Abstract
Background
Previous studies have reported that hyperthyroid and hypothyroid women experience menstrual irregularities more often compared with euthyroid women, but reasons for this are not well understood and studies on thyroid hormones among euthyroid women are lacking. In a prospective cohort study of euthyroid women, this study characterized the relationship between thyroid hormone concentrations and prospectively-collected menstrual function outcomes.
Methods
Between 2004–2014, 86 euthyroid premenopausal women not lactating or taking hormonal medications participated in a study measuring menstrual function. Serum thyroid hormones were measured before the menstrual function study began. Women then collected first morning urine voids and completed daily bleeding diaries every day for three cycles. Urinary estrogen and progesterone metabolites (estrone 3-glucuronide (E13G) and pregnanediol 3-glucuronide (Pd3G)) and follicle-stimulating hormone were measured and adjusted for creatinine (Cr).
Results
Total thyroxine (T4) concentrations were positively associated with Pd3G and E13G. Women with higher (vs. lower) T4 had greater luteal phase maximum Pd3G (Pd3G=11.7 μg/mg Cr for women with high T4 vs. Pd3G=9.5 and 8.1 μg/mg Cr for women with medium and low T4, respectively) and greater follicular phase maximum E13G (E13G=41.7 ng/mg Cr for women with high T4 vs. E13G=34.3 and 33.7 ng/mg Cr for women with medium and low T4, respectively).
Conclusions
Circulating thyroid hormone concentrations were associated with subtle differences in menstrual cycle function outcomes, particularly sex steroid hormone levels in healthy women. Results contribute to the understanding of the relationship between thyroid function and the menstrual cycle, and may have implications for fertility and chronic disease.
Keywords: menstrual cycle function, thyroid hormones, estrogen, progesterone, euthyroid, women’s health
Introduction
Menstrual cycle function is determined by a complex endocrine axis that controls the ovaries and endometrium and represents the underlying hormonal milieu of the female reproductive system. Menstrual cycle function characteristics such as cycle length have been found to be associated with reproductive health and fertility.1, 2 Therefore, menstrual cycle characteristics provide non-invasive markers of endocrine and reproductive health, making them useful for epidemiologic research.
The menstrual cycle is governed by a network of gonadotropins (e.g., luteinizing hormone (LH) and follicle-stimulating hormone (FSH)) and sex steroid hormones (e.g., estrogens and progesterone); key constituents of the hypothalamic-pituitary-gonadal axis. This system is closely related to the hypothalamic-pituitary-thyroid axis, which controls thyroid function.3 However, the relationship between thyroid function and female reproductive physiology is complex. Women are more likely to develop thyroid disease than men, and incidence is greatest during times of hormonal flux, such as menopause, puberty, and pregnancy, which may indicate a role of estrogens.4
Both hypothyroid and hyperthyroid women have been reported to have a greater prevalence of menstrual disturbances compared with euthyroid women.5–7 Specifically, hypothyroid women are more likely to experience oligomenorrhea and menorrhagia;6, 8, 9 and hyperthyroid women are more likely to experience hypomenorrhea compared with euthyroid women.5, 8 However, much of the research on this topic to date has been limited to clinic-based studies that compare women with severe forms of thyroid disease (e.g., thyrotoxicosis and myxedema) with euthyroid women, and few have measured thyroid hormones.6 In addition, many of these studies have relied upon self-reported data of menstrual cycle outcomes assessed at one time-point, and have often consolidated different outcomes together as menstrual “disturbances” or “irregularities”.8, 10 Therefore, the intricacies of the relationship between thyroid function and menstrual physiology remain unclear.
The purpose of this study was to characterize the relationship between serum thyroid hormones and specific menstrual cycle function outcomes using prospectively collected menstrual function data and urinary hormone levels across several cycles in a sample of euthyroid, premenopausal women. The associations between serum thyroid hormone concentrations and several indicators of menstrual cycle function were evaluated across multiple cycles: cycle-specific characteristics, several multi-day gonadotropin and steroid hormone measures, as well as day-specific gonadotropin and steroid hormone levels throughout the cycle.
Methods
Study population
The Michigan Polybrominated Biphenyl (PBB) Registry is a study of the long-term health outcomes of a population that was exposed to persistent organic pollutants in the early 1970s through a contamination of the food supply.11 A subset of women in this cohort were recruited to participate in a longitudinal study on menstrual cycle function.
Participants for the menstrual cycle function study were recruited between 2004–2006 and 2013–2014. To be eligible, women had to be premenopausal, not pregnant or lactating, not currently taking hormonal medications, and never diagnosed with or treated for cancer. Participation entailed a blood draw; completion of a health questionnaire with details on medical history (including report of physician-diagnosed thyroid disease), current medication use, behaviors, and demographics; and menstrual cycle function monitoring which included daily urine collections and daily diaries (see Menstrual cycle function protocol). This study was approved by the Institutional Review Boards at Emory University and the State Health Department of Michigan.
Thyroid hormones
Thyroid hormone levels were measured in serum of study participants without a history of thyroid disease and who were not currently taking thyroid-disrupting medication. Thyroid-stimulating hormone (TSH), total and free thyroxine (T4), and total and free triiodothyronine (T3) were analyzed at the Emory Clinical Translational Research Laboratory with the Beckman Coulter Access II chemiluminescent immunoassay analyzer (Beckman Coulter, Brea, CA). Analyses were conducted as directed by the manufacturer. Daily quality control samples were run before and after all study samples, and blinded controls were run throughout. If QC samples were more than two standard deviations from the expected values, the assay was repeated. Coefficients of variation for thyroid hormone assays are shown in Table S1.
Menstrual cycle function protocol
Women were asked to keep daily menstrual diaries and collect first morning urine void samples every day for at least three menstrual cycles (defined as four menstrual periods or 18 weeks for oligomenorrheaic or amenorrheic women). The diaries included daily information on bleeding, menstrual cramping, stress, smoking and alcohol consumption, exercise habits, and medication use (Figure S1). In urine samples from women recruited in 2004–2006, the estrogen and progesterone metabolites, estrone 3-glucuronide (E13G) and pregnanediol 3-glucuronide (Pd3G), respectively, were measured in a 17-day window around expected ovulation (preceding the last 4 days of the cycle). In women with sufficient data during the luteal-follicular transition (n=29 of 32 women recruited between 2004–2006), E13G, Pd3G, and FSH were also measured in this 10-day window, which included menses onset (5 days before menses onset through cycle day 4 of the next cycle). Day of ovulation was based on identifying a day of luteal transition (DLT), which was determined by an algorithm examining changes in the ratio of E13G to Pd3G.12 In urine samples from women recruited in 2013–2014, E13G and Pd3G were measured every day and FSH was measured in the same 10-day window during the luteal-follicular transition, which included menses onset. Ovulatory cycles were again defined the presence of a DLT and were validated against urinary LH measurements conducted in a 10-day mid-cycle window due to the common clinical use of LH as a marker of ovulation.13
Urinary E13G and Pd3G were measured in triplicate using competitive double-antibody time-resolved fluoroimmunoassays.14 Urinary LH and FSH were assayed in duplicate using immunofluorometric assays (PerkinElmer, Waltham, MA, USA; Cat. Nos. A031–101 and A017–201, respectively) modified and validated for analyzing urine samples.15, 16 Urinary creatinine (Cr) was measured using a Vitros 250 Chemistry Analyzer (Ortho-Clinical Diagnostics, Raritan, NJ, USA).17, 18 Urinary endocrine concentrations were divided by Cr concentrations to normalize for sample dilution.
