Abstract
The study aimed to determine the prevalence of occult Hepatitis B virus (HBV) infection among HIV infected persons, and to evaluate the use of a pooling strategy to detect occult HBV infection in the setting of HIV infection. Five hundred and two HIV positive individuals were tested for HBV, occult HBV, and Hepatitis C and D with serologic and nucleic acid testing (NAT). We also evaluated a pooled NAT strategy for screening occult HBV infection among the HIV-positive individuals. The prevalence of HBV infection among HIV positive individuals was 32 (6.4%) and occult HBV prevalence was 10%. The pooling HBV NAT had a sensitivity of 66.7% and specificity of 100%, compared to HBV DNA NAT of individual samples. In conclusion, this study found a high prevalence of occult HBV infection among our HIV infected population. We also demonstrated that pooled HBV NAT is highly specific, moderately sensitive and cost-effective. Since conventional HBV viral load assays are expensive in resource-limited settings such as India, pooled HBV DNA NAT might be a good way for detecting occult HBV infection and will reduce HBV associated complications.
Keywords: HBV, Occult HBV, HIV, pooled NAT, resource-limited settings
INTRODUCTION
Globally, 240 million people have chronic hepatitis B virus infection (HBV) (1), over 185 million have hepatitis C virus (HCV) (2) and over 36.7 million HIV (3). Since HIV, HBV and HCV often share same routes of transmission, co-infections are common, and these confections can greatly increase disease progression. One such co-infection that is often missed is HIV with occult HBV infection, which is defined as presence of HBV DNA in serum or liver despite the absence of HBsAg in the blood (4). Occult HBV infection cannot be identified by conventional assays, such as HBsAg testing, and requires HBV DNA nucleic acid testing (NAT); however, the cost of NAT is very high, thus most countries, like India, do not screen for the condition, despite its potential impact on a person’s health. To see if we could overcome this problem, we adapted a strategy of pooled NAT and evaluated the same as a cost effective approach for the diagnosis of OBI, wherein samples from a group of individuals will be pooled and tested for HBV DNA. If found to be positive, individual samples will be tested. The performance of different pooling strategy has been widely studied for HIV (5–7). An important advantage with this strategy is that, pooling PCR is cost effective and efficient for prevalence up to 30% for any infection tested.
METHODS
Study Population
We tested stored serum samples collected from HIV positive individuals, who attended the YRG centre for AIDS Research and Education (YRG CARE) for HIV testing between 2009 and 2010 (n=502). No participant had past history of HBV vaccination. Samples collected from the participants were processed within 6 hours and stored at −75°C till testing. At the time of testing, each untouched aliquot was retrieved from the freezer and thawed once prior to serological testing and NAT. The study was approved by the institutional review board (IRB) of YRG CARE and UCSD.
Laboratory Assays
HBsAg ELISA was performed using Genedia HBsAg ELISA 3.0, and anti-HCV ELISA was performed using Genedia HCV ELISA 3.0 (Green Cross Medical Science, Chungbuk, Korea). Hepatitis B (HB) serology assays included antibody to HBsAg (anti-HBs), antibody to hepatitis B core antigen (anti-HBc), hepatitis B envelope antigen (HBeAg), and the corresponding antibody (anti-HBe) were performed with Diasorin kits (Diasorin S.p.A. Saluggia, Italy), as per the manufacturer’s instructions. Hepatitis D antibody was also performed with a Diasorin kit (Diasorin S.p.A. Saluggia, Italy), as per manufacturer’s instructions. Occult HBV infection was identified by testing each sample individually for HBV DNA by the Abbott RealTime PCR (0.5mL) protocol (Abbott Molecular Inc., Des Plaines, IL, USA). Absolute CD4 T cell count was measured by Pan Leukocyte Gating (PLG) in a Beckman Coulter (Miami Florida Inc.USA) Cytomics FC500.
Pooling NAT (PCR) to identify Occult HBV infection
Since HBV infection can be missed with the serological tests described above, we evaluated the use of minipool HBV NAT to identify ‘Occult HBV’ infection, which is defined as HBsAg negative and HBV DNA positive. To try to increase testing efficiency, we applied minipool methods, as previously described (8, 9) with slight modifications. Briefly, minipools of five samples were prepared by combining 200 µL from each sample, constituting 1000 µL of sample in each minipool. Sample selection for constructing minipools was random and the assay performer was blinded to the already available HBV viral load results. To test the minipool, 500 µL from each pool was then tested for HBV DNA by Abbott real time PCR. If any minipool was found to be positive, each sample in the pool was individually deconvoluted, as per Dorfmann’s method (10). Results from minipool testing were compared to individual testing from each person’s sample to determine the test characteristics of the minipool NAT (Ref supplementary file).
