Abstract
Purpose
Cerebral venous oxygenation (Yv) is an important physiological parameter and has potential clinical application in many brain diseases. T2-relaxation-under-spin-tagging (TRUST) is a commonly used MRI method to measure Yv. Harmonization of this technique across MRI vendors is important for dissemination and multi-center studies of brain oxygenation and metabolism as a disease biomarker.
Methods
TRUST pulse sequence components and imaging parameters were carefully matched between two major MRI vendors, Philips and Siemens. Each subject (N=10) was scanned on both scanners within a 2.5-hour period. On each scanner, the subject was scanned in two sessions to assess inter-session reproducibility. A hyperoxia challenge was also included in both sessions and on both scanners to evaluate the sensitivity of the technique to Yv changes. Measured Yv values, confidence interval of Yv estimates (εYv), as well as intra-session and inter-session coefficient of variation (CoV) of Yv, were compared between the two scanners.
Results
Yv measured on the two vendors were highly compatible and strongly correlated (R2=0.957). Yv changes associated with hyperoxia challenge were significant on both scanners (P<0.001) and were also correlated across scanners (P=0.007). Intra-session and inter-session CoV of measured Yv were less than 3% and showed no difference between scanners. εYv were less than 1% on both scanners and showed no difference between scanners when echo times were matched on the two scanners.
Conclusion
This work suggests that harmonized TRUST MRI can yield highly compatible Yv measurements across different vendors.
Keywords: venous oxygenation, TRUST, reproducibility, vendor, variation
INTRODUCTION
Cerebral venous oxygenation (Yv) is an important physiological parameter of the brain’s oxygen homeostasis. Yv is defined as the fraction of oxygenated hemoglobin in the venous blood, and can be combined with other physiological measures such as arterial oxygenation and cerebral blood flow to estimate key physiological markers such as oxygen extraction fraction (OEF) and cerebral metabolic rate of oxygen (CMRO2) (1). Previous studies have suggested that Yv, OEF, and CMRO2 may represent a key biomarker in many physiological and pathological conditions such as aging (2–4), multiple sclerosis (5), and Alzheimer’s disease (6,7).
Traditionally, measurement of Yv, OEF, and CMRO2 in humans is a niche market of Positron Emission Tomography using 15O-labeled tracers (8,9). However, the invasive and complex procedures, the exposure to radiation, and the need of an onsite cyclotron have been the major obstacles for the broad application of this technology. Over the past decade, several MRI techniques have been proposed to measure Yv without contrast agent by exploiting the association between blood oxygenation and MRI characteristics such as phase (10,11), susceptibility (12–16), T2* (17,18), and T2 (19–25). Among these techniques, T2-relaxation-under-spin-tagging (TRUST) (19,26) has been one of the most widely used and published methods. This sequence only takes 1.2 min, has been validated with pulse oximetry (27), and has demonstrated its sensitivity to a number of diseases (5,6,28–32).
However, in the era of “big-data” medicine, virtually all major studies of diseases and clinical trials are based on a multi-site setting and MRI techniques must make themselves scalable across sites and vendors in order to adapt to this new trend. The goal of the present work is therefore to conduct a harmonization study to evaluate compatibility of TRUST results across two major vendors of MRI. Each participant was scanned on both scanners within a 2.5-hour period. On each scanner, the participant was scanned multiple times to allow the assessment of intra-session and inter-session reproducibility. A hyperoxia challenge was also included on both scanners to allow the evaluation of sensitivity to Yv change.
METHODS
Pulse sequence
The basic principles of TRUST MRI have been described in previous reports (19,26). Briefly, TRUST oximetry is based on the well-known relationship between Yv and T2 relaxation time of the blood (33). To separate the venous blood from surrounding brain tissues, the TRUST sequence uses radiofrequency (RF) pulses to label the inflow venous blood, and acquires labeled and control images by alternating the RF pulses. Subtraction of the labeled images from the control yields difference images with pure venous blood signal in the superior sagittal sinus (SSS). To obtain T2 of the venous blood, non-slice-selective T2-preparation pulses with varying numbers are applied to modulate the blood signal, monoexponential fitting of which then yields blood T2. A calibration plot specifying the relationship between Yv and T2 is then used to convert T2 to Yv (27).
To evaluate the TRUST technique across vendors, the sequence was implemented on two platforms, a 3T Philips system (Achieva with Quasar Dual gradients, software release 5.1.7, Philips Healthcare, Best, The Netherlands) and a 3T Siemens system (Prisma with 80/200 gradients, software version VE11B, Siemens Healthcare, Erlangen, Germany). Details of the RF and gradient components are described in the Supporting Methods.
MRI experiment
The study protocol was approved by the Johns Hopkins University Institutional Review Board. Written informed consent was obtained from each participant. Ten healthy volunteers (24.0±3.2 years old, 6 male, 4 female) participated in this study. To minimize physiological fluctuations, the subjects were instructed to refrain from the consumption of coffee, tea, alcohol and cigarettes 2 hours prior to the scanning.