Menstrual cycle function outcomes
Menstrual cycle function outcomes included cycle-level characteristics: cycle length, bleed length and intensity, and follicular and luteal phase lengths; multi-day hormonal outcomes calculated as 3-day geometric means (GM) of FSH, Pd3G, and E13G at various times throughout the cycle (Table S2); and day-specific E13G, Pd3G, and FSH levels throughout the cycle. Anovulation was also of interest but we did not have the power to examine this potential association. Four cycles from 3 different women showed evidence of anovulation, which constituted 1.6% of cycles that had sufficient mid-cycle samples to detect potential ovulation.
Menses onset was defined as the first of 2 consecutive days of bleeding, only one of which could be spotting, and which was preceded by 3 consecutive days of non-bleeding or spotting.19 Cycle length was defined as the first day of menses through the day before menses onset in the next cycle. In order to characterize within-woman variability in menstrual cycle length, we calculated the standard deviation of cycle length for each woman who contributed ≥2 cycles. Bleed intensity was the arithmetic mean of self-reported bleeding (0=no bleeding, 1=spotting, 2=light, 3=moderate, 4=heavy) over the course of menses. The follicular phase was defined as the first day of menses through the day of ovulation. The luteal phase was the day after ovulation through the day before menses onset.20
The hormonal outcomes were adapted from definitions proposed by Baird et al. that were shown to be related to conception.21 Maximum 3-day GMs were calculated for the follicular phase and the luteal phase only when no samples were missing during each relevant timeframe; thus sample sizes for each analysis varied by outcome (mostly due to women who missed single days of urine collection).
Statistical analysis
Among women who did not report a history of physician-diagnosed thyroid disease and/or current thyroid medication use, we explored the distribution of each thyroid hormone in univariate analyses and by each covariate stratum. TSH was log-transformed to approximate a normal distribution.
For models where there was one outcome per cycle (cycle-level characteristics and 3-day GMs), we fit linear mixed models with a random effect for woman in order to account for the intra-individual correlations among multiple menstrual cycles per woman. We evaluated associations between each individual thyroid hormone and each menstrual cycle outcome, adjusting for age as a fixed effect. Thyroid hormones were fit as fixed effects and were expressed continuously in all our final models, with both a linear and quadratic term to allow for flexibility with each outcome. Estimates were output for the 12.5th (low), 50th (medium) and 87.5th (high) percentile of each hormone. We also adjusted for body mass index (BMI) and smoking, but because results did not change, we present results only controlling for age. For cycle-level characteristics, we generated predicted means of each characteristic for a woman of mean age in our study (38 years). For the 3-day GM outcomes, we present comparisons (via β-coefficients) between the low (12.5th percentile) and medium (50th percentile) and high (87.5th percentile) and medium (50th percentile) thyroid hormone levels. Medium was treated as the reference because as it has been reported that both hypothyroid and hyperthyroid women experience menstrual cycle disruption compared to euthyroid women,7 we aimed to assess impacts at the lower end as well as the higher end of each thyroid hormone distribution and compare them with median levels
We also examined the associations between each thyroid hormone and day-specific E13G, Pd3G, and FSH by fitting linear mixed models with random effects for woman and cycle to account for the nesting of days within cycles and cycles within women. E13G, Pd3G, and FSH levels were adjusted for creatinine and natural log-transformed. Models for E13G and Pd3G were aligned by the estimated day of ovulation ± 10 days and for E13G, Pd3G, and FSH by menses onset (5 days before menses onset through cycle day 4 of the next cycle). We adjusted for age and present the predicted daily log-transformed E13G, Pd3G, and FSH levels by each thyroid hormone category (low (minimum-25th percentile), medium (25th percentile–75th percentile), and high (75th percentile-maximum) over each relevant timeframe for a 38-year old (mean-aged) woman.
Results
A total of 93 women had their blood drawn, completed a questionnaire, and collected menstrual cycle function data consisting of both urine samples and corresponding daily diaries. Seven women (7.5%) reported a previous thyroid disease diagnosis (n=5 hypothyroid, n=1 Hashimoto’s thyroiditis, and n=1 type not specified), were currently taking thyroid medication, and were thus excluded from further analyses. The remaining women (n=86) collected data for a total of 423 cycles and 65.0% of these were complete cycles (n=275), meaning that diary data indicated a start and end to the cycle and daily menstrual function monitoring (urine samples and diary entries) occurred during the vast majority of days during the cycle. Among these 275 cycles, 64.4% had no missing urine samples; 24.0% had between 1 and 3 missing samples, and the remainder (11.6%) were missing four or more. Individual women contributed between 1 and 9 complete cycles, with a median of 3 complete cycles (interquartile range=3–4). Women were between 18 and 53 years old (mean=38 years), and a substantial proportion (40.7%) between 36–40 years (Table 1). Most women had completed at least some college (84.7%), had previously been pregnant (77.9%), and were never smokers (59.3%).
Table 1.
