Abstract
Background
Despite improvements in perinatal care, Bronchopulmonary Dysplasia (BPD) in extremely premature infants has not decreased. Postnatal surfactant therapy provides symptomatic relief from Respiratory Distress Syndrome, but it does not translate into a reduction in BPD. Therefore, the search for effective interventions to prevent BPD continues.
Objectives
Since PPARγ agonists have been demonstrated to promote neonatal lung maturation and injury repair, we hypothesized that a combined PPARγ agonist [pioglitazone (PGZ)] and synthetic lung surfactant [surfactant protein B (SP-B) mimic B-YL] formulation would stimulate lung maturation and block hyperoxia-induced neonatal lung injury more effectively than either modality alone.
Methods
One-day old Sprague-Dawley rat pups were administered PGZ+B-YL via nebulization every 24h up to 72h. The pups were exposed to either 21% or 95% O2, following which pups were sacrificed and lungs examined for markers of lung maturation [(PPARγ, SP-C, and cholinephosphate cytidylyltransferase (CCT-α) by Western analysis), and (H3)triolein uptake] and injury repair (bronchoalveolar lavage cell count and protein content; LEF-1, Fibronectin, ALK5, and β-catenin protein levels by Western analysis].
Results
The markers of alveolar epithelial-mesenchymal maturation (SP-C, CCT-α, and triolein uptake) increased significantly in the PGZ+B-YL group, more than with either drug alone. Similarly, markers of hyperoxia-induced lung injury were blocked effectively with PGZ+B-YL treatment.
Conclusions
Nebulized PPARγ agonist PGZ with synthetic lung surfactant accelerates lung maturation and prevents neonatal hyperoxia-induced lung injury more than with either modality alone, potentially preventing BPD more effectively.
Keywords: Bronchopulmonary Dysplasia, Chronic lung disease, Lung maturation, Prematurity, Respiratory Distress Syndrome
Introduction
Despite improvements in perinatal care, Bronchopulmonary Dysplasia (BPD) in extremely premature infants has not decreased [1,2]. The use of postnatal surfactant provides symptomatic relief from Respiratory Distress Syndrome (RDS), but it does not translate into a reduction in BPD. This is not surprising, since the administration of surfactant alone does not address the fundamental issue in RDS, i.e., lung immaturity [3,4]. In contrast, PPARγ agonists recapitulate the natural cellular and molecular pathways that determine normal lung structure, function, and homeostasis. In multiple in vitro and in vivo experimental models of neonatal lung injury, PPARγ agonists, administered either parenterally [5] or locally [6], enhance lung maturation and prevent neonatal lung injury [5–7]. We reasoned that combining lung surfactant with a PPARγ agonist will complement each other’s benefits and the net effect on lung injury reduction would be greater than with either intervention alone. Therefore, we hypothesized that a combined PPARγ agonist + surfactant formulation effectively blocks neonatal lung injury and that this effect is greater than with either modality alone.
We used a new and innovative surfactant protein B (SP-B) peptide mimic B-YL developed for aerosolized delivery specifically in a hyperoxic environment. Using the N-terminal (residues 1-25) and C-terminal (residues 63-78) α-helical sequences of native SP-B, connected with a short β-sheet loop [8], we replaced the cysteine and methionine residues with tyrosine and leucine, respectively, to minimize inactivation by oxidative stress. Based on homology templating of the linear amino acid sequence (NH2-FPIPLPYYWLYRALIKRIQALIPKGGRLLPQLVYRLVLRYS-COOH), a 3D structure of SP-B peptide mimic B-YL is shown in Fig. 1. As for the choice of PPARγ agonist, due to the superior safety and efficacy of nebulized pioglitazone (PGZ) in blocking hyperoxia-induced neonatal lung injury [5], PGZ was used to combine with the synthetic surfactant B-YL. Using a neonatal rat model, we planned to administer a highly effective, stable, and oxidant resistant synthetic lung surfactant (B-YL) and simultaneously enhance lung maturation via PGZ to prevent hyperoxia-induced neonatal lung injury.
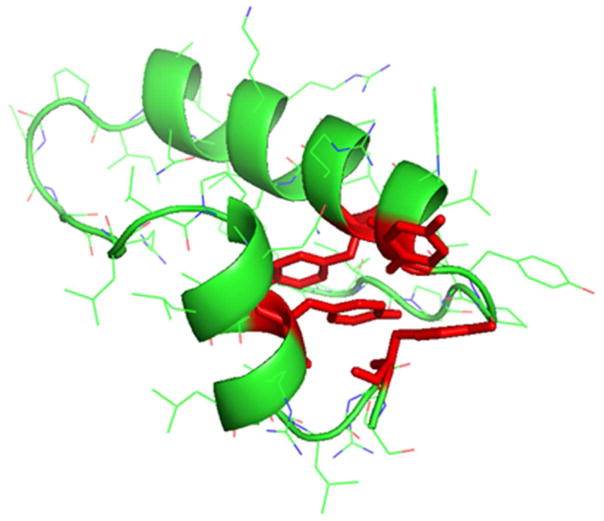
Fig. 1. Predicted molecular model of B-YL derived from the primary amino acid sequence using the homology templating program i-Tasser [26].
