Abstract
A new study reveals that B cells restrict the transendothelial migration of T cells in physiological inflammation in response to adiponectin, but that this mechanism is compromised in autoimmunity and is hence a novel avenue for therapy development.
Autoimmune disease is on the rise worldwide, and it represents a group of over 100 medical conditions, some with high prevalence, in which the immune system fails to distinguish self from non-self, resulting in immune-mediated tissue damage. The healthy immune system fine-tunes responses through myriad mechanisms, including regulation of lymphocyte trafficking to sites of inflammation. In this issue of Nature Medicine, Chimen et al.1 reveal a previously unknown activity of B cells that prevents memory T cells from undergoing transendothelial migration (TEM) from blood into inflamed tissues (Fig. 1). This effect presumably tempers the inflammatory cascade by reducing the release of T cell–derived cytokines and further immune cell recruitment. In individuals with autoimmune diseases, however, the process malfunctions.
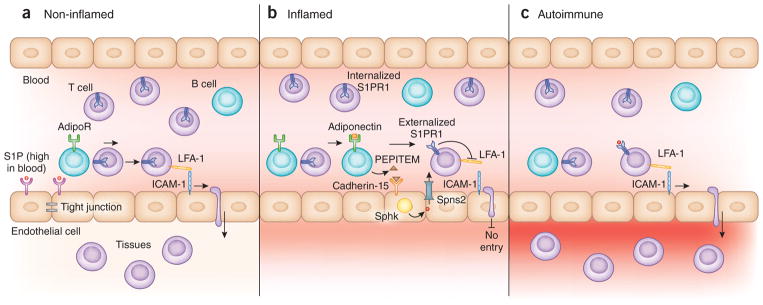
Figure 1.
The role of B cells in S1P-mediated regulation of T cell trafficking. (a) In non-inflamed tissues, high S1P concentration in blood results in S1PR1 downregulation on circulating T cells, and interactions between T cell LFA-1 and endothelial cell (EC) ICAM-1 facilitate T cell TEM into non-inflamed tissues. S1P activation of endothelial cell S1PR1 promotes endothelial cell tight junctions, maintaining vascular integrity. (b) Chimen et al.1 show that in inflamed tissues adiponectin stimulates B cells through AdipoRs, inducing them to release PEPITEM. PEPITEM binds to its receptor, cadherin-15, on endothelial cells. Cadherin-15 activation leads to production of S1P by the enzyme sphingosine kinase 1. S1P is exported to the extracellular space through the Spns2 transporter. Adherent T cells stimulated by inflammatory signals upregulate S1PR1, which allows them to bind the S1P released locally from endothelial cells. As a result of S1P signaling, T cell LFA-1 and endothelial cell ICAM-1 are reduced; T endothelial cell TEM is blocked, and inflammation is restrained. (c) In autoimmune tissues, B cells lack adiponectin receptors and thus fail to initiate the adiponectin–PEPITEM axis. Interactions between T cell LFA-1 and endothelial cell ICAM-1 facilitate inappropriate T cell TEM into inflamed tissues, thereby intensifying inflammation.
The authors made their discovery while investigating adiponectin’s effect on lymphocyte migration1. Adiponectin is an adipokine involved in metabolism, adipogenesis and diabetes. Recently, adiponectin and its receptors AdipoR1 and AdipoR2 were shown to inhibit leukocyte recruitment to inflamed endothelium2. Furthermore, low levels of circulating adiponectin have been observed in some diseases, including diabetes3. To explore adiponectin’s influence on lymphocyte TEM in detail, the authors carried out in vitro transmigration assays, isolating peripheral blood lymphocytes (PBLs) from healthy individuals and following their movement across confluent monolayers of TNF-α– and IFN-γ–stimulated endothelial cells by video-microscopy.
They found that adiponectin inhibited cytokine-induced transmigration of memory T cells across the endothelium without influencing T cell recruitment1. Although the authors considered a direct effect on T cells to be the most plausible explanation for their observations, this possibility was ruled out1. Further investigation revealed that adiponectin’s effect was mediated through B cells, which (unlike T cells) expressed high AdipoR levels. Depleting B cells from PBL mixtures released the adiponectin-induced blockade on migration, whereas adding back B cells or conditioned medium from adiponectin-treated B cells restored the blockade.
