Abstract
Background
The ability of radiation to enhance anti-tumor immunity under specific experimental conditions is well established. Here, we explore pre-clinical data and the rationale for combining different radiation doses and fractions with immune checkpoint blockade immunotherapy.
Methods
Literature review
Results
The ability of high-dose or hypofractionated radiation to enhance anti-tumor immunity resulting in additive or synergistic tumor control when combined with checkpoint blockade is well studied. Whether low-dose, daily fractionated radiation does the same is less well studied and available data suggests it may be immunosuppressive.
Conclusions
While daily fractionated radiation is well established as the standard of care for the treatment of patients with head and neck cancer, how this radiation schema alters anti-tumor immunity needs further study. That radiation doses and fractions alter anti-tumor immunity differently has profound implications in the rational design of clinical trials investigating whether radiation can enhance response rates to immune checkpoint blockade.
Keywords: radiation, immunity, microenvironment, checkpoint, fractionation
Introduction
Immunotherapy for head and neck squamous cell carcinoma (HNSCC) has emerged as a feasible treatment option for many patients with Food and Drug Administration approval of programmed death (PD) pathway immune checkpoint blockade (ICB) (1, 2). Yet, only a small subset of patients with recurrent/metastatic HNSCC demonstrate durable responses. Higher response rates are achieved in other cancer types with combinations of checkpoint inhibitors, but with significant immune-related toxicity(3). Given evidence that PD-based ICB primarily relies upon reversal of adaptive immune resistance to exert a therapeutic effect(4), much interest has been placed on finding other treatment modalities that enhance anti-tumor immunity to use in combination with PD-1-based ICB.
Fractionated, low-dose external beam ionizing radiation is a mainstay of treatment for both early and advanced HNSCC(5). Greater than two-thirds of all patients with HNSCC will receive IR at some point during their treatment(6). Significant pre-clinical data suggests that IR is additive or synergistic with different forms of immunotherapy, including checkpoint inhibition. However, close inspection reveals that most combinations demonstrating a significant combinatorial effect utilize high-dose single or hypo-fractionated IR regimens, with mixed results observed with combinations utilizing low-dose fractionated regimens, potentially due to immune suppression following many fractions of daily IR(7–11). Here, we review the historical contexts for the use of daily fractionated IR in HNSCC, how an anti-tumor immune response develops, how IR alters the function of individual components of this response, and the preclinical and clinical data supporting the combination of IR and ICB.
Why do we use fractionated IR for head and neck cancer?
Historically the anti-tumor effects of IR have been attributed to its direct tumor cell cytotoxic effects. Many well performed, prospective clinical trials have established improved survival and treatment tolerability in patents with locoregionally advanced HNSCC following fractionated IR – with the most common treatment schema being 2Gy/day fractions, 5 days/week for 35 total days (70Gy total), though various accelerated and hyperfractionation schedules have been studied(5, 12). In this context of upfront treatment of advanced HNSCC, several principles have emerged to potentially explain why fractionated IR controls tumor growth. Commonly referred to as the “4R’s of fractionated radiotherapy,”(13) these include repair (fractionated IR gives normal tissues, which repair faster than tumor tissues, time to repair between doses), repopulation (based on hypothesis that damaged tumor cells will be replaced by non-damaged tumor cells between fractions), reoxygenation (IR requires oxygen for production of free radicals and fractionation allows for variation of hypoxic regions within tumors over time) and redistribution (fractionation allows more tumor cells to cycle into G2/M of the cell cycle where they are the most sensitive to OR).
In our new era of using immunotherapy to reverse adaptive immune resistance in HNSCC, how different dose and fractionation IR schemas alter anti-tumor immunity must be considered. Daily, low dose, fractionated IR for HNSCC results in peripheral lymphopenia and the degree of drop in peripheral lymphocyte levels correlates to disease-free survival after treatment with either IR alone or IR plus chemotherapy(14–16). Does this mean that how we give IR to patients with advanced HNSCC is immunosuppressive? How IR alters anti-tumor immunity at the level of the tumor microenvironment (TME) can be very complex and peripheral lymphopenia may not be a good surrogate measure of local anti-tumor immunity. To begin to understand these complex differences, we must understand how an effective anti-tumor immune response develops and how IR alters the function of these critical cell types within the TME.
What effect does ionizing radiation have on the tumor microenvironment?
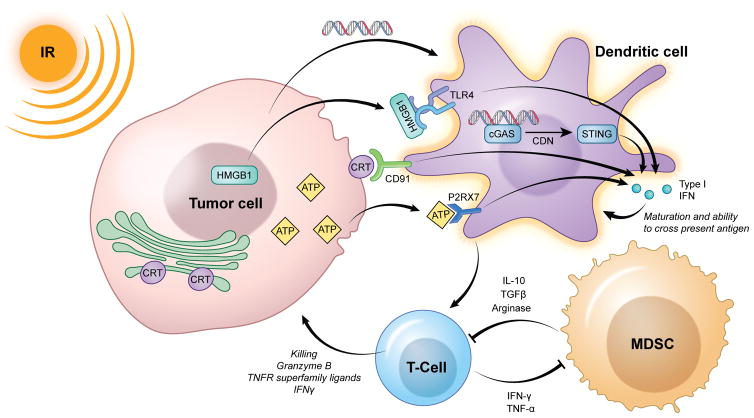
While hematopoietic cells are exquisitely sensitive to low doses of IR, the cumulative effects of different IR doses and fractionation schemas on these cells as they circulate through the TME are less well understood. Immune modulation within the TME in response to IR is complex due to circulation and tumor re-population of immune cells, changes in tumor oxygenation, and numerous direct effects that IR may have on tumor and stromal cells. Here, we review known alterations induced by IR on cellular subsets present within the tumor microenvironment. Critical known alterations in the function of these cellular subsets following IR are summarized in Figure 1.
