Abstract
Pathological pain is a hyperexcitability disorder. Since the excitability of a neuron is set and controlled by a complement of ion channels it expresses, in order to understand and treat pain, we need to develop a mechanistic insight into the key ion channels controlling excitability within the mammalian pain pathways and how these ion channels are regulated and modulated in various physiological and pathophysiological settings. In this review, we will discuss the emerging data on the expression in pain pathways, functional role and modulation of a family of voltage‐gated K+ channels called ‘M channels’ (KCNQ, Kv7). M channels are increasingly recognized as important players in controlling pain signalling, especially within the peripheral somatosensory system. We will also discuss the therapeutic potential of M channels as analgesic drug targets.
Linked Articles
This article is part of a themed section on Recent Advances in Targeting Ion Channels to Treat Chronic Pain. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.12/issuetoc/
Abbreviations
- BBB
blood–brain barrier
- BNB
blood‐nerve barrier
- DH
dorsal horn
- DRG
dorsal root ganglion
- G9a
euchromatic histone‐lysine N‐methyltransferase 2
- PSNL
partial sciatic nerve ligation
- REST
repressor element 1‐silencing transcription factor
- SAR
structure–activity relationship
- SIN3A
SIN3 transcription regulator family member A
- TRPV1
transient receptor potential cation channel subfamily V member 1
- WDR
wide dynamic range
- XE991
10,10‐bis(4‐Pyridinylmethyl)‐9(10H)‐anthracenone dihydrochloride
Introduction
Pathological pain is a vast and unmet clinical problem, which brings about poor quality of life for sufferers and puts a colossal burden on healthcare systems worldwide. In European countries, national annual economic costs of chronic pain run into billions and amounts to 3–10% of gross domestic products (Breivik et al., 2013). The available statistics suggest that in the United States, total incremental costs of health care due to pain reaches up to hundreds of billions annually, which is higher than the combined costs of cancer and diabetes (Anonymous, 2016). It is clear that other parts of the world are affected to a similar degree, so pain is indeed a worldwide health, societal and economic concern. Yet, despite years of research and investment, there is no ultimate clinical solution to pathological pain and opioids, known to humanity since ancient times, are still a gold standard. While in recent decades there has been a dramatic progress in our understanding of the molecular and cellular mechanisms of pain, the rational design of new therapies based on this mounting knowledge is painstakingly slow. Part of the problem is that most targets for current analgesics are within the CNS and, thus, often cause cognitive and behavioural side effects and are subject to tolerance and addiction issues. Current strategies for peripheral analgesia involve the local application of fairly non‐specific drugs (such as the non‐specific http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=82 blockers, e.g. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2623) or drugs that are specific for broadly expressed targets [e.g. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2536, a conotoxin inhibitor of N‐type http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=80 (Cav2.2 channels)]. These strategies can be robust but often result in the complete loss of sensory and motor fibre activity. Drug discovery for new analgesics mostly focuses on targets that are ‘specific’ for nociceptive neurons, but this has also proven difficult due to either widespread expression patterns [such as, for example with http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=507 (Lee et al., 2015)] or the existence of closely related isoforms in other tissues (such as with the voltage‐gated Na+ channels; Wood, 2013). Therefore, new ideas for therapies and new molecular targets for these therapies are urgently needed.
Here, we review the current literature on the expression in pain pathways, functional role and a potential for therapeutic targeting of a family of K+ channels called ‘M channels’ (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=560&familyId=81&familyType=IC which are increasingly recognized as one of the important mechanisms controlling nociceptive fibre activity. Several earlier reviews on this or related topics are available (Munro and Dalby‐Brown, 2007; Gribkoff, 2008; Rivera‐Arconada et al., 2009; Wickenden and McNaughton‐Smith, 2009; Du and Gamper, 2013; Wang and Li, 2016), so here, we will focus on recent developments in the field as well as on the emerging understanding of the sites of analgesic efficacy of M channel potentiating drugs (‘openers’) within the mammalian nervous system. Since most of the available evidence concerns the M channels expressed within the peripheral somatosensory system, we will mainly focus on the role of M channels in peripheral pain pathways.
M channels: fit to control
M channels (Kv7.1–Kv7.5 coded for by KCNQ1‐KCNQ5 genes) are voltage‐gated K+ channels with interesting biophysical properties affecting neuronal excitability (Figure 1; Delmas and Brown, 2005; Gamper and Shapiro, 2015). In particular, they have a very negative threshold for activation, do not inactivate and have slow activation and deactivation kinetics. If the density of Kv7 channels is sufficiently large (relative to the density of ‘leak’ channels, or other channels that open around the resting potential), they may contribute to the resting potential of a neuron (Delmas and Brown, 2005; Huang and Trussell, 2011; Du et al., 2014). As a result, M channel inhibition causes depolarization, a reduction in the threshold current and rheobase and an increase in input resistance, while their activation hyperpolarizes neuronal plasma membrane and reduces input resistance, making the neuron more resistant to firing action potentials (Figure 1; Gamper and Shapiro, 2015). Numerous reports indeed confirm that acute Kv7 channel inhibition leads to increased excitability of various types of central and peripheral neurons while loss‐of‐function mutations in KCNQ genes results in debilitating excitability disorders such as epilepsy, deafness and cardiac arrhythmia (reviewed in Jentsch, 2000; Delmas and Brown, 2005; Gamper and Shapiro, 2015).
Figure 1.
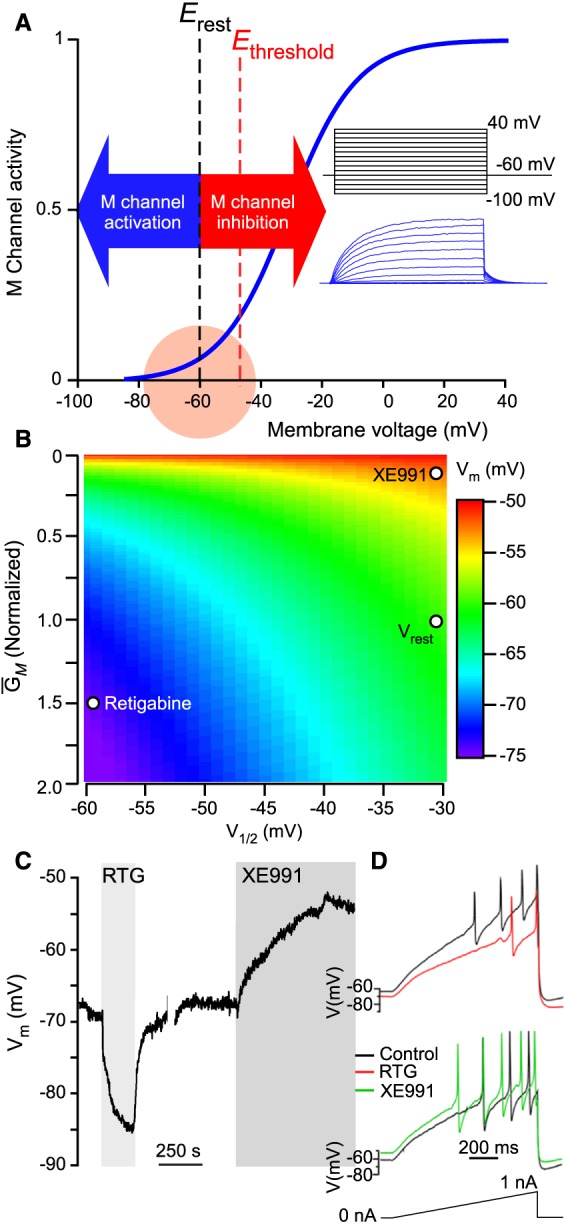
Role of M channels in sensory neurons from a biophysical perspective. (A) Schematized voltage‐dependence of an M channel (Kv7.2/Kv7.3) with relation to the resting membrane potential and firing threshold of a generalized C‐fibre nociceptor. Inset shows M currents recorded in a CHO cell, overexpressing Kv7.2 and Kv7.3, in response to the voltage steps depicted in the diagram above the traces. (B) Simulation of the effect of modulation of M channel (Kv7.2/Kv7.3) activity with an ‘opener’, retigabine and blocker, XE991, on steady‐state membrane potential. Steady‐state membrane potential (Vm) arises when net current across the plasma membrane is zero (in this example, for only ‘M’ and leak currents). Varying M channel voltage‐dependence and maximum conductance (GM) shifts Vm (leak currents were kept constant). Parameters for Vrest, retigabine and XE991 were obtained from Linley et al. (2012b). (C) Current‐clamp recording of the effect of retigabine and XE991 on the Vm recorded from a cultured small‐diameter rat nociceptor in current‐clamp mode (modified from Du et al., 2014, with permission). (D) Effects of retigabine and XE991 on the firing threshold and frequency of a cultured small‐diameter mouse nociceptor; stimulus ramp is shown below the traces.
M channels in neurons are inhibited by stimulation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=2 (M receptors) and, therefore, serve as a mechanism for the excitatory action of the neurotransmitter http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=294 (Brown and Adams, 1980). By now, it is firmly established that http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=13, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=15 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=17 receptors, as well as many other GPCRs of a similar type (e.g. http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=42&familyId=10&familyType=GPCR, purinergic http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=52 and angiotensin II http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=6 receptors), inhibit M channels (Shapiro et al., 1994; Cruzblanca et al., 1998; Filippov et al., 2006; Zaika et al., 2006, 2007; Linley et al., 2008; Gamper and Shapiro, 2015) and cause excitatory actions in neurons of different types, including the nociceptors (Crozier et al., 2007; Linley et al., 2008; Liu et al., 2010). These GPCRs are coupled to Gq/11 type of Gα and to PLC. The signalling pathways linking the Gq/11‐coupled receptor activation with the M channel inhibition have been a subject of intense research over the past few decades and are largely outside the scope of this review (in‐depth reviews on this topic are available; Gamper and Shapiro, 2007, 2015; Hernandez et al., 2008; Hille et al., 2015; Greene and Hoshi, 2016). The major mediators of the inhibition are believed to be (i) the depletion of membrane phosphoinositide http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2387, a factor that is necessary for M channel activity (Suh and Hille, 2002; Zhang et al., 2003; Li et al., 2005); (ii) Ca2+ release from the IP3‐sensitive Ca2+ stores (Cruzblanca et al., 1998; Gamper and Shapiro, 2003; Kosenko and Hoshi, 2013); and (iii) http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=286&familyType=ENZYME‐mediated M channel phosphorylation (Hoshi et al., 2003; Zhang et al., 2011b). This GPCR‐mediated M channel suppression is at the core of the role these channels play in the development of persistent inflammatory pain (see below).
