Abstract
Background and Purpose
The pathophysiological role of α6‐subunit‐containing GABAA receptors, which are mainly expressed in cerebellar granule cells, remains unclear. Recently, we demonstrated that hispidulin, a flavonoid isolated from a local herb that remitted a patient's intractable motor tics, attenuated methamphetamine‐induced hyperlocomotion in mice as a positive allosteric modulator (PAM) of cerebellar α6GABAA receptors. Here, using hispidulin and a selective α6GABAA receptor PAM, the pyrazoloquinolinone Compound 6, we revealed an unprecedented role of cerebellar α6GABAA receptors in disrupted prepulse inhibition of the startle response (PPI), which reflects sensorimotor gating deficits manifested in several neuropsychiatric disorders.
Experimental Approach
PPI disruptions were induced by methamphetamine and NMDA receptor antagonists in mice. Effects of the tested compounds were measured in Xenopus oocytes expressing recombinant α6β3γ2SGABAA receptors.
Key Results
Hispidulin given i.p. or by bilateral intracerebellar (i.cb.) injection rescued PPI disruptions induced by methamphetamine, ketamine, MK‐801 and phencyclidine. Intracerebellar effects of hispidulin were mimicked by Ro15‐4513 and loreclezole (two α6GABAA receptor PAMs), but not by diazepam (an α6GABAA receptor‐inactive benzodiazepine) and were antagonized by furosemide (i.cb.), an α6GABAA receptor antagonist. Importantly, Compound 6 (i.p.) also rescued methamphetamine‐induced PPI disruption, an effect prevented by furosemide (i.cb.). Both hispidulin and Compound 6 potentiated α6β3γ2SGABAA receptor‐mediated GABA currents.
Conclusions and Implications
Positive allosteric modulation of cerebellar α6GABAA receptors rescued disrupted PPI by attenuating granule cell activity. α6GABAA receptor‐selective PAMs are potential medicines for treating sensorimotor gating deficits in neuropsychiatric disorders. A mechanistic hypothesis is based on evidence for cerebellar contributions to cognitive functioning including sensorimotor gating.
Abbreviations
- α6GABAA receptors
α6‐subunit‐containing GABAA receptors
- DCN
deep cerebellar nuclei
- MIH
methamphetamine‐induced hyperlocomotion
- PAM
positive allosteric modulator
- PCP
phencyclidine
- PFC
prefrontal cortex
- PPI
prepulse inhibition of the startle response
- TS
Tourette syndrome
Introduction
Some years ago, we identified a patient with intractable motor tic disorders who responded well to a herbal remedy consisting of the leaf juice of Clerodendrum inerme (L.) Gaertn (C. inerme), without apparent haemotoxicity, hepatic toxicity or renal toxicity (Fan et al., 2009). Tic disorders and Tourette syndrome (TS), an idiopathic spectrum of motor tic disorders, are attributed to an over‐reactive dopaminergic system in the cortico–thalamic–striatal circuit (Singer, 2005). Therefore, using a hyper‐dopaminergic behaviour model, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4803‐induced hyperlocomotion (MIH), we identified the flavonoid hispidulin (6‐methoxy‐4′,5,7‐trihydoxyflavonoid) (Figure 1A) as an active constituent of the ethanol extract of C. inerme leaves (Huang et al., 2015).
Figure 1.
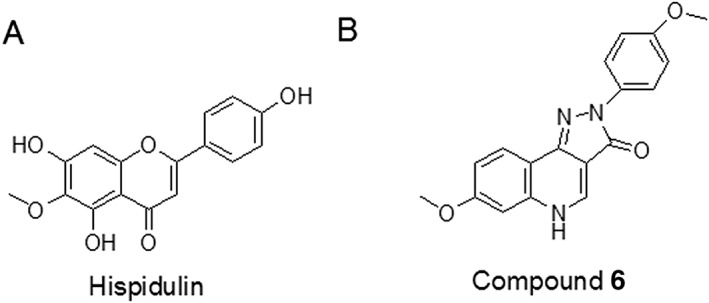
Chemical structures of hispidulin and Compound 6.
Hispidulin has micromolar affinity for the benzodiazepine site of human http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=72 (Kavvadias et al., 2003) and is a positive allosteric modulator (PAM) of oocyte‐expressed recombinant GABAA receptors comprising the α1β2γ2S, α2β2γ2S, α3β2γ2S, α5β2γ2S or α6β2γ2S subunits (Kavvadias et al., 2004). It is noteworthy that hispidulin is a PAM of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=409&familyId=72&familyType=IC‐containing GABAA receptors (α6GABAA receptors) that are mainly expressed in the granule cells of the cerebellum (Gutierrez et al., 1996; Pirker et al., 2000). Among 92 neurotransmitter receptors, enzymes and transporters, we found that hispidulin displayed micromolar affinity only at GABAA receptors and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2472 (Liao et al., 2016). Further, hispidulin alleviated MIH in mice by acting as a PAM at cerebellar α6GABAA receptors, but not as a COMT inhibitor, at doses without benzodiazepine‐like effects, such as sedation, anxiolysis and motor impairment (Liao et al., 2016).
In addition to motor tics, TS patients also manifest a sensorimotor gating deficit, which may contribute to their premonitory urges (Leckman et al., 1993) and can be measured by a disruption in the prepulse inhibition of the startle response (PPI) (Swerdlow et al., 2001). In addition to tic disorders/TS, PPI disruptions are also manifested in patients with several neuropsychiatric disorders, such as obsessive–compulsive disorder (Swerdlow et al., 1993), attention deficit disorder (Ornitz et al., 1992), panic disorder (Ludewig et al., 2002), nocturnal enuresis (Freitag et al., 2006), Huntington's disease (Swerdlow et al., 1995), premenstrual dysphoric disorder (Kask et al., 2008), mania in bipolar disorder (Giakoumaki et al., 2007), antisocial personality disorder (Kumari et al., 2005) and schizophrenia (Braff et al., 1978). Especially in patients with schizophrenia, PPI disruption is a well‐known endophenotype manifestation (Braff et al., 1978).
In PPI disruption mouse models induced by methamphetamine and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75 blockers, based on hyper‐dopaminergic and hypo‐glutamatergic hypotheses of schizophrenia, respectively (Geyer and Moghaddam, 2002), the ethanolic extract of C. inerme was also effective (Chen et al., 2012). Here, we have investigated whether the effects of C. inerme extracts on PPI disruptions could also be attributed to hispidulin and whether PAM effects on α6GABAA receptors and/or inhibitory effects on COMT were involved. To substantiate whether PAM action at cerebellar α6GABAA receptors can rescue PPI disruptions, we also investigated the effects of the pyrazoloquinolinone Compound 6 (Figure 1B), which, in contrast to hispidulin, is a highly selective PAM at α6β2γ2 and α6β3γ2GABAA receptors (Varagic et al., 2013). Finally, we examined whether effects of hispidulin and Compound 6 could be prevented by an intracerebellar (i.cb.) microinjection of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4839, a selective α6GABAA receptor antagonist.
Methods
Animal experiments
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of National Taiwan University, College of Medicine (NTUMC) and Meijo University, Japan. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). All animal procedures were performed in male ICR mice (6‐8 week‐old; BioLASCO Taiwan Co., Ltd. Ilan, Taiwan) as reported in our previous studies conducted in NTUMC (Chen et al., 2012; Huang et al., 2015) except the experiments with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4282 which were conducted in male 6 week‐old ddY mice (Nihon SLC, Shizuoka, Japan) at Meijo University. Animals were randomly divided into several groups as needed. The experimenters were blind to the treatment(s) in each group.
The PPI test
Except for the experiments with the PPI disruption model induced by PCP (PCP‐PPI), all PPI tests were performed with two acoustic stimulation protocols (71–115 and 77–115 dB) as reported previously with a PPI apparatus (SR‐LAB; San Diego Instruments, San Diego, CA, USA) consisting of a startle chamber equipped with various programming acoustic stimulations (Chen et al., 2012). Briefly, the mouse was placed in the startle chamber for a 4 min acclimation period with a 65 dB background noise. There were four types of startle trials: PULSEALONE (115 dB, 20 ms), two types of PREPULSE + PULSE (71 + 115 and 77 + 115 dB, interval: 120 ms) and NOSTIM (no stimulation with background 65 dB only). In each session, the mouse received four NOSTIM trials first, followed by four types of trials randomly given 14 times (56 trials in total) and finally four PULSEALONE trials. The magnitude of PPI (PPI%) was determined by the summarized startle responses in PULSEALONE and PREPULSE + PULSE trials, according to the equation: (PULSEALONE − PREPULSE + PULSE) ∕ PULSEALONE × 100%. To induce PPI disruptions, mice were injected i.p. (pretreatment) with 2 mg·kg−1 methamphetamine (METH‐PPI) or 30 mg·kg−1 http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4233 (Ketamine‐PPI) for 10 min, or with 0.3 mg·kg−1 http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2403 MK‐PPI), 15 min before receiving the PPI test, unless stated otherwise.