Characteristics of 86 premenopausal euthyroid women in the Michigan Polybrominated Biphenyl Registry menstrual cycle function study by thyroid hormone levelsa (2004–2014)
| N (%) | TSH (μIU/ml) | Total T4 (μg/dl) | |
|---|---|---|---|
|
|
|||
| Age (years)b | |||
| 18–25 | 8 (9.3) | 1.4 | 8.0 |
| 26–30 | 7 (8.1) | 1.3 | 9.7 |
| 31–35 | 11 (12.8) | 1.6 | 9.3 |
| 36–40 | 35 (40.7) | 1.6 | 9.2 |
| 41–45 | 9 (10.5) | 1.5 | 8.1 |
| 46–54 | 16 (18.6) | 1.1 | 9.2 |
| Education | |||
| High school | 13 (15.3) | 1.4 | 8.9 |
| Some college or technical school | 40 (47.1) | 1.4 | 9.0 |
| College graduate or more | 32 (37.7) | 1.5 | 9.1 |
| Missing | 1 | ||
| Income | |||
| <$50,000/year | 41 (48.2) | 1.5 | 9.0 |
| ≥$50,000/year | 44 (51.8) | 1.4 | 9.0 |
| Missing | 1 | ||
| Parity | |||
| 0 prior pregnancies | 19 (22.1) | 1.5 | 8.6 |
| 1–2 prior pregnancies | 28 (32.6) | 1.5 | 9.1 |
| 3 or more prior pregnancies | 39 (45.4) | 1.4 | 9.2 |
| Body mass index categoryc | |||
| Normal | 34 (39.5) | 1.6 | 8.8 |
| Overweight | 23 (26.7) | 1.4 | 9.0 |
| Obese | 29 (33.7) | 1.3 | 9.3 |
| Smoking statusd | |||
| Never smoker | 51 (59.3) | 1.5 | 9.1 |
| Former smoker | 12 (14.0) | 1.2 | 9.2 |
| Current smoker | 23 (26.7) | 1.4 | 8.8 |
| Study phase | |||
| 2004–2006 | 30 (34.9) | 1.8 | 9.5 |
| 2013–2014 | 56 (65.1) | 1.3 | 8.8 |
| Average behavior over study period | |||
| Weekly Exercise | |||
| 0 times | 28 (32.6) | 1.3 | 9.3 |
| 1–3 times | 29 (33.7) | 1.6 | 8.8 |
| >3 times | 29 (33.7) | 1.5 | 8.9 |
| Stresse | |||
| Low | 27 (31.4) | 1.5 | 8.9 |
| Moderate | 32 (37.2) | 1.4 | 8.6 |
| High | 27 (31.4) | 1.4 | 9.6 |
| Daily Smoking | |||
| None | 63 (73.3) | 1.5 | 9.1 |
| 1–9 cigarettes/day | 12 (14.0) | 1.4 | 9.3 |
| ≥10 cigarettes/day | 11 (12.8) | 1.4 | 8.4 |
| Weekly alcohol consumption | |||
| 0 servings | 25 (29.1) | 1.2 | 9.0 |
| 1–3 servings | 42 (48.8) | 1.6 | 9.2 |
| ≥4 servings | 19 (22.1) | 1.5 | 8.7 |
Geometric means calculated for TSH, arithmetic means calculated for total T4
Age when participant started the menstrual cycle function study
Body mass index (kg/m2) =weight (lb)/[height (in)]2 × 703 and defined as: normal: 18.5–24.9; overweight: 25.0–29.9; obese: ≥30.0
Based on the questionnaire responses and daily diaries during the study
Stress determination is based on a combination of duration and intensity
Table 2 shows the distribution of thyroid hormones among the 86 women never diagnosed with thyroid disease. Hormone levels for one woman (TSH=0.06 μIU/ml, total T4= 12.1 μg/dl, total T3= 148 ng/dl, free T4= 1.24 ng/dl, free T3= 3.73 pg/ml) met the clinical definition of hyperthyroidism. All others had hormones within the euthyroid range.
Table 2.
Distribution of thyroid hormones among premenopausal women in the menstrual cycle function study (2004–2014) without self-reported thyroid disease (N=86)
| Thyroid hormones | Minimum | 25th percentile | Median | 75th percentile | Maximum | Mean/GMa |
|---|---|---|---|---|---|---|
| Thyroid-stimulating hormone (μIU/mL) | 0.06 | 1.1 | 1.4 | 2.0 | 7.6 | 1.4 |
| Total T4 (μg/dL) | 5.9 | 8.2 | 8.9 | 9.9 | 13.1 | 9.0 |
| Total T3 (ng/dL) | 62 | 92 | 107 | 123 | 148 | 108 |
| Free T4 (ng/dL) | 0.50 | 0.70 | 0.77 | 0.84 | 1.2 | 0.8 |
| Free T3 (pg/mL) | 2.2 | 3.0 | 3.2 | 3.6 | 5.0 | 3.3 |
Abbreviations: TSH, thyroid-stimulating hormone; T4, thyroxine; T3, triiodothyronine; GM, geometric mean
Geometric means are reported for TSH; arithmetic means are reported for all other hormones
Women aged 18–29 had longer cycles and follicular phases and greater cycle length variability than women in their thirties (Table S3). Bleed length and intensity decreased with age. Former and current smokers had shorter cycles compared with never smokers. Obese women had longer cycles than overweight and normal weight women. Overweight and obese women had shorter bleeds than normal weight women. Women aged 41–54 had higher FSH in the late luteal phase and early follicular phase, and lower Pd3G and E13G at various timeframes across the cycle compared with younger women (Table S4). Current smokers showed similar patterns compared with never and former smokers even after controlling for age. No consistent patterns between participant characteristics and thyroid hormones were observed (Table S5).
Thyroid hormone levels were not associated with cycle or phase lengths and effect estimates were small (Table 3). However, there was a suggestion that lower free T4 was associated with decreased cycle length compared with higher free T4. Although the predicted mean cycle lengths increased with increasing free T4 (low: 28.2 days (95% confidence interval [CI] 23.9, 32.5; medium: 31.1 days (95% CI 27.6, 34.5); high: 32.1 days (95% CI 27.6, 36.5)), the differences were small and the confidence intervals overlapped substantially. These subtle differences in cycle length were due to variation in follicular phase length, which showed a similar pattern with free T4, while luteal phase lengths remained consistent across groups. Bleed length and intensity were not associated with thyroid hormone levels. Although adjusting for smoking status and BMI did not influence the effect estimates for thyroid hormones (data not shown), the predicted cycle-level characteristics were different between smoking and BMI strata, which suggests that these factors were independently associated with these menstrual cycle characteristics. In addition, results did not change when we excluded the one woman who met the clinical definition of hyperthyroidism based on her thyroid hormone assay results (data not shown).
Table 3.