The secondary structure of the SP-B peptide mimic B-YL is that of a helix hairpin with the helical elements shown as green ribbons connected by a bend domain shown as a green tube. The helix hairpin structure is stabilized by the interaction of the tyrosine side chains in red highlight at the N and C terminal domains that emulate the disulfide interactions and the Saposin protein fold of the native SP-B sequence.
Materials and Methods
Synthetic surfactant
B-YL peptide was synthesized on a Symphony Multiple Peptide Synthesizer (Protein Technologies, Tucson, AZ) using a FastMoc™ protocol on a H-Ser(OtBu)-HMPB NovaPEG resin, as described previously [8,9]. Purified B-YL peptide was freeze-dried and its mass confirmed by MALDI-TOF mass spectrometry. B-YL surfactant was formulated to contain 35 mg/ml of 5:3:2 DPPC:POPC:POPG combined with 3.0% (by wt) of B-YL. Synthetic surfactant components were combined in organic solvent (e.g., chloroform for lipids and TFE for peptide), dried under nitrogen, exposed to house vacuum to remove residual solvent, resuspended by hand- vortexing in 0.15 M NaCl adjusted to pH 7.0, heated to 65°C intermittently for 30 min, and refrigerated for >12 h prior to use. PGZ was mixed into B-YL surfactant as an ethanol suspension (4 mg/ml pure ethanol), followed by removal of the ethanol by evaporation. The surface tension lowering ability of surfactant preparations was assessed under dynamic compression at 37°C by captive bubble surfactometry [8]. We routinely analyze surfactant samples of 1 μL (35 mg phospholipids/mL) in ~1.5 ml of Goerke’s buffer with 10% sucrose (~ 25 μg surfactant/mL) in the captive bubble surfactometer and perform all measurements in quadruplicate. Lipids only are used as negative control and the clinical surfactant Infasurf® as positive control.
Animal Protocol and Study Design
All studies were approved by the Institutional Review Board and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Briefly, following our previously described protocol, one-day-old ad libitum breastfeeding rat pups were exposed to either normoxia (21% O2) or hyperoxia (95% O2) for 72 h [6,7]. Hyperoxia exposed animals were administered diluent, B-YL surfactant [100 mg/kg body weight, (BW)], PGZ (1 mg/kg), or PGZ (1 mg/kg) + B-YL surfactant (100 mg/kg) in a 1 ml volume via nebulization over 30 min every 24 h up to 72 h. Aerosolization of B-YL surfactant and PGZ was achieved with a vibrating mesh nebulizer (Aeroneb Pro® nebulizer, Aerogen Inc., Mountain View, CA)[9–10], which aerosolizes liquid formulations without affecting compound’s composition and concentration. This small volume (10 ml maximum), median diameter nebulizer delivers a low velocity aerosol with a particle size <3.0 μm and a flow rate >0.1 ml/min, is FDA-approved for human use, and does not need a gas source [11]. Control (normoxia group) pups were administered the diluent similarly via nebulization.
All pups were killed at 72 h, and lungs were either snap-frozen for Western analysis of markers of lung homeostasis [SP-C and cholinephosphate cytidylyltransferase (CCT)-α] and injury repair (LEF-1 and Fibronectin) or cultured as explants to determine the rate of [3H] triolein uptake, a functional marker of surfactant phospholipid synthesis. A separate set of animals was used for bronchoalveolar lavage (BAL) collection. Retrieved BAL solutions were centrifuged at 400g for 10 min. Sediments were analyzed for total cell counts and supernatants were snap-frozen in liquid nitrogen for subsequent analysis of inflammatory markers, such as IL-6 and interferon (IF)-γ (ELISA kits from R&D Systems).
Western analysis
Following previously described methods [6,7,12], Western analysis was performed on lung tissue to determine protein levels of alveolar epithelial (SP-C and CCTα) and alveolar mesenchymal (PPAR-γ) differentiation and injury repair (LEF-1 and Fibronectin) markers. Primary antibodies (all from Santa Cruz Biotechnology, Dallas, TX, USA) used included: PPARγ (1:500), SP-C (1:250), CCT-α (1:200), LEF1 (1:200), Fibronectin (1:250), BAX (1:500), Bcl-2 (1:200), and GAPDH (1:10,000), which was from Millipore, Billerica, MA, USA.
Immunofluorescence
Tissue immunofluorescence staining for ALK5 (1:100) and β-catenin (1:100) was performed following previously described methods [7].
