Abstract
Background
Most patients with juvenile myelomonocytic leukemia (JMML) are curable only with allogeneic hematopoietic cell transplantation (HCT). However, the current standard conditioning regimen, busulfan-cyclophosphamide-melphalan (Bu-Cy-Mel), may be associated with higher risks of morbidity and mortality. ASCT1221 was designed to test whether the potentially less-toxic myeloablative conditioning regimen containing busulfan-fludarabine (Bu-Flu) would be associated with equivalent outcomes.
Procedure
Twenty-seven patients were enrolled on ASCT1221 from 2013–2015. Pre- and post-HCT (starting Day +30) mutant allele burden was measured in all and pre-HCT therapy was administered according to physician discretion.
Results
Fifteen patients were randomized (6 to Bu-Cy-Mel and 9 to Bu-Flu) after meeting diagnostic criteria for JMML. Pre-HCT low-dose chemotherapy did not appear to reduce pre-HCT disease burden. Two patients, however, received aggressive chemotherapy pre-HCT and achieved low disease-burden state; both are long-term survivors. All 4 patients with detectable mutant allele burden at Day +30 post-HCT eventually progressed, as compared to 2 of 9 patients with unmeasurable allele burden (p=0.04). The 18-month event-free-survival of the entire cohort was 47% (95% CI, 21–69%); and was 83% (95% CI, 27–97%) and 22% (95% CI, 03–51%) for Bu-Cy-Mel and Bu-Flu, respectively (p=0.04). ASCT1221 was terminated early due to concerns that the Bu-Flu arm had inferior outcomes.
Conclusions
The regimen of Bu-Flu is inadequate to provide disease control in patients with JMML who present to HCT with large burdens of disease. Advances in molecular testing may allow better characterization of biologic risk, pre-HCT responses to chemotherapy, and post-HCT management.
Keywords: Juvenile Myelomonocytic Leukemia, Hematopoietic Cell Transplantation, Conditioning Regimens, Mutant Allele Burden
INTRODUCTION
Juvenile myelomonocytic leukemia (JMML) is classified as an overlapping myeloproliferative/myelodysplastic neoplasm, with a median age of diagnosis of <2 years.1,2 Patients frequently present with high disease burdens and severe symptoms, including massive hepatosplenomegaly, pulmonary infiltrates, fevers, and rash.3 The precise role of pre-transplant chemotherapy to attempt to minimize disease-burden is not yet determined.1,4 The majority of patients are now molecularly diagnosed via identification of mutations in PTPN11, NRAS, KRAS, RRAS, RRAS2, CBL, SETBP1, or NF1.5
With few exceptions, long-term event-free survival (EFS) has only been achieved following allogeneic hematopoietic cell transplantation (HCT).6 The most common conditioning regimen utilized is busulfan, cyclophosphamide, and melphalan (Bu-Cy-Mel).1 Young children with JMML are known to be at risk for transplant-related mortality (TRM), with reported rates of 10–22% in patients with JMML,1,2,7 Identification of a less-toxic conditioning regimen may help improve short- and long-term outcomes. The conditioning regimen of busulfan and fludarabine (Bu-Flu) had decreased toxicity and equivalent survival compared with the Bu-Cy-Mel regimen in a non-randomized study of patients mainly with acute myeloid leukemia (AML) in remission.8 Pharmacokinetic analysis of busulfan demonstrated that adding a third alkylating agent increases the risk of acute toxicities, including sinusoidal obstruction syndrome (SOS) and graft-versus-host disease (GVHD).9
Here we report the findings of the randomized Phase II Children’s Oncology Group (COG) study ASCT1221 in patients with de novo JMML. The objective was to pick the winner of two different conditioning regimens on the basis of the one with the lowest risk of TRM, as long as EFS was comparable.
METHODS
Eligibility
ASCT1221 (registered at www.clinicaltrials.gov as NCT 01824693) activated in June 2013 and approved by the National Cancer Institute Central Institutional Review Board (IRB) and local center IRBs. Legal guardians signed written informed consent. Patients with suspected JMML were eligible for Part 1 of the study to confirm the diagnosis. Central review of clinical characteristics, pathology, cytogenetics (including G-banding and interphase FISH analysis for monosomy 7), and molecular testing was performed to ensure that patients met consensus criteria for JMML10 prior to proceeding to HCT on Part 2 of the study. Patients with germline mutations in PTPN11 or who had progressed to AML (>20% blasts) were excluded.
Pre-HCT Therapy
ASCT1221 did not mandate the use of, or a particular approach to, pre-HCT therapy (including the potential use of splenectomy), which was left to the discretion of the treating physician. The use of pre-HCT chemotherapy was captured and defined as: none, low-dose (including treatment with 6-mercaptopurine [6-MP] and/or cis-retinoic acid), or AML-like (cytarabine ≥1 g/m2/day +/− other agents).
Conditioning and Transplant
All patients who met eligibility criteria (appropriate donor identified, adequate organ function, absence of uncontrolled infection) could proceed to HCT per the treating physician. Donor selection was per treating physician and included matched siblings (MSD), adult unrelated donors (URD), and umbilical cord blood (UCB), but not haploidentical-related donors. Allele-level HLA-matching at ≥7/8 loci was required for URD. For UCB, ≥4/6 serologic HLA-matching at HLA-A and –B (with allele level at HLA-DR), and a dose ≥5×107 nucleated cells/kg were required. Stratified randomization occurred based on donor type and the presence of PTNP11 mutations, which have been considered high-risk.11–13
Conditioning regimen A (Bu-Cy-Mel) was intravenous busulfan (initial 3.2–4 mg/kg/day, with 1–2 therapeutic dose adjustments to achieve a cumulative area under the curve [AUC] of 59–98 mg*h/L, or 3600–6000 μM/L*Min per day) on Days −8 to −5, cyclophosphamide (60 mg/kg/dose with prophylactic mesna 60 mg/kg/day) on Days −4 to −3, and melphalan (140 mg/m2, or 4.67 mg/kg for patients <10 kg) on Day −1. Conditioning regimen B (Bu-Flu) was intravenous busulfan (as above) on Days −8 to −5, and fludarabine (40 mg/m2/dose, or 1.33 mg/kg/dose for patients <10 kg) on Days −5 to −2. Rabbit anti-thymocyte globulin (2 mg/kg/dose) was added to recipients of cells from URD (on Days −4 to −1) or UCB (on Days −8 to −5).