Cost analysis
Cost effectiveness was evaluated by the following formula:
Statistical analysis
All demographic data were recorded as median and inter-quartile range (IQR) for continuous variable and as percentage for categorical variables. Chi-square test was performed to find the association between serological status and a particular group. Test characteristics of sensitivity, specificity, positive predictive value and negative predictive value were calculated, and relative efficiency was calculated, as described by May et al (11). GraphPad Prism version 5.0 was used for statistical analysis.
RESULTS
Characteristics of HIV infected individuals with positive HBV markers
We tested stored serum samples, collected from 502 HIV positive individuals who presented for HIV testing at a large testing center in India. Most samples collected for testing were largely from men (n=406, 80.9%), with a median age of 36 years (IQR 31–40) and median CD4 T cell count of 410 cells/mm3 (IQR 231–565). Among 502 participants, 207 (41.2%) reported heterosexual risk behavior and 295 (58.8%) reported injection drug usage. Among 502 tested, 32 (6.4%) were HBV (HBsAg) positive. Among the 32 persons with HBV infection, the majority were male, n=30 (94%) with median age of 36 years (IQR 33–40). Median HBV DNA viral load was 1,863 IU mL−1 (IQR 341–58,712,974 IU mL−1) and 7 (22%) had undetectable viral load. All persons with HBV infection were positive for anti-HBc and 1 (3%) was positive for anti-HBs. HBeAg positivity was seen in 7 (22%) individuals; individuals who were positive for HBeAg had viral load of > 7.5 log10IU mL−1, indicating HBeAg as a marker of active infection. Anti-HBe was detected in 19 (59%) individuals, all but two had viral loads less than 3.4 log10 IU mL−1, and all individuals positive for anti-HBe were negative for HBeAg. For completeness, we also tested for anti-HCV in all HIV infected person, and this was detected in 27 (84%) HBV positive individuals. We also tested for anti-HDV in half (n=17) of the HIV-HBV infected persons and HDV was detected in over a third of them (6/17) (Table 1).
Table 1.
Comparison of occult and overt HBV infection.
| Characteristics | Occult HBV (n=27) |
Overt HBV (n=32) |
P value |
|---|---|---|---|
|
| |||
| Age (IQR) | 35 (30–39) | 36 (33–40) | 0.7 |
|
| |||
| Sex | |||
|
| |||
| Male (%) | 37 (100%) | 30 (94%) | 0.5 |
|
| |||
| Mode of Transmission | |||
|
| |||
| Heterosexual (%) | 2 (7.4%) | 4 (12.5%) | 0.7 |
| Blood (%) | 25 (92.6%) | 28 (87.5%) | |
|
| |||
| CD4 T cell count (cells/µL), median (IQR)* | 451 (304–597) | 463 (367–577) | 0.5 |
|
| |||
| HBV viral load (IU mL−1) | 37 (21–131) | 1,863 (341–58,712,974) | < 0.0001 |
|
| |||
| Serological markers | |||
|
| |||
| Anti-HBc | 26 (96.3%) | 31 (96.9%) | 1.0 |
|
| |||
| Anti-HBs | 7 (25.9%) | 1 (3.1%) | 0.02 |
|
| |||
| HBeAg | 7 (25.9%) | 7 (21.9%) | 0.8 |
|
| |||
| Anti-HBe | 6 (22.2%) | 19 (59.4%) | 0.008 |
|
| |||
| Anti-HCV | 25 (92.6%) | 27 (84.4%) | 0.4 |
|
| |||
| Anti-HDV# | Nil | 6 (35.3%) | 0.002 |
Results are reported as frequency (%) and median (IQR)
Overt HBV is defined as HBsAg positive individuals
Tested for 22 HBV infected and 21occult HBV infected individuals
anti-HDV was tested for 17 HBV infected and all occult HBV infected individuals
Occult HBV
We randomly selected serum samples from 270 HBsAg negative HIV positive participants and these were tested for HBV DNA with the Abbott HBV DNA Realtime viral load assay, to identify occult HBV infection. 27 (10%) were found to have occult HBV, with median HBV viral load of 37 IU mL−1 (IQR 21–131). Median age of individuals with occult HBV infection was 35 years (IQR 30–39), and all were male (Table 1). Of those with detectable HBV DNA, 26/27, 96% were anti-HBc positive and 7/27, 25.9% were anti-HBs positive. None were HBeAg positive and 6/27 (22.2%) were anti-HBe positive. Interestingly, markers such as HBV viral load, anti-HBe and anti-HDV were significantly associated with overt HBV infection (HBsAg+) (p <0.05), whereas anti-HBs was significantly associated with occult HBV infection (p <0.05) (Table 1). Further, among those with occult HBV, none were positive for HDV and 25 (92.6%) were positive for anti-HCV.