Real time qRT-PCR for lung cytokine assay
Tissue RNA extraction and q-RT-PCR were performed according to previously described methods [5]. RT-PCR primers used included IL6: F5′-CTTCCTACCCCAACTTCCAA-3′ and R5′-ACCACAGTGAGGAATGTCCA-3′ (191 bp); Interferon-γ: F5′-CCAAGTTCGAGGTGAACAAC-3′; R5′-ACTCCTTTTCCGCTTCCT-3′ (110 bp). The normalization control was 18S ribosomal RNA. The relative fold-change for each gene was calculated using the ΔΔCT method.
Choline incorporation in saturated phosphatidylcholine assay
[3H]choline (NEN Dupont, Boston, MA) incorporation into saturated phosphatidylcholine, which is the major surface-active lipid substrate of surfactant was determined in cultured lung explants following our previously described protocol [12].
Triglyceride uptake assay
[3H]triolein (PerkinElmer, Boston, MA, USA) uptake, a key marker of alveolar lipofibroblast (LIF) function, was used to quantitate triglyceride uptake by fetal rat lung explants using our previously described protocol [12].
Statistical analysis
Student t-test and ANOVA, in combination with the Tukey’s post-hoc test, as appropriate, were used to detect group differences. The number of samples for each analysis was 4–6, from three separate sets of experiments, i.e., N=3. Results are expressed as means ± SEM.
Results
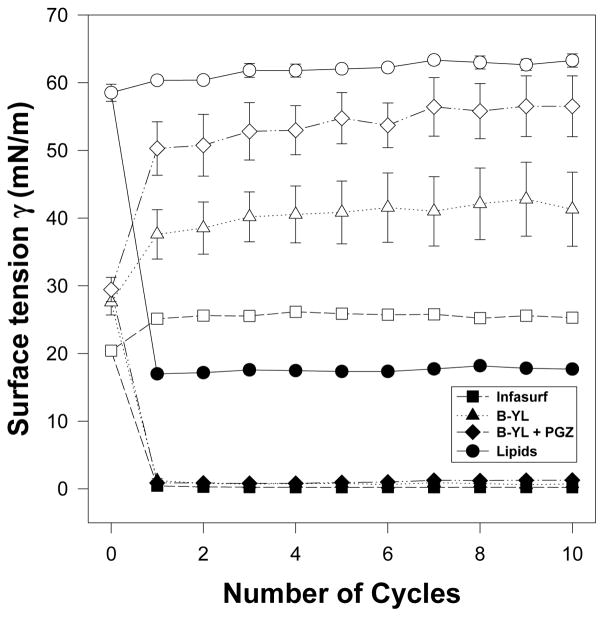
We first determined whether combining PGZ with B-YL affects B-YL’s bioactivity by measuring its surface activity with captive bubble surfactometry (CBS) [8,10,13] in the presence and absence of PGZ [B-YL surfactant (100 mg/kg) and PGZ (1 mg/kg)] and compared with a clinical surfactant (Infasurf®) as positive and lipids alone (DPPC:POPC: POPG 5:3:2 wt:wt:wt) as negative controls, respectively. Clinical surfactant contains both SP-B and SP-C in contrast with B-YL surfactant that only contains a highly active SP-B peptide mimic. Minimum surface tension values of B-YL surfactant ± PGZ were similar to those of Infasurf (Fig. 2).
Fig. 2. Comparison of B-YL, PGZ+B-YL, lipids alone, and Infasurf® on surface tension reduction activity.
Surface activity of the B-YL (100 mg/kg) was measured with captive bubble surfactometry in the presence and absence of PGZ (1 mg/kg) and compared with a clinical surfactant (Infasurf®) as positive control and lipids alone (DPPC:POPC:POPG 5:3:2 wt:wt:wt) as negative control. Minimum surface tension values are indicated with black symbols and maximum values with similar white symbols. Minimum surface tension values of B-YL surfactant ± PGZ were similar to those of Infasurf®. Values are mean ± SEM of N=4–5.