In the PCP‐PPI disruption model, three PPI protocols, 69–120, 73–120 and 81–120 dB, were used, and PCP (1 mg·kg−1) was injected s.c. into ddY mice 10 min before the PPI test (Zou et al., 2008).
Bilateral intracerebellar microinjection
Bilateral i.cb. microinjection was performed as described previously (Liao et al., 2016). Briefly, the mouse was, under anaesthesia, implanted with two 24‐gauge stainless‐steel guide cannulas, respectively, directed towards the right and left lateral cerebella (−6.4 mm caudal, ±1.5 mm lateral and −1.0 mm ventral from bregma) (Paxinos and Franklin, 2001). Seven days after the cannulation, mice received bilateral i.cb. microinjections, which were performed by slowly infusing 0.5 μL (each side) drug solution for 30 s with a 60 s hold time through a microinfusion pump (KDS311; KD Scientific Inc., Holliston, MA, USA). The microinjection site was confirmed by the positive staining with Trypan blue.
Electrophysiological measurements at recombinant α6β3γ2GABAA and α6β3δGABAA receptors
Two‐electrode voltage‐clamp recordings were performed in Xenopus laevis oocytes expressing recombinant α6β3γ2GABAA receptors as described previously (Varagic et al., 2013). To obtain α6β3γ2GABAA receptors with efficient incorporation of the γ2 subunit and thus consistent PAM responses of tested compounds, we utilized concatenated subunits as described previously (Minier and Sigel, 2004), and the constructs were a kind gift from E. Sigel (University of Bern, Institute for Biochemistry and Molecular Medicine. Bern, Switzerland). Specifically, cRNA coding for one triple concatemer γ2‐β3‐α6 was co‐injected in oocytes with cRNA coding for one double concatemer β3‐α6 in a 1:1 ratio, instead of non‐concatenated α6, β3 and γ2 subunits. The PAM effect of the tested compound on α6β3γ2GABAA receptors was measured by the increment of the Cl− current induced by GABA at EC3–5, that is, the concentration needed to elicit 3–5% of the maximum GABA current in each oocyte.
Because the arrangement of the δ‐subunit‐containing GABAA receptors is still not clear, subunit concatenation for α6β3δGABAA receptors is not a viable option. To determine whether drugs also modulate these receptors, mRNA of the individual subunits was mixed and injected as described previously (Mirheydari et al., 2014). We collected data only from cells that displayed at least 500% enhancement of the GABA EC10 currents in response to the subunit‐selective compound, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=4184 (4‐chloro‐N‐[2‐(thiophen‐2‐yl)imidazo[1,2‐a]pyridin‐3‐yl]benzamide), which is an established method to test incorporation of the δ subunit.
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Data are expressed as the mean ± SEM. Statistical comparisons among groups were analysed by ANOVA with Tukey's post hoc test, and differences between two groups were analysed by Student's t‐test. Differences were considered significant if P < 0.05.
Materials
Hispidulin was purchased from Tocris Bioscience (Bristol, UK) or isolated from the ethanol extract of C. inerme leaves as reported previously (Chen et al., 2012; Huang et al., 2015) with the structure identified by NMR and mass spectroscopy. Isolated hispidulin displayed the same efficacy in alleviating MIH as commercial hispidulin purchased from Tocris Bioscience (Huang et al., 2015). Compound 6 was synthesized as reported previously (Zhang et al., 1995). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5466, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4296, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3364 and furosemide were purchased from Tocris Bioscience, (+)MK‐801 hydrogen maleate, methamphetamine and OR‐486 from Sigma‐Aldrich (St. Louis, MO, USA) and ketamine from Parke‐Davis (Taoyuan, Taiwan). Diazepam, methamphetamine and ketamine were purchased under the approval from Food and Drug Administration, the Ministry of Health and Welfare, Taiwan. PCP hydrochloride was synthesized according to the method of Maddox et al. (1965), and its purity has been confirmed by the melting point and UV spectrum.
Methamphetamine, ketamine, MK‐801 and PCP were dissolved in normal saline. Hispidulin, Compound 6, OR‐486 and diazepam, when given by i.p. injection, were dissolved in a vehicle containing 20% DMSO, 20% Cremophor® EL (polyoxyethylene castor; Sigma‐Aldrich) and 60% normal saline. Hispidulin, Ro15‐4513, loreclezole, diazepam and furosemide, when administered by i.cb. microinjection, were dissolved in DMSO as reported previously (Liao et al., 2016). All drugs were administered 10 min before the test session unless stated otherwise. The i.p. or s.c. injection volume was 10 mL·kg−1. For oocyte experiments, hispidulin, Compound 6 and OR‐486 were dissolved in DMSO as stock solutions. From the stock solutions, the compounds were diluted to final concentrations in the Xenopus Ringer solution, where the final concentration of DMSO was less than 0.1% (Varagic et al., 2013).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2017), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c)
Results
Hispidulin rescued METH‐PPI disruption at doses not eliciting benzodiazepine‐like effects
In the METH‐PPI disruption model, a hyper‐dopaminergic model that was responsive to antipsychotic drugs and the C. inerme ethanol extract (Chen et al., 2012), hispidulin was tested at 1, 4, 10 and 50 mg·kg−1 (i.p.). In the METH‐PPI group, PPI magnitudes elicited by 71–115 and 77–115 dB were significantly lower than in control mice (Figure 2A, E, F), suggesting that METH disrupts PPI. Hispidulin at 4 mg·kg−1 (i.p.) and above significantly reduced METH‐PPI disruption. At 10 mg·kg−1, it restored PPI magnitudes elicited by 71–115 and 77–115 dB, respectively, to control levels (Figure 2A). Increasing the dose to 50 mg·kg−1, hispidulin also completely reversed the disruption in METH‐PPI (Figure 2A). Note that hispidulin at doses below 100 mg·kg−1 did not exert benzodiazepine‐like effects (Liao et al., 2016),
Figure 2.
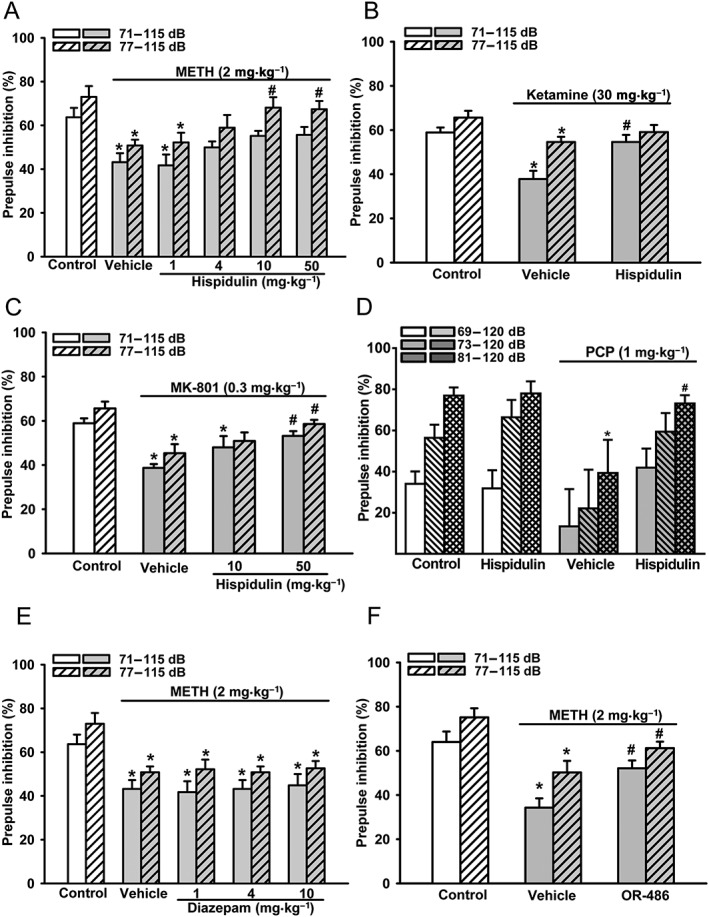
Effects of hispidulin, diazepam and OR‐486 on disruptions of prepulse inhibition of acoustic startle response (PPI) in mice. PPI disruptions were induced by methamphetamine (METH‐PPI), ketamine (KI‐PPI), MK‐801 (MK‐PPI) or PCP (PCP‐PPI). (A) Effects of hispidulin (1, 4, 10 and 50 mg·kg−1, i.p.) on METH‐PPI. (B) The effect of hispidulin (10 mg·kg−1, i.p.) on Ketamine‐PPI. (C) Effects of hispidulin (10 and 50 mg·kg−1, i.p.) on MK‐PPI. (D) The effect of hispidulin (10 mg·kg−1, i.p.) on PCP‐PPI. (E) Effects of diazepam (1, 4 and 10 mg·kg−1, i.p.), an α6GABAA receptor‐inactive classical benzodiazepine, on METH‐PPI. (F) The effect of OR‐486 (10 mg·kg−1, i.p.), a selective COMT inhibitor, on METH‐PPI. The magnitude of PPI was measured by the inhibition ratio of the startle response in mice receiving a 115 dB acoustic stimulation with and without a prepulse of 71 dB (71–115 dB) or 77 dB (77–115 dB) 120 ms ahead, except in the PCP‐PPI experiment where three startle protocols, 69–120, 73–120 and 81–120 dB, were used. Mice were i.p. pretreated with the tested drug or vehicle for 10 min, followed by methamphetamine (2 mg·kg−1, i.p.), ketamine (30 mg·kg−1, i.p.) or PCP (1 mg·kg−1, s.c.) for 10 min, or MK‐801 (0.3 mg·kg−1, i.p.), 15 min, before the PPI test. The control group of mice was treated with normal saline 10 min before the PPI test. Data shown are means ± SEM; n = 8. * P < 0.05, significantly different from the control group; # P < 0.05, significantly different from the vehicle group; one‐way ANOVA with Tukey's post hoc test.