Each subject was scanned on a Philips and a Siemens 3T scanner on the same day, within a period of 2.5 hours. The two scanners are physically adjacent to each other with a distance of less than 5 minutes of walking. The order of the Philips and Siemens scans were counterbalanced across subjects with gender matching, i.e., 3 male and 2 female subjects completed the Philips scan first and the rest completed the Siemens scan first. Both Philips 3T and Siemens 3T used a 32-channel head coil.
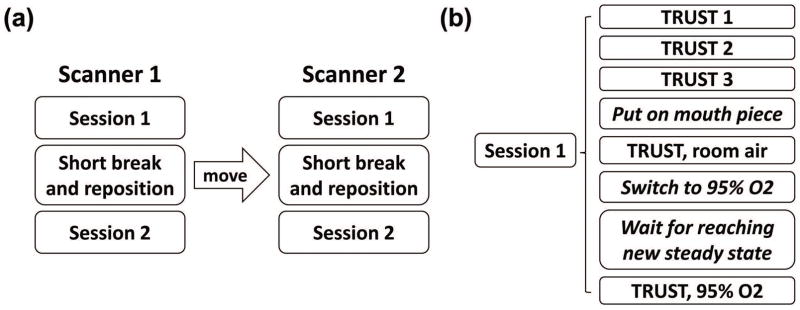
The procedure performed on each subject is illustrated in Figure 1. On each scanner, each subject was scanned twice with a short break (2–5 minutes) and repositioning between the two identical sessions (Figure 1a). This allows the quantification of inter-session variability. Within each session (Figure 1b), the subject first underwent 3 TRUST scans, which allows the quantification of intra-session variability. Then, the subject was pulled out of the scanner for placement of nose clip and mouthpiece, and entered the scanner again. The subject breathed through the mouthpiece for the remainder of the session. One TRUST scan was acquired while the subject breathed room air to provide a normoxic measure of Yv. Then the inhaled air was switched to hyperoxic gas and 3 minutes were waited to allow the brain’s physiology (34) to reach a new steady state, before another TRUST scan was performed to obtain a hyperoxic measure of Yv (Figure 1b). The procedures for hyperoxia challenge are described in the Supporting Methods. This allows the comparison of the sensitivity of the TRUST technique to blood oxygenation change across scanners.
Figure 1.
Illustration of the experimental design. (a) each subject was scanned twice on Scanner 1 with a short break and repositioning between two identical sessions, then the subject was escorted to Scanner 2 to repeat the same procedure; (b) each session began with 3 TRUST scans while the subject breathed room air without mouth piece. Then the subject was pulled out of the scanner for placement of nose clip and mouthpiece, and entered the scanner again. One TRUST scan was acquired while the subject breathed room air through mouthpiece; then the inhaled air was switched to hyperoxic gas, and one TRUST scan was acquired after the subject’s physiology reached a new steady state.
TRUST imaging parameters on both scanners are described in the Supporting Methods. All relevant parameters were matched except for echo time (TE), which was 4ms on Philips with a bandwidth of 4.5kHz/pixel and 7ms on Siemens with a bandwidth of 3.3kHz/pixel. This difference was attributed to the additional navigators present in the Siemens echo-planar imaging (EPI) module. The discrepancy in TE is not expected to affect the accuracy of the T2 estimation, but may have an impact on the precision. Therefore, as a supplemental study, we modified the Siemens EPI module to reduce the TE to 4ms and then recruited six new subjects (28.3±6.0 years old, 2 females, 4 males), in whom we performed three TRUST scans on Philips and another three on Siemens.
Data processing
The TRUST MRI data were processed with in-house MATLAB (Mathworks, Natick, MA) scripts, following the procedures described previously (as detailed in the Supporting Methods) (19,26). Yv and confidence interval of the estimated Yv (εYv) were obtained from each dataset.
Yv differences measured between vendors can be attributed to both pulse sequence differences and physiological fluctuations. To focus our attention on the contribution from pulse sequence differences, we minimized the influence of physiological fluctuations by performing a correction of the measured Yv using a within-subject relationship between Yv and end-tidal CO2 (EtCO2):
| [1] |
where Yv|corrected and Yv|uncorrected are Yv measured on either Philips or Siemens scanner before and after correction, respectively; EtCO2 is the EtCO2 recorded simultaneously with the corresponding TRUST scans; is the EtCO2 averaged between Philips and Siemens scanners. The coefficient α denotes the dependence of venous oxygenation on EtCO2. Previous studies have reported that value of α is between 1 and 2%/mmHg (35,36). In this study, since we measured Yv and EtCO2 twice on each scanner, we were able to calculate the value of α from the ratio between Yv and EtCO2 differences, which was found to be 1.6%/mmHg. For comparison, we also showed data using uncorrected Yv in the supporting figures and tables.
Statistical analysis
A paired Student’s t-test was utilized to compare the measured Yv and εYv between the scanners, separately under room-air (i.e. normoxia) and hyperoxia conditions. Correspondence between Yv measured on the Philips scanner and that on the Siemens scanner was evaluated with Pearson correlation. Bland-Altman plot was used to assess the dependence of the inter-scanner difference on Yv value. In addition, to assess the sensitivity of TRUST in detecting Yv changes under physiological challenges, Yv differences (ΔYv) between hyperoxia and normoxia conditions (both through mouthpiece) were calculated and compared between scanners.