Using proteomics, the authors identified a peptide released from B-cells after adiponectin stimulation, which they named PEPITEM for PEPtide Inhibitor of Trans-Endothelial Migration1 (Fig. 1). Described for the first time in this study, PEPITEM is a small peptide derived from the 14.3.3.ζδ protein by proteolytic cleavage. A biotin-conjugated PEPITEM was used to identify its unknown receptor, endothelial cell cadherin-15, an adhesion protein expressed in myoblasts but not previously recognized as part of the endothelial cell expressed gene repertoire.
When searching for a mechanism by which PEPITEM influenced T-cell TEM, the authors focused on sphingosine-1-phosphate (S1P), a bioactive sphingolipid known to orchestrate T cell trafficking1. S1P controls T cell migration through S1P receptors (S1PRs) which respond to S1P gradients between tissues and blood/lymph4. S1P/S1PR1 signaling is a regulator of T cell trafficking, influencing the entry, retention and egress of naïve and antigen-experienced T cells to and from bone marrow, lymphoid organs and peripheral tissues under homeostatic and inflammatory conditions4 (Fig. 1).
Chimen et al.1 found that treatment with an S1PR1 antagonist made T cells impervious to the effects of PEPITEM or adiponectin, whereas addition of exogenous S1P at levels found in blood blocked T cell TEM. Further, upon stimulation by PEPITEM, cadherin-15 induced S1P biosynthesis via sphingosine kinase 1, which was upregulated in response to tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ). Either chemical inhibition of sphingosine kinase or knockdown of the S1P transporter Spns2 blocked PEPITEM’s effect on T cell transmigration.
Blood S1P levels are constitutively high, and S1PR1 is desensitized and internalized in T cells under these conditions. How, then, does a minor change in local S1P levels create a recognizable signal, and how can adherent T cells bathed in blood sense the gradient if they lack S1PR1 expression? The authors rationalized that most circulating S1P is protein-bound and inactive; adherent T cells could, therefore, potentially detect a pulse of free S1P at the endothelial cell apical surface1,5. Interestingly, this suggests that effective ‘S1P gradients’ may act on a microenvironmental scale. Second, the authors demonstrated that memory T cells upregulate surface S1PR1 expression (sustained even in the presence of high S1P concentrations) under inflammation-like conditions1.
T cell TEM requires strong interactions between the integrin receptor LFA-1 and endothelial ICAM-1. Interestingly, S1P signaling reduced the affinity of LFA-1 for ICAM-1 under inflammatory conditions, which appears to be the fundamental mechanism by which S1P halts T cell entry into inflamed tissues (Fig. 1)1. S1P’s influence on LFA-1/ICAM-1 interactions is implicated in other aspects of T-cell trafficking, including T cell migration from peripheral tissues into afferent lymphatics6. Thus, this could represent a central mechanism by which S1P signaling regulates T cell migration.
A strength of the study is the inclusion of convincing evidence demonstrating the PEPITEM-induced T cell blockade is an endogenous phenomenon. By using a zymosan-induced peritonitis model, the authors showed that mice lacking B cells exhibited greater T cell trafficking into inflamed peritoneal cavities compared to controls, whereas delivery of PEPITEM to the former reduced T cell trafficking1. PEPITEM administration also impeded mouse T cell trafficking to infectious foci in a Salmonella model of bacteremia, to hepatic sinusoids after ischemia and reperfusion injury, to ocular infiltrates in endotoxin-induced autoimmune uveitis, and to salivary glands in a virally induced model of Sjogren’s syndrome. In contrast, PEPITEM had no impact on B cell recruitment.
Notably, B cells isolated from patients with type I diabetes (T1D) and rheumatoid arthritis had lower AdipoR expression than controls, and their T cell TEM was not inhibited by adiponectin1. In both T1D-affected individuals and controls, AdipoR expression levels correlated with the amount of adiponectin-induced PEPITEM released by B cells. PEPITEM treatment, however, could reduce TEM of the patients’ T cells. Patients with T1D also had lower plasma PEPITEM levels than controls1. In the elderly, a population at risk for autoimmune diseases, the process was also compromised, as their B cells exhibited reduced AdipoR expression compared to younger individuals. Altogether, these results suggest this immunoregulatory mechanism is compromised in autoimmunity and aging.
