Abstract
Microglia as immune cells of the central nervous system (CNS) play significant roles not only in pathology but also in physiology, such as shaping of the CNS during development and its proper maintenance in maturity. Emerging research is showing a close association between microglia and neurovasculature that is critical for brain energy supply. In this review, we summarize the current literature on microglial interaction with the vascular system in the normal and diseased brain. First, we highlight data that indicate interesting potential involvement of microglia in developmental angiogenesis. Then we discuss the evidence for microglial participation with the vasculature in neuropathologies from brain tumors to acute injuries such as ischemic stroke to chronic neurodegenerative conditions. We conclude by suggesting future areas of research to advance the field in light of current technical progress and outstanding questions.
Keywords: Microglia, Neurovasculature, Angiogenesis, Stroke, Neurodegeneration
[1] Introduction
Microglia are innate immune cells of the brain that are fully engaged in central nervous system (CNS) functions in normal and pathological conditions (Hanisch and Kettenmann, 2007; Casano and Peri, 2015). Depending on various methods used and regions examined, microglia make up 5–15% of brain cells (Pelvig et al., 2008; Lyck et al., 2009) and many of their properties distinguish them as a unique cell type from other brain cells as confirmed by recent transcriptome studies (Hickman et al., 2013; Zhang et al., 2014; Bennett et al., 2016). Neurons and macroglia like astrocyte and oligodendrocytes are derived from the neuroectoderm, while microglia originate from the embryonic yolk sac, then migrate and colonize the neuroepithelium (Prinz and Priller, 2014; Reemst et al., 2016). In the mature brain, microglia are exquisitely tiled and share non-overlapping territories with a small central soma and multiple elaborate processes (Davalos et al., 2005; Nimmerjahn et al., 2005; Wu et al., 2007). Unlike peripheral macrophages and circulating monocytes, microglia keep a fairly low turnover rate (Lawson et al., 1992; Askew et al., 2017; Tay et al., 2017) without contributions from peripheral sources such as circulating monocytes or blood-derived macrophages in the healthy condition (Ajami et al., 2007; Ajami et al., 2011; Gu et al., 2016).
One of the most notable features of microglia is their robust morphological plasticity by which they perform CNS surveillance (Davalos et al., 2005; Nimmerjahn et al., 2005). With remarkably dynamic processes, they frequently interact with neuronal elements including somata (Li et al., 2012; Eyo et al., 2014), axons (Baalman et al., 2015), dendrites and synapses (Wake et al., 2009; Tremblay et al., 2010; Eyo et al., 2015; Eyo et al., 2017) by which they sense and monitor neural activity and thus modulate neural circuit processing (Chen et al., 2010; Paolicelli and Gross, 2011; Parkhurst et al., 2013; Schafer et al., 2013; Squarzoni et al., 2014). Microglia are also the primary phagocytes of the brain and therefore engage and engulf excess neural material during early development (Marin-Teva et al., 2004; Petersen and Dailey, 2004; Wakselman et al., 2008; Svahn et al., 2013; Eyo et al., 2016) and in select neurogenic niches in the mature brain (Sierra et al., 2010; Sierra et al., 2014; Abiega et al., 2016; Fourgeaud et al., 2016). Thus, microglial roles in shaping brain development and function by regulating neuronal activity and phagocytic clearance have been well established and continue to be explored.
In addition to functioning in the healthy brain, microglia participate in aberrant neurological conditions from acute injury to neurodegenerative diseases (Ransohoff and Perry, 2009; Nayak et al., 2014; Peng et al., 2016; Eyo et al., 2017). Here, they are hotly debated to promote either neuroprotective or neurotoxic functions. They can ameliorate pathology as is the case with acute experimental seizures where seizure behaviors are worsened with microglial elimination or microglial P2Y12 receptor depletion (Mirrione et al., 2010; Eyo et al., 2014) and ischemic stroke where they are presumed to reduce infarct size and promote functional recovery (Lalancette-Hebert et al., 2007; Narantuya et al., 2010; Faustino et al., 2011). Conversely, they can also promote disease progression as in the case of neuropathic pain (Peng et al., 2016). It is noteworthy to point out that microglial functions are not always straight-forward in diseases. For example, while studies may suggest neuroprotective microglial functions in ischemia there is also evidence for neurotoxic activity in the disease (Wu et al., 2012; Tian et al., 2016). Interestingly, microglia have also been implicated in the pathogenesis of psychiatric disorders such as pathological grooming in mice which is suggested to mimic obsessive compulsive disorder (OCD) in humans (Chen et al., 2010). Together, these studies and many others indicate that microglia are significant cellular components in the brain orchestrating critical events during normal development, the maintenance of the mature CNS and during pathology.
It has been long recognized that microglia physically interact with the neurovasculature and interest in the physiological significance of these interactions are increasing (Arnold and Betsholtz, 2013; Dudvarski Stankovic et al., 2016). The brain vasculature, which includes a complex arrangement of arteries, veins and capillaries, distributes essential substances like oxygen and glucose to the brain while eliminating waste products like CO2 (Daneman and Prat, 2015). The brain, though constituting only about 2% of the body’s weight, demands about 20% of the body’s energy (Magistretti and Allaman, 2015). This fact implies a need for efficient and controlled delivery of blood to the brain, which is especially critical for optimal synaptic and thus cognitive function. While astrocytes are now established as critical regulators of the neurovasculature (MacVicar and Newman, 2015), microglia have increasingly been implicated in neurovascular development and complexity and thus indirectly regulate brain function. This review will summarize the current literature on the role for microglia in neurovascular function. Our assessment will begin with the relevant literature on the current conclusions regarding microglial roles in developmental angiogenesis and conclude with the insights provided by studies investigating microglial function in pathological conditions including cerebral tumors and neurodegenerative diseases.
While the focus of this review is not on microglial-astrocyte or microglial-pericyte interactions, it should be noted that there is growing evidence for interactions between microglia and these cells as components of the neurovascular unit. For example, microglia promote astrocyte differentiation (Nakanishi et al., 2007). However, astrocytes also regulate microglial phenotypes by paracrine signaling of released molecules (Rezaie et al., 2002; Bohlen et al., 2017) indicating that bidirectional regulation between microglia and astrocytes could have consequences on vascular development and integrity. Pericytes also serve as cytokine responsive cells and amplify microglia activation (Matsumoto et al., 2014) and are suggested to function as immune suppressors (Hurtado-Alvarado et al., 2014). This might imply a regulation by pericytes of the microglial phenotype on the uninjured brain. They have been recently reported to be a source of microglia especially following ischemia (Ozen et al., 2014; Sakuma et al., 2016). Moreover, activated microglia promote pericytes cell death via ROS production (Ding et al., 2017) and may thus compromise blood vessel integrity since pericytes make up the basement membranes of the vasculature. Therefore, although we don’t highlight the various interactions between these cellular components of the neurovascular unit and microglia in the following pages, these interactions cannot be ignored.
[2] Microglia and Blood Vessels in Normal Physiology
[2.1] Microglial Colonization of the CNS Precedes the Development of, but then Closely Associates with Blood Vessels
Several studies have documented that microglial emergence precedes the carefully orchestrated formation of the cerebral blood vessel network in the developing brain. This is the case in the mouse retina (Rymo et al., 2011) as well as hindbrain (Fantin et al., 2010). Consistent with these results from mice, avian data using chick-quail chimeras also revealed that yolk sac derived macrophages that give rise to microglia invade the CNS prior to and independent of neurovascular development (Cuadros et al., 1993; Kurz and Christ, 1998).