GVHD prophylaxis was tacrolimus (starting on Day −1 with goal level of 5–12 ng/mL; taper starting by Day +60 and complete by Day +98 for MSD; taper starting by Day +100 and complete by Day +180 for URD/UCB), and mycophenolate mofetil (15 mg/kg/dose every 8 hours; from Day +1 to Day +30 for MSD, or Day +45 for URD/UCB).
Response Criteria and Definitions
Disease response was defined according to international consensus criteria.14 Mutant allele burden was measured at diagnosis, prior to HCT and at regular intervals after HCT, with the first measurement occurring at Day +30. Primary graft failure was defined as failure to achieve an absolute neutrophil count of ≥500/mm3 after 42 days or <5% donor cells in bone marrow by Day +42, without evidence of JMML. SOS was defined by the Baltimore criteria.15 Grade 3/4 toxicities were collected using CTCAEv4.0. Acute (aGVHD) and chronic GVHD were scored using consensus criteria.16,17 For the purpose of this analysis, clinical high-risk disease at diagnosis was defined as being >2 years of age,11,13,18 platelets <40 × 109/L,18 or having a hemoglobin F elevated for age.1,18 Biologic high-risk disease was defined as having any cytogenetic abnormality,2,13 secondary mutations,19 or DNA-methylation designation of intermediate or high.20
Correlative Biologic Studies
JMML Mutation Testing
All patients enrolled on ASCT1221 were sequenced to determine their JMML mutation status in real-time, using a CLIA-certified assay at diagnosis, pre-HCT, at defined time-points post-HCT, and at relapse (when applicable). The entire coding sequence of Ras-pathway genes including NF1, NRAS, KRAS, PTPN11 and CBL were analyzed and the allele fraction of each mutation was reported to the treating physician. All patients were analyzed on the Ion-Torrent platform using a tumor-normal approach to distinguish germline from somatic mutations, as described previously.21 Tumor samples were obtained from mononuclear cells from blood or bone marrow. Germline samples were obtained from buccal swabs or bone marrow fibroblasts.
UCSF500 Sequencing
After ASCT1221 was closed, our group reported on the presence of secondary mutations in 11 additional genes, including SETBP1, JAK3, ASXL1 and SH2B3. Patients who were randomized and received a HCT on study had diagnostic material sequenced using the UCSF500 assay. This assay compares tumor to normal for approximately 500 genes that are commonly altered in cancer. Twelve of 15 transplanted patients had samples (buccal or bone marrow fibroblasts and relapse, when available) evaluated. Analysis and determination of pathogenic variants was performed, as previously described.21
Exome Sequencing
Three of 15 transplanted patients who had relapse samples available were analyzed using whole exome sequencing (WES) with a trio approach (normal-diagnosis-relapse). Analysis and determination of pathogenic variants and copy number were performed, as previously described.21,22
DNA Methylation Analysis
Fourteen of 15 transplanted patients had diagnostic tumor DNA available for genome-wide DNA methylation analysis performed using the Illumina 450k BeadChip platform. Patient samples from ASCT1221 were compared to a cohort of 39 distinct JMML patients, and using a “nearest centroid neighbor” approach were classified with a low, intermediate or high methylation-cluster designation, as previously described.20
Statistical Analysis
ASCT1221 was powered to accrue 54 patients in each arm in order to pick-the-winner of regimens, Arm A and Arm B in terms of TRM and EFS, with the outcome determined after all eligible patients had ≥18 months of follow-up, as patients with JMML rarely relapse >18 months post-HCT.1,2 An interim analysis of outcomes at 2 years after the start of the study was planned to look at rates of graft failure, relapse, and TRM. Primary response measures were TRM, EFS, overall survival (OS), and relapse/disease progression as of June 30, 2017. The Kaplan-Meier method was used to estimate probabilities of EFS and OS. Events were defined as relapse/progression or TRM. The probabilities of relapse/progression, TRM, SOS, and GVHD were estimated using the method of cumulative incidence that accounts for competing events (i.e. death). The significance of observed differences in proportions was tested using the Fisher’s exact test. Clopper-Pearson was used to calculate confidence intervals. Comparison of median between arms was done using Wilcoxon rank-sum test. All tests were two-sided with P values <0.05 considered statistically significant.
RESULTS
Patient Characteristics
Thirty patients were enrolled, however 3 patients were determined to not meet diagnostic criteria for JMML (Supplemental Figure S1). The baseline characteristics of the remaining 27 patients are listed in Table 1. No patient had evidence of neurofibromatosis type-1; 96.3% of patients had a canonical Ras-pathway mutation. High-risk disease was noted retrospectively in 15%, 19%, and 47% of patients based on the presence of cytogenetic abnormalities, secondary mutations, or DNA hypermethylation, respectively.2,13,19,20
TABLE 1.
