Abstract
The proverbial role of microglia during brain development is shifting from passive members of the brain’s immune system to active participants that are able to dictate enduring outcomes. Despite these advances, little attention has been paid to one of the most critical components of early brain development- sexual differentiation. Mounting evidence suggests that the normal developmental functions microglia perform- cell number regulation and synaptic connectivity- may be involved in the sex-specific patterning of the brain during these early sensitive periods, and may have lasting sex-dependent and sex-independent effects on behavior. In this review, we outline the known functions of microglia during developmental sensitive periods, and highlight the role they play in the establishment of sex differences in brain and behavior. We also propose a framework for how researchers can incorporate microglia in their study of sex differences and vice versa.
Keywords: Microglia, sexual differentiation, sex differences, critical (sensitive) period, brain development, neuroimmunology, behavior
INTRODUCTION
Development is a word of such simplicity it cannot capture the complexity it is meant to define. The span of events from a single cell to a fully mature and reproductively capable organism, consist of dynamic and irreversible fate decisions coordinated in space and time. The development of the brain is arguably one of the longest spans, with development beginning early embryonically and maturation continuing post-puberty in the form of honing neuronal connections and synaptic refinement. Unlike the essential organs, heart, lungs, kidneys etc., which must be mature at birth in order to support life, the brain is remarkably undeveloped and responsive to both internal and external cues. A central goal of neuroscience is to identify and understand those cues in both the healthy individual and those suffering environmental or genetic insult.
In recent years a surprising new player has been added to the list of change agents acting on the developing brain- microglia. Although of non-neuronal origin, these innate immune cells are active partners in neuronal maturation. They are continuously active, surveying their environment and responding to neural activity (Nimmerjahn et al., 2005; Davalos et al., 2005). As regulators of developmental events like cell genesis, cell death, synaptogenesis, and synaptic pruning, these cells are able to impact the developmental trajectory of the brain and ultimately, behavior.
A key feature of many of the brain’s developmental processes is the presence of critical periods (Hensch, 2004). A critical period is a window of time in which a normal developmental event must take place in response to internal or external stimuli. Once this window closes, the normal trajectory of development cannot be restored via the original stimulus required. During these periods, the brain’s architecture is shaped in such a way that will endure throughout the organism’s life. Sensory systems of the brain, such as the visual and auditory systems, are some of the most well known for their critical periods during development. It has only recently been discovered that microglia are essential to activity-dependent refinement of the visual system during its critical period of development (Stevens et al., 2007; Schafer et al., 2012). Given the varied and dynamic roles that microglia play during development, it is not surprising that they might be regulators of other critical periods.
Sexual differentiation of the brain occurs during a classic critical period defined by the onset and offset of sensitivity to gonadal steroids. The cellular mechanisms of sexual differentiation are diverse and brain region specific, yet all function to coordinate brain development in a male-typical or female-typical manner. Evidence is rapidly accumulating that suggests immune cells may be far more important to establishing sex differences in the brain than previously thought (McCarthy et al., 2015).
The goal of this review is to examine the role of microglia during critical periods in development, with a particular focus on those during which sex differences in the brain are established. We will discuss how microglia orchestrate early developmental processes and the resulting impact on behavior later in life. We also present a conceptual framework for how future studies could begin to consider biological sex as a factor for studying microglial function during development.
SEXUAL DIFFERENTIATION OF THE BRAIN
Sexual differentiation of the brain is a developmental process whereby physiological and behavioral phenotypes are modified to match gonadal phenotype. For instance in mammals, animals with testis are exposed to high levels of androgens and their metabolites which act on the brain to determine male-typical sexual and aggressive behaviors and physiology. Conversely, animals with ovaries will develop a feminized brain phenotype that supports ovulation and reproductive behaviors associated with internal fertilization, pregnancy and lactation (McCarthy et al., 2017).
In the rodent, the critical period for masculinization of the brain begins around embryonic day 16–18 when the testes produce high levels of circulating testosterone, which decreases within hours after birth, closing this critical period (Weisz & Ward, 1980; Konkle & McCarthy, 2004). Testosterone and its androgenic metabolites are able to sexually differentiate several endpoints in the brain; however, most are driven by estradiol synthesized from testosterone by the aromatase enzyme in the brain (McCarthy, 2008; Zuloaga et al., 2008). In female rodents, there is a sensitive period for sexual differentiation of the brain that extends past the critical period in males, continuing through the first postnatal week. During this period, the female brain is sensitive to hormone exposure and masculinization can be induced by exogenous administration of testosterone or estradiol. These two examples demonstrate the difference between critical and sensitive periods: in critical periods, normal developmental processes must be established in a short time or else they are lost, while in sensitive periods, the brain remains sensitive for longer to particular inputs that it is not sensitive to prior to and after that period of time (McCarthy, 2017).
Sexually differentiated endpoints are diverse, varying drastically between brain regions. Hormone exposure may drive differential cell genesis and survival, cell death, synaptogenesis, synaptic pruning, and cellular migration. Ultimately, these processes result in lasting differences in brain region size, cellular phenotype and complexity, and synaptic connectivity between males and females (Arnold, 2009; Forger et al., 2016) (Figure 1). Actions of gonadal hormones during this time are collectively referred to as “organizational”, and considered to be a form of early life programming, also referred to as the “organizational hypothesis” (Phoenix et al., 1959).
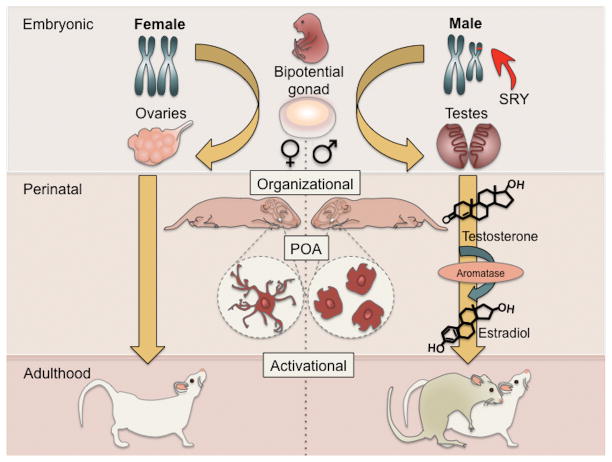
Figure 1. Sexual differentiation of the brain and behavior.