Hispidulin rescued PPI disruptions induced by NMDA channel blockers
Hispidulin also rescued PPI disruptions induced by NMDA channel blockers. In the ketamine disruption model (Figure 2B), PPI magnitudes elicited by 71–115 and 77–115 dB were reduced by ketamine (30 mg·kg−1, i.p.). Hispidulin, at 10 mg·kg−1 (i.p.), completely inhibited ketamine‐induced PPI disruption (Figure 2B). In the MK‐PPI disruption model (Figure 2C), PPI magnitudes elicited by 71–115 and 77–115 dB were reduced by MK‐801 (0.3 mg·kg−1, i.p.) and hispidulin at 50 mg·kg−1 (i.p.) restored PPI magnitudes elicited by 71–115 and 77–115 dB to control levels (Figure 2C).
Hispidulin also significantly reversed the PPI disruption induced by PCP (Figure 2D). As shown in Figure 2D, the PPI magnitude elicited by 81–120 dB in ddY mice was reduced by PCP (1 mg·kg−1, s.c.) and was restored by hispidulin (10 mg·kg−1, i.p.) to control levels. Notably, hispidulin (10 mg·kg−1, i.p.) per se did not affect PPI in control mice (without PCP treatment) (Figure 2D).
Diazepam did not rescue METH‐PPI disruption at motor‐impairing doses
In addition to α6GABAA receptors, hispidulin is also a PAM of other benzodiazepine‐sensitive GABAA receptors (Kavvadias et al., 2004). We, therefore, examined whether diazepam, a typical benzodiazepine ineffective at α6GABAA receptors, would affect METH‐PPI disruption. Diazepam, at doses up to 10 mg·kg−1 (i.p.) that displayed significant sedative, anxiolytic and motor‐impairing activities in ICR mice (Liao et al., 2016), did not rescue METH PPI disruption (Figure 2E). Thus, it is unlikely that hispidulin reversed PPI disruptions via acting at diazepam‐sensitive GABAA receptors.
OR‐486 rescued METH‐PPI disruption
Hispidulin displayed micromolar affinity only at GABAA receptors and COMT in a binding affinity screening of 92 different neurotransmitter receptors, enzymes and transporters (Liao et al., 2016). To investigate the contribution of COMT inhibition to the effect of hispidulin on PPI disruptions, we examined the effect of OR‐486, a selective COMT inhibitor (Nissinen et al., 1988), on METH‐PPI disruption. OR‐486 was 2.75 times more potent than hispidulin in inhibiting COMT activity in a concurrent assay (Liao et al., 2016). OR‐486, at the same dose (10 mg·kg−1, i.p.) as hispidulin, also partly reversed METH‐PPI disruption in mice (Figure 2F). Therefore, inhibition of COMT by hispidulin may also contribute to its ability to restore PPI disruption.
Intracerebellar hispidulin rescued METH‐PPI, Ketamine‐PPI and MK‐PPI disruptions
We further investigated whether α6GABAA receptors, which are mainly expressed in cerebellar granule cells (Gutierrez et al., 1996; Pirker et al., 2000), could be the target of hispidulin. For this, hispidulin (10 nmol) was administered by bilateral microinjections into the cerebellum (i.cb.) of mice and, as shown in Figure 3, it restored the PPI disruption induced by methamphetamine (Figure 3A) or ketamine (Figure 3B). In the MK‐PPI model, i.cb. hispidulin was also effective, although a higher dose (50 nmol) was needed to completely reverse the PPI disruption (Figure 3C).
Figure 3.
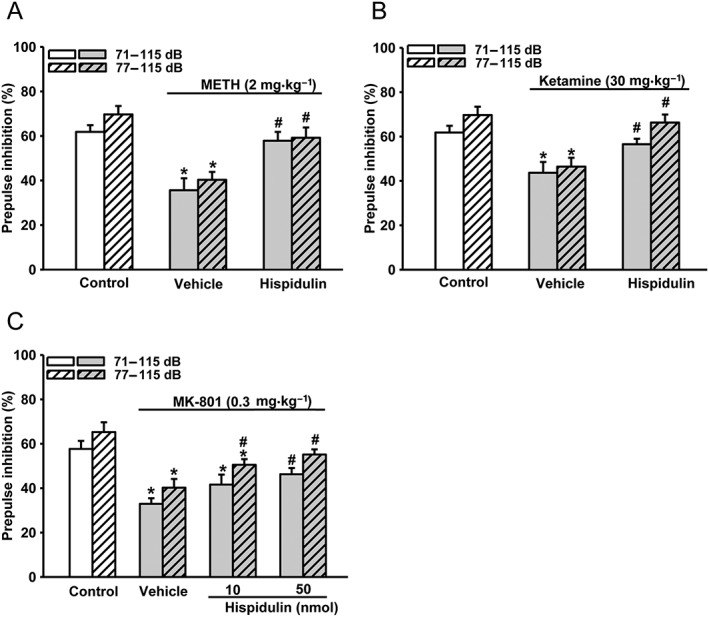
Effects of i.cb. microinjection of hispidulin on disruptions of METH‐PPI, Ketamine‐PPI and MK‐PPI. Hispidulin (10 or 50 nmol) or vehicle was administered by microinjection at the lateral cerebellum (i.cb.) bilaterally 10 min before injection of (A) methamphetamine (METH; 2 mg·kg−1, i.p.), (B) ketamine (30 mg·kg−1, i.p.) or (C) MK‐801 (0.3 mg·kg−1, i.p.). PPI was measured and analysed as in Figure 2. Data shown are means ± SEM; n = 8. * P < 0.05, significantly different from the control group; # P < 0.05, significantly different from the vehicle group.
Hispidulin (i.cb.)‐induced PPI restoration was mimicked by α6GABAA receptor PAMs and prevented by an α6GABAA receptor antagonist
In a second set of experiments, we used a pharmacological approach with diazepam, Ro15‐4513, loreclezole and furosemide to confirm that hispidulin was acting as a PAM at α6GABAA receptors in the cerebellum. Diazepam is a classical benzodiazepine that is ineffective at α6GABAA receptors (Derry et al., 2004). Ro15‐4513, although being a negative allosteric modulator at the benzodiazepine site of diazepam‐sensitive GABAA receptors, is a PAM at diazepam‐insensitive GABAA receptors, including the α6GABAA receptors (Hadingham et al., 1996; Knoflach et al., 1996). Loreclezole is a PAM at the majority of β2/3‐containing GABAA receptors independent of the α‐subunit type (Wingrove et al., 1994). Furosemide is a non‐competitive selective antagonist of α6GABAA receptors (Korpi et al., 1995).
Bilateral i.cb. microinjections of Ro15‐4513 (10 nmol) and loreclezole (10 nmol), but not diazepam (10 nmol), significantly reversed METH‐PPI disruption (Figure 4A). These effects of Ro15‐4513, loreclezole and hispidulin were prevented by i.cb. co‐microinjection of furosemide (10 nmol) (Figure 4B). However, i.cb. furosemide per se affected neither METH‐PPI disruption (Figure 4A) nor the PPI in control mice without disruption (Supporting Information Figure S1). Similarly, i.cb. Ro15‐4513 (10 nmol) and hispidulin (10 nmol), but not diazepam (10 nmol), were also effective (Figure 4C) in the Ketamine‐PPI disruption model. Effects of Ro15‐4513 and hispidulin were also prevented by i.cb. furosemide (10 nmol), in this model, (Figure 4D). These results suggest that hispidulin rescues METH‐PPI and Ketamine‐PPI disruptions by acting as a PAM of α6GABAA receptors in the cerebellum.
Figure 4.