Prediction of occult HBV prevalence, by selecting samples based on serological markers
In this study, we selected HBsAg negative samples for occult HBV identification. If anti-HBc or anti-HBs positive participants were selected for occult HBV testing only, we might have missed a few occult HBV infections and may have underestimated occult prevalence. Based on our analyses in our population, if only anti-HBc positive persons were selected for occult HBV testing, 26 would have had occult HBV infection. If anti-HBs positive persons were also selected for occult HBV identification, 7 additional people who have been identified (Table 2).
Table 2.
Predicting occult HBV infection based on serological markers.
| Marker | HBV DNA positive | Predicted occult prevalence |
Actual occult prevalence |
|---|---|---|---|
| anti-HBc | N=26 | 9.6% | 10% |
| Anti-HBs | N=7 | 2.6% |
Minipool NAT to identify Occult HBV infection
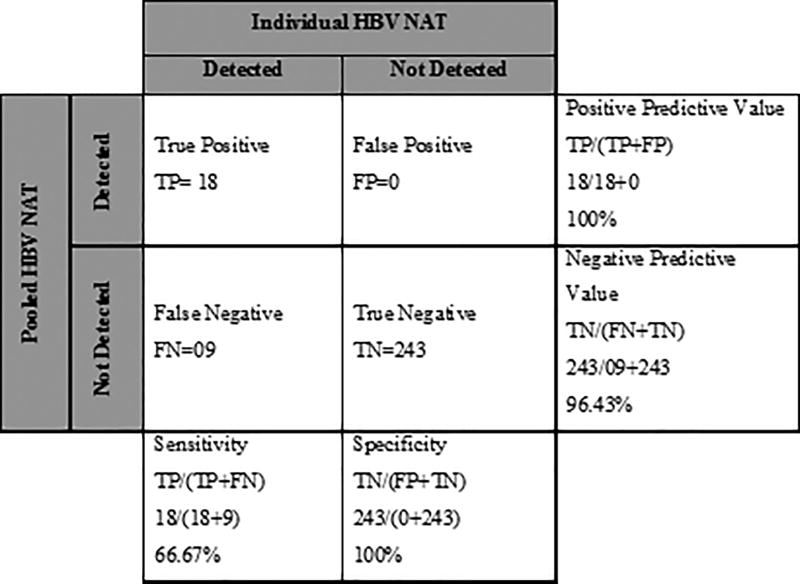
To identify occult HBV infection using the minipool strategy, we tested a different aliquot of a sample collected from the same person and prepared 54 minipools with each pool comprising 5 samples. Of the 54 pools tested, 13 pools had a detectable level of HBV DNA. All 13 pools were deconvoluted, as per Dorfman’s method (10) and identified HBV DNA in 18 samples, with a 6.7% prevalence. Overall, the minipool NAT assay showed a sensitivity and specificity of 66.7% and 100% respectively, positive predictive value of the assay was 100% and negative predictive value of the assay was 96.4% (Figure 1), when compared to testing each sample individually. We then estimated the cost-saving of the pooling strategy versus individual HBV viral load tests to identify occult HBV infection. For the pooled and deconvolution testing, we ran 119 tests to screen the 270 individuals. The cost per test was $85, so $10,115 was spent in the minipool testing and $22,950 in the individual testing (Table 3), so the cost saving was 56%.
Figure 1.
Table 3.