A male fetus carries a gene on the SRY gene on the Y chromosome and this gene codes for a protein called Testis Determining Factor which differentiates the biopotential gonad into testes. The testes synthesize testosterone which is converted to estradiol in some areas of the brain by the enzyme aromatase. In the preoptic area, estradiol exposure in males and females ultimately triggers microglia to assume an ameboid morphology and begin producing prostaglandin (PGE2) which acts on neurons to organize a 2-fold higher synaptic density pattern that is necessary for male copulatory behavior later in life. Post-puberty and in adulthood the organized (masculinized) POA is activated by circulating gonadal steroids to enable male sexual behavior.
MICROGLIA AND SENSITIVE PERIODS OF DEVELOPMENT
Microglia are derived from embryonic yolk-sac macrophage precursors and enter the brain as early as embryonic day 9.5 (E9.5) in the rodent (Alliot et al 1999; Ginhoux et al 2010; Schulz et al., 2012; Kierdorf et al 2013). Once in the brain, these cells continue to migrate, proliferate, and mature throughout development, until microglia attain their adult phenotype by the end of the third postnatal week (Ajami et al., 2007; Swinnen et al., 2013; Elmore et al., 2014; Nikodemova et al., 2015). By this time, microglia are tiled throughout the brain yet exhibit great regional variation in density and morphology, and sustain their numbers through local self-renewal (Ajami et al., 2007; Elmore et al., 2014; Bruttger et al., 2015; Doorn et al., 2015; Grabert et al., 2016; De Biase et al., 2017).
Microglia and sex differences
Microglia are highly dynamic and present in appreciable numbers throughout much of the brain perinatally. Their morphologies range from amoeboid to highly ramified and phagocytic phenotypes; such variation is likely reflective of both their developmental maturity as well as their signaling and physical functions in given brain regions.
Sex differences in microglia are not apparent until after the onset of the prenatal androgen surge and vary by brain region and age, suggesting a role for hormones in the diversity of microglia-mediated developmental processes (Schwarz et al., 2012) (Figure 2). Two particular features have been noted to differ markedly between males and females in rodent models of development- microglial number and morphology.
Figure 2. Microglial morphology is variable during the critical period for sexual differentiation.
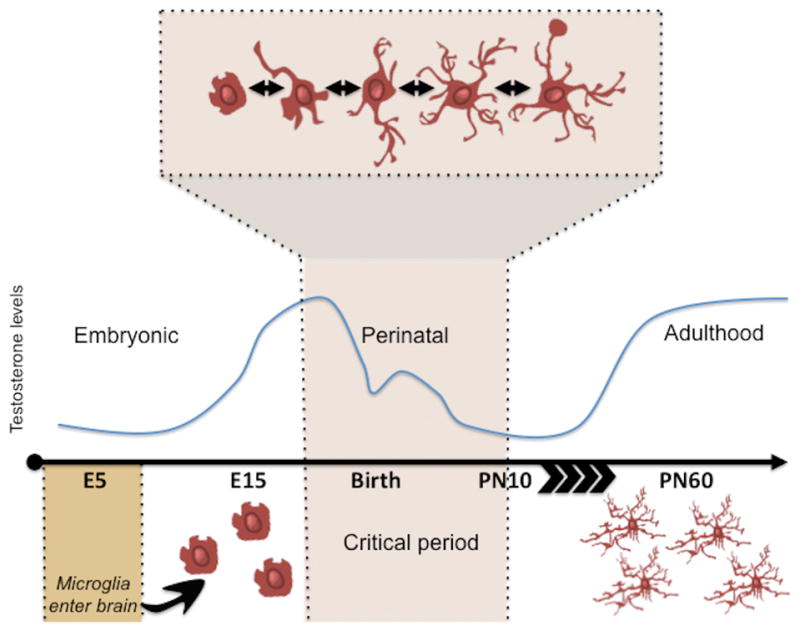
Microglia originate in the yolk sac and migrate into the brain during embryogenesis. These cells are capable of morphological plasticity at all stages of life during and after insult. Under normal conditions, however, microglia exhibit an especially dynamic array of morphologies during the critical period for sexual differentiation of the brain. These morphologies range from (left to right above) ameboid, stout, transitioning, ramified and phagocytic. The critical period for sexual differentiation of the brain is defined by the surge in testicular hormones and their metabolites in males perinatally. Sex differences in microglial morphologies during the critical period have been identified in many regions of the brain, however, these differences often disappear, or are even reversed, at the close of the critical period for sexual differentiation.
On the day of birth, female rats have more amoeboid and stout microglia in a number of brain regions, including the paraventricular nucleus of the hypothalamus (PVN), hippocampus, and amygdala, when compared to male rats. This sex difference quickly reverses, as males display more amoeboid and stout microglia than females four days later in the parietal cortex, hippocampus, and amygdala (Schwarz et al., 2012). By contrast, male rat pups have more microglia per unit area than females in the developing preoptic area (POA), and nearly twice as many amoeboid microglia as females from PN0-2 (Lenz et al., 2013). Microglia in the male POA are the major source of high levels of the inflammatory prostaglandin, PGE2, which is both necessary and sufficient for the masculinization of POA synaptic density and male sexual behavior in adulthood. This microglial sex difference is hormonally programmed, as treating females with a masculinizing dose of estradiol increases both the number of microglia and their amoeboid morphology (Lenz et al., 2013).
It is currently unknown whether sex differences in microglial number and morphology during development are a result of differential maturation or due to intrinsic differences in microglial function. Differences in microglial number may arise from a combination of timing of migration and/or local proliferation within a given brain region. Moreover, differences in morphology may be reflective of relative functional or maturational state, or driven by local cues from the developing brain region in which they reside. Amoeboid and stout morphologies are characteristic of immature microglia during development (Monier et al., 2007; Rigato et al., 2011; Swinnen et al., 2013), and in many brain regions, amoeboid microglia make up a greater proportion of the population in males than females (Schwarz et al., 2011; Lenz et al., 2013). Interestingly, in the developing POA, all microglia co-express traditional pro-inflammatory (M1) and alternative activation (M2) markers, despite males having more amoeboid microglia and higher pro-inflammatory PGE2 signaling (Lenz et al., 2013). These findings corroborate those of others who observe similar developmental co-expression of the M1 and M2 phenotypes (Crain et al., 2013; Cunningham et al., 2013), which strongly suggests the morphologies observed in the developing brain are not as easily classified by the same phenotypic markers used to analyze microglia in adulthood and after insult. Much more research is required in this area to fully interpret and classify the relationship between microglial morphology, function, and region-specific brain development during the perinatal period.
RNA sequencing of microglia, in an attempt to understand their developmental trajectory, revealed three distinct stages in microglial development. These stages, identified as early (E10.5–E14), pre- (E14-PN9), and adult (4 weeks and older), consisted of discrete transcriptional patterns reflective of differing microglial function throughout brain development (Matcovitch-Natan et al., 2016). While only males were analyzed in this study, identification of distinct and functional periods of microglial development nevertheless opens the door for further investigation. What remains to be determined is if these perinatal transcriptional patterns are caused by the natural hormonal surge in males and whether they exist or differ in females. Such findings may shed light on early life interactions between the brain’s immune system and sex, and how these factors work together to differentially influence brain development.
Alternatively, the maturation of the brain itself may exert regulatory control over microglia through changes in regional microenvironment. Longitudinal MRI confirms that regions of the brain vary in maturational trajectory in boys and girls (Geidd, 2004). Analogous studies have been done in rodents where other markers of maturation, such as depolarizing GABA, differ in neonatal male and female rat pups (Nunez & McCarthy, 2009). It is possible that microglia in less mature brain regions are themselves less mature and could therefore differ in males and females at a particular time of development as a consequence, not a cause, of maturational events.
Microglia and the regulation of cell number
Microglia regulate cell number during development, performing a dual role facilitating cell proliferation and differentiation, while also actively phagocytosing dead and stressed, but viable cells. This delicate balance of life and death is critical during early brain development in order to establish a healthy template from which to build on through experience in the remainder of the organism’s life.
Neural progenitor cells (NPCs) are intimately linked to microglial function. NPCs coordinate colonization by microglia early in brain development, and in turn, microglia produce factors that promote proliferation and differentiation of neural progenitors (Arnò et al., 2014). In the early postnatal subventricular zone, microglia produce cytokines that act as trophic factors for normal neurogenesis and oligodendrogenesis (Shigemoto-Mogami et al., 2014). Similarly, in the developing cortex microglia support healthy neurons in layer V through production of IGF-1. Microglial inhibition or depletion increases the number of apoptotic layer V neurons, and appropriate neuronal support appears to require microglia-neuron crosstalk through the fractalkine receptor, CX3CR1 (Ueno et al., 2013). In vitro, the presence of microglia or microglia-conditioned media enhances proliferation of cultured NPCs, astrogenesis, and neuronal maturation (Morgan et al., 2004; Antony et al., 2011).
Much like how microglia colonize early neurogenic niches, microglial colonization patterns often chronologically match patterns of programmed cell death (PCD) at many developmental stages (Perry et al., 1985; Ashwell 1990). Microglia facilitate the removal of dead and dying cells from the developing and adult brain (Ferrer et al., 1990; Bessis et al., 2007; Sierra et al., 2010). Apoptotic neurons release several “find me” signals, all of which function to attract microglial migration to the site of cell death (Ravichandran, 2011). However, while PCD does facilitate microglial colonization in some brain regions, migration is not entirely dependent on localized apoptosis as a cue (Eyo et al., 2015, Xu et al., 2016).
While initial observations suggested microglia only passively phagocytose dead and dying cells during development, more recent evidence suggests microglia are far more active in cell death, capable of inducing it in otherwise viable cells. In the early postnatal cerebellum, microglia induce cell death in Purkinje cells through a targeted “superoxide burst”; this process occurs during a critical period in cerebellar development in which Purkinje cells migrate to their final locations and refine their synaptic patterning (Marín-Teva et al., 2004). While the majority of the Purkinje cells targeted by microglia also expressed markers indicative of programmed cell death (such as activated caspase-3), when microglia were depleted from cerebellar slice cultures in vitro, Purkinje cell survival increased (Marín-Teva et al., 2004). Microglia in the postnatal hippocampus eliminate cells in a similar fashion, contacting caspase-3+ neurons and producing superoxide ions in a CD11b/DAP12 dependent mechanism (Wakselman et al., 2008). Again, preventing superoxide production by DAP12 deficiency or CD11b antibody blockade, decreases the number of caspase-3+ cells. These data suggest that in brain regions characterized by normal programmed cell death, microglia may act independently to facilitate elimination of cells.
NPCs in the cerebral cortex are also phagocytosed by microglia as a means of controlling cortical cell number. However, very few of the NPCs being engulfed by microglia express markers of cell death, and when microglia are inhibited or depleted, the number of cortical NPCs increases (Cunningham et al., 2013). Thus, microglial phagocytic function may be independent from cell death, providing further evidence for developmental “phagoptosis” or the phagocytosis of viable cells (Brown & Neher, 2012; 2014).
Given the evidence for microglia regulation of cell number in development, sex differences in cell number may also arise from microglia-mediated mechanisms. A recent examination of the neonatal rat hippocampus revealed females have nearly twice the number of phagocytic microglia as males (Nelson et al., 2017). Prior to the onset of fetal hormone production (at E20), phagocytic morphologies did not differ between the sexes. Moreover, treating newborn female pups with a masculinizing dose of estradiol eliminated the observed sex difference. Other parameters of microglial morphology (classified as amoeboid, transitioning, or ramified) did not differ between the sexes suggesting that phagocytic morphology occurred independent of traditional morphology or activation classifications. Further analysis found that newborn cells (marked by BrdU labeling) and Sox2+ progenitor cells were targeted by a subset of microglia for phagocytosis (Nelson et al., 2017). As the number of newborn cells in the developing hippocampus is also sexually dimorphic (Zhang et al., 2008; Bowers et al., 2010), it is tempting to speculate that microglial phagocytosis might be a determining factor of newborn cell number. The relationship between newborn cells and phagocytic microglia is inverse; males have more newborn cells postnatally (Zhang et al., 2008; Bowers et al., 2010) but also have fewer phagocytic microglia (Nelson et al., 2017). However, further study is needed to functionally link microglia and newborn cells in the hippocampus, as these observations are correlational and from different laboratories.