Clinical and Molecular Characteristics at Time of Diagnosis for Eligible Patients
| Total Enrolled | 27 |
|---|---|
| Male Sex | 17 (63%) |
| Median Age at Diagnosis (range) | 13.3 months (0.7–148 months) |
| Splenomegaly at Diagnosis | 24 (89%) |
| Median WBC Count, × 109/L (range) | 26.9 (7–122) |
| Median Absolute Monocyte Count, × 106/L(range) | 4430 (1120–96,000) |
| Median Platelet Count, × 109/L (range) | 46 (10–227) |
| Median Peripheral Blood Blasts (range) | 2% (0–13%) |
| Median Bone Marrow Blasts (range) | 3% (0–16%) |
| Median Hemoglobin F (range) | 8% (1–66%) |
| RAS-Pathway Mutation Identified | |
| PTPN11 | 11 (41%) p.E76K (N=8); p.E76G (N=1); p.D61Y (N=1); p.E69K (N=1); |
| KRAS | 6 (22%) p.G13D (N=3); p.G13Y (N=1); p.G12D (N=1); p.G12V (N=1) |
| NRAS | 3 (11%) p.G12D (N=2); p.G13D (N=1) |
| RRAS | 1 (4%) p.Q87L (N=1) |
| RRAS2 | 1 (4%) p.Q72L (N=1) |
| CBL | 4 (15%) p.C404R (N=1); p.Y371H (N=1); pY371splice_site (N=1); |
| None | 1 (4%) |
| Median Mutant Allele Burden (range)* | 43.5% (8.1–50.2%) |
| Cytogenetic Abnormality | |
| Monosomy 7 | 3 (11%) |
| Other [t(3;5)] | 1 (4%) |
| None | 23 (85%) |
| Secondary Mutations | 5 (19%) |
| DNA Hypermethylation (Transplanted Patients Only) | |
| Low | 7 (47%) |
| Intermediate | 4 (27%) |
| High | 3 (20%) |
| Not Available | 1 (6%) |
| Median IgG, mg/dL (range) | 1045 (15–2990) |
| Direct Antibody Test (Coomb’s) Positive | 5/23 (22%) |
| Elevated IgG for Age or DAT Positive | 5/11 (46%) with RAS mutation; 7/16 (44%) without RAS mutation |
For patients with mutations in PTPN11, KRAS, NRAS, or RRAS
One patient withdrew consent shortly after enrollment. Two patients (with KRAS p.G12D and KRAS p.G13D) were not eligible for Part 2 of the study due to progression to AML. Thus, 24 patients were eligible to proceed to HCT, of which 15 patients were randomized (Table 2). One patient (with PTPN11 p.E76K) refused HCT and died of progressive disease. Four patients (with NRAS p.G12D, CBL p.Y371H, CBL p.Y371splice_site, and without an identifiable mutation) were intentionally observed without HCT per physician preference, all without signs of progression at last follow-up. Four patients were treated with non-protocol HCT, three after randomization was suspended.
TABLE 2.
Risk and Transplant Characteristics by Treatment Assignment
| Risk Factors & HCT Characteristics | Arm A: Busulfan-Cyclophosphamide-Melphalan (N = 6) | Arm B: Busulfan-Fludarabine (N = 9) |
|---|---|---|
| High-Risk Clinicallyˆ | 3 (50%) | 7 (78%) |
| Age >2 years at Diagnosis | 1 (16%) | 4 (44%) |
| Platelets <40 × 109/L at Diagnosis | 2 (33%) | 4 (44%) |
| Hemoglobin F elevated for age | 2 (33%) | 6 (67%) |
| High-Risk Biologically* | 2 (33%) | 6 (67%) |
| Cytogenetic Abnormality | 1 (16%) | 2 (22%) |
| Secondary Mutations | 1 (16%) | 3 (33%) |
| DNA Methylation (Intermediate or High) | 2 (33%) | 5 (63%)1 |
| Time from Diagnosis to HCT (range) | 1.4 months (0.7–3.5 months) |
1.2 months (0.5–3.7 months) |
| Pre-HCT Therapy | ||
| None | 2 (66%) | 4 (45%) |
| Low-Dose | 4 (67%) | 3 (33%) |
| AML-Like | 0 | 2 (22%) |
| Median Pre-HCT Allele Burden (range) | 39.4% (23%-46.8%) | 46.9% (0–50.3%) |
| Donor | ||
| HLA-Matched Related | 2 (33%) | 3 (33%) |
| 8/8 HLA-Matched Unrelated | 3 (50%) | 5 (56%) |
| <8/8 HLA-Mismatched Unrelated | 1 (16%) | 1 (11%) |
| Stem Cell Source | ||
| Bone Marrow | 6 (100%) | 7 (78%) |
| Peripheral Blood | 0 | 1 (11%) |
| Umbilical Cord Blood | 0 | 1 (11%) |
| Busulfan Cumulative AUC (mg*h/L) | 71 (58–87) | 68 (62–80) |
Defined as the presence of at least 1 of the following: a) age >2 years at diagnosis; b) platelets <40 × 109/L at diagnosis; c) hemoglobin F elevated for age
Defined as presence of at least 1 of the following: a) cytogenetic abnormality; b) secondary mutation; or c) DNA Methylation Intermediate or High
Of 8 patients tested
Baseline characteristics between Arm A (Bu-Cy-Mel) and Arm B (Bu-Flu) were notable for 33% (2/6) of patients in Arm A retrospectively identified to have high-risk biological features compared to 67% (6/9) of patients in Arm B (p=0.31). In the 15 randomized patients, mutant allele burden was measured at the time of diagnosis and within 2 weeks of start of conditioning. As shown in Figure 1, the use of low-dose pre-HCT chemotherapy did not impact mutant allele burden compared to no chemotherapy, however, the two patients given AML-like chemotherapy achieved low mutant allele burdens (0 and 1.4%) upon count recovery. No patient underwent splenectomy prior to HCT.
FIGURE 1.
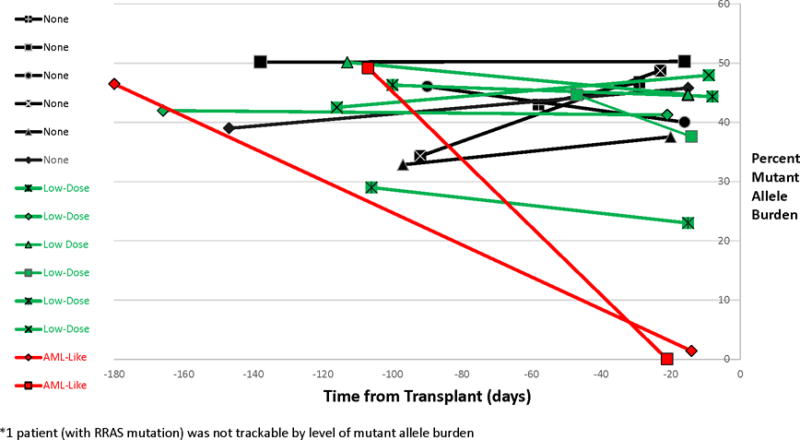
Allele Burden by Pre-Transplant Therapy
HCT Toxicities & GVHD
Cumulative busulfan exposure was similar between the 2 arms (p=0.64). Day 100 TRM occurred in one patient (7% overall), who was assigned to Arm B. This patient had significant disease burden at time of HCT, and subsequently developed SOS and multi-system organ failure on Day+12 post-HCT. In total, SOS developed in 50% (3/6) and 22% (2/9) of patients on Arms A and B, respectively (p=0.33). Other notable grade 3–4 toxicities that occurred prior to Day 100 included oral mucositis (33% in both Arms), and febrile neutropenia (33% in both Arms). No SOS or Grade 3–4 toxicities were reported in 2/6 (33%) and 4/9 (44%) of patients in Arms A and Arm B, respectively.