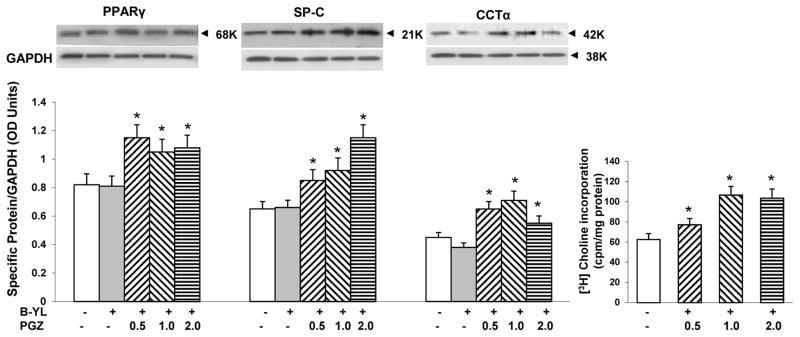
Based on the ampholytic properties, the addition of B-YL to a PGA is not expected to interfere with PGZ’s PPARγ agonist activity [14]. Therefore, we next confirmed that the addition of B-YL to PGZ did not interfere with its PPARγ agonist activity, and, hence its lung maturation effect. Embryonic day 19 fetal rat lung explants were cultured for 24 h with or without B-YL [100 mg/kg fetal body weight (BW)] alone or with PGZ (0.5, 1 or 2 mg/kg BW)). Following which PPARγ, SP-C, and CCT-α protein levels, along with choline incorporation into disaturated phosphatidylcholine (DSPC), all markers of lung maturation, were determined. As shown in Fig. 3, explants treated with PGZ+B-YL demonstrated the expected increases in PPARγ, SP-C, and CCT-α protein levels along with an increase in choline incorporation into DSPC, indicating that combining B-YL with PGZ does not affect PGZ’s lung maturation activity.
Fig. 3. Effect of B-YL on PGZ’s PPARγ agonist activity.
Embryonic day 19 fetal rat lung explants, cultured for 24 h, under either control condition (without B-YL or PGZ supplementation) or treated with B-YL (100 mg/kg BW) alone or with PGZ (0.5, 1 or 2 mg/kg BW) + B-YL (100 mg/kg BW), demonstrated significant increases in PPARγ, SP-C, and CCT-α protein levels, as determined by Western analysis, along with a significant increase in [3H]choline incorporation into disaturated phosphatidylcholine (p <0.05 vs. control; N=3).
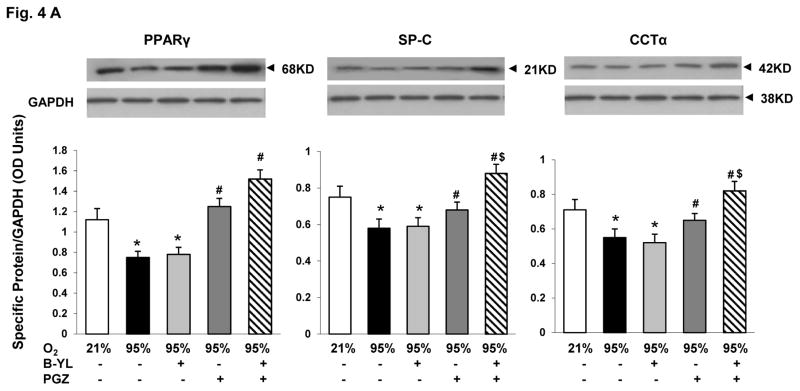
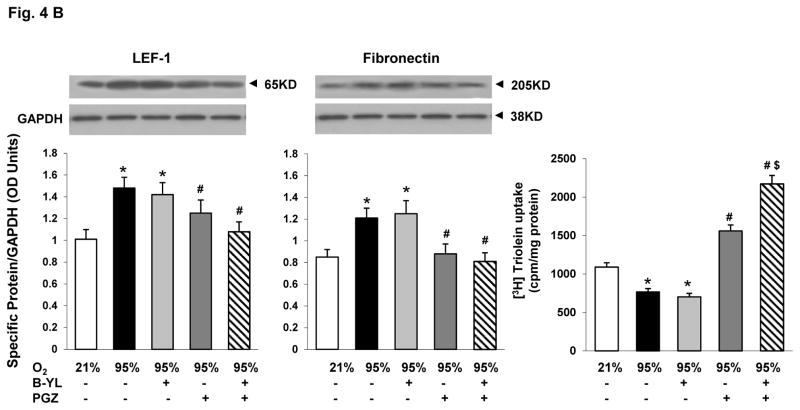
Having confirmed that combining B-YL and PGZ does not interfere with the surface tension reducing and lung maturational activity, respectively, of B-YL and PGZ, under in vitro conditions, we next determined if this combinatorial approach prevents hyperoxia-induced neonatal lung injury under in vivo conditions. Postnatal day (PND) 1 rat pups were exposed to either normoxia or hyperoxia up to 72 h. Hyperoxia-induced decrease in PPARγ (A), SP-C (B), CCT-α (C) and increase in LEF-1 (D) and Fibronectin (E) protein levels were blocked by the concomitant administration of either PGZ or PGZ+B-YL. This was true also for hyperoxia-induced decrease in triolein uptake (F). As evidenced by the effects on the protein levels of SP-C and CCT-α, and triolein uptake, PGZ+B-YL administration had a more robust effect; In contrast, B-YL administration alone had no effect on any of these parameters.