Microglia are also likely to play a role in sexual differentiation of the anteroventral periventricular nucleus (AVPV) of the POA although this has not yet been tested directly. The AVPV is a cluster of cells important for regulating the sex-specific luteinizing hormone release patterns necessary for reproduction and is approximately 2x larger in females than males, with a higher cell density (Murakami & Arai, 1989; Sumida et al., 1993). This sex difference is largely due to hormonally driven cell death, which through inhibition of constitutively active tumor necrosis factor alpha (TNFalpha) signaling, induces higher rates of cell death in the male AVPV (Arai et al., 1996; Krishnan et al., 2009). Microglia are the major producer of TNFalpha in the brain, and trigger cell death in developing motor neurons in a TNFalpha-dependent manner (Sedel et al., 2004). As such, microglia are potential drivers of the sexual differentiation of the AVPV.
Microglia and synaptic development, maturation, and connectivity
Microglia are found in higher densities in discrete developing axonal tracts and axon outgrowth is highly dependent upon microglia. Microglia both promote axonal outgrowth and phagocytose aberrant axons to facilitate a proper balance of projections. Microglial depletion (genetic deletion of Pu.1 or antibody blockade of the colony-stimulating factor 1 receptor; CSF1R) or activation (lipopolysaccharide; LPS) results in an over or under abundance of dopaminergic projections in the developing mouse brain, respectively (Squarzoni et al., 2014). Similarly, microglial depletion (Pu.1 knockout) or deficiency (DAP12 mutant) leads to defasciculation of the corpus callosum, likely through a lack of trophic support (Pont-Lezica et al., 2014).
Microglial interactions with synapses are more complex, being largely driven by neuronal activity, which they assess through an array of receptors tuned to signals associated with synaptic firing (Pockock & Kettenmann, 2007). Neurotransmission induces microglial motility and process outgrowth toward the active synapse resulting in brief and rapid contacts with the synapse (Davalos et al., 2005; Nimmerjahn et al., 2005; Fontainhas et al., 2011; Li et al., 2012; Dissing-Olesen et al., 2014). It is estimated microglia are able to survey their local environment in approximately 1 hour (Wake et al., 2009).
Developmental microglia-synapse interactions result in the functional maturation and remodeling of synaptic connectivity. Microglia actively engulf weaker synaptic inputs to facilitate the appropriate segregation of eye-specific retinal inputs to the LGN during the critical period for maturation of the visual system. Disrupting this process, pharmacologically or genetically, results in aberrant connectivity (Stevens et al., 2007; Schafer et al., 2012). In the visual cortex, engulfment is preferential towards transient and smaller spines, and is modulated by alterations in sensory experience (Tremblay et al., 2010). Similar findings occur in vitro, where microglia depletion or their addition to hippocampal neurons increases or decreases the number of excitatory synapses, respectively (Ji et al., 2013). These findings are likely mediated by contact-dependent synaptic pruning, as preventing physical interaction with hippocampal neurons in vitro increases the number of dendritic spines and functional synapses through the production of interleukin 10 (Lim et al., 2013). Microglial contact with dendrites in the developing somatosensory cortex induces cytoskeletal rearrangement and filopodia formation during periods of high synaptogenesis (Miyamoto et al., 2016). These findings suggest the initial development and further refinement of synaptic connectivity likely depends on a microglia-mediated balance between eliminating weak or unnecessary synapses and promoting the growth of new ones.
Evidence for sex-specific regulation of dendritic spines can be found in the POA where microglia increase spinogenesis through inflammatory signaling, specifically in males. Perinatal estradiol increases the production of the synthesizing enzymes for PGE2- COX1 and COX2- in neurons shortly after birth, increasing levels of PGE2 in the POA. Microglia greatly amplify the PGE2 signal in a feed forward mechanism, such that a single injection of PGE2 into female pups is sufficient to initiate this cascade and masculinize their adult brain architecture and behavior (Wright & McCarthy, 2009). PGE2 binds the EP2 and EP4 receptors, both of which are g-protein coupled receptors that activate the adenylyl cyclase pathway (Amateau & McCarthy, 2004; Wright et al., 2008). Increased cAMP in neurons and astrocytes results in increased AMPA receptor membrane trafficking, and in neurons, further activation of the newly inserted AMPA receptors results in dendritic spine induction and stabilization (Lenz et al., 2011). The end result of this process is a 2x greater density of dendritic spines on neurons in the male POA. This entire process can be prevented by pharmacologically inhibiting microglia postnatally, disrupting the PGE2 feed forward signaling process and preventing masculinization of POA dendritic synapse density (Lenz et al., 2013).
Lasting impacts of early microglial function
The diverse array of microglial functions- control of cell number and differentiation, synaptogenesis and pruning- work in coordination to facilitate development and maturation of neural circuits. Alterations to these processes, at the molecular, cellular, or circuit level, are likely to have significant and lasting impacts on both brain and behavior. Studies examining long-term effects of early microglia function often use two approaches- genetic deletions or mutation of microglia specific genes, or microglia depletion (Frost & Schafer, 2016; Paolicelli & Ferretti, 2017). Deletion or mutation of specific genes offers the ability to study the native function of a particular protein; however, these models are often constitutive mutants so lasting effects on parameters such as behavior become difficult to associate with the deficient microglia function rather than an accumulated effect from a life time of deficiency. Nonetheless, genetic mutants provide some of the most comprehensive and compelling evidence to suggest that early microglial dysfunction impacts synaptic maturation and later life behavior.
Genetic deletion of microglia-specific CX3CR1 (CX3CR1 KO) delays colonization of the rodent barrel cortex, which normally occurs during a critical period for the maturation of thalamocortical projections. As a result, glutamatergic synapse maturation is delayed (Hoshiko et al., 2012). CX3CR1 KO mice also have fewer microglia in the hippocampus postnatally, resulting in an abundance of immature synapses and delayed maturation of synaptic structure and function (Paolicelli et al., 2011). In mature animals, these deficits manifest as impairments in long-term potentiation, learning-dependent memory, social interactions, and increased self-grooming behaviors (Rogers et al., 2011; Zhan et al., 2014).
