Abstract
Background
Basal cell carcinoma (BCC) has a characteristic stroma, but less is known about the dermal characteristics associated with melanoma in situ (MIS) and actinic keratosis (AK).
Materials and methods
Dermal changes were studied in 301 specimens of AK, BCC and MIS. Subsequently, blinded images of dermal changes from 90 randomly selected cases of those entities were used to assess the predictive value of the dermal changes. Agreement with the final diagnosis was calculated using kappa coefficient (κ).
Results
Fibromyxoid stroma was present in 82% of BCC cases; fibrous stroma was seen in 25% of BCC, 58% of MIS and 35.6% of AK specimens (p <0.05). A lichenoid inflammatory infiltrate was frequently associated with AK and a perifollicular infiltrate with periadnexal fibrosis with MIS. Blinded evaluation of images of the dermal changes associated with the tumors yielded the correct diagnosis in (54.4, 41.1 and 27.8%; average 41.2%) by the three appraisers. Coefficient of agreement in blinded imaged evaluation with the actual diagnosis was higher in the BCC and MIS compared with AK (κ = 0.37, p = 0.0001; κ = 0.2, p = 0.0005 and κ = −0.06, p = 0.84, respectively).
Conclusion
Dermal features may be helpful in predicting the correct diagnosis when tumor is not visible.
Keywords: cancer, malignant melanoma, non-melanoma skin cancer, pathology, pathology dermatopathology
Melanoma in situ (MIS), actinic keratosis (AK) and superficial basal cell carcinoma (BCC) are among the most common lesions encountered by dermatopathologists.1 – 4 It is widely accepted that BCC has stroma that creates a unique environment conducive to tumor growth.1,2 Stromal features in BCC are well described in the literature, but little has been written about the dermal characteristics of MIS and AK.5,6 We sought to evaluate dermal changes in MIS and AK in comparison to superficial BCC.
Materials and methods
A retrospective data search was performed at the Ackerman Academy of Dermatopathology, NY, USA, for ‘AK’, ‘MIS’ and ‘superficial BCC’. Three hundred and one specimens, diagnosed in the Academy in 2009–2011, were randomly selected including 101 specimens of AK, 100 specimens of BCC and 100 specimens of MIS. Exclusion criteria were as follows: the section was severely fragmented; it showed a collision tumor; it was too superficial to include a sufficient amount of dermis or it was a re-excision specimen that included a postsurgical scar or any history of previous local treatment. In the MIS group, exclusion criteria included the presence of obvious regression (presence of dense lichenoid lymphocytic infiltrate and/or dermal fibrosis associated with a diminished number or absence of melanocytes in the epidermis).7 The MIS cases in our study corresponded to lentiginous melanoma on sun damaged skin.
Histopathological changes were evaluated in hematoxylin/eosin (H&E) stained sections. We evaluated stromal changes as follows:
Normal dermis – stromal changes were absent. Presence of solar elastosis or angiofibroplasia characteristic of stasis was considered as normal age/site related changes;
Fibrosis – dense red stroma with the presence of red thick bundles of collagen;
Fibromyxoid stroma – loose amphophilic stroma with increased mucin, fine collagen bundles and dilated vessels.
The inflammatory infiltrate was classified as perivascular, interstitial, perifollicular or lichenoid. The growth pattern of each BCC was recorded.
Statistical evaluation of collected data was performed using the Statistica 10.0 program (StatSoft, Tulsa, OK). Differences between study variables were evaluated using the chi-square test (3 × 2 and 2 × 2 tables). Specificity, sensitivity, positive and negative likelihood ratios (LR+ and LR−) were calculated for each of the features. An LR+ more than 10 was considered as convincing diagnostic evidence, LR+ 5–10 as probable diagnostic evidence and LR+ less than 5 as low level diagnostic evidence. LR− less than 0.1 was strongly associated with absence of the diagnosis, 0.1–0.5 – had moderate association and more than 0.5 – weak association.8
To determine how easily these findings could be applied in practice, 90 cases (30 cases of each entity: BCC, MIS and AK) were randomly selected and dermal changes were photographed. Photomicrographs included only dermal changes, without any visible tumor. The images were presented to three independent observers who were blinded to the diagnosis. Each observer made independent predictions about the diagnosis, based only on the dermal changes in images provided to them and using the criteria noted above. Their predictions were compared with the actual diagnosis of each lesion and agreement with the actual diagnosis was evaluated statistically. Fleiss kappa coefficient (κ) was used for the estimation of the agreement with the actual diagnosis. Interpretation of the coefficient is as follows: <0: poor agreement; 0.01–0.20: slight agreement; 0.21–0.40: fair agreement; 0.41–0.60: moderate agreement; 0.61–0.80: substantial agreement and 0.81–0.99: almost perfect agreement.9 The p-value of kappa was used to evaluate, if the agreement was random or not (p-value more than 0.05 was considered to be due to the chance alone).