M channel expression in peripheral nociceptive pathways
Peripheral somatosensory neurons are the longest cells in our body (on par with motor neurons) and have a complex, pseudo‐unipolar morphology (schematized in Figure 2). A somatosensory neuron can be subdivided into at least four specialized compartments: (i) a peripheral ending within the innervated tissue (skin, epithelia, muscles, etc.); (ii) a very long axon (fibre); (iii) a central terminal synapsing in the spinal cord; and (iv) a cell body within sensory ganglia. All four compartments have distinct functional roles and distinct anatomical locations (e.g. central terminals are within the CNS while other parts belong to the PNS). Functional M channels are expressed in all these compartments. Thus, dorsal root ganglion (DRG) and trigeminal ganglion (TG) neuron cell bodies generate sizable M currents (Passmore et al., 2003; Rose et al., 2011; King and Scherer, 2012; Zheng et al., 2013; Du et al., 2014), whereas M channel openers reduce and M channel blockers increase somatic excitability of these sensory neurons (Figure 1B, C; Liu et al., 2010; Linley et al., 2012a,b; Du et al., 2014). Functional expression of M channels is confirmed in peripheral sensory axons (Devaux et al., 2004; Rose et al., 2011; Roza et al., 2011; King and Scherer, 2012; Passmore et al., 2012; Peiris et al., 2017), including human sural nerve C fibres (Lang et al., 2008) and human colonic afferents (Peiris et al., 2017). Functional M channels are also reported in nociceptive dorsal roots/central terminals (Rivera‐Arconada and Lopez‐Garcia, 2006) as well as in nociceptive peripheral nerve endings (Linley et al., 2008; Liu et al., 2010; Passmore et al., 2012; Vetter et al., 2013). The relative expression levels and relative functional activity of M channels at these distinct compartments of a peripheral somatosensory neuron are hard to evaluate at present; in case of myelinated fibres, it has been reported that http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=561 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=562 subunits are concentrated at the nodes of Ranvier (Devaux et al., 2004; Roza et al., 2011; King and Scherer, 2012).
Figure 2.
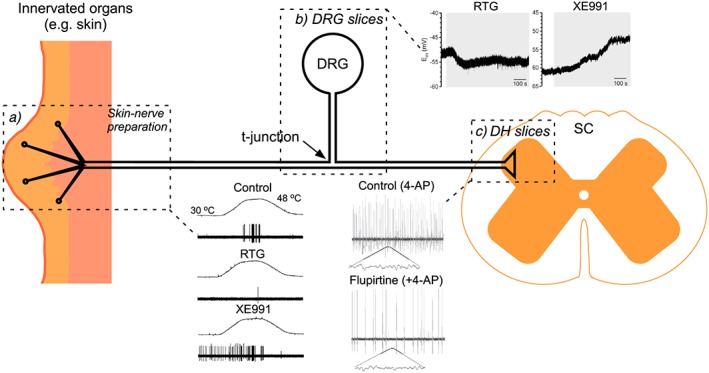
Simplified schematic of a nociceptive neuron. Dotted boxes encircle areas where functional M channel activity has been recorded in situ using slices (spinal cord, DRG) or skin‐nerve preparations. Examples of recordings from such preparations are also shown as follows: (a) the effects of retigabine and XE991 on heat‐induced Aδ fibre activity recorded in skin‐saphenous‐nerve preparation (modified from Passmore et al., 2012, with permission); (b) the effect of the same compounds on the resting membrane potential of a capsaicin‐sensitive neuron recorded from the DRG ‘slice’ using a sharp electrode recording (modified from Du et al., 2014, with permission); (c) the effect of flupirtine on the 4‐AP‐induced excitatory activity recorded extracellularly from substantia gelatinosa in rat spinal cord (modified from Visockis and King, 2013, with permission). Abbreviations: 4‐AP, 4‐aminopiridine; RTG, retigabine, SC, spinal cord.
The Kv7 subunit expression profiles in fibres with different sensory modalities are still poorly characterized and even controversial. It is logical to expect that nociceptors, which have intrinsically high firing thresholds, would express more functional M channels as compared to low‐threshold, non‐nociceptive neurons (e.g. low‐threshold mechanoreceptors, proprioceptors, etc.) as the M current is one of the neuronal mechanisms that raises threshold current (see Figure 1); however, this is yet to be confirmed experimentally. The original report describing M channel expression in DRG (Passmore et al., 2003) documented the expression of Kv7.2, Kv7.3 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=564 in nociceptive and non‐nociceptive DRG neurons with Kv7.2 and Kv7.3 being predominant subunits. In our studies, we found that Kv7.2 was indeed more robustly expressed in small‐diameter DRG neurons and non‐myelinated fibres with less abundant (although still detectable) expression in larger neurones (Rose et al., 2011; Huang et al., 2016a). Other studies also reported expression of Kv7.2 in nociceptive C‐ and Aδ fibres (Wladyka et al., 2008; Passmore et al., 2012); in addition, the expression of Kv7.5 was found in larger, myelinated fibres (Wladyka et al., 2008). However King and Scherer (2012) came to the opposite conclusion with regard to Kv7.2 and Kv7.5 expression: they found that Kv7.2 is expressed at higher levels in larger, presumably non‐nociceptive, neurons while small‐diameter DRG neuron cell bodies and C fibres expressed lower levels of Kv7.2 but high levels of Kv7.5.
The pharmacological profile of M currents recorded from cultured nociceptive DRG neurons speaks against a strong contribution of Kv7.5, since native M currents in DRG neurons display reasonable sensitivity to http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2596 (Passmore et al., 2003, 2012; Linley et al., 2008, 2012a; Rose et al., 2011), as do the heterologousely overexpressed Kv7.2 and Kv7.3 channels, while Kv7.5 has a weaker sensitivity to this blocker (Schroeder et al., 2000). This, however, does not exclude the possibility that Kv7.5 contribute to some extent, as channels expressed in cultured DRG neurons may not be equivalent to those expressed in native tissue. Future studies will have to resolve this controversy as well as establishing the exact Kv7 subunit expression profiles in somatosensory fibres of different modalities. This is indeed an important task as it can hold the key to the development of subunit‐specific M channel openers, optimized for targeting channels in peripheral fibres for pain relief (see below).
In addition to the above findings, in another study it was demonstrated that a defined subpopulation of cutaneous mechanoreceptors (rapidly adapting hair follicles and Meissner corpuscles) specifically expresses http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=563 (Heidenreich et al., 2012).
M channels and peripheral fibre excitability in vitro, in silico and in vivo
There are very clear and undisputed effects of M channel activity on the somatic excitability of cultured DRG and TG neurons (Passmore et al., 2003; Crozier et al., 2007; Linley et al., 2008, 2012a,b; Liu et al., 2010; Mucha et al., 2010; King et al., 2014; Figure 1C). Yet, since cultured sensory neurons are axotomized and are grown in artificial conditions, their physiology may not necessarily accurately represent those of native neurons, since gene expression may be different. Therefore, a higher level of confidence regarding the functional role of M channels in sensory neuron excitability is gained from acute preparations, such as skin‐nerve preparations and sectioning of acutely dissected ganglia/nerves.
M channel activity and excitability of spinal nociceptors
Depolarization by the M channel inhibitor, XE991 and hyperpolarization by an M channel activator (‘opener’) http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2601 has been recorded from http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2486‐sensitive neurons using sharp‐electrode recordings from acute DRG slices (Du et al., 2014). In a skin‐saphenous‐nerve preparation, retigabine markedly reduced noxious heat responses of the mechano‐heat Aδ‐ and some C‐fibres (the latter to a somewhat lesser extent), while XE991 increased noxious heat sensitivity and induced ongoing activity at 32°C in Aδ fibres (Passmore et al., 2012). A similar skin‐saphenous‐nerve preparation has been used to demonstrate that M channel inhibition (e.g. by XE991, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2422 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2471) amplified cold transduction by a specific population of C fibre nociceptors: http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=500‐expressing C‐mechano‐cold fibres (Vetter et al., 2013). In the same fibres, retigabine inhibited cold‐evoked activity. In another study, the multi‐unit recordings of the lumbar splanchnic nerve in a colon‐nerve preparation demonstrated that the noxious distension‐induced firing was reduced by retigabine and increased by XE991 (Peiris et al., 2017). Collectively, these studies indicate that M channel activity does strongly affect the excitability of acutely isolated spinal Aδ and C fibres.
Although indicative, in vitro tests fall short of demonstrating how strongly, if at all, native M channel activity contributes to peripheral nociceptive transmission in vivo. Yet there is growing evidence suggesting that this is indeed the case. Thus, plantar hind paw injections of the M channel blocker, XE991, induce moderate pain in rats (Linley et al., 2008; Liu et al., 2010). In accord with this, plantar injections of XE991 increased firing of wide dynamic range (WDR) dorsal horn (DH) neurons in response to both mechanical and thermal stimulation of the hind paw in spinal cord recordings in anaesthetized animals (Passmore et al., 2012). Moreover, hind paw injection of retigabine reduced thermal sensitivity in freely behaving animals (Huang et al., 2016a) and also greatly reduced pain produced by local (hind paw) injections of the inflammatory mediator http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=649 (Liu et al., 2010; Huang et al., 2016a).
A recent computational study provided a biophysical foundation to these and similar observations by utilizing a multi‐component model of rat C fibre that included a geometrically realistic nociceptive nerve ending in the skin (Barkai et al., 2017). When sufficiently pronounced, M channel inhibition resulted in ‘spontaneous firing’. Whereas almost complete inhibition was required for spike generation in the soma, a less dramatic decrease (to just below 37%) was required to produce ‘spontaneous’ firing in a nociceptive terminal. The modelling also showed that M current actively prevented excitation of nociceptive terminals in response to a range of subthreshold depolarizations and fairly large (up to 15 mV) ‘spontaneous’ fluctuations of resting membrane potential. When reduced to 50% of ‘basal’ density, M current still prevented spontaneous firing, but such a reduction dramatically potentiated responses to noxious stimulation (e.g. with simulated ‘capsaicin’).