Although microglia and the neurovasculature do not appear in the developing CNS at the same time, when they do appear, they often display physical interactions. Early studies documented the localization of microglial cells with the vasculature in the developing rodent brain (Ashwell et al., 1989) and were sometimes referred to as “pericytic macrophages” (Thomas, 1999). This has been repeatedly confirmed in subsequent studies in the brain proper (Arnoux et al., 2013) and suggests relevant (perhaps bi-directional) communication between microglia and the vasculature. Moreover, these observations provided support for an early hypothesis that microglia migrate into the brain via blood vessels (Perry et al., 1985). Subsequent results, however, suggest that circulating cells do not infiltrate the brain parenchyma in the healthy CNS (Ajami et al., 2007) dampening enthusiasm for this early hypothesis. The observation of microglial proximity to blood vessels is especially noticeable during development. For example, in both the developing human and mouse retina, microglia are closely opposed to blood vessels (Checchin et al., 2006; Rymo et al., 2011). Furthermore, “juxtavascular” microglia were observed by real time confocal imaging to migrate along the walls of cerebral blood vessels in brain slices (Grossmann et al., 2002). These microglia exhibited a greater likelihood of migration than non-juxtavascular microglia. Although such observations were determined in an excised tissue context, whether the observations are an artifact of tissue excision or representative of the native developmental brain environment will have to be elucidated using more robust in vivo imaging approaches. Moreover, the precise function of this migration along blood vessels is not clear.
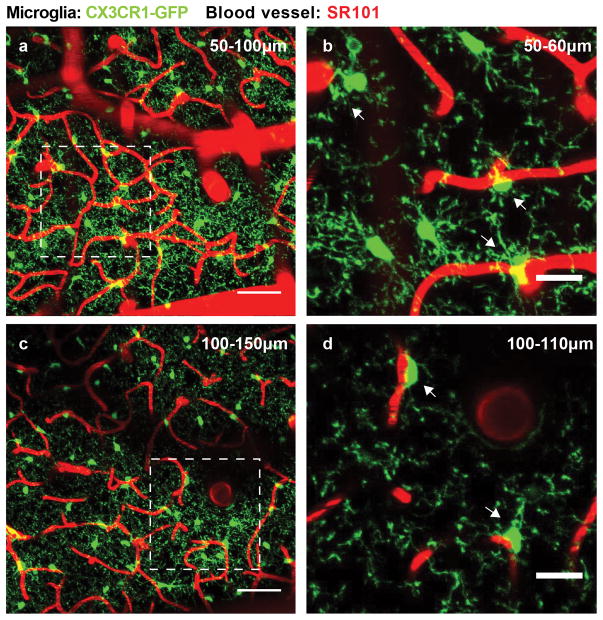
Less work has been done to provide details on microglial-vascular interactions in the mature brain. Nevertheless, interest in functional interactions between microglia and the neurovasculature continues to mount. Our initial studies using in vivo two photon imaging in adult mice has revealed that microglia maintain robust physical contact with elements of the neurovasculature (Fig. 1). Work is underway to adequately characterize these interesting microglial interactions with the neurovascular system and determine their functional significance in physiology and pathology.
Figure 1. Microglia and blood vessels exist in close proximity in the adult brain.
Z-stack images from the somatosensory cortex of a transgenic CX3CR1-GFP+/− mouse injected with SR101 (red) to label the neurovasculature. a–b, Low magnification images of ramified microglia and blood vessels in 50 μm z-stack images. Scale bar: 50 μm. c–d, High magnification images of ramified microglia and blood vessels in 10 μm z-stack images of the corresponding boxed regions in (a) and (b). Physical contact between microglia somata and capillaries are indicated with arrows. Scale bar: 20 μm.
[2.2] Microglia Promote Angiogenesis
One of the increasingly recognized functions of microglia is that they participate in the formation of new blood vessels or angiogenesis. This has especially been documented in pathological contexts (see next section) and presumes prior mechanistic similarities in earlier development. We will now consider some of the evidence that have been provided for this from both genetic and pharmacological approaches.
Support for the involvement of microglia in developmental angiogenesis from genetic approaches was first provided by the genetic depletion of macrophage colony stimulating factor, otherwise known as mCSF. This gene controls the development and survival of cells of the monocytic lineage including brain microglia (Cecchini et al., 1994). In mice genetically deficient of mCSF, the development of microglia and other monocyte-derived cells are lacking. Interestingly, the complexity of the developing retinal vasculature in mCSF knockout mice was also reduced suggesting roles for microglial mCSF signaling in angiogenesis in the retina. The deficiency in vasculature complexity was transient as differences were only observed in development but not in adulthood. Therefore, there might be complementary non-mCSF or microglia-independent mechanisms for retinal angiogenesis (Kubota et al., 2009).
Similar to mCSF, the PU.1 gene controls hematopoietic cell differentiation and genetic interruption of its function prevents microglial development (Scott et al., 1994; McKercher et al., 1996). Interestingly, PU.1 mutant mice also exhibit less elaborate blood vessel complexity (Fantin et al., 2010). Of course, given the limitation that these genetic approaches target non-microglial cells, the deficiency in vascular complexity could result from contributions from non-microglial population. Therefore, more selective microglial elimination techniques will have to be employed in future studies to adequately determine microglia-specific roles in developmental angiogenesis. However, it is worth noting that the challenge of ablating microglia in early development is not trivial and has yet to be accomplished at the time of this writing.
Pharmacological evidence suggesting microglial contributions to developmental angiogenesis has been multiply documented. First, consistent with a role for mCSF, its pharmacological inhibition using neutralizing antibodies to mCSF also reduced blood vessel complexity (Kubota et al., 2009). This pharmacological approach also suffers from the limitation that mCSF could target non-microglial cells that express mCSF receptors. Second, selective depletion of microglia using clodronate liposomes by which microglia die upon their uptake liposomes, resulted in reduced vascularization in the developing mouse retina (Checchin et al., 2006). Yet, as with the genetic approach, future studies will have to specifically target microglia selectively and do so especially in an in vivo context. Together, these results consistently suggest that microglia promote developmental angiogenesis.
[3] Microglia and Blood Vessels in Pathology
Although microglial roles in blood vessel development and maintenance have not received intense research attention as discussed above, more research has been directed to its function in various pathologies from brain tumors to acute brain injuries like ischemia to chronic neurodegenerative diseases. Current data on these findings will be discussed in this section. The relevance of findings from such studies is that understanding microglial roles in these pathological contexts could (i) inform future therapies that can be developed in the treatment directed towards ameliorating the progression of these pathologies and (ii) inform researchers on candidate mechanisms to identify factors by which microglia may regulate developmental angiogenesis and neurovascular physiology.