Predicted mean values and 95% confidence intervals (95% CI) of menstrual cycle function outcomes from regression modelsa for associations with serum thyroid hormone levelsb among Michigan women without thyroid disease, 2004–2014
| Cycle length | Bleed length | Bleed intensityc | Follicular phase length | Luteal phase length | Cycle length standard deviation (per woman) | |
|---|---|---|---|---|---|---|
|
| ||||||
| n=78 women n=268 cycles |
n=81 women n=343 cycles |
n=79 women n=329 cycles |
n=74 women n=211 cycles |
n=74 women n=207 cycles |
n=70 women | |
|
| ||||||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| TSHd,e | ||||||
| Low | 30.4 (26.0, 34.8) | 5.7 (5.2, 6.2) | 2.3 (2.2, 2.4) | 17.5 (12.7, 22.3) | 12.9 (12.1, 13.8) | 4.9 (2.9, 7.0) |
| Medium | 30.5 (27.1, 34.0) | 5.6 (5.3, 6.0) | 2.2 (2.2, 2.3) | 17.5 (13.8, 21.3) | 13.0 (12.4, 13.6) | 4.8 (3.1, 6.4) |
| High | 29.7 (25.3, 34.0) | 5.6 (5.1, 6.1) | 2.1 (2.0, 2.3) | 16.7 (12.0, 21.4) | 12.9 (12.1, 13.7) | 3.9 (1.9, 6.0) |
| Total T4f | ||||||
| Low | 30.0 (25.2, 34.7) | 5.6 (5.1, 6.1) | 2.2 (2.1, 2.4) | 16.3 (10.7, 21.9) | 12.7 (11.7, 13.7) | 4.5 (2.3, 6.7) |
| Medium | 30.9 (27.2, 34.5) | 5.7 (5.3, 6.1) | 2.3 (2.2, 2.4) | 18.1 (14.1, 22.2) | 13.0 (12.3, 13.6) | 4.8 (3.0, 6.5) |
| High | 30.0 (25.7, 34.4) | 5.6 (5.1, 6.1) | 2.2 (2.1, 2.3) | 17.4 (12.6, 22.2) | 13.1 (12.3, 14.0) | 4.4 (2.2, 6.5) |
| Total T3g | ||||||
| Low | 30.5 (25.4, 35.6) | 5.7 (5.2, 6.2) | 2.3 (2.1, 2.4) | 17.0 (11.3, 22.8) | 13.4 (12.4, 14.3) | 4.7 (2.3, 7.2) |
| Medium | 31.0 (26.7, 35.3) | 5.6 (5.1, 6.1) | 2.2 (2.1, 2.3) | 18.1 (13.4, 22.8) | 12.9 (12.1, 13.7) | 4.1 (2.0, 6.1) |
| High | 29.1 (24.1, 34.1) | 5.6 (5.0, 6.2) | 2.2 (2.1, 2.4) | 16.4 (11.0, 21.8) | 12.6 (11.6, 13.6) | 4.7 (2.3, 7.1) |
| Free T4h | ||||||
| Low | 28.2 (23.9, 32.5) | 5.5 (5.0, 6.1) | 2.3 (2.2, 2.4) | 15.4 (10.7, 20.2) | 12.9 (12.0, 13.7) | 4.0 (1.9, 6.1) |
| Medium | 31.1 (27.6, 34.5) | 5.7 (5.3, 6.0) | 2.2 (2.1, 2.3) | 17.9 (14.1, 21.7) | 13.0 (12.4, 13.6) | 4.8 (3.2, 6.5) |
| High | 32.1 (27.6, 36.5) | 5.7 (5.2, 6.2) | 2.2 (2.1, 2.3) | 19.0 (14.0, 23.9) | 13.0 (12.2, 13.8) | 4.9 (2.8, 7.1) |
| Free T3i | ||||||
| Low | 29.6 (24.9, 34.2) | 5.4 (4.9, 5.9) | 2.2 (2.1, 2.3) | 16.6 (11.5, 21.7) | 13.2 (12.3, 14.0) | 3.5 (1.2, 5.7) |
| Medium | 30.4 (26.8, 34.1) | 5.6 (5.3, 6.0) | 2.2 (2.2, 2.3) | 17.2 (13.2, 21.2) | 13.1 (12.5, 13.8) | 4.6 (2.9, 6.4) |
| High | 30.7 (26.3, 35.1) | 5.9 (5.3, 6.4) | 2.3 (2.1, 2.4) | 17.7 (12.9, 22.5) | 12.6 (11.8, 13.5) | 5.5 (3.3, 7.7) |
Abbreviations: TSH, thyroid-stimulating hormone; T4, thyroxine; T3, triiodothyronine
All models control for age; in cycle length and follicular phase models, age is fit using quadratic term
Thyroid hormones modeled continuously and estimates output at the low (12.5th percentile), medium (50th percentile), and high (87.5th percentile) levels
Average bleeding score where women rated their daily menstrual bleeding on the following scale: 0=no bleeding, 1=spotting, 2=light, 3=moderate, 4=heavy
TSH modeled as natural log-transformed in models
Low=0.80, Medium=1.4, High=3.0 μIU/ml
Low=7.5, Medium=8.9, High=10.5 μg/dl
Low=85, Medium=107, High=134 ng/dl
Low=0.65, Medium=0.77, High=0.86 ng/dl
Low=2.9, Medium=3.2, High=3.8 pg/ml
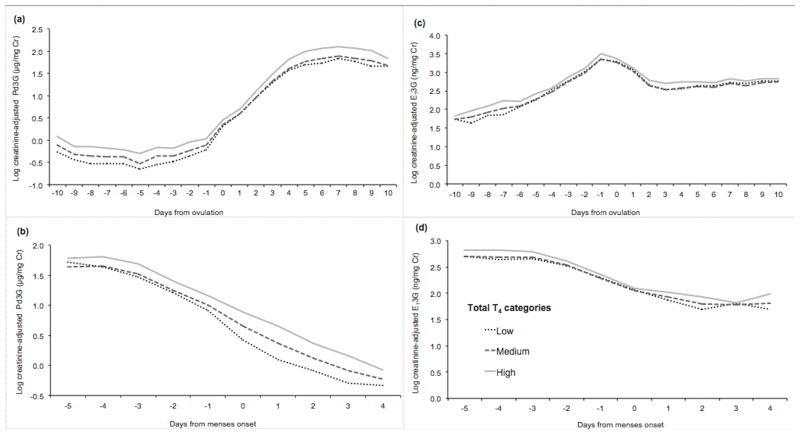
Table 4 shows the associations between thyroid hormone concentrations and the multi-day hormonal outcomes for FSH, Pd3G, and E13G. Total T4 was consistently associated with greater Pd3G and E13G across various 3-day intervals (follicular phase maximum E13G and luteal phase maximum Pd3G, preovulatory E13G, and mid-luteal phase Pd3G and E13G). Estimated differences in E13G and Pd3G were largest between those with high and medium total T4 levels. This pattern was also apparent across the cycle when considered in day-level analyses (Figure 1). Women with high levels of total T4 had greater predicted concentrations of Pd3G throughout the follicular and luteal phases compared with those with medium and low T4, and this difference was smallest around the time of ovulation (Figure 1, panel A). Furthermore, lower total T4 was associated with lower Pd3G at various points during the cycle, as evidenced by lower 3-day GMs though not statistically significant, as well as lower daily levels throughout the cycle, especially in the follicular phase (Figure 1, panels A and B). Total T4 was also positively associated with E13G throughout the cycle (Figure 1, panels C and D). Women with higher total T4 concentrations had greater E13G levels compared with those with lower total T4 concentrations, although differences were smaller than for Pd3G. Free T4 was not associated with these endocrine levels.