Figure 1. Summary of known innate immune signaling alterations following IR within the tumor microenvironment.
IR induces the release or surface translocation of several innate immune receptor ligands (HMGB1, ATP, CRT) in a process known as immunogenic cell death, that result in type I IFN production from antigen presenting cells (dendritic cells). Recent evidence also has demonstrated the importance of DNA sensing through cGAS, also resulting in STING-dependent type I IFN production. Type I IFN is critical for the maturation of dendritic cells, allowing cross-presentation of antigen and initiation of adaptive immunity. Activated T-cells in turn eliminate antigen positive target cells, but also help to reduce local immunosuppression through effector cytokine (IFNγ, TNFα)-dependent reduction in MDSCs. Whether IR can directly reduce the viability or function of immunosuppressive cells such as MDSCs, or whether this effect is secondary through T-cell effector cytokines, remains unclear.
DCs
Dendritic cells are effective antigen-capturing cells in their immature form. Upon encountering maturation signals, they differentiate into effective antigen-presenting dendritic cells and become specialized in stimulating T cells through expression of appropriate costimulatory molecules. DC maturation van be triggered by a variety of “danger” signals (damage associated molecular patterns, or DAMP) released by pathogens as well as damaged or stressed host cells(17, 18). IR may induce immunogenic cell death leading to increased tumor cell surface calreticulin and release of DAMP such as high-mobility group box 1 (HMGB1) and ATP(19, 20). Calreticulin on the surface of tumor cells or cellular debris increases phagocytosis by dendritic cells while HGMB1 acts as a chemoattractant and activator of immature dendritic cells. These alterations appear to activate DCs, though effects appear to be both IR dose, fractionation and model dependent. In vitro, immature dendritic cells co-incubated with supernatant from SC480 colorectal tumor cells irradiated with 2Gyx5 or 5Gyx3 increased expression of DC maturation markers CD80 and CD83 and expression of pro-inflammatory cytokines IL-12p70, IL-8, IL-6, TNFα(21). However, direct exposure of DCs isolated from PBMC to 30Gyx1 reduced expression of CD86, CD80, and HLA-DR with resulting decreased capacity for stimulating T-cell proliferation(22). In vivo results more consistently demonstrate enhanced DC function following IR. Lugade et al. demonstrated an increased accumulation and activation of DC within the tumor draining lymph node (TDLN) when B16-OVA tumor cells were exposed to either 15Gyx1 or 3Gyx5 with greater effects observed with 15Gyx1(10). Similar results were observed by Lee et al. after B16-SIY tumors were exposed to 20Gyx1(9).
Strong evidence for the importance of functional DCs within the TME following IR comes from studies in genetically altered mice with dysfunctional DCs or type I IFN responses. Cytosolic sensing of DNA within DCs and subsequent STING-dependent production of type I IFN appears to be critical for cross-priming of antigen-specific T-cell responses, and any alteration of this DNA sensing pathway or type I IFN response within host cells abrogates tumor control after IR(23–25). Cumulatively, pre-clinical evidence suggests that while direct IR exposure may be detrimental to DCs, IR may enhance immunogenic tumor cell death and indirectly activate DCs within the tumor microenvironment through enhanced antigen release, availability of DAMP and ultimately STING-dependent type I IFN signaling resulting in enhanced antigen cross-presentation.
T-lymphocytes
While NK cells and even innate immune cells can exert anti-tumor effects(26, 27), T-lymphocytes are largely credited with having the ability to detect and eradicate malignant cells. Lymphocytes are highly sensitive to IR-induced death and lymphopenia is a side effect of fractionated radiotherapy, and this effect appears to be fractionation dependent(16, 28). Yet, cumulative effects of therapeutic IR on lymphocyte activation within the TME are diverse. Summarized in Table I, most studies evaluating the effects of IR on T-lymphocyte function within the TME describe some degree of anti-tumor activation, though similar to the effects of IR on DC function, these effects seem to be dose/fractionation and model dependent. For example, Lee et al. demonstrated primary tumor growth control or rejection of established B16-SIY melanomas with 20Gyx1 but not 5Gyx4(9); whereas results from Dewan et al. revealed that both 20Gyx1, 8Gyx3 and 6Gyx5 all control the primary growth of TSA mammary carcinomas and MC38 colon carcinomas(7). Increased recruitment of CD8 T-cells after 12Gyx2 IR treatment of breast carcinomas was dependent on induced release of CXCL16 from tumor cells(29). Some consistent trends do emerge from the existing pre-clinical studies on the effects of IR of T-lymphocytes. Tumor growth control after IR in immunocompetent mouse models appears to be partially or totally dependent on the presence and function of CD8+ cells(9, 11, 23), suggesting that CD8 T-lymphocytes play a critical role in the cumulative effect of IR on tumors. Clearly dose and fractionation schedules of IR have an impact on primary and abscopal tumor control as several studies have demonstrated control of tumor growth or rejection of established tumors after single high dose IR but not after fractionated IR(9–11). Overall, fewer studies have evaluated the impact of low-dose, daily fractionated IR on anti-tumor immunity. This has obvious implications for the study of HNSCC, as these patients are treated with 35 daily fractions of 1.8–2.0Gy. Pre-clinical studies evaluating T-lymphocyte tumor repopulation after different doses and fractionation schemes of IR are lacking and may provide information critical to the design of therapeutic regimens utilizing IR to activate or enhance anti-tumor immunity.
Table I.
