse effects which showed less withdrawals with cefazolin vs. ASP (RR 0.27 95% CI 0.16–0.47, P < 0.00001, Figure 6).
Figure 2.
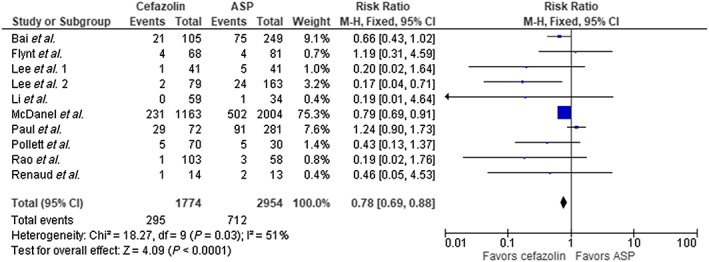
Mortality cefazolin vs. antistaphylococcal penicillin (ASP). CI, confidence interval; df, degrees of freedom
Figure 3.
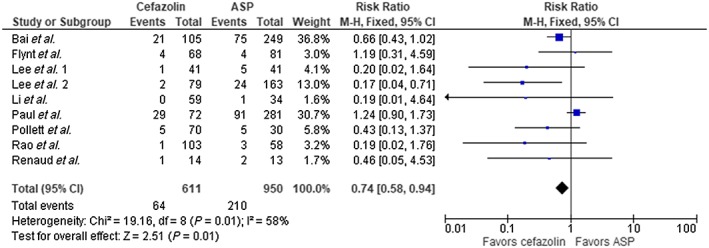
Mortality cefazolin vs. antistaphylococcal penicillin (ASP), excluding McDanel et al. 14. CI, confidence interval; df, degrees of freedom
Figure 4.
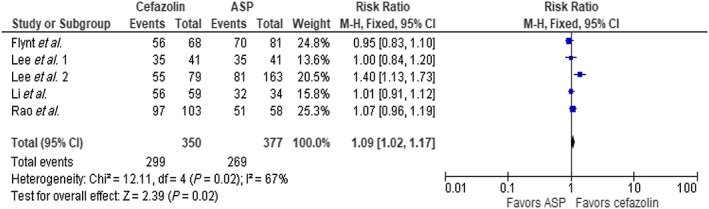
Clinical cure, cefazolin vs. antistaphylococcal penicillin (ASP). CI, confidence interval; df, degrees of freedom
Figure 5.
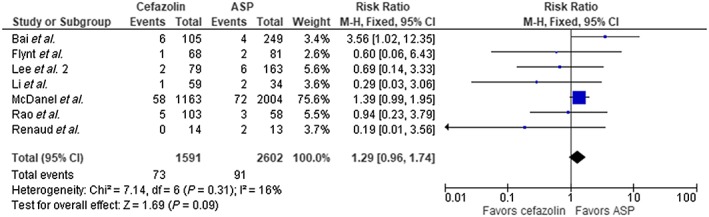
Relapse cefazolin vs. antistaphylococcal penicillin (ASP). CI, confidence interval; df, degrees of freedom
Figure 6.
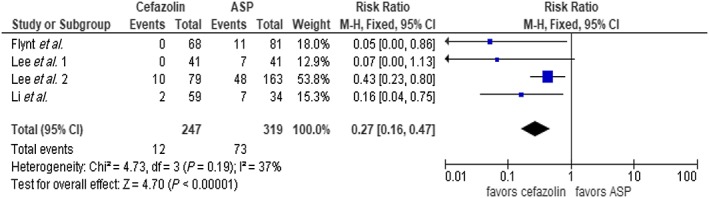
Withdrawal from adverse effects cefazolin vs. antistaphylococcal penicillin (ASP). CI, confidence interval; df, degrees of freedom
Discussion
The results of our meta‐analysis show that cefazolin is more effective in affecting clinical cure and safer than ASP in MSSA bacteraemias linked to a variety of infection sources. A mortality difference was noted in favour of cefazolin vs. ASP. The absolute difference was 7.4% which gives a number needed to treat of approximately 14. In other words, treating 14 patients with cefazolin in place of ASP will result in one additional life saved in MSSA bacteraemias from various infections.