Table 4.
β-Coefficients and predicted mean hormone levelsa and 95% confidence intervals (95% CI) from regression modelsb for associations between serum thyroid hormone levelsc and urinary reproductive hormone concentrations among Michigan women without thyroid disease, 2004–2014
| Early follicular FSH (mIU/mg Cr) | Late luteal FSH (mIU/mg Cr) | Luteal phase maximum Pd3G (μg/mg Cr) | Mid-luteal Pd3G (μg/mg Cr) | Follicular phase maximum E13G (ng/mg Cr) | Luteal phase maximum E13G (ng/mg Cr) | Early follicular E13G (ng/mg Cr) | Preovulatory E13G (ng/mg Cr) | Mid-luteal E13G (ng/mg Cr) | |
|---|---|---|---|---|---|---|---|---|---|
| n=73 women | n=72 women | n=62 women | n=75 women | n=48 women | n=64 women | n=73 women | n=74 women | n=75 women | |
| n=186 cycles | n=186 cycles | n=137 cycles | n=207 cycles | n=90 cycles | n=136 cycles | n=220 cycles | n=202 cycles | n=207 cycles | |
| TSHd,e | |||||||||
| Low (vs. Medium) | +0.2 (−1.3, 1.7) | +0.0 (−1.4, 1.3) | − −0.2 (−1.3, 0.8) | −0.2 (−1.1, 0.7) | +1.0 (−5.0, 7.0) | −0.9 (−4.1, 2.4) | +0.2 (−0.8, 1.2) | +0.5 (−2.3, 3.3) | −0.4 (−2.4, 1.6) |
| Medium | 8.4 (6.4, 10.3) | 4.9 (3.1, 6.6) | 9.6 (8.2, 11.0) | 8.2 (7.1, 9.4) | 38.0 (32.7, 43.3) | 30.1 (25.7, 34.4) | 9.3 (8.0, 10.6) | 28.8 (25.2, 32.4) | 19.6 (17.0, 22.3) |
| High (vs. Medium) | −0.7 (−3.1, 1.7) | −0.4 (−2.6, 1.7) | +0.7 (−1.0, 2.4) | +0.6 (−0.8, 2.0) | −3.5 (−9.0, 2.0) | −0.3 (−5.5, 5.0) | −0.8 (−2.4, 0.8) | −2.2 (−6.5, 2.2) | −0.2 (−3.4, 2.9) |
| Total T4f | |||||||||
| Low (vs. Medium) | −0.8 (−3.5, 1.8) | −0.9 (−3.3, 1.4) | −1.4 (−3.2, 0.4) | −0.9 (−2.3, 0.6) | −0.6 (−6.8, 5.5) | −0.9 (−7.1, 5.2) | −0.5 (−2.2, 1.3) | −0.9 (−5.5, 3.6) | −0.3 (−3.7, 3.1) |
| Medium | 8.1 (6.0, 10.2) | 4.8 (2.9, 6.6) | 9.5 (8.1, 10.9) | 7.9 (6.7, 9.0) | 34.3 (29.4, 39.2) | 28.5 (23.9, 33.2) | 9.2 (7.8, 10.6) | 25.9 (22.2, 29.5) | 17.8 (15.1, 20.5) |
| High (vs. Medium) | +1.0 (−1.4, 3.3) | +0.7 (−1.3, 2.7) | +2.2 (0.7, 3.8) | +2.2 (1.0, 3.4) | +7.4 (2.3, 12.4) | +3.2 (−2.0, 8.4) | −0.1 (−1.7, 1.5) | +5.8 (1.9, 9.8) | +3.8 (0.9, 6.6) |
| Total T3g | |||||||||
| Low (vs. Medium) | +1.9 (−1.1, 4.8) | +1.6 (−1.1, 4.2) | −0.8 (−3.1, 1.5) | −1.0 (−2.8, 0.7) | −2.9 (−10.5, 4.7) | −4.0 (−11.1, 3.1) | −1.7 (−3.6, 0.3) | −4.3 (−9.6, 0.9) | −1.9 (−5.7, 1.9) |
| Medium | 7.5 (5.0, 10.0) | 4.3 (2.1, 6.5) | 9.8 (8.1, 11.5) | 8.4 (6.9, 9.8) | 35.5 (29.6, 41.5) | 28.7 (23.4, 34.0) | 9.4 (7.7, 11.0) | 26.8 (22.5, 31.2) | 17.8 (14.6, 20.9) |
| High (vs. Medium) | −0.1 (−3.8, 3.6) | −0.6 (−3.9, 2.8) | +1.0 (−1.6, 3.5) | +1.2 (−1.0, 3.4) | +9.2 (−1.5, 19.8) | +6.6 (−1.3, 14.5) | +0.7 (−1.7, 3.2) | +8.3 (1.7, 14.9) | +6.8 (2.1, 11.5) |
| Free T4h | |||||||||
| Low (vs. Medium) | −1.2 (−3.6, 1.3) | −0.7 (−2.8, 1.4) | −0.2 (−1.8, 1.5) | −0.6 (−1.9, 0.6) | +1.2 (−6.9, 9.3) | +0.2 (−5.0, 5.3) | −0.4 (−2.0, 1.2) | −1.0 (−5.4, 3.4) | −0.5 (−3.4, 2.4) |
| Medium | 8.3 (6.3, 10.3) | 4.9 (3.2, 6.7) | 9.8 (8.5, 11.2) | 8.5 (7.3, 9.7) | 35.8 (30.5, 41.2) | 30.1 (25.8, 34.5) | 9.4 (8.1, 10.7) | 28.6 (24.9, 32.2) | 19.6 (17.0, 22.3) |
| High (vs. Medium) | +0.6 (−1.1, 2.4) | +0.2 (−1.3, 1.7) | +0.1 (−1.1, 1.3) | +0.4 (−0.6, 1.3) | +1.4 (−4.0, 6.8) | −1.1 (−4.8, 2.6) | −0.3 (−1.4, 0.9) | −0.2 (−3.3, 3.0) | −0.1 (−2.3, 2.1) |
| Free T3i | |||||||||
| Low (vs.Medium) | −0.9 (−3.1, 1.2) | −0.7 (−2.7, 1.3) | −1.2 (−2.7, 0.3) | −1.4 (−2.7, −0.2) | −2.7 (−7.9, 2.4) | −3.1 (−7.9, 1.6) | −2.0 (−3.4, −0.6) | −5.4 (−9.3, −1.6) | −3.1 (−5.9, −0.3) |
| Medium | 8.7 (6.6, 10.8) | 5.1 (3.3, 7.0) | 10.3 (8.9, 11.7) | 8.8 (7.7, 10.0) | 37.2 (32.0, 42.5) | 29.0 (24.6, 33.4) | 9.4 (8.0, 10.7) | 28.5 (25.0, 32.1) | 19.4 (16.8, 22.0) |
| High (vs. Medium) | −0.3 (−2.7, 2.2) | −0.3 (−2.5, 1.8) | +0.3 (−1.4, 1.9) | +0.7 (−0.5, 2.0) | +2.6 (−3.3, 8.4) | +5.2 (0.0, 10.4) | +1.5 (−0.1, 3.1) | +5.2 (1.4, 8.9) | +3.3 (0.6, 6.0) |
Predicted mean hormone levels presented for medium level and β-coefficients presented for low and high levels representing mean estimated differences between low and medium levels and high and medium levels, respectively
All models control for age
Thyroid hormones modeled continuously and estimates output at the low (12.5th percentile), medium (50th percentile), and high (87.5th percentile) levels
TSH modeled as natural log-transformed
Low=0.80, Medium=1.4, High=3.0 μIU/ml
Low=7.5, Medium=8.9, High=10.5 μg/dl
Low=85, Medium=107, High=134 ng/dl
Low=0.65, Medium=0.77, High=0.86 ng/dl
Low=2.9, Medium=3.2, High=3.8 pg/ml
Figure 1.
