Abstract
Purpose
The purpose of this study was to estimate fetal O2 delivery rate in vivo across a range of gestational ages (GA). Toward this, a calibration equation for T2-based oximetry was derived.
Methods
Umbilical cord blood of varying hematocrit (Hct) and oxygen saturation (HbO2) levels was prepared and T2 measured using a T2-prepared balanced steady-state free precession (T2-bSSFP) sequence at 1.5T. The relationship between blood R2=1/T2, HbO2 and Hct was established based on the model R2 = (1 − Hct)R2,plasma + Hct R2,RBC + k · Hct · (1 − Hct) · (1 − HbO2)2. Experimental R2, HbO2 and Hct levels were fit to the model yielding values of k, R2,plasma and R2,RBC (R2 of plasma and erythrocytes). Umbilical vein (UV) T2 measured in vivo was then converted to HbO2 yielding, together with blood flow rate (BFR), fetal O2 delivery rate in 22 pregnancies (GA 30±3 weeks).
Results
Constants derived from the fit (R2 = 0.94) were: k=83.1s−1, R2,plasma = 1.1s−1, R2,RBC = 12.9s−1. R2,RBC and k were found to be larger than those obtained for adult blood, likely due to differences in dominant hemoglobin type. Data suggest that the use of adult blood calibration could entail errors up 10% in fetal blood HbO2. Average UV BFR (89.5±17.2 mL/min/kg), HbO2 (84±7%,) and fetal O2 delivery rate (15.1±3.8 mL O2/min/kg) were independent of GA. Fetal O2 delivery rate agreed well with results obtained with invasive methods at term.
Conclusion
The present work describes strategies for measuring UV BFR and HbO2 in vivo and estimates fetal O2 delivery rate noninvasively with quantitative MRI during the second and third trimesters of pregnancy.
Keywords: fetal oxygen delivery rate, T2-based oximetry, fetal blood, oxygen saturation
Introduction
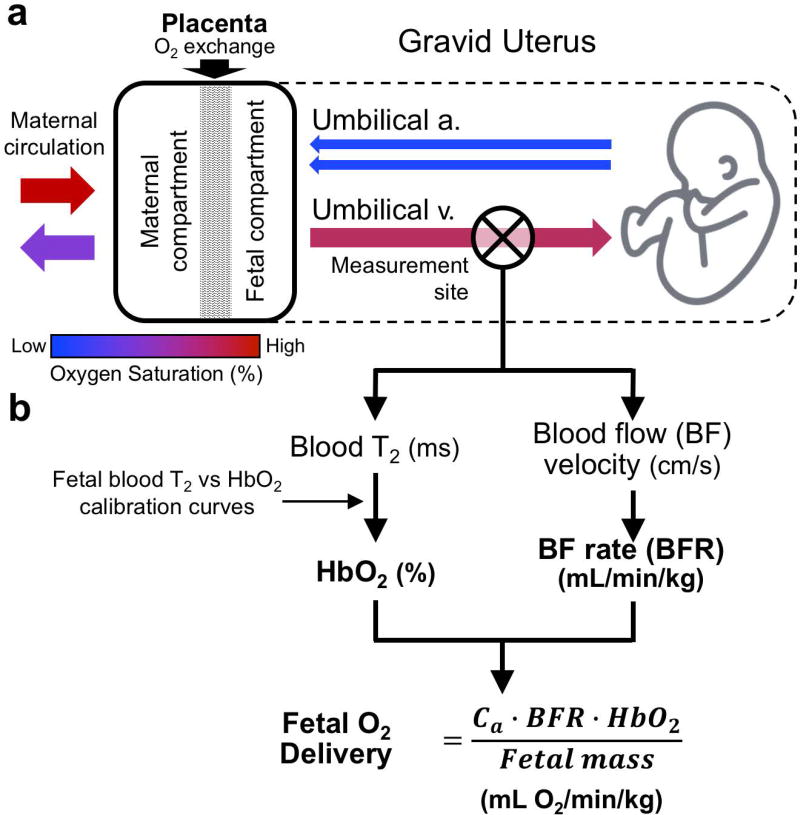
The placenta is a transient organ present during pregnancy. The main function of the placenta is to provide a nurturing environment for fetal development and sustainability. One of the chief functions of the placenta is to transport O2 between the maternal and fetal circulations (1), which is established at the end of the first trimester (2). However, in this process, maternal and fetal blood never mix; fetal capillaries are embedded inside chorionic villi, a structure analogous to the alveoli in the lung. Highly oxygenated maternal blood enters the intervillous space in the placenta through the spiral arteries. Oxygen is then transferred to the fetal circulation by diffusion and delivered to the fetus via the umbilical vein (UV). Hemoglobin O2 saturation (HbO2) at the UV is only on the order of 70–80% (3,4), low HbO2 is compensated for by high fetal cardiac output. After fetal O2 extraction, deoxygenated blood (HbO2 40–60%) (5,6) returns to the placenta through the umbilical arteries, where carbon dioxide diffuses into the maternal compartment of the placenta and are then removed through the maternal venous system (Figure 1). Hence, the proper establishment of the utero-placental and placental-fetal circulations is critical to the viability of the fetus.
Figure 1.
(a) Schematic of oxygen transport between maternal and fetal circulation. Maternal arterial circulation delivers highly oxygenated blood to the placenta, however, maternal and fetal blood are never in direct contact. O2 diffuses from the maternal to the fetal compartment and is delivered to the fetus via the umbilical vein (UV). After O2 extraction deoxygenated blood returns to the placenta via umbilical arteries, where carbon dioxide and waste products diffuse to the maternal compartment and are removed by the maternal venous system. Fetal O2 delivery rate may be used as means to evaluate placental function and (b) is estimated as the product of the oxygen carrying capacity of hemoglobin (Ca), hemoglobin (Hb) concentration, blood flow rate (BFR) and oxygen saturation (HbO2) at the UV, normalized by fetal mass. All of the above parameters can be determined via quantitative MRI methods.
Placental dysfunction is widely accepted as the cause of common adverse pregnancy outcomes. However, the etiology of abnormal placental development remains unknown. Importantly, clinically available tools are inadequate for evaluating placental function. Therefore, methods that allow quantification of O2 delivery rate to the fetus throughout pregnancy in vivo would be of great interest to maternal-fetal medicine as they could provide new insight into placental and fetal development. Fetal O2 delivery rate in physiological units normalized to fetal mass (mL O2/min/kg), is defined as:
| [1] |
where Ca is the oxygen carrying capacity of fetal hemoglobin in units of mL O2/mL (Ca = 1.33 mL O2/g Hb · [Hb], where [Hb] is the blood hemoglobin concentration in g/mL blood), BFR is the umbilical vein (UV) blood flow rate (in mL/min) and HbO2 is oxygen saturation measured at the UV. Quantitative MRI allows for the estimation of BFR and fetal mass from widely used MR techniques such as phase-contrast MRI (PC-MRI) and structural MR images (7,8). Further, HbO2 can be estimated via T2-based oximetry, which requires a calibration equation to convert blood T2 values to HbO2 (9).
The present authors have previously derived a calibration equation for human adult blood using a T2-prepared bSSFP (T2-bSSFP) sequence (10). However, due to differences in the biophysical (11), rheological (12) and biochemical properties between fetal and adult hemoglobin, the calibration equation derived from adult blood may not be accurate for fetal blood. Therefore, the purpose of this study was twofold: first to measure T2 of human fetal blood samples of varying Hct for a wide range of HbO2 levels using a T2-bSSFP sequence to derive a calibration equation, and second, to estimate fetal O2 delivery rate in vivo across a range of gestational ages (GA) with quantitative MRI.
Methods
Ex Vivo Blood Sample Preparation
Fetal whole blood (average 30±18mL, range 10–70mL) was collected from the umbilical cord of 13 fetuses at term (GA>37 weeks), yielding a total of 77 3mL fetal blood samples. Upon delivery, the umbilical cord was clamped on one end and dissected from the newborn. Subsequently, the placenta was immediately taken to a laboratory and placed on a container that was raised approximately 50cm. The container had a perforation at the base through which the umbilical cord was inserted. Removal of the clamp then allowed blood to flow and be collected into open 7mL Vacutainer glass tubes, each containing 12mg of K3 EDTA (Becton, Dickinson and Company, New Jersey, USA). Tubes were capped and inverted to mix with the EDTA agent to prevent clotting. Lastly, the specimens were stored at 4°C before being transported to the hematology laboratory for preparation (to achieve the desired HbO2 and Hct levels). The Institutional Review Board of the University of Pennsylvania approved the study, and all subjects gave oral and written informed consent.
Both, Hct and HbO2 levels of the blood samples, were varied as described in (10). Briefly, an extended range of Hct values was achieved by separating plasma and RBC via centrifugation. Both components were then redistributed, resulting in samples of various Hct. Subsequently, HbO2 was varied by exposing samples to N2 gas at 37°C for different durations. Samples were taken every 10 minutes with a 3mL syringe, HbO2 and hemoglobin (Hb) concentration were measured with a clinical blood gas analyzer (ABL 700 series; Radiometer, Copenhagen, Denmark). Syringes were sealed using a vinyl plastic putty (Critoseal; Fisher Scientific, Pennsylvania, USA) and parafilm. Complete blood count was performed (HEMAVET HV950FS, Drew Scientific, Florida, USA) to calculate the Hct to Hb ratio for each Hct preparation. Sealed syringes were kept at 37°C and placed in a cylindrical container filled with distilled water at the same temperature for the MRI. All samples were prepared and scanned within 24hrs of delivery.
Hemoglobin Composition of Fetal Blood
Clarified hemolysates were prepared by hypotonic lysis followed by centrifugation using standard methods. Approximately 20 uL of lysates from experimental samples were analyzed for identification of fetal and adult hemoglobin levels by cation-exchange high-performance liquid chromatography (HPLC) using previously described methods, with slight modifications (13–16). A Hitachi D-7000 Series (Hitachi Instruments, Inc., San Jose, CA), and a weak cation-exchange column (Poly CAT A: 35 mm × 4.6 mm, Poly LC, Inc., Columbia, MD) were used. Hemoglobin isotype peaks were eluted with a linear gradient of phase B from 0% to 80% at A410nm (Mobile Phase A: 20 mM Bis-Tris, 2 mM KCN, pH 6.95; Phase B:20 mM Bis-Tris, 2 mM KCN, 0.2 M sodium chloride, pH 6.55). Clarified lysates from normal human red blood cells (RBCs, exclusively Hb A), as well as a commercial standard containing fetal and adult hemoglobin, together with hemoglobin types S and C (FASC reference material, Trinity Biotech USA Inc., Jamestown, NY, USA) were utilized as reference isotypes (Supporting Figure S1). Fetal hemoglobin fraction was estimated as the ratio of the amplitude of the fetal hemoglobin peak to the total hemoglobin, defined at the sum of fetal and adult hemoglobin as all samples were of typical development fetuses and hemoglobin types S and C were not present.
Ex vivo MRI Experiments
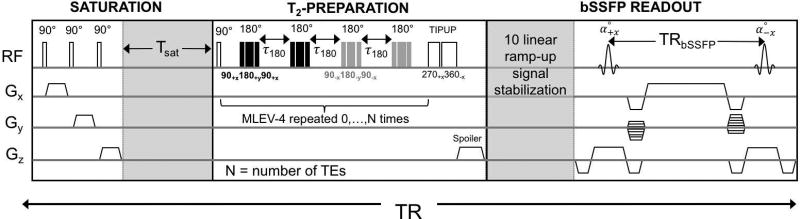
Experiments were conducted at 1.5T (Siemens Avanto, Erlangen, Germany) with a 10-channel head coil. The T2-bSSFP sequence and data reconstruction have been previously described in (10). Briefly, this sequence consists of three parts: spin saturation, T2-preparation and bSSFP readout (Figure 2). The magnetization is nulled by a saturation pulse and allowed to partially recover during the Tsat period prior to each T2-preparation, which consist of non-selective 90° RECT excitation pulse, followed by n MLEV-4 type refocusing pulses (n = 0, 1, 2, 3, 4) with inter-refocusing pulse interval τ180=12ms, each followed by a composite tip-up (270x360−x) pulse. Lastly, bSSFP signal encoding is preceded by 10 linear ramp-up pulses for signal stabilization and 14 reference lines are collected for phase correction during partial-Fourier reconstruction in linear order.
Figure 2.
T2-prepared bSSFP sequence consisting of saturation pulses to null the magnetization after each T2-preparation, T2-preparation with composite refocusing pulses in MLEV-4 pattern repeated to achieve TE values of 0, 48, 96, 144 and 192ms at constant interpulse interval (τ180=12ms), and bSSFP encoding with linear ramp-up signal stabilization and partial Fourier sampling.
These methods were used to derive a calibration equation to relate fetal blood T2 to HbO2 and Hct. Imaging parameters: TR = 4400 ms, TSat = 4000 ms, bSSFP sequence parameters: TE/TR = 1.9/3.8 ms, field of view (FOV) = 160×160 mm2, acquisition matrix 128×94, voxel size = 1.25×1.25×5 mm3, T2-preparations TEs of 0, 48, 96, 144 and 192ms, flip angle (FA) = 60°, partial-Fourier acquisition and total scan duration = 22 s. T2 of the blood samples was extracted using a three-parameter fit to the equation S(t) = S0e−t/T2 + C, with C representing the steady-state amplitude of the bSSFP signal.