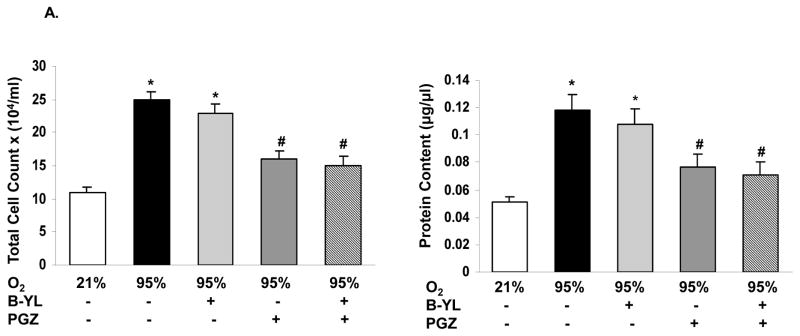
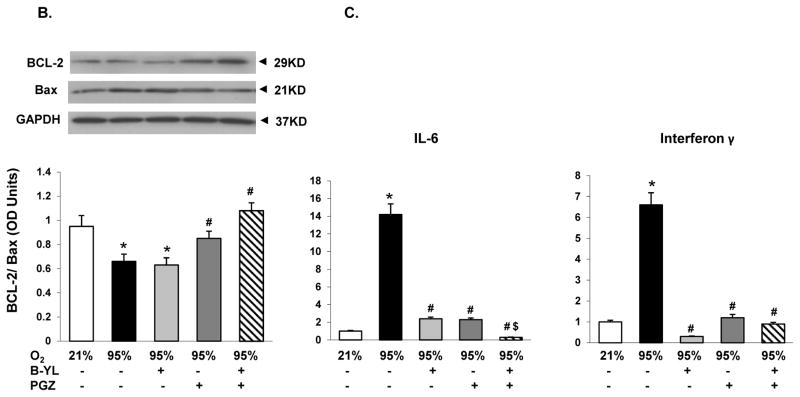
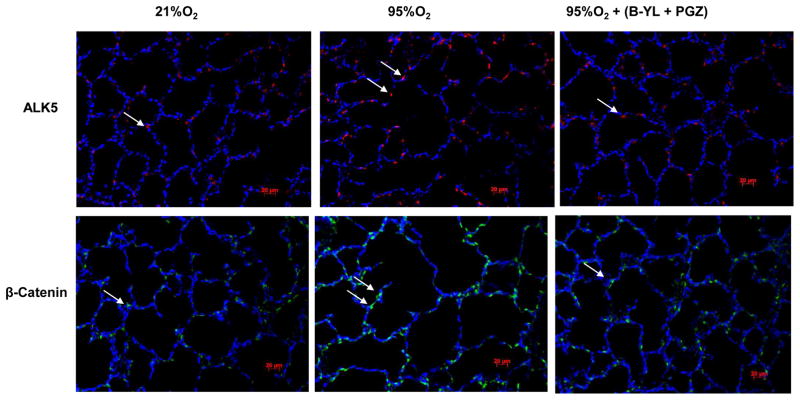
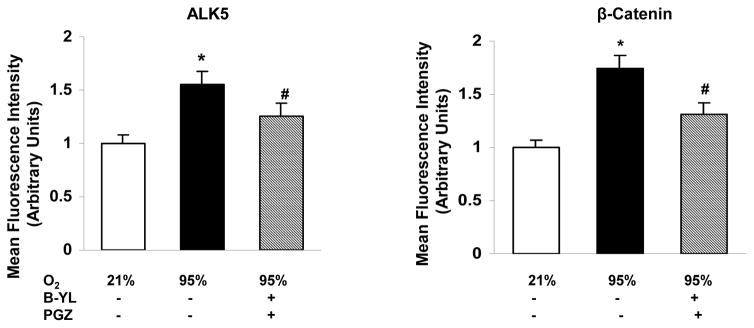
Blockage of hyperoxia-induced neonatal lung injury with combined PGZ+B-YL nebulization is also evidenced by the normalization of hyperoxia induced alterations in BAL cell count and protein content (Fig. 5A), as well as whole lung apoptosis (BcL2/Bax) (Fig. 5B) and lung inflammatory markers IL-6 and Interferon (IF)-γ (Fig. 5C). Interestingly, even though B-YL administration alone did not have any effect on most molecular markers of lung maturation and injury repair; it did suppress the hyperoxia-induced increase in IL-6 and IF-γ expression. Lastly, since hyperoxia-induced neonatal lung injury has been previously shown to be mediated via TGF-β and Wnt activations, lung sections from control, hyperoxia, and hyperoxia + B-YL+PGZ groups were stained for ALK5 and β-catenin, two key intermediates of TGF-β and Wnt pathway activations, respectively. In line with the data on other markers of lung injury, hyperoxia-induced increase in ALK5 and β-catenin staining was blocked in the B-YL+PGZ group (Fig. 6).