Summary of pre-clinical studies evaluating the effects of ionizing radiation on T-lymphocyte function within the tumor immune microenvironment.
| Title and reference | Authors | Journal | Year | Model | IR | Results | Mechanism |
|---|---|---|---|---|---|---|---|
| Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects | Ruocco et al | J Clin Invest. | 2012 | 4T1 | 12Gyx2 | Motility arrest of CD8 TIL within tumor after IR | Increased tumor cell ICAM-1 after IR |
| Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas | Zeng et al | Int J Radiat Oncol Biol Phys. | 2013 | GL261 | 10Gyx1 | Increased CD8 TIL infiltration | Increased tumor cell MHC class I, ICAM-1 and CXCL16 expression after IR |
| Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment | Lee et al | Blood | 2009 | B16-SIY | 20Gyx1 5Gyx4 |
Tumor control and rejection after single high dose IR but not after fractionated IR | CD8 dependent tumor rejection after IR |
| Maximizing tumor immunity with fractionated radiation | Schaue et al | Int J Radiat Oncol Biol Phys. | 2012 | B16-OVA | 3Gyx5 5Gyx3 7.5Gyx2 15Gyx1 |
7.5Gyx2 resulted in better tumor control than other IR regimens | 7.5Gyx2 resulted in highest peripheral IFNγ producing cell:Treg ratio |
| Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor | Lugade et al | J Immunol. | 2005 | B16-OVA | 15Gyx1 3Gyx5 |
Enhanced tumor control with single high dose compared to fractionated IR | Increased IFNγ producing and cytotoxic cells in the tumor of mice treated with 15Gyx1 |
| STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors | Deng et al. | Immunity | 2014 | MC38 | 20Gyx1 | Single high dose of IR induces control of established MC38 tumors | Tumor control after single high dose IR abrogated with CD8 cell depletion |
| Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions | Filatenkov et al. | Clin Cancer Res. | 2015 | CT26 MC38 |
30Gyx1 3Gyx10 |
Rejection of established CT26 and MC38 tumors after single high dose IR but not after fractionated IR | Tumor rejection after single high dose IR abrogated with CD8 cell depletion |
Mediators of immunosuppression within the tumor microenvironment
While T-lymphocyte responses rely upon the presence and recognition of tumor-associated or -specific antigen, most tumors likely harbor many genetic alterations that result in a number of neoantigens with a high degree of clonality(30). Taking this and antigen-independent NK cytotoxicity into account(31), it is likely that the ability of solid tumors to develop a directly immunosuppressive microenvironment plays a critical role in the outgrowth of clinically relevant malignancies(32, 33). This immunosuppressive tumor microenvironment can be mediated by tumor, stromal and infiltrating immune cells. Tumor cell-intrinsic mechanisms include downregulation of MHC class I and antigen-processing machinery, genetic alterations leading to insensitivity to granzyme B and TNFR superfamily-induced apoptosis, and increased expression of cell surface molecules that inhibit CTLs (programmed death-ligand 1; PD-L1). Tumor cells secrete immunosuppressive cytokines such as TGFβ and IL-10 that inhibit DC activation and T-lymphocyte function. Tumor cells also express chemokines that drive the recruitment of hematopoietic cells into the tumor that are immunosuppressive. These include myeloid derived suppressor cells (MDSCs), M2-polarized tumor-associated macrophages (M2TAMs), and regulatory CD4+ T-lymphocytes (Tregs). Via mechanisms such as local nutrient depletion, cytokine and immune checkpoint expression and generation of reactive oxygen species, these cell types potently suppress effector CTL and NK function.
MDSCs
Deng et al. demonstrated that 12Gyx1 IR can significantly reduce the accumulation of Gr1+ MDSC within TUBO tumors(34). Mechanistically, this appeared to be due to loss of MDSC viability following exposure to TNFα released from IR-activated CD8 TIL within the TME. Alternatively, Filatenkov et al demonstrated that IFNγ released from CD8+ TIL was critical for significant reduction in MDSC after 30Gyx1 IR treatment(11). Clearly, alterations in the tumor cytokine milieu appear to influence the presence and activity of MDSC. Crittenden et al. reported that 20Gyx3 treatment of Panc02 tumors transiently reduced peripheral accumulation of CD11b+ myeloid cells, though tumor infiltration was not assessed in these experiments(35). Studies evaluating the effects of low dose, daily fractionated IR on peripheral or tumor accumulation of MDSCs are lacking.
Tregs
Irradiation of TUBO tumors with 12Gyx1 did not significantly alter tumor infiltration of Tregs(34). Conversely, in an intracranial glioma model, 10Gyx1 did reduce infiltration of Tregs into the brain microenvironment(36). Interestingly, 8Gyx3 IR treatment of MC38 colon carcinomas did not significantly reduce Treg accumulation of primary treated tumors but did decrease Treg accumulation in contralateral untreated tumors(37). A commonly cited manuscript details IR dose-dependent increased percentages of CD25+FoxP3+ cells within the CD4+ splenocyte compartment with single doses ranging from 5–15Gy, but this study did not evaluate tumor accumulation of Tregs(8). Again, studies evaluating the effects of low dose, daily fractionated IR on peripheral or tumor accumulation of Tregs are lacking.
TAMs
Treatment of Panc02 tumors with 20Gyx3 IR resulted in increased accumulation of CD11b+ cells that express immunosuppressive markers of M2-polarization such as arginase and IL-10(38). Similarly, exposure of TRAMP-C1 tumors to 25Gyx1 or 4Gyx15 results in selective accumulation of arginase, iNOS and COX2 expressing macrophages in areas of tumor hypoxia(39, 40). Conversely, vascular normalization and accumulation of antigen-specific CD8 TIL was enhanced in insulinomas following a single dose of 2Gy. This recruitment was dependent upon the presence of radiation-induced mature macrophages within the TME(41). Understanding how different IR doses and schemas alter macrophage function challenging given their high plasticity and multiple functions.