M channel activity and excitability of trigeminal nociceptors
Kv7.2 immunoreactivity (Abd‐Elsayed et al., 2015) and sizable M currents were reported in small‐diameter TG neurons in culture (Linley et al., 2012a, b; Ooi et al., 2013; Abd‐Elsayed et al., 2015) and in the whole‐cell patch clamp recordings from acute whole‐mount trigeminal ganglia (Kanda and Gu, 2017). These studies showed that, similar to spinal neurons, M channels are involved in setting resting membrane potential and rheobase in cold‐sensing TG nociceptors (Abd‐Elsayed et al., 2015) and also in most neurons that are sensitive to cold but are not primarily cold‐sensing (Kanda and Gu, 2017). Interestingly, it was also reported that inhibition of M channel activity by cold contributes to cold sensitivity of the latter type of neurons. Retigabine (i.p.) alleviated cold hyperalgesia in two orofacial neuropathic pain models (Abd‐Elsayed et al., 2015), further supporting the active role M channels play in the control of trigeminal nociceptor excitability in vivo. In addition to the above studies, Linley et al. (2012a) analysed the unexpected potentiation of M channel activity by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2098 in TG and DRG neurons and found that, mechanistically, the modulation was similar in both types of sensory neurons.
M channels in nociceptive mechanotransduction
The studies discussed above established a role for M channels in modulating the sensitivity of peripheral nociceptive fibres to heat and cold. However, the role of M channels in mechanical sensitivity is less certain. In skin‐saphenous‐nerve preparations from naïve CD1 mice, retigabine had no significant effect on firing in response to mechanical stimulation of either slowly or rapidly adapting mechano‐sensitive Aδ and C fibres, while two out of four rapidly adapting Aδ fibres responded to XE991 with increased firing rate (Roza and Lopez‐Garcia, 2008). However, in the same study, retigabine strongly suppressed firing in axotomized mechanically‐sensitive fibres of either type. Mice with genetic deletion of Kcnq2 in cells derived from the neural crest (including DRG neurons) displayed both thermal and mechanical hyperalgesia (King et al., 2014). Consistently, in another study, mice with global genetic deletion of Kv7.3 (Kcnq3 −/−) and down‐regulation of Kv7.2 (Kcnq2 +/−) displayed increased firing rates of mechanosenstive D‐hairs (Aδ‐low‐threshold mechanoreceptors) in response to mechanical stimulation (Schutze et al., 2016). Yet the effect was modest and no obvious behavioural phenotype was reported (although this could have been due to only partial loss of Kv7.2). Finally, it was also reported that peripheral nerve endings of rapidly adapting cutaneous hair follicles and Meissner corpuscle mechanoreceptors from mice and humans abundantly express Kv7.4 (Heidenreich et al., 2012). Moreover, mice with genetic deletion of Kcnq4 displayed increased sensitivity of rapidly adapting mechanoreceptors to low‐frequency vibration. In accord with these findings, humans with DFNA2 (a slowly progressing, autosomal dominant form of hearing loss) due to loss‐of‐function mutations in KCNQ4 were better than age‐matched controls at detecting low frequency skin vibration (Heidenreich et al., 2012). Altogether, these data suggest that the contribution of Kv7 subunits to the modulation of mechanical sensitivity varies in different types of mechanoreceptors; however, elucidation of the exact role of M channels in the mechanosensitivity of peripheral afferents (and how it might change in pathological pain states) requires further research.
M channel suppression as a general mechanism for peripheral nerve hyperexcitability
The findings discussed above established that (i) M channels control the excitability of neurons that express them at sufficient levels; (ii) loss of M channel function results in an overexcitable neuron; and (iii) at least some peripheral nociceptive fibres express sufficient levels of functional Kv7 subunits for these to influence fibre excitability. The most logical projection from these conclusions is that inhibition, down‐regulation or any other form of loss of M channel function in nociceptive fibres might result in increased excitability, nociception and pain. On the other hand, peripheral M channel activation could be analgesic. Below, we will consider growing experimental evidence supporting the above considerations.
Inflammatory pain
As mentioned earlier, many Gq/11‐coupled GPCRs were shown to acutely and potently inhibit M channel activity. Tissue inflammation brings about a specific environment, in which inflamed/damaged tissue as well as cells involved in the immune response release various chemical factors (inflammatory mediators; e.g. prostaglandins, leukotrienes, interleukins, bradykinin, substance P, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1713, growth factors, proteases, protons and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2509) in order to orchestrate defensive and reparative processes or as a result of the loss of tissue integrity (Serhan and Savill, 2005; Ferrero‐Miliani et al., 2007). These same factors often ‘sensitize’ or even directly excite nociceptive afferents triggering inflammatory pain and hyperalgesia. Many of the receptors of these inflammatory mediators are GPCRs that are coupled to the Gq/11‐PLC signalling pathway (similar to muscarinic M1 receptors). These include B2 receptors (Couture et al., 2001; Petho and Reeh, 2012), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=262 receptors (Kuhn et al., 1996), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=348 (PAR2; Bushell et al., 2006), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=340 (Breyer et al., 1996) and P2Y (Chen and Chen, 1997) receptors (reviewed in Linley et al., 2010). Bradykinin can be considered as one of the prototypic Gq/11 coupled inflammatory mediators as it is released upon tissue damage and inflammation; its receptors are abundantly expressed in nociceptors; and bradykinin injections or topical applications to wounds/blisters are very painful (Keele, 1967; Dray and Perkins, 1993; Liu et al., 2010; Petho and Reeh, 2012; Linley et al., 2012a). Bradykinin is so active that it has been branded ‘the most potent endogenous algogenic substance ever identified’ (Dray and Perkins, 1993).
Bradykinin is known to inhibit M channels in expression systems and sympathetic and sensory neurons (Cruzblanca et al., 1998; Gamper and Shapiro, 2003; Zhang et al., 2003; Liu et al., 2010; Linley et al., 2012a). Thus, in cultured DRG neurons, bradykinin inhibited M current by 50–60% and increased their excitability, effects that were mimicked by XE991 and antagonized by a retigabine analogue, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2598 (Liu et al., 2010; Linley et al., 2012a). In accord with these findings, hind paw injections of bradykinin produced prominent pain‐like (‘nocifensive’) behaviour in rats, an effect that can be attenuated by retigabine or mimicked by XE991 (Liu et al., 2010; Zhang et al., 2015; Huang et al., 2016a). Bradykinin was recently also shown to robustly excite colonic afferents, and this effect was also abolished by retigabine and partially mimicked by XE991 (Peiris et al., 2017). It has to be noted that M current inhibition is not the sole excitatory action of bradykinin in DRG neurons; B2 receptor stimulation also results in the activation of the excitatory chloride channel, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=130#708 (Liu et al., 2010; Jin et al., 2013), sensitization of TRPV1 (Sugiura et al., 2002) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=485http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=485 (Bandell et al., 2004) channels as well some additional effects, including trafficking of pro‐excitatory channels (Huang et al., 2016b; reviewed in Petho and Reeh, 2012).
Similarly to bradykinin receptors, stimulation of PAR2 (Linley et al., 2008) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=152 (Crozier et al., 2007) receptors also inhibits M channels in nociceptive DRG neurons, an effect that contributes to the inflammatory pain and itch mediated by these inflammatory GPCRs.
Neuropathic pain
Neuropathic pain is defined as ‘pain caused by a lesion or disease of the somatosensory nervous system’ (International Association for the Study of Pain, 2017); it is caused by injuries or degeneration within peripheral or central somatosensory pathways. Among chronic pain conditions, neuropathic pain is one of the most debilitating and also one of the most resilient to treatment as it often responds poorly to medication (O'Connor and Dworkin, 2009). Neuropathy is associated with spontaneous and evoked pains that often persist over long periods of time (Campbell and Meyer, 2006). Both peripheral and central mechanisms contribute to the development of neuropathic pain. A state of sustained pathological overexcitability of nociceptive afferents (‘peripheral sensitization’) is considered necessary for the development of most types of neuropathic pain.
When peripheral axons are severed (e.g. by trauma), the nerve stump forms a swelling or endbulb, which then sprouts thin processes, which may re‐connect severed nerve endings, if these are in close enough proximity. If regeneration is obstructed, the endbulb, aborted sprouts and surrounding glia become a bulky tissue formation called a neuroma (Fawcett and Keynes, 1990; Devor, 2006). Both the neuroma (Govrin‐Lippmann and Devor, 1978; Wall and Devor, 1983) and cell bodies of damaged nerves (Wall and Devor, 1983; Amir and Devor, 1997; Liu et al., 2000) generate spurious ectopic firing, which may cause pain on its own but also feeds into the spinal wind‐up mechanisms. The neuropathic deformation of the injured nerve is associated with large‐scale remodelling of the injured and perhaps also neighbouring, uninjured fibres within the affected nerve and the corresponding sensory ganglion. One of the striking features of such remodelling is a marked down‐regulation of several key K+ channel genes (including Kcnq2 and Kcnq5) (Rose et al., 2011; King and Scherer, 2012; Du and Gamper, 2013; Cisneros et al., 2015; Laumet et al., 2015). Such a loss of K+ channels is expected to result in depolarized and more excitable fibres, which is indeed one of the characteristic of neuropathic afferents and, also, of the DRG neurons from mice with the genetic deletion of Kv7.2 from sensory neurons (King et al., 2014). The mechanisms underlying the down‐regulation of K+ channel genes following nerve injury are under intense scrutiny, and several molecular players have been identified. Thus, we found that a down‐regulation of Kcnq2 expression triggered by the partial sciatic nerve ligation (PSNL) in rat DRG is dependent on repressor element 1‐silencing transcription factor (REST, also known as NRSF) (Rose et al., 2011). Kcnq genes have functional repressor element 1 (NRSE) binding sites (Mucha et al., 2010; Iannotti et al., 2012). Accordingly, viral overexpression of REST in DRG neurons strongly suppressed M current density and increased their tonic excitability (Mucha et al., 2010). REST inhibits transcription by recruiting co‐repressor complexes SIN3A/B and REST corepressor 1; these complexes, in turn, modify target gene regions through chromatin‐modifying enzymes, including histone deacetylases 1/2 (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=8481/2), the histone demethylase http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2669 and the histone methylase http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=871#2652 (Ooi and Wood, 2007; Willis et al., 2016). Interestingly, inhibition or genetic deletion of G9a in DRG was shown to abolish the down‐regulation of Kv7.2 [as well as three other K+ channels: http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=541, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=553 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=380 (KCa1.1)] induced by neuropathic injury (Laumet et al., 2015). Inhibition or deletion of G9a also markedly reduced neuropathic hyperalgesia. Notably, baseline REST levels in neurons are low, but neuropathic injury and inflammation triggers REST expression (Mucha et al., 2010; Uchida et al., 2010; Rose et al., 2011; Willis et al., 2016), which, in turn, drives the neuropathic remodelling.
Of note is the fact that M channel expression in neuropathic fibres is reduced, but not abolished (Rose et al., 2011; Cisneros et al., 2015). Accordingly, M channel openers are still efficacious in alleviating neuropathic pain symptoms, presumably by acting on the remaining Kv7 channels to stabilize the E rest and reduce firing in the affected fibres. Thus, retigabine strongly suppressed the spontaneous activity of neuropathic fibres (Bernal et al., 2016), and thermal hyperalgesia produced by PSNL in rats was effectively alleviated by an injection of flupirtine directly into the neuroma (an effect that was reversed by XE991) (Rose et al., 2011). It has to be noted that an increase in Kv7.2‐positive nodes of Ranvier in nerve‐end neuromas of mice with neuropathic injury (saphenous nerve transection) has also been reported (Roza et al., 2011; Cisneros et al., 2015). Thus, while down‐regulated in the soma and elsewhere, remaining M channels may actually be pooled at the sites of ectopic activity (e.g. at neuroma nerve stubs), as a compensatory mechanism to offset spurious nerve activity. Such an effect would represent an additional mechanism for anti‐nociceptive efficacy of peripherally applied M channel openers.
Cancer pain
Tumours often trigger pain via a combination of inflammatory, neuropathic, ischaemic and tissue compression mechanisms and also by chemical mediators released by cancer cells (Prinsloo et al., 2013). Systemic administration of flupirtine displayed analgesic efficacy in a rat model of prostate bone metastasis (Kolosov et al., 2012), suggesting that a general dampening of excitability within the pain pathways via M channel potentiation might have potential as a treatment for cancer pain.
As in the case of neuropathic pain, a marked down‐regulation of Kv7.2 and Kv7.3 channel abundance and a reduction of M current amplitudes were reported in DRG neurons in a rat model of bone cancer pain (Zheng et al., 2013); these effects were also accompanied by a hyperexcitable state of the DRG neurons. In a follow‐up study, the same group also showed that the excitability of the C‐fibre‐synapsed WDR neurons in the dorsal horn is also enhanced in the rats developing bone cancer and this effect was also attributed, at least in part, to the M channel suppression (although in the latter study, it was not clear, whether the M current was down‐regulated in the C‐fibre terminals or in the WDR neurons themselves, or at both locations) (Cai et al., 2015). Systemic (Zheng et al., 2013) or intrathecal (Cai et al., 2015) retigabine alleviated mechanical allodynia and thermal hyperalgesia induced by bone cancer and also reduced hyperexcitability of both DRG and WDR DH neurons. Similar to the neuropathic pain models (Rose et al., 2011), despite the down‐regulation of M channel activity/expression, retigabine and flupirtine were still efficacious in alleviating cancer pain even when applied locally (e.g. intrathecally).
M channel openers
Due to the extensive expression of Kv7 channels in the nervous and cardiovascular systems and their strong effects on excitability, the therapeutic potential of these channels is not limited to pain but also includes epilepsy, anxiety (Gribkoff, 2008), stroke (i.e. as a protection against excitotoxicity) (Gamper et al., 2006; Bierbower et al., 2015), smooth muscle disorders (Stott et al., 2014) and other diseases. It is therefore not surprising that there is a large drive, both in industry and academia, to identify potent and selective M channel openers. Conveniently, M channels present an unusually rich substrate for pharmacological modulation, and literally thousands of openers acting via various distinct mechanisms have already been identified. World Intellectual Property Organization lists well over 2 hundred patents related to M channel openers. Progress in M channel pharmacology has been discussed in several recent reviews (Gribkoff, 2008; Miceli et al., 2008; Du and Gamper, 2013; Grunnet et al., 2014); known mechanisms of potentiation and binding sites for major classes of M channel openers were reviewed by us recently (Du and Gamper, 2013); therefore, here we will just briefly outline the main classes of M channel openers, which display analgesic activity, as well as some major recent developments.
The prototypical M channel openers retigabine (ezogabine) and flupirtine (katadolon, awegal) belong to triaminopyridines (Figure 3A, B); these compounds are potent but relatively non‐selective Kv7 openers that activate all Kv7 subunits except of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=560, with Kv7.3 being the most sensitive subunit (Tatulian et al., 2001; Tatulian and Brown, 2003; Schenzer et al., 2005; Wuttke et al., 2005). Flupirtine has been used clinically in Europe since 1984 as a centrally acting nonopioid analgesic for postoperative, traumatic and cancer pain; it also has muscle‐relaxant activity. The analgesic efficacy of flupirtine is comparable to that of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2713s such as ibuprofen (Miceli et al., 2008). Although flupirtine was reported to affect http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=72 (Miceli et al., 2008), little direct evidence exists and it is believed that its analgesic effect is mainly related to its activity as an M channel opener (Devulder, 2010). Retigabine is a clinically approved antiepileptic drug (Stafstrom et al., 2011), but it also consistently displays analgesic efficacy in multiple animal pain models such as temporomandibular joint pain (Xu et al., 2010), visceral pain (Hirano et al., 2007), carrageenan‐induced hyperalgesia (Passmore et al., 2003), trigeminal neuropathy (Abd‐Elsayed et al., 2015), bone cancer pain (Zheng et al., 2013). The clinical use of retigabine has been recently restricted to adjunctive treatment of drug‐resistant partial onset seizures (and only if other treatments have failed or could not be tolerated; Daniluk et al., 2016) due to significant risks of skin discoloration, retinal pigmentation, urinary retention, sedation and QTc prolongation (Daniluk et al., 2016). Nevertheless, retigabine remains a useful research tool and a ‘benchmark’ drug in animal pain models. Its mechanism of action is also fairly well understood. The binding site of retigabine resides within the cytosolic region between S5 and S6 transmembrane domains of Kv7 channels and contains a critical tryptophan (W236 in Kv7.2), which is absent in Kv7.1 (Schenzer et al., 2005; Wuttke et al., 2005). Biophysically triaminopyridines induce a large shift in channel voltage‐dependence towards negative voltages (i.e. retigabine shifts half‐maximal activation voltage of Kv7.2/Kv7.3 multimers by more than −30 mV); additionally, these compounds increase macroscopic steady‐state K+ conductance at saturating voltages (Tatulian et al., 2001; Tatulian and Brown, 2003; Schenzer et al., 2005; Wuttke et al., 2005; Linley et al., 2012b).
Figure 3.
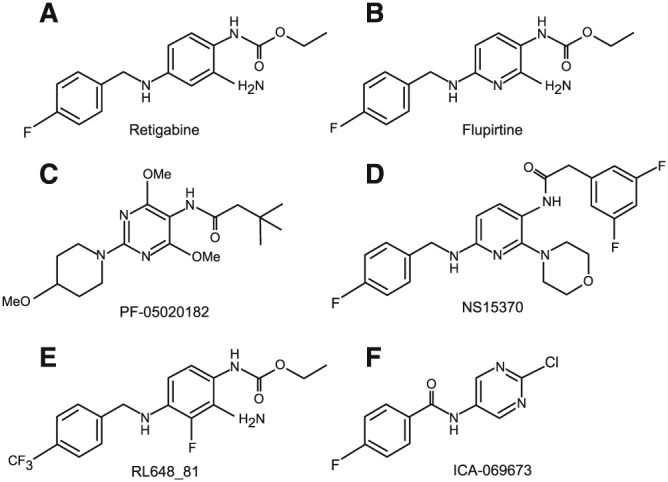
Chemical structures of retigabine and its analogues.
Kv7 channels are widely expressed within the CNS, PNS and vasculature, yet their subunit distribution is not uniform. While Kv7.2/Kv7.3 multimeric channels are believed to dominate the CNS, Kv7.4 is highly expressed specifically in auditory pathways (as well as in a subpopulation of rapidly adapting cutaneous mechanoreceptors; Heidenreich et al., 2012; see above). Kv7.1 is mostly expressed in the heart and epithelia (but not in neurons), while vascular smooth muscles more abundantly express Kv7.5, Kv7.4 and their multimers (reviewed in Gamper and Shapiro, 2015). Therefore, openers such as retigabine, which discriminate poorly between Kv7 subunits, are likely to have multiple ‘on‐target’ side effects. Drugs with higher subunit selectivity ‘tailored’ towards specific cell types are needed to achieve more specific pharmacological actions. Indeed, recent efforts in academia and industry have been focused on discovering new drugs or the refinement of existing compounds towards better selectivity and higher potency.
Several groups have performed structure–activity relationship (SAR) analyses of retigabine to produce more potent and selective derivatives. Thus, compounds PF‐05020182 (Figure 3C) (Davoren et al., 2015) and NS15370 (Figure 3D) (Dalby‐Brown et al., 2013), which are ~10 and ~30 fold more potent than retigabine, were synthesized; these are ineffective against Kv7.1 but do activate Kv7.4 and Kv7.5 channels with comparable potency to that of Kv7.2/Kv7.3. Thanos Tzounopoulos' group used the SAR approach to generate several improved retigabine derivatives. By introducing a CF3‐group at the 4‐position of the benzylamine moiety and a fluorine at the 3‐position of the aniline ring (Figure 3E), they generated a compound (RL648_81) which is over 15 times more potent than retigabine at Kv7.2/Kv7.3 channels (EC50 0.2 μM vs. ~3 μM) and does not affect Kv7.4 and Kv7.5 (Kumar et al., 2016). Another recent compound, ICA‐069673 (and related derivatives such as ICA‐27243 and ztz series compounds), displays remarkable selectivity for Kv7.2 over Kv7.3 (Figure 3F) (Wickenden et al., 2008; Gao et al., 2010; Wang et al., 2016).
Other M channel openers include acrylamides known as compounds (S)‐1 and (S)‐2 (Wu et al., 2004a,b,c, 2013; L'Heureux et al., 2005) developed by Bristol‐Myers Squibb. Acrylamides share the site of action and specificity profile with retigabine (Bentzen et al., 2006) and display analgesic efficacy in rodent models of migraine (Wu et al., 2004a), inflammatory and neuropathic pain (Wu et al., 2013). A QO series of openers is based on pyrazolo[1,5‐a]pyrimidin‐7(4H)‐one (Jia et al., 2011; Qi et al., 2011; Zhang et al., 2012; Teng et al., 2016); the lead compound, QO‐58 (and QO‐58‐lysine, which has improved bioavailability), has analgesic efficacy in neuropathic and inflammatory pain models (Zhang et al., 2012; Teng et al., 2016). QO‐58 potentiates all Kv7 channels except Kv7.3. Benzimidazoles, such as compound B1 (Zhang et al., 2011a; Du and Gamper, 2013), have high selectivity for Kv7.2 over Kv7.3, Kv7.4 and Kv7.5. Like the QO series, benzimidazoles do not require W236 for their action on Kv7 channels (reviewed in Du and Gamper (2013). In addition, adamantyl derivatives (Fritch et al., 2010), N‐phenilanthranilic acid derivatives, for example, meclofenamic acid, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2714 and related compounds (Peretz et al., 2005; Peretz et al., 2007; Brueggemann et al., 2009; Peretz et al., 2010), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4319 (Zhang et al., 2015) and a Rho kinase inhibitor http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5181 (Zhang et al., 2016) were identified as M channel openers. Of these, fasudil is perhaps unique as it selectively activates Kv7.4 and Kv7.4/Kv7.5 channels but not the other Kv7 subunits. A zinc coordination complex, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2597, has also been suggested to display Kv7 channel opener activity (Xiong et al., 2007), but it appears that the channel potentiation in this case is produced by intracellular zinc, whereas pyrithione acts as a zinc ionophore (Gao et al., 2017).
Where is the site of analgesic action of M channel openers?
M channel openers display analgesic efficacy when administered peripherally (e.g. plantar paw injections) (Linley et al., 2008; Liu et al., 2010; Huang et al., 2016a), injected into the neuroma site (Rose et al., 2011), focally applied to DRG (Du et al., 2014) or administered intrathecally (Cai et al., 2015) or systemically (reviewed in Du and Gamper (2013). Interestingly, one recent study has demonstrated that even when given orally, the analgesic action of retigabine is almost exclusively peripheral (Hayashi et al., 2014). Thus, most central effects of oral retigabine (anticonvulsant effect, impaired motor coordination, reduced exploratory behaviour) were effectively precipitated by central (i.c.v.) application of XE991. Yet the analgesic effect was not affected. The likelihood therefore is that the main site of analgesic action of retigabine is within the peripheral fibres.
As discussed above, functional M channels have been shown to be present in all major sensory nerve compartments (peripheral and central terminals, soma, axon). Thus, theoretically, M channel activation can suppress the generation, propagation and transmission of nociceptive signals to the CNS.
Experimental data suggest that potentiation of M channel activity at the nociceptive terminals in the skin with retigabine, flupirtine or other openers does indeed reduce basal sensitivity to thermal and mechanical stimuli (Passmore et al., 2012; Vetter et al., 2013; Huang et al., 2016a) and alleviates the nociceptive effects of various peripherally applied algogenic stimuli (Linley et al., 2008; Liu et al., 2010; Passmore et al., 2012; Vetter et al., 2013; Hayashi et al., 2014; Huang et al., 2016a; Peiris et al., 2017). This suggests that nociceptive nerve endings are indeed one of the sites for the analgesic efficacy of the openers.
Intrathecal application of M channel openers also produces an analgesic effect (Cai et al., 2015), suggesting that the transmission of nociceptive signals to the spinal cord can also be controlled by M channels. Yet, in this case, it is hard to pinpoint the site of action exactly, as DRG, dorsal roots, central terminals of the afferent fibres and the spinal circuitry are all exposed to an intrathecally applied drug.
Interestingly, a focal injection of flupirtine onto un‐injured sciatic nerve did not significantly affect noxious heat sensitivity of the plantar surface of the paw (Rose et al., 2011), suggesting that the propagation of peripheral nociceptive signals through the axons is perhaps less affected by M channel activity, as compared to their initiation or transmission. However, there is one site within the axon of a somatosensory neuron where propagation of the peripherally‐derived action potentials can be effectively disrupted by M channel activation, and it is the point at which the axon, emanating from the cell body, bifurcates into the peripheral and central branches, the t‐junction (Figures 2 and 4A). The bifurcation of an afferent axon is a point of lowest safety factor for action potential propagation (Stoney, 1985; Luscher et al., 1994; Debanne, 2004; Gemes et al., 2013; Du et al., 2014; Sundt et al., 2015), and both experiments and computer modelling suggest that hyperpolarization of a t‐junction (e.g. with retigabine) can result in the failure of an action potential to propagate (Du et al., 2014; Sundt et al., 2015; Figure 4).
Figure 4.
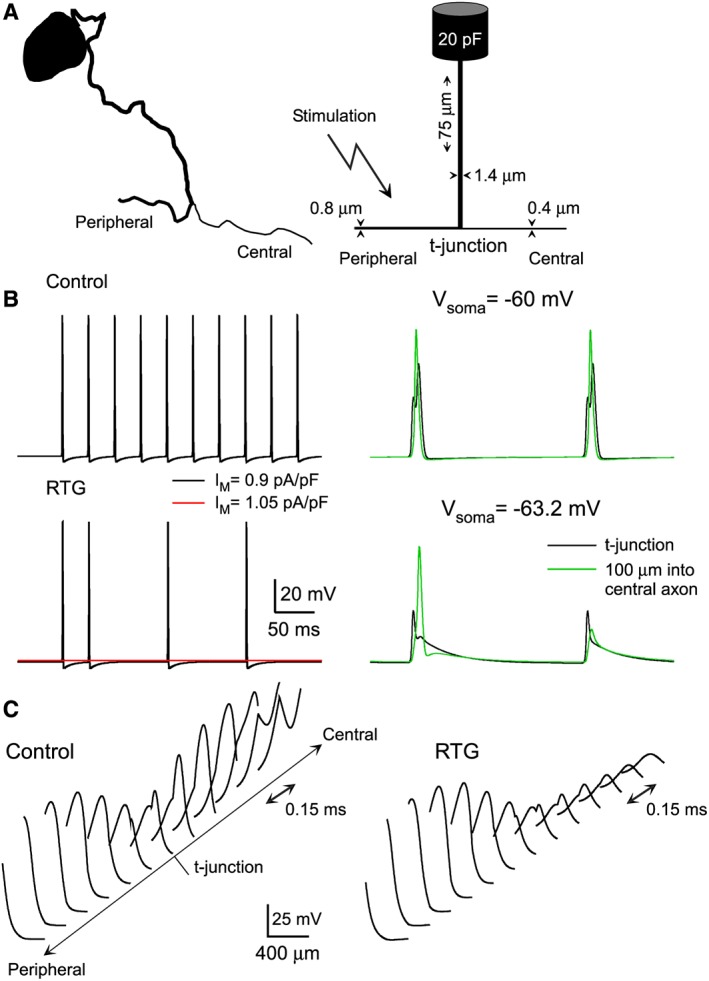
Biophysical model of a small‐diameter unmyelinated DRG neuron. (A) Shown on the left is a drawing of soma and stem axon of small‐diameter cat DRG neuron (based on micrograph from Ha, 1970); on the right are the morphological dimensions of the model neuron. (B) Enhancing the activity of M channels with virtual retigabine (−30 mV shift in activation curve, 1.5 fold increase in conductance density) hyperpolarized the t‐junction and reduced (at initial IM density of 0.9 pA/pF) or abolished (at initial IM density of 1.05 pA/pF) spike propagation in the model neuron. (C) Retigabine reduces the likelihood of spike generation in the central axon. Action potential amplitude is plotted as function of distance along 100 μm of peripheral and central axon sections with the t‐junction in the middle. In control conditions, spike amplitude decreases when approaching the t‐junction, due to the impedance load of the bifurcation. On the central side of the t‐junction, a delayed action potential develops and grows with distance from the t‐junction. When retigabine is added, the spike amplitude in the peripheral axon is not significantly affected; in contrast, the spike fails on the central side of the t‐junction and its decay is recorded (modified from Du et al., 2014, with permission).
In order to better understand the filtering role of the t‐junction, we recently developed a computational model of a small‐diameter, unmyelinated mammalian DRG neuron (Du et al., 2014; Sundt et al., 2015; Figure 4) based on available anatomical data (Ha, 1970; Suh et al., 1984; Hoheisel and Mense, 1987). The model revealed that due to the electrotonically short stem axon (Figure 4A), somatic/perisomatic hyperpolarization in combination with the increased membrane conductance, produced by ‘M channel’ potentiation (or by other similar manoeuvres), further lowered the safety factor at the t‐junction and, as a result, interfered with action potential propagation (Figure 4B, C). Without a t‐junction, the safety factor in the axon is relatively high, and comparable ion channel modulation, for example, in the central axon distal to the t‐junction, had no effect on spike transmission. The model explains well why an injection of flupirtine into the sciatic nerve did not significantly affect pain thresholds (Rose et al., 2011). Whereas, as predicted by the model, focal infusion of retigabine directly on to the L5 DRG in vivo (via an implanted cannula) strongly alleviated pain produced by a hind paw injection of bradykinin (Du et al., 2014).
In summary, current evidence suggests that the antinociceptive effect of M channel openers is mostly localized to peripheral nociceptive fibres, and within these, the action is most likely localized to the sites of action potential initiation (peripheral nerve endings, ectopic sites), propagation (the t‐junctions) and transmission (central terminals).
Conclusions and future perspectives
The fifteen or so years since the first report of the presence of functional M channels in peripheral somatosensory neurons (Passmore et al., 2003) has seen rapid progress in our understanding of the role these channels play in nociception. It is now firmly established that (i) the M current is an important control mechanism, which ‘clamps’ E rest of nociceptors and sets their resting excitability parameters (e.g. threshold current); (ii) M channel openers consistently display analgesic efficacy in most experimental pain models; and (iii) both acute inhibition of M channel activity or long‐term down‐regulation of M channel abundance (e.g. via the epigenetic mechanisms) often drives nociceptors into an overexcitable state, which is associated with acute or chronic pain. M channels are therefore a validated drug target for the treatment of pain, and many pharmaceutical companies are investing in the development of new M channel openers. There are, however, a few issues that hamper the progress. Particularly, (i) there is still no clarity with regards to the M channel subunit expression profile in known subpopulations of sensory neurons and (ii) we are still short of the set of truly subunit‐selective M channel openers with good drug‐like properties. Both these shortcomings should be solved in order to be able to target M channels in crucial subpopulations of nociceptors more specifically.
Another consideration regarding possible refinement of current drug design strategies arises from the fact that while current therapeutic M channel openers are designed for the systemic application (i.e. oral), the analgesic efficacy of such drugs is most likely peripheral. It is also important to point out that the dorsal roots and the central terminals of the nociceptive fibres are protected by the blood–brain barrier (BBB) within the spinal cord, whereas the afferent axons within the peripheral nerves are protected by the blood‐nerve barrier (BNB). Yet spinal ganglia themselves have no such barrier and are exposed to the circulation to a significant extent (Devor, 1999). Since somatic/perisomatic M channels are capable of controlling action potentials propagating through the t‐junctions of nociceptors, it seems logical to propose that M channel openers with poor BBB/BNB permeability can still have analgesic efficacy due to their action within peripheral ganglia, yet the central side effects of such compounds could be significantly reduced. Future research will test if such a strategy can be implemented.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c,d).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The work in our laboratories is supported by Medical Research Council (MR/K021303/1 to N.G.); National Natural Science Foundation of China (31571088 to X.D.; 81201642 to H.G.; 31270882 to H.Z.); Key Basic Research Project of Applied Basic Research Program of Hebei Province (16967712D to X.D.); and the National Basic Research Program (2013CB531302 to H.Z.).