Fig. 5. Nebulized PGZ+B-YL blocks hyperoxia-induced alterations in selective markers of neonatal lung injury more effectively than either modality alone.
Postnatal day one rat pups were exposed to hyperoxia ± B-YL, PGZ, or PGZ+B-YL for 72 h. Hyperoxia-induced alterations in bronchoalveolar lavage total cell count(A) and protein content (B), apoptosis marker BcL2/Bax ratio (C) and inflammatory markers IL-6 and interferon-γ (D) were blocked by concomitant administration of either PGZ or PGZ+B-YL. *, p<0.05 vs. cont; #, p<0.05 vs. 95% O2; cont; $, p< 0.05 vs. PGZ N=3.
Fig. 6. Nebulized PGZ+B-YL blocks hyperoxia-induced activations of TGF-β and Wnt signaling pathways.
Postnatal day one rat pups were exposed to hyperoxia ± PGZ+B-YL for 72h. Hyperoxia-induced activation in TGF-β [determined by ALK5 protein levels, red staining) and Wnt (determined by β-catenin protein levels, green staining) signaling was blocked by concomitant PGZ+B-YL administration. Representative immunostaining pictures and relative mean fluorescence values are shown; N=3.
Discussion
Using both in vitro and ex vivo studies we found that combining PGZ with the SP-B mimic B-YL doesn’t interfere with PGZ’s PPARγ’s agonist activity and B-YL’s surface tension lowering activity. Furthermore, in a neonatal rat model of hyperoxia-induced lung injury, nebulized combined PGZ+B-YL formulation complemented each other’s benefits as reflected by a greater net reduction in lung injury compared with either intervention alone. For example, on hyperoxia exposure, there was relatively greater preservation of alveolar homeostatic markers PPARγ, SP-C, CCT-α, and triolein uptake, suggesting potentially greater protection against hyperoxia-induced neonatal lung injury with the PGZ+B-YL formulation than with either component alone.
Prematurity is the underlying cause of lung immaturity leading to RDS. Various approaches including antenatal steroids, administration of exogenous surfactant, and non-invasive and gentler modes of ventilation have shown only limited benefits in preventing RDS and BPD. Only antenatal steroid therapy has been consistently shown to enhance lung maturation and decrease the incidence and severity of RDS. However, it is differentially more effective in females [15,16], is associated with significant side effects, and does not result in a reduction in BPD. In contrast, in experimental models, administration of PPARγ agonists has been shown reduce lung injury and enhance fetal/neonatal lung maturation safely in both males and females [17,18].
One of the biggest impediments towards finding an effective preventive/therapeutic intervention against BPD has been a failure to target the fundamental cellular and molecular pathways centrally involved in lung development and injury repair [3]. Combining surfactant with a PPARγ agonist aims to not only reduce the manifestations of RDS acutely, but also to recapitulate the natural cellular and molecular pathways that i) determine the normal lung structure, function, and homeostasis; ii) are disrupted by oxotrauma, inflammation, and volutrauma; and iii) have been shown to be normalized by PPARγ agonists in multiple in vitro and in vivo models [4–7,18]. This approach is based on the paradigm that the developing lung mesenchyme, dominated by Wnt signaling, the default pathway for muscle development, inhibits C/EBPα and PPARγ, thereby actively inhibiting the adipogenic programming of the developing alveolar mesenchyme [19]. During lung morphogenesis, the mesenchymal default Wnt pathway is inhibited and the adipogenic pathway is dis-inhibited, resulting in the formation of lipid-laden alveolar interstitial adepithelial fibroblasts~LIFs [19]. Lipofibroblasts are critical for lung homeostasis and injury repair, since they actively provide triglyceride substrate to ATII cells for surfactant synthesis, support ATII cell growth and differentiation, and act as an important defense against oxidant lung injury [19,20]. On exposure to volutrauma, oxotrauma, andr infection, pulmonary LIFs transdifferentiate to a myogenic phenotype, i.e., myofibroblasts (MYFs) [18,21]. Myofibroblasts do not support ATII cell growth or differentiation, whereas LIFs do [20], hence the critical importance of maintaining the proper balance of LIFs and MYFs to direct lung development. Previous studies have demonstrated that all of the above mentioned BPD inducers cause downregulation of alveolar LIF PPARγ expression[18], and thus inhibit normal lung development, but PPARγ agonists mitigate and in some cases even reverse the lung damage [18,20–22]. However, it is important to point out that although we have focused on PPARγ’s role in LIF biology, it also exerts a prominent injury repair effect via a variety of other mechanisms [23].
Other advantages of our approach include the potential delivery of the nebulized PGZ+B-YL formulation directly to the lung, which should result in maximum therapeutic effect, limit systemic side effects, and circumvent the issues pertaining to bioavailability and first-pass metabolism if administered orally. Furthermore, aerosolized delivery of PGZ+B-YL eliminates intubation and the related complications. However, detailed pharmacokinetics studies are needed to determine the bio-distribution of PGZ and B-YL in the lung, blood, and other organs and to determine if there are any systemic effects before the translation of the proposed approach to bedside.