Tumor vasculature
At baseline, most solid tumors display disorganized and highly leaky tumor vasculature that ultimately contributes to tumor hypoxia and increased interstitial pressure – both of which are highly detrimental to the function of effector immune cells(42, 43). Multiple groups have demonstrated that single low dose (2 Gy) or high dose (15 Gy) IR can normalize/stabilize tumor vasculature and increase expression of VCAM on endothelial cells required for leukocyte adhesion, likely in a type II IFN-dependent fashion(41, 44). Intermediate doses of IR (5–10Gy) appear to similarly normalize tumor vasculature resulting in decreased vessel leakiness and better tumor oxygenation(45, 46). However, higher individual doses of IR (>10Gy) appear to lead to vessel instability and eventual collapse, promoting tumor hypoxia(47, 48). Enhanced understanding of how different doses and fraction of IR ultimately alter the ability of effector immune cells to penetrate into tumor parenchyma through normalized vasculature is critical given the exquisite sensitivity of these cell types to hypoxia(42).
Tumor stroma
Mounting evidence suggests that cancer associated fibroblasts (CAFs) influence the behavior of malignancies both through both providing mitogenic signals to tumor cells and through local immunosuppression(49, 50). Some groups have demonstrated that CAFs appear to be highly resistant to the cytotoxic effects of IR, even at high doses(51, 52). However, Grinde et al. demonstrated that greater engraftment kinetics when CAFs were mixed with tumor cells before transplantation were abrogated when the CAFs were irradiated prior to the mixture(53). This effect was the same between 18Gyx1 and 6Gyx3 schemas. These data suggest that IR potentially alters CAF viability and function, but more direct studies on how IR alters the immunosuppressive function of CAFs are needed. Of great interest are a series of projects in the Schreiber group that have elegantly detailed the necessity of eliminating CAFs to achieve complete tumor rejection(54, 55). In the model system used by this group, 10Gyx1 induced enough antigen release from tumor cells that CAFs cross presenting released tumor antigen were eliminated by adoptively transferred CTLs and this irradiation was required for sensitization of the CAFs to immune killing(56). Clearly immune elimination of both tumor and stromal cells is critical for tumor rejection.
Direct effects on tumor cells
IR causes DNA damage, and could induce the formation of new mutations that could lead to the expression of neoantigens in irradiated cells. Riets et al. demonstrated that not only does IR induce expression of MHC class I on the surface of tumor cells, it increases the intracellular pool of peptides available for loading onto MHC class I in an mTOR-dependent fashion(57). Some of these differentially presented peptides appeared to be derived from proteins selectively upregulated by irradiation. This suggests that if irradiation led to the formation of neoepitopes unique to irradiated cells, the MHC presentation pathways required for CTL recognition may also be upregulated by IR. Others have demonstrated upregulation of MHC class I on the surface of tumor cells both in vitro and in vivo in mechanisms often dependent on increased levels of type II IFN(44, 58).
Immunogenic cell death (ICD), as defined by Zitvogel and Kroemer et al., includes the cell surface expression or release of molecules known to stimulate innate immune receptors to activate the innate arm of the immune system after a cytotoxic insult(19, 59). This includes increased expression of cell surface calreticulin (binds CD91) and release of HMGB1 (binds TLR4) and ATP (binds P2RX7). Whether IR induces pure ICD is unclear, but more substantial evidence exists that IR can induce tumor cells death associated with one or more ICD components or release of other innate immunity activating molecules(17, 60, 61). In addition to IR inducing innate immune activation through induction of different components of ICD, more recent work has highlighted the importance of cytosolic sensing of DNA (released from dying tumor cells) in DCs through the STING receptor. Type I IFN production serves as the critical link between activation of innate and adaptive immunity through activation of antigen cross-presentation by CD8+ DCs. Induction of type I IFN responses and subsequent T-cell mediated tumor control following 20Gyx1 IR was completely abrogated in mice with STING deficient immune cells(23–25). Recent work by Vanpuille-Box et al. has demonstrated that higher single doses of irradiation (>12–18Gy in different cell lines) induces expression of an exonuclease (trex1) that degrades DNA accumulation in the cytosol after IR and prevents cGAS and STING-dependent type I IFN responses(62). In addition to emphasizing importance of STING-dependent type I IFN responses following IR, such work demonstrates how a better understanding of how tumor cells respond to IR in the context of immune activation can critically inform the way we combine IR with immune activating treatments.
When damage following IR is not sufficient to directly induce cell death, irradiated tumor cells appear to be more sensitive to CTL mediated lysis. Garnett et al. demonstrated in a panel of CEA+ colon carcinoma lines that sublethal IR doses of 10 or 20Gy enhanced tumor cell susceptibility to CTL lysis(58). Such “immunogenic modulation” to enhance CTL lysis after sublethal IR in vitro has been demonstrated in many cancer cell types(63, 64) and appears to mechanistically be due to enhanced antigen presentation on MHC class I, enhanced ICAM-mediated tumor:T-cell interaction and enhanced cell surface calreticulin exposure.
Some of the most powerful data demonstrating enhanced antigen-specific immune responses after IR comes from studies on antigen-spread following peptide vaccination. Following single-peptide vaccination of tumors expressing multiple MHC class I-restricted antigens, 8Gyx1 IR treatment induces the formation of T-cell responses against multiple antigens resulting in rejection or control of both locally treated and distant untreated tumors(65, 66). This data suggests that IR enhances the presentation of multiple antigens, leading to the development of a polyclonal T-cell response against antigens not attributable to the peptide vaccine directly. This concept was reinforced by a recent study in B16–F10 melanoma tumors demonstrating increased diversity of TCR clones in CD8+ TIL from irradiated compared to non-irradiated tumors(67).