[3.1] Microglia and Blood Vessels in Brain Tumors
In the last two decades of angiogenesis research, it has become clear that vascular endothelial growth factor (VEGF) is a predominant regulator in the development and patterning of blood vessels (Thomas, 1996). This has been confirmed in multiple systems including the mouse brain (Ruhrberg et al., 2002; Haigh et al., 2003; Raab et al., 2004), the mouse retina (Stalmans et al., 2002; Haigh et al., 2003) and the quail neural tube (James et al., 2009). As with developmental angiogenesis, the tumor environment is pro-angiogenic and thus seems to promote the development of new blood vessels (otherwise termed “neovascularization”), which is important for (i) the delivery of blood and its accompanying nutrients to the tumor and (ii) the metastasis of tumor cells from the original tumor site to novel sites (Lorger, 2012). On this account, brain tumors possess a characteristically increased density of blood vessels (Lopes, 2003) some of which could be malformed and potentially leaky. Factors that promote the neovascularization of tumors are therefore generally considered detrimental to the outcome for the patient while those that conversely limit tumorigenic neovascularization are considered beneficial.
While astrocytes are considered to be a predominant cell type that secrete VEGF for angiogenesis (Pierce et al., 1995), selectively abrogating astrocyte-derived VEGF did not significantly alter the developmental angiogenesis (Scott et al., 2010; Weidemann et al., 2010) suggesting that either compensatory mechanisms are in play or astrocyte-derived VEGF is not critical for blood vessel development. Although microglial VEGF roles in developmental angiogenesis are not clear, microglia are now known to express some VEGF isoforms (Zhang et al., 2014) suggesting the capacity for regulating vascularization.
In human tumors, microglial/macrophage density was increased with a corresponding increase in tumorigenic neovascularization (Nishie et al., 1999; Brandenburg et al., 2016) and macrophages were increasingly associated with blood vessels (Leek et al., 1996; Brandenburg et al., 2016). Since microglia are the macrophages of the CNS, the implication that macrophages induce angiogenesis in tumor environments (Kobayashi et al., 1994; Sunderkotter et al., 1994; Polverini, 1997; Wang et al., 2013; Qin et al., 2015) could also apply to microglia (Wyckoff et al., 2004). Interestingly, despite the fact that it is difficult to distinguish brain resident microglia from infiltrated macrophages molecularly, recent evidence suggest that the pro-angiogenic function is predominantly carried out by microglia rather than macrophages in brain tumors (Brandenburg et al., 2016). Furthermore, factors derived from microglia are known to facilitate tumor progression (Sliwa et al., 2007). Specifically, microglia release tumor necrosis factor-alpha (TNFα) in the tumorigenic environment (Hattermann et al., 2014; Hwang et al., 2016), which in turn regulates the release of VEGF from glioma cell lines (Ryuto et al., 1996) that is critical for neovascularization. Thus either by the direct release of VEGF or the indirect release of other factors that increase its expression, microglia participate in the promotion of tumor angiogenesis. Finally, since VEGF serves as a chemoattractant to microglia (Forstreuter et al., 2002), its release in tumors could serve as an attractive signal to microglia towards blood vessels in an autocrine (if microglia are the source) or paracrine (if other cells are the source) manner.
In addition to microglial VEGF signaling, microglia release matrix metalloproteinases (MMPs) in vascularizing the tumor environment. Since MMPs function to degrade and remodel the extracellular matrix, their function is pro-angiogenic and thought to be recruited by the tumor for the promotion of tumor expansion (Egeblad and Werb, 2002; Rao, 2003). Microglia express several MMPs along with other cells of the brain (Hickman et al., 2013; Zhang et al., 2014; Holtman et al., 2015). MMP expression such as MT1-MMP (or MMP14), has been detected on human and murine microglia while tumor cells fail to express the protein (Markovic et al., 2009). Moreover, microglial depletion using either clodronate liposomes which eliminated MMP expression or genetic ablation both resulted in a reduction in tumor invasion (Markovic et al., 2009) confirming a pro-tumorigenic role for the microglial-derived MMP. Consistent with a role for microglial release of MT1-MMP in promoting tumor-induced angiogenesis, upregulation of the gene during tumor progression was correlated with increased neovascularization (Gabrusiewicz et al., 2011). These results indicate that microglial MMP activity promotes tumor progression by facilitating vascularization in the tumor environment.
Other microglial factors have been identified that promote tumor growth. For example, the microglial Na(+)/H(+) exchanger isoform 1 (NHE1) was recently identified as a respectable target to ameliorate tumor progression (Zhu et al., 2016). The mechanisms investigated suggested a regulation of tumor cell migration and proliferation. However, it would be interesting to determine whether the exchanger promotes tumor invasiveness by angiogenic mechanisms as well. Recently, CXCL2, a cytokine which is predominantly expressed by microglia in the brain parenchyma (Hickman et al., 2013; Zhang et al., 2014; Holtman et al., 2015), was shown to promote tumor-induced angiogenesis (Brandenburg et al., 2016). These results indicate that microglia facilitate blood vessel formation in a tumorigenic environment and some of the regulatory factors include growth factors, proteases, transporters and cytokines.
[3.2] Microglia and Blood Vessels in Ischemic Stroke
Stroke is the fifth leading cause of mortality in United States and a leading cause of disability (Talwalkar and Uddin, 2015). Many aspects of the role of microglia in ischemic contexts have been extensively reviewed (Ma et al., 2016). Microglia respond earliest following an ischemic insult and serve as the first line of defense to the injury (Morioka et al., 1991; Weinstein et al., 2010). Microglial accumulation is also one of the earliest cellular signatures in cerebral ischemia (Gelderblom et al., 2009). Ischemia induced by photothrombosis revealed that microglial dynamic activity is closely associated with capillary blood flow around its cell body (Masuda et al., 2011). The dynamics of microglial processes is suppressed around the capillary with decreased blood flow, suggesting microglial surveillance is inhibited during ischemia, which is consistent with evidence from the developing brain (Eyo and Dailey, 2012).
Microglia become closely associated with blood vessel after ischemia by forming perivascular clusters and phagocytic structures (Jolivel et al., 2015). The accumulation of microglia around the vasculature subsequently led to the disintegration of the vessels which included their upregulation of phagocytic CD68 expression in the penumbra area. Accumulation of microglia with blood vessels also correlated with the invasion of blood-borne molecules during reperfusion (Jolivel et al., 2015). Interestingly, a selective inactivation of microglial CX3CR1 that has been reported to regulate microglial migration (Cardona et al., 2006; Liang et al., 2009) and significantly reduce blood extravasation (Jolivel et al., 2015). This may be a mechanism for neuroprotection in stroke since several studies have indicated reduced stroke pathology in CX3CR1-deficient mice (Denes et al., 2008; Jolivel et al., 2015).
Furthermore, perivascular microglia might also contribute to cerebral ischemia by releasing microglia-specific cytokines that are known to compromise vascular integrity in ischemia (Sprague and Khalil, 2009). For example, IL-1β and TNFα, both known to increase the permeability of the blood brain barrier (BBB) (Tsao et al., 2001; Mayhan, 2002; Sibson et al., 2002; Wang et al., 2014; Richter et al., 2017) are released by microglia early during ischemia (Lambertsen et al., 2012) to promote the compromise of the BBB. Later in the progression of ischemia, microglia also release VEGF (Xie et al., 2013) known to promote angiogenesis in stroke (Zhang et al., 2000) suggesting that they could play some reparative functions by inducing neovascularization in later stages of stroke that contrasts with their earlier function. Of note, even IL-1β has been reported to have pro-angiogenic functions as well (Giulian et al., 1988) which may also be recruited in the latter repair following ischemic injury. Together, these results suggest that in the stroke context, microglia breakdown extant blood vessels, partially through cytokine insults and partially through phagocytic engulfment early on following the insult, and then contribute to building new vasculature later on. Future work will have to more precisely and adequately test this hypothesis.