Results
The location of BCC cases in our series was as follows: head and neck – 56 cases (56%), chest – 26 cases (26%), back – 8 cases (8%), upper extremity – 7 cases (7%) and shin – 3 cases (3%). The location of MIS in our study was on the head and neck – 85 cases (85%), back – 1 case (1%), chest – 11 cases (11%), forearm – 1 case (1%), calf – 2 cases (2%). Lesions of AK in our series were located on the head and neck – 97 cases (97%), forearm – 3 cases (3%), arm – 1 case (1%).
Dermal changes were seen in 96 (96%) cases of BCC. In order to find the best predictor to differentiate the three neoplasms, a chi-square test (3 × 2 tables) was performed (results are presented in Table 1).
Table 1.
Characteristics of dermal changes in superficial BCC, MIS and AK
| Features | Group 1 BCC n (%) | Group 2 LM n (%) | Group 3 AK n (%) | p-value* |
|---|---|---|---|---|
| Dermal fibrosis | 25 (25) | 58 (58) | 36 (35.6) | <0.0001 |
| Mucinous stroma | 82 (82) | 0 | 0 | <0.0001 |
| Perivascular/interstitial infiltrate | 60 (60) | 5 (5) | 9 (8.9) | <0.0001 |
| Lichenoid infiltrate | 8 (8) | 4 (4) | 33 (32.7) | <0.0001 |
| Perifollicular infiltrate | 1 (1) | 17 (17) | 3 (3) | <0.0001 |
| Widened vessels | 41 (41) | 9 (9) | 11 (10.9) | <0.0001 |
BCC, basal cell carcinoma; MIS, melanoma in situ; AK, actinic keratosis.
<0.05 is considered statistically significant.
The best predictor of superficial BCC was fibromyxoid stroma, which was seen in 82 cases (82%). It was specific for superficial BCC and was not seen in MIS or AK cases (sensitivity 0.82, specificity 1, LR+ = infinity and LR− = 0.18; Table 1). We noted that in 12 cases fibromyxoid stroma was seen at the lateral or deep margin of the specimen and BCC nests were found in the subsequent serial sections in these cases (Fig. 1), suggesting that stroma at the margin should be interpreted as a ‘positive margin’. In eight cases tumor was found in the deeper sections, it was of the superficial type, similar to that seen in original sections; in two cases nodular subtype of BCC was found; and in the remaining two cases it was not possible to define the BCC subtype. Perivascular infiltrate and an increased number of dilated vessels were also seen more frequently in superficial BCC, but the diagnostic value of those features was lower (sensitivity 0.60, specificity 0.93, LR+ 8.61, LR− 0.43 for perivascular infiltrate and sensitivity 0.41, specificity 0.90, LR+ 4.12 and LR− 0.66 for vessel prolferations).
Fig. 1.
Superficial basal cell carcinoma. Nests of basaloid cells, surrounded by fibromyxoid stroma with a prominent lymphoid infiltrate. Fibromyxoid stroma extended to the lateral margin, and deeper sections (shown) subsequently showing tumor involving the margin; hematoxylin/eosin, ×200.
Fibrous stroma was present in 25 cases of superficial BCC (25%). In 11 of those cases, the fibrous stroma was noted in combination with fibromyxoid stroma. In such situations, fibromyxoid stroma was located around the nests of BCC, while the fibrous stroma was at the lateral margin or below the tumor (Fig. 2). In 4 of 25 cases of BCC with fibrous stroma (16%), underlying deeper tumor was found in subsequent sections cut from the block. In two of these specimens, aggressive infiltrative type of BCC was seen in the deeper sections (Fig. 2A,B).