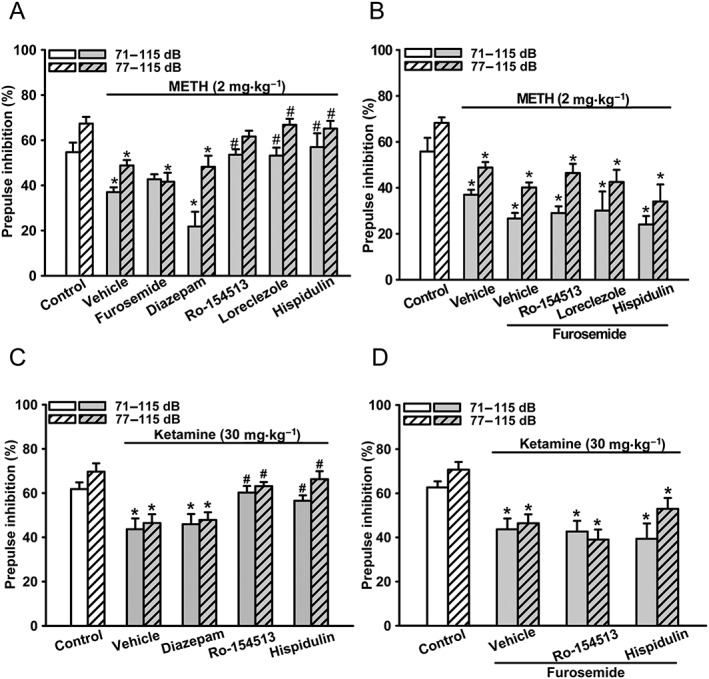
Effects of i.cb. microinjection of hispidulin, Ro15‐4513, loreclezole or diazepam on METH‐PPI and Ketamine‐PPI disruptions and their interactions with i.cb. furosemide. Hispidulin, Ro15‐4513 (an α6 GABAA receptor‐active benzodiazepine), loreclezole (an anticonvulsant triazole derivative effective at most GABAA receptors), diazepam (an α6GABAA receptor‐inactive classical benzodiazepine) and furosemide (an α6GABAA receptor‐selective allosteric antagonist) were i.cb. administered at 10 nmol (A, C) without or (B, D) with i.cb. co‐administration of furosemide 10 min before administration of (A, B) methamphetamine (2 mg·kg−1, i.p.) or (C, D) ketamine (30 mg·kg−1, i.p.). PPI was measured and analysed as in Figure 2. Data shown are means ± SEM; n = 8. * P < 0.05, significantly different from the control group; # P < 0.05, significantly different from the vehicle group.
Compound 6 reversed METH‐PPI disruption and this reversal was prevented by i.cb. furosemide
To directly support the conclusion that PAM action at cerebellar α6GABAA receptors can rescue PPI disruption, we examined whether Compound 6, a highly selective α6GABAA receptor PAM identified recently (Varagic et al., 2013), can rescue METH‐PPI disruption. Indeed, Compound 6, at 3 and 10 mg·kg−1 (i.p.), significantly reversed METH‐PPI disruption in mice (Figure 5A). Effects of 3 and 10 mg·kg−1 were not significantly different, suggesting that the maximal effective dose of Compound 6 is 3 mg·kg−1 or lower. Importantly, these effects of Compound 6 (10 mg·kg−1) given systemically, was prevented by i.cb. furosemide (10 nmol) (Figure 5B), suggesting that the cerebellar α6GABAA receptors were the target of Compound 6 administered systemically.
Figure 5.
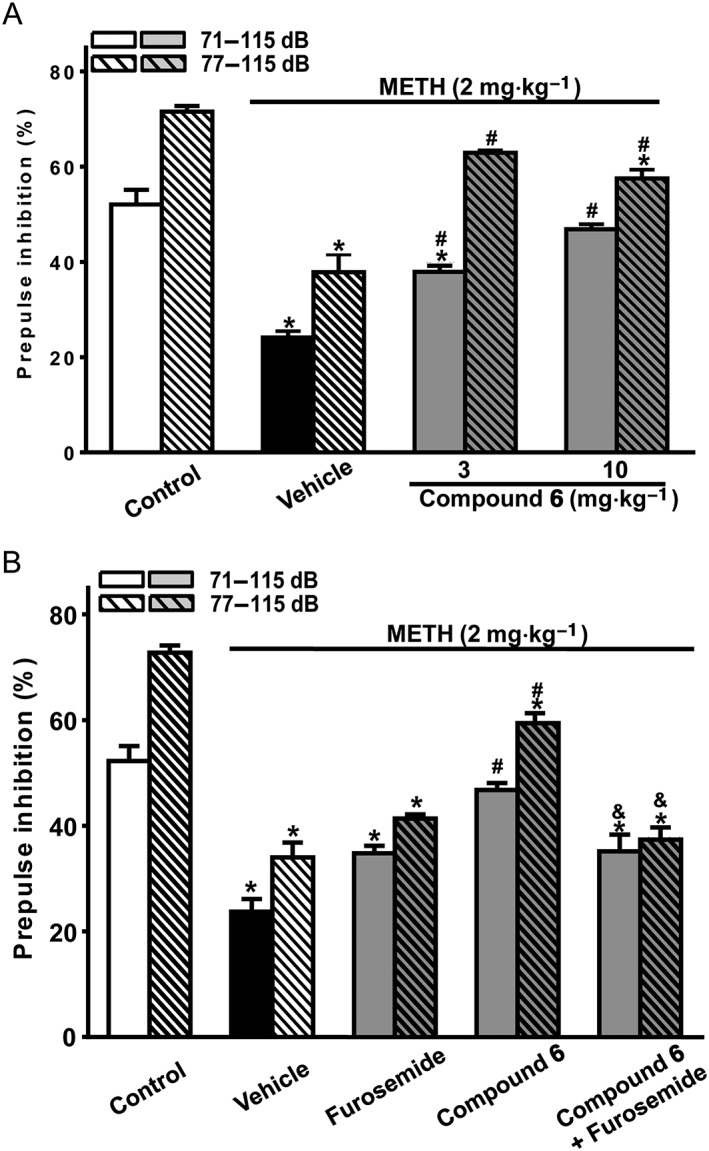
Effects of Compound 6 (i.p.) on METH‐PPI disruption in mice with and without i.cb. pretreatment with furosemide. Compound 6 (3 or 10 mg·kg−1) or vehicle was administered by i.p. injection 10 min before injection of methamphetamine (2 mg·kg−1, i.p.). Furosemide was administered by i.cb. bilaterally alone or 5 min before Compound 6 (10 mg·kg−1, i.p.) was given. PPI was measured and analysed as in Figure 2. Data shown are means ± SEM; n = 8. * P < 0.05, significantly different from the control group, # P < 0.05, significantly different from the vehicle group, & P < 0.05, significantly different from the Compound 6 alone group.
Hispidulin and Compound 6 effectively rescued already established METH‐PPI disruption
The results described above suggested that hispidulin and Compound 6 could prevent PPI disruptions as both compounds were administered before mice were treated with METH or NMDA channel blockers. To further examine whether they are effective after PPI disruptions had been established, a clinically more relevant condition, we treated mice with Compound 6 or hispidulin 10 min after methamphetamine (2 mg·kg−1 i.p), when hyperlocomotion was clearly present in these mice (Liao et al., 2016). As shown in Figure 6, both hispidulin and Compound 6, applied after methamphetamine, effectively reversed the PPI disruptions, as they did when they were applied before methamphetamine. In contrast, neither hispidulin nor Compound 6 per se affected PPI after injections of saline (the vehicle of methamphetamine).
Figure 6.
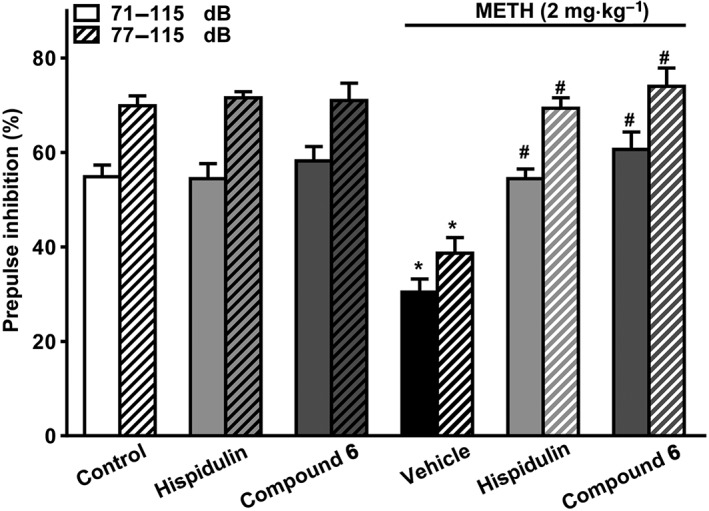
Effects of hispidulin and Compound 6 on PPI in mice without and with methamphetamine pretreatment. Mice without or with methamphetamine (2 mg·kg−1, i.p.) pretreatment for 10 min were i.p. injected with hispidulin (10 mg·kg−1), Compound 6 (10 mg·kg−1) or vehicle 10 min before performing the PPI test. PPI was measured and analysed as in Figure 2. Data shown are means ± SEM; n = 6. * P < 0.05, significantly different from the control group; # P < 0.05, significantly different from the vehicle group.