It is unclear if these results can be applied to all patients with MSSA bacteraemia. In our cohort, the percentage of ICU admissions was relatively low apart from one trial 11. This could indicate the percentage of patients with more severe, or deep seated MSSA infections (e.g. undrained abscesses) were uncommon. In addition, the percentage of patients with endocarditis was relatively infrequent in our cohort (9–11.4% incidence). It is possible that cefazolin would be less efficacious than ASP in these settings since more severe infection may predispose to an inoculum effect to cefazolin, which could result in treatment failure. Inoculum effect is the phenomena of rising minimum inhibitory concentrations in the presence of increasing bacterial load (inoculum). Cases of cefazolin treatment failures have been reported in patients with endocarditis who were subsequently treated successfully with ASP 16, 17, 18. A series of patients undergoing hemodialysis and MSSA bacteraemia exhibited an inoculum effect in three of six cefazolin failures 19. Contrary to this, none of the other six patients successfully treated with cefazolin displayed this effect. A larger investigation compared the outcome of 65 patients with MSSA bacteraemia with documented cefazolin inoculum effect to 48 patients without this effect 20. They showed that those who exhibited the effect were more likely to have persistent bacteraemia than the control group despite high dose cefazolin used in the majority of patients (≥6 g day–1). There was a trend towards more treatment failures, antibiotic changes due to clinical failure, and overall mortality in the group exhibiting an inoculum effect, but these differences were not statistically significant. This study may have been underpowered to show such differences. Another report described 303 cases of MSSA bacteraemia and demonstrated that cefazolin may be used successfully in high‐inoculum infections if the isolate is sensitive to either clindamycin or erythromycin 21.
Our analysis demonstrated that fewer patients on cefazolin stopped therapy due to adverse events than those receiving ASP, although only four trials were included. These results are consistent with other observational studies that compared ASP to cefazolin in the outpatient treatment of MSSA infections. One trial compared the tolerability of nafcillin (366 patients) to cefazolin (119 patients) in the outpatient treatment of MSSA infections from various sources 22. Premature discontinuation rates were 33.8% and 6.7% for nafcillin and cefazolin, respectively (P < 0.001). Over 7% of patients on nafcillin who stopped therapy prematurely were switched to cefazolin. More patients receiving nafcillin had drug‐emergent events, which included rash, renal impairment and liver abnormalities. Another trial evaluated the safety of cefazolin (205 patients) to both nafcillin (94 patients) and oxacillin (157 patients) in the outpatient treatment of various MSSA infections 23. They showed withdrawal rates from side effects to be more common with nafcillin (5.4%) than cefazolin (2.5%), although this was not statistically significant. The withdrawal rate with oxacillin was comparable to cefazolin.
Cost of therapy can be a consideration in choosing between cefazolin and ASP. The average wholesale price of nafcillin and oxacillin, 12 g day–1 is $158.40 and $174, respectively. Cloxacillin is no longer on the market in the USA. This is in contrast to the average wholesale price for cefazolin 6 g day–1, which is $29.70. This does not include the costs involved with pharmacy preparation and nursing administration since ASP are typically dosed 6 times a day vs. 3 times for cefazolin 24. In the setting of haemodialysis, cefazolin is administered after dialysis which is typically three times weekly. Multiply the cost differences over a prolonged course, which is often needed for MSSA bacteraemias, and the differences are magnified significantly.
The major limitation of our analysis includes the retrospective nature of nine of the 10 trials. This can lead to selection bias with patients and introduce confounding variables that could affect the outcomes. For instance, sicker patients may have been included in the ASP groups, but we noted that disease severity was roughly comparable between groups based on various metrics. One trial did do propensity matching in an attempt to ameliorate these pitfalls. All trials included a relatively small sample of patients apart from one trial involving Veteran Affairs medical centres 14. This large trial showed a mortality benefit of cefazolin vs. ASP and could have slanted our results to favour cefazolin, although our subgroup analysis suggest this not to be the case. Our funnel plot shows some asymmetry which could suggest publication bias and limit the validity or our findings (Figure 7). To our knowledge, there are no randomized controlled trials that compare cefazolin to ASP for MSSA bacteraemia. In addition, the diverse nature of the infections among trials with the minority of patients treated for endocarditis and/or with ICU admission can limit the applicability of our results to sicker patients with MSSA bacteraemia.