Daily predicted log-transformed creatinine-adjusted Pd3G (panels A and B) and E13G (panels C and D) levels across the menstrual cycle by total T4 concentrations.
Total and free T3 were also associated with both Pd3G and E13G levels. In particular, higher total and free T3 were associated with greater concentrations of E13G at various timeframes throughout the cycle. For example, preovulatory levels of E13G were greater for women with high total T3 (E13G=35.1 ng/mg Cr, 95% CI 33.4, 50.0) compared with those with medium total T3 (E13G=26.8 ng/mg Cr, 95% CI 22.5, 31.2). Similarly, those with high free T3 had greater E13G (33.7 ng/mg Cr, 95% CI 32.3, 42.6) than those with medium free T3 (28.5 ng/mg Cr, 95% CI: 25.0, 32.1). However, associations of thyroid hormones with FSH were less apparent.
Comment
Principal findings
In a cohort of premenopausal euthyroid women, thyroid hormone levels were evaluated in relation to prospectively collected menstrual cycle function outcomes. To our knowledge, this is the first study to assess these relationships. Greater total T4 was associated with higher Pd3G and E13G levels throughout the cycle, and lower total T4 was associated with lower Pd3G especially during the follicular phase. Total and free T3 were also positively correlated with Pd3G and E13G levels at several times during the cycle. There was also a suggestion that free T4 was associated with shorter cycle and follicular phase length. These observations were consistent when controlling for multiple potential confounders, excluding an outlier with biochemical evidence of undiagnosed hyperthyroidism, and considering hormonal outcomes as 3-day means as well as day-specific measures.
Interpretation
Studying the relationship between thyroid hormones and menstrual cycle function is complex because while it has been repeatedly noted in clinical studies that women with thyroid diseases experience disruption of their menstrual cycles, it is also well understood that steroid hormones can affect thyroid hormones. For example, during pregnancy, elevated estrogen levels lead to increased total T4 due to thyroxine-binding globulin (TBG).22 In this study, total T4 was positively correlated with E13G and Pd3G throughout the cycle. Although this study was prospective in design and serum samples for measuring thyroid hormones were collected before the menstrual cycle function monitoring began, it may be possible that pre-existing steroid hormone levels led to a state of greater total T4 concentrations, perhaps due to greater levels of TBG. Therefore, even given this longitudinal study design, it is difficult to parse out the directionality of these relationships.
One study that conducted a post-hoc analysis of a randomized controlled trial found that women on progesterone therapy had increased free T4 after therapy compared with those who received placebo.23 This observed correlation between progesterone and thyroxine could be due to a shared pathway within metabolic processes for increasing basal body temperature and energy expenditure. These hormones are also both transported by albumin, although albumin carries only about 10% of circulating thyroxine, with its main transporter being TBG.24
The positive association between total T4 and E13G is consistent with previous observations of higher plasma estrogen levels throughout the menstrual cycle among hyperthyroid women compared with euthyroid controls.25, 26 It has been hypothesized that this may be due to T4-induced increases in sex-hormone binding globulin, which leads to increases in serum levels of estrogen and decreased clearance rates.5, 26 However, another potential mechanism may be the increased androgen production and subsequent increased conversion of androgens to estrogens that occurs among hyperthyroid women.27 In this study, a similar association was noted despite the significant differences in study design and population: women had thyroid hormone levels within the euthyroid range and urinary estrogen metabolites were measured as opposed to plasma or serum estradiol concentrations.
If the relationships between thyroid hormones and menstrual cycle function are causal, these findings may be relevant to exogenous factors that influence thyroid function. For example, to the extent that environmental toxicants and personal behaviors such as smoking may affect thyroid function,28, 29 menstrual cycle outcomes and female reproduction may also in turn be affected through this pathway. However, in this study, personal exposures such as smoking were not strongly related to thyroid hormone concentrations. Alternatively, it is possible that if an exogenous factor affected both thyroid function and menstrual cycle function independently, this could induce an association between thyroid hormones and menstrual cycle function or make the associations vary across strata of this other factor. Women in this study were exposed to a persistent organic pollutant (PBB) and most (89%) had detectable levels of PBBs in their blood. However, when controlling for PBB in models or restricting analyses to women with PBB levels below the median, results did not change (data not shown). In conjunction with the results being consistent with those of other studies conducted among unexposed women, this strengthens the hypothesis that there is a direct and causal relationship between thyroid hormones and menstrual cycle function.
Strengths of the study
This study benefited from prospective collection of menstrual cycle function data over multiple cycles per woman. This type of monitoring has several advantages over self-report of “usual” menstrual characteristics in that it improves accuracy and precision,19, 30 thereby reducing the likelihood of misclassification, and it incorporates a degree of within-woman variability due to observations over multiple cycles. In addition, the assessment of menstrual cycle function was comprehensive; several reproductive endocrine analytes were measured in addition to cycle characteristics. Finally, age, smoking, and BMI were related to cycle characteristics as well as several reproductive hormone levels, as reported in the literature.31–34 These observations lend additional support to the validity of the menstrual cycle function data.
