Summary
In aggressive lymphomas, discrepancies in survival reported from experimental and observational studies may reflect selective non-enrolment of high-risk patients in trials. We examined the association between time from diagnosis to chemotherapy and overall survival in diffuse large B-cell (DLBCL), Burkitt (BL), mantle cell (MCL) and peripheral T-cell lymphoma (PTCL), using National Cancer Data Base records of 130,549 patients treated in 2004–2014. Across the histologies, patients who started chemotherapy within 7 days of diagnosis had more often high International Prognostic Index (IPI) or advanced-stage disease. The discrepancy in 3-year survival between groups treated within 7 or >30 days from diagnosis ranged from 14% in BL to 30% in MCL. After adjusting for the IPI, time to treatment was significantly associated with shorter overall survival. Using the group treated >30 days from diagnosis as reference, patients treated within 7 days had a hazard ratio of 1.38 (95% confidence interval [CI], 1.28–1.48) in DLBCL, 1.42 (95%CI, 1.22–1.66) in BL, 2.23 (95%CI, 1.79–2.78) in MCL and 1.46 (95%CI, 1.18–1.81) in PTCL. Time from diagnosis to treatment may reflect high-risk features uncaptured by standard prognostic assessments. Clinical trials should accommodate patients who need urgent therapy to improve external validity and detect treatment effects in high-risk groups.
Keywords: diffuse large B-cell lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma, Burkitt lymphoma, chemotherapy
Introduction
Aggressive subtypes of non-Hodgkin lymphoma (NHL), which include diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma (BL), peripheral T-cell lymphoma (PTCL) and many cases of mantle cell lymphoma (MCL), are often characterized by rapid growth and symptoms related to tumour bulk or systemic effