Abstract
The presence of a conserved mechanism for mitochondrial calcium uptake in trypanosomatids was crucial for the molecular identification of the mitochondrial calcium uniporter (MCU), a long-sought channel present in most eukaryotic organisms. Since then, research efforts to elucidate the role of MCU and its regulatory elements in different biological models have multiplied. MCU is the pore-forming subunit of a multimeric complex (the MCU complex or MCUC) and its predicted structure in trypanosomes is simpler than in mammalian cells, lacking two of its subunits and probably possessing other unidentified components. MCU protein has been characterized in Trypanosoma brucei and Trypanosoma cruzi, the causative agents of African and American trypanosomiasis, respectively. Contrary to its mammalian homolog, TbMCU was found to be essential for cell growth and survival, while its paralog MCUb is an essential protein in T. cruzi. These findings could be further exploited for chemotherapeutic purposes. The emergence of new molecular tools for the genetic manipulation of trypanosomatids has been determinant for the functional characterization of the MCUC components in these organisms. However, further research has to be done to determine the role of each component in intracellular calcium signaling and cell bioenergetics. In this mini-review we summarize the original results on mitochondrial calcium uptake in trypanosomes, how did they contribute to the molecular identification of the MCU, and the functional characterization of the MCUC subunits that has so far been studied in these peculiar eukaryotes.
Keywords: Calcium signaling, Cell bioenergetics, Mitochondrial calcium uniporter complex, Trypanosomes
1. Introduction
Calcium ion (Ca2+) is an important second messenger that regulates a vast repertoire of cellular processes (Clapham, 2007). In mammalian cells, cytosolic free Ca2+ concentration is strictly maintained in the range of 20-100 nM. There, Ca2+ is sequestered by soluble calcium-binding proteins within different intracellular compartments, or can be directly removed from cytosol through the activation of different Ca2+ extrusion mechanisms (Ca2+-ATPase and Na+/Ca2+ exchangers) present in the plasma membrane or in intracellular Ca2+ stores (Clapham, 2007). Among these compartments, mitochondria play a key role in intracellular Ca2+ homeostasis (Rizzuto et al., 2012).
Mitochondrial Ca2+ homeostasis is important for cell bioenergetics by stimulation of aerobic metabolism through activation of mitochondrial dehydrogenases. It is also involved in pathways that lead to cellular life/death decisions (autophagy, necrosis, apoptosis) and in the control of cytosolic Ca2+ concentration (cytosolic free Ca2+ buffering) through rapid uptake upon Ca2+ release from endoplasmic reticulum (ER) and the sarcoplasmic reticulum (SR) or upon entry through the plasma membrane (Patron et al., 2013). In most eukaryotic organisms, including trypanosomatids, the mitochondrial calcium uniporter complex (MCUC) mediates mitochondrial Ca2+ uptake.
Trypanosomes are flagellated protists belonging to one of the oldest branches of eukaryotic cells containing mitochondria. They have a single mitochondria extended all over the cellular body, exhibiting peculiar characteristics (Docampo et al., 2014). The hallmark of these members of Kinetoplastida order, is the presence of a network of circular DNA, the kinetoplast or kDNA, inside a large mitochondrion, containing thousands of concatenated DNA circles, several of which correspond to maxicircles that encode a few mitochondrial genes, and exhibit a very complex mechanism of DNA replication. Mitochondrial mRNAs are subjected to RNA editing, a process that was first described in trypanosomes and then observed in other eukaryotic organisms. tRNAs are imported into mitochondria of trypanosomatids. Some of the main complexes of the electron transport chain are absent or not functional in certain trypanosomes (i.e. Phytomonas spp. and T. brucei bloodstream forms) (Docampo et al., 2014). ATP synthase activity has been found to work in reverse (as an ATPase) in T. brucei bloodstream forms, a feature that allows them to maintain their mitochondrial membrane potential in the absence of oxidative phosphorylation and complete pathways of the tricarboxylic acid cycle (Docampo et al., 2014; Nolan and Voorheis, 1992; Vercesi et al., 1992). A similar mechanism has been suggested in Phytomonas spp. where the presence of MCU complex orthologs in the absence of functional complexes of the electron transport chain suggests that they also utilize the ATP synthase in reverse to maintain a mitochondrial membrane potential that drives Ca2+ uptake through the MCU (Porcel et al., 2014). However, the mitochondrial Ca2+ uptake mechanism is very conserved among trypanosomatids and mammalian cells, and this observation (Docampo and Vercesi, 1989a; Docampo and Vercesi, 1989b) together with the fact that MCU activity is absent in the budding yeast Sccharomyces cerevisiae (Carafoli et al., 1970), led to the molecular identification in 2011 of the MCU (Baughman et al., 2011; De Stefani et al., 2011), the pore-forming subunit of the MCU complex (MCUC). In this work we review the original results on mitochondrial Ca2+ uptake in trypanosomes, how did they contribute to the molecular identification of the MCU, and the functional characterization of the MCUC subunits of trypanosomatids performed through genetic manipulation and modulation of their expression.
2. The molecular identification of the Mitochondrial Calcium Uniporter (MCU)
MCU-mediated mitochondrial Ca2+ uptake was first observed in rat kidney mitochondria (Deluca and Engstrom, 1961; Vasington and Murphy, 1962), and for many years this channel was thought to be absent in other eukaryotes such as insects, plants and unicellular organisms, including yeast (Carafoli et al., 1970). This scenario changed in 1989 when mitochondrial Ca2+ uptake with similar characteristics to that mediated by MCU in mammalian cells was reported in Trypanosoma cruzi (Docampo and Vercesi, 1989a; Docampo and Vercesi, 1989b), the causative agent of Chagas disease. The MCU activity observed in T. cruzi exhibited low-affinity electrogenic Ca2+ transport, was inhibited by ruthenium red, and had high capacity as observed by successive Ca2+ addition to digitonin-permeabilized T. cruzi epimastigotes in the presence of succinate as mitochondrial substrate (Docampo and Vercesi, 1989a) (Figure 1A). MCU-mediated Ca2+ uptake was later reported in other kinetoplastids such as Leishmania spp. (Benaim et al., 1990; Vercesi and Docampo, 1992; Vercesi et al., 1990), Crithidia fasciculata (Vercesi et al., 1990) and Trypanosoma brucei (Moreno et al., 1992; Vercesi et al., 1992; Vercesi et al., 1993). The presence of MCU in vertebrates and kinetoplastids, together with the fact that this channel is absent in yeast, was used for the in silico identification of proteins involved in mitochondrial Ca2+ uptake (Perocchi et al., 2010), from which MICU1 (mitochondrial calcium uptake 1) appeared to be a promising candidate according to an RNAi screen of the selected genes. In this study the following criteria were used to identify MICU1 from MitoCarta database: inner mitochondrial membrane localization, ubiquitous expression in mammalian tissues and presence of homologs in vertebrates and kinetoplastids but not in S. cerevisiae. However, MICU1 exhibits a single putative transmembrane domain and therefore was unlikely to be the pore-forming subunit itself. Later on, the inclusion of an additional criterion, the presence of at least two predicted transmembrane α-helixes in the primary sequence, led to find 14 MCU-candidate human proteins, from which the product of ccdc109a gene was finally identified as the MCU (De Stefani et al., 2011). Using a different approach, the same gene was simultaneously identified as encoding the human MCU by another group (Baughman et al., 2011), searching for genes closely related to MICU1 by evolutionary co-occurrence and co-expression profiles. Gene knockdown and overexpression confirmed that ccdc109a encodes MCU and its orthologs were successively characterized in different organisms (Reviewed in (Docampo and Lukes, 2012; Mammucari et al., 2017; Patron et al., 2013).
Figure 1. Mitochondrial calcium uniporter (MCU) in Trypanosoma cruzi.
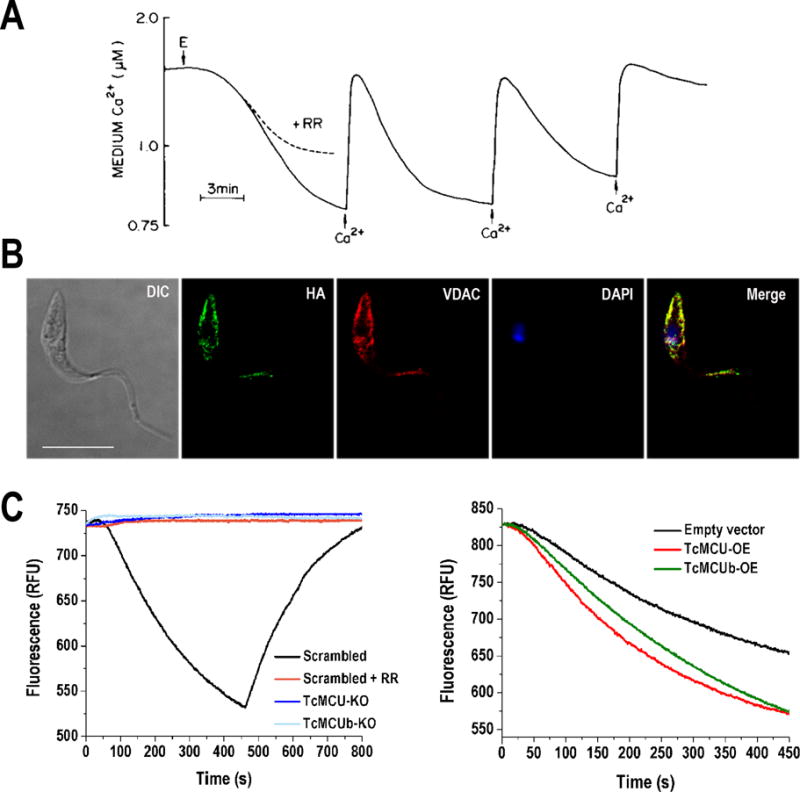