Figure 7.
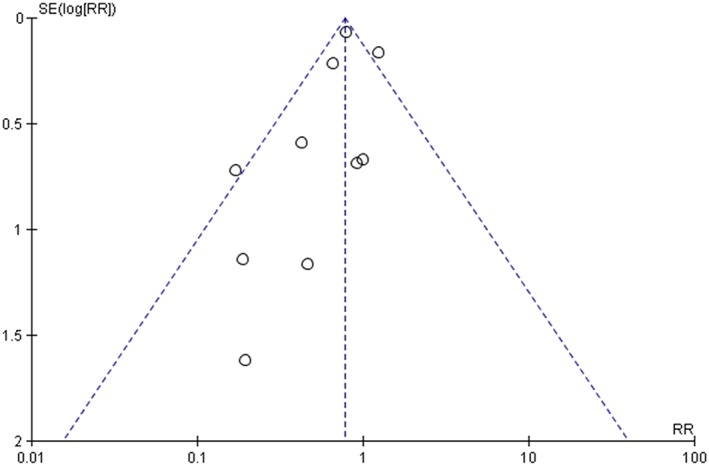
Funnel plot (mortality data)
Conclusion
Our analysis showed that cefazolin is superior to ASP in terms of clinical cure, safety and mortality in the treatment of MSSA bacteraemia from various sources. This is tempered by the retrospective nature of most trials, moderate heterogeneity, and possibly publication bias. Conducting randomized controlled trials of this comparison in MSSA bacteraemia would greatly clarify this issue.
Competing Interests
There are no competing interests to declare.
Rindone, J. P. , and Mellen, C. K. (2018) Meta‐analysis of trials comparing cefazolin to antistaphylococcal penicillins in the treatment of methicillin‐sensitive Staphylococcus aureus bacteraemia. Br J Clin Pharmacol, 84: 1258–1266. doi: 10.1111/bcp.13554.
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
References
- 1. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39: 309–317. [DOI] [PubMed] [Google Scholar]
- 2. Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, et al Clinical practice guidelines for the diagnosis and management of intravascular catheter‐related infections: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, et al Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132: 1435–1486. [DOI] [PubMed] [Google Scholar]
- 4. Sanford Guide to Antimicrobial Therapy. Available at http://www.webedition-dva-sanfordguide.com (last accessed 1 May 2017).
- 5. Dahlgren A. Adverse drug reactions in home care patients receiving nafcillin or oxacillin. Am J Health Syst Pharm 1997; 54: 1176–1179. [DOI] [PubMed] [Google Scholar]
- 6. Renaud CJ, Lin X, Subramanian S, Fisher DA. High‐dose cefazolin on consecutive hemodialysis in anuric patients with staphylococcal bacteremia. Hemodial Int 2011; 15: 63–68. [DOI] [PubMed] [Google Scholar]
- 7. Paul M, Zemer‐Wassercug N, Talker O, Lishtzinsky Y, Lev B, Samra Z, et al Are all beta‐lactams similarly effective in the treatment of methicillin‐sensitive Staphylococcus aureus bacteraemia? Clin Microbiol Infect 2011; 17: 1581–1586. [DOI] [PubMed] [Google Scholar]
- 8. Lee S, Gynu‐Choe P, Song KH, Park SW, Kim HB, Kim NJ, et al Is cefazolin inferior to nafcillin for the treatment of methicillin‐susceptible Staphylococcus aureus bacteremia? Antimicrob Agents Chemother 2011; 55: 5122–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li J, Echevarria KL, Hughes DW, Cadena JA, Bowling JE, Lewis JS. Comparison of cefazolin versus oxacillin for the treatment of complicated bacteremia cause by methicillin‐susceptible Staphylococcus aureus . Antimicrob Agents Chemother 2014; 58: 5117–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bai AD, Showler A, Burry L, Steinberg M, Ricciuto DR, Fernandes T, et al Comparative effectiveness of cefazolin versus cloxacillin as definitive antibiotic therapy for MSSA bacteraemia: results from a large multicentre cohort study. J Antimicrob Chemother 2015; 70: 1539–1546. [DOI] [PubMed] [Google Scholar]