Fig. 2.
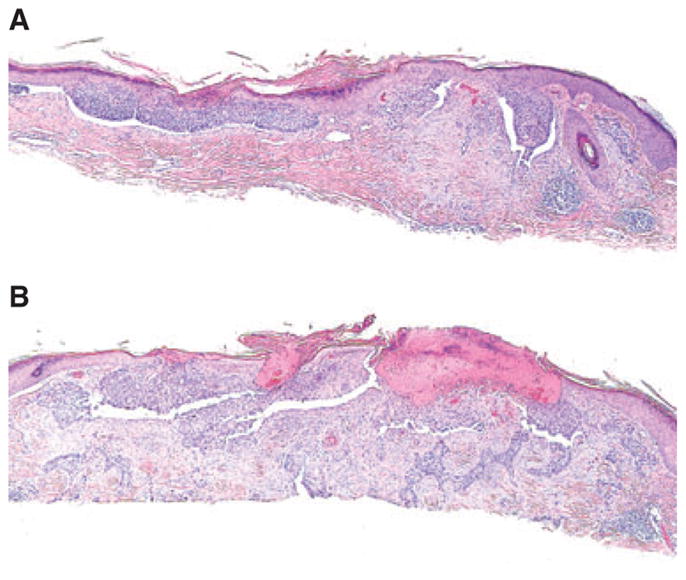
A) Superficial basal cell carcinoma (BCC) with fibrous stroma at the bottom. B) BCC, infiltrative type in the subsequent sections of the same specimen; hematoxylin/eosin, ×200.
Fibrous stroma was also noted in any BCC occurring on the shin. As fibrosis and nodular angioplasia on the shin may be related to stasis in this location, we did not include them in our calculations of BCC stromal changes. (Fig. 3).
Fig. 3.
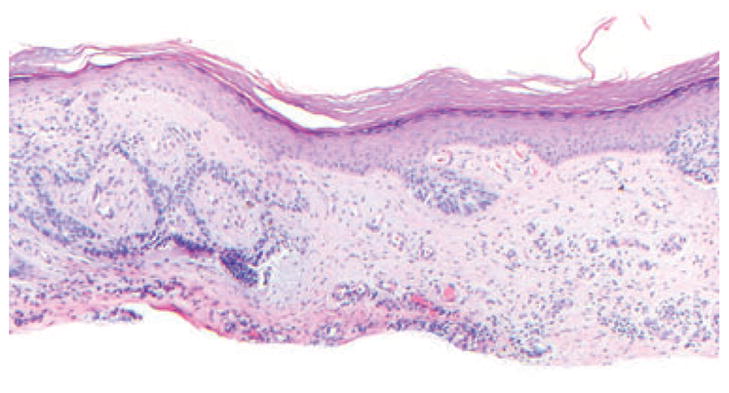
Superficial basal cell carcinoma (BCC) on the shin. Fibrosis at the bottom, nodular angioplasia – which may represent stasis-associated changes in this location; hematoxylin/eosin, ×100.
We performed the chi-square test (2 × 2 tables) to compare the strength of association of these features with MIS and AK groups. Fibrotic changes in the dermis were statistically more frequent in MIS and AK as compared with superficial BCC and were seen in 58 and 36% of these cases, respectively (p <0.0001) (Fig. 4A,B and 5). A unique feature of the fibrosis, associated with MIS, was its extension along follicles [seen in 23 (23%) cases]. The sensitivity, specificity, LR+ and LR− of fibrotic dermal change to differentiate MIS from AK were 0.58, 0.64, 1.63 and 0.65, respectively.
Fig. 4.
Melanoma in situ with fibrous stroma with parallel collagen bundles located under the tumor and extending to the lateral margin. A and B) Hematoxylin/eosin, ×100.
Fig. 5.
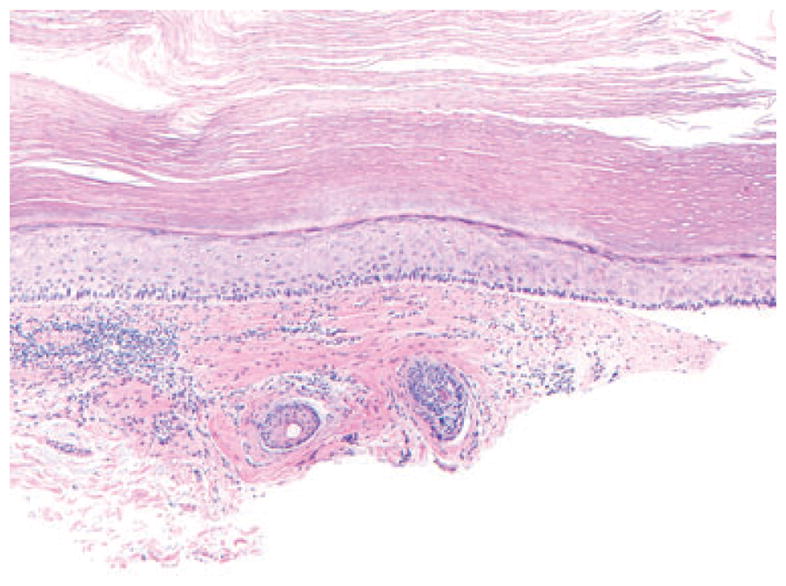
Actinic keratosis with red collagenous fibrosis extending to the deep margin; hematoxylin/eosin, ×200.
Lichenoid inflammation showed the strongest association with AK (p <0.0001). The sensitivity, specificity, LR+ and LR− of lichenoid inflammation to differentiate AK from MIS were 0.33, 0.96, 8.17 and 0.70, respectively. In contrast, a perifollicular infiltrate was more frequently seen in MIS (sensitivity 0.17, specificity 0.97, LR+ 5.72 and LR− 0.86).
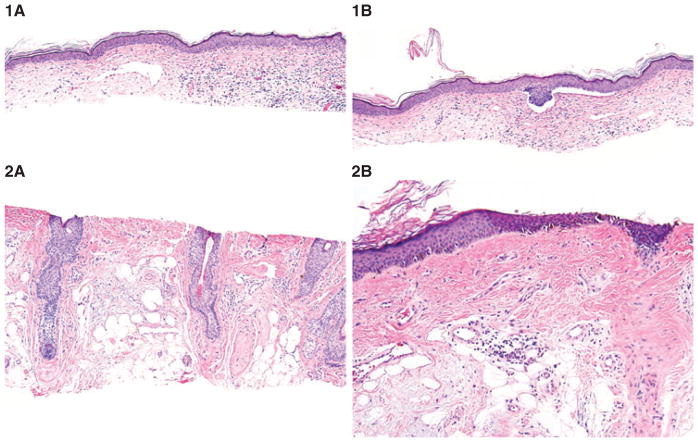
Blinded evaluation of images of the dermal changes associated with the tumors yielded the correct diagnosis in 49/90, 37/90 and 25/90 of cases (54.4%, 41.1% and 27.8%; average 41.2%) by the three appraisers. Overall agreement of three appraisers was κ = 0.15, p = 0.0001. The agreement with the final diagnosis in BCC cases was higher than in MIS (κ = 0.38, p = 0.0001 and κ = 0.20, p = 0.0005, respectively). In AK cases the level of agreement was that of chance alone (κ = −0.06, p = 0.84). In addition, all three appraisers were able to successfully narrow down their differential diagnosis between MIS and AK in 9, 3 and 42 cases, respectively (Fig. 6).
Fig. 6.