A) Mitochondrial Ca2+ uptake by digitonin-permeabilized T. cruzi epimastigotes (E) in the presence of succinate. T. cruzi mitochondria has the ability to take up Ca2+ in successive additions and as in mammalian cells, mitochondrial Ca2+ uptake by MCU is inhibited by ruthenium red (RR). B) Fluorescence microscopy of endogenously tagged TcMCU-3xHA in T. cruzi epimastigotes confirms the localization of TcMCU in mitochondria. TcMCU-3xHA was detected with monoclonal anti-HA antibodies (green) whereas anti-TbVDAC (T. brucei voltage dependent anion channel) was used as mitochondrial marker (red). Yellow signal in merge indicates co-localization. Nucleus and kinetoplast were labeled with DAPI (blue). Bars, 10 μm. C) Ca2+ uptake by digitonin-permeabilized TcMCU- and TcMCUb-knockout (KO) cells. Scrambled, scrambled control cells in absence or presence of 5 μM ruthenium red (RR). The reaction was started after adding 50 μM digitonin in the presence of 5 mM succinate and 20 μM CaCl2. The variation of Calcium Green-5N fluorescence is showed in relative fluorescence units (RFU), in which a decreasing extramitochondrial Ca2+ concentration (or increasing mitochondrial [Ca2+]) is observed as a decrease in fluorescence. Addition of the uncoupler carbonyl cyanide p-trifluoromethoxylhydrazone (FCCP) releases mitochondrial Ca2+ taken up, which is evidenced by the increase of fluorescence. D) Ca2+ uptake by digitonin-permeabilized TcMCU- and TcMCUb-overexpressing (OE) and control (epimastigotes transfected with empty pTREX vector) cells. Conditions were as panel C. The image in Panel A has been reproduced with permission of (Docampo and Vercesi, 1989a).
3. Structure of the mitochondrial calcium uniporter complex
In mammalian cells, the MCU complex is a 480 kDa multimeric structure located at the inner mitochondrial membrane (IMM), conformed by the pore-forming protein (MCU) and its regulatory subunits (Figure 2A). The electrochemical gradient across the IMM (mitochondrial membrane potential, ΔΨm) provides the driving force for MCU-mediated mitochondrial Ca2+ uptake. However, basal mitochondrial Ca2+ concentration ([Ca2+]m) is in the nanomolar range, due to the low Ca2+ affinity of the channel. Inositol 1,4,5-trisphosphate (InsP3) -mediated Ca2+ release from ER generates high (micromolar range) cytosolic Ca2+ concentration ([Ca2+]cyt) at microdomains of closed proximity between ER and mitochondria, that stimulates mitochondrial Ca2+ uptake through the high-capacity MCU complex. The MCU complex capacity and Ca2+ sensitivity is tissue-specific regulated by its different subunits (Reviewed in (Docampo and Lukes, 2012; Mammucari et al., 2017)).
Figure 2. Mitochondrial calcium uniporter complex (MCUC) organization.
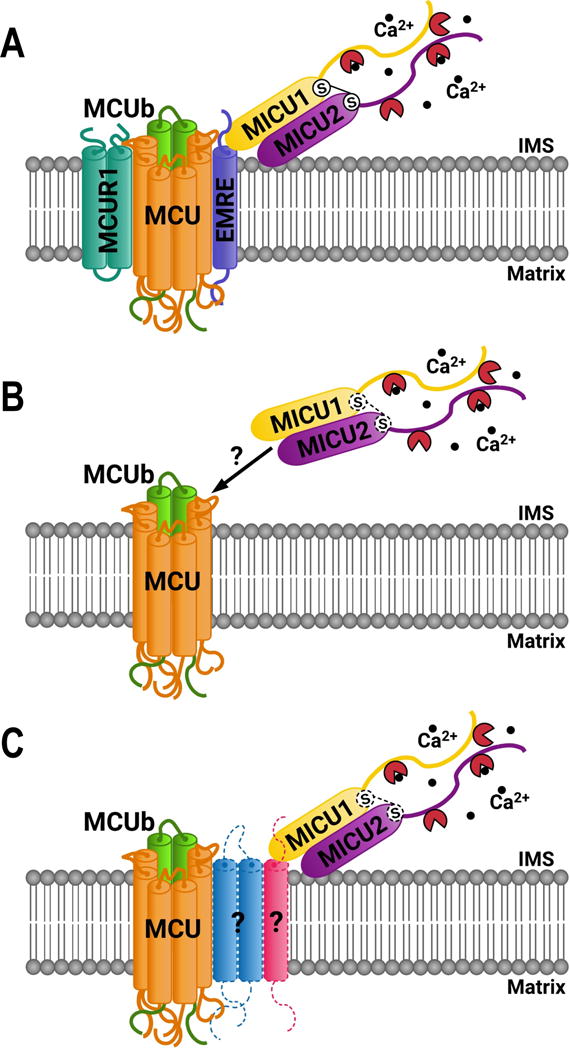
A) In mammals the MCUC is constituted by the pore-forming subunit MCU, and regulator proteins MCUb, MICU1, MICU2, EMRE and MCUR1. B) MCUC in trypanosomes seems to be simpler than those of mammals. No orthologs for EMRE and MCUR1 subunit has been identified, or other subunits that could interact with MICU1 and MICU2. Although there are C-terminal cysteine residues in MICU1 and MICU2 that could form a disulfide bridge, dimer formation between these proteins have not been confirmed yet. C) Hypothetical model of the MCUC in trypanosomes showing possible additional subunits of the complex that remain unidentified, indicated by question marks. IMS: inter membrane space. Black balls: Ca2+ ions. Dark red circular sector: EF hand domains.