[3.3] Microglia and Blood Vessels in Neurodegeneration
[3.3.1] Alzheimer’s disease
Alzheimer’s disease (AD) represents the most common neurodegenerative disease and is especially fatal in the aging population (Ballard et al., 2011). Characterized by amyloid beta (Aβ) deposits in the brain as a histopathological hallmark, microglial reactivity in the AD brain is also well known (Heneka et al., 2015; Yeh et al., 2017) but the specific contribution of microglia remain hotly debated (Gold and El Khoury, 2015; Malm et al., 2015). Here, we focus on some of the evidence that among other things, microglia in the AD context participates in vascular abnormalities that occur in the disease.
As with previous discussions, VEGF expression is increased in AD in response to Aβ deposition (Kalaria et al., 1998; Tarkowski et al., 2002). Since Aβ is deposited in and/or around blood vessels in addition to the parenchyma (Okamoto et al., 2009; Hickman and El Khoury, 2010), VEGF, among other chemoattractant, may mobilize microglia to surround blood vessels. This is consistent with a robust perivascular accumulation of microglia in AD (Ryu and McLarnon, 2009; Giannoni et al., 2016). In the 5xFAD model of AD, a longitudinal assessment of AD pathology using intravital two-photon microscopy revealed overlapping regions of neurovascular defects and Aβ plaques, which correlated with increased microglial activation (Giannoni et al., 2016). Whether the microglial reactivity in perivascular regions was a cause, consequence or an independent correlating factor with the histopathological vascular defects was not determined.
However, in an experimental model of AD where Aβ is injected into the hippocampus, there is a corresponding increase in neovascularization and microglial activation (Zand et al., 2005). Furthermore, in this condition, the BBB becomes leaky and this has been correlated with BBB-associated astrocytes and microglia (Ryu and McLarnon, 2006). Indeed, application of minocycline to inhibit microglial activation in this context significantly reduced the Aβ-induced defect in BBB integrity (Ryu and McLarnon, 2006). This result was further supported by an alternative approach using CD11b antibodies to block microglial function, which resulted in reduced neurovascular deficits from Aβ injection (Ryu and McLarnon, 2009). Although the precise mechanisms for microglial action remain to be elucidated, one of the mechanisms that may be proposed to be employed by microglia in promoting vascular pathology in Aβ pathology is through the purinergic P2X7 receptor (P2X7R) signaling (Ryu and McLarnon, 2008). Microglia are recognized as a predominant cell that expresses the P2X7R in the brain (Hickman et al., 2013; Zhang et al., 2014; Holtman et al., 2015) despite the lingering controversy of neuronal P2X7R expression (Illes et al., 2017; Miras-Portugal et al., 2017). In this light, since pharmacological inhibition of P2X7Rs improved defects induced by Aβ treatment such as aberrant vascular function (Ryu and McLarnon, 2008), it is tempting to speculate that a primary cellular component of action occurred through microglia.
MMPs expressed by microglia and released in AD pathology have also been documented to contribute to the progression of AD (Kim and Joh, 2012). However, whether MMP action is specifically through microglia or has any specific effect on AD-induced vascular aberrations remains to be determined. Finally, microglia also release several cytokines including TNFα and IL-1β during AD pathology (Cameron and Landreth, 2010; Mandrekar-Colucci and Landreth, 2010; Wang et al., 2015) that can also compromise the vasculature as discussed above (see section 3.2 above). On the basis of the above, microglia are thus thought to promote vascular pathology including a breakdown of the BBB in Aβ pathology like AD.
[3.3.2] Multiple Sclerosis and Parkinson’s disease
Multiple sclerosis (MS) is a progressive neurodegenerative autoimmune disease characterized by demyelination, brain atrophy and chronic inflammation (Lassmann et al., 2001; Vos et al., 2005). As with other conditions discussed above, angiogenesis has been reported in animal models of MS (Seabrook et al., 2010; Girolamo et al., 2014). In addition, BBB integrity is compromised in early stages of the disease leading to increased leukocyte infiltration and accumulation of blood products such as fibrinogen into the brain (Vos et al., 2005; van Horssen et al., 2007). Real time in vivo imaging revealed that leaked fibrinogen serves to attract microglia towards blood vessels in the early stages of animal models of MS. This attraction continues into more severe stages of the disease (Davalos et al., 2012). However, although microglia in general are thought to promote MS pathology (Heppner et al., 2005), it’s precise role in vascular abnormalities of the disease such as BBB compromise and angiogenesis have not been clarified and should be a focus of future studies.
Parkinson’s disease (PD) is a progressive neurodegenerative motor disease and is characterized by the loss of dopaminergic neurons in the substantia nigra (Kalia and Lang, 2015). Activation of the immune system and especially microglia is well known for disease progression (Whitton, 2007; Luo et al., 2017). As with the AD and MS, neovascularization (Barcia et al., 2005; Desai Bradaric et al., 2012) and a compromised BBB (Brochard et al., 2009; Gray and Woulfe, 2015) have been reported in PD/animal models of PD. However, whether and how microglia may be involved in regulating neurovascular changes that occur during PD has not been adequately explored.
[4] Outstanding Questions and Future Direction
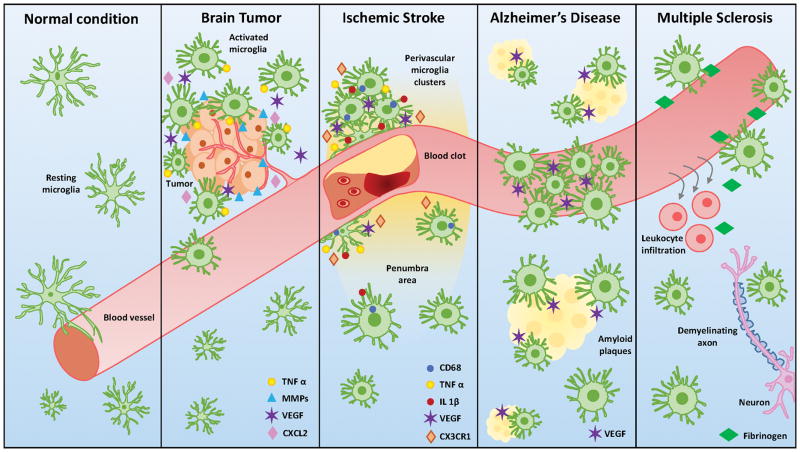
The previous sections have documented the progress in understanding microglial engagement with the neurovascular system in health and disease (Fig. 2). However, at least three avenues of research should be employed to address outstanding questions. First is the question of molecular and mechanistic details of microglial regulation of vascular function in the developing and mature brain. While compelling evidence support an important role for microglia in maintaining vasculature function in the brain, the basis of communication between microglia and the neurovasculature is still elusive at this moment. More basic research would lead to a better understanding of microglia-vascular interaction that can be harnessed to treat vascular pathologies of the brain. Precise roles of cytokines, free radicals, purines and proteases should be investigated in these various contexts. Relevant intracellular signaling pathways by which microglia receive signals from and send signals to the vasculature remain to be identified. Furthermore, future research should be directed to reveal the extent and details of microglial physical and dynamic interactions with elements of the neurovasculature and how they may differ between for example, capillaries, arteries and/or veins. The advent of real time two photon imaging can be used to accomplish this.