Du, X. , Gao, H. , Jaffe, D. , Zhang, H. , and Gamper, N. (2018) M‐type K+ channels in peripheral nociceptive pathways. British Journal of Pharmacology, 175: 2158–2172. doi: 10.1111/bph.13978.
References
- Abd‐Elsayed AA, Ikeda R, Jia Z, Ling J, Zuo X, Li M et al (2015). KCNQ channels in nociceptive cold‐sensing trigeminal ganglion neurons as therapeutic targets for treating orofacial cold hyperalgesia. Mol Pain 11: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Devor M (1997). Spike‐evoked suppression and burst patterning in dorsal root ganglion neurons of the rat. J Physiol 501 (Pt 1): 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous (2016). Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Mil Med 181: 397–399. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ et al (2004). Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–857. [DOI] [PubMed] [Google Scholar]
- Barkai O, Goldstein RH, Caspi Y, Katz B, Lev S, Binshtok AM (2017). The role of kv7/m potassium channels in controlling ectopic firing in nociceptors. Front Mol Neurosci 10: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzen BH, Schmitt N, Calloe K, Dalby Brown W, Grunnet M, Olesen SP (2006). The acrylamide (S)‐1 differentially affects Kv7 (KCNQ) potassium channels. Neuropharmacology 51: 1068–1077. [DOI] [PubMed] [Google Scholar]
- Bernal L, Lopez‐Garcia JA, Roza C (2016). Spontaneous activity in C‐fibres after partial damage to the saphenous nerve in mice: effects of retigabine. Eur J Pain 20: 1335–1345. [DOI] [PubMed] [Google Scholar]
- Bierbower SM, Choveau FS, Lechleiter JD, Shapiro MS (2015). Augmentation of M‐type (KCNQ) potassium channels as a novel strategy to reduce stroke‐induced brain injury. J Neurosci 35: 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivik H, Eisenberg E, O'Brien T, Openminds (2013). The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 13: 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyer MD, Jacobson HR, Breyer RM (1996). Functional and molecular aspects of renal prostaglandin receptors. J Am Soc Nephrol 7: 8–17. [DOI] [PubMed] [Google Scholar]
- Brown DA, Adams PR (1980). Muscarinic suppression of a novel voltage‐sensitive K+ current in a vertebrate neurone. Nature 283: 673–676. [DOI] [PubMed] [Google Scholar]
- Brueggemann LI, Mackie AR, Mani BK, Cribbs LL, Byron KL (2009). Differential effects of selective cyclooxygenase‐2 inhibitors on vascular smooth muscle ion channels may account for differences in cardiovascular risk profiles. Mol Pharmacol 76: 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell TJ, Plevin R, Cobb S, Irving AJ (2006). Characterization of proteinase‐activated receptor 2 signalling and expression in rat hippocampal neurons and astrocytes. Neuropharmacology 50: 714–725. [DOI] [PubMed] [Google Scholar]
- Cai J, Fang D, Liu XD, Li S, Ren J, Xing GG (2015). Suppression of KCNQ/M (Kv7) potassium channels in the spinal cord contributes to the sensitization of dorsal horn WDR neurons and pain hypersensitivity in a rat model of bone cancer pain. Oncol Rep 33: 1540–1550. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA (2006). Mechanisms of neuropathic pain. Neuron 52: 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Chen WC (1997). P2Y receptor linked to phospholipase C: stimulation of neuro 2A cells by UTP and ATP and possible regulation by protein kinase C subtype epsilon. J Neurochem 69: 1409–1416. [DOI] [PubMed] [Google Scholar]
- Cisneros E, Roza C, Jackson N, Lopez‐Garcia JA (2015). A new regulatory mechanism for Kv7.2 protein during neuropathy: enhanced transport from the soma to axonal terminals of injured sensory neurons. Fron Cell Neurosci 9: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture R, Harrisson M, Vianna RM, Cloutier F (2001). Kinin receptors in pain and inflammation. Eur J Pharmacol 429: 161–176. [DOI] [PubMed] [Google Scholar]
- Crozier RA, Ajit SK, Kaftan EJ, Pausch MH (2007). MrgD activation inhibits KCNQ/M‐currents and contributes to enhanced neuronal excitability. J Neurosci 27: 4492–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzblanca H, Koh DS, Hille B (1998). Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3‐sensitive Ca2+ stores in rat sympathetic neurons. Proc Natl Acad Sci U S A 95: 7151–7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby‐Brown W, Jessen C, Hougaard C, Jensen ML, Jacobsen TA, Nielsen KS et al (2013). Characterization of a novel high‐potency positive modulator of Kv7 channels. Eur J Pharmacol 709: 52–63. [DOI] [PubMed] [Google Scholar]
- Daniluk J, Cooper JA, Stender M, Kowalczyk A (2016). Survey of physicians' understanding of specific risks associated with retigabine. Drugs Real World Outcomes 3: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoren JE, Claffey MM, Snow SL, Reese MR, Arora G, Butler CR et al (2015). Discovery of a novel Kv7 channel opener as a treatment for epilepsy. Bioorg Med Chem Lett 25: 4941–4944. [DOI] [PubMed] [Google Scholar]
- Debanne D (2004). Information processing in the axon. Nat Rev Neurosci 5: 304–316. [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA (2005). Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 6: 850–862. [DOI] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS (2004). KCNQ2 is a nodal K+ channel. J Neurosci 24: 1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M (1999). Unexplained peculiarities of the dorsal root ganglion. Pain Suppl 6: S27–S35. [DOI] [PubMed] [Google Scholar]
- Devor M (2006). Response of nerves to injury in relation to neuropathic pain In: McMahon SB, Koltzenburg M. (eds). Wall and Melzack's Textbook of Pain, 6th edn. Elsevier Churchill Livingstone, pp. 905–927. [Google Scholar]
- Devulder J (2010). Flupirtine in pain management: pharmacological properties and clinical use. CNS Drugs 24: 867–881. [DOI] [PubMed] [Google Scholar]
- Dray A, Perkins M (1993). Bradykinin and inflammatory pain. Trends Neurosci 16: 99–104. [DOI] [PubMed] [Google Scholar]
- Du X, Gamper N (2013). Potassium channels in peripheral pain pathways: expression, function and therapeutic potential. Curr Neuropharmacol 11: 621–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Hao H, Gigout S, Huang D, Yang Y, Li L et al (2014). Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. Pain 155: 2306–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Keynes RJ (1990). Peripheral nerve regeneration. Annu Rev Neurosci 13: 43–60. [DOI] [PubMed] [Google Scholar]
- Ferrero‐Miliani L, Nielsen OH, Andersen PS, Girardin SE (2007). Chronic inflammation: importance of NOD2 and NALP3 in interleukin‐1beta generation. Clin Exp Immunol 147: 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov AK, Choi RC, Simon J, Barnard EA, Brown DA (2006). Activation of P2Y1 nucleotide receptors induces inhibition of the M‐type K+ current in rat hippocampal pyramidal neurons. J Neurosci 26: 9340–9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritch PC, McNaughton‐Smith G, Amato GS, Burns JF, Eargle CW, Roeloffs R et al (2010). Novel KCNQ2/Q3 agonists as potential therapeutics for epilepsy and neuropathic pain. J Med Chem 53: 887–896. [DOI] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS (2003). Calmodulin mediates Ca2+‐dependent modulation of M‐type K+ channels. J Gen Physiol 122: 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS (2007). Regulation of ion transport proteins by membrane phosphoinositides. Nat Rev Neurosci 8: 921–934. [DOI] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS (2015). KCNQ Channels In: Zheng J, Trudeau MC. (eds). Handbook of Ion Channels, 1st edn. CRC Press: Boca Raton, FL, pp. 275–306. [Google Scholar]
- Gamper N, Zaika O, Li Y, Martin P, Hernandez CC, Perez MR et al (2006). Oxidative modification of M‐type K+ channels as a mechanism of cytoprotective neuronal silencing. EMBO J 25: 4996–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Boillat A, Huang D, Liang C, Peers C, Gamper N (2017). Intracellular zinc activates KCNQ channels by reducing their dependence on phosphatidylinositol 4,5‐bisphosphate. Proc Natl Acad Sci U S A 114: E6410–£6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Zhang T, Wu M, Xiong Q, Sun H, Zhang Y et al (2010). Isoform‐specific prolongation of Kv7 (KCNQ) potassium channel opening mediated by new molecular determinants for drug‐channel interactions. J Biol Chem 285: 28322–28332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemes G, Koopmeiners A, Rigaud M, Lirk P, Sapunar D, Bangaru ML et al (2013). Failure of action potential propagation in sensory neurons: mechanisms and loss of afferent filtering in C‐type units after painful nerve injury. J Physiol 591: 1111–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govrin‐Lippmann R, Devor M (1978). Ongoing activity in severed nerves: source and variation with time. Brain Res 159: 406–410. [DOI] [PubMed] [Google Scholar]
- Greene DL, Hoshi N (2016). Modulation of Kv7 channels and excitability in the brain. Cell Mol Life Sci 74: 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribkoff VK (2008). The therapeutic potential of neuronal Kv7 (KCNQ) channel modulators: an update. Expert Opin Ther Targets 12: 565–581. [DOI] [PubMed] [Google Scholar]
- Grunnet M, Strobaek D, Hougaard C, Christophersen P (2014). Kv7 channels as targets for anti‐epileptic and psychiatric drug‐development. Eur J Pharmacol 726: 133–137. [DOI] [PubMed] [Google Scholar]
- Ha H (1970). Axonal bifurcation in the dorsal root ganglion of the cat: a light and electron microscopic study. J Comp Neurol 140: 227–240. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Iwata M, Tsuchimori N, Matsumoto T (2014). Activation of peripheral KCNQ channels attenuates inflammatory pain. Mol Pain 10: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich M, Lechner SG, Vardanyan V, Wetzel C, Cremers CW, De Leenheer EM et al (2012). KCNQ4 K+ channels tune mechanoreceptors for normal touch sensation in mouse and man. Nat Neurosci 15: 138–145. [DOI] [PubMed] [Google Scholar]
- Hernandez CC, Zaika O, Tolstykh GP, Shapiro MS (2008). Regulation of neural KCNQ channels: signalling pathways, structural motifs and functional implications. J Physiol 586: 1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, Dickson EJ, Kruse M, Vivas O, Suh BC (2015). Phosphoinositides regulate ion channels. Biochim Biophys Acta 1851: 844–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Kuratani K, Fujiyoshi M, Tashiro N, Hayashi E, Kinoshita M (2007). Kv7.2–7.5 voltage‐gated potassium channel (KCNQ2‐5) opener, retigabine, reduces capsaicin‐induced visceral pain in mice. Neurosci Lett 413: 159–162. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Mense S (1987). Observations on the morphology of axons and somata of slowly conducting dorsal root ganglion cells in the cat. Brain Res 423: 269–278. [DOI] [PubMed] [Google Scholar]
- Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N et al (2003). AKAP150 signaling complex promotes suppression of the M‐current by muscarinic agonists. Nat Neurosci 6: 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Huang S, Gao H, Liu Y, Qi J, Chen P et al (2016a). Redox‐dependent modulation of T‐type Ca2+ channels in sensory neurons contributes to acute anti‐nociceptive effect of substance P. Antioxid Redox Signal 25: 233–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Liang C, Zhang F, Men H, Du X, Gamper N et al (2016b). Inflammatory mediator bradykinin increases population of sensory neurons expressing functional T‐type Ca2+ channels. Biochem Biophys Res Commun 473: 396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Trussell LO (2011). KCNQ5 channels control resting properties and release probability of a synapse. Nat Neurosci 14: 840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti FA, Barrese V, Formisano L, Miceli F, Taglialatela M (2012). Specification of skeletal muscle differentiation by repressor element‐1 silencing transcription factor (REST)‐regulated Kv7.4 potassium channels. Mol Biol Cell 24: 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Association for the Study of Pain [online]. Available at: http://www.iasp-pain.org/Taxonomy?navItemNumber=576#Neuropathicpain (accessed 17 July 2017).