The MCU protein contains two transmembrane domains (TMD) separated by a short hydrophilic region enriched in acidic amino acids (the DIME motif), with both the N- and the C-terminus facing the mitochondrial matrix. Sequence analysis led to predict that the MCU structure is conserved in all the species where it is present, including trypanosomatids (Docampo et al., 2014). The negatively charged residues of the DIME loop conform the Ca2+ selectivity filter (Mammucari et al., 2017; Patron et al., 2013). A tetrameric organization has been proposed in mammalian MCU (Raffaello et al., 2013), while a pentameric structure has been proposed in Caernohabditis elegans (Oxenoid et al., 2016). Mammalian MCU has not been found to be essential in a mixed genetic background of C57BL/6 and CD1 mice, while the absence of MCU was observed to be embryonically lethal in a C57BL/6 pure background (Pendin et al., 2014) and MCU−/− CD1 mice exhibited a defective skeletal muscle performance (Pan et al., 2013).
An MCU paralog named MCUb has been also described in mammalian cells. This MCU complex subunit shares 50% similarity with MCU and also contains two TMDs (Mammucari et al., 2017). It has been reported that MCUb exerts an inhibitory effect on MCU, acting as a dominant negative subunit of the MCU complex (Raffaello et al., 2013). In addition, MCU/MCUb ratio varies among different tissues, suggesting that MCUb plays a tissue-specific regulatory role (Mammucari et al., 2017).
EMRE (essential MCU regulator) is a 10 kDa subunit of the mammalian MCU complex. EMRE contains a single TMD and it has been found to be necessary for MCU activity and interaction with MICU1 and MICU2 (scaffold protein) (Sancak et al., 2013). The essentiality of this subunit has been widely debated, as its absence in mice does not generate an evident phenotype (Liu et al., 2016). However, reconstitution of human MCU in S. cerevisiae requires co-expression of EMRE. It is currently accepted that EMRE plays a regulatory role in the MCU complex, in addition to its scaffold role to keep in place MCU and MICU proteins (Mammucari et al., 2017).
MICU1 and MICU2 proteins are EF-hand containing subunits of the MCU complex located at the inter membrane space. These two paralogs are considered the “gatekeepers” of the uniporter, forming a heterodimer where they exert opposite roles: MICU1 plays a stimulatory role while MICU2 has an inhibitory effect on mitochondrial Ca2+ uptake (Patron et al., 2014). MICU2 inhibitory effect prevails at low [Ca2+]cyt while MICU1 activates the channel opening at high [Ca2+]cyt, through conformational changes induced by Ca2+ binding to its EF-hand motifs. MICU1 and MICU2 expression levels are dependent on each other and they act together to establish the threshold of [Ca2+]cyt response (Mammucari et al., 2017; Patron et al., 2014). More recently the MICU1–MICU2 heterodimer was reconstituted to demonstrate that it binds Ca2+ cooperatively with high affinity (Kamer et al., 2017). In this work the authors demonstrated that both proteins are stabilized by Ca2+ and that MICU1-MICU2 interaction complex with Ca2+ serves as an on-off switch of the MCU complex, leading to a sharp control of the channel, that is able to directly respond to cytosolic Ca2+ changes (Kamer et al., 2017).
Finally, an additional regulatory subunit of the MCU complex has been debated to be part of it, the MCUR1, an IMM protein that also possesses two TMDs. MCUR1 was initially reported as a positive modulator of MCU (Mallilankaraman et al., 2012). However, a role as cytochrome c oxidase assembly factor was found later on, with implications in the collapse of ΔΨm that could explain the decrease in mitochondrial Ca2+ uptake observed upon MCUR1 silencing (Paupe et al., 2015). However, recent data points out to the fact that MCUR1 effect on ΔΨm is negligible, as demonstrated in experiments where ΔΨm was strictly monitored and MCUR1 silencing significantly decreased MCU activity (Vais et al., 2015).