- 11. Rao SN, Rhodes NJ, Lee BJ, Scheetz MH, Hanson AP, Segreti J, et al Treatment outcomes of cefazolin versus oxacillin for deep‐seated methicillin‐susceptible Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 2015; 59: 5232–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pollett S, Baxi SM, Rutherford GW, Doernberg SB, Bacchetti P, Chambers HF. Cefazolin versus nafcillin for methicillin‐sensitive Staphylococcus aureus bloodstream infection in a California tertiary medical center. Antimicrob Agents Chemother 2016; 60: 4684–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flynt LK, Kenney RM, Zervos MJ. The safety and economic impact of cefazolin versus nafcillin for the treatment of methicillin‐susceptible Staphylococcus aureus bloodstream infections. Infect Dis Ther 2017; 6: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McDanel JS, Roghmann MC, Perencevich EN, Ohl ME, Goto M, Livorsi DJ, et al Comparative effectiveness of cefazolin versus nafcillin or oxacillin for treatment of methicillin‐susceptible Staphylococcus aureus infections complicated by bacteremia: A nationwide cohort study. Clin Infect Dis 2017; 65: 100–106. [DOI] [PubMed] [Google Scholar]
- 15. Lee S, Song KH, Jung SI, Park WB, Lee SH, Kim YS, et al Comparative outcomes of cefazolin versus nafcillin for methicillin‐susceptible Staphylococcus aureus bacteraemia: a prospective multicenter cohort study in Korea. Clin Microbiol Infect 2018; 24: 152–158. [DOI] [PubMed] [Google Scholar]
- 16. Bryant RE, Alford RH. Unsuccessful treatment of staphylococcal endocarditis with cefazolin. JAMA 1977; 237: 569–570. [PubMed] [Google Scholar]
- 17. Nannini EC, Singh KV, Murray BE. Relapse of type A beta‐lactamase‐producing Staphylococcus aureus native valve endocarditis during cefazolin therapy: revisiting the issue. Clin Infect Dis 2003; 37: 1194–1198. [DOI] [PubMed] [Google Scholar]
- 18. Fernandez‐Guerrero ML, de Gorgolas M. Cefazolin therapy for Staphylococcus aureus bacteremia. Clin Infect Dis 2005; 41: 127. [DOI] [PubMed] [Google Scholar]
- 19. Nannini EC, Stryjewski ME, Singh KV, Bourgogne A, Rude TH, Corey GR, et al Inoculum effect with cefazolin among clinical isolates of methicillin‐susceptible Staphylococcus aureus: frequency and possible cause of cefazolin treatment failure. Antimicrob Agents Chemother 2009; 53: 3437–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee S, Kwon KT, Kim HI, Chang HH, Lee JM, Choe PG, et al Clinical implications of cefazolin inoculum effect and beta‐lactamase type on methicillin‐susceptible Staphylococcus aureus bacteremia. Microb Drug Resist 2014; 20: 568–574. [DOI] [PubMed] [Google Scholar]
- 21. Song KH, Jung SI, Lee S, Park S, Kiem SM, Lee SH, et al Characteristics of cefazolin inoculum effect‐positive methicillin‐susceptible Staphylococcus aureus infection in a multicentre bacteraemia cohort. Eur J Clin Microbiol Infect Dis 2017; 36: 285–294. [DOI] [PubMed] [Google Scholar]
- 22. Youngster I, Shenoy ES, Hooper DC, Nelson SB. Comparative evaluation of the tolerability of cefazolin and nafcillin for the treatment of methicillin‐susceptible Staphylococcus aureus infections in the outpatient setting. Clin Infect Dis 2014; 59: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wynn M, Dalovisio JR, Tice AD, Jiang X. Evaluation of the efficacy and safety of outpatient parenteral antimicrobial therapy for infections with methicillin‐sensitive Staphylococcus aureus . S Med J 2005; 98: 590–595. [DOI] [PubMed] [Google Scholar]
- 24. Wolters Kluwer . In: Drug Facts and Comparisons, ed Kastrup EK. St Louis, 2017; 2997–2999. [Google Scholar]