Blinded evaluation of the dermal images of stromal changes. 1A) Fibromyxoid dermal changes with interstitial inflammatory infiltrate and widended tortuous vessels are suggestive of basal cell carcinoma; hematoxylin/eosin, ×100. 1B) Further sections reveal superficial basal cell carcinoma; hematoxylin/eosin, ×100. 2A) Dermal changes show red fibrosis pushing the solar elastosis. Although the differentiation between melanoma and actinic keratosis is not unequivocally possible, fibrosis along the hair follicles suggests melanoma in situ; hematoxylin/eosin, ×100. 2B) Further sections reveal irregular single melanocytes and dermal nests, suggestive of melanoma in situ (lentigo maligna type); hematoxylin/eosin, ×100,
Discussion
BCC, AK and MIS are among the most frequent tumors seen in proximity to the epidermis, usually affecting sun exposed areas in elderly people.2 – 6 They are often located on the face and, as small biopsies are commonly taken from such locations, dermatopathologists sometimes have to deal with specimens that contain dermal changes but no visible tumor. Recognizing the patterns of dermal changes associated with these entities may alert the pathologists to order deeper sections or to recommend a second biopsy.10 In the unblinded portion of our study, a fibromyxoid stroma was strongly predictive of BCC. Eosinophilic dermal fibrosis, often with a lichenoid infiltrate, was characteristic of AK. Eosinophilic fibrosis that frequently extended along follicles or was associated with a perifollicular infiltrate was associated with MIS. In the blinded assessment, there was some variability in how observers interpreted the stroma. Each observer read the criteria, but no concordance training was performed prior to the blinded portion of the study. This may have resulted in lower concordance with the final diagnosis when compared with the unblinded portion of the study.
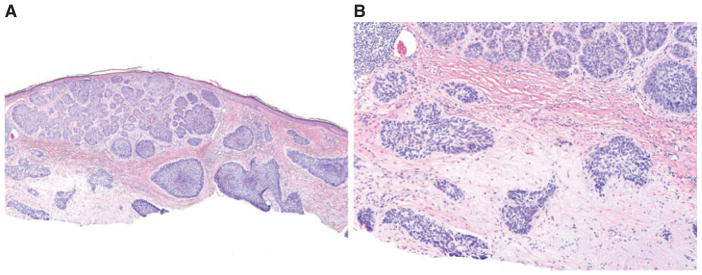
Fibromyxoid stroma, associated with BCC, has been cited as a useful clue for diagnosing BCC in cases where no tumor cells are seen.11 In addition, presence of stroma at the margin suggests incomplete removal of the tumor and has been cited as an indication for re-excision by some dermatopathologists.10 – 12 Fibromyxoid stroma has been reported in about 76% of all forms of BCC,2 and was seen in 82% of all superficial BCC in our series. Characteristic features of this stroma include presence of mucin, a sparse lymphoid infiltrate and increased number of dilated vessels.2 Of all the variables studied, only presence of fibromyxoid stroma was highly associated with BCC (LR = infinity), and this feature showed 100% specificity. While fibromyxoid stroma is described most often in association with BCC, fibrous stroma may also be present.13 An eosinophilic fibrous stroma may be associated with more aggressive BCC subtypes, such as infiltrative or micronodular BCC.14 Fibrous stroma in morpheaform BCC has some peculiar features, such as high types I and III procollagen mRNA levels and the presence of activated fibroblasts, that are positively associated with more aggressive patterns of tumor growth.15,16 Kaur et al. reported that fibrous stroma was seen in only 7% of ‘low-risk’ types of BCC (superficial and nodular types) and in 25% of ‘high-risk’ BCC (morpheaform, infiltrative and micronodular) in their series,2 but in our study we found a higher incidence (25%) of fibrous stroma in superficial BCC. Moreover, in 2 out of 25 cases of superficial BCC with fibrous stroma in our series, a more aggressive BCC variant was found in subsequent deeper sections. This suggests that the presence of fibrous stroma adjacent to or at the base of a specimen showing a nonaggressive variant may indicate an underlying more aggressive variant of BCC (Fig. 7A,B).
Fig. 7.
Fibrosis, underlying superficial nests of nodular basal cell carcinoma (BCC) with underlying micronodular BCC nests. A) Hematoxylin/eosin, ×100; B) hematoxylin/eosin, ×200.
Dilatation of vessels was statistically more frequent in BCC stroma, as compared with AK or LM (p <0.0001). These vessels may correspond clinically to telangiectasias seen on the surface of BCC.