Compound 6 had no significant effect on COMT or psychoactive neurotransmitter receptors/transporters
Because inhibition of COMT can also contribute to the restoration of PPI by hispidulin (Figure 2F), we further examined whether Compound 6 could inhibit COMT activity. In an assay of the COMT activity (PT #: 1203067) conducted by Eurofins Panlabs (Beitou, Taipei, Taiwan). (https://www.eurofinspanlabs.com), as used for hispidulin (Liao et al., 2016), Compound 6 at concentrations up to 10 μM displayed only 4% inhibition of COMT activity (Study No. AB61114; Eurofins Panlabs).
We also conducted a receptor binding screen for Compound 6 on a panel of assays of 46 receptors, transporters and channels, including the hERG channel, by the Psychoactive Drug Screening Program of the National Institute of Mental Health. Compound 6 at 10 μM did not display significant (>50%) inhibition of the radioligand binding on almost all of the tested targets (Supporting Information Table S1).
Hispidulin, but not OR‐486, positively modulated α6β3γ2GABAA receptors where Compound 6 is a potent and selective PAM
Hispidulin has previously been reported as a PAM at α6β2γ2GABAA receptors (Kavvadias et al., 2004), and we demonstrated that Compound 6 is a highly selective PAM at GABAA receptors consisting of α6β2γ2 or α6β3γ2 subunits (Varagic et al., 2013). To investigate a possible effect of OR‐486 on α6GABAA receptors, under the same conditions as hispidulin and Compound 6, here we compared the effects of all three compounds at α6β3γ2GABAA receptors expressed in Xenopus oocytes with concatenated subunits. Compound 6 (10 μM) increased GABA currents (at EC3–5) by more than eight‐fold (Figure 7), which was identical to its PAM effect on α6β3γ2GABAA receptors in our previous study, using non‐concatenated subunit expression (Varagic et al., 2013) (diamond symbols in Figure 7), supporting the validity of data obtained from concatenated α6β3γ2GABAA receptors. At these concatenated α6β3γ2GABAA receptors, hispidulin (0.3–10 μM) increased GABA currents in a concentration‐dependent manner, but its efficacy was lower than that of Compound 6 at 10 μM (Figure 7). In contrast, OR‐486 did not elicit significant modulatory effects on these receptors (Figure 7), even at 10 μM.
Figure 7.
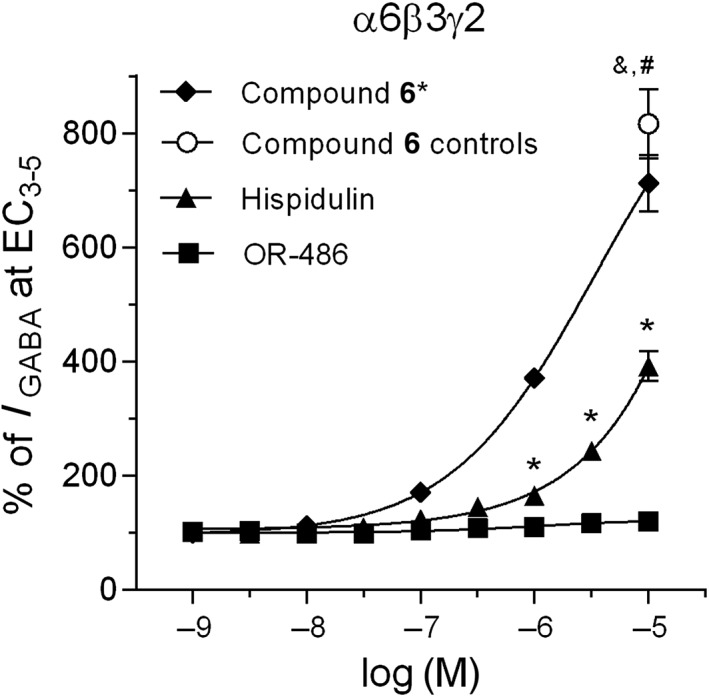
Effects of hispidulin and OR‐486, in comparison with Compound 6, on GABA currents of recombinant α6β3γ2GABAA receptors expressed in Xenopus oocytes. Concatenated recombinant α6β3γ2GABAA receptors were expressed in Xenopus laevis oocytes. The ordinate indicates the modulatory effect of hispidulin, OR‐486 or Compound 6 on the GABA current elicited by GABA at EC3–5 (the effective concentration that induced 3–5% of maximum GABA currents), expressed as % of the control GABA current (I GABA, EC3–5). Hispidulin (30 nM–10 μM) and OR‐486 (1 nM–10 μM) were tested. Compound 6, serving as the positive control of the tested oocytes, was tested at 10 μM. The concentration–response curve of Compound 6 (Compound 6 *) was taken from Varagic et al. (2013). Data are means ± SEM for the Compound 6, hispidulin and OR‐486 groups obtained from seven, five and six cells, respectively, which were prepared from three separate cell batches. * P < 0.05, hispidulin significantly different from solvent; one‐way ANOVA with Dunnett's post hoc test, & P < 0.05, Compound 6 significantly different from solvent; paired t‐test, # P < 0.05, Compound 6 significantly different from hispidulin; unpaired t‐test.
Hispidulin, in contrast to Compound 6, did not modulate α6β3δGABAA receptors
The γ2‐subunit‐containing and δ‐subunit‐containing α6GABAA receptors mediate phasic and tonic inhibition of cerebellar granule cells respectively (Nusser et al., 1998). Although Compound 6 is a highly selective PAM at α6β2/3γ2GABAA receptors (Varagic et al., 2013), it may also modulate the δ‐subunit‐containing α6GABAA receptors. We therefore examined whether Compound 6 and hispidulin modulated α6β3δGABAA receptors. In recombinant α6β3δGABAA receptors, the GABA current levels were too low to measure at the EC3–5 of GABA. Even at the EC10, GABA currents in most of oocytes were too low to be detectable. Nevertheless, in two independent experiments with successful recordings of EC10 GABA currents, Compound 6 at 3 μM increased GABA currents to 270 and 270% of control and at 10 μM to 440 and 500%. As compared with α6β2/3γ2GABAA receptors where 10 μM Compound 6 increased GABA currents to 800 ± 70% of control (Varagic et al., 2013), Compound 6 is a weaker PAM in α6β3δGABAA receptors. In a concurrent assay with the same oocytes expressing α6β3δGABAA receptors, GABA currents were 95% and 105% in the presence of 3 μM hispidulin and were 100% and 130% with 10 μM hispidulin. This indicates that hispidulin at concentrations up to 10 μM did not significantly affect α6β3δGABAA receptors and, therefore, modulation of only the α6β2/3γ2GABAA receptors is necessary and sufficient to account for the restoration of PPI by hispidulin and Compound 6.
Discussion
Hispidulin and Compound 6 rescued PPI disruptions as PAMs at cerebellar α6β2/3γ2GABAA receptors
Here, we demonstrated that hispidulin, a flavonoid and benzodiazepine site ligand, significantly restored PPI disruptions in several mouse models based on the hyper‐dopaminergic and hypo‐glutamatergic hypotheses of schizophrenia. Several lines of evidence indicate that hispidulin restored disrupted PPI by acting as a PAM at cerebellar α6β2/3γ2GABAA receptors. First, hispidulin restored the disrupted PPI after systemic administration of a dose which had no benzodiazepine‐like effects (Liao et al., 2016). Second, diazepam, a typical benzodiazepine ineffective at α6GABAA receptors, did not affect PPI disruption. Third, direct microinjection of hispidulin into the cerebellum restored PPI disruptions, and this effect was mimicked by i.cb. α6GABAA receptor PAMs, such as Ro15‐4513 or loreclezole, or systemic administration of the α6GABAA receptor‐selective PAM, Compound 6, but not by i.cb. diazepam. Fourth, effects of all α6GABAA receptor PAMs on PPI disruption were prevented by i.cb. furosemide, an allosteric α6GABAA receptor antagonist. Fifth, hispidulin seemed to restore PPI disruptions via α6β2/3γ2GABAA receptors, as it did not modulate α6β3δGABAA receptors. Sixth, combining the target screening results of hispidulin (Liao et al., 2016) and Compound 6 (Supporting Information Table S1) and their effects on COMT activity, the only common target of hispidulin and Compound 6 in restoring PPI seems to be the α6β2/3γ2GABAA receptor.
COMT inhibition contributes to hispidulin‐induced, but not Compound 6‐induced, PPI restoration
In addition to modulating α6GABAA receptors, hispidulin is also a COMT inhibitor (Liao et al., 2016). The selective COMT inhibitor OR‐486 also restored the METH‐PPI disruption model. This effect does not involve α6GABAA receptors as OR‐486 did not modulate α6β3γ2GABAA receptors. Therefore, COMT inhibition may also contribute to hispidulin‐induced PPI restoration. In contrast, Compound 6 did not inhibit COMT, excluding the contribution of COMT inhibition in its restoration of PPI.