Since we performed multiple sessions on each scanner and multiple scans during each session, we calculated intra-session and inter-session coefficient of variation (CoV) for data from each scanner. Intra-session and inter-session CoVs were compared between the scanners using a paired t-test. A P value of less than 0.05 was considered statistically significant.
We also calculated inter-scanner CoV by pairing runs between scanners.
RESULTS
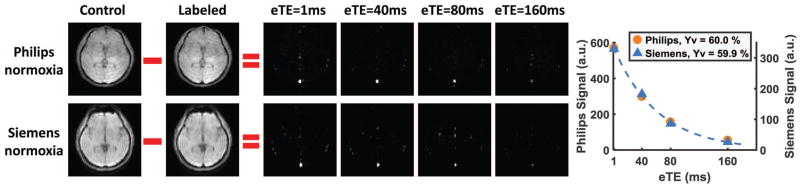
Figure 2 shows TRUST MRI data from a representative subject, including both Philips and Siemens results under normoxic state. The subtraction of control and labeled images revealed a strong venous signal, the intensity of which decays with effective echo time (eTE). The plots on the far right display quantitative signal values in the sagittal sinus as a function of eTE. Representative data of the same subject under hyperoxic state are shown in Supporting Figure S1. Table 1 summarizes the measured Yv across all participants. The values of Yv are in excellent agreement with previous reports under similar physiological states (19,26,34).
Figure 2.
Representative data from one participant under normoxia. Both Philips and Siemens data are shown. The control and labeled images are only shown for eTE=1ms. Difference images at all 4 eTEs are shown. Strong venous signals in the difference images can be seen. The plots on the right show averaged signal intensities within the SSS as a function of eTE, as well as their monoexponential fitting. The Yv values converted from the T2 are also shown.
Table 1.
Summary of Yv and εYv results from all participants (Mean±SE, N=10).
| Normoxia | Hyperoxia | |||
|---|---|---|---|---|
|
|
|
|||
| Philips | Siemens | Philips | Siemens | |
| Yv (%) | 59.2±1.1 | 60.0±0.9 | 73.8±1.3 | 75.2±1.4 |
| εYv (%) | 0.5±0.05 | 0.7±0.1 | 0.5±0.1 | 0.8±0.1 |
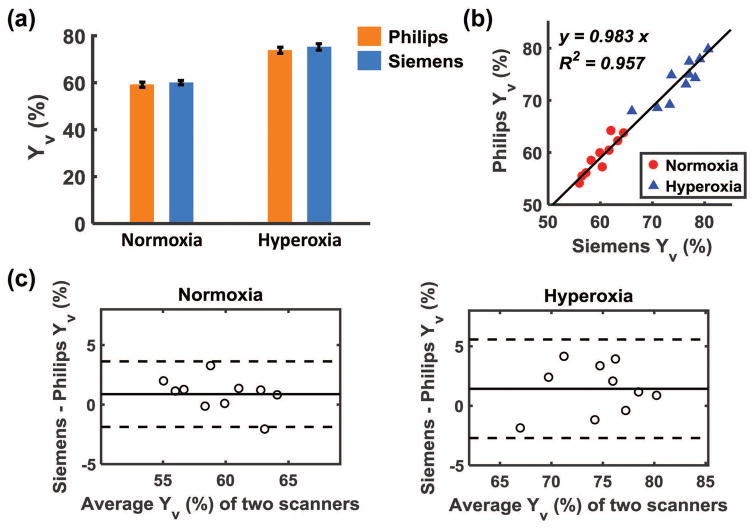
Figure 3a shows a comparison of Yv values measured on Philips and Siemens scanners, to examine whether Yv obtained from these two scanners manifests a systematic difference. There was not a significant difference between Philips and Siemens measurements under either normoxic or hyperoxic state, suggesting that both measures have comparable accuracy. Figure 3b shows a scatter plot of Yv obtained from the two scanners across subjects, with normoxic data shown in red and hyperoxic data in blue. It can be seen that there was a strong correlation between these measures under each physiologic state as well as when studied together (P<0.001 for all correlation coefficients). Figure 3c shows Bland-Altman plots comparing Philips and Siemens Yv measurements under normoxia and hyperoxia, demonstrating consistency between the two platforms. The results shown in Figure 3 are based on data after EtCO2 correction, to separate scanner differences from physiological fluctuations. Results from uncorrected data are shown in Supporting Figure S2, which are qualitatively similar to the corrected results although the inter-scanner correlation becomes weaker.
Figure 3.
Comparison of Yv values measured on Philips and Siemens scanners. (a) Yv from both scanners under normoxia and hyperoxia conditions. (b) scatter plot of Yv measured on the two scanners for each subject. Each dot represents data from one subject under normoxia (red circle) or hyperoxia (blue triangle). The solid line indicates the fitted linear regression curve. (c) Bland-Altman plots comparing Yv measurements on Philips and Siemens scanners under normoxia and hyperoxia, respectively. The solid line indicates the average difference between Siemens and Philips measurements. The dashed lines indicate the 95% confidence interval.