Three patients developed Grade I aGVHD, while no patient developed Grade II aGVHD. The cumulative incidence of Grade III-IV aGVHD by 100 days was 27% (95% CI, 8–51%), with no difference between Arms (p=0.48). However, despite the presence of severe aGVHD, 3/4 patients eventually relapsed. Chronic GVHD occurred in only 1 patient total (in Arm A), for an 18-month cumulative incidence of 7% (95% CI, 0.33–28%).
Disease Progression Post-HCT
All patients had evidence of primary neutrophil recovery. One patient in Arm B had secondary graft loss (no detectable donor chimerism) in conjunction with JMML progression. For the other 13 patients, the median initial whole blood chimerism was 100% (range, 98–100%) for patients in Arm A, compared to 97% (range, 51–100%) in Arm B (p=0.03). Patient-level risk-characteristics and outcomes are shown in Table 3. The cumulative incidence of disease progression by 18-months was 17% (95% CI, 0.4–55%) and 55% (95% CI, 16–83%) for patients on Arms A and B, respectively (p=0.19).
TABLE 3.
Patient-Level Data on Biologic Risk, Pre-HCT Therapy, Conditioning Regimen, and Outcome
| UPN | Clinical Risk | Driver Mutation | Additional Mutation(s) | Cytogenetics | Methylation Status | Biologic Risk | Pre-HCT Chemo | Regimen | Outcome* |
|---|---|---|---|---|---|---|---|---|---|
| 1 | High | KRAS (p.G13D) | None | Normal | Low | Standard | None | Bu-Flu | Relapsed |
| 2 | Standard | KRAS (p.G13Y) | None | Normal | Low | Standard | None | Bu-Flu | Relapsed |
| 3 | High | RRAS (p.Q87L) | DNMT3A p.R882C | Monosomy 7 | Low | High | Low-Dose | Bu-Flu | Relapsed |
| 4 | Standard | PTPN11 (p.E76K) | None | Normal | Intermediate | High | AML-Like | Bu-Flu | Disease-Free |
| 5 | High | PTPN11 (p.E76K) | None | Normal | Intermediate | High | None | Bu-Flu | Relapsed |
| 6 | High | NRAS (p.G12D) | None | t(3;5) | Intermediate | High | AML-Like | Bu-Flu | Disease-Free |
| 7 | High | PTPN11 (p.E76K) | CBL c.1227+4C>T | Normal | High | High | Low-Dose | Bu-Flu | Relapsed |
| 8 | High | PTPN11 (p.E76K) | NF1 (p.0splice_site) | Normal | High | High | None | Bu-Flu | Relapsed |
| 9 | High | PTPN11 (p.E76K) | None | Normal | Not Available | Standard | Low-Dose | Bu-Flu | TRM |
| 10 | High | KRAS (p.G13D) | None | Normal | Low | Standard | None | Bu-Cy-Mel | Relapsed |
| 11 | High | KRAS (p.G12V) | None | Normal | Low | Standard | Low-Dose | Bu-Cy-Mel | Disease-Free |
| 12 | Standard | NRAS (p.G13D) | None | Normal | Low | Standard | Low-Dose | Bu-Cy-Mel | Disease-Free |
| 13 | Standard | PTPN11 (p.E69K) | None | Normal | Low | Standard | Low-Dose | Bu-Cy-Mel | Disease-Free |
| 14 | Standard | PTPN11 (p.E76K) | None | Monosomy 7 | Intermediate | High | Low-Dose | Bu-Cy-Mel | Disease-Free |
| 15 | High | PTPN11 (p.E76G) | JAK3 p.R657Q | Normal | High | High | None | Bu-Cy-Mel | Disease-Free |
At time of last follow-up. Relapsed patients include patients with refractory disease and/or who were demonstrated to have JMML at time of count “recovery” from HCT.
Two general patterns of disease progression were noted (Figure 2). Two patients (both in Arm B) entered HCT with large disease burdens (clinically and molecularly). Although both recovered neutrophils (and 1 had Grade III aGVHD), they also continued to have clinical and molecular signs of residual JMML and were scored as non-responders. Two patients (both in Arm B) appeared to be in clinical remission post-HCT, however, they continued to have low mutant allele burden (0.8–1.9%) measured at Day +30 post-HCT. Both eventually progressed clinically, with a corresponding increase in their molecular burden, and were scored as relapses. In contrast, nine patients had no evidence of mutant allele burden at Day +30 post-HCT, and only 2 of them (one in Arm A and 1 in Arm B) progressed (p=0.04).
FIGURE 2.
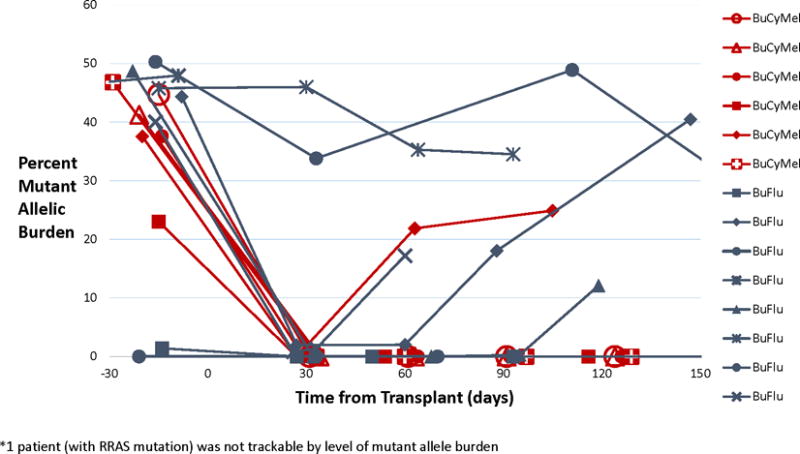
Allele Burden by Conditioning Regimen
Monosomy 7 was present at diagnosis in 13% (2/15) patients. Of the three relapsed patients who were analyzed using WES, two developed chromosome 7 derangements (Supplemental Figure S2). Neither had monosomy 7 detected (by FISH, conventional cytogenetics, nor WES) at diagnosis, implying that clonal evolution contributed to relapse in these patients.