Hispidulin is a constituent of C. inerme, effective in attenuating both MIH and PPI disruptions
Hispidulin alleviated MIH by acting as a PAM at cerebellar α6GABAA receptors (Liao et al., 2016). Here, we demonstrate that hispidulin also rescued PPI disruptions via cerebellar α6GABAA receptors at a similar dose. The extract of C. inerme from which we isolated hispidulin (Huang et al., 2015) also alleviated MIH and restored PPI (Chen et al., 2012), suggesting hispidulin is the active constituent of the extract, in both models, by modulation of cerebellar α6GABAA receptors.
In contrast, COMT inhibition only contributes to hispidulin‐induced PPI restoration (this study) but not MIH alleviation (Liao et al., 2016). Although both PPI deficit and MIH are hyper‐dopaminergic behavioural models, their underlying circuitries and mechanisms are different. In the striatum, which is involved in hyperlocomotion (Nelson and Kreitzer, 2014), COMT plays a minor role in dopamine clearance compared with synaptic uptake by the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=927. In contrast, dopamine transporters are sparse in the prefrontal cortex (PFC), which is involved in PPI processing and, therefore, COMT activity is crucial in regulating dopamine levels in the PFC (Tunbridge et al., 2006; Sagud et al., 2010). In addition, reduced dopaminergic activity in the PFC leads to impaired PPI (Koch and Bubser, 1994; Ellenbroek et al., 1996), and there is an inverted‐U relationship between the dopamine level and cognitive functions in PFC (Farrell, 2012): PPI and cognitive functions are improved at low but inhibited at high dopamine levels. However, increased dopaminergic activity in the nucleus accumbens (a part of the ventral striatum) also impairs PPI (Swerdlow et al., 2007; Swerdlow et al., 2013). This may explain why methamphetamine, by inducing massive release of dopamine in both PFC and nucleus accumbens, causes PPI disruption whereas COMT inhibitors, by increasing dopamine levels in the PFC only, can restore PPI.
How can a cerebellar α6GABAA receptor PAM restore PPI disruptions?
To the best of our knowledge, this is the first study demonstrating that positive allosteric modulation of cerebellar α6GABAA receptors can rescue disrupted PPI. Several reports in the literature shed some light on a possible explanation (Figure 8).
Figure 8.
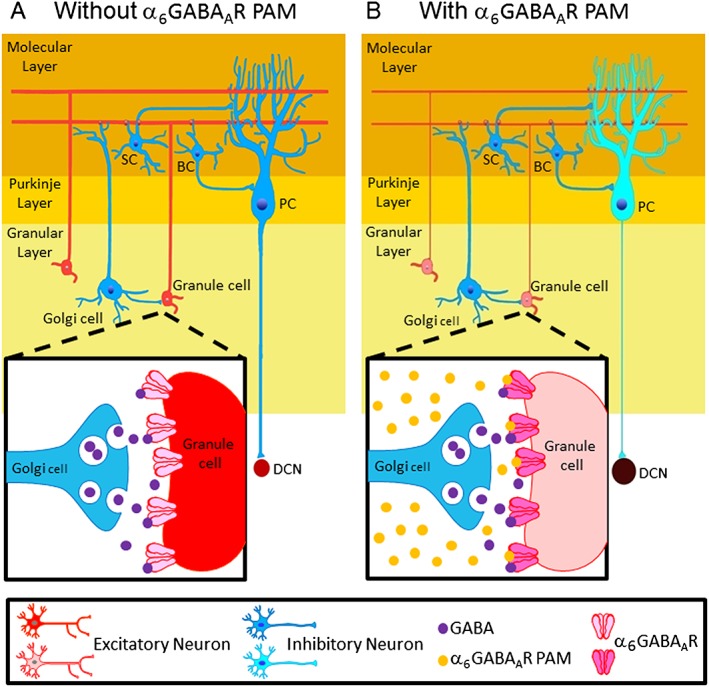
A proposed model for how an α6GABAA receptor PAM affects canonical cerebellar circuits and ultimately leads to the restoration of prepulse inhibition. Schemes of the basic circuit of the cerebellar cortex, which consists of molecular, Purkinje and granule layers, (A) before and (B) after treatment with an α6GABAA receptor PAM, such as hispidulin or Compound 6. The excitatory (red) and inhibitory (blue) neurons in the cerebellar cortex (the yellow part) that may be involved in the action of α6GABAA receptor PAMs, including Golgi cells that form GABAergic synapses onto granule cells, where the α6GABAA receptors are located synaptically and extrasynaptically (enlarged squares). Granule cells form excitatory synapses on dendrites of PCs and also send feedforward inhibition onto PCs indirectly through GABAergic interneurons such as basket cells (BCs) and stellate cells (SCs). PCs are GABAergic output neurons in the cerebellar cortex, providing an inhibitory control onto the downstream DCN. Thinner lines and lighter colours represent reduced neurotransmission and neuronal activity, respectively, after α6GABAA receptor PAM treatment. α6GABAA receptor PAMs act by enhancing Golgi‐GABAergic transmission to granule cells, which then decreases PC activity as a result of the attenuated excitatory inputs from granule cells and this sequence of events ultimately decreases the inhibitory output from the cerebellar cortex. This will activate the DCN, to increase dopamine levels in the PFC, leading to the restoration of prepulse inhibition.
α6GABAA receptors are mainly located at cerebellar Golgi cell–granule cell synapses and extrasynaptic sites (Gutierrez et al., 1996; Pirker et al., 2000), mediating phasic and tonic inhibition of granule cells via the γ2‐subunit‐containing and δ‐subunit‐containing ones respectively (Nusser et al., 1998). Granule cells are important excitatory interneurons in the cerebellar cortex, providing a direct excitatory input onto Purkinje cells (PCs). PCs are the major output neurons of the cerebellar cortex, providing an inhibitory control on the downstream deep cerebellar nuclei (DCN) (Sacchetti et al., 2005). By enhancing GABAergic inhibition of granule cells, α6GABAA receptor PAMs may reduce the activity of PCs, ultimately enhancing the activity of DCN and downstream DCN‐innervated brain regions (Figure 8).
The diagram shown in Figure 8, however, does not take into account the dynamics of the system. Both PCs and DCN neurons spontaneously fire tens of action potentials per second in vivo when animals are not engaged in cerebellar behaviours and firing rates can be increased by synaptic excitation and decreased by synaptic inhibition (Pugh and Raman, 2009). Due to their specific ion channels, even a short excitation can drive DCN neurons into a depolarization block. Their firing usually resumes only after an active hyperpolarization, likely to be due to the inhibitory GABAergic input from PCs (Pugh and Raman, 2009).
Granule cells, in addition to activating PCs, also increase feedforward and lateral inhibition of PCs. Feedforward inhibition limits the excitation of directly activated PCs, whereas lateral inhibition reduces the activity of other PCs, leading to increased spike precision. In addition, phasic, but not tonic, inhibition also plays an important role in shaping the timing and precision of granule cell firing (Nieus et al., 2014). Therefore, α6β2/3γ2GABAA receptor‐selective PAMs would only modulate those granule cells that are activated and thus increase spike precision and synchronization of associated PCs, which is important for coupling to a specific DCN neuronal output. DCN neurons preferentially relay the spike timing of synchronized PCs to downstream premotor and other brain areas (Pugh and Raman, 2009; Person and Raman, 2012; Najac and Raman, 2015).
Increasing evidence suggests that the cerebellum, aside from its role in motor coordination, contributes to cognitive functioning. This probably involves the connections of PCs in the posterior lateral cerebellum to DCN neurons distinct from those controlling motor activity that convey the information from the cerebellar cortex to the PFC via the thalamus (Caligiore et al., 2016). Therefore, the cerebellum has been proposed to be involved in the pathophysiology of neuropsychiatric disorders (Phillips et al., 2015; Caligiore et al., 2016). For example, patients with schizophrenia had lower emotion‐induced cerebellar activity (Mothersill et al., 2015) and reduced glucose consumption in the dorsal PFC than normal subjects (Andreasen et al., 1996) when performing a memory task, suggesting a low activity of this connection. At the cellular level, reduced GABA‐synthesizing enzyme has been reported in post mortem schizophrenia cerebellar tissues, as well as at cerebellar Golgi–granule synapses, of rats chronically treated with PCP (Bullock et al., 2008), an animal model for schizophrenia (Grayson et al., 2016). These results suggest that patients with schizophrenia have impaired Golgi‐inhibitory control and overactive granule cells and thus overactive PCs in the cerebellar cortex.
The circuits involved in PPI are complex, especially the role of the cerebellum in PPI regulation is far from clear (Takeuchi et al., 2001). Nevertheless, PPI was enhanced in mice with impaired excitatory transmission at PCs, elicited by a targeted deletion of PC‐specific glutamate receptors (Takeuchi et al., 2001). If a reduced excitatory input at PCs via granule cells causes an enhanced PPI (Takeuchi et al., 2001), then overactive granule cells, possibly driven by a multitude of unfiltered information contributing to cognitive impairments in patients with schizophrenia (Lewis et al., 2004; Bullock et al., 2008), may contribute to PPI disruptions.