Relaxation Model and Analysis
The relationship between blood relaxation rate R2 (1/T2) and HbO2 was first established by Wright et al (9) who resorted to a semi-empirical rendition of the Luz-Meiboom equation for two-site chemical exchange (17):
| [2] |
Here, R2o is the relaxation rate of fully oxygenated blood and K represents the relaxivity. This model was subsequently expanded by van Zijl et al to incorporate the effect of Hct on blood R2o and K (18):
| [3] |
It can then be observed that R2o scales linearly with Hct (19,20), while the relaxivity K depends on both Hct and the empirical parameter k. The relaxation rates of blood R2 were plotted versus (1 − HbO2)2 and Hct, and the data fit to the model described by Equations [3] and [4] yielding constants k, R2,plasma, and R2,RBC.
In vivo MRI Studies
The purpose of these studies was to quantify fetal O2 delivery rate in the second and third trimester in vivo. Exclusion criteria included: known uterine fibroids, pre-gestational diabetes, connective tissue disorders affecting vascular development (e.g. lupus or vasculitis), body mass index greater than 35 before or at the start of pregnancy, known major structural fetal anomaly or genetic syndrome, and any history of oophorectomy. Toward this goal 30 pregnant women (GA: 30±3 weeks, range 23–35 weeks) were scanned using two 4-element body matrix coils at 1.5T (Siemens Avanto, Erlangen, Germany) in supine position. Fetal O2 delivery rate in physiological units (mL O2/min/kg) was obtained as the product of Ca of fetal hemoglobin, BFR and HbO2 with the latter two quantities measured at the UV, divided by fetal mass (Figure 1). Half-Fourier single-shot turbo spin-echo (HASTE) imaging with the following parameters was used to identify the umbilical cord: TR/TE = 405/60 ms, FOV 430×430 mm2, and voxel size = 1.7×1.7×4 mm3, bandwidth = 475 Hz/pixel.
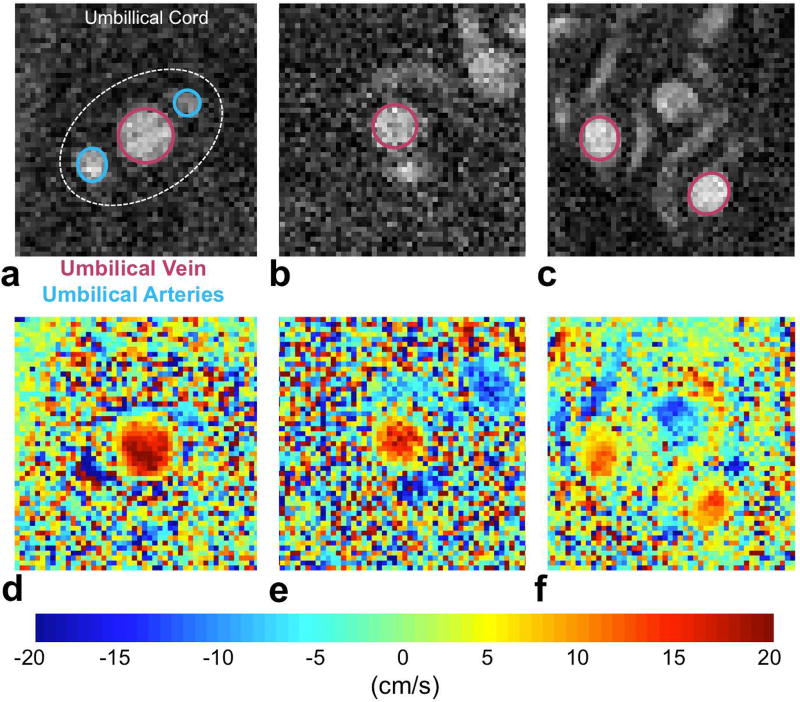
Blood flow velocity was measured using PC-MRI during a single breath hold with the following imaging parameters: VENC = 20cm/s, TR/TE = 10.6/6.5 ms, TE = 6.5 ms, FOV 200×200 mm2, acquisition matrix = 320×320, voxel size = 0.625×0.625×5 mm3, FA = 20°, bandwidth = 260 Hz/pixel, resulting in a total scan time of 9 s. In some instances, fetal motion during PC-MRI acquisition resulted in displacement of the umbilical cord in the scanning plane causing motion artifacts that could be observed on the magnitude and complex difference intensity images. In such case, PC-MRI was repeated multiple times to obtain two motion-artifact free datasets. Blood flow rate was then estimated as the product of flow velocity (averaged from two phase-contrast MRI datasets) and UV cross-sectional area. The flow velocity and cross-sectional area, used were calculated as the average from two artifact free datasets. The regions of interest (ROIs) of the UV were manually traced on the magnitude PC-image using Osirix (Pixmeo SARL, Bernex, Switzerland).
Umbilical vein HbO2 was estimated as described above for the in vitro experiments (10) using the following imaging parameters: TR = 3900 ms, TSat = 3000 ms, bSSFP TE/TR = 1.9/3.8 ms, FOV = 307×307 mm2, matrix size = 384×206 (partial Fourier), voxel size = 0.8×0.8×5 mm3, FA = 60°, number of acquisitions = 5, resulting in a total scan time of 1:40 mins. Therefore, T2-bSSFP data were acquired three times: at the prescribed plane and 8mm above and below. Imaging planes were chosen where the umbilical cord was abutting the fetus or where it could be visualized in at least three sequential localizer images. No motion correction was implemented. To account for displacement of the UV between T2-bSSFP images corresponding to specific echo times, the ROI used to estimate the average signal at each TE and repetition was manually repositioned. An elliptical ROI of the UV was initially traced on the first echo image, but was appropriately repositioned in subsequent images if deemed necessary (without modifying the shape of the initial ROI). Typical ROI placement correction was 2–3 pixels. Care was taken to avoid inclusion of Wharton’s jelly (tissue embedding the umbilical vessels) in the ROI.
Fetal volume was estimated by manually segmenting the HASTE coronal images using Osirix (Pixmeo SARL, Geneva, Switzerland). Total fetal volume was estimated as the product of the total number of pixels that belonged to the fetus segmentation and the volume of a voxel element in dm3. Fetal volume (in dm3) was then converted to mass using the previously determined relationship: mass (kg) = 1.03 · volume (dm3) + 0.12 (kg) (7).