Post-HCT Survival
The estimated 18-month post-HCT EFS of the entire cohort was 47% (95% CI, 21–69%), with a median follow-up time of 29 months (range, 13–44 months) in surviving patients. As shown in Figure 3, the estimated 18-month EFS was 83% (95% CI, 27–97%) and 22% (95% CI, 03–51%) in Arms A and B, respectively (p=0.04). When this imbalance in events became apparent, the Data and Safety Monitoring Committee suspended further randomization and ASCT1221 was closed. Patients who relapsed/progressed post-HCT were taken off-study and treated per physician-discretion. The estimated 18-month post-HCT OS was 64% (95% CI, 34–83%) overall, with 83% (95% CI, 27–97%) and 48% (95% CI, 12–77%) in Arms A and B, respectively (p=0.26).
FIGURE 3.
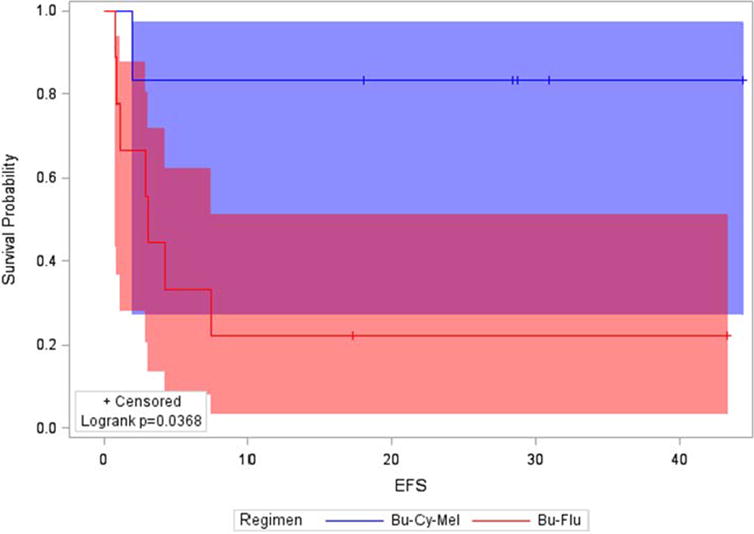
Estimated 18-month Event-Free Survival by Treatment Arm
Patients with biological high-risk disease (classified retrospectively) had a similar 18-month EFS compared to standard-risk disease: 50% (95% CI, 15–77%) and 43% (95% CI, 10–73%), respectively (p=0.41), though small numbers limits ASCT1221’s power to address this question. The 18-month EFS was 17% (95% CI, 0.77–52%) in patients not treated with any chemotherapy, 57% (17–84%) in patients who were given low-dose chemotherapy, and 100% in the 2 patients treated with AML-like chemotherapy (p=0.08).
DISCUSSION
JMML is a rare disease of early childhood that is typically curable only with HCT. However, 5-year EFS with current approaches remain sub-optimal, ranging from 39–53%.1,2 On ASCT1221, we tested two conditioning regimens (busulfan-cyclophosphamide-melphalan versus busulfan-fludarabine) in a randomized fashion. While the overall 18-month EFS of 47% was similar to that of other reported JMML HCT cohorts, ASCT1221 was suspended early for excessive events in the Bu-Flu arm.
Conclusions regarding the optimal conditioning regimen are limited by small numbers, possible imbalances in disease severity between the arms, and differing approaches to pre-HCT chemotherapy; the latter two potentially having a substantial impact on post-HCT outcomes. Nevertheless, ASCT1221 is the first prospective trial wherein all transplanted patients were molecularly characterized and confirmed to have JMML, an important consideration given that there are known mimics of the disease, such as Wiskott-Aldrich Syndrome23 and Infantile Osteopetrosis.24
In regards to disease severity, to date a consensus risk-stratification system for patients with JMML is lacking. High-risk features that have been reported in some cohorts include older age,1,2,11,13,18 mutations in PTPN11,11,13 presence of monosomy 7 or other cytogenetic abnormalities,2,11,13 low platelet counts,18,19 elevated HgbF,1,18 increased blasts or an AML-like gene-expression profile,1,13 DNA hyper-methylation,20,25,26 and the presence of >1 somatic mutation in genes such as SETBP1, SH2B3, ASXL1, JAK3, and others.19,27,28 The exact weighting of these different factors in determining an overall risk-status is not yet known. ASCT1221 was designed to stratify only by PTPN11 status and donor type, and not on age or newer biologic risk-factors. Nevertheless, the better outcomes with the more intensive Bu-Cy-Mel supports the hypothesis that treatment-delivered remains an important prognostic factor, in that standard-risk patients may still relapse/progress if not adequately challenged with aggressive chemotherapy either pre-HCT or during conditioning.