What is the preclinical evidence for radiation + checkpoint inhibition?
The rational combination of IR and PD-based immunotherapy stems from a fundamental understanding of the mechanism of PD-based checkpoint inhibition and evidence that IR may actually induce an innate and adaptive anti-tumor immune response, as described above. PD-based ICB reverses adaptive immune resistance(68). To our knowledge, there is no data to suggest that PD-1 or PD-L1 mAb treatment can induce a de novo immune response(69). If baseline or treatment-induced anti-tumor immunity is present within an organism and being held back by PD-1/PD-L1 signaling, then PD-blockade can potentially block this signaling and unleash this existing immune response. If another therapy, such as IR, can actually induce an immune response and there is evidence that this induced immune response is being blocked by the induced expression of PD-pathway components, then the combination of this therapy and PD-based ICB is rational. Evidence that IR can induce expression of PD pathway components is substantial. Deng et al. demonstrated that 20Gyx1 IR treatment of TUBO tumors increased PD-L1 expression on tumor cells and tumor-infiltrating immune cells(34). Dovedi et al. found similar increases of PD-L1 expression on CT26 tumors cells following 2Gyx5(70). This increased PD-L1 expression is very likely to be linked to overall increases in local IFN(44) that then drives PD-L1 expression, consistent with adaptive immune resistance.
The principles underlying enhanced anti-tumor immunity following CTLA-4-based checkpoint inhibition are different. As opposed to PD-1/PD-L1 expression in response to IFN and immune activation as a mechanism of adaptive immune resistance, CTLA-4 appears to be constitutively expressed at varying levels on both effector CD8 TIL and tumor infiltrating Tregs. Blockade of CTLA4 signaling with CTLA-4 mAb both blocks the negative signal mediated by CTLA-4 on effector CD8 TIL but also results in macrophage-dependent ADCC elimination of CTLA-4+ Tregs(71–73). Both mechanisms are required to enhance anti-tumor immunity(73). Subsequently, evidence suggests that CTLA-4 ICB can actually activate an immune response, as opposed to just unblocking a pre-existing response(72, 73). While CTLA-4 ICB is still simply a tool to enhance anti-tumor immunity, the mechanism of how it may be additive or synergistic with IR is likely different than when IR is combined with PD-based ICB.
Table II details studies that have combined IR with either PD or CTLA-4 ICB in syngeneic pre-clinical models. General trends from these reports include additive or synergistic effects between IR and ICB that is CD8+ cell dependent, often with immune-mediated rejection of tumors that results in immunologic memory. Some studies demonstrate an abscopal effect – or control of a distant untreated tumor. While rarely occurring with IR or ICB alone, abscopal control of distant tumors following combination therapy provides strong evidence for the development of systemic anti-tumor immunity. One significant study elegantly demonstrated that combination 20Gyx1 IR plus CTLA-4 ICB leads to increased PD-L1 expression on tumor cells(67). Tumor rejection rates could be significantly enhanced by reversing adaptive immune resistance with the addition of PD-based ICB to IR plus CTLA-4 mAb, reinforcing many of the principles discussed above.
Table II.
Summary of pre-clinical studies combining ionizing radiation and immune checkpoint blockade in syngeneic mouse models of cancer.
| Title and reference | Authors | Journal | Year | Model | IR | ICB | Results |
|---|---|---|---|---|---|---|---|
| Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody23 | Dewan et al. | Clin Cancer Res. | 2009 | TSA, MC38 | 20Gy × 1, 8Gy × 3, 6Gy × 5 | CTLA-4 mAb | Best tumor control with 8Gyx3+CTLA-4 mAb; abscopal control of distant tumor only observed with 8Gyx3+CTLA-4 mAb |
| Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer | Demaria et al. | Clin Cancer Res. | 2005 | 4T1 | 12Gy × 1 | CTLA-4 mAb | Combo significantly enhanced survival of 4T1 tumor-bearing mice and slowed formation of lung metastases |
| Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice | Deng et al. | J Clin Invest. | 2014 | TUBO, MC38 | 12Gy × 1 | PD-L1 mAb | Combo significantly enhanced survival of TUBO or MC38 tumor-bearing mice; CD8+ cell dependent |
| Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas | Zeng et al. | Int J Radiat Oncol Biol Phys. | 2013 | GL261 | 10Gy × 1 | PD-1 mAb | Combo significantly enhanced survival of GL261 intracranial tumor-bearing mice; cured mice rejected challenge with original cell line indicating immunologic memory |
| Abscopal effects of radiotherapy are enhanced by combined immunostimulatory mAbs and are dependent on CD8 T cells and cross-priming | Rodriguez-Ruiz et al. | Cancer Res. | 2016 | MC38, 4T1, B16-OVA | 8Gyx3 | PD-1 mAb and 41BB mAb | Combo significantly reduced primary tumor growth; combo resulted in abscopal control of distant tumors; abscopal effect was CD8+ cell dependent; abscopal effect dependent on type I IFN signaling and DCs |
| Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade | Dovedi et al. | Cancer Res. | 2014 | CT26, 4434, 4T1 | 2Gy × 5 | PD-1 mAb or PD-L1 mAb | Combo induced rejection in 66–80% of tumor-bearing mice, concurrent treatment needed for result; result CD8+ cells and IFNγ dependent |
| Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects | Ruocco et al. | J Clin Invest. | 2012 | 4T1 | 12Gy × 2 | CTLA-4 mAb | Combo enhanced primary tumor growth control; dependent upon arrest of TIL within tumor in an MHC class I and ICAM dependent fashion |
| Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. | Twyman-Saint Victor, et al. | Nature | 2015 | B16–F10, TSA, PDA.4662 | 20Gyx1, 8Gyx3 | CTLA-4 mAb and PD-1 mAb | The addition of PD-1 mAb to IR plus CTLA-4 significantly enhanced tumor growth control and rates of tumor rejection |
| DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity | Vanpouille-Box, et al. | Nat Commun | 2017 | TSA | 30Gyx1, 8Gyx3 | CTLA4 mAb | 8Gyx3 but not 30Gyx1 synergized with CTLA-4 mAb to control primary and abscopal tumors |
What is the clinical evidence for radiation + checkpoint inhibition?