Synaptic deficiencies and alterations in spine numbers are observed in other genetic mutant models (Roumier et al. 2004; Kim et al., 2016) and are common phenotypes associated with alterations in microglial function. Such deficiencies are corroborated by studies depleting microglia to examine their role in modulating circuit function and behavior. Conditional genetic microglia depletion in mature animals induces learning-dependent memory deficits, likely through impairments in synaptic transmission and spine remodeling (Parkhurst et al., 2013). Other behavioral impairments occur following pharmacological microglia depletion using inhibitors of the colony stimulating factor 1 receptor (CSF-1R). CSF-1R is necessary for microglia viability, and systemic treatment with its inhibitors achieves nearly complete microglia depletion, with no lasting microglia impairment following repopulation (Elmore et al., 2014; 2015). Using this approach, adult mice depleted of microglia display deficits in spatial memory and abnormal social behavior (Torres et al., 2016); however, the behavioral effects of microglia depletion are reversed after microglia repopulate the brain (Elmore et al., 2015; Torres et al., 2016). Such a transient impact of microglial depletion on behavior underscores the highly dynamic role microglia play. It seems that microglia not only participate in synaptic refinement during critical periods of development, but also dynamically modulate social and learning-dependent behaviors by altering synaptic remodeling.
While the above studies underscore the importance of microglia in experience-dependent modulation of synapses and behavior in mature animals, the functional outcome of developmental microglia actions has received far less attention until recently. It now seems that microglia have two very different, but complementary roles in the developing and mature animal. At maturity, microglia facilitate experience-dependent synaptic remodeling that has profound impacts on learning-dependent and social behaviors. Behavioral abnormalities observed in these experimental conditions are limited to the period of dysfunction or depletion, that is, they do not persist once microglia function is restored. In development, however, microglia are far more important in facilitating the organization of brain architecture with significant and lasting implications for later life behavior.
Selective microglia depletion during discrete epochs of development allows for the assessment of how early life microglia function ultimately shapes the animal’s behavior later in life. One approach for temporary depletion of microglia is intracerebral injections of the dominant negative ATP analog, clodronate, packaged into liposomes for ready uptake by phagocytosing cells (Buiting & Van Rooijen, 1994). This approach facilitates studying microglia with more precise temporal control as it allows a rapid and transient depletion of the microglia population.
Liposomal clodronate treatment of male and female pups on PN0, 2 and 4 reduces microglial numbers by 50–80% within 24 hours with a full repopulation by PN10 (VanRyzin et al., 2016). Temporary microglial depletion during development produces significant and long lasting changes to various aspects of behavior in both sexes, which become apparent within a short time period. Within days after depletion, treated pups display impaired nest seeking behavior and reduced numbers of ultrasonic vocalizations, two ethologically relevant early pro-social behaviors. By the end of the second postnatal week, once the microglia had repopulated, clodronate-treated animals were hyper-locomotive compared to controls. Once juveniles, prior depletion produced drastic reductions in innate fear and anxiety-like behaviors, social, and working memory. When all of these behavioral alterations are considered together, transient postnatal microglia depletion induces two primary phenotypes- behavioral disinhibition and hyper-locomotion- which persist throughout life (VanRyzin et al., 2016).
These findings are remarkably similar to those observed in a similar study, which found the behavioral deficits after neonatal microglial depletion persist well into adulthood. Both male and female adult rats displayed reduced anxiety-like and despair behavior when tested in the open field, elevated plus maze, and forced swim test (Nelson & Lenz, 2017). Together, these data provide intriguing evidence implicating microglia in early life programming of various affective behaviors. Future research is needed to dissect the microglial contribution to establishing these behavioral circuits during early postnatal life.
While our findings in juvenile animals indicate sex-independent functions of microglia during sensitive periods in development, sex-dependent reproductive behaviors in adults require microglia in males but not females. Postnatal microglial depletion drastically reduces the expression of adult male sex behavior, impairing both motivation and execution; females, however, are completely unaffected and display normal proceptive and receptive behaviors, indicating divergence in microglia’s roles in the formation of behavioral circuitry (VanRyzin et al., 2016). Male-typical sex behavior requires microglia for appropriate development. Insults (reductions in microglia number or alterations to microglia signaling) drastically alter this trajectory (Lenz et al., 2013; VanRyzin et al., 2016). Yet the neural underpinnings of female sex behavior appear to develop independent of microglia during this period. The remarkable divergence in microglia involvement could provide a valuable vantage point for identifying novel developmental pathways between the sexes.
CONCLUSIONS AND FUTURE DIRECTIONS
Recent advances in our understanding of how microglia regulate brain development have shed light on a number of normal physiological processes that occur in a time- and brain region-dependent manner. As we continue to investigate the molecular and cellular interactions governing these processes, and how these developmental functions eventually influence brain architecture and behavior, it is essential to consider sex as a factor in the study of microglia and development. Similarly, studies on sex differences in brain and behavior would likely benefit from the inclusion of microglia as a key factor.
Reframing the study of microglia and sensitive periods
How exactly might microglia mediate sensitive periods and sculpt sex differences in development? Many studies focus on processes that appear to be specific and intrinsic to non-microglial cells, and for good reason, as microglia make up only 5–15% of the total cell population in the adult human brain (Pelvig et al., 2008; Lyck et al., 2009). Neurons, astrocytes, and even oligodendrocytes constitute the majority of the brain; however, despite their small number and size, microglia have asserted themselves as regulators of sensitive periods in development.
Most sexually differentiated processes, such as neurogenesis and programmed cell death, have thus far been studied without consideration of microglia. We now know that microglia differ markedly between the sexes during development and play a larger role in these processes than originally thought. If we are to better understand developmental processes in the brain, particularly sexual differentiation, a greater focus must be placed on understanding sex differences in the microglial colonization and morphology from a functional perspective. Doing so is likely to uncover new and potentially sexually dimorphic functions of these cells.