Treating physicians were allowed to choose whether or not to administer any pre-HCT chemotherapy, therefore patients presented to HCT with markedly-different disease burdens. We noted that the use of 6-MP, while capable of reducing some of the clinical symptoms, had minimal effect on mutant allele burden, supporting in vitro data that JMML myeloid progenitors are often resistant to 6-MP.29 Some patients were not treated with any chemotherapy pre-HCT, on the basis of the EWOG-MDS/EBMT study results that patients who received either no therapy or low-dose chemotherapy compared to AML-like chemotherapy had nearly-identical 5-year EFS, relapse, and TRM on univariate analysis.1 However, since the choice of pre-HCT chemotherapy in the EWOG-MDS/EBMT study was left to the treating physician’s discretion, patients with more aggressive disease may have been more intensively pre-treated. Furthermore, the EWOG-MDS/EBMT study included 10% of patients with AML transformed out of JMML, which had 0% 5-year EFS. It is possible that inclusion of these very high-risk patients (who may have received AML-like chemotherapy) might have obscured a potential benefit to intensive chemotherapy in a “standard” patient with JMML. In the 2013 EUROCORD/CIBMTR retrospective UCB study (excluding patients with >20% blasts) there was improved EFS in patients who received AML-like therapy compared to none or low-dose chemotherapy (55% vs. 32%; p=0.048).2 COG AAML0122 evaluated the efficacy of a pre-transplant window therapy with a farnesyl transferase inhibitor followed by two cycles of intensive AML-type chemotherapy using fludarabine and cytarabine.30 For patients who achieved a complete response (defined as normalization of WBC count and resolution of organomegaly) there was a trend towards improved 5-year EFS (54% vs. 33%, p=0.144) and 5-year relapse rate (38% vs. 57%, p=0.154); though AAML0122 was not powered to address this question. The Japanese Pediatric MDS Study Group’s JMML HCT report included three patients who received AML-like chemotherapy prior to HCT; all remained relapse-free.7 The European community is currently testing the potential role of azacytidine in decreasing pre-HCT disease burden with the goal of improving post-HCT outcomes.31,32
An established principle of pediatric allogeneic HCT for hematologic malignancies is that patients who come to HCT with lower disease burden have improved post-HCT outcomes, typically due to lower relapse rates. Therefore, for acute lymphoblastic leukemia,33 AML,34 and myelodysplastic syndrome with excess blasts,35–38 pre-HCT chemotherapy aiming to achieve the best possible remission status is considered standard-of-care. This also holds for the adult JMML-like disease chronic myelomonocytic leukemia.39 One potential explanation may be that the alloreactive graft-versus-leukemia (GVL) effect is typically incapable of controlling large disease burdens.40 Although the numbers were small, we noted that all patients who developed GVHD (a surrogate for GVL) on the intensive Arm A (Bu-Cy-Mel) remained disease-free at 18 months, while all patients on Arm B (Bu-Flu) who developed GVHD still relapsed. As such, the more intensive Bu-Cy-Mel regimen may be required to provide optimal de-bulking when patients present to HCT with large disease burdens. Conversely, the less-intensive Bu-Flu regimen, or other less-toxic regimens, may still be a potential option for patients who receive effective pre-HCT chemotherapy, with the caveat that our numbers are too small to make any definitive conclusions. Finally, we did not test the regimen which adds Melphalan to Busulfan and Fludarabine, and this could potentially be as efficacious as Bu-Cy-Mel.7
Finally, the particular Bu-Flu regimen used on ASCT1221 administered busulfan and fludarabine on separate days. However, in vitro data suggests improved killing of AML cells when the two are administered together.41 In addition, the patients on the Bu-Flu arm had a low cumulative busulfan exposure (median 68 mg×h/L) compared to the recently-identified optimal AUC of 78–101 mg×h/L when using a single alkylator,9 which also may have contributed to poor outcomes in this group. Despite these modest busulfan levels, SOS rates were high in both Arms, suggesting that JMML patients may need additional SOS prophylaxis.
In conclusion, JMML patients with high pre-HCT disease burden have a poor outcome when treated with a conditioning regimen of busulfan-fludarabine. Advances in molecular testing now allow better characterization of pre-HCT responses to chemotherapy. In addition, improved understanding of precise pre-HCT risk-stratification will potentially facilitate future risk-adapted treatment approaches. Further efforts at studying novel approaches to improving the outcomes of rare patients with JMML will likely require international cooperation, as well as detailed molecular risk-profiling to ensure that comparisons between regimens are valid.
Supplementary Material
SUPPLEMENTAL FIGURE S1. Flow Diagram of Patient Enrollment, Allocation, and Analysis.
SUPPLEMENTAL FIGURE S2. Acquisition of Bone Marrow Chromosome 7 Derangements at Relapse in Two Patients as Detected by Copy Number Analysis on Whole Exome Sequencing
Acknowledgments
The authors thank Bryan Langholz, Mark Krailo, Lu Chen, and Todd Alonzo for additional statistical support and advice. Research reported in this publication was supported by the Children’s Oncology Group and the National Clinical Trials Network (NCTN) of the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under grants number U10CA98543, U10CA98413, U10CA180886, U10CA180899, CA21765, CA36401, and U01GM92666. Further support from the St. Baldrick’s Foundation, the Leukemia and Lymphoma Society (ES, MLL) and NIH/NCI 5P30CA082103 (AO). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Abbreviations Key
- JMML
Juvenile Myelomonocytic Leukemia
- HCT
Hematopoietic Cell Transplantation
- Bu-Cy-Mel
Busulfan-Cyclophosphamide-Melphalan
- Bu-Flu
Busulfan-Fludarabine
- EFS
Event-Free Survival
- AML
Acute Myeloid Leukemia
- SOS
Sinusoidal Obstruction Syndrome
- GVHD
Graft-Versus-Host Disease
- COG
Children’s Oncology Group
- TRM
Transplant-Related Mortality
- 6-MP
6-Mercaptopurine
- MSD
Matched Sibling Donor
- URD
Unrelated Donor
- UCB
Umbilical Cord Blood
- AUC
Area Under Curve
- WES
Whole Exome Sequencing
- EWOG-MDS/EBMT
European working Group of MDS and JMML in Childhood / European Society for Blood and Marrow Transplantation
- CIBMTR
Center for International Blood and Marrow Transplant Research
- GVL
Graft-Versus-Leukemia
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Contributor Information
Christopher C. Dvorak, University of California San Francisco
Prakash Satwani, Columbia University.
Elliot Stieglitz, University of California San Francisco.
Mitchell S. Cairo, Maria Fareri Children’s Hospital, Westchester Medical Center, New York Medical College
Ha Dang, University of Southern California.
Qinglin Pei, Children’s Oncology Group.