Comparison of Individual PCR and Pooling NAT.
| Individual PCR | Pooling NAT PCR |
|---|---|
| Reagent cost per reaction 85$ | |
| 270 tests performed | 119 tests performed (54 pools+65 deconvoluted tests from positive pools) |
| $22,950 | $10,115 |
| Advantage | |
| Gold Standard | Comparatively cheaper |
| Disadvantage | |
| Expensive | Less sensitive |
DISCUSSION
Since HBV infection is known to have a negative impact on HIV infection by doubling the events of AIDS or death (12), we investigated HBV infection among individuals with HIV infection in our South Indian population. The prevalence of HBV infection among HIV patients in our study was 6.3% and this is comparable to the global prevalence (3.6%) (13), as well as Indian HBV prevalence data in the general population (2 – 8%) (14–16). The prevalence of HIV-HBV coinfection in India from recent studies varies widely from (5.3%–11.3%) (17–21), which is consistent with our data. We saw a decreasing trend of HIV-HBV coinfection compared to the past decade, where the prevalence of HIV-HBV coinfection was 22.2% (22) and 30.4% (23). We further evaluated the presence of other hepatitis viral infections and found that the prevalence of HBV-HCV coinfection (5.4%) among HIV positive individuals was slightly higher than other reports from India, where the prevalence ranged from 0 – 2.5% (24–27), possibly because all HBV-HCV co-infected individuals were injection drug users. HIV-HBV-HDV infection was found in 35% of our HIV-HBV co-infected participants, which is in line with previous studies from our centre and other centers, where HDV infection among HIV/HBV coinfection was 36% (28, 29).
Occult HBV infection is clinically significant, especially in HIV infected and other immunosuppressed conditions where it can accelerate disease progression. So far, only a few groups have studied occult HBV infection in the Indian population, especially among HIV infected persons. In this study, we identified occult HBV infection among 10% of HIV positive participants. Results from other studies suggest that, occult HBV prevalence among HIV patients ranges widely from 0 – 89% (30–33). These rates depend on a number of factors like the endemicity of HBV infection, types of NAT used (some studies have used a qualitative assay, which is less sensitive), and using NAT only for anti-HBc (34) and anti-HBs (35) positive participants.
We next measured HBeAg, a marker of active HBV replication, among occult HBV participants, and all were negative. However, markers of HBV recovery, anti-HBe and anti-HBs were seen in 6/27 and 7/27 participants, respectively. This indicated a considerable proportion of occult HBV infections may be in the ‘recovery phase’, and follow-up data on these individuals may reveal whether they lose HBV DNA after some time (36).
Pooling NAT has been demonstrated to be a practical and effective means of analyzing large numbers of samples for a particular infection where the expected positivity is low (37). This technique has demonstrated accuracy and cost-effectiveness, as it can reduce the processing and reagent costs. In this study, we adapted a pooling NAT strategy to identify the prevalence of occult HBV infection and to define its test characteristics. We found that, the prevalence of occult HBV infection was 6.7% in pooled NAT (18/270) and10% by individual sample testing. Test specificity and positive predictive value were both 100% and sensitivity and negative predictive value was 66.7% and 96.4%, respectively. The low sensitivity was likely due to the inability of the assay to identify 9 participants, who had low levels of HBV DNA (all <15 IU mL−1).Of note, these very low levels of HBV DNA detected by individual testing could represent false positivity of the individual testing, but since this was the gold standard of our tests, we cannot be certain. Altogether, the pooling NAT methods would have missed 9 cases of occult HBV infection but would have cost 56% less than testing each person individually. Future strategies aimed at increasing sensitivity, such as increasing sample volume and viral concentration before performing pooling NAT, could enhance the sensitivity of the assay.
In conclusion, the prevalence of occult HBV infection among HIV participants was 10%, and the newly developed pooled HBV NAT was highly specific leading to cost savings in our setting, but the sensitivity of this method at low levels of HBV DNA was still low. Since conventional HBV viral load assays are expensive and occult HBV infection in the setting of HIV can have severe disease consequences, this pooled NAT method assay should be evaluated further.
Supplementary Material
Acknowledgments
The authors sincerely thank the study participants and appreciate the cooperation and assistance received from YRG CARE staff. This research was supported by the University of California, San Diego, Center for AIDS Research (CFAR), an NIH-funded program (AI036214) and EB015365. We also thank DST INSPIRE, New Delhi for providing financial assistance to TRD and CSIR, New Delhi for providing financial assistance to DS. This work received ethical approval from the Institutional Review Board of YRG CARE, Chennai, India.