As alluded to above, B-YL is an innovative surfactant developed specifically to resist hyperoxia-induced inactivation [24]. It is both stable and highly active in its native state and after addition of PGZ during in vitro testing (Fig. 2). Moreover, it has a low viscosity, aerosolizes easily, and can be produced inexpensively, all highly relevant attributes for its translation to clinical practice. Since B-YL+ PGZ formulation effectively blocked lung injury, even following an extremely high O2 exposure, i.e., 95% O2 exposure for 72h, a scenario which is rarely used in contemporary neonatal practice, we are confident that it will also be effective against the usual lower levels of hyperoxia exposure frequently encountered in neonatal intensive care units. While PGZ was used as a prototype PPARγ agonist in this study, other PPARγ agonists might prove to be safer and even more effective than PGZ. It is also worth noting that the lung maturational effect of PPARγ agonists is not restricted only to PGZ, but is also seen with other PPARγ agonists such as curcumin, troglitazone, RGZ, and PGJ2 [4–7,18,21,25].
Fig. 4. Nebulized PGZ+B-YL blocks hyperoxia-induced alterations in selective markers of neonatal lung maturation more effectively than either modality alone.
Postnatal day one rat pups were exposed to hyperoxia ± B-YL, PGZ, or PGZ+B-YL for 72 h. Hyperoxia-induced decrease in PPARγ (A), SP-C (B), CCT-α (C) and increase in LEF-1 (D) and Fibronectin (E) protein levels were blocked by concomitant administration of either PGZ or PGZ+B-YL. This was also true for hyperoxia-induced decrease in triolein uptake (F). PGZ+B-YL administration had a more robust effect, as evidenced by the effects on the protein levels of SP-C and CCT-α and the triolein uptake; however, B-YL administration alone had no effect on any of these parameters. *, p<0.05 vs. control; #, p<0.05 vs. 95% O2; control; $, p< 0.05 vs. PGZ; N=3.
Acknowledgments
Grant Support: NIH (HD51857, HL107118, HD071731, and HL127237), TRDRP (17RT-0170, and 23RT-0018), and Bill & Melinda Gates Foundation (OPP1112090)
Footnotes
The authors declare no conflicts of interest.
Reference List
- 1.Maitre NL, Ballard RA, Ellenberg JH, Davis SD, Greenberg JM, Hamvas A, Pryhuber GS Prematurity and Respiratory Outcomes Program. Respiratory consequences of prematurity: evolution of a diagnosis and development of a comprehensive approach. J Perinatol. 2015;35(5):313–321. doi: 10.1038/jp.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin RJ, Fanaroff AA. The preterm lung and airway: past, present, and future. Pediatr Neonatol. 2013;54(4):228–234. doi: 10.1016/j.pedneo.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Silva DM, Nardiello C, Pozarska A, Morty RE. Recent advances in the mechanisms of lung alveolarization and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2015;309(11):L1239–72. doi: 10.1152/ajplung.00268.2015. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Santos J, Sakurai R, Shin E, Cerny L, Torday JS, Rehan VK. Peroxisome proliferator-activated receptor gamma agonists enhance lung maturation in a neonatal rat model. Pediatr Res. 2009;65(2):150–155. doi: 10.1203/PDR.0b013e3181938c40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morales E, Sakurai R, Husain S, Paek D, Gong M, Ibe B, Li Y, Husain M, Torday JS, Rehan VK. Nebulized PPARgamma agonists: a novel approach to augment neonatal lung maturation and injury repair in rats. Pediatr Res. 2014;75(5):631–640. doi: 10.1038/pr.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, Rehan VK. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L1031–L1041. doi: 10.1152/ajplung.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakurai R, Villarreal P, Husain S, Liu J, Sakurai T, Tou E, Torday JS, Rehan VK. Curcumin protects the developing lung against long-term hyperoxic injury. Am J Physiol Lung Cell Mol Physiol. 2013;305(4):L301–L311. doi: 10.1152/ajplung.00082.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walther FJ, Waring AJ, Hernandez-Juviel JM, Gordon LM, Wang Z, Jung CL, Ruchala R, Clark AP, Smith WM, Sharma S, Notter RH. Critical structural and functional roles for the N-terminal insertion sequence in surfactant protein B analogs. PLoS One. 2010;5(1):e8672. doi: 10.1371/journal.pone.0008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walther FJ, Hernandez-Juviel JM, Waring AJ. Aerosol delivery of synthetic lung surfactant. PeerJ. 2014;2:e403. doi: 10.7717/peerj.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubus JC, Vecellio L, De Monte M, Fink JB, Grimbert D, Montharu J, Valat C, Behan N, Diot P. Aerosol deposition in neonatal ventilation. Pediatr Res. 2005;58(1):10–14. doi: 10.1203/01.PDR.0000156244.84422.55. [DOI] [PubMed] [Google Scholar]