Yun Gao, Children’s Oncology Group.
Donna Wall, Hospital for Sick Children.
Tali Mazor, University of California San Francisco.
Adam B. Olshen, University of California San Francisco
Joel S. Parker, University of North Carolina
Samir Kahwash, Nationwide Children’s Hospital.
Betsy Hirsch, University of Minnesota.
Susana Raimondi, St. Jude Children’s Research Hospital.
Neil Patel, Children’s Hospital of Philadelphia.
Micah Skeens, Nationwide Children’s Hospital.
Todd Cooper, Seattle Children’s Hospital.
Parinda A. Mehta, Cincinnati Children’s Hospital Medical Center
Stephan A. Grupp, Children’s Hospital of Philadelphia
Mignon L. Loh, University of California San Francisco
References
- 1.Locatelli F, Nollke P, Zecca M, et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005;105(1):410–419. doi: 10.1182/blood-2004-05-1944. [DOI] [PubMed] [Google Scholar]
- 2.Locatelli F, Crotta A, Ruggeri A, et al. Analysis of risk factors influencing outcomes after cord blood transplantation in children with juvenile myelomonocytic leukemia: a EUROCORD, EBMT, EWOG-MDS, CIBMTR study. Blood. 2013;122(12):2135–2141. doi: 10.1182/blood-2013-03-491589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arico M, Biondi A, Pui CH. Juvenile myelomonocytic leukemia. Blood. 1997;90(2):479–488. [PubMed] [Google Scholar]
- 4.Bergstraesser E, Hasle H, Rogge T, et al. Non-hematopoietic stem cell transplantation treatment of juvenile myelomonocytic leukemia: a retrospective analysis and definition of response criteria. Pediatr Blood Cancer. 2007;49(5):629–633. doi: 10.1002/pbc.21038. [DOI] [PubMed] [Google Scholar]
- 5.Chang TY, Dvorak CC, Loh ML. Bedside to bench in juvenile myelomonocytic leukemia: insights into leukemogenesis from a rare pediatric leukemia. Blood. 2014;124(16):2487–2497. doi: 10.1182/blood-2014-03-300319. [DOI] [PubMed] [Google Scholar]
- 6.Niemeyer CM, Arico M, Basso G, et al. Chronic myelomonocytic leukemia in childhood: a retrospective analysis of 110 cases. European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS) Blood. 1997;89(10):3534–3543. [PubMed] [Google Scholar]
- 7.Yabe M, Ohtsuka Y, Watanabe K, et al. Transplantation for juvenile myelomonocytic leukemia: a retrospective study of 30 children treated with a regimen of busulfan, fludarabine, and melphalan. Int J Hematol. 2015;101(2):184–190. doi: 10.1007/s12185-014-1715-7. [DOI] [PubMed] [Google Scholar]
- 8.Bartelink I, van Reij E, Gerhardt C, et al. Fludarabine and exposure-targeted busulfan compares favorably with busulfan/cyclophosphamide based regimens in pediatric HCT: maintaining efficacy with less toxicity. Biol Blood Marrow Transplant. 2014;20(3):345–353. doi: 10.1016/j.bbmt.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Bartelink IH, Lalmohamed A, van Reij EML, et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: a multicentre, retrospective cohort analysis. The Lancet Haematology. 2016;3(11):e526–e536. doi: 10.1016/S2352-3026(16)30114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dvorak C, Loh M. Juvenile myelomonocytic leukemia: Molecular pathogenesis informs current approaches to therapy & hematopoietic cell transplantation. Front Pediatr. 2014;2(25) doi: 10.3389/fped.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida N, Yagasaki H, Xu Y, et al. Correlation of clinical features with the mutational status of GM-CSF signaling pathway-related genes in juvenile myelomonocytic leukemia. Pediatr Res. 2009;65(3):334–340. doi: 10.1203/PDR.0b013e3181961d2a. [DOI] [PubMed] [Google Scholar]
- 12.Park H, Lee S, Sung K, et al. Gene mutations in the Ras pathway and the prognostic implication in Korean patients with juvenile myelomonocytic leukemia. Ann Hematol. 2012;91(4):511–517. doi: 10.1007/s00277-011-1326-9. [DOI] [PubMed] [Google Scholar]
- 13.Bresolin S, Zecca M, Flotho C, et al. Gene expression-based classification as an independent predictor of clinical outcome in juvenile myelomonocytic leukemia. J Clin Oncol. 2010;28(11):1919–1927. doi: 10.1200/JCO.2009.24.4426. [DOI] [PubMed] [Google Scholar]
- 14.Niemeyer CM, Loh ML, Cseh A, et al. Criteria for evaluating response and outcome in clinical trials for children with juvenile myelomonocytic leukemia. Haematologica. 2015;100(1):17–22. doi: 10.3324/haematol.2014.109892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones RJ, Lee KS, Beschorner WE, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44(6):778–783. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Leisenring WM, Martin PJ, Petersdorf EW, et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood. 2006;108(2):749–755. doi: 10.1182/blood-2006-01-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e381. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passmore SJ, Chessells JM, Kempski H, Hann IM, Brownbill PA, Stiller CA. Paediatric myelodysplastic syndromes and juvenile myelomonocytic leukaemia in the UK: a population-based study of incidence and survival. Br J Haematol. 2003;121(5):758–767. doi: 10.1046/j.1365-2141.2003.04361.x. [DOI] [PubMed] [Google Scholar]
- 19.Stieglitz E, Taylor-Weiner AN, Chang TY, et al. The genomic landscape of juvenile myelomonocytic leukemia. Nat Genet. 2015;47(11):1326–1333. doi: 10.1038/ng.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stieglitz E, Mazor T, Olshen AB, et al. Genome-wide DNA Methylation is Predictive of Outcome in Juvenile Myelomonocytic Leukemia. Nat Commun. 2017;8(1):2127. doi: 10.1038/s41467-017-02178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kline C, Joseph N, Grenert J, et al. Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro Oncol. 2017;19(5):699–709. doi: 10.1093/neuonc/now254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva G, Siegel M, Mose L, et al. SynthEx: a synthetic-normal-based DNA sequencing tool for copy number alteration detection and tumor heterogeneity profiling. Genome Biol. 2017;18(1):66. doi: 10.1186/s13059-017-1193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimi A, Kamachi Y, Imai K, et al. Wiskott-Aldrich syndrome presenting with a clinical picture mimicking juvenile myelomonocytic leukaemia. Pediatr Blood Cancer. 2013;60(5):836–841. doi: 10.1002/pbc.24359. [DOI] [PubMed] [Google Scholar]