Microglia themselves may be sexually dimorphic- that is, the very function of microglia may be different between the sexes and perform different roles during developmental sensitive periods. In these cases, microglial function may be the result of chromosome composition (XY vs XX in mammals) or of developmental exposure to gonadal hormones. Gonadal hormones may act directly on microglia or modify the local environment to elicit a sex-dependent microglial response. Differentiating between these two possibilities is paramount to understanding the generation of sex differences and how these processes may go awry. An example of this is in the developing POA, where microglia in the male brain respond to a hormonally initiated cascade of events which results in the subsequent masculinization of the POA (Lenz et al. 2013). Both male and female microglia are similar in their ability to participate in this cascade, as masculinization can be induced in females or disrupted in males (Lenz et al. 2013, VanRyzin et al. 2016). Only after careful consideration of this signaling cascade can we begin to see that microglia are the executioners, not the source, of this phenomenon.
Alternatively, microglia may have completely differing roles in development between the sexes. These cells may perform one function in the developing female brain and perform a completely different function in the developing male brain to achieve a common endpoint. Indeed, such a precedent exists in adult animals; microglia mediate mechanical pain hypersensitivity in male mice, whereas female mice use T lymphocytes (Sorge et al., 2015).
Future Directions
As the study of microglia and sensitive periods continues to advance, it is important to remember that each of these developmental processes may be affected by exogenous insult, resulting in lasting changes in the brain. Early life stress, inflammation or immune challenge, and even environmental insult can significantly alter microglial functions during development (for reviews, see Bilbo & Schwarz, 2009; 2012; Paolicelli & Ferretti, 2017). There is growing appreciation that these early insults not only disrupt early microglial function, but may also impart lasting changes to microglial activity throughout life. Enduring microglial alterations may be, in part, epigenetic in nature (Garden, 2013; Netea et al., 2016). Epigenetic alterations to the microglia may program how these cells interact with the brain later in life and have significant consequences for behavior. For example, neonatal handling of rat pups induces long-term increases in expression of the anti-inflammatory cytokine, interleukin-10 (IL-10), by decreasing microglia-specific IL-10 gene methylation. The increased anti-inflammatory signaling promotes resilience to the reinstatement of morphine-induced conditioned place preference (Schwarz et al., 2011) and induces robust down regulation of many pro-inflammatory genes in the nucleus accumbens in adulthood (Lacagnina et al., 2017).
The X chromosome represents another convergence between immune function and epigenetic regulation, as it contains a large number of immune-related genes (Fish 2008; Bianchi et al., 2012). This makes X-chromosome inactivation essential to appropriate immune function in females. To this point, inhibiting DNA methyltransferase activity in the developing female POA results in a significant enrichment in immune-related genes (Nugent et al., 2015). These data suggest that epigenetic regulation by methylation serves to dampen immune function in the developing female as a natural part of sexual differentiation.
The last decade has been enormously exciting in the arena of microglia investigation. Tremendous strides have been made in elucidating fundamental principles of these mysterious cells, along side startling and unexpected discoveries. The potential that treatments and interventions specific to microglia will offer new and effective therapies is growing in probability but it is clear there is still much to be learned about these small, infrequent but oh so powerful cells.
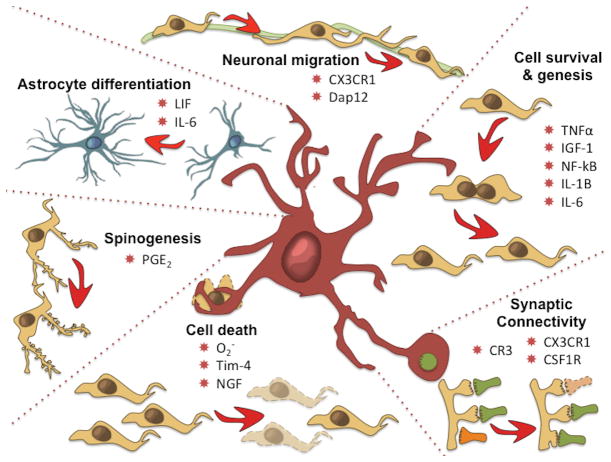
Figure 3. Microglia modulate sexually differentiated endpoints.
During the critical period for sexual differentiation microglia help to modulate fundamental processes from cell genesis to migration, differentiation, synaptogenesis and cell death, all of which differ in males and females in at least one brain region. Abbreviations: CR3, complement receptor 3; DAP12, DNAX-activation protein 12; Insulin-like growth factor 1; IL, interleukin; LIF, leukemia inhibitory factor; NF-kB, nuclear factor-kappaB; NGF, nerve growth factor; Tim-4, Tcell, immunoglobulin, mucin 4.
Acknowledgments
This work was supported by RO1MH52716 and R01DA039062 to MMM and F31NS093947 to LAP
Footnotes
Conflict of interest statement: No conflicts of interest
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FMV. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Antony JM, Paquin A, Nutt SL, Kaplan DR, Miller FD. Endogenous microglia regulate development of embryonic cortical precursor cells. J Neurosci Res. 2011;89:286–298. doi: 10.1002/jnr.22533. [DOI] [PubMed] [Google Scholar]
- Arai Y, Sekine Y, Murakami S. Estrogen and apoptosis in the developing sexually dimorphic preoptic area in female rats. Neurosci Res. 1996;25:403–407. doi: 10.1016/0168-0102(96)01070-x. [DOI] [PubMed] [Google Scholar]
- Arnò B, Grassivaro F, Rossi C, Bergamaschi A, Castiglioni V, Furlan R, Greter M, Favaro R, Comi G, Becher B, Martino G, Muzio L. Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat Commun. 2014;5:5611. doi: 10.1038/ncomms6611. [DOI] [PubMed] [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell K. Microglia and cell death in the developing mouse cerebellum. Brain Res Dev Brain Res. 1990;55:219–230. doi: 10.1016/0165-3806(90)90203-b. [DOI] [PubMed] [Google Scholar]
- Bessis A, Béchade C, Bernard D, Roumier A. Microglia control of neuronal death and synaptic properties. Glia. 2007;55:233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- Bianchi I, Lleo A, Gershwin ME, Invernizzi P. The X chromosome and immune associated genes. J Autoimmun. 2012;38:187–192. doi: 10.1016/j.jaut.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33:267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers JM, Waddell J, McCarthy MM. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol. Biol Sex Differ. 2010;1:8. doi: 10.1186/2042-6410-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Eaten alive! Cell death by primary phagocytosis: “phagoptosis”. Trends Biochem Sci. 2012;37:325–332. doi: 10.1016/j.tibs.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15:209–216. doi: 10.1038/nrn3710. [DOI] [PubMed] [Google Scholar]