Footnotes
Disclosures
None declared conflict of interest
References
- 1.Ott J, Stevens G, Groeger J, Wiersma S. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 2.Mohd Hanafiah K, Groeger J, Flaxman A, Wiersma S. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 3.UNAIDS Global report. 2013 http://files.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
- 4.De Mitri M, Cassini R, Bernardi M. Hepatitis B virus-related hepatocarcinogenesis: Molecular oncogenic potential of clear or occult infections. European Journal of Cancer. 2010;46(12):2178–2186. doi: 10.1016/j.ejca.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Tilghman M, Tsai D, Buene T, et al. Pooled Nucleic Acid Testing to Detect Antiretroviral Treatment Failure in HIV-Infected Patients in Mozambique. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2015;70(3):256–261. doi: 10.1097/QAI.0000000000000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Zyl G, Preiser W, Potschka S, Lundershausen A, Haubrich R, Smith D. Pooling Strategies to Reduce the Cost of HIV-1 RNA Load Monitoring in a Resource-Limited Setting. Clinical Infectious Diseases. 2010;52(2):264–270. doi: 10.1093/cid/ciq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilghman M, May S, Pérez-Santiago J, et al. A Combined Screening Platform for HIV Treatment Failure and Resistance. PLoS ONE. 2012;7(4):e35401. doi: 10.1371/journal.pone.0035401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharti A, Letendre S, Patra K, Vinetz J, Smith D. Malaria Diagnosis by a Polymerase Chain Reaction-Based Assay Using a Pooling Strategy. American Journal of Tropical Medicine and Hygiene. 2009;81(5):754–757. doi: 10.4269/ajtmh.2009.09-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilghman M, Guerena D, Licea A, et al. Pooled Nucleic Acid Testing to Detect Antiretroviral Treatment Failure in Mexico. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;56(3):e70–e74. doi: 10.1097/QAI.0b013e3181ff63d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorfman R. The Detection of Defective Members of Large Populations. The Annals of Mathematical Statistics. 1943;14(4):436–440. [Google Scholar]
- 11.May S, Gamst A, Haubrich R, Benson C, Smith D. Pooled Nucleic Acid Testing to Identify Antiretroviral Treatment Failure During HIV Infection. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2010;53(2):194–201. doi: 10.1097/QAI.0b013e3181ba37a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun H, Roediger M, Hullsiek K, et al. Hepatitis B Virus Coinfection Negatively Impacts HIV Outcomes in HIV Seroconverters. The Journal of Infectious Diseases. 2011;205(2):185–193. doi: 10.1093/infdis/jir720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schweitzer A, Horn J, Mikolajczyk R, Krause G, Ott J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. The Lancet. 2015;386(10003):1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 14.Abraham P. Viral Hepatitis in India. Clinics in Laboratory Medicine. 2012;32(2):159–174. doi: 10.1016/j.cll.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Tyagarajan SP, Jayaram S, Mohanavalli S. Prevalence of HBV in general population in India. Hepatitis B in India–Problem and Prevention. N. 1996:5–13. [Google Scholar]
- 16.Sarin SK, Singal AK. Hepatitis B in India problems and prevention. 1996:73. [Google Scholar]
- 17.Amin U, Girish N, Shruthi N, Rajendran R. Seroprevalence of Hepatitis B and C Co-infection in HIV Infected Patients: A Study in a Teritiary Care Centre From South India. International Journal of Contemporary Microbiology. 2016;2(1):26. [Google Scholar]
- 18.Sharma A, Halim J, Jaggi T, et al. Time trends of seroepidemiology of hepatitis C virus and hepatitis B virus coinfection in human immunodeficiency virus-infected patients in a Super Specialty Hospital in New Delhi, India: 2012–2014. Indian Journal of Sexually Transmitted Diseases and AIDS. 2016;37(1):33. doi: 10.4103/2589-0557.176214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha D, Pal A, Biswas A, et al. Characterization of Treatment-Naive HIV/HBV Co-Infected Patients Attending ART Clinic of a Tertiary Healthcare Centre in Eastern India. PLoS ONE. 2013;8(8):e73613. doi: 10.1371/journal.pone.0073613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guha S, Sarkar J, Saha D, et al. Baseline characteristics of HIV & hepatitis B virus (HIV/HBV) co-infected patients from Kolkata, India. Indian Journal of Medical Research. 2016;143(5):636. doi: 10.4103/0971-5916.187113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parande MV, Mantur BG, Parande AM, et al. Seroprevalence of human immunodeficiency virus & hepatitis B virus co-infection in Belgaum, southern India. Indian journal of medical research. 2013 Sep;138(3):364. [PMC free article] [PubMed] [Google Scholar]