Results
Ex-vivo Umbilical Cord blood T2 vs HbO2 Calibration
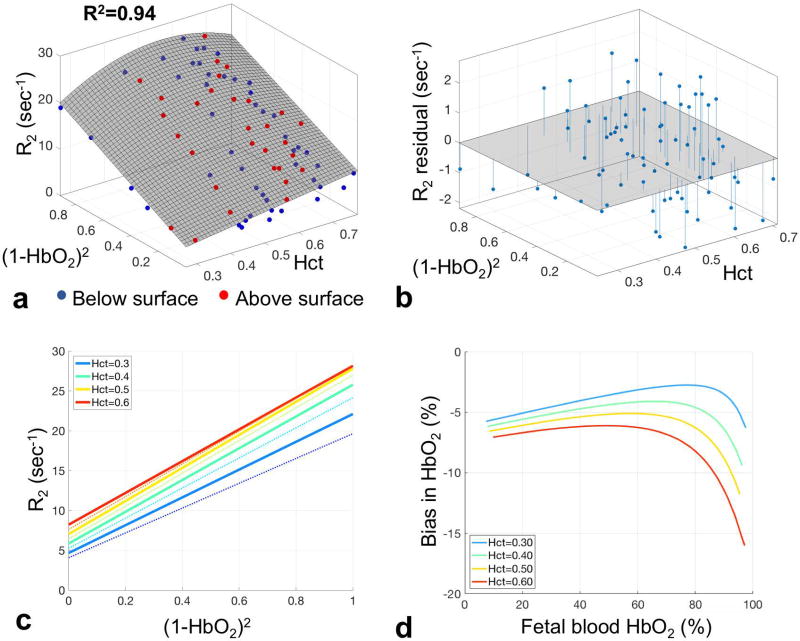
Fetal hemoglobin fraction could be estimated in samples from 8 of the 12 pregnancies (all >36 weeks) included in the study, yielding an average of 80±7% (range 68–90%). No correlation was observed between fetal hemoglobin fraction, and either blood T2 or HbO2. The experimental transverse relaxation rates R2 from all 77 samples plotted versus (1−HbO2)2 (with HbO2 having been obtained by blood gas analysis), and Hct, are depicted in Figure 3a. The Hct to Hb ratio was found to be 3.62±0.12 for these samples. The surface obtained by 3-parameter fitting the data to Equations [2] and [3] was then used to estimate constants k, R2,plasma and R2,RBC. Decay rates increase with decreasing HbO2 (depicted as increasing (1−HbO2)2 in Figure 3c), as expected. The coefficient of determination of the fit was R2=0.94 and the root mean square error between data points and predicted surface was 1.6 s−1. The fitted value of k was 83.1 s−1 (95% CI 77.2–89.0) yielding the following relationship between R2o and Hct: R2o = 1.1(1 − Hct) + 12.9Hct = 11.8Hct + 1.1 [s−1] for Hct values in the range of 0.24 to 0.71, corresponding to R2,plasma = 1.1 (95% CI −0.85–3.06) and R2,RBC = 12.9 s−1 (95% CI 9.30–16.50). Figure 3c depicts profile lines through the surface in Figure 3a for a range of Hct values. Note the increase in slope (relaxivity K) with increasing Hct up to a value of 0.5, beyond which K decreases due to the quadratic Hct term in Equation [3]. The intercept R2o however, continues to increase as a function of Hct (Equation [3]) (19,20) as a result of the well-known increase in relaxation rate with protein concentration.
Figure 3.
(a) Blood R2 as a function of the square of deoxyhemoglobin fraction (1 − HbO2)2 and Hct from 77 fetal blood samples, measured with a T2-prepared bSSFP sequence along with fitted surface R2 = R2o(Hct) + k · Hct · (1 − Hct) · (1 − HbO2)2 in gray; R2=0.94. Red and blue markers represent data points above and below the fitted surface, respectively. (b) Plot of the residuals of R2 measurements w.r.t the fitted surface of panel (a). (c) Profiles of R2 versus (1 − HbO2)2 derived from data of panel A. Thin lines correspond to profiles across the fitted surface in panel A for Hct values in the range of 0.25–0.65. Thick lines correspond to Hct values of 0.3, 0.4, 0.5 and 0.6. The corresponding K and R2o values are 17.4, 19.9, 20.8, and 19.9 s−1 and 215, 171 142, 122 ms, respectively. (d) Plot of bias in fetal blood HbO2 estimation introduced by using the adult blood calibration equation to convert fetal blood T2 to HbO2 values, for the same range of Hct values.
Table 1 shows the fitted values of k, R2,plasma, and R2,RBC for previously reported adult (10) and fetal calibration equations. R2,plasma was found to be lower in fetal blood (1.1 s−1 vs 1.5 s−1), consistent with the lower viscosity of fetal plasma (see Table 1). While R2,RBC and k, which are a function of the intra- to extracellular frequency shift and exchange lifetime (9), were found to be 11% larger for fetal blood than that obtained previously for adult blood, potentially due to increased RBC volume, decreased deformability and differences in hemoglobin types (12,21,22). The average bias in HbO2 estimates introduced by using the adult blood calibration and T2-prep bSSFP sequence (as opposed to that for fetal blood) is 4 – 7% depending on the Hct, in the range of expected values at the umbilical vessels (Figure 3d). This bias varies across the range of HbO2 due to the quadratic relationship between HbO2 and blood R2.
Table 1.
Fitted parameters of the calibration equations to convert fetal and blood T2 to HbO2 values.
| Parameter | Adult(18) | Fetal |
|---|---|---|
| k (s−1) | 74.2 | 83.1 |
| R2,plasma (s−1) | 1.5 | 1.1 |
| R2,RBC (s−1) | 11.6 | 12.9 |
| Hematocrit range (%) | 0.23–0.53 | 0.24–0.71 |
| R2 | 0.95 | 0.94 |
| RMSE (s−1) | 1.1 | 1.6 |
| RMSD (%) | 5.4% | 6.9% |
Fetal O2 Delivery Rate
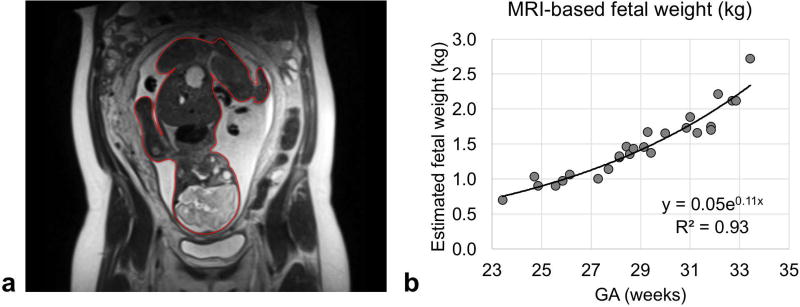
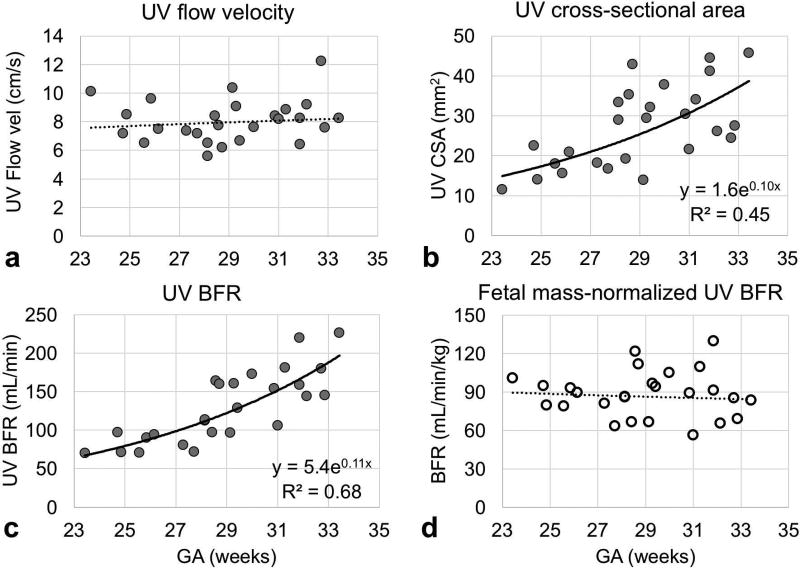
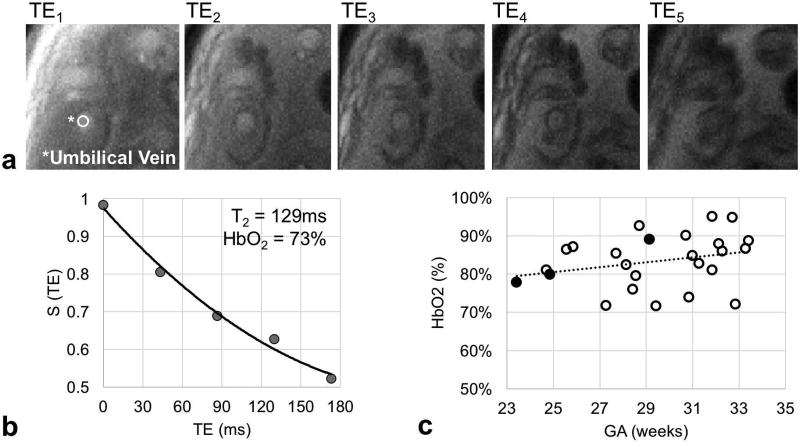
Fetal mass in the subjects studied ranged from 0.7 to 2.7 kg and could be fit to an exponential y = 0.05e0.11x with y = mass in kg and x = GA in weeks (R2=0.93, p-value<0.001, Figure 4). This empirically-derived relationship and is in good agreement with population median values (23). Umbilical vein BFR data were successfully acquired in 26 of the 30 participants enrolled. Representative magnitude and velocity images used for the estimation of BFR are shown in Figure 5. Flow velocity at the UV was not found to be significantly correlated with GA (average 8.1±1.5 cm/s, Figure 6a). In agreement with previous reports (24), the cross-sectional area was found to increase with GA (R2=0.45, p-value<0.05, Figure 6b), resulting in a significant increase in BFR as pregnancy progresses (R2=0.68, p-value<0.05, Figure 6c). However, after normalization with respect to fetal mass, this relationship became non-significant (average BFR 88.2±18.1 mL/min/kg, Figure 6d). Umbilical vein oximetry data were successfully acquired in 26 of the 30 enrolled participants (average ROI diameter 7±4 pixels, range 5–9 pixels). Representative images of the five echoes acquired using T2-bSSFP and corresponding fitted T2 decay are shown in Figures 7a and 7b. Oxygen saturation at the UV was quantified based on the values of k, R2,plasma, and R2,RBC determined above. The value of Hct used for each participant was the population average for GA (25). Oxygen saturation at the UV was found to be independent of GA (Figure 7c), average HbO2 being 84±7% (T2=138±8ms), which is in good agreement with previously reported values measured using invasive methods (26).
Figure 4.
(a) Representative coronal half-Fourier single-shot turbo spin-echo (HASTE) image of a gravid uterus. Red contour depicts the segmentation of the fetus on a single image. Segmentations of the entire fetal body were used to estimate fetal mass from volume information. Fetal volume was then converted to mass using the relationship determined by Baker et al: mass (kg) = 1.03 · volume (dm3) + 0.12 (kg). (b) Plot of fetal mass in kg versus gestational age (GA).
Figure 5.
Examples of phase-contrast (PC) MRI (a–c) magnitude images and (d–f) velocity maps of the umbilical vein (UV) of three different participants. Data were acquired during a single breath hold and flow velocity measurements were the average of two datasets without fetal motion, as observed in the complex difference intensity images.
Figure 6.
Plots of the parameters used to extract fetal mass-normalized blood flow rate (BFR, mL/min/kg). The regions of interest of the umbilical vein used to estimate (A) flow velocity (cm/s) and (b) cross-sectional area (mm2) were manually traced on phase-contrast (PC) MRI images. (c) Blood flow rate (mL/min) was estimated as the product of flow velocity and cross-sectional area and (d) was normalized to fetal mass.
Figure 7.
(a) Example of images used to estimate umbilical vein blood T2. TE: echo time. (b) Plot of a T2 decay of the umbilical vein (UV) blood signal in vivo. T2 was estimated with a three-parameter fit S(t) = S0e−t/T2 + C, including TE correction. (c) Oxygen saturation (HbO2) at the UV was measured across gestational ages (GAs) in 26 participants in vivo. Open points indicate cases in which UV regions of interest (ROIs) are 6 or more pixels in diameter. Solid points indicate cases when ROIs are only 5 pixels in diameter.
Fetal O2 delivery rate was estimated as the product of Ca of fetal hemoglobin, BFR and HbO2 at the UV. The value of Ca (11) was scaled to the concentration of Hb based on the GA population average (25). Both BFR and HbO2 data were successfully acquired in 22 of the 30 participants enrolled.
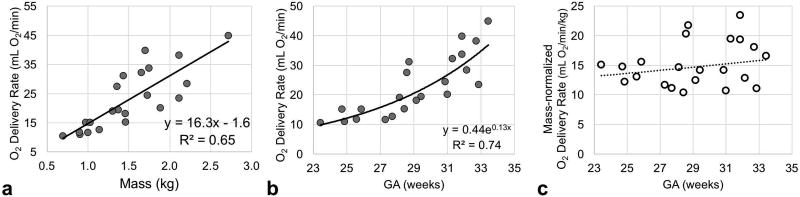
In Figure 8a fetal O2 delivery rate (mL O2/min) is plotted against MRI-based fetal weight; indicating a significant relationship between these two measurements (R2=0.65, p-value<0.01) with a slope of 16.3mL O2/min/kg. Oxygen delivery in units of mL O2/min significantly increased as pregnancy progressed as a result of increasing BFR (R2=0.76, p-value<0.001, Figure 8b). However, after normalization to fetal mass O2 delivery rate was found to be invariant throughout GA (average 15.1±3.8 mL O2/min/kg; Figure 8c).
Figure 8.
Plot of (a) fetal O2 delivery rate versus MRI based fetal mass, (b) estimated fetal O2 delivery rate and (c) normalized with fetal mass across gestational ages (GAs) measured in vivo with quantitative MRI methods.
Discussion
The purpose of this study was to demonstrate the feasibility of estimating fetal O2 delivery rate in vivo across a range of gestational ages with quantitative MRI at 1.5T. Phase-contrast MRI and T2-based oximetry were used to estimate UV BFR and HbO2, respectively. In order to attain this objective, a calibration equation had to be derived using fetal blood from umbilical cord blood samples to convert blood T2 to HbO2 values based on T2 measurements with a T2-prepared balanced SSFP sequence since the biophysical, chemical, and rheological properties of fetal and adult blood differ from each other. For instance, a large fraction of total Hb present in the fetal circulation is fetal hemoglobin (22), which is has a higher oxygen affinity than adult hemoglobin. In addition, fetal hematocrit in the third trimester of pregnancy is considerably higher than the normal physiological range in adults (25). This property is counterbalanced by reduced fetal plasma viscosity (12). These differences between adult and fetal blood prompted the derivation of a calibration equation for converting fetal blood T2 to HbO2 as a means to estimate fetal O2 transport. As shown in Figure 3c the error incurred, if instead the adult calibration curve were used, could be as large as 10%. If average HbO2 and fetal O2 delivery were estimated utilizing the calibration curve generated for adult blood would result in an underestimation of 4±3% (HbO2 units, range 1–10%) and 1±1 mL O2/min (range 0–4 mL O2/min), respectively. Although these values are similar to each other, in 6 cases it was not possible to convert fetal blood T2 to HbO2 values using the adult blood calibration equation as the measured blood T2 was larger than T2o, resulting in invalid HbO2 estimations.
The relationship between T2 and HbO2 depends on magnetic field strength, inter-refocusing interval of the T2-preparation and readout strategy. In some previously reported T2-HbO2 calibration pulse sequences the center of k-space is scanned immediately after T2 preparation (9,27). In that situation, the spatial encoding is not relevant and the established calibration equation can be used as long as the T2-preparation parameters are identical to those used to determine that particular calibration. This would also be the case for centric encoding with bSSFP readout, which, however, is not recommended since it causes artifacts due to undesirable k-space modulation. Sequential encoding is therefore preferable as implemented in the present work. For this reason, the center of k-space is not acquired immediately after T2-preparation, and the sequence employed to determine the calibration equation for conversion of blood T2 to HbO2 ex vivo needs to match the one used to conduct the in vivo studies. In the present work, bSSFP spatial encoding was based on a standard Siemens sequence consisting of a 10-pulse cycle ramp-up catalyzation and linear ordering that included the acquisition of 14 reference lines before traversing the center of k-space in the 25th pulse cycle. Between the end of the T2-preparation and the acquisition of the center of k-space the MRI T2-prepared signal decays as a function of T2/T1. Therefore, in order to utilize the calibration equation described above both the spatial encoding and T2-preparation parameters must be closely matched to those presented here which, however, should be straightforward to achieve.
Fetal blood samples used to establish the aforementioned calibration equation were obtained after delivery at term (GA>37 weeks). In the present study we have used this equation to extract HbO2 information from fetuses of average GA of 30±3 weeks (range 23–35 weeks). Changes in fetal blood composition throughout gestation may influence the accuracy of the HbO2 estimation. These changes include variations in the fetal hemoglobin fraction (22), which in the blood from eight of the umbilical cords used averaged 87%. However, since at earlier GA this fraction is even larger, any contribution from adult hemoglobin can be ignored. Further, total Hb concentration, Hct (25) and total plasma protein content (28) increase throughout pregnancy. These changes lead to progressively higher fetal whole-blood viscosity, which would affect R2,plasma and thus whole-blood R2 (as well as R1). However, raised whole-blood viscosity throughout gestation has been shown to be mainly attributable to hematocrit (29). Therefore, the effect of the variations in fetal blood composition, except those resulting from changes in hematocrit and hemoglobin concentration throughout gestation can be assumed to be negligible.
In the present study, population average Hct values were used as means to estimate subject-specific hematocrit in vivo (25) since Hct measurements on fetal blood is only possible upon delivery and therefore would not account for variations during pregnancy. However, it is known that the longitudinal relaxation rate, 1/T1, scales linearly with Hct (19,30). This relationship has recently been used to convert fetal whole-blood T1 to Hct in the context of calibrating spin-echo T2 measurements of fetal blood to HbO2 (27). The empirically determined association between blood water T1 and Hct may improve the accuracy of future T2-based oximetry studies.
Fetal O2 delivery has been previously measured in humans at late gestation (36 weeks of GA) with quantitative MRI (8) and at term (38–42 weeks of GA) using a combination of ultrasound and invasive methods (26) Sun et al utilized PC-MRI with metric-optimized gating (31) to measure BFR and T2-based oximetry to estimate HbO2 at the fetal abdominal insertion of the UV (8). In contrast, the BFR data reported by Acharya et al, were based on Doppler ultrasound, obtained within 24 hours prior to delivery (26). In the latter study oxygen saturation of the umbilical vessels was measured immediately after delivery with blood gas analysis, along with neonatal mass (26). However, to the best of the present authors’ knowledge, fetal O2 delivery in humans in vivo had not previously been reported across a range of GAs.
The average UV BFR values reported by the above two studies somewhat differ from those in the present work: 89.5±17.2 mL/min/kg, versus 129±28 mL/min/kg (8), 67±30 mL/min/kg (26). The differences in GAs among these three studies may to some extent account for the discrepancies in BFR. Although in the present study no changes in BFR were found across GAs, this result cannot be extrapolated to the full-term case due to the limited number of study subjects. In fact, fetal mass-normalized BFR has been previously reported to decrease towards term (23,32). Differences in BFR may then be related to the different methods used to measure flow velocity.