Figure 2. Schematic diagram of microglial interaction with the neurovascular system in physiology and pathology.
Resting microglia, as depicted in normal condition, are characterized with ramified cellular processes and close proximity of blood vessel. Activated microglia under diseased conditions are featured with larger somata and enriched in the dysfunctional core of associated diseases. Molecules, like chemokines, cytokines & growth factors, included in interaction of microglia and neurovascular system are indicated in each panel as normal condition, brain tumor, ischemic stroke, Alzheimer’s disease and multiple sclerosis.
A second aspect that should guide future research is the question of specific microglial populations that may orchestrate neurovascular development and maintenance (or disintegration) in health and disease. RNA sequencing and transcriptional profiling data suggests a rich heterogeneity of microglia with distinctive molecular profiles (Zhang et al., 2014; Grabert et al., 2016; Keren-Shaul et al., 2017). In light of these results, it would be interesting to determine whether there is a microglial subtype specifically responsible for the maintenance/modulation of the neurovascular system in various brain regions. Morphological and molecular characterizations of such region specific microglia-vascular interaction would have to be determined. In addition, perivascular microglia have been identified in the literature but whether they are molecularly distinct from other resident microglia will need to be clarified to better understand their function.
Finally, while evidence is mounting for promising roles of microglia in angiogenesis and the maintenance of the BBB integrity, a lot of work remains to be done to determine precise microglial involvement in these processes especially in pathology. Prior work did not adequately ascertain microglial involvement because of the lack of specificity in the approaches. Approaches that potentially targeted microglia and peripheral monocytes/macrophages indiscriminately may have led to faulty conclusions in microglia-neurovascular interactions. Minocycline was mostly used to inhibit microglial activation in studying microglia-vascular interaction, however, it also has other non-microglial and perhaps direct neuromodulatory effects (Huang et al., 2010). Moreover, future studies are needed to differentiate the direct microglial interaction with neurovascular system from the indirect effect of microglia-neuron or microglia-astrocyte communication. Continual advancements in microglial-specific genetic and pharmacological tools in general cell ablation and specific protein deletion has now set the stage for better studies to adequately address these concerns.
Acknowledgments
This work is supported by the grants from the National Institute of Health (R01NS088627 and R21DE025689). We thank Dr. Junting Liu from Wu lab at Rutgers for the technical assistance in microglia-neurovascular imaging in our initial studies.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- Abiega O, Beccari S, Diaz-Aparicio I, Nadjar A, Laye S, Leyrolle Q, Gomez-Nicola D, Domercq M, Perez-Samartin A, Sanchez-Zafra V, Paris I, Valero J, Savage JC, Hui CW, Tremblay ME, Deudero JJ, Brewster AL, Anderson AE, Zaldumbide L, Galbarriatu L, Marinas A, Vivanco MD, Matute C, Maletic-Savatic M, Encinas JM, Sierra A. Correction: Neuronal Hyperactivity Disturbs ATP Microgradients, Impairs Microglial Motility, and Reduces Phagocytic Receptor Expression Triggering Apoptosis/Microglial Phagocytosis Uncoupling. PLoS Biol. 2016;14:e1002554. doi: 10.1371/journal.pbio.1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Arnold T, Betsholtz C. Correction: The importance of microglia in the development of the vasculature in the central nervous system. Vasc Cell. 2013;5:12. doi: 10.1186/2045-824X-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoux I, Hoshiko M, Mandavy L, Avignone E, Yamamoto N, Audinat E. Adaptive phenotype of microglial cells during the normal postnatal development of the somatosensory “Barrel” cortex. Glia. 2013;61:1582–1594. doi: 10.1002/glia.22503. [DOI] [PubMed] [Google Scholar]
- Ashwell KW, Hollander H, Streit W, Stone J. The appearance and distribution of microglia in the developing retina of the rat. Vis Neurosci. 1989;2:437–448. doi: 10.1017/s0952523800012335. [DOI] [PubMed] [Google Scholar]
- Askew K, Li KZ, Olmos-Alonso A, Garcia-Moreno F, Liang YJ, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, Sierra A, Molnar Z, Cragg MS, Garaschuk O, Perry VH, Gomez-Nicola D. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Reports. 2017;18:391–405. doi: 10.1016/j.celrep.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baalman K, Marin MA, Ho TS, Godoy M, Cherian L, Robertson C, Rasband MN. Axon initial segment-associated microglia. J Neurosci. 2015;35:2283–2292. doi: 10.1523/JNEUROSCI.3751-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- Barcia C, Bautista V, Sanchez-Bahillo A, Fernandez-Villalba E, Faucheux B, Poza y Poza M, Fernandez Barreiro A, Hirsch EC, Herrero MT. Changes in vascularization in substantia nigra pars compacta of monkeys rendered parkinsonian. J Neural Transm (Vienna) 2005;112:1237–1248. doi: 10.1007/s00702-004-0256-2. [DOI] [PubMed] [Google Scholar]
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016;113:E1738–1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen CJ, Bennett FC, Tucker AF, Collins HY, Mulinyawe SB, Barres BA. Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron. 2017;94:759–773. e758. doi: 10.1016/j.neuron.2017.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg S, Muller A, Turkowski K, Radev YT, Rot S, Schmidt C, Bungert AD, Acker G, Schorr A, Hippe A, Miller K, Heppner FL, Homey B, Vajkoczy P. Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathol. 2016;131:365–378. doi: 10.1007/s00401-015-1529-6. [DOI] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer’s disease. Neurobiol Dis. 2010;37:503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Casano AM, Peri F. Microglia: multitasking specialists of the brain. Dev Cell. 2015;32:469–477. doi: 10.1016/j.devcel.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard JW, Stanley ER. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006;47:3595–3602. doi: 10.1167/iovs.05-1522. [DOI] [PubMed] [Google Scholar]
- Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, Capecchi MR. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadros MA, Martin C, Coltey P, Almendros A, Navascues J. First appearance, distribution, and origin of macrophages in the early development of the avian central nervous system. J Comp Neurol. 1993;330:113–129. doi: 10.1002/cne.903300110. [DOI] [PubMed] [Google Scholar]
- Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, Deerinck TJ, Smirnoff DS, Bedard C, Hakozaki H, Gonias Murray S, Ling JB, Lassmann H, Degen JL, Ellisman MH, Akassoglou K. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28:1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- Desai Bradaric B, Patel A, Schneider JA, Carvey PM, Hendey B. Evidence for angiogenesis in Parkinson’s disease, incidental Lewy body disease, and progressive supranuclear palsy. J Neural Transm (Vienna) 2012;119:59–71. doi: 10.1007/s00702-011-0684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Zhang M, Gu R, Xu G, Wu H. Activated microglia induce the production of reactive oxygen species and promote apoptosis of co-cultured retinal microvascular pericytes. Graefes Arch Clin Exp Ophthalmol. 2017;255:777–788. doi: 10.1007/s00417-016-3578-5. [DOI] [PubMed] [Google Scholar]