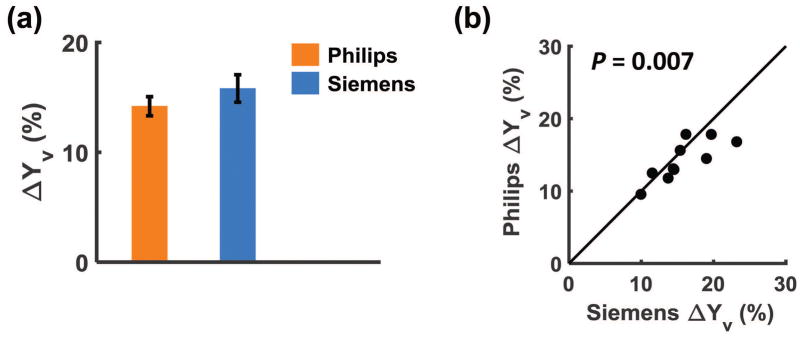
Figure 4a shows Yv changes associated with hyperoxia challenge. ΔYv was found to be 14.2±0.9% (P<0.001) on Philips and 15.8±1.3% (P<0.001) on Siemens, showing no significant difference between scanners. Figure 4b shows a scatter plot of Yv changes of each individual, revealing a significant correlation (P=0.007) between results measured on the two scanners. Results of uncorrected data are shown in Supporting Table S1.
Figure 4.
(a) Yv changes associated with hyperoxia challenge. Measurements on both scanners revealed a significant increase in oxygenation. But no significant difference was seen between Philips and Siemens scanners. (b) scatter plot of Yv changes of each individual. The solid line indicates the identity line.
We also evaluated intra-session, inter-session, and inter-scanner CoV of Yv on the Philips and Siemens scanners. The measured intra-session CoV of 1.35±0.28% on Philips and 2.19±0.30% on Siemens are in good agreement with a previous single-scanner study that reported a CoV of 1.88% (37). The inter-session CoV of 1.69±0.27% on Philips and 2.76±0.44% on Siemens observed in the present study are smaller than that reported in the previous study, which reported a value of 5.1% (37). This is likely because the sessions in Liu et al. (37) were performed on different days whereas those in the present study were done on the same day. Intra-session and inter-session CoV on the Siemens scanner were slightly higher than the respective values on the Philips, although the differences were not statistically significant. The inter-scanner CoV was found to be 2.57±0.28%. Results of uncorrected data are shown in Supporting Table S1.
εYv, which is an index of the estimation uncertainty in the monoexponential fitting, is summarized in Table 1. It was found that, for both normoxic and hyperoxic conditions, TRUST data from the Siemens scanner had a significantly higher εYv than that on the Philips (P<0.01). We attributed this difference to the longer TE used on the Siemens scanner (7ms), compared to that on the Philips (4ms).
To further elucidate the reason for the difference in εYv, and to some extent CoV, across scanners, we conducted a supplemental study (N=6) after further matching the TE on Siemens to that on Philips (i.e. TE=4ms on both scanners). εYv from Philips and Siemens scanners were found to be 0.42±0.05% and 0.40±0.03%, respectively. Intra-session CoV was 1.63±0.32% and 1.43±0.34% for Philips and Siemens scanners, respectively. No difference in εYv or intra-session CoV were found between scanners. These findings support the notion that shorter TE helps improve the precision of TRUST Yv measurement.
DISCUSSION
The present work performed a harmonization of TRUST MRI across two major vendors, in an effort to facilitate potential dissemination and provide benchmarks for future multi-site studies. To our knowledge, this is the first report of cross-vendor studies of MRI-based oximetry. Through matching of pulse sequence details across platforms, we showed that Yv measured on Philips and Siemens MRI systems can provide highly compatible results, with strong correlation across scanners. We further showed that Yv measured on both systems can reliably detect oxygenation changes associated with physiological challenges. Assessment of test-retest reproducibility revealed that the CoV values were less than 3% regardless of the measurement conditions (Philips or Siemens scanners, normoxic or hyperoxic physiological states, intra-session, inter-session, or inter-scanner comparisons).
Brain oximetry provides a basis for the measurement of brain oxygenation consumption and energy metabolism, and offers a potential window into non-invasive assessment of brain function and dysfunction. Compared to the conventional blood-oxygenation-level-dependent functional techniques, oximetry and metabolism techniques are more quantitative and are directly related to brain physiology, and thus have gained increasing attention in recent literature. Indeed, novel MRI oximetry and metabolism techniques have found clinical utility in a large number of diseases, including Alzheimer’s disease (6), multiple sclerosis (5), moyamoya (28), sickle cell disease (28,29), hepatic encephalopathy (30), end-stage renal disease (32), and cognitive aging (2). However, as some of these promising applications are expanded into further testing of their value as a clinically useful biomarker, multi-site/multi-vendor studies or trials represent a critical step in this path. Our previous work has demonstrated the reliability of brain oximetry across different sites on a single vendor (38). The present work suggests that across multiple vendors the technique also has an excellent reproducibility.