Overall, fetal mass estimates are in excellent agreement with those expected in the second trimester of pregnancy (33), but smaller by approximately 0.4kg in the third trimester (Supporting Figure S2a). Before normalizing to fetal mass, reported BFR for a GA of 35 weeks was approximately 400 mL/min (8) and 247 mL/min (26), compared to 230 mL/min in the present study (33). Our BFR results thus are comparable to those reported by Acharya et al measured with Doppler ultrasound, which has been shown to accurately quantify blood flow measurements when performed appropriately (34). Similarly, BFR data across GAs are in excellent agreement with those measured by Bellotti et al (23), also acquired by Doppler ultrasound (Supporting Figure S2b). Based on the population average values of UV BFR (mL/min) and fetal mass (kg), fetal mass-normalized BFR (mL/min/kg) across GAs can be estimated. Supporting Figure S2c shows that BFR results are in fair agreement with expected values. The difference in shape between the plots depicted in Supporting Figure S2c (open circles) are due to the fact that fetal growth rate is larger than the rate of BFR increase rate towards term (1). However, in the present study the estimated growth rates for both parameters were the same (0.11, Figure 4b and Figure 6c) potentially due to the limited number of subjects in late GAs. Finally, the bias in BFR values between the present study and those reported by Sun et al may be due to differences in the methods. Future work is necessary to determine the accuracy of these three different methodologies in measuring BFR at the umbilical vessels.
The values for HbO2 reported by Acharya et al (26) are ~18% lower than those estimated in the present study, which conform to other observations suggesting a decrease in HbO2 toward the end of pregnancy (35). Therefore, this apparent disparity may be caused by the lower GAs of the participants in the present study and differences in measurement methods. Nevertheless, our HbO2 estimates (84±7%) are in good agreement with those reported previously (80–82%) from invasive (1,3,4) and, more recently, MRI-based oximetry (8) for second and third trimesters of pregnancies. Despite differences in BFR and HbO2, the estimated values of fetal O2 delivery calculated here (15.3±3.7 mL O2/min/kg) are in good agreement with those measured with invasive methods by Acharya et al (13.4±6.9 mL O2/min/kg) (13), but lower than those by Sun et al (20.4±4.2 mL O2/min/kg) (8).
The present calibration equation and methods may, in theory, also be applied to quantifying fetal O2 consumption rate to study fetal O2 metabolism, which can be estimated via Fick’s principle:
| [4] |
Here, AvO2 is the arteriovenous difference in HbO2 between umbilical vein and arteries. As presented herein, it is feasible to estimate UV HbO2, however, the measurement of blood T2 in the umbilical arteries is difficult due to the small vessel size. For example, the typical diameter of the umbilical arteries is ≤4mm at 36 weeks of gestation (36), therefore limiting the number of pixels across the vessel diameter and possibly compromising the accuracy of the measured T2. An alternative approach is to measure T2 in the fetal abdominal aorta from which the umbilical arteries branch off, thereby yielding a surrogate of umbilical artery HbO2 as shown in a recent study involving third trimester pregnancies (8). Future work will focus on assessing the feasibility of T2-based oximetry to estimate fetal O2 consumption in the second and third trimesters of pregnancy.
In conclusion, the derived calibration curve for fetal blood should ensure improved accuracy in fetal-blood oximetry. Finally, this work demonstrates the feasibility of noninvasively measuring fetal O2 delivery in vivo with a combination of T2-based oximetry and phase-contrast flow imaging in the umbilical circulation.
Supplementary Material
Acknowledgments
The authors would like to thank their clinical coordinators, Hope Pappas and Julia Siegal for their invaluable assistance with blood collection and subject enrollment. This work was supported by NIH grants U01-HD087180, R01-MD009124, R01-HL122754, UL1TR001878, and K25-HL111422
References
- 1.Creasy RK, Resnik R, Greene MF, Iams JD, Lockwood CJ. Creasy and Resnik's maternal-fetal medicine : principles and practice. xxiv. Philadelphia, PA: Elsevier/Saunders; 2014. p. 1294. [Google Scholar]
- 2.Wang Y, Zhao S. Vascular Biology of the Placenta, Integrated Systems Physiology: from Molecules to Function to Disease. San Rafael (CA): 2010. [Google Scholar]
- 3.Brinkman CR., 3rd Umbilical blood flow and fetal oxygen consumption. Clin Obstet Gynecol. 1970;13(3):565–78. doi: 10.1097/00003081-197009000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Arikan GM, Scholz HS, Petru E, Haeusler MC, Haas J, Weiss PA. Cord blood oxygen saturation in vigorous infants at birth: what is normal? BJOG. 2000;107(8):987–94. doi: 10.1111/j.1471-0528.2000.tb10401.x. [DOI] [PubMed] [Google Scholar]
- 5.Weiner CP, Williamson RA. Evaluation of severe growth retardation using cordocentesis--hematologic and metabolic alterations by etiology. Obstet Gynecol. 1989;73(2):225–9. [PubMed] [Google Scholar]
- 6.Siggaard-Andersen O, Huch R. The oxygen status of fetal blood. Acta Anaesthesiol Scand Suppl. 1995;107:129–35. doi: 10.1111/j.1399-6576.1995.tb04347.x. [DOI] [PubMed] [Google Scholar]
- 7.Baker PN, Johnson IR, Gowland PA, Hykin J, Harvey PR, Freeman A, Adams V, Worthington BS, Mansfield P. Fetal weight estimation by echo-planar magnetic resonance imaging. Lancet. 1994;343(8898):644–5. doi: 10.1016/s0140-6736(94)92638-7. [DOI] [PubMed] [Google Scholar]