- Bruttger J, Karram K, Wörtge S, Regen T, Marini F, Hoppmann N, Klein M, Blank T, Yona S, Wolf Y, Mack M, Pinteaux E, Müller W, Zipp F, Binder H, Bopp T, Prinz M, Jung S, Waisman A. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity. 2015;43:92–106. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Buiting Am, Van Rooijen N. Liposome mediated depletion of macrophages: an approach for fundamental studies. J Drug Target. 1994;2:357–362. doi: 10.3109/10611869408996810. [DOI] [PubMed] [Google Scholar]
- Crain JM, Nikodemova M, Watters JJ. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J Neurosci Res. 2013;91:1143–1151. doi: 10.1002/jnr.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Martinex-Cerdeño V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Schuebel KE, Fusfeld ZH, Jair K, Hawes IA, Cimbro R, Zhang HY, Liu QR, Shen H, Xi ZX, Goldman D, Bonci A. Local cues establish and maintain region-specific phenotypes of basal ganglia microglia. Neuron. 2017 doi: 10.1016/j.neuron.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissing-Olesen L, LeDue JM, Rungta RL, Hefendehl JK, Choi HB, MacVicar BA. Activation of neuronal NMDA receptors triggers transient ATP-mediated microglial process outgrowth. J Neurosci. 2014;34:10511–10527. doi: 10.1523/JNEUROSCI.0405-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorn KJ, Brevé JJ, Drukarch B, Boddeke HW, Huitinga I, Lucassen PJ, van Dam AM. Brain region-specific gene expression profiles in freshly isolated rat microglia. Front Cell Neurosci. 2015;9:84. doi: 10.3389/fncel.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MR, Lee RJ, West BL, Green KN. Characterizing newly repopulated microglia in the adult mouse: impacts on animal behavior, cell morphology, and neuroinflammation. PLoS One. 2015;10:e0122912. doi: 10.1371/journal.pone.0122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Miner SA, Weiner JA, Dailey ME. Developmental changes in microglial mobilization are independent of apoptosis in the neonatal mouse hippocampus. Brain Behav Immun. 2015;55:49–59. doi: 10.1016/j.bbi.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Bernet E, Soriano E, del Rio T, Fonseca M. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39:451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- Fish EN. The x-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–734. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, Fariss RN, Li W, Wong WT. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One. 2011;6:e15973. doi: 10.1371/journal.pone.0015973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Forger NG, Strahan JA, Castillo-Ruiz A. Cellular and molecular mechanisms of sexual differentiation in the mammalian nervous system. Front Neuroendocrinol. 2016;40:67–86. doi: 10.1016/j.yfrne.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JL, Schafer DP. Microglia: Architects of the Developing Nervous System. Trends Cell Biol. 2016;8:587–97. doi: 10.1016/j.tcb.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA. Epigenetics and the modulation of neuroinflammation. Neurotherapeutics. 2013;10:782–788. doi: 10.1007/s13311-013-0207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanely ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016;19:504–516. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, Alter MD, Block CS, Sullivan H, Bolton JL, Bilbo SD. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia. 2017;65:1504–1520. doi: 10.1002/glia.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hoshiko M, Arnoux I, Avignone E, Yamamoto N, Audinat E. Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J Neurosci. 2012;32:15106–15111. doi: 10.1523/JNEUROSCI.1167-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K, Akgul G, Wollmuth LP, Tsirka SE. Microglia actively regulate the number of functional synapses. PLoS One. 2013;8:e56293. doi: 10.1371/journal.pone.0056293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Emy D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Holscher C, Muller DN, Luckrow B, Brocker T, Debowski K, Fritz G, Opdenakker G, Diefenbach A, Biber K, Heikenwalder M, Geissmann F, Rosenbauer F, Prinz M. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8- dependent pathways. Nat Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Cho MH, Shim WH, Kim JK, Jeon EY, Kim DH, Yoon SY. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle A, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152:223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Intlekofer KA, Aggison LK, Petersen SL. Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc Natl Acad Sci U S A. 2009;106:16692–16697. doi: 10.1073/pnas.0906293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina MJ, Kopec AM, Cox SS, Hanamsagar R, Wells C, Slade S, Grace PM, Watkins LR, Levin ED, Bilbo SD. Opioid self-administration is attenuated by early-life experience and gene therapy for anti-inflammatory IL-10 in the nucleus accumbens of male rats. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Wright CL, Martin RC, McCarthy MM. Prostaglandin E2 regulates AMPA receptor phosphorylation and promotes membrane insertion in preoptic area neurons and glia during sexual differentiation. PLoS One. 2011;6:e18500. doi: 10.1371/journal.pone.0018500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. J Neurosci. 2013;33:2761–2772. doi: 10.1523/JNEUROSCI.1268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23:1189–1202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Lim SH, Park E, You B, Jung Y, Park AR, Park SG, Lee JR. Neuronal synapse formation induced by microglia and interleukin 10. PLoS One. 2013;8:e81218. doi: 10.1371/journal.pone.0081218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyck L, Santamaria ID, Pakkenberg B, Chemnitz J, Schrøder HD, Finsen B, Gundersen HJ. An empirical analysis of the precision of estimating the numbers of neurons and glia in human neocortex using a fractionator-design with sub sampling. J Neurosci Methods. 2009;182:143–156. doi: 10.1016/j.jneumeth.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Marín-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada González F, Perrin P, Keren-Shaul H, Gury M, Lara-Astaiso D, Thaiss CA, Cohen M, Bahar Halpern K, Baruch K, Deczkowska A, Lorenzo-Vivas E, Itzkovitz S, Elinav E, Sieweke MH, Schwartz M, Amit I. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353:aad8670. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Pickett LA, VanRyzin JW, Kight KE. Surprising origins of sex differences in the brain. Horm Behav. 2015;76:3–10. doi: 10.1016/j.yhbeh.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, de Vries GJ, Forger NG. Sexual differentiation of the brain: A fresh look at mode, mechanisms, and meaning. In: Pfaff DW, Joels M, editors. Hormones, Brain and Behavior. Cambridge, MA: Academic Press; 2017. pp. 3–32. [Google Scholar]