The ‘adiponectin–PEPITEM axis’ expands an ever-growing list of mechanisms by which B cells are recognized to modulate immune responses independently of antibody production7. Effector B cells amplify humoral and cellular immunity through pro-inflammatory cytokine production. Conversely, regulatory B cells restrain immune responses through transforming growth factor beta 1 (TGF-β1) and interleukin 10 (IL-10) secretion, suppression of T helper 1 (TH1)-, TH17- and TH2-mediated responses, and induction/maintenance of regulatory T cells8. Furthermore, regulatory B cells are known to have immunosuppressive functions, but PEPITEM, an immune regulator, is also derived primarily from these plasma B cells, which contradicts their ascribed role as culprits in autoimmunity and reveals them as ‘double agents’ in immune regulation.
The identification of the adiponectin and PEPITEM axis raises additional questions. What is the source of adiponectin? What regulates adiponectin release under inflammatory conditions? Does adiponectin induce additional effects on B cell development, function, expansion, or cytokine repertoire? How and where is PEPITEM produced and possibly exported? Do other cell types produce PEPITEM-like peptides? Furthermore, what is its half-life, and what off-target effects may occur with PEPITEM delivery? Can PEPITEM mediate biology in myoblasts and/or other cadherin-15–expressing cells, and is cadherin-15 expressed at different levels in specific endothelial beds during homeostasis and/or inflammation?
In addition, the role of S1P/S1PR interactions in vascular development and integrity are well described9. What impact might PEPITEM have on vascular permeability during homeostasis, inflammation, infection and shock? S1PR1 antagonists are known to induce vascular leak10. Conversely, what effects do S1PR modulators and B cell depletion strategies currently used to treat autoimmune disease have on T cell TEM, as revealed by this study? Although in the present study adiponectin’s influence on S1P signaling is mediated through a paracrine effect, in other systems AdipoR activation promoted ceramide degradation and S1P synthesis in a cell-autonomous manner via ceramidase activation, suggesting that these two important pathways link in multiple ways11.
S1P-targeted therapies are effective for multiple sclerosis and are being tested in other autoimmune diseases12. The potential of harnessing the adiponectin–PEPITEM axis for similar therapeutic purposes is tantalizing. Identifying other disease contexts (atherosclerosis, cancer, trauma, infection, shock, transplantation, graft-versus-host disease) in which this pathway or its dysfunction plays a role, and defining which axis components are affected will be key. The fact that cadherin-15 is expressed at low levels on normal endothelium but upregulated on inflammatory endothelial cells may confer specificity to PEPITEM or agents that could be developed to target this pathway including cadherin-15–specific antibodies or chimeric antigen receptor T cells. The authors’ discovery of the adiponectin–PEPITEM pathway and its dysfunction in autoimmune diseases thus offers a novel strategy for potentially restoring immune homeostasis in autoimmunity and other inflammatory conditions.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Chimen M, et al. Nat Med. 2015;21 doi: 10.1038/nm.3842. XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamauchi T, Kadowaki T. Cell Metab. 2013;17:185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Shin HJ, Ding EL, van Dam RM. J Am Med Assoc. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 4.Garris CS, Blaho VA, Hla T, Han MH. Immunology. 2014;142:347–353. doi: 10.1111/imm.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thuy AV, Reimann CM, Hemdan NY, Graler MH. Cell Physiol Biochem. 2014;34:158–171. doi: 10.1159/000362992. [DOI] [PubMed] [Google Scholar]
- 6.Ledgerwood LG, et al. Nat Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 7.Lund FE. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauri C, Bosma A. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 9.Xiong Y, Hla T. Curr Top Microbiol Immunol. 2014;378:85–105. doi: 10.1007/978-3-319-05879-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanna MG, et al. Nat Chem Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 11.Tao C, Sifuentes A, Holland WL. Best Pract Res Clin Endocrinol Metab. 2014;28:43–58. doi: 10.1016/j.beem.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kappos L, et al. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]