4. The role of MCU complex in trypanosomes
The molecular identification of the gene encoding the human MCU made possible to find its orthologs in many model organisms, including trypanosomes. In fact, one gene encoding the MCU was identified in each trypanosome for which the genome sequence was available (Docampo et al., 2014). The predicted MCU of trypanosomatids also exhibits a mitochondrial targeting signal (MTS) and two TMD separated by a short loop containing the DIME motif (Docampo et al., 2014; Huang et al., 2013b). The protein localizes in mitochondria, as confirmed by immunofluorescence analysis in T. brucei bloodstream forms (BSF) and procyclic forms (PCF) (Huang et al., 2013b) and by CRISPR/Cas9-mediated endogenous tagging and overexpression in T. cruzi epimastigotes (Chiurillo et al., 2017; Lander et al., 2016) (Figure 1B). Orthologs of MCUb, MICU1 and MICU2 subunits of the MCU complex have also been identified in trypanosomes, while MCUR1 and EMRE appear to be absent in these organisms (Figure 2B) (Docampo et al., 2014). These two last subunits seemed to have been acquired more recently in evolution, while MICU2 orthologs have not been identified in Leishmania spp. (Docampo et al., 2014).
The role of MCU was first investigated in T. brucei PCF and BSF, in experiments where MCU was downregulated by RNAi and conditional knockout, respectively (Huang et al., 2013b). These experiments demonstrated that MCU alone is responsible for mitochondrial Ca2+ uptake in digitonin-permeabilized cells and that this protein is essential for parasite survival in vitro and in vivo. As reported in mammalian cells, TbMCU silencing induced a decrease in mitochondrial Ca2+ uptake without affecting the ΔΨm. This downregulation in PCF also increased the AMP/ATP ratio and induced autophagy, which can be interpreted as the survival mechanism previously observed in mammalian cells. This phenotype was stronger when T. brucei PCF were cultured in proline-rich/glucose-depleted medium, emulating the nutrient conditions of its invertebrate vector, the tse tse fly. Under these conditions, PCF consume more proline and their energy metabolism mainly relies in oxidative phosphorylation (Huang et al., 2013b). The first reaction of the metabolic conversion of proline into glutamate is catalyzed by proline dehydrogenase (PRODH), an enzyme that in trypanosomes possesses an EF-hand domain that is absent in its mammalian homolog. Thus, mitochondrial Ca2+ could be important for its activation (Docampo et al., 2014; Huang et al., 2013b). In contrast, in regular culture medium (glucose-rich condition) mitochondrial Ca2+ could activate pyruvate dehydrogenase (PDH) phosphatase, which stimulates PDH and induces pyruvate oxidation (Docampo et al., 2014; Huang et al., 2013b). In this regard, an ortholog of PDH E1α with putative phosphorylation sites is present en trypanosomes (Docampo et al., 2014), and a putative pyruvate dehydrogenase phosphatase (PDP) has been identified by mass spectrometry in T. brucei (Gunasekera et al., 2012).
MCU downregulation in T. brucei BSF completely blocked mitochondrial Ca2+ uptake in the presence of ATP, without affecting the mitochondrial membrane potential (Huang et al., 2013b). This phenotype was partially rescued when threonine was added to the culture medium. Threonine dehydrogenase probably allows BSF to bypass the need for activation of Ca2+ -stimulated PDH in TbMCU knockout parasites, for generation of acetyl-CoA and intramitochondrial fatty acid synthesis, a pathway that is essential in this parasite stage (Docampo et al., 2014; Huang et al., 2013b).
The overexpression of TbMCU in PCF resulted in an increase of mitochondrial Ca2+ uptake, leading to mitochondrial Ca2+ overload, increased sensitivity to pro-apoptotic agents, reactive oxygen species (ROS) generation and cell death, in agreement with results in vertebrate cells (Docampo et al., 2014; Huang et al., 2013b).
Using the CRISPR/Cas9 technology we previously adapted for genome editing in T. cruzi (Lander et al., 2015) we investigated the role of TcMCU and its paralog TcMCUb (Chiurillo et al., 2017), for which the mammalian ortholog has been found to exert a dominant negative effect on MCU, regulating in that way its activity (Raffaello et al., 2013). Ablation of TcMCU and TcMCUb by CRISPR/Cas9 led to the conclusion that both proteins are necessary for mitochondrial Ca2+ uptake, while only TbMCUb is essential for parasite survival, suggesting an additional role for this subunit in cell bioenergetics (Chiurillo et al., 2017). TcMCU and TcMCUb knockout (TcMCU-KO and TcMCUb-KO) permeabilized epimastigotes are unable to take up Ca2+, as compared with control cells in the presence of mitochondrial substrates and Calcium green-5N probe (Figure 1C). In contrast, overexpression of both genes resulted in a significant increase in the ability of mitochondria to accumulate Ca2+ (Figure 1D). While TcMCU-KO parasites exhibited normal growth, unaffected percentage of metacyclogenesis (ability to differentiate into infective metacyclic trypomastigote forms), normal infectivity of tissue-cultured host cells, and intracellular replication, TcMCUb-KO parasites resulted in epimastigotes showing a significant growth defect, lower metacyclogenesis and respiration rates, reduced mitochondrial mass, increased autophagy under starvation conditions and impaired infectivity.