- Jentsch TJ (2000). Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci 1: 21–30. [DOI] [PubMed] [Google Scholar]
- Jia C, Qi J, Zhang F, Mi Y, Zhang X, Chen X et al (2011). Activation of KCNQ2/3 potassium channels by novel pyrazolo[1,5‐a]pyrimidin‐7(4H)‐one derivatives. Pharmacology 87: 297–310. [DOI] [PubMed] [Google Scholar]
- Jin X, Shah S, Liu Y, Zhang H, Lees M, Fu Z et al (2013). Activation of the Cl‐ channel ANO1 by localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci Signal 6: ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Gu JG (2017). Effects of cold temperatures on the excitability of rat trigeminal ganglion neurons that are not for cold sensing. J Neurochem 141: 532–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele CA (1967). The chemistry of pain production. Proc R Soc Med 60: 419–422. [PMC free article] [PubMed] [Google Scholar]
- King CH, Lancaster E, Salomon D, Peles E, Scherer SS (2014). Kv7.2 regulates the function of peripheral sensory neurons. J Comp Neurol 522: 3262–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CH, Scherer SS (2012). Kv7.5 is the primary Kv7 subunit expressed in C‐fibers. J Comp Neurol 520: 1940–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosov A, Goodchild CS, Williams ED, Cooke I (2012). Flupirtine enhances the anti‐hyperalgesic effects of morphine in a rat model of prostate bone metastasis. Pain Med 13: 1444–1456. [DOI] [PubMed] [Google Scholar]
- Kosenko A, Hoshi N (2013). A change in configuration of the calmodulin‐KCNQ channel complex underlies Ca2+‐dependent modulation of KCNQ channel activity. PLoS One 8: e82290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn B, Schmid A, Harteneck C, Gudermann T, Schultz G (1996). G proteins of the Gq family couple the H2 histamine receptor to phospholipase C. Mol Endocrinol 10: 1697–1707. [DOI] [PubMed] [Google Scholar]
- Kumar M, Reed N, Liu R, Aizenman E, Wipf P, Tzounopoulos T (2016). Synthesis and evaluation of potent KCNQ2/3‐specific channel activators. Mol Pharmacol 89: 667–677. [DOI] [PubMed] [Google Scholar]
- Lang PM, Fleckenstein J, Passmore GM, Brown DA, Grafe P (2008). Retigabine reduces the excitability of unmyelinated peripheral human axons. Neuropharmacology 54: 1271–1278. [DOI] [PubMed] [Google Scholar]
- Laumet G, Garriga J, Chen SR, Zhang Y, Li DP, Smith TM et al (2015). G9a is essential for epigenetic silencing of K+ channel genes in acute‐to‐chronic pain transition. Nat Neurosci 18: 1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Hong S, Cui M, Sharma PK, Lee J, Choi S (2015). Transient receptor potential vanilloid type 1 antagonists: a patent review (2011–2014). Expert Opin Ther Pat 25: 291–318. [DOI] [PubMed] [Google Scholar]
- L'Heureux A, Martel A, He H, Chen J, Sun LQ, Starrett JE et al (2005). (S,E)‐N‐[1‐(3‐heteroarylphenyl)ethyl]‐3‐(2‐fluorophenyl)acrylamides: synthesis and KCNQ2 potassium channel opener activity. Bioorg Med Chem Lett 15: 363–366. [DOI] [PubMed] [Google Scholar]
- Li Y, Gamper N, Hilgemann DW, Shapiro MS (2005). Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol (4,5)‐bisphosphate. J Neurosci 25: 9825–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley JE, Ooi L, Pettinger L, Kirton H, Boyle JP, Peers C et al (2012a). Reactive oxygen species are second messengers of neurokinin signaling in peripheral sensory neurons. Proc Natl Acad Sci U S A 109: E1578–E1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley JE, Pettinger L, Huang D, Gamper N (2012b). M channel enhancers and physiological M channel block. J Physiol 590: 793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley JE, Rose K, Ooi L, Gamper N (2010). Understanding inflammatory pain: ion channels contributing to acute and chronic nociception. Pflugers Arch 459: 657–669. [DOI] [PubMed] [Google Scholar]
- Linley JE, Rose K, Patil M, Robertson B, Akopian AN, Gamper N (2008). Inhibition of M current in sensory neurons by exogenous proteases: a signaling pathway mediating inflammatory nociception. J Neurosci 28: 11240–11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H et al (2010). The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M‐type K+ channels and activation of Ca2+‐activated Cl− channels. J Clin Invest 120: 1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CN, Wall PD, Ben‐Dor E, Michaelis M, Amir R, Devor M (2000). Tactile allodynia in the absence of C‐fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain 85: 503–521. [DOI] [PubMed] [Google Scholar]
- Luscher C, Streit J, Quadroni R, Luscher HR (1994). Action potential propagation through embryonic dorsal root ganglion cells in culture. I. Influence of the cell morphology on propagation properties. J Neurophysiol 72: 622–633. [DOI] [PubMed] [Google Scholar]
- Miceli F, Soldovieri MV, Martire M, Taglialatela M (2008). Molecular pharmacology and therapeutic potential of neuronal Kv7‐modulating drugs. Curr Opin Pharmacol 8: 65–74. [DOI] [PubMed] [Google Scholar]
- Mucha M, Ooi L, Linley JE, Mordaka P, Dalle C, Robertson B et al (2010). Transcriptional control of KCNQ channel genes and the regulation of neuronal excitability. J Neurosci 30: 13235–13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro G, Dalby‐Brown W (2007). Kv7 (KCNQ) channel modulators and neuropathic pain. J Med Chem 50: 2576–2582. [DOI] [PubMed] [Google Scholar]
- O'Connor AB, Dworkin RH (2009). Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 122: S22–S32. [DOI] [PubMed] [Google Scholar]
- Ooi L, Gigout S, Pettinger L, Gamper N (2013). Triple cysteine module within M‐type K+ channels mediates reciprocal channel modulation by nitric oxide and reactive oxygen species. J Neurosci 33: 6041–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi L, Wood IC (2007). Chromatin crosstalk in development and disease: lessons from REST. Nat Rev Genet 8: 544–554. [DOI] [PubMed] [Google Scholar]
- Passmore GM, Reilly JM, Thakur M, Keasberry VN, Marsh SJ, Dickenson AH et al (2012). Functional significance of M‐type potassium channels in nociceptive cutaneous sensory endings. Front Mol Neurosci 5: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore GM, Selyanko AA, Mistry M, Al‐Qatari M, Marsh SJ, Matthews EA et al (2003). KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci 23: 7227–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris M, Hockley JR, Reed DE, Smith ESJ, Bulmer DC, Blackshaw LA (2017). Peripheral KV7 channels regulate visceral sensory function in mouse and human colon. Mol Pain 13: 1744806917709371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A, Degani N, Nachman R, Uziyel Y, Gibor G, Shabat D et al (2005). Meclofenamic acid and diclofenac, novel templates of KCNQ2/Q3 potassium channel openers, depress cortical neuron activity and exhibit anticonvulsant properties. Mol Pharmacol 67: 1053–1066. [DOI] [PubMed] [Google Scholar]
- Peretz A, Pell L, Gofman Y, Haitin Y, Shamgar L, Patrich E et al (2010). Targeting the voltage sensor of Kv7.2 voltage‐gated K+ channels with a new gating‐modifier. Proc Natl Acad Sci U S A 107: 15637–15642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A, Sheinin A, Yue C, Degani‐Katzav N, Gibor G, Nachman R et al (2007). Pre‐ and postsynaptic activation of M‐channels by a novel opener dampens neuronal firing and transmitter release. J Neurophysiol 97: 283–295. [DOI] [PubMed] [Google Scholar]
- Petho G, Reeh PW (2012). Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet‐activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev 92: 1699–1775. [DOI] [PubMed] [Google Scholar]
- Prinsloo S, Gabel S, Lyle R, Cohen L (2013). Neuromodulation of cancer pain. Integr Cancer Ther 13: 30–37. [DOI] [PubMed] [Google Scholar]
- Qi J, Zhang F, Mi Y, Fu Y, Xu W, Zhang D et al (2011). Design, synthesis and biological activity of pyrazolo[1,5‐a]pyrimidin‐7(4H)‐ones as novel Kv7/KCNQ potassium channel activators. Eur J Med Chem 46: 934–943. [DOI] [PubMed] [Google Scholar]
- Rivera‐Arconada I, Lopez‐Garcia JA (2006). Retigabine‐induced population primary afferent hyperpolarisation in vitro. Neuropharmacology 51: 756–763. [DOI] [PubMed] [Google Scholar]
- Rivera‐Arconada I, Roza C, Lopez‐Garcia JA (2009). Enhancing M currents: a way out for neuropathic pain? Front Mol Neurosci 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K, Ooi L, Dalle C, Robertson B, Wood IC, Gamper N (2011). Transcriptional repression of the M channel subunit Kv7.2 in chronic nerve injury. Pain 152: 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza C, Castillejo S, Lopez‐Garcia JA (2011). Accumulation of Kv7.2 channels in putative ectopic transduction zones of mice nerve‐end neuromas. Mol Pain 7: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza C, Lopez‐Garcia JA (2008). Retigabine, the specific KCNQ channel opener, blocks ectopic discharges in axotomized sensory fibres. Pain 138: 537–545. [DOI] [PubMed] [Google Scholar]