Limitations of the data
This study had some limitations as well. First, it was limited by small sample size even though there were multiple cycles per woman. The detailed data on menstrual cycle function is reflected in a demanding study protocol, which was a contributing factor to the small number of women who participated in the study. This limited sample size prevented thorough investigation of potential effect measure modification by several characteristics that are known to affect menstrual cycle function parameters, such as age. It is theoretically possible that the associations between thyroid hormones and menstrual cycle function may differ by proximity to menopause. Given the wide age range in this study, upon further review, two women may have been perimenopausal (one woman aged 52 years was amenorrheic during the study and another aged 54 years had consistently short cycles). However, both women showed hormonal evidence of ovulation and when excluded, were not influential observations in analyses. Future studies with larger sample sizes may consider stratifying by age to evaluate whether thyroidal impacts on menstrual cycle function may vary. Second, serum reproductive hormones were not measured; instead relying on urinary FSH and metabolites of estrogen and progesterone. Although these are less clinically interpretable, this made it possible to obtain serial measures every day over a three-month period, resulting in comprehensive data on menstrual cycle function.
Third, a potentially important caveat to this research is that although particular thyroid hormones were associated with certain menstrual cycle function outcomes, it is possible that these associations are not independent due to the thyroid’s negative feedback loop which results in functional relationships between hormones.35 Therefore, the observed associations with each thyroid hormone may not be isolated and, rather, the ability to detect certain associations over others is likely related to low statistical power given the limited sample size. Furthermore, because the measurement of menstrual cycle function was comprehensive and included both cycle characteristics and hormone measurements, this allowed the fitting of several models that tested associations between multiple thyroid hormones and a vast array of menstrual cycle function outcomes. This allowed the opportunity to observe whether there was consistency among measures within and across menstrual cycles and look for overall patterns of associations between the interrelated exposures and outcomes.
Conclusions
Thyroid hormone levels within the euthyroid range were associated with several menstrual cycle function outcomes among a sample of healthy premenopausal women. In particular, T4 and T3 were positively related to urinary estrogen and progesterone metabolite levels across the menstrual cycle. This suggests that even within normal ranges, thyroid hormones may exert effects on the female reproductive system, perhaps through mechanisms involving gonadotropins and steroid hormones, specifically estrogens and progesterone. This research contributes to furthering the understanding of the relationships between thyroid function, menstrual and reproductive physiology, and the endocrine axes that govern each.
Supplementary Material
Figure S1. Menstrual cycle function diary card
Table S1. Coefficients of variation for thyroid hormone assays
Table S2. Multi-day hormonal outcomes
Table S3. Predicted mean values and 95% confidence intervals (95% CI) of menstrual cycle function outcomes from regression models for associations with personal characteristics among Michigan women without thyroid disease, 2004–2014
Table S4. Predicted mean values and 95% confidence intervals (95% CI) of creatinine-adjusted hormone outcomes from regression models for associations with personal characteristics among Michigan women without thyroid disease, 2004–2014
Table S5. Thyroid hormone levels by population characteristics among women without self-reported thyroid disease (n=86)
Acknowledgments
Funding was provided by the National Institute of Environmental Health Sciences, National Institutes of Health (Grants R01ES012014, P30ES019776, R21ES023927, R01ES024790, and R01ES08341), the Environmental Protection Agency (Agreement Number R825300), the Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive, Perinatal, & Pediatric Training Grant (T32HD052460), and the National Institute for Occupational Safety and Health Environmental and Occupational Epidemiology Training Grant (5T03OH008609-10). This work was additionally supported by the Laney Graduate School and the Livingston Fellowship at Emory University.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health (NIOSH). Mention of any company or product does not constitute endorsement by NIOSH.
References
- 1.Harlow SD, Ephross SA. Epidemiology of menstruation and its relevance to women’s health. Epidemiologic Reviews. 1995;17:265–286. doi: 10.1093/oxfordjournals.epirev.a036193. [DOI] [PubMed] [Google Scholar]
- 2.Small CM, Manatunga AK, Klein M, Feigelson HS, Dominguez CE, McChesney R, et al. Menstrual cycle characteristics: associations with fertility and spontaneous abortion. Epidemiology. 2006;17:52–60. doi: 10.1097/01.ede.0000190540.95748.e6. [DOI] [PubMed] [Google Scholar]
- 3.Dittrich R, Beckmann MW, Oppelt PG, Hoffmann I, Lotz L, Kuwert T, et al. Thyroid hormone receptors and reproduction. Journal of Reproductive Immunology. 2011;90:58–66. doi: 10.1016/j.jri.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Adlersberg MA, Burrow GN. Focus on primary care. Thyroid function and dysfunction in women. Obstetrical and Gynecological Survey. 2002;57:S1–7. doi: 10.1097/00006254-200203001-00001. [DOI] [PubMed] [Google Scholar]
- 5.Krassas GE, Pontikides N, Kaltsas T, Papadopoulou P, Batrinos M. Menstrual disturbances in thyrotoxicosis. Clinical Endocrinology. 1994;40:641–644. doi: 10.1111/j.1365-2265.1994.tb03016.x. [DOI] [PubMed] [Google Scholar]
- 6.Krassas GE, Pontikides N, Kaltsas T, Papadopoulou P, Paunkovic J, Paunkovic N, et al. Disturbances of menstruation in hypothyroidism. Clinical Endocrinology. 1999;50:655–659. doi: 10.1046/j.1365-2265.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- 7.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocrine Reviews. 2010;31:702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 8.Joshi JV, Bhandarkar SD, Chadha M, Balaiah D, Shah R. Menstrual irregularities and lactation failure may precede thyroid dysfunction or goitre. Journal of Postgraduate Medicine. 1993;39:137–141. [PubMed] [Google Scholar]
- 9.Thomas R, Reid RL. Thyroid disease and reproductive dysfunction: a review. Obstetrics and Gynecology. 1987;70:789–798. [PubMed] [Google Scholar]