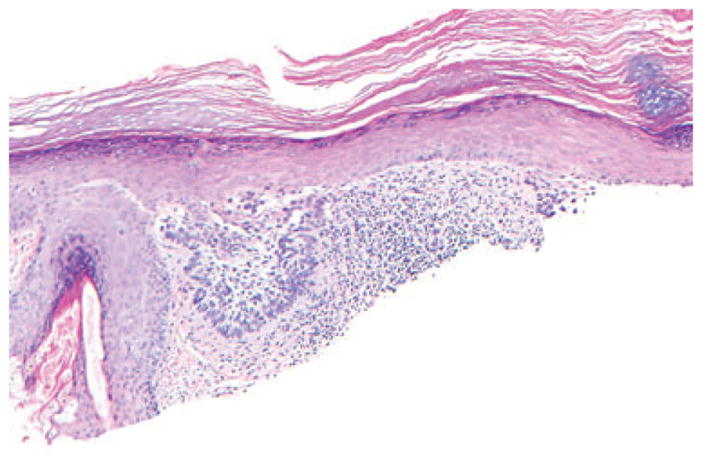
While fibrotic changes in the dermis could be a marker for more aggressive variants of BCC, they were not specific for BCC and were seen more frequently in MIS and AKs. We also noted that the pattern of the associated lymphoid infiltrate and perifollicular extension of the fibrosis may be predictive of the associated tumor. The eosinophilic fibrosis underlying MIS often displaced solar elastosis downward. Cases of melanoma in our series showed no invasive foci anywhere in the lesion and melanocytes in the epidermis remained preserved throughout. Melanophages, although present in some specimens, were few and spaced far apart. Lichenoid inflammation was seen only in 4% cases of MIS in our series, and was present only focally. The reason for the presence of fibrosis in MIS is unknown. We excluded the cases with known previous treatments, which could possibly lead to fibrosis, for example, treatment with liquid nitrogen. As desmoplastic melanoma often arises in conjunction with the lengtio maligna type of MIS, the in situ lesions may share the ability to stimulate collagen production. Ability to activate fibroblast growth factor (FGF) by nevi and melanomas has been studied by Reed et al.17 They showed that increased expression of FGF was seen in all metastatic and invasive melanomas, 22 of 25 ‘nevi with architectural disorder and cytologic atypia’ and 7 of 20 MIS.17 Further studies should examine whether melanocytes in lentiginous types of melanoma have a greater propensity to induce fibrosis as compared with other types of melanoma. The flat-bottomed fibrotic change in lentiginous MIS should be differentiated from the concentric fibrosis of Clark’s/dysplastic nevi.5 Concentric fibrosis was only occasionally seen in our MIS specimens (Fig. 8).
Fig. 8.
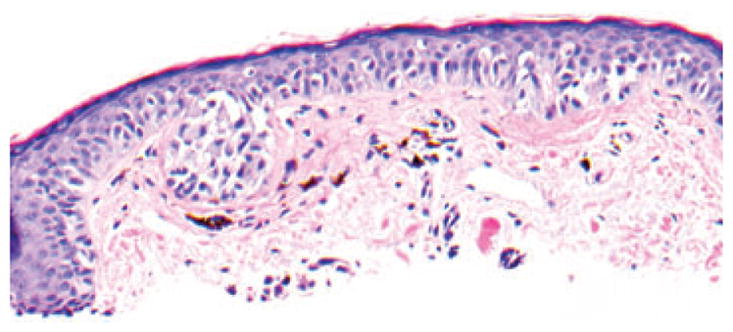
Thin fibrosis around irregular melanocytic nests and single melanocytes of melanoma in situ, mimicking concentric fibrosis of dysplastic nevus; hematoxylin/eosin, ×200, cropped image.
Examples of MIS and AK may occur in collision18 and an intriguing question is whether any differences in the associated dermal changes might predict an associated MIS. The majority of the actinic keratoses in our study showed no dermal changes, while 36% were associated with fibrotic dermal changes. In contrast, 58% of MIS had red fibrotic dermal changes which were more likely to be associated with perifollicular lymphoid infiltrates and to show follicular extension. It should be noted that presence of fibrosis has also been previously noted in hypertrophic AK on the hands with protracted rubbing mentioned as a possible cause.6
In conclusion, the presence of fibromyxoid stroma had a 100% specificity and infinite LR+ as well as high sensitivity for associated BCC. While fibrotic dermal changes can be seen in all three neoplasms, the pattern varied and frequency was greatest in association with MIS. Fibrosis in MIS is more likely to show adnexal extension, and fibrotic dermal change underlying BCC may be a clue to an underlying more aggressive growth pattern. A diffuse interstitial lymphoid infiltrate was most common in BCC (60% of cases) and a lichenoid infiltrate obscuring the dermal-epidermal junction was associated with AK in 33% of cases. During blind evaluation of images, dermal features showed some potential to be helpful in predicting the correct diagnosis, especially in MIS and BCC cases.