Several case reports have demonstrated control of non-irradiated tumors following irradiation of target lesions with hypofractionated IR in the presence of systemic CTLA-4 mAb (Table III). While abscopal tumor control cannot be completely attributed to radiation given that patients are receiving systemic CTLA-4 mAb, many of these reports demonstrate some degree of abscopal control of non-irradiated tumors in the setting of progression while receiving CTLA-4 mAb, suggesting a critical role for irradiation in the induction of systemic immunity. To date, no clinical data describing results following combination IR and ICB in head and neck cancer has been published. However, many clinical trials specific for HNSCC or in solid tumors that include HNSCC are underway (Table IV).
Table III.
Case reports documenting possible abscopal tumor control following treatment with ionizing radiation and immune checkpoint blockade.
| Title | Authors | Journal | Year | Tumor | RTx | Immunotherapy | Results |
|---|---|---|---|---|---|---|---|
| Immunologic correlates of the abscopal effect in a patient with melanoma.51 | Postow et al. | N Engl J Med. | 2012 | Metastatic melanoma | 9.5Gy × 3 | Ipilimumab | After progression of disease on ipilimumab alone, regression of non-irradiated metastatic melanoma lesions after irradiation of paraspinal masses for palliation |
| An Abscopal Response to Radiation and Ipilimumab in a Patient with Metastatic Non–Small Cell Lung Cancer.52 | Golden et al. | Cancer Immunol Res | 2013 | Lung adenocCA | 6Gy × 5 | Ipilimumab | Reduction in size of multiple non-irradiated metastatic deposits following irradiation of a single liver metastasis plus ipilimumab |
| A systemic complete response of metastatic melanoma to local radiation and immunotherapy.53 | Hiniker et al. | Transl Oncol. | 2012 | Metastatic melanoma | 18Gy × 3 | Ipilimumab | Resolution of non-irradiated metastatic deposits after irradiation to two liver metastases plus ipilimumab |
| Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy.54 | Grimaldi et al. | Onco-immunol | 2014 | Metastatic melanoma | various | Ipilimumab | Stable disease or partial response in non-irradiated metastatic deposits following irradiation of individual metastases inpatient who failed ipilimumab alone |
Table IV.
Current clinical trials designed to evaluate the effect of combination ionizing radiation and immune checkpoint blockade.
| Institution/Study ID | Tumor site/stage | Primary study aim | RT | ICB | Inclusion criteria |
|---|---|---|---|---|---|
| University of Pittsburgh/NCT01935921 | PULA HNSCC | DLTs | 2Gy × 35 | CTLA-4 mAb | Stage III/IVB excluding T1N1 histologically confirmed SCC or undifferentiated carcinoma |
| Sanford Health/NCT02586207 | PULA HNSCC | DLTs | 2Gy × 35 | PD-1 mAb | Stage III/IVB HNSCC histologically confirmed, measurable disease, no prior RT |
| RTOG (multicenter)/NCT02764593 | PULA HNSCC | DLTs | 2Gy × 35 | PD-1 mAb | Stage III/IVB HNSCC histologically confirmed, measurable disease, no prior RT |
| University of Cincinnati/NCT02759575 | PULA HNSCC | DLTs | 2Gy × 35 | PD-1 mAb | Stage III or IV SCC of the larynx, measurable disease, no prior RT |
| University of Cincinnati/NCT02641093 | Adjuvant for resected HNSCC | DLTs, DFS | 2Gy × 30 | PD-1 | Any T stage with at least N2 disease, T4 disease any N stage, T3 oral cavity any N stage, clinical evidence of extra-capsular extension on scans, willing to undergo definitive resection with neck dissection |
| Groupe Oncologie Radiotherapie Tete et Cou/NCT02707588 | Locally-advanced HNSCC | LRC of ICB+IMRT vs cetuximab+IMRT | 2.12Gy × 33 | PD-1 | Histologically confirmed previously untreated locally advanced HNSCC |
| UNC Lineberger Cancer Center/NCT02609503 | Locally-advanced HNSCC | PFS | 2Gy × 35 | PD-1 | Histologically confirmed stage III–IV (non-metastatic) HNSCC, ineligible for high-dose cisplatin therapy, no prior curative attempts |
| University of Maryland/NCT02289209 | Locoregional inoperable recurrence or second primary HNSCC | PFS | 1.2Gy BID for 5 days a week for 5 weeks | PD-1 | Histologically confirmed locoregional recurrence or second primary HNSCC which is unresectable or the patient is unwilling to undergo resection, received only prior RT with curative intent, at least 1 measurable area of disease within previously radiated field |
| University of California, San Diego/NCT02843165 | Metastatic disease including HNSCC | ORR of ICB alone vs ICB+SBRT | 9.5Gy × 3 | PD-1, PD-L1, CTLA-4 mAb | At least 1 lesion treatable by SBRT, histologic confirmation of malignancy (primary or metastatic), no prior radiotherapy to treatment site |
| Sidney Kimmel Cancer Center at Thomas Jefferson University/NCT02318771 | Recurrent/metastatic HNSCC, RCC, Mel, NSCLC | Immune correlates | 8Gy × 1, 4Gy × 5 | PD-1 mAb | Histologically-confirmed recurrent/metastatic HNSCC, RCC, melanoma, or lung CA with at least 1 measurable lesion in addition to the index lesion |
| Memorial Sloan Kettering/NCT02684253 | Metastatic HNSCC | ORR of ICB alone vs ICB+SBRT | 9Gy × 3 | PD-1 | Histologically confirmed metastatic HNSCC, at least 2 lesions |
How different IR dose and fractionation schemas alter local anti-tumor immunity to be additive or synergistic with ICB is a critical question. While the majority of pre-clinical data suggests that individual large or hypofractionated IR doses appear to enhance local anti-tumor immunity to a greater degree than daily fractionated IR, we must remember that our preclinical models simply serve as models for what may happen in patients with HNSCC. Despite this pre-clinical data, several institutions are moving forward with HNSCC trials investigating ICB combined with both standard, low-dose, daily fractionated (Table IV trials 1–7) and higher-dose hypofractionated IR (Table IV trials 9–11). Clinical and immune correlative data emerging form these trials in the coming years as they mature will be very informative and should help guide the design of large phase trials designed to more clearly define the role of combination IR and ICB in both recurrent/metastatic and previously untreated, locally advanced HNSCC.