This work demonstrated that the inter-session CoV of Yv measurement is 2.2% on average. This CoV range is larger than some of the anatomical MRI measurements such as brain volumetry which has a CoV of less than 1% for whole brain, gray and white matter (39), and is also higher than that in diffusion tensor imaging (DTI) measurements such as whole brain fractional anisotropy, which has a CoV of about 1% (40–42). However, it should be noted that the inter-session CoV of TRUST is considerably lower than other common physiological MRI measurements such as arterial spin labeling (ASL), which has a CoV of approximately 10% (43–45). The inter-scanner CoV of TRUST is about 2.6%, and is better than that of brain volumetry which was found to be around 4% for whole brain measures (39). This level of inter-scanner CoV of TRUST is comparable to that of DTI measurements (42), but is drastically lower than that of ASL (~15% (43)) or functional MRI (~20% (46)).
The confidence interval estimate, εYv, in our data fitting provides an assessment of the reliability of the data. εYv was found to be less than 1% on both scanners. This level of high fitting confidence allowed us to reliably detect oxygenation changes induced by hyperoxia challenge on an individual level, i.e. a Yv increase was detected in all subjects. A confidence interval of <1% is also remarkably less than disease-related changes of approximately 9% in stroke (47), 7% in moyamoya (28), 6–11% in sickle cell (28,29), 5% in mountain disease (31), 6% in multiple sclerosis (5), 6% in Alzheimer (6), 10% in hepatic encephalopathy (30), and 11% in end-stage renal disease (32). Therefore, this level of precision may allow sensitive detection of disease-related abnormalities on an individual level, a step beyond the traditional group-level analysis.
Harmonization of MRI protocols across vendors is a topic of interest in virtually all MRI techniques. Due to the differences in hardware specifications and software programming environments across scanners, it is not possible to match every component between two sequences. In our experience, the most important components for TRUST MRI are the pulse type, width, and power of the T2-preparation RF pulses. In this study, T2-preparation on both scanners used composite block pulses with each 90° element having a power of 13μT and a width of 0.44ms. This level of B1 power is expected to be feasible on most clinical 3T systems. Should a researcher use a pulse of different width, a correction on the measured T2 is recommended before using the calibration plot to estimate Yv, as a longer T2-preparation pulse is known to yield longer T2 estimation (25,48). The shape and power of the labeling pulses are also of some significance, as they determine whether the blood is effectively labeled. Poorly matched labeling RF pulses could result in a difference in contrast-to-noise ratio of the TRUST signal. Additionally, as shown in the present study, the acquisition module is relevant and the TE of the EPI echo train should be kept minimal, e.g. 4ms, by using high parallel imaging factors as well as aggressive partial Fourier factor. Other components such as the RF shape of the post-saturation pulses and the zeroth-moment of the dephasing gradients are less critical in our experience.
The present study has primarily focused on harmonizing pulse sequences for T2 measurement, but has not considered the calibration model that is used to convert T2 to oxygenation (27), which is also an important topic in T2-based MR oximetry. The calibration model is mainly affected by hematocrit and cell morphometric factors such as size, shape, and aggregation pattern. In particular, when applying the method to patients with abnormally low hematocrit, e.g. anemic patients, or atypical red blood cells, e.g. sickle cell patients or neonatal patients, caution is needed to select the most appropriate calibration model, which is an active area of research (49–51).
There are a few limitations in this study. First, due to a lack of other vendors’ MRI scanners (such as General Electric, Toshiba, etc.) in the authors’ institution, only Philips and Siemens scanners were included in the present study. For better scalability of the technique, our future efforts will be directed toward the implementation of a matched TRUST sequence on other MRI vendors. Second, to separate the effect of scanner differences from physiological fluctuations, we used a method to correct the EtCO2 effect on Yv. This may complicate the data interpretation. Therefore, we have also shown uncorrected Yv results in the supporting figures and tables.
CONCLUSION
The present work evaluated the measurement of venous oxygenation using TRUST MRI across two major vendors. The results demonstrated that the estimated Yv values were compatible across scanners, with excellent test-retest reproducibility for intra-session, inter-session, and inter-scanner comparisons. The sequence was able to detect hyperoxia-induced Yv changes on each subject for both scanners. These findings suggest that TRUST MRI has the potential to be used as a non-invasive imaging biomarker in brain diseases.
Supplementary Material
Supporting Figure S1. Representative data from one participant under hyperoxia. Both Philips and Siemens data are shown. The control and labeled images are only shown for eTE=1ms. Difference images at all 4 eTEs are shown. Strong venous signals in the difference images can be seen. The plots on the right show averaged signal intensities within the SSS as a function of eTE, as well as their monoexponential fitting. The Yv values converted from the T2 are also shown.