- Dudvarski Stankovic N, Teodorczyk M, Ploen R, Zipp F, Schmidt MH. Microglia-blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol. 2016;131:347–363. doi: 10.1007/s00401-015-1524-y. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Eyo U, Dailey ME. Effects of oxygen-glucose deprivation on microglial mobility and viability in developing mouse hippocampal tissues. Glia. 2012;60:1747–1760. doi: 10.1002/glia.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Gu N, De S, Dong H, Richardson JR, Wu LJ. Modulation of microglial process convergence toward neuronal dendrites by extracellular calcium. J Neurosci. 2015;35:2417–2422. doi: 10.1523/JNEUROSCI.3279-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Miner SA, Weiner JA, Dailey ME. Developmental changes in microglial mobilization are independent of apoptosis in the neonatal mouse hippocampus. Brain Behav Immun. 2016;55:49–59. doi: 10.1016/j.bbi.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Murugan M, Wu LJ. Microglia-Neuron Communication in Epilepsy. Glia. 2017;65:5–18. doi: 10.1002/glia.23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Peng J, Murugan M, Mo M, Lalani A, Xie P, Xu P, Margolis DJ, Wu LJ. Regulation of Physical Microglia-Neuron Interactions by Fractalkine Signaling after Status Epilepticus. eNeuro. 2017:3. doi: 10.1523/ENEURO.0209-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ. Neuronal Hyperactivity Recruits Microglial Processes via Neuronal NMDA Receptors and Microglial P2Y12 Receptors after Status Epilepticus. J Neurosci. 2014;34:10528–10540. doi: 10.1523/JNEUROSCI.0416-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino JV, Wang X, Johnson CE, Klibanov A, Derugin N, Wendland MF, Vexler ZS. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci. 2011;31:12992–13001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstreuter F, Lucius R, Mentlein R. Vascular endothelial growth factor induces chemotaxis and proliferation of microglial cells. J Neuroimmunol. 2002;132:93–98. doi: 10.1016/s0165-5728(02)00315-6. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Traves PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, Callaway P, Zagorska A, Rothlin CV, Nimmerjahn A, Lemke G. TAM receptors regulate multiple features of microglial physiology. Nature. 2016;532:240–244. doi: 10.1038/nature17630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011;6:e23902. doi: 10.1371/journal.pone.0023902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Giannoni P, Arango-Lievano M, Neves ID, Rousset MC, Baranger K, Rivera S, Jeanneteau F, Claeysen S, Marchi N. Cerebrovascular pathology during the progression of experimental Alzheimer’s disease. Neurobiol Dis. 2016;88:107–117. doi: 10.1016/j.nbd.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Girolamo F, Coppola C, Ribatti D, Trojano M. Angiogenesis in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol Commun. 2014;2:84. doi: 10.1186/s40478-014-0084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Woodward J, Young DG, Krebs JF, Lachman LB. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci. 1988;8:2485–2490. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M, El Khoury J. beta-amyloid, microglia, and the inflammasome in Alzheimer’s disease. Semin Immunopathol. 2015;37:607–611. doi: 10.1007/s00281-015-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016;19:504–516. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MT, Woulfe JM. Striatal blood-brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab. 2015;35:747–750. doi: 10.1038/jcbfm.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann R, Stence N, Carr J, Fuller L, Waite M, Dailey ME. Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia. 2002;37:229–240. [PubMed] [Google Scholar]
- Gu N, Peng J, Murugan M, Wang X, Eyo UB, Sun D, Ren Y, DiCicco-Bloom E, Young W, Dong H, Wu LJ. Spinal Microgliosis Due to Resident Microglial Proliferation Is Required for Pain Hypersensitivity after Peripheral Nerve Injury. Cell Rep. 2016;16:605–614. doi: 10.1016/j.celrep.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N, Wagner EF, Betsholtz C, Nagy A. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hattermann K, Sebens S, Helm O, Schmitt AD, Mentlein R, Mehdorn HM, Held-Feindt J. Chemokine expression profile of freshly isolated human glioblastoma-associated macrophages/microglia. Oncol Rep. 2014;32:270–276. doi: 10.3892/or.2014.3214. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- Hickman SE, El Khoury J. Mechanisms of mononuclear phagocyte recruitment in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2010;9:168–173. doi: 10.2174/187152710791011982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16:1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman IR, Noback M, Bijlsma M, Duong KN, van der Geest MA, Ketelaars PT, Brouwer N, Vainchtein ID, Eggen BJ, Boddeke HW. Glia Open Access Database (GOAD): A comprehensive gene expression encyclopedia of glia cells in health and disease. Glia. 2015;63:1495–1506. doi: 10.1002/glia.22810. [DOI] [PubMed] [Google Scholar]
- Huang WC, Qiao Y, Xu L, Kacimi R, Sun X, Giffard RG, Yenari MA. Direct protection of cultured neurons from ischemia-like injury by minocycline. Anat Cell Biol. 2010;43:325–331. doi: 10.5115/acb.2010.43.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-Alvarado G, Cabanas-Morales AM, Gomez-Gonzalez B. Pericytes: brain-immune interface modulators. Front Integr Neurosci. 2014;7:80. doi: 10.3389/fnint.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JS, Jung EH, Kwon MY, Han IO. Glioma-secreted soluble factors stimulate microglial activation: The role of interleukin-1beta and tumor necrosis factor-alpha. J Neuroimmunol. 2016;298:165–171. doi: 10.1016/j.jneuroim.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Illes P, Khan TM, Rubini P. Neuronal P2X7 Receptors Revisited: Do They Really Exist? J Neurosci. 2017;37:7049–7062. doi: 10.1523/JNEUROSCI.3103-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JM, Gewolb C, Bautch VL. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development. 2009;136:833–841. doi: 10.1242/dev.028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivel V, Bicker F, Biname F, Ploen R, Keller S, Gollan R, Jurek B, Birkenstock J, Poisa-Beiro L, Bruttger J, Opitz V, Thal SC, Waisman A, Bauerle T, Schafer MK, Zipp F, Schmidt MH. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol. 2015;129:279–295. doi: 10.1007/s00401-014-1372-1. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Cohen DL, Premkumar DR, Nag S, LaManna JC, Lust WD. Vascular endothelial growth factor in Alzheimer’s disease and experimental cerebral ischemia. Brain Res Mol Brain Res. 1998;62:101–105. doi: 10.1016/s0169-328x(98)00190-9. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell. 2017;169:1276–1290. e1217. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Kim YS, Joh TH. Matrix metalloproteinases, new insights into the understanding of neurodegenerative disorders. Biomol Ther (Seoul) 2012;20:133–143. doi: 10.4062/biomolther.2012.20.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Nagaura T, Kimura I, Kimura M. Interferon-gamma-activated macrophages enhance angiogenesis from endothelial cells of rat aorta. Immunopharmacology. 1994;27:23–30. doi: 10.1016/0162-3109(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206:1089–1102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz H, Christ B. Embryonic CNS macrophages and microglia do not stem from circulating, but from extravascular precursors. Glia. 1998;22:98–102. [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32:1677–1698. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med. 2001;7:115–121. doi: 10.1016/s1471-4914(00)01909-2. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Gordon S. Turnover of Resident Microglia in the Normal Adult-Mouse Brain. Neuroscience. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23:1189–1202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Liang KJ, Lee JE, Wang YD, Ma W, Fontainhas AM, Fariss RN, Wong WT. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Invest Ophthalmol Vis Sci. 2009;50:4444–4451. doi: 10.1167/iovs.08-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes MB. Angiogenesis in brain tumors. Microsc Res Tech. 2003;60:225–230. doi: 10.1002/jemt.10260. [DOI] [PubMed] [Google Scholar]