Complementation of TcMCU-KO epimastigotes with an exogenous TcMCU gene copy but not with the gene mutated in the essential residues of the DIME motif Asp223 and Glu226, was able to restore mitochondrial Ca2+ uptake, confirming that these two conserved acidic residues are indeed necessary for TcMCU-mediated Ca2+ uptake (Chiurillo et al., 2017). However, a mutant including a substitution at the acidic residue Asp219, which was expected to be a critical residue for Ca2+ transport, was able to rescue Ca2+ uptake in TcMCU-KO epimastigotes.
Overexpression of the TcMCUb paralog in TcMCU-KO epimastigotes did not restore the mitochondrial Ca2+ uptake, suggesting that TcMCUb cannot replace TcMCU. Another interesting result found in T. cruzi is that human MCU was unable to restore mitochondrial Ca2+ transport in TcMCU-KO cells (Chiurillo et al., 2017). Furthermore, TcMCU alone could not reconstitute mitochondrial Ca2+ uptake in yeast mitochondria. As EMRE and MCUR1 orthologs are absent in T. cruzi, this result suggests that other unidentified subunits of the MCU complex could be required for TcMCU to reconstitute mitochondrial Ca2+ uptake in yeast. Whether one or more additional subunits are present in the MCUC of trypanosomatids, and if one of them bridges MICU1 and MICU2 to MCU should be further investigated (Figure 2C). Finally, the overexpression of TcMCUb did not result in a dominant negative effect on the T. cruzi MCU complex (Chiurillo et al., 2017), in contrast to the results reported in its mammalian ortholog (Raffaello et al., 2013).
A peculiar characteristic of Ca2+ signaling in trypanosomes is the presence of acidocalcisomes, acidic, electron-dense, phosphate and calcium-rich compartments that were first described in trypanosomes (Docampo et al., 1995; Vercesi et al., 1994). The inositol 1,4,5-triphosphate receptor (IP3R) of T. brucei and T. cruzi is located in this organelle instead of the ER (Huang et al., 2013a; Lander et al., 2016). Therefore, InsP3 -mediated Ca2+ release from acidocalcisomes could generate high [Ca2+]cyt at microdomains of close proximity between acidocalcisomes and mitochondria, stimulating mitochondrial Ca2+ uptake through the MCU complex. Thus, the existence of such microdomains can be predicted in trypanosomes and should be further investigated.
2. Concluding remarks
In conclusion, the mitochondrial Ca2+ uptake mechanism of trypanosomes was determinant to elucidate the molecular identity of the MCU. This channel seems to be simpler in trypanosomes than in higher eukaryotes, although the presence of additional unidentified subunits cannot be ruled out. Different elements of the MCU complex are essential in these parasites. While T. brucei MCU was the first essential MCU homolog ever reported, the T. cruzi MCUb paralog was in turn found to be an essential subunit of the complex in this organism. The structural and functional differences between the MCU complex of mammalian cells and trypanosomes can be explored in the future for the search of alternative anti-parasitic chemotherapies. The emergence of the CRISPR/Cas9 technology has significantly improved the ability to perform genetic interventions in trypanosomatids, which has been exploited for the functional characterization of the MCU subunits. The biochemical and physiological characteristics of the MCU complex in trypanosomes represent an excellent example of their relevance as biological models.
Acknowledgments
Funding
Funding for this work was provided by the São Paulo Research Foundation (FAPESP), Brazil (2013/50624-0), and the U.S. National Institutes of Health (grants AI107663 and AI104120). N.L. and M.A.C. are postdoctoral fellows of FAPESP (grants 2014/08995-4 and 2014/13148-9, respectively). M.S.B. is a Master’s fellow of FAPESP (grant 2015/25709-8).