Supporting Figure S2. Comparison of Yv values measured on Philips and Siemens scanners, without EtCO2 correction. (a) Yv from both scanners under normoxia and hyperoxia conditions. (b) scatter plot of Yv measured on the two scanners for each subject. Each dot represents data from one subject under normoxia (red circle) or hyperoxia (blue triangle). The solid line indicates the fitted linear regression curve. (c) Bland-Altman plots comparing Yv measurements on Philips and Siemens scanners under normoxia and hyperoxia, respectively. The solid line indicates the average difference between Siemens and Philips measurements. The dashed lines indicate the 95% confidence interval.
Supporting Table S1: Summary of ΔYv, intra-session, inter-session and inter-scanner CoVs, without EtCO2 correction.
Supporting Table S2: Summary of EtCO2 and EtO2 from all participants (Mean±SE, N=10). There was no difference in EtCO2 or EtO2 between scanners or sessions (P>0.05). Hyperoxia increased EtO2 (P<0.001) as expected, while maintaining the EtCO2 values (P=0.68). The absence of EtCO2 change was attributed to the addition of 2.5% CO2 into the hyperoxic gas mixture to offset the hyperventilation effect.
Acknowledgments
Grant Sponsors: NIH R01 MH084021, NIH R01 NS067015, NIH R01 AG042753, NIH R01 AG047972, NIH R21 NS095342, NIH R21 NS085634, and NIH P41 EB015909.
References
- 1.Xu F, Ge Y, Lu H. Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (CMRO2) by MRI. Magn Reson Med. 2009;62:141–148. doi: 10.1002/mrm.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng SL, Dumas JA, Park DC, Liu P, Filbey FM, McAdams CJ, Pinkham AE, Adinoff B, Zhang R, Lu H. Age-related increase of resting metabolic rate in the human brain. Neuroimage. 2014;98:176–183. doi: 10.1016/j.neuroimage.2014.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21:1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchal G, Rioux P, Petit-Taboue MC, Sette G, Travere JM, Le Poec C, Courtheoux P, Derlon JM, Baron JC. Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol. 1992;49:1013–1020. doi: 10.1001/archneur.1992.00530340029014. [DOI] [PubMed] [Google Scholar]
- 5.Ge Y, Zhang Z, Lu H, Tang L, Jaggi H, Herbert J, Babb JS, Rusinek H, Grossman RI. Characterizing brain oxygen metabolism in patients with multiple sclerosis with T2-relaxation-under-spin-tagging MRI. J Cereb Blood Flow Metab. 2012;32:403–412. doi: 10.1038/jcbfm.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas BP, Sheng M, Tseng BY, Tarumi T, Martin-Cook K, Womack KB, Cullum MC, Levine BD, Zhang R, Lu H. Reduced global brain metabolism but maintained vascular function in amnestic mild cognitive impairment. J Cereb Blood Flow Metab. 2017;37:1508–1516. doi: 10.1177/0271678X16658662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishii K, Kitagaki H, Kono M, Mori E. Decreased medial temporal oxygen metabolism in Alzheimer’s disease shown by PET. J Nucl Med. 1996;37:1159–1165. [PubMed] [Google Scholar]
- 8.Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25:177–187. [PubMed] [Google Scholar]
- 9.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan AP, Bilgic B, Gagnon L, Witzel T, Bhat H, Rosen BR, Adalsteinsson E. Quantitative oxygenation venography from MRI phase. Magn Reson Med. 2014;72:149–159. doi: 10.1002/mrm.24918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan AP, Benner T, Bolar DS, Rosen BR, Adalsteinsson E. Phase-based regional oxygen metabolism (PROM) using MRI. Magn Reson Med. 2012;67:669–678. doi: 10.1002/mrm.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haacke EM, Lai S, Reichenbach JR, Kuppusamy K, Hoogenraad FGC, Takeichi H, Lin WL. In vivo measurement of blood oxygen saturation using magnetic resonance imaging: A direct validation of the blood oxygen level-dependent concept in functional brain imaging. Human Brain Mapping. 1997;5:341–346. doi: 10.1002/(SICI)1097-0193(1997)5:5<341::AID-HBM2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 13.He X, Yablonskiy DA. Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn Reson Med. 2007;57:115–126. doi: 10.1002/mrm.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain V, Langham MC, Wehrli FW. MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab. 2010;30:1598–1607. doi: 10.1038/jcbfm.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Seara MA, Techawiboonwong A, Detre JA, Wehrli FW. MR susceptometry for measuring global brain oxygen extraction. Magn Reson Med. 2006;55:967–973. doi: 10.1002/mrm.20892. [DOI] [PubMed] [Google Scholar]