- Lorger M. Tumor microenvironment in the brain. Cancers (Basel) 2012;4:218–243. doi: 10.3390/cancers4010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Jian C, Liao Y, Huang Q, Wu Y, Liu X, Zou D, Wu Y. The role of microglia in multiple sclerosis. Neuropsychiatr Dis Treat. 2017;13:1661–1667. doi: 10.2147/NDT.S140634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyck L, Santamaria ID, Pakkenberg B, Chemnitz J, Schroder HD, Finsen B, Gundersen HJG. An empirical analysis of the precision of estimating the numbers of neurons and glia in human neocortex using a fractionator-design with sub-sampling. Journal of Neuroscience Methods. 2009;182:143–156. doi: 10.1016/j.jneumeth.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2016 doi: 10.1016/j.pneurobio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Newman EA. Astrocyte regulation of blood flow in the brain. Cold Spring Harb Perspect Biol. 2015:7. doi: 10.1101/cshperspect.a020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86:883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Malm TM, Jay TR, Landreth GE. The evolving biology of microglia in Alzheimer’s disease. Neurotherapeutics. 2015;12:81–93. doi: 10.1007/s13311-014-0316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar-Colucci S, Landreth GE. Microglia and inflammation in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2010;9:156–167. doi: 10.2174/187152710791012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Markovic DS, Vinnakota K, Chirasani S, Synowitz M, Raguet H, Stock K, Sliwa M, Lehmann S, Kalin R, van Rooijen N, Holmbeck K, Heppner FL, Kiwit J, Matyash V, Lehnardt S, Kaminska B, Glass R, Kettenmann H. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci U S A. 2009;106:12530–12535. doi: 10.1073/pnas.0804273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Croom D, Hida H, Kirov SA. Capillary blood flow around microglial somata determines dynamics of microglial processes in ischemic conditions. Glia. 2011;59:1744–1753. doi: 10.1002/glia.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto J, Takata F, Machida T, Takahashi H, Soejima Y, Funakoshi M, Futagami K, Yamauchi A, Dohgu S, Kataoka Y. Tumor necrosis factor-alpha-stimulated brain pericytes possess a unique cytokine and chemokine release profile and enhance microglial activation. Neurosci Lett. 2014;578:133–138. doi: 10.1016/j.neulet.2014.06.052. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Cellular mechanisms by which tumor necrosis factor-alpha produces disruption of the blood-brain barrier. Brain Res. 2002;927:144–152. doi: 10.1016/s0006-8993(01)03348-0. [DOI] [PubMed] [Google Scholar]
- McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, Maki RA. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- Miras-Portugal MT, Sebastian-Serrano A, de Diego Garcia L, Diaz-Hernandez M. Neuronal P2X7 Receptor: Involvement in Neuronal Physiology and Pathology. J Neurosci. 2017;37:7063–7072. doi: 10.1523/JNEUROSCI.3104-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirrione MM, Konomos DK, Gravanis I, Dewey SL, Aguzzi A, Heppner FL, Tsirka SE. Microglial ablation and lipopolysaccharide preconditioning affects pilocarpine-induced seizures in mice. Neurobiol Dis. 2010;39:85–97. doi: 10.1016/j.nbd.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka T, Kalehua AN, Streit WJ. The microglial reaction in the rat dorsal hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab. 1991;11:966–973. doi: 10.1038/jcbfm.1991.162. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007;25:649–658. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- Narantuya D, Nagai A, Sheikh AM, Masuda J, Kobayashi S, Yamaguchi S, Kim SU. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PLoS One. 2010;5:e11746. doi: 10.1371/journal.pone.0011746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M, Iwaki T, Kuwano M. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5:1107–1113. [PubMed] [Google Scholar]
- Okamoto Y, Ihara M, Fujita Y, Ito H, Takahashi R, Tomimoto H. Cortical microinfarcts in Alzheimer’s disease and subcortical vascular dementia. Neuroreport. 2009;20:990–996. doi: 10.1097/WNR.0b013e32832d2e6a. [DOI] [PubMed] [Google Scholar]
- Ozen I, Deierborg T, Miharada K, Padel T, Englund E, Genove G, Paul G. Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol. 2014;128:381–396. doi: 10.1007/s00401-014-1295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Gross CT. Microglia in development: linking brain wiring to brain environment. Neuron Glia Biol. 2011;7:77–83. doi: 10.1017/S1740925X12000105. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiology of Aging. 2008;29:1754–1762. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Peng J, Gu N, Zhou L, UBE, Murugan M, Gan WB, Wu LJ. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun. 2016;7:12029. doi: 10.1038/ncomms12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Petersen MA, Dailey ME. Diverse microglial motility behaviors during clearance of dead cells in hippocampal slices. Glia. 2004;46:195–206. doi: 10.1002/glia.10362. [DOI] [PubMed] [Google Scholar]
- Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverini PJ. Role of the macrophage in angiogenesis-dependent diseases. EXS. 1997;79:11–28. doi: 10.1007/978-3-0348-9006-9_2. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nature Reviews Neuroscience. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Qin T, Wang C, Chen X, Duan C, Zhang X, Zhang J, Chai H, Tang T, Chen H, Yue J, Li Y, Yang J. Dopamine induces growth inhibition and vascular normalization through reprogramming M2-polarized macrophages in rat C6 glioma. Toxicol Appl Pharmacol. 2015;286:112–123. doi: 10.1016/j.taap.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Raab S, Beck H, Gaumann A, Yuce A, Gerber HP, Plate K, Hammes HP, Ferrara N, Breier G. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost. 2004;91:595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- Reemst K, Noctor SC, Lucassen PJ, Hol EM. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Frontiers in Human Neuroscience. 2016:10. doi: 10.3389/fnhum.2016.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie P, Trillo-Pazos G, Greenwood J, Everall IP, Male DK. Motility and ramification of human fetal microglia in culture: an investigation using time-lapse video microscopy and image analysis. Exp Cell Res. 2002;274:68–82. doi: 10.1006/excr.2001.5431. [DOI] [PubMed] [Google Scholar]