Electrical stimulation at DCN can evoke dopamine efflux in the PFC (Mittleman et al., 2008) and decreased PFC dopaminergic activity leads to impaired PPI (Koch and Bubser, 1994; Ellenbroek et al., 1996). Therefore, α6GABAA receptor PAMs may restore disrupted PPI by reducing granule cell activity and enhancing synchronization of PCs, which is important for activating appropriate DCN neurons, and then increase dopamine levels in the PFC, leading to PPI restoration. Further experiments will have to investigate this tentative scheme providing a possible explanation for the present observations. However, our findings that hispidulin and Compound 6 only restored disrupted PPI but did not affect PPI in control animals (Figure 2D, Figure 6) are consistent with the synchronizing activity of α6GABAA receptor PAMs: they will not change synchronization of adequately synchronized PCs in control animals.
In conclusion, using hispidulin and a structurally unrelated pyrazoloquinolinone Compound 6, we demonstrated that positive allosteric modulation of cerebellar α6GABAA receptors, for which so far no function was known, could prevent or treat PPI disruptions. Moreover, we provided a tentative explanation for this finding. PPI disruptions are considered sensorimotor gating deficits that are usually manifested in patients with several neuropsychiatric disorders, including but not limited to tic disorders and schizophrenia. Therefore, this study may pave the way for the development of α6GABAA receptor‐selective PAMs as a novel treatment for sensorimotor gating deficits in these neuropsychiatric disorders. Compound 6, which was systemically effective and thus blood–brain barrier permeable and is devoid of HERG channel activity (and thus lacking cardiotoxicity, see Supporting Information Table S1), may serve as a lead compound for the future drug development.
Author contributions
L.‐C.C., H.‐J.L., M.E., H.‐L.C., J.‐F.C., A.M. and T.N. designed the study; H.‐J.L., H.‐L.C., J.‐F.C., M.T. and A.M. acquired and analysed the data; L.‐C.C., W.‐J.H., D.E.K., C.W. and J.C. provided the compounds; M.E., P.‐C.F., A.M. and T.M. participated in discussing the data and writing the manuscript; and L.‐C.C., M.E., W.S. and T.N. wrote and finalized the article, which all authors reviewed and approved for publication.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Intra‐cerebellar (i.cb.) microinjection of furosemide alone did not affect PPI. Furosemide (10 nmol) or vehicle was administered by bilateral i.cb. microinjection. PPI was measured and analysed as in Figure 2. Note that there is no significant difference in the magnitude of PPI elicited by either 70–115 dB or 77–115 dB. N = 6.
Table S1 Radioligand displacement studies conducted by the Psychoactive Drugs Screening Program, National Institute of Medical Health to determine the binding affinity of Compound 6 over a panel of 46 receptors, channels and transporters.
Acknowledgements
This study was mainly supported by the National Research Program for Biopharmaceuticals (NSC 100‐2325‐B002‐050, NSC 101‐2325‐B002‐048, NSC 102‐2325‐B002‐047, MOST 103‐2325‐B002‐037, MOST 104‐2325‐B002‐010 and MOST 105‐2325‐B002‐004) to L.‐C.C. and by the research grant (MOST 106‐2911‐I‐002‐514 to L.‐C.C., NSC 102‐2320‐B038‐019‐MY3 to W.‐J.H.) from the National Science Council/ the Ministry of Science and Technology, Taiwan, as well as the Innovative Research Grant (NHRI‐EX107‐10733NI) from National Health Research Institutes, Taiwan, to L.‐C.C from Taiwan. It was also supported by the Taiwan‐Austria bilateral international grant (MOST 104‐2923‐B‐002‐006‐MY3) from the Ministry of Science and Technology to L.‐C.C. from Taiwan as well as by the Austrian Science Fund (FWF I 2306) to M.E. from Austria. It was also supported by Grants‐in‐Aids for Scientific Research (26460240 and 15K08218, 16K10195, 17H04252) from the Japan Society for the Promotion of Science, the Private University Research Branding Project from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Research Grants from Takeda Science Foundation, the Nakatomi Foundation and the Smoking Research Foundation, Japan, to TN from Japan as well as by the R01 grants (R01 NS076517 and R01 MH096463) from the National Institutes of Health, and the Shimadzu Analytical Facility of Southeastern Wisconsin to J.C. from USA. We appreciate the support from the Psychoactive Drug Screening Program, which is funded by the National Institute of Mental Health and run by Dr. Bryan Roth at the University of North Carolina. We thank the help from Ms. Kuan‐Ling Lu in preparing this manuscript and the support from Behavior Core, Neurobiology and Cognitive Center, National Taiwan University.
Chiou, L.‐C. , Lee, H.‐J. , Ernst, M. , Huang, W.‐J. , Chou, J.‐F. , Chen, H.‐L. , Mouri, A. , Chen, L.‐C. , Treven, M. , Mamiya, T. , Fan, P.‐C. , Knutson, D. E. , Witzigmann, C. , Cook, J. , Sieghart, W. , and Nabeshima, T. (2018) Cerebellar α6‐subunit‐containing GABAA receptors: a novel therapeutic target for disrupted prepulse inhibition in neuropsychiatric disorders. British Journal of Pharmacology, 175: 2414–2427. doi: 10.1111/bph.14198.
References
- Alexander SPH, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. Br J Pharmacol 174: S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. Br J Pharmacol 174: S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL et al (1996). Schizophrenia and cognitive dysmetria: a positron‐emission tomography study of dysfunctional prefrontal–thalamic–cerebellar circuitry. Proc Natl Acad Sci U S A 93: 9985–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L (1978). Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology 15: 339–343. [DOI] [PubMed] [Google Scholar]
- Bullock WM, Cardon K, Bustillo J, Roberts RC, Perrone‐Bizzozero NI (2008). Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am J Psychiatry 165: 1594–1603. [DOI] [PubMed] [Google Scholar]
- Caligiore D, Pezzulo G, Baldassarre G, Bostan AC, Strick PL, Doya K , et al (2016). Consensus paper: towards a systems‐level view of cerebellar function: the interplay between cerebellum, basal ganglia, and cortex. Cerebellum: 10.1007/s12311‐12016‐10763‐12313. [DOI] [PMC free article] [PubMed]
- Chen HL, Lee HJ, Huang WJ, Chou JF, Fan PC, Du JC et al (2012). Clerodendrum inerme leaf extract alleviates animal behaviors, hyperlocomotion, and prepulse inhibition disruptions, mimicking Tourette syndrome and schizophrenia. Evid Based Complement Alternat Med 2012: 284301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry JM, Dunn SM, Davies M (2004). Identification of a residue in the gamma‐aminobutyric acid type A receptor alpha subunit that differentially affects diazepam‐sensitive and ‐insensitive benzodiazepine site binding. J Neurochem 88: 1431–1438. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Budde S, Cools AR (1996). Prepulse inhibition and latent inhibition: the role of dopamine in the medial prefrontal cortex. Neuroscience 75: 535–542. [DOI] [PubMed] [Google Scholar]
- Fan PC, Huang WJ, Chiou LC (2009). Intractable chronic motor tics dramatically respond to Clerodendrum inerme (L.) Gaertn. J Child Neurol 24: 887–890. [DOI] [PubMed] [Google Scholar]
- Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ (2012). COMT Val(158)Met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol Psychiatry 71: 538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM, Rohling D, Seifen S, Pukrop R, von Gontard A (2006). Neurophysiology of nocturnal enuresis: evoked potentials and prepulse inhibition of the startle reflex. Dev Med Child Neurol 48: 278–284. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Moghaddam B (2002). Animal models relevant to schizophrenia disorders In: Davis KL, Charney D, Coyle JT, Nemeroff C. (eds). Neuropsychopharmacology: The Fifth Generation of Progress. American College of Neuropsychopharmacology: Brentwood, TN, USA: pp. 689–701. [Google Scholar]
- Giakoumaki SG, Roussos P, Rogdaki M, Karli C, Bitsios P, Frangou S (2007). Evidence of disrupted prepulse inhibition in unaffected siblings of bipolar disorder patients. Biol Psychiatry 62: 1418–1422. [DOI] [PubMed] [Google Scholar]
- Grayson B, Barnes SA, Markou A, Piercy C, Podda G, Neill JC (2016). Postnatal phencyclidine (PCP) as a neurodevelopmental animal model of schizophrenia pathophysiology and symptomatology: a review. Curr Top Behav Neurosci 29: 403–428. [DOI] [PubMed] [Google Scholar]
- Gutierrez A, Khan ZU, De Blas AL (1996). Immunocytochemical localization of the alpha 6 subunit of the gamma‐aminobutyric acidA receptor in the rat nervous system. J Comp Neurol 365: 504–510. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji DJ et al (1996). Cloning of cDNAs encoding the human gamma‐aminobutyric acid type A receptor alpha 6 subunit and characterization of the pharmacology of alpha 6‐containing receptors. Mol Pharmacol 49: 253–259. [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2017). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WJ, Lee HJ, Chen HL, Fan PC, Ku YL, Chiou LC (2015). Hispidulin, a constituent of Clerodendrum inerme that remitted motor tics, alleviated methamphetamine‐induced hyperlocomotion without motor impairment in mice. J Ethnopharmacol 166: 18–22. [DOI] [PubMed] [Google Scholar]