- 11.Finer NN, Merritt TA, Bernstein G, Job L, Mazela J, Segal R. An open label, pilot study of Aerosurf(R) combined with nCPAP to prevent RDS in preterm neonates. J Aerosol Med Pulm Drug Deliv. 2010;23(5):303–309. doi: 10.1089/jamp.2009.0758. [DOI] [PubMed] [Google Scholar]
- 12.Rehan VK, Wang Y, Sugano S, Santos J, Patel S, Sakurai R, Boros LG, Lee WP, Torday JS. In utero nicotine exposure alters fetal rat lung alveolar type II cell proliferation, differentiation, and metabolism. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L323–3. doi: 10.1152/ajplung.00071.2006. [DOI] [PubMed] [Google Scholar]
- 13.Walther FJ, Waring AJ, Hernandez-Juviel JM, Gordon LM, Schwan AL, Jung C-L, Chang Y, Wang Z, Notter RH. Dynamic surface activity of a fully synthetic phospholipase-resistant lipid/peptide lung surfactant. PLos One. 2007;2(10):e1039. doi: 10.1371/journal.pone.0001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giaginis C, Theocharis S, Tsantili-Kakoulidou A. Investigation of the lipophilic behaviour of some thiazolidinediones. Relationships with PPAR-gamma activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857(2):181–187. doi: 10.1016/j.jchromb.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Effect of antenatal dexamethasone administration on the prevention of respiratory distress syndrome. Am J Obstet Gynecol. 1981;141(3):276–287. [PubMed] [Google Scholar]
- 16.Papageorgiou AN, Colle E, Farri-Kostopoulos E, Gelfand MM. Incidence of respiratory distress syndrome following antenatal betamethasone: role of sex, type of delivery, and prolonged rupture of membranes. Pediatrics. 1981;67(5):614–617. [PubMed] [Google Scholar]
- 17.Lee C, Sakurai R, Shen H, Rehan V. Antenatal PPAR-γ pioglitazone stimulates fetal lung maturation equally in males and females. Abstract #53, Western Society of Pediatric Research; Jan 2017; Carmel, CA. [Google Scholar]
- 18.Torday JS, Rehan VK. Developmental cell/molecular biologic approach to the etiology and treatment of bronchopulmonary dysplasia. Pediatr Res. 2007;62(1):2–7. doi: 10.1203/PDR.0b013e31806772a1. [DOI] [PubMed] [Google Scholar]
- 19.Torday JS, Rehan VK. Up-regulation of fetal rat lung parathyroid hormone-related protein gene regulatory network down-regulates the Sonic Hedgehog/Wnt/betacatenin gene regulatory network. Pediatr Res. 2006;60(4):382–388. doi: 10.1203/01.pdr.0000238326.42590.03. [DOI] [PubMed] [Google Scholar]
- 20.Torday JS, Torres E, Rehan VK. The role of fibroblast transdifferentiation in lung epithelial cell proliferation, differentiation, and repair in vitro. Pediatr Pathol Mol Med. 2003;22(3):189–207. doi: 10.1080/pdp.22.3.189.207. [DOI] [PubMed] [Google Scholar]
- 21.Rehan VK, Torday JS. The lung alveolar lipofibroblast: an evolutionary strategy against neonatal hyperoxic lung injury. Antioxid Redox Signal. 2014;21(13):1893–1904. doi: 10.1089/ars.2013.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehan VK, Sakurai R, Wang Y, Santos J, Huynh K, Torday JS. Reversal of nicotine-induced alveolar lipofibroblast-to-myofibroblast transdifferentiation by stimulants of parathyroid hormone-related protein signaling. Lung. 2007;185(3):151–159. doi: 10.1007/s00408-007-9007-0. [DOI] [PubMed] [Google Scholar]
- 23.Reddy RC, Rehan VK, Roman J, Sime PJ. PPARs: Regulators and Translational Targets in the Lung. PPAR Res. 2012;2012:342924. doi: 10.1155/2012/342924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walther FJ, Gordon LM, Waring AJ. Design of surfactant protein B peptide mimics based on the saposin fold for synthetic lung surfactants. Biomed Hub. 2016 Sep-Dec;1(3) doi: 10.1159/000451076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakurai R, Li Y, Torday JS, Rehan VK. Curcumin augments lung maturation, preventing neonatal lung injury by inhibiting TGF-beta signaling. Am J Physiol Lung Cell Mol Physiol. 2011;301(5):L721–L730. doi: 10.1152/ajplung.00076.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.http://zhanglab.ccmb.med.umich.edu/I-TASSER