- Schenzer A, Friedrich T, Pusch M, Saftig P, Jentsch TJ, Grotzinger J et al (2005). Molecular determinants of KCNQ (Kv7) K+ channel sensitivity to the anticonvulsant retigabine. J Neurosci 25: 5051–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ (2000). KCNQ5, a novel potassium channel broadly expressed in brain, mediates M‐type currents. J Biol Chem 275: 24089–24095. [DOI] [PubMed] [Google Scholar]
- Schutze S, Orozco IJ, Jentsch TJ (2016). KCNQ potassium channels modulate sensitivity of skin down‐hair (D‐hair) mechanoreceptors. J Biol Chem 291: 5566–5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Savill J (2005). Resolution of inflammation: the beginning programs the end. Nat Immunol 6: 1191–1197. [DOI] [PubMed] [Google Scholar]
- Shapiro MS, Wollmuth LP, Hille B (1994). Angiotensin II inhibits calcium and M current channels in rat sympathetic neurons via G proteins. Neuron 12: 1319–1329. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Grippon S, Kirkpatrick P (2011). Ezogabine (retigabine). Nat Rev Drug Discov 10: 729–730. [DOI] [PubMed] [Google Scholar]
- Stoney SD Jr (1985). Unequal branch point filtering action in different types of dorsal root ganglion neurons of frogs. Neurosci Lett 59: 15–20. [DOI] [PubMed] [Google Scholar]
- Stott JB, Jepps TA, Greenwood IA (2014). K(V)7 potassium channels: a new therapeutic target in smooth muscle disorders. Drug Discov Today 19: 413–424. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Tominaga M, Katsuya H, Mizumura K (2002). Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol 88: 544–548. [DOI] [PubMed] [Google Scholar]
- Suh B, Hille B (2002). Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5‐bisphosphate synthesis. Neuron 35: 507–520. [DOI] [PubMed] [Google Scholar]
- Suh YS, Chung K, Coggeshall RE (1984). A study of axonal diameters and areas in lumbosacral roots and nerves in the rat. J Comp Neurol 222: 473–481. [DOI] [PubMed] [Google Scholar]
- Sundt D, Gamper N, Jaffe DB (2015). Spike propagation through the dorsal root ganglia in an unmyelinated sensory neuron: a modeling study. J Neurophysiol 114: 1340–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatulian L, Brown DA (2003). Effect of the KCNQ potassium channel opener retigabine on single KCNQ2/3 channels expressed in CHO cells. J Physiol 549: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatulian L, Delmas P, Abogadie FC, Brown DA (2001). Activation of expressed KCNQ potassium currents and native neuronal M‐type potassium currents by the anti‐convulsant drug retigabine. J Neurosci 21: 5535–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng BC, Song Y, Zhang F, Ma TY, Qi JL, Zhang HL et al (2016). Activation of neuronal Kv7/KCNQ/M‐channels by the opener QO58‐lysine and its anti‐nociceptive effects on inflammatory pain in rodents. Acta Pharmacol Sin 37: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Ma L, Ueda H (2010). Epigenetic gene silencing underlies C‐fiber dysfunctions in neuropathic pain. J Neurosci 30: 4806–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter I, Hein A, Sattler S, Hessler S, Touska F, Bressan E et al (2013). Amplified cold transduction in native nociceptors by M‐channel inhibition. J Neurosci 33: 16627–16641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visockis V, King AE (2013). M‐channels modulate network excitatory activity induced by 4‐aminopyridine in immature rat substantia gelatinosa in vitro. Brain Res 1513: 9–16. [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M (1983). Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain 17: 321–339. [DOI] [PubMed] [Google Scholar]
- Wang AW, Yang R, Kurata HT (2016). Sequence determinants of subtype‐specific actions of KCNQ channel openers. J Physiol 595: 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JJ, Li Y (2016). KCNQ potassium channels in sensory system and neural circuits. Acta Pharmacol Sin 37: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickenden AD, Krajewski JL, London B, Wagoner PK, Wilson WA, Clark S et al (2008). N‐(6‐chloro‐pyridin‐3‐yl)‐3,4‐difluoro‐benzamide (ICA‐27243): a novel, selective KCNQ2/Q3 potassium channel activator. Mol Pharmacol 73: 977–986. [DOI] [PubMed] [Google Scholar]
- Wickenden AD, McNaughton‐Smith G (2009). Kv7 channels as targets for the treatment of pain. Curr Pharm Des 15: 1773–1798. [DOI] [PubMed] [Google Scholar]
- Willis DE, Wang M, Brown E, Fones L, Cave JW (2016). Selective repression of gene expression in neuropathic pain by the neuron‐restrictive silencing factor/repressor element‐1 silencing transcription (NRSF/REST). Neurosci Lett 625: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wladyka CL, Feng B, Glazebrook PA, Schild JH, Kunze DL (2008). The KCNQ/M‐current modulates arterial baroreceptor function at the sensory terminal in rats. J Physiol 586: 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN (2013). Results in analgesia‐‐Darwin 1, pharma 0. N Engl J Med 369: 2558–2560. [DOI] [PubMed] [Google Scholar]
- Wu YJ, Boissard CG, Chen J, Fitzpatrick W, Gao Q, Gribkoff VK et al (2004a). (S)‐N‐[1‐(4‐cyclopropylmethyl‐3,4‐dihydro‐2H‐benzo[1,4]oxazin‐6‐yl)‐ethyl]‐3‐(2‐f luoro‐phenyl)‐acrylamide is a potent and efficacious KCNQ2 opener which inhibits induced hyperexcitability of rat hippocampal neurons. Bioorg Med Chem Lett 14: 1991–1995. [DOI] [PubMed] [Google Scholar]
- Wu YJ, Conway CM, Sun LQ, Machet F, Chen J, Chen P et al (2013). Discovery of (S,E)‐3‐(2‐fluorophenyl)‐N‐(1‐(3‐(pyridin‐3‐yloxy)phenyl)ethyl)‐acrylamide as a potent and efficacious KCNQ2 (Kv7.2) opener for the treatment of neuropathic pain. Bioorg Med Chem Lett 23: 6188–6191. [DOI] [PubMed] [Google Scholar]
- Wu YJ, He H, Sun LQ, L'Heureux A, Chen J, Dextraze P et al (2004b). Synthesis and structure‐activity relationship of acrylamides as KCNQ2 potassium channel openers. J Med Chem 47: 2887–2896. [DOI] [PubMed] [Google Scholar]
- Wu YJ, Sun LQ, He H, Chen J, Starrett JE Jr, Dextraze P et al (2004c). Synthesis and KCNQ2 opener activity of N‐(1‐benzo[1,3]dioxol‐5‐yl‐ethyl, N‐[1‐(2,3‐dihydro‐benzofuran‐5‐yl)‐ethyl, and N‐[1‐(2,3‐dihydro‐1H‐indol‐5‐yl)‐ethyl acrylamides. Bioorg Med Chem Lett 14: 4533–4537. [DOI] [PubMed] [Google Scholar]
- Wuttke TV, Seebohm G, Bail S, Maljevic S, Lerche H (2005). The new anticonvulsant retigabine favors voltage‐dependent opening of the Kv7.2 (KCNQ2) channel by binding to its activation gate. Mol Pharmacol 67: 1009–1017. [DOI] [PubMed] [Google Scholar]
- Xiong Q, Sun H, Li M (2007). Zinc pyrithione‐mediated activation of voltage‐gated KCNQ potassium channels rescues epileptogenic mutants. Nat Chem Biol 3: 287–296. [DOI] [PubMed] [Google Scholar]
- Xu W, Wu Y, Bi Y, Tan L, Gan Y, Wang K (2010). Activation of voltage‐gated KCNQ/Kv7 channels by anticonvulsant retigabine attenuates mechanical allodynia of inflammatory temporomandibular joint in rats. Mol Pain 6: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaika O, Lara LS, Gamper N, Hilgemann DW, Jaffe DB, Shapiro MS (2006). Angiotensin II regulates neuronal excitability via phosphatidylinositol 4,5‐bisphosphate‐dependent modulation of Kv7 (M‐type) K+ channels. J Physiol 575: 49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaika O, Tolstykh GP, Jaffe DB, Shapiro MS (2007). Inositol triphosphate‐mediated Ca2+ signals direct purinergic P2Y receptor regulation of neuronal ion channels. J Neurosci 27: 8914–8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Thimmapaya R, Zhang XF, Anderson DJ, Baranowski JL, Scanio M et al (2011a). KCNQ2/3 openers show differential selectivity and site of action across multiple KCNQ channels. J Neurosci Methods 200: 54–62. [DOI] [PubMed] [Google Scholar]
- Zhang F, Mi Y, Qi JL, Li JW, Si M, Guan BC et al (2012). Modulation of Kv7 potassium channels by a novel opener pyrazolo[1,5‐a]pyrimidin‐7(4H)‐one compound QO‐58. Br J Pharmacol 168: 1030–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T et al (2003). PIP2 activates KCNQ channels, and its hydrolysis underlies receptor‐mediated inhibition of M currents. Neuron 37: 963–975. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bal M, Bierbower S, Zaika O, Shapiro MS (2011b). AKAP79/150 signal complexes in G‐protein modulation of neuronal ion channels. J Neurosci 31: 7199–7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, An H, Li J, Zhang Y, Liu Y, Jia Z et al (2016). Selective activation of vascular Kv 7.4/Kv 7.5 K+ channels by fasudil contributes to its vasorelaxant effect. Br J Pharmacol 173: 3480–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang H, Zhou N, Xu J, Si M, Jia Z et al (2015). Tannic acid modulates excitability of sensory neurons and nociceptive behavior and the Ionic mechanism. Eur J Pharmacol 764: 633–642. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Fang D, Liu M, Cai J, Wan Y, Han JS et al (2013). Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain 154: 434–448. [DOI] [PubMed] [Google Scholar]