- 16.Xu B, Liu T, Spincemaille P, Prince M, Wang Y. Flow compensated quantitative susceptibility mapping for venous oxygenation imaging. Magn Reson Med. 2014;72:438–445. doi: 10.1002/mrm.24937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao D, Li Y, Liu P, Peng SL, Pillai JJ, Lu H. Three-dimensional mapping of brain venous oxygenation using R2* oximetry. Magn Reson Med. 2017 doi: 10.1002/mrm.26763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.An H, Lin W. Quantitative measurements of cerebral blood oxygen saturation using magnetic resonance imaging. J Cereb Blood Flow Metab. 2000;20:1225–1236. doi: 10.1097/00004647-200008000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med. 2008;60:357–363. doi: 10.1002/mrm.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin Q, Grgac K, van Zijl PC. Determination of whole-brain oxygen extraction fractions by fast measurement of blood T2 in the jugular vein. Magn Reson Med. 2011;65:471–479. doi: 10.1002/mrm.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oja JM, Gillen JS, Kauppinen RA, Kraut M, van Zijl PC. Determination of oxygen extraction ratios by magnetic resonance imaging. J Cereb Blood Flow Metab. 1999;19:1289–1295. doi: 10.1097/00004647-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Guo J, Wong EC. Venous oxygenation mapping using velocity-selective excitation and arterial nulling. Magn Reson Med. 2012;68:1458–1471. doi: 10.1002/mrm.24145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolar DS, Rosen BR, Sorensen AG, Adalsteinsson E. QUantitative Imaging of eXtraction of oxygen and TIssue consumption (QUIXOTIC) using venular-targeted velocity-selective spin labeling. Magn Reson Med. 2011;66:1550–1562. doi: 10.1002/mrm.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnamurthy LC, Liu P, Ge Y, Lu H. Vessel-specific quantification of blood oxygenation with T2-relaxation-under-phase-contrast MRI. Magn Reson Med. 2014;71:978–989. doi: 10.1002/mrm.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain V, Magland J, Langham M, Wehrli FW. High temporal resolution in vivo blood oximetry via projection-based T2 measurement. Magn Reson Med. 2013;70:785–790. doi: 10.1002/mrm.24519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu F, Uh J, Liu P, Lu H. On improving the speed and reliability of T2-relaxation-under-spin-tagging (TRUST) MRI. Magn Reson Med. 2012;68:198–204. doi: 10.1002/mrm.23207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H, Xu F, Grgac K, Liu P, Qin Q, van Zijl P. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn Reson Med. 2012;67:42–49. doi: 10.1002/mrm.22970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watchmaker JM, Juttukonda MR, Davis LT, et al. Hemodynamic mechanisms underlying elevated oxygen extraction fraction (OEF) in moyamoya and sickle cell anemia patients. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16682509:271678X16682509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan LC, Gindville MC, Scott AO, et al. Non-invasive imaging of oxygen extraction fraction in adults with sickle cell anaemia. Brain. 2016;139:738–750. doi: 10.1093/brain/awv397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng G, Lu H, Yu W, et al. Severity-specific alterations in CBF, OEF and CMRO2 in cirrhotic patients with hepatic encephalopathy. Eur Radiol. 2017 doi: 10.1007/s00330-017-4809-9. [DOI] [PubMed] [Google Scholar]
- 31.Smith ZM, Krizay E, Guo J, Shin DD, Scadeng M, Dubowitz DJ. Sustained high-altitude hypoxia increases cerebral oxygen metabolism. J Appl Physiol. 2013;114:11–18. doi: 10.1152/japplphysiol.00703.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng G, Wen J, Lu H, et al. Elevated global cerebral blood flow, oxygen extraction fraction and unchanged metabolic rate of oxygen in young adults with end-stage renal disease: an MRI study. Eur Radiol. 2016;26:1732–1741. doi: 10.1007/s00330-015-3968-9. [DOI] [PubMed] [Google Scholar]
- 33.van Zijl PC, Eleff SM, Ulatowski JA, Oja JM, Ulug AM, Traystman RJ, Kauppinen RA. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nat Med. 1998;4:159–167. doi: 10.1038/nm0298-159. [DOI] [PubMed] [Google Scholar]
- 34.Xu F, Liu P, Pascual JM, Xiao G, Lu H. Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. J Cereb Blood Flow Metab. 2012;32:1909–1918. doi: 10.1038/jcbfm.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng SL, Ravi H, Sheng M, Thomas BP, Lu H. Searching for a truly “iso-metabolic” gas challenge in physiological MRI. J Cereb Blood Flow Metab. 2017;37:715–725. doi: 10.1177/0271678X16638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu F, Uh J, Brier MR, Hart J, Jr, Yezhuvath US, Gu H, Yang Y, Lu H. The influence of carbon dioxide on brain activity and metabolism in conscious humans. J Cereb Blood Flow Metab. 2011;31:58–67. doi: 10.1038/jcbfm.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu P, Xu F, Lu H. Test-retest reproducibility of a rapid method to measure brain oxygen metabolism. Magn Reson Med. 2013;69:675–681. doi: 10.1002/mrm.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu P, Dimitrov I, Andrews T, et al. Multisite evaluations of a T2-relaxation-under-spin-tagging (TRUST) MRI technique to measure brain oxygenation. Magn Reson Med. 2016;75:680–687. doi: 10.1002/mrm.25627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huppertz HJ, Kroll-Seger J, Kloppel S, Ganz RE, Kassubek J. Intra- and interscanner variability of automated voxel-based volumetry based on a 3D probabilistic atlas of human cerebral structures. Neuroimage. 2010;49:2216–2224. doi: 10.1016/j.neuroimage.2009.10.066. [DOI] [PubMed] [Google Scholar]