- 24.Strauss A, Furlan I, Steinmann S, et al. Unmistakable Morphology? Infantile Malignant Osteopetrosis Resembling Juvenile Myelomonocytic Leukemia in Infants. J Pediatr. 2015;167(2):486–488. doi: 10.1016/j.jpeds.2015.04.064. [DOI] [PubMed] [Google Scholar]
- 25.Olk-Batz C, Poetsch A, Nöllke P, et al. Aberrant DNA methylation characterizes juvenile myelomonocytic leukemia with poor outcome. Blood. 2011;117(18):4871–4880. doi: 10.1182/blood-2010-08-298968. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi H, Muramatsu H, Okuno Y, et al. Aberrant DNA Methylation Is Associated with a Poor Outcome in Juvenile Myelomonocytic Leukemia. PLoS ONE. 2016;10(12):e0145394. doi: 10.1371/journal.pone.0145394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stieglitz E, Troup CB, Gelston LC, et al. Subclonal mutations in SETBP1 confer a poor prognosis in juvenile myelomonocytic leukemia. Blood. 2015;125(3):516–524. doi: 10.1182/blood-2014-09-601690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi H, Okuno Y, Muramatsu H, et al. Exome sequencing identifies secondary mutations of SETBP1 and JAK3 in juvenile myelomonocytic leukemia. Nat Genet. 2013;45(8):937–941. doi: 10.1038/ng.2698. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda K, Nakazawa Y, Iwashita C, et al. Myeloid progenitors with PTPN11 and nonRAS pathway gene mutations are refractory to treatment with 6-mercaptopurine in juvenile myelomonocytic leukemia. Leukemia. 2014;28(7):1545–1548. doi: 10.1038/leu.2014.58. [DOI] [PubMed] [Google Scholar]
- 30.Stieglitz E, Ward AF, Gerbing RB, et al. Phase II/III trial of a pre-transplant farnesyl transferase inhibitor in juvenile myelomonocytic leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62(4):629–636. doi: 10.1002/pbc.25342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cseh A, Niemeyer CM, Yoshimi A, et al. Bridging to transplant with azacitidine in juvenile myelomonocytic leukemia: a retrospective analysis of the EWOG-MDS study group. Blood. 2015;125(14):2311–2313. doi: 10.1182/blood-2015-01-619734. [DOI] [PubMed] [Google Scholar]
- 32.Flotho C, Sommer S, Lubbert M. DNA-hypomethylating agents as epigenetic therapy before and after allogeneic hematopoietic stem cell transplantation in myelodysplastic syndromes and juvenile myelomonocytic leukemia. Semin Cancer Biol. 2017 doi: 10.1016/j.semcancer.2017.10.011. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Pulsipher MA, Carlson C, Langholz B, et al. IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood. 2015;125(22):3501–3508. doi: 10.1182/blood-2014-12-615757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter RB, Gyurkocza B, Storer BE, et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2015;29(1):137–144. doi: 10.1038/leu.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, et al. Transplantation in Remission Improves the Disease-Free Survival of Patients with Advanced Myelodysplastic Syndromes Treated with Myeloablative T Cell-Depleted Stem Cell Transplants from HLA-Identical Siblings. Biol Blood Marrow Transplant. 2008;14(4):458–468. doi: 10.1016/j.bbmt.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warlick ED, Cioc A, DeFor T, Dolan M, Weisdorf D. Allogeneic Stem Cell Transplantation for Adults with Myelodysplastic Syndromes: Importance of Pretransplant Disease Burden. Biol Blood Marrow Transplant. 2009;15(1):30–38. doi: 10.1016/j.bbmt.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Festuccia M, Deeg H, Gooley T, et al. Minimal Identifiable Disease and the Role of Conditioning Intensity in Hematopoietic Cell Transplantation for Myelodysplastic Syndrome and Acute Myelogenous Leukemia Evolving from Myelodysplastic Syndrome. Biol Blood Marrow Transplant. 2016;22(7):1227–1233. doi: 10.1016/j.bbmt.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waespe N, Van Den Akker M, Klaassen R, et al. Response to treatment with azacitidine in children with advanced myelodysplastic syndrome prior to hematopoietic stem cell transplantation. Haematologica. 2016;101(12):1508–1515. doi: 10.3324/haematol.2016.145821. 2016 Dec;101(12):1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Symeonidis A, van Biezen A, de Wreede L, et al. Achievement of complete remission predicts outcome of allogeneic haematopoietic stem cell transplantation in patients with chronic myelomonocytic leukaemia. A study of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Br J Haematol. 2015;171(2):239–246. doi: 10.1111/bjh.13576. [DOI] [PubMed] [Google Scholar]
- 40.Inagaki J, Fukano R, Nishikawa T, et al. Outcomes of immunological interventions for mixed chimerism following allogeneic stem cell transplantation in children with juvenile myelomonocytic leukemia. Pediatr Blood Cancer. 2013;60(1):116–120. doi: 10.1002/pbc.24259. [DOI] [PubMed] [Google Scholar]
- 41.Andersson BS, Valdez BC, de Lima M, et al. Clofarabine ± Fludarabine with Once Daily i.v. Busulfan as Pretransplant Conditioning Therapy for Advanced Myeloid Leukemia and MDS. Biol Blood Marrow Transplant. 2011;17(6):893–900. doi: 10.1016/j.bbmt.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE S1. Flow Diagram of Patient Enrollment, Allocation, and Analysis.
SUPPLEMENTAL FIGURE S2. Acquisition of Bone Marrow Chromosome 7 Derangements at Relapse in Two Patients as Detected by Copy Number Analysis on Whole Exome Sequencing