Conclusions
The emergence of checkpoint inhibitors as an FDA-approved, off-the-shelf immunotherapy with reasonable safety profiles has helped usher in the current age of immunotherapy for cancer. With our enhanced mechanistic understanding of how these drugs work has come the realization that combination with other anti-cancer therapies that have the capacity to induce immune responses is likely needed to meaningfully enhance response rates. Based upon extensive pre-clinical data, IR fills this role well. There is a tendency however to combine new therapies (checkpoint inhibitors) with current standard-of-care therapies (low-dose daily fractionated IR, in the case of HNSCC) without supporting pre-clinical data. Indeed, the majority of published pre-clinical data supports that single high dose or hypofractionated IR enhances local anti-tumor immunity and is either additive or synergistic with either PD-based or CTLA-4-based ICB. However, pre-clinical data supporting the combination of low dose, daily fractionated IR with ICB is at best lacking and at worst negative. Clearly, mechanistic pre-clinical studies investigating how different radiation schemas perform head-to-head when combined with ICB are needed to inform the data-driven design of clinical trials. While many current clinical trials combining IR and ICB are designed to assess safety as a primary endpoint, secondary immune correlative and clinical response outcomes will certainly assist in the design of future trials aimed at enhancing response rates for patients with HNSCC.
Acknowledgments
Funding: This work was supported by the Intramural Research Program of the NIH, NIDCD, project number ZIA-DC000087 (CTA). MM was supported by through the National Institutes of Health Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Newport Foundation, The American Association for Dental Research, The Howard Hughes Medical Institute, and the Colgate-Palmolive Company, as well as other private donors.
Footnotes
Meetings at which this work was presented: None
References
- 1.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–65. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 2.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benci JL, Xu B, Qiu Y, et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167(6):1540–1554. e12. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368(9538):843–54. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 6.Caudell JJ, Torres-Roca JF, Gillies RJ, et al. The future of personalised radiotherapy for head and neck cancer. Lancet Oncol. 2017;18(5):e266–e273. doi: 10.1016/S1470-2045(17)30252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83(4):1306–10. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 11.Filatenkov A, Baker J, Mueller AM, et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res. 2015;21(16):3727–39. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48(1):7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 13.Perez B. Principles and practice of radiation oncology. Vol. 5. Philadelphia: Lippincott, William and Wilkins; 2008. [Google Scholar]
- 14.Kuo P, Bratman SV, Shultz DB, et al. Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin Cancer Res. 2014;20(21):5558–69. doi: 10.1158/1078-0432.CCR-14-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campian JL, Sarai G, Ye X, Marur S, Grossman SA. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2014;36(12):1747–53. doi: 10.1002/hed.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crocenzi T, Cottam B, Newell P, et al. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J Immunother Cancer. 2016;4:45. doi: 10.1186/s40425-016-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol. 2012;39(3):323–39. doi: 10.1053/j.seminoncol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33:445–74. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- 19.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 20.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81(1):59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 21.Kulzer L, Rubner Y, Deloch L, et al. Norm- and hypo-fractionated radiotherapy is capable of activating human dendritic cells. J Immunotoxicol. 2014;11(4):328–36. doi: 10.3109/1547691X.2014.880533. [DOI] [PubMed] [Google Scholar]
- 22.Cao MD, Chen ZD, Xing Y. Gamma irradiation of human dendritic cells influences proliferation and cytokine profile of T cells in autologous mixed lymphocyte reaction. Cell Biol Int. 2004;28(3):223–8. doi: 10.1016/j.cellbi.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Deng L, Liang H, Xu M, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41(5):843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamond MS, Kinder M, Matsushita H, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuertes MB, Kacha AK, Kline J, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208(10):2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Sullivan T, Saddawi-Konefka R, Vermi W, et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med. 2012;209(10):1869–82. doi: 10.1084/jem.20112738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muntasell A, Ochoa MC, Cordeiro L, et al. Targeting NK-cell checkpoints for cancer immunotherapy. Curr Opin Immunol. 2017;45:73–81. doi: 10.1016/j.coi.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Heylmann D, Rodel F, Kindler T, Kaina B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim Biophys Acta. 2014;1846(1):121–9. doi: 10.1016/j.bbcan.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181(5):3099–107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–9. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gras Navarro A, Bjorklund AT, Chekenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front Immunol. 2015;6:202. doi: 10.3389/fimmu.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis RJ, Van Waes C, Allen CT. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol. 2016;58:59–70. doi: 10.1016/j.oraloncology.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16(1):3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crittenden MR, Savage T, Cottam B, et al. The peripheral myeloid expansion driven by murine cancer progression is reversed by radiation therapy of the tumor. PLoS One. 2013;8(7):e69527. doi: 10.1371/journal.pone.0069527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–9. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Ruiz ME, Rodriguez I, Garasa S, et al. Abscopal Effects of Radiotherapy Are Enhanced by Combined Immunostimulatory mAbs and Are Dependent on CD8 T Cells and Crosspriming. Cancer Res. 2016;76(20):5994–6005. doi: 10.1158/0008-5472.CAN-16-0549. [DOI] [PubMed] [Google Scholar]