- Richter F, Eitner A, Leuchtweis J, Bauer R, Lehmenkuhler A, Schaible HG. Effects of interleukin-1ss on cortical spreading depolarization and cerebral vasculature. J Cereb Blood Flow Metab. 2017;37:1791–1802. doi: 10.1177/0271678X16641127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymo SF, Gerhardt H, Wolfhagen Sand F, Lang R, Uv A, Betsholtz C. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS One. 2011;6:e15846. doi: 10.1371/journal.pone.0015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG. Minocycline or iNOS inhibition block 3-nitrotyrosine increases and blood-brain barrier leakiness in amyloid beta-peptide-injected rat hippocampus. Exp Neurol. 2006;198:552–557. doi: 10.1016/j.expneurol.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG. Block of purinergic P2X(7) receptor is neuroprotective in an animal model of Alzheimer’s disease. Neuroreport. 2008;19:1715–1719. doi: 10.1097/WNR.0b013e3283179333. [DOI] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med. 2009;13:2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryuto M, Ono M, Izumi H, Yoshida S, Weich HA, Kohno K, Kuwano M. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J Biol Chem. 1996;271:28220–28228. doi: 10.1074/jbc.271.45.28220. [DOI] [PubMed] [Google Scholar]
- Sakuma R, Kawahara M, Nakano-Doi A, Takahashi A, Tanaka Y, Narita A, Kuwahara-Otani S, Hayakawa T, Yagi H, Matsuyama T, Nakagomi T. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J Neuroinflammation. 2016;13:57. doi: 10.1186/s12974-016-0523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61:24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Powner MB, Gandhi P, Clarkin C, Gutmann DH, Johnson RS, Ferrara N, Fruttiger M. Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS One. 2010;5:e11863. doi: 10.1371/journal.pone.0011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- Seabrook TJ, Littlewood-Evans A, Brinkmann V, Pollinger B, Schnell C, Hiestand PC. Angiogenesis is present in experimental autoimmune encephalomyelitis and pro-angiogenic factors are increased in multiple sclerosis lesions. J Neuroinflammation. 2010;7:95. doi: 10.1186/1742-2094-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Blamire AM, Perry VH, Gauldie J, Styles P, Anthony DC. TNF-alpha reduces cerebral blood volume and disrupts tissue homeostasis via an endothelin- and TNFR2-dependent pathway. Brain. 2002;125:2446–2459. doi: 10.1093/brain/awf256. [DOI] [PubMed] [Google Scholar]
- Sierra A, Beccari S, Diaz-Aparicio I, Encinas JM, Comeau S, Tremblay ME. Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast. 2014;2014:610343. doi: 10.1155/2014/610343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwa M, Markovic D, Gabrusiewicz K, Synowitz M, Glass R, Zawadzka M, Wesolowska A, Kettenmann H, Kaminska B. The invasion promoting effect of microglia on glioblastoma cells is inhibited by cyclosporin A. Brain. 2007;130:476–489. doi: 10.1093/brain/awl263. [DOI] [PubMed] [Google Scholar]
- Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F, Garel S. Microglia Modulate Wiring of the Embryonic Forebrain. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.07.042. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, Hicklin D, Anderson DJ, Gardiner T, Hammes HP, Moons L, Dewerchin M, Collen D, Carmeliet P, D’Amore PA. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- Svahn AJ, Graeber MB, Ellett F, Lieschke GJ, Rinkwitz S, Bennett MR, Becker TS. Development of ramified microglia from early macrophages in the zebrafish optic tectum. Dev Neurobiol. 2013;73:60–71. doi: 10.1002/dneu.22039. [DOI] [PubMed] [Google Scholar]
- Talwalkar A, Uddin S. Trends in emergency department visits for ischemic stroke and transient ischemic attack: United States, 2001–2011. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- Tarkowski E, Issa R, Sjogren M, Wallin A, Blennow K, Tarkowski A, Kumar P. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer’s disease and vascular dementia. Neurobiol Aging. 2002;23:237–243. doi: 10.1016/s0197-4580(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Tay TL, Mai D, Dautzenberg J, Fernandez-Klett F, Lin G, Sagar, Datta M, Drougard A, Stempfl T, Ardura-Fabregat A, Staszewski O, Margineanu A, Sporbert A, Steinmetz LM, Pospisilik JA, Jung S, Priller J, Grun D, Ronneberger O, Prinz M. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci. 2017;20:793–803. doi: 10.1038/nn.4547. [DOI] [PubMed] [Google Scholar]
- Thomas KA. Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem. 1996;271:603–606. doi: 10.1074/jbc.271.2.603. [DOI] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: on the role of pericytes and perivascular cells. Brain Res Brain Res Rev. 1999;31:42–57. doi: 10.1016/s0165-0173(99)00024-7. [DOI] [PubMed] [Google Scholar]
- Tian DS, Li CY, Qin C, Murugan M, Wu LJ, Liu JL. Deficiency in the voltage-gated proton channel Hv1 increases M2 polarization of microglia and attenuates brain damage from photothrombotic ischemic stroke. J Neurochem. 2016;139:96–105. doi: 10.1111/jnc.13751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao N, Hsu HP, Wu CM, Liu CC, Lei HY. Tumour necrosis factor-alpha causes an increase in blood-brain barrier permeability during sepsis. J Med Microbiol. 2001;50:812–821. doi: 10.1099/0022-1317-50-9-812. [DOI] [PubMed] [Google Scholar]
- van Horssen J, Brink BP, de Vries HE, van der Valk P, Bo L. The blood-brain barrier in cortical multiple sclerosis lesions. J Neuropathol Exp Neurol. 2007;66:321–328. doi: 10.1097/nen.0b013e318040b2de. [DOI] [PubMed] [Google Scholar]
- Vos CM, Geurts JJ, Montagne L, van Haastert ES, Bo L, van der Valk P, Barkhof F, de Vries HE. Blood-brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiol Dis. 2005;20:953–960. doi: 10.1016/j.nbd.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SC, Yu CF, Hong JH, Tsai CS, Chiang CS. Radiation therapy-induced tumor invasiveness is associated with SDF-1-regulated macrophage mobilization and vasculogenesis. PLoS One. 2013;8:e69182. doi: 10.1371/journal.pone.0069182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WY, Tan MS, Yu JT, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 2015;3:136. doi: 10.3978/j.issn.2305-5839.2015.03.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jin S, Sonobe Y, Cheng Y, Horiuchi H, Parajuli B, Kawanokuchi J, Mizuno T, Takeuchi H, Suzumura A. Interleukin-1beta induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS One. 2014;9:e110024. doi: 10.1371/journal.pone.0110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, Dorrell MI, Simon MC, Haase VH, Friedlander M, Johnson RS. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58:1177–1185. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JR, Koerner IP, Moller T. Microglia in ischemic brain injury. Future Neurol. 2010;5:227–246. doi: 10.2217/fnl.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Vadakkan KI, Zhuo M. ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia. 2007;55:810–821. doi: 10.1002/glia.20500. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Wu G, Akhavan Sharif MR, Baker A, Jia Y, Fahey FH, Luo HR, Feener EP, Clapham DE. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat Neurosci. 2012;15:565–573. doi: 10.1038/nn.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- Xie L, Mao X, Jin K, Greenberg DA. Vascular endothelial growth factor-B expression in postischemic rat brain. Vasc Cell. 2013;5:8. doi: 10.1186/2045-824X-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FL, Hansen DV, Sheng M. TREM2, Microglia, and Neurodegenerative Diseases. Trends Mol Med. 2017;23:512–533. doi: 10.1016/j.molmed.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Zand L, Ryu JK, McLarnon JG. Induction of angiogenesis in the beta-amyloid peptide-injected rat hippocampus. Neuroreport. 2005;16:129–132. doi: 10.1097/00001756-200502080-00011. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Carney KE, Pigott VM, Falgoust LM, Clark PA, Kuo JS, Sun D. Glioma-mediated microglial activation promotes glioma proliferation and migration: roles of Na+/H+ exchanger isoform 1. Carcinogenesis. 2016;37:839–851. doi: 10.1093/carcin/bgw068. [DOI] [PMC free article] [PubMed] [Google Scholar]