References
- 1.Furue M. Epithelial tumor, invasion and stroma. Ann Dermatol. 2011;23:125. doi: 10.5021/ad.2011.23.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur P, Mulvaney M, Carlson JA. Basal cell carcinoma progression correlates with host immune response and stromal alterations a histologic analysis. Am J Dermatopathol. 2006;28:293. doi: 10.1097/00000372-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Forman SB, Ferringer TC, Peckham SJ, et al. Is superficial spreading melanoma still the most common form of malignant melanoma? J Am Acad Dermatol. 2008;58:1013. doi: 10.1016/j.jaad.2007.10.650. [DOI] [PubMed] [Google Scholar]
- 4.Cockerell CJ. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”) J Am Acad Dermatol. 2000;42:11. doi: 10.1067/mjd.2000.103344. [DOI] [PubMed] [Google Scholar]
- 5.King R. Lentiginous melanoma. Arch Pathol Lab Med. 2011;135:337. doi: 10.5858/2009-0538-RA.1. [DOI] [PubMed] [Google Scholar]
- 6.Billano RA, Little WP. Hypertrophic actinic keratosis. J Am Acad Dermatol. 1982;7:484. doi: 10.1016/s0190-9622(82)80251-x. [DOI] [PubMed] [Google Scholar]
- 7.Massi G, LeBoit PE. Histological diagnosis of nevi and melanoma. Darmstadt: Steinkopff Verlag; 2004. Regressing and regressed melanoma; p. 617. [Google Scholar]
- 8.Sonis J. How to use and interpret interval likelihood ratios. Fam Med. 1999;31:432. [PubMed] [Google Scholar]
- 9.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159. [PubMed] [Google Scholar]
- 10.Haupt HM, Stern JB, Dilaimy MS. Basal cell carcinoma: clues to its presence in histologic sections when the initial slide is nondiagnostic. Am J Surg Path. 2000;24:1294. doi: 10.1097/00000478-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Ackerman A, Guo Y, Vitale P. Clues to diagnosis in dermatopathology. Chicago: ASCP Press; 1992. p. 256. [Google Scholar]
- 12.Leboit P. Stroma, interrupted. Am J Dermpath. 2001;23:67. doi: 10.1097/00000372-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Ackerman A, Reddy V, Soyer P. Neoplasms with follicular differentiation. 2. New York: Ardor Shribendi; 2001. Trichoblastic carcinoma; p. 625. [Google Scholar]
- 14.Lesack K, Naugler C. Morphometric characteristics of basal cell carcinoma peritumoral stroma varies among basal cell carcinoma subtypes. BMC Dermatol. 2012;12:1. doi: 10.1186/1471-5945-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moy RL, Moy LS, Matsuoka LY, Bennett RG, Uitto J. Selectively enhanced procollagen gene expression in sclerosing (morphea-like) basal cell carcinoma as reflected by elevated pro alpha 1(I) and pro alpha 1(III) procollagen messenger RNA steady-state levels. J Invest Dermatol. 1988;90:634. doi: 10.1111/1523-1747.ep12560782. [DOI] [PubMed] [Google Scholar]
- 16.Abbas O, Richards JE, Mahalingam M. Fibroblast-activation protein a single marker that confidently differentiates morpheaform/ infiltrative basal cell carcinoma from desmo-plastic trichoepithelioma. Mod Pathol. 2010;23:1535. doi: 10.1038/modpathol.2010.142. [DOI] [PubMed] [Google Scholar]
- 17.Reed JA, McNutt NS, Albino AP. Differential expression of basic fibroblast growth factor (bFGF) in melanocytic lesions demonstrated by in situ hybridization. Implications for tumor progression. Am J Pathol. 1994;144:329. [PMC free article] [PubMed] [Google Scholar]
- 18.Dalton SR, Gardner TL, Libow LF, Elston DM. Contiguous lesions in lentigo maligna. J Am Acad Dermatol. 2005;52:859. doi: 10.1016/j.jaad.2004.11.063. [DOI] [PubMed] [Google Scholar]