- 8.Sun L, Macgowan CK, Sled JG, Yoo SJ, Manlhiot C, Porayette P, Grosse-Wortmann L, Jaeggi E, McCrindle BW, Kingdom J, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131(15):1313–23. doi: 10.1161/CIRCULATIONAHA.114.013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright GA, Hu BS, Macovski A. 1991 I.I. Rabi Award. Estimating oxygen saturation of blood in vivo with MR imaging at 1.5 T. J Magn Reson Imaging. 1991;1(3):275–83. doi: 10.1002/jmri.1880010303. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Soto AE, Abdulmalik O, Langham MC, Schwartz N, Lee H, Wehrli FW. T2-prepared balanced steady-state free precession (bSSFP) for quantifying whole-blood oxygen saturation at 1.5T. Magn Reson Med. 2017 doi: 10.1002/mrm.26835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregory IC. The oxygen and carbon monoxide capacities of fetal and adult blood. J Physiol. 1974;236(3):625–34. doi: 10.1113/jphysiol.1974.sp010456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rampling MW, Whittingstall P, Martin G, Bignall S, Rivers RP, Lissauer TJ, Bailey PC. A comparison of the rheologic properties of neonatal and adult blood. Pediatr Res. 1989;25(5):457–60. doi: 10.1203/00006450-198905000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Abdulmalik O, Safo MK, Lerner NB, Ochotorena J, Daikhin E, Lakka V, Santacroce R, Abraham DJ, Asakura T. Characterization of hemoglobin bassett (alpha94Asp-->Ala), a variant with very low oxygen affinity. Am J Hematol. 2004;77(3):268–76. doi: 10.1002/ajh.20184. [DOI] [PubMed] [Google Scholar]
- 14.Abdulmalik O, Safo MK, Chen Q, Yang J, Brugnara C, Ohene-Frempong K, Abraham DJ, Asakura T. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br J Haematol. 2005;128(4):552–61. doi: 10.1111/j.1365-2141.2004.05332.x. [DOI] [PubMed] [Google Scholar]
- 15.Abdulmalik O, Safo MK, Seeholzer SH, Asakura T, Hasbrouck NC, Russell JE. Hb Baden: structural and functional characterization. Am J Hematol. 2010;85(11):848–52. doi: 10.1002/ajh.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdulmalik O, Ghatge MS, Musayev FN, Parikh A, Chen Q, Yang J, Nnamani I, Danso-Danquah R, Eseonu DN, Asakura T, et al. Crystallographic analysis of human hemoglobin elucidates the structural basis of the potent and dual antisickling activity of pyridyl derivatives of vanillin. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 11):920–8. doi: 10.1107/S0907444911036353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luz Z, Meiboom S. Nuclear Magnetic Resonance study of the protolysis of trimethylammonium ion in aqueous solution. The Journal of Chemical Physics. 1963;39:366–370. [Google Scholar]
- 18.van Zijl PC, Eleff SM, Ulatowski JA, Oja JM, Ulug AM, Traystman RJ, Kauppinen RA. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nat Med. 1998;4(2):159–67. doi: 10.1038/nm0298-159. [DOI] [PubMed] [Google Scholar]
- 19.Bryant RG, Marill K, Blackmore C, Francis C. Magnetic-Relaxation in Blood and Blood-Clots. Magnetic Resonance in Medicine. 1990;13(1):133–144. doi: 10.1002/mrm.1910130112. [DOI] [PubMed] [Google Scholar]
- 20.Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochimica et Biophysica Acta (BBA) - General Subjects. 1982;714(2):265–270. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- 21.Riopel L, Fouron JC, Bard H. Blood viscosity during the neonatal period: the role of plasma and red blood cell type. J Pediatr. 1982;100(3):449–53. doi: 10.1016/s0022-3476(82)80458-7. [DOI] [PubMed] [Google Scholar]
- 22.Galacteros F, Guilloud-Bataille M, Feingold J. Sex, gestational age, and weight dependancy of adult hemoglobin concentration in normal newborns. Blood. 1991;78(4):1121–4. [PubMed] [Google Scholar]
- 23.Bellotti M, Pennati G, De Gasperi C, Battaglia FC, Ferrazzi E. Role of ductus venosus in distribution of umbilical blood flow in human fetuses during second half of pregnancy. Am J Physiol Heart Circ Physiol. 2000;279(3):H1256–63. doi: 10.1152/ajpheart.2000.279.3.H1256. [DOI] [PubMed] [Google Scholar]
- 24.Boito S, Struijk PC, Ursem NT, Stijnen T, Wladimiroff JW. Umbilical venous volume flow in the normally developing and growth-restricted human fetus. Ultrasound Obstet Gynecol. 2002;19(4):344–9. doi: 10.1046/j.1469-0705.2002.00671.x. [DOI] [PubMed] [Google Scholar]
- 25.Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics. 2009;123(2):e333–7. doi: 10.1542/peds.2008-2654. [DOI] [PubMed] [Google Scholar]
- 26.Acharya G, Sitras V. Oxygen uptake of the human fetus at term. Acta Obstet Gynecol Scand. 2009;88(1):104–9. doi: 10.1080/00016340802460339. [DOI] [PubMed] [Google Scholar]
- 27.Portnoy S, Osmond M, Zhu MY, Seed M, Sled JG, Macgowan CK. Relaxation properties of human umbilical cord blood at 1.5 Tesla. Magn Reson Med. 2017;77(4):1678–1690. doi: 10.1002/mrm.26231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linderkamp O, Versmold HT, Riegel KP, Betke K. Contributions of red cells and plasma to blood viscosity in preterm and full-term infants and adults. Pediatrics. 1984;74(1):45–51. [PubMed] [Google Scholar]
- 29.Christensen RD, Baer VL, Gerday E, Sheffield MJ, Richards DS, Shepherd JG, Snow GL, Bennett ST, Frank EL, Oh W. Whole-blood viscosity in the neonate: effects of gestational age, hematocrit, mean corpuscular volume and umbilical cord milking. J Perinatol. 2014;34(1):16–21. doi: 10.1038/jp.2013.112. [DOI] [PubMed] [Google Scholar]
- 30.Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med. 2004;52(3):679–82. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- 31.Jansz MS, Seed M, van Amerom JF, Wong D, Grosse-Wortmann L, Yoo SJ, Macgowan CK. Metric optimized gating for fetal cardiac MRI. Magn Reson Med. 2010;64(5):1304–14. doi: 10.1002/mrm.22542. [DOI] [PubMed] [Google Scholar]
- 32.Acharya G, Wilsgaard T, Rosvold Berntsen GK, Maltau JM, Kiserud T. Reference ranges for umbilical vein blood flow in the second half of pregnancy based on longitudinal data. Prenat Diagn. 2005;25(2):99–111. doi: 10.1002/pd.1091. [DOI] [PubMed] [Google Scholar]
- 33.Alexander GR, Kogan MD, Himes JH. 1994–1996. U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3(4):225–31. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 34.Hoyt K, Hester FA, Bell RL, Lockhart ME, Robbin ML. Accuracy of volumetric flow rate measurements: an in vitro study using modern ultrasound scanners. J Ultrasound Med. 2009;28(11):1511–8. doi: 10.7863/jum.2009.28.11.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soothill PW, Nicolaides KH, Rodeck CH, Campbell S. Effect of gestational age on fetal and intervillous blood gas and acid-base values in human pregnancy. Fetal Ther. 1986;1(4):168–75. doi: 10.1159/000262264. [DOI] [PubMed] [Google Scholar]
- 36.Persutte WH, Lenke RR. Transverse umbilical arterial diameter: technique for the prenatal diagnosis of single umbilical artery. J Ultrasound Med. 1994;13(10):763–6. doi: 10.7863/jum.1994.13.10.763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.