- 40.Vollmar C, O’Muircheartaigh J, Barker GJ, Symms MR, Thompson P, Kumari V, Duncan JS, Richardson MP, Koepp MJ. Identical, but not the same: intra-site and inter-site reproducibility of fractional anisotropy measures on two 3. 0T scanners. Neuroimage. 2010;51:1384–1394. doi: 10.1016/j.neuroimage.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heiervang E, Behrens TE, Mackay CE, Robson MD, Johansen-Berg H. Between session reproducibility and between subject variability of diffusion MR and tractography measures. Neuroimage. 2006;33:867–877. doi: 10.1016/j.neuroimage.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 42.Magnotta VA, Matsui JT, Liu D, et al. Multicenter reliability of diffusion tensor imaging. Brain Connect. 2012;2:345–355. doi: 10.1089/brain.2012.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mutsaerts HJ, van Osch MJ, Zelaya FO, et al. Multi-vendor reliability of arterial spin labeling perfusion MRI using a near-identical sequence: implications for multi-center studies. Neuroimage. 2015;113:143–152. doi: 10.1016/j.neuroimage.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 44.Petersen ET, Mouridsen K, Golay X. The QUASAR reproducibility study, Part II: Results from a multi-center Arterial Spin Labeling test-retest study. Neuroimage. 2010;49:104–113. doi: 10.1016/j.neuroimage.2009.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Wang DJ, Detre JA. Test-retest reliability of arterial spin labeling with common labeling strategies. J Magn Reson Imaging. 2011;33:940–949. doi: 10.1002/jmri.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman L, Glover GH, Fbirn C. Reducing interscanner variability of activation in a multicenter fMRI study: controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. Neuroimage. 2006;33:471–481. doi: 10.1016/j.neuroimage.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Yamauchi H, Fukuyama H, Nagahama Y, Nabatame H, Nakamura K, Yamamoto Y, Yonekura Y, Konishi J, Kimura J. Evidence of misery perfusion and risk for recurrent stroke in major cerebral arterial occlusive diseases from PET. J Neurol Neurosurg Psychiatry. 1996;61:18–25. doi: 10.1136/jnnp.61.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foltz WD, Stainsby JA, Wright GA. T2 accuracy on a whole-body imager. Magn Reson Med. 1997;38:759–768. doi: 10.1002/mrm.1910380512. [DOI] [PubMed] [Google Scholar]
- 49.Bush A, Borzage M, Detterich J, Kato RM, Meiselman HJ, Coates T, Wood JC. Empirical model of human blood transverse relaxation at 3 T improves MRI T2 oximetry. Magn Reson Med. 2017;77:2364–2371. doi: 10.1002/mrm.26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bush A, Coates T, Meiselman HJ, Wood JC. Can We Trust TRUST Venous Oximetry in Sickle Cell Disease?. Proceedings of the 25th Annual Meeting of ISMRM; Honolulu, Hawaii, USA. 2017. p. 1898. [Google Scholar]
- 51.Liu P, Chalak LF, Krishnamurthy LC, Mir I, Peng SL, Huang H, Lu H. T1 and T2 values of human neonatal blood at 3 Tesla: Dependence on hematocrit, oxygenation, and temperature. Magn Reson Med. 2016;75:1730–1735. doi: 10.1002/mrm.25775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure S1. Representative data from one participant under hyperoxia. Both Philips and Siemens data are shown. The control and labeled images are only shown for eTE=1ms. Difference images at all 4 eTEs are shown. Strong venous signals in the difference images can be seen. The plots on the right show averaged signal intensities within the SSS as a function of eTE, as well as their monoexponential fitting. The Yv values converted from the T2 are also shown.
Supporting Figure S2. Comparison of Yv values measured on Philips and Siemens scanners, without EtCO2 correction. (a) Yv from both scanners under normoxia and hyperoxia conditions. (b) scatter plot of Yv measured on the two scanners for each subject. Each dot represents data from one subject under normoxia (red circle) or hyperoxia (blue triangle). The solid line indicates the fitted linear regression curve. (c) Bland-Altman plots comparing Yv measurements on Philips and Siemens scanners under normoxia and hyperoxia, respectively. The solid line indicates the average difference between Siemens and Philips measurements. The dashed lines indicate the 95% confidence interval.
Supporting Table S1: Summary of ΔYv, intra-session, inter-session and inter-scanner CoVs, without EtCO2 correction.
Supporting Table S2: Summary of EtCO2 and EtO2 from all participants (Mean±SE, N=10). There was no difference in EtCO2 or EtO2 between scanners or sessions (P>0.05). Hyperoxia increased EtO2 (P<0.001) as expected, while maintaining the EtCO2 values (P=0.68). The absence of EtCO2 change was attributed to the addition of 2.5% CO2 into the hyperoxic gas mixture to offset the hyperventilation effect.