- McCarthy MM. Fast, furious and enduring: Sensitive versus critical periods in sexual differentiation of the brain. Physiol Behav. 2017 doi: 10.1016/j.physbeh.2017.10.030. pii: S0031-9384(17)30380-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y, Nabekura J. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 2016;7:12540. doi: 10.1038/ncomms12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier A, Adle-Biassette H, Delezoide AL, Evrard P, Gressens P, Verney C. Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J Neuropathol Exp Neurol. 2007;66:372–382. doi: 10.1097/nen.0b013e3180517b46. [DOI] [PubMed] [Google Scholar]
- Morgan SC, Taylor DL, Pocock JM. Microglia release activators of neuronal proliferation mediated by activation of mitogen-activated protein kinase, phosphatidylinositol-3-kinase/Akt and delta-Notch signalling cascades. J Neurochem. 2004;90:89–101. doi: 10.1111/j.1471-4159.2004.02461.x. [DOI] [PubMed] [Google Scholar]
- Murakami S, Arai Y. Neuronal death in the developing sexually dimorphic periventricular nucleus of the preoptic area in the female rat: effect of neonatal androgen treatment. Neurosci Lett. 1989;102:185–190. doi: 10.1016/0304-3940(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Nelson LH, Lenz KM. Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats. Behav Brain Res. 2017;316:279–293. doi: 10.1016/j.bbr.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LH, Warden S, Lenz KM. Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain Behav Immun. 2017;64:11–22. doi: 10.1016/j.bbi.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O’Neill LA, Xavier RJ. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Kimyon RS, De I, Small AL, Collier LS, Watters JJ. Microglial numbers attain adult levels after undergoing a rapid decrease in cell number in the third postnatal week. J Neuroimmunol. 2015;278:280–288. doi: 10.1016/j.jneuroim.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;208:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18:690–697. doi: 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez JL, McCarthy MM. Resting intracellular calcium concentration, depolarizing Gamma-Aminobutyric Acid and possible role of local estradiol synthesis in the developing male and female hippocampus. Neurosience. 2009;158(2):623–634. doi: 10.1016/j.neuroscience.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Guistetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Ferretti MT. Function and dysfunction of microglia during brain development: consequences for synapses and neural circuits. Front Synaptic Neurosci. 2017;9:9. doi: 10.3389/fnsyn.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging. 2008;29:1754–1762. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing actions of prenatally administered testosterone proprionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Pont-Lezica L, Beumer W, Colasse S, Drexhage H, Versnel M, Bessis A. Microglia shape corpus callosum axon tract fasciculation: functional impact of prenatal inflammation. Eur J Neurosci. 2014;39:1551–1557. doi: 10.1111/ejn.12508. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigato C, Buckinx R, Le-Corrronc H, Rigo JM, Legendre P. Pattern of invasion of the embryonic mouse spinal cord by microglial cells at the time of the onset of functional neuronal networks. Glia. 2011;59:675–695. doi: 10.1002/glia.21140. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC, Gemma C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 2011;31:16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumier A, Béchade C, Poncer JC, Smalla KH, Tomasello E, Vivier E, Gundelfinger ED, Triller A, Bessis A. Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. J Neurosci. 2004;24:11421–11428. doi: 10.1523/JNEUROSCI.2251-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Hutchinson MR, Bilbo SD. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci. 2011;31:17835–47. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedel F, Béchade C, Vyas S, Triller A. Macrophage-derived tumor necrosis factor alpha, an early developmental signal for motoneuron death. J Neurosci. 2004;24:2236–2246. doi: 10.1523/JNEUROSCI.4464-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci. 2014;34:2231–2243. doi: 10.1523/JNEUROSCI.1619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F, Garel S. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8:1271–1279. doi: 10.1016/j.celrep.2014.07.042. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Sumida H, Nishizuka M, Kano Y, Arai Y. Sex differences in the anteroventral periventricular nucleus of the preoptic area and in the related effects of androgen in prenatal rats. Neurosci Lett. 1993;151:41–44. doi: 10.1016/0304-3940(93)90040-r. [DOI] [PubMed] [Google Scholar]
- Swinnen N, Smolders S, Avila A, Notelaers K, Paesen R, Ameloot M, Brône B, Legendre P, Rigo JM. Complex invasion pattern of the cerebral cortex by microglial cells during development of the mouse embryo. Glia. 2013;61:150–163. doi: 10.1002/glia.22421. [DOI] [PubMed] [Google Scholar]
- Torres L, Danver J, Ji K, Miyauchi JT, Chen D, Anderson ME, West BL, Robinson JK, Tsirka SE. Dynamic microglia modulation of spatial learning and social behavior. Brain Behav Immun. 2016;55:6–16. doi: 10.1016/j.bbi.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e100527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME. The role of microglia at synapses in the healthy CNS: novel insights from recent imaging studies. Neuron Glia Biol. 2011;7:67–76. doi: 10.1017/S1740925X12000038. [DOI] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. Layer V cortical neurons require microglia support for survival during postnatal development. Nat Neurosci. 2013;16:543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- VanRyzin JW, Yu SJ, Perez-Pouchoulen M, McCarthy MM. Temporary depletion of microglia during the early postnatal period induces lasting sex-dependent and sex-independent effects on behavior in rats. eNeuro. 2016:3. doi: 10.1523/ENEURO.0297-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S, Béchade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Wright CL, Burks SR, McCarthy MM. Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev Neurobiol. 2008;68:1406–1419. doi: 10.1002/dneu.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, McCarthy MM. Prostaglandin E2-induced masculinization of brain and behavior requires protein kinase A, AMPA/kainate, and metabotropic glutamate receptor signaling. J Neurosci. 2009;29:13274–13282. doi: 10.1523/JNEUROSCI.3603-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B. Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol. 2015;36:605–613. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang T, Wu Y, Jin W, Wen Z. Microglia colonization of developing zebrafish midbrain is promoted by apoptotic neuron and lysophosphatidylcholine. Dev Cell. 2016;38:214–222. doi: 10.1016/j.devcel.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, Gross CT. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Konkle AT, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: what we’ve learned from the testicular feminization mutation. Horm Behav. 2008;53:613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]