- 22.Sud A, Singh J, Dhiman RK, et al. Hepatitis B virus co-infection in HIV infected patients. Tropical gastroenterology: official journal of the Digestive Diseases Foundation. 2001;22(2):90–2. [PubMed] [Google Scholar]
- 23.Tankhiwale SS, Khadase RK, Jalgoankar SV. Seroprevalence of anti-HCV and hepatitis B surface antigen in HIV infected patients. Indian journal of medical microbiology. 2003 Oct 1;21(4):268. [PubMed] [Google Scholar]
- 24.Chandra N, Joshi N, Raju YS, et al. Hepatitis B and/or C co-infection in HIV infected patients: A study in a tertiary care centre from south India. Indian Journal of Medical Research. 2013 Dec 1;138(6):950. [PMC free article] [PubMed] [Google Scholar]
- 25.Ahuja S, Malhotra S, Chauhan A, et al. Seroprevalence of Hepatitis B and C Co infection in HIV Positive Patients from a Tertiary Care Hospital. JIMSA. 2013 Apr;26:91. [Google Scholar]
- 26.Padmapriyadarsini C, Chandrabose J, Victor L, et al. Hepatitis B or hepatitis C co-infection in individuals infected with human immunodeficiency virus and effect of anti-tuberculosis drugs on liver function. Journal of postgraduate medicine. 2006 Apr 1;52(2):92. [PubMed] [Google Scholar]
- 27.Hussain T, Kulshreshtha K, Sinha S, Yadav V, Katoch V. HIV, HBV, HCV, and syphilis co-infections among patients attending the STD clinics of district hospitals in Northern India. International Journal of Infectious Diseases. 2006;10(5):358–363. doi: 10.1016/j.ijid.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Saravanan S, Madhavan V, Velu V, et al. High Prevalence of Hepatitis Delta Virus among Patients with Chronic Hepatitis B Virus Infection and HIV-1 in an Intermediate Hepatitis B Virus Endemic Region. Journal of the International Association of Providers of AIDS Care (JIAPAC) 2013;13(1):85–90. doi: 10.1177/2325957413488166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakraborty P, Kailash U, Jain A, et al. Seroprevalence of hepatitis D virus in patients with hepatitis B virus-related liver diseases. Indian Journal of Medical Research. 2005 Sep 1;122(3):254. [PubMed] [Google Scholar]
- 30.Núñez M, Ríos P, Pérez-Olmeda M, Soriano V. Lack of ‘occult’ hepatitis B virus infection in HIV-infected patients. AIDS. 2002;16(15):2099–2101. doi: 10.1097/00002030-200210180-00024. [DOI] [PubMed] [Google Scholar]
- 31.Hofer M, Joller-Jemelka H, Grob P, Lüthy R, Opravil M. Frequent chronic hepatitis B virus infection in HIV-infected patients positive for antibody to hepatitis B core antigen only. European Journal of Clinical Microbiology & Infectious Diseases. 1998;17(1):6–13. 15. doi: 10.1007/BF01584356. [DOI] [PubMed] [Google Scholar]
- 32.Piroth L, Grappin M, Buisson M, Duong M, Portier H, Chavanet P. Hepatitis B Virus Seroconversion in HIV-HBV Coinfected Patients Treated With Highly Active Antiretroviral Therapy. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2000;23(4):356–357. doi: 10.1097/00126334-200004010-00013. [DOI] [PubMed] [Google Scholar]
- 33.Shire N, Rouster S, Stanford S, et al. The Prevalence and Significance of Occult Hepatitis B Virus in a Prospective Cohort of HIV-Infected Patients. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2007;44(3):309–314. doi: 10.1097/QAI.0b013e31802e29a9. [DOI] [PubMed] [Google Scholar]
- 34.Martinez M, Kok C, Baleriola C, Robertson P, Rawlinson W. Investigation of Occult Hepatitis B Virus Infection in Anti-HBc Positive Patients from a Liver Clinic. PLOS ONE. 2015;10(3):e0117275. doi: 10.1371/journal.pone.0117275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su H, Zhang Y, Xu D, et al. Occult Hepatitis B Virus Infection in Anti-HBs-Positive Infants Born to HBsAg-Positive Mothers in China. PLoS ONE. 2013;8(8):e70768. doi: 10.1371/journal.pone.0070768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsui J, French A, Seaberg E, et al. Prevalence and Long-Term Effects of Occult Hepatitis B Virus Infection in HIV-Infected Women. Clinical Infectious Diseases. 2007;45(6):736–740. doi: 10.1086/520989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Z, Mitchell R, Gutman J, et al. Pooled PCR testing strategy and prevalence estimation of submicroscopic infections using Bayesian latent class models in pregnant women receiving intermittent preventive treatment at Machinga District Hospital, Malawi, 2010. Malaria Journal. 2014;13(1):509. doi: 10.1186/1475-2875-13-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.