- Kask K, Gulinello M, Backstrom T, Geyer MA, Sundstrom‐Poromaa I (2008). Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology 33: 2283–2290. [DOI] [PubMed] [Google Scholar]
- Kavvadias D, Monschein V, Sand P, Riederer P, Schreier P (2003). Constituents of sage (Salvia officinalis) with in vitro affinity to human brain benzodiazepine receptor. Planta Med 69: 113–117. [DOI] [PubMed] [Google Scholar]
- Kavvadias D, Sand P, Youdim KA, Qaiser MZ, Rice‐Evans C, Baur R et al (2004). The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood–brain barrier and exhibits anticonvulsive effects. Br J Pharmacol 142: 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scheurer L, Luddens H, Hamilton BJ et al (1996). Pharmacological modulation of the diazepam‐insensitive recombinant gamma‐aminobutyric acidA receptors alpha 4 beta 2 gamma 2 and alpha 6 beta 2 gamma 2. Mol Pharmacol 50: 1253–1261. [PubMed] [Google Scholar]
- Koch M, Bubser M (1994). Deficient sensorimotor gating after 6‐hydroxydopamine lesion of the rat medial prefrontal cortex is reversed by haloperidol. Eur J Neurosci 6: 1837–1845. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Kuner T, Seeburg PH, Luddens H (1995). Selective antagonist for the cerebellar granule cell‐specific gamma‐aminobutyric acid type A receptor. Mol Pharmacol 47: 283–289. [PubMed] [Google Scholar]
- Kumari V, Das M, Hodgins S, Zachariah E, Barkataki I, Howlett M et al (2005). Association between violent behaviour and impaired prepulse inhibition of the startle response in antisocial personality disorder and schizophrenia. Behav Brain Res 158: 159–166. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Walker DE, Cohen DJ (1993). Premonitory urges in Tourette's syndrome. Am J Psychiatry 150: 98–102. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Volk DW, Hashimoto T (2004). Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 174: 143–150. [DOI] [PubMed] [Google Scholar]
- Liao YH, Lee HJ, Huang WJ, Fan PC, Chiou LC (2016). Hispidulin alleviated methamphetamine‐induced hyperlocomotion by acting at alpha6 subunit‐containing GABAA receptors in the cerebellum. Psychopharmacology (Berl) 233: 3187–3199. [DOI] [PubMed] [Google Scholar]
- Ludewig S, Ludewig K, Geyer MA, Hell D, Vollenweider FX (2002). Prepulse inhibition deficits in patients with panic disorder. Depress Anxiety 15: 55–60. [DOI] [PubMed] [Google Scholar]
- Maddox VH, Godefroi EF, Parcell RF (1965). The synthesis of phencyclidine and other 1‐arylcyclohexylamines. J Med Chem 8: 230–235. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minier F, Sigel E (2004). Positioning of the alpha‐subunit isoforms confers a functional signature to gamma‐aminobutyric acid type A receptors. Proc Natl Acad Sci U S A 101: 7769–7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirheydari P, Ramerstorfer J, Varagic Z, Scholze P, Wimmer L, Mihovilovic MM et al (2014). Unexpected properties of delta‐containing GABAA receptors in response to ligands interacting with the alpha + beta‐site. Neurochem Res 39: 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittleman G, Goldowitz D, Heck DH, Blaha CD (2008). Cerebellar modulation of frontal cortex dopamine efflux in mice: relevance to autism and schizophrenia. Synapse 62: 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothersill O, Knee‐Zaska C, Donohoe G (2015). Emotion and theory of mind in schizophrenia – investigating the role of the cerebellum. Cerebellum 15: 357–368. [DOI] [PubMed] [Google Scholar]
- Najac M, Raman IM (2015). Integration of Purkinje cell inhibition by cerebellar nucleo‐olivary neurons. J Neurosci 35: 544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Kreitzer AC (2014). Reassessing models of basal ganglia function and dysfunction. Annu Rev Neurosci 37: 117–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieus TR, Mapelli L, D'Angelo E (2014). Regulation of output spike patterns by phasic inhibition in cerebellar granule cells. Front Cell Neurosci 8: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissinen E, Linden IB, Schultz E, Kaakkola S, Mannisto PT, Pohto P (1988). Inhibition of catechol‐O‐methyltransferase activity by two novel disubstituted catechols in the rat. Eur J Pharmacol 153: 263–269. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P (1998). Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18: 1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz EM, Hanna GL, de Traversay J (1992). Prestimulation‐induced startle modulation in attention‐deficit hyperactivity disorder and nocturnal enuresis. Psychophysiology 29: 437–451. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ (2001). The Mouse Brain in Stereotaxic Coordinates, Second edn. Academic Press: London. [Google Scholar]
- Person AL, Raman IM (2012). Synchrony and neural coding in cerebellar circuits. Front Neural Circuits 6: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JR, Hewedi DH, Eissa AM, Moustafa AA (2015). The cerebellum and psychiatric disorders. Front Public Health 3: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G (2000). GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815–850. [DOI] [PubMed] [Google Scholar]
- Pugh JR, Raman IM (2009). Nothing can be coincidence: synaptic inhibition and plasticity in the cerebellar nuclei. Trends Neurosci 32: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Scelfo B, Strata P (2005). The cerebellum: synaptic changes and fear conditioning. Neuroscientist 11: 217–227. [DOI] [PubMed] [Google Scholar]
- Sagud M, Muck‐Seler D, Mihaljevic‐Peles A, Vuksan‐Cusa B, Zivkovic M, Jakovljevic M et al (2010). Catechol‐O‐methyl transferase and schizophrenia. Psychiatr Danub 22: 270–274. [PubMed] [Google Scholar]
- Singer HS (2005). Tourette's syndrome: from behaviour to biology. Lancet Neurol 4: 149–159. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL (1993). A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol Psychiatry 33: 298–301. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Hines SR, Herrera SD, Weber M, Breier MR (2013). Opposite effects of tolcapone on amphetamine‐disrupted startle gating in low vs. high COMT‐expressing rat strains. Pharmacol Biochem Behav 106: 128–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Karban B, Ploum Y, Sharp R, Geyer MA, Eastvold A (2001). Tactile prepuff inhibition of startle in children with Tourette's syndrome: in search of an “fMRI‐friendly” startle paradigm. Biol Psychiatry 50: 578–585. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR (1995). Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington's disease. J Neurol Neurosurg Psychiatry 58: 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Bongiovanni MJ, Neary AC, Tochen LS, Saint Marie RL (2007). Strain differences in the disruption of prepulse inhibition of startle after systemic and intra‐accumbens amphetamine administration. Pharmacol Biochem Behav 87: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Kiyama Y, Nakamura K, Tsujita M, Matsuda I, Mori H et al (2001). Roles of the glutamate receptor epsilon2 and delta2 subunits in the potentiation and prepulse inhibition of the acoustic startle reflex. Eur J Neurosci 14: 153–160. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR (2006). Catechol‐O‐methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry 60: 141–151. [DOI] [PubMed] [Google Scholar]
- Varagic Z, Ramerstorfer J, Huang S, Rallapalli S, Sarto‐Jackson I, Cook J et al (2013). Subtype selectivity of alpha + beta‐site ligands of GABAA receptors: identification of the first highly specific positive modulators at alpha6beta2/3gamma2 receptors. Br J Pharmacol 169: 384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove PB, Wafford KA, Bain C, Whiting PJ (1994). The modulatory action of loreclezole at the gamma‐aminobutyric acid type A receptor is determined by a single amino acid in the beta 2 and beta 3 subunit. Proc Natl Acad Sci U S A 91: 4569–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Zhang W, Liu R, Harris B, Skolnick P, Cook JM (1995). Synthesis of novel imidazobenzodiazepines as probes of the pharmacophore for “diazepam‐insensitive” GABAA receptors. J Med Chem 38: 1679–1688. [DOI] [PubMed] [Google Scholar]
- Zou H, Zhang C, Xie Q, Zhang M, Shi J, Jin M et al (2008). Low dose MK‐801 reduces social investigation in mice. Pharmacol Biochem Behav 90: 753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Intra‐cerebellar (i.cb.) microinjection of furosemide alone did not affect PPI. Furosemide (10 nmol) or vehicle was administered by bilateral i.cb. microinjection. PPI was measured and analysed as in Figure 2. Note that there is no significant difference in the magnitude of PPI elicited by either 70–115 dB or 77–115 dB. N = 6.
Table S1 Radioligand displacement studies conducted by the Psychoactive Drugs Screening Program, National Institute of Medical Health to determine the binding affinity of Compound 6 over a panel of 46 receptors, channels and transporters.