- 10.Krassas GE. Thyroid disease and female reproduction. Fertility and Sterility. 2000;74:1063–1070. doi: 10.1016/s0015-0282(00)01589-2. [DOI] [PubMed] [Google Scholar]
- 11.Fries GF. The PBB episode in Michigan: an overall appraisal. Critical Reviews in Toxicology. 1985;16:105–156. doi: 10.3109/10408448509056268. [DOI] [PubMed] [Google Scholar]
- 12.Baird DD, Weinberg CR, Wilcox AJ, Mcconnaughey DR. Using the Ratio of Urinary Estrogen and Progesterone Metabolites to Estimate Day of Ovulation. Statistics in Medicine. 1991;10:255–266. doi: 10.1002/sim.4780100209. [DOI] [PubMed] [Google Scholar]
- 13.Lynch KE, Mumford SL, Schliep KC, Whitcomb BW, Zarek SM, Pollack AZ, et al. Assessment of anovulation in eumenorrheic women: comparison of ovulation detection algorithms. Fertility and Sterility. 2014;102:511–518. e512. doi: 10.1016/j.fertnstert.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesner JS, Knecht EA, Krieg EF, Barnard G, Mikola HJ, Kohen F, et al. Validations of time-resolved fluoroimmunoassays for urinary estrone 3-glucuronide and pregnanediol 3-glucuronide. Steroids. 1994;59:205–211. doi: 10.1016/0039-128x(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 15.Kesner JS, Knecht EA, Krieg EF. Time-resolved immunofluorometric assays for urinary luteinizing hormone and follicle stimulating hormone. Analytica Chimica Acta. 1994;285:13–22. [Google Scholar]
- 16.Kesner JS, Knecht EA, Krieg E, Wilcox AJ, O’Connor JF. Detecting pre-ovulatory luteinizing hormone surges in urine. Human Reproduction. 1998;13:15–21. doi: 10.1093/humrep/13.1.15. [DOI] [PubMed] [Google Scholar]
- 17.Findlay J, Wu A, Knott V, Mauck L, Frickey P, Norton G. Clinical Chemistry. Amer Assoc Clinical Chemistry; 2101 L Street NW, Suite 202, Washington, DC 20037–1526: 1985. Development of a Kodak Ektachem® clinical chemistry slide for CK-B activity; pp. 1000–1000. [Google Scholar]
- 18.Mauck J, Mauck L, Novros J, Norton G, Toffaletti J. Clinical Chemistry. Amer Assoc Clinical Chemistry; 2101 L Street NW, Suite 202, Washington, DC 20037-1526: 1986. Development of a single slide Kodak Ektachem thin-film assay for serum and urine creatinine; pp. 1197–1198. [Google Scholar]
- 19.Jukic AM, Weinberg CR, Wilcox AJ, McConnaughey DR, Hornsby P, Baird DD. Accuracy of reporting of menstrual cycle length. American Journal of Epidemiology. 2008;167:25–33. doi: 10.1093/aje/kwm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Windham GC, Elkin E, Fenster L, Waller K, Anderson M, Mitchell PR, et al. Ovarian hormones in premenopausal women: variation by demographic, reproductive and menstrual cycle characteristics. Epidemiology. 2002;13:675–684. doi: 10.1097/00001648-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Baird DD, Weinberg CR, Zhou HB, Kamel F, McConnaughey DR, Kesner JS, et al. Preimplantation urinary hormone profiles and the probability of conception in healthy women. Fertility and Sterility. 1999;71:40–49. doi: 10.1016/s0015-0282(98)00419-1. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan MM. Clinical perspectives in the diagnosis of thyroid disease. Clinical Chemistry. 1999;45:1377–1383. [PubMed] [Google Scholar]
- 23.Sathi P, Kalyan S, Hitchcock C, Pudek M, Prior J. Progesterone therapy increases free thyroxine levels—data from a randomized placebo-controlled 12-week hot flush trial. Clinical Endocrinology. 2013;79:282–287. doi: 10.1111/cen.12128. [DOI] [PubMed] [Google Scholar]
- 24.Oppenheimer JH. Role of plasma proteins in the binding, distribution and metabolism of the thyroid hormones. New England Journal of Medicine. 1968;278:1153–1162. doi: 10.1056/NEJM196805232782107. [DOI] [PubMed] [Google Scholar]
- 25.Ridgway EC, Longcope C, Maloof F. Metabolic clearance and blood production rates of estradiol in hyperthyroidism. Journal of Clinical Endocrinology and Metabolism. 1975;41:491–497. doi: 10.1210/jcem-41-3-491. [DOI] [PubMed] [Google Scholar]
- 26.Akande EO, Hockaday TD. Plasma oestrogen and luteinizing hormone concentrations in thyrotoxic menstrual disturbance. Proceedings of the Royal Society of Medicine. 1972;65:789–790. doi: 10.1177/003591577206500933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Southren AL, Olivo J, Gordon GG, Vittek J, Brener J, Rafii F. The conversion of androgens to estrogens in hyperthyroidism. Journal of Clinical Endocrinology and Metabolism. 1974;38:207–214. doi: 10.1210/jcem-38-2-207. [DOI] [PubMed] [Google Scholar]
- 28.Zoeller TR. Environmental chemicals targeting thyroid. Hormones (Athens) 2010;9:28–40. doi: 10.14310/horm.2002.1250. [DOI] [PubMed] [Google Scholar]
- 29.Asvold BO, Bjoro T, Nilsen TI, Vatten LJ. Tobacco smoking and thyroid function: a population-based study. Archives of Internal Medicine. 2007;167:1428–1432. doi: 10.1001/archinte.167.13.1428. [DOI] [PubMed] [Google Scholar]
- 30.Small CM, Manatunga AK, Marcus M. Validity of self-reported menstrual cycle length. Annals of Epidemiology. 2007;17:163–170. doi: 10.1016/j.annepidem.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Rowland AS, Baird DD, Long S, Wegienka G, Harlow SD, Alavanja M, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13:668–674. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Harlow SD, Campbell BC. Host factors that influence the duration of menstrual bleeding. Epidemiology. 1994;5:352–355. doi: 10.1097/00001648-199405000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Harlow SD, Matanoski GM. The association between weight, physical activity, and stress and variation in the length of the menstrual cycle. American Journal of Epidemiology. 1991;133:38–49. doi: 10.1093/oxfordjournals.aje.a115800. [DOI] [PubMed] [Google Scholar]
- 34.Windham GC, Mitchell P, Anderson M, Lasley BL. Cigarette smoking and effects on hormone function in premenopausal women. Environmental Health Perspectives. 2005;113:1285–1290. doi: 10.1289/ehp.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jameson JL, De Groot LJ. Endocrinology: adult and pediatric. Elsevier Health Sciences; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Menstrual cycle function diary card
Table S1. Coefficients of variation for thyroid hormone assays
Table S2. Multi-day hormonal outcomes
Table S3. Predicted mean values and 95% confidence intervals (95% CI) of menstrual cycle function outcomes from regression models for associations with personal characteristics among Michigan women without thyroid disease, 2004–2014
Table S4. Predicted mean values and 95% confidence intervals (95% CI) of creatinine-adjusted hormone outcomes from regression models for associations with personal characteristics among Michigan women without thyroid disease, 2004–2014
Table S5. Thyroid hormone levels by population characteristics among women without self-reported thyroid disease (n=86)