- 38.Crittenden MR, Cottam B, Savage T, Nguyen C, Newell P, Gough MJ. Expression of NF-kappaB p50 in tumor stroma limits the control of tumors by radiation therapy. PLoS One. 2012;7(6):e39295. doi: 10.1371/journal.pone.0039295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiang CS, Fu SY, Wang SC, et al. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front Oncol. 2012;2:89. doi: 10.3389/fonc.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai CS, Chen FH, Wang CC, et al. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int J Radiat Oncol Biol Phys. 2007;68(2):499–507. doi: 10.1016/j.ijrobp.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 41.Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 42.McNamee EN, Korns Johnson D, Homann D, Clambey ET. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol Res. 2013;55(1–3):58–70. doi: 10.1007/s12026-012-8349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamzah J, Jugold M, Kiessling F, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453(7193):410–4. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 44.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180(5):3132–9. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 45.Sonveaux P, Dessy C, Brouet A, et al. Modulation of the tumor vasculature functionality by ionizing radiation accounts for tumor radiosensitization and promotes gene delivery. FASEB J. 2002;16(14):1979–81. doi: 10.1096/fj.02-0487fje. [DOI] [PubMed] [Google Scholar]
- 46.Crokart N, Jordan BF, Baudelet C, et al. Early reoxygenation in tumors after irradiation: determining factors and consequences for radiotherapy regimens using daily multiple fractions. Int J Radiat Oncol Biol Phys. 2005;63(3):901–10. doi: 10.1016/j.ijrobp.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 47.Song CW, Lee YJ, Griffin RJ, et al. Indirect Tumor Cell Death After High-Dose Hypofractionated Irradiation: Implications for Stereotactic Body Radiation Therapy and Stereotactic Radiation Surgery. Int J Radiat Oncol Biol Phys. 2015;93(1):166–72. doi: 10.1016/j.ijrobp.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS) Radiat Res. 2012;177(3):311–27. doi: 10.1667/rr2773.1. [DOI] [PubMed] [Google Scholar]
- 49.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang X, Lin Y, Shi Y, et al. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016;76(14):4124–35. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 51.Gorchs L, Hellevik T, Bruun JA, et al. Cancer-associated fibroblasts from lung tumors maintain their immunosuppressive abilities after high-dose irradiation. Front Oncol. 2015;5:87. doi: 10.3389/fonc.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hellevik T, Pettersen I, Berg V, et al. Cancer-associated fibroblasts from human NSCLC survive ablative doses of radiation but their invasive capacity is reduced. Radiat Oncol. 2012;7:59. doi: 10.1186/1748-717X-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grinde MT, Vik J, Camilio KA, Martinez-Zubiaurre I, Hellevik T. Ionizing radiation abrogates the pro-tumorigenic capacity of cancer-associated fibroblasts co-implanted in xenografts. Sci Rep. 2017;7:46714. doi: 10.1038/srep46714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10(3):294–8. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- 55.Spiotto MT, Schreiber H. Rapid destruction of the tumor microenvironment by CTLs recognizing cancer-specific antigens cross-presented by stromal cells. Cancer Immun. 2005;5:8. [PubMed] [Google Scholar]
- 56.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204(1):49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64(21):7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 59.Obeid M, Panaretakis T, Tesniere A, et al. Leveraging the immune system during chemotherapy: moving calreticulin to the cell surface converts apoptotic death from “silent” to immunogenic. Cancer Res. 2007;67(17):7941–4. doi: 10.1158/0008-5472.CAN-07-1622. [DOI] [PubMed] [Google Scholar]
- 60.Wattenberg MM, Fahim A, Ahmed MM, Hodge JW. Unlocking the combination: potentiation of radiation-induced antitumor responses with immunotherapy. Radiat Res. 2014;182(2):126–38. doi: 10.1667/RR13374.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubner Y, Wunderlich R, Ruhle PF, et al. How does ionizing irradiation contribute to the induction of anti-tumor immunity? Front Oncol. 2012;2:75. doi: 10.3389/fonc.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gameiro SR, Malamas AS, Bernstein MB, et al. Tumor Cells Surviving Exposure to Proton or Photon Radiation Share a Common Immunogenic Modulation Signature, Rendering Them More Sensitive to T Cell-Mediated Killing. Int J Radiat Oncol Biol Phys. 2016;95(1):120–30. doi: 10.1016/j.ijrobp.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5(2):403–16. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64(12):4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 66.Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm. 2012;27(1):12–22. doi: 10.1089/cbr.2012.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–68. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 71.Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1(1):32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 73.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206(8):1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]