Abbreviations
- ER
endoplasmic reticulum
- SR
sarcoplasmic reticulum
- MCUC
mitochondrial calcium uniporter complex
- MCU
mitochondrial calcium uniporter
- MICU1
mitochondrial calcium uptake 1
- IMM
inner mitochondrial membrane
- ΔΨm
mitochondrial membrane potential
- [Ca2+]m
mitochondrial calcium ion concentration
- InsP3
inositol 1,4,5-trisphosphate
- [Ca2+]cyt
cytosolic calcium ion concentration
- TMD
transmembrane domains
- MCUb
mitochondrial calcium uniporter b
- EMRE
essential MCU regulator
- MICU2
mitochondrial calcium uptake 2
- MCUR1
mitochondrial calcium uniporter regulator 1
- BSF
bloodstream forms
- PCF
procyclic forms
- CRISPR/Cas9
clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9
- PRODH
proline dehydrogenase
- PDH
pyruvate dehydrogenase
- PDP
pyruvate dehydrogenase phosphatase
- IP3R
inositol 1,4,5-triphosphate receptor
References
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaim G, Bermudez R, Urbina JA. Ca2+ transport in isolated mitochondrial vesicles from Leishmania braziliensis promastigotes. Mol Biochem Parasitol. 1990;39:61–68. doi: 10.1016/0166-6851(90)90008-a. [DOI] [PubMed] [Google Scholar]
- Carafoli E, Balcavage WX, Lehninger AL, Mattoon JR. Ca2+ metabolism in yeast cells and mitochondria. Biochim Biophys Acta. 1970;205:18–26. doi: 10.1016/0005-2728(70)90057-5. [DOI] [PubMed] [Google Scholar]
- Chiurillo MA, Lander N, Bertolini MS, Storey M, Vercesi AE, Docampo R. Different Roles of Mitochondrial Calcium Uniporter Complex Subunits in Growth and Infectivity of Trypanosoma cruzi. MBio. 2017;8 doi: 10.1128/mBio.00574-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca HF, Engstrom GW. Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci U S A. 1961;47:1744–1750. doi: 10.1073/pnas.47.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, Lukes J. Trypanosomes and the solution to a 50-year mitochondrial calcium mystery. Trends Parasitol. 2012;28:31–37. doi: 10.1016/j.pt.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, Scott DA, Vercesi AE, Moreno SN. Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi. Biochem J. 1995;310(Pt 3):1005–1012. doi: 10.1042/bj3101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, Vercesi AE. Ca2+ transport by coupled Trypanosoma cruzi mitochondria in situ. J Biol Chem. 1989a;264:108–111. [PubMed] [Google Scholar]
- Docampo R, Vercesi AE. Characteristics of Ca2+ transport by Trypanosoma cruzi mitochondria in situ. Archives of biochemistry and biophysics. 1989b;272:122–129. doi: 10.1016/0003-9861(89)90202-6. [DOI] [PubMed] [Google Scholar]
- Docampo R, Vercesi AE, Huang G. Mitochondrial calcium transport in trypanosomes. Mol Biochem Parasitol. 2014;196:108–116. doi: 10.1016/j.molbiopara.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekera K, Wuthrich D, Braga-Lagache S, Heller M, Ochsenreiter T. Proteome remodelling during development from blood to insect-form Trypanosoma brucei quantified by SILAC and mass spectrometry. BMC Genomics. 2012;13:556. doi: 10.1186/1471-2164-13-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Bartlett PJ, Thomas AP, Moreno SN, Docampo R. Acidocalcisomes of Trypanosoma brucei have an inositol 1,4,5-trisphosphate receptor that is required for growth and infectivity. Proc Natl Acad Sci U S A. 2013a;110:1887–1892. doi: 10.1073/pnas.1216955110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Vercesi AE, Docampo R. Essential regulation of cell bioenergetics in Trypanosoma brucei by the mitochondrial calcium uniporter. Nat Commun. 2013b;4:2865. doi: 10.1038/ncomms3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer KJ, Grabarek Z, Mootha VK. High-affinity cooperative Ca(2+) binding by MICU1-MICU2 serves as an on-off switch for the uniporter. EMBO Rep. 2017;18:1397–1411. doi: 10.15252/embr.201643748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander N, Chiurillo MA, Storey M, Vercesi AE, Docampo R. CRISPR/Cas9-mediated endogenous C-terminal Tagging of Trypanosoma cruzi Genes Reveals the Acidocalcisome Localization of the Inositol 1,4,5-Trisphosphate Receptor. J Biol Chem. 2016;291:25505–25515. doi: 10.1074/jbc.M116.749655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander N, Li ZH, Niyogi S, Docampo R. CRISPR/Cas9-Induced Disruption of Paraflagellar Rod Protein 1 and 2 Genes in Trypanosoma cruzi Reveals Their Role in Flagellar Attachment. MBio. 2015;6:e01012. doi: 10.1128/mBio.01012-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, Liu J, Holmstrom KM, Menazza S, Parks RJ, Fergusson MM, Yu ZX, Springer DA, Halsey C, Liu C, Murphy E, Finkel T. MICU1 Serves as a Molecular Gatekeeper to Prevent In Vivo Mitochondrial Calcium Overload. Cell Rep. 2016;16:1561–1573. doi: 10.1016/j.celrep.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, Miller R, Kolesar JE, Molgo J, Kaufman B, Hajnoczky G, Foskett JK, Madesh M. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, Gherardi G, Rizzuto R. Structure, Activity Regulation, and Role of the Mitochondrial Calcium Uniporter in Health and Disease. Front Oncol. 2017;7:139. doi: 10.3389/fonc.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno SN, Docampo R, Vercesi AE. Calcium homeostasis in procyclic and bloodstream forms of Trypanosoma brucei. Lack of inositol 1,4,5-trisphosphate-sensitive Ca2+ release. J Biol Chem. 1992;267:6020–6026. [PubMed] [Google Scholar]
- Nolan DP, Voorheis HP. The mitochondrion in bloodstream forms of Trypanosoma brucei is energized by the electrogenic pumping of protons catalysed by the F1F0-ATPase. Eur J Biochem. 1992;209:207–216. doi: 10.1111/j.1432-1033.1992.tb17278.x. [DOI] [PubMed] [Google Scholar]
- Oxenoid K, Dong Y, Cao C, Cui T, Sancak Y, Markhard AL, Grabarek Z, Kong L, Liu Z, Ouyang B, Cong Y, Mootha VK, Chou JJ. Architecture of the mitochondrial calcium uniporter. Nature. 2016;533:269–273. doi: 10.1038/nature17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, Aponte AM, Gucek M, Balaban RS, Murphy E, Finkel T. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabo I, De Stefani D, Rizzuto R. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell. 2014;53:726–737. doi: 10.1016/j.molcel.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron M, Raffaello A, Granatiero V, Tosatto A, Merli G, De Stefani D, Wright L, Pallafacchina G, Terrin A, Mammucari C, Rizzuto R. The mitochondrial calcium uniporter (MCU): molecular identity and physiological roles. J Biol Chem. 2013;288:10750–10758. doi: 10.1074/jbc.R112.420752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupe V, Prudent J, Dassa EP, Rendon OZ, Shoubridge EA. CCDC90A (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell Metab. 2015;21:109–116. doi: 10.1016/j.cmet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Pendin D, Greotti E, Pozzan T. The elusive importance of being a mitochondrial Ca(2+) uniporter. Cell Calcium. 2014;55:139–145. doi: 10.1016/j.ceca.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcel BM, Denoeud F, Opperdoes F, Noel B, Madoui MA, Hammarton TC, Field MC, Da Silva C, Couloux A, Poulain J, Katinka M, Jabbari K, Aury JM, Campbell DA, Cintron R, Dickens NJ, Docampo R, Sturm NR, Koumandou VL, Fabre S, Flegontov P, Lukes J, Michaeli S, Mottram JC, Szoor B, Zilberstein D, Bringaud F, Wincker P, Dollet M. The streamlined genome of Phytomonas spp. relative to human pathogenic kinetoplastids reveals a parasite tailored for plants. PLoS Genet. 2014;10:e1004007. doi: 10.1371/journal.pgen.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabo I, Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, Goldberger O, Mootha VK. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vais H, Tanis JE, Muller M, Payne R, Mallilankaraman K, Foskett JK. MCUR1, CCDC90A, Is a Regulator of the Mitochondrial Calcium Uniporter. Cell Metab. 2015;22:533–535. doi: 10.1016/j.cmet.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasington FD, Murphy JV. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem. 1962;237:2670–2677. [PubMed] [Google Scholar]
- Vercesi AE, Docampo R. Ca2+ transport by digitonin-permeabilized Leishmania donovani. Effects of Ca2+, pentamidine and WR-6026 on mitochondrial membrane potential in situ. Biochem J. 1992;284(Pt 2):463–467. doi: 10.1042/bj2840463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercesi AE, Docampo R, Moreno SN. Energization-dependent Ca2+ accumulation in Trypanosoma brucei bloodstream and procyclic trypomastigotes mitochondria. Mol Biochem Parasitol. 1992;56:251–257. doi: 10.1016/0166-6851(92)90174-i. [DOI] [PubMed] [Google Scholar]
- Vercesi AE, Macedo DV, Lima SA, Gadelha FR, Docampo R. Ca2+ transport in digitonin-permeabilized trypanosomatids. Mol Biochem Parasitol. 1990;42:119–124. doi: 10.1016/0166-6851(90)90119-7. [DOI] [PubMed] [Google Scholar]
- Vercesi AE, Moreno SN, Bernardes CF, Meinicke AR, Fernandes EC, Docampo R. Thapsigargin causes Ca2+ release and collapse of the membrane potential of Trypanosoma brucei mitochondria in situ and of isolated rat liver mitochondria. J Biol Chem. 1993;268:8564–8568. [PubMed] [Google Scholar]
- Vercesi AE, Moreno SN, Docampo R. Ca2+/H+ exchange in acidic vacuoles of Trypanosoma brucei. Biochem J. 1994;304(Pt 1):227–233. doi: 10.1042/bj3040227. [DOI] [PMC free article] [PubMed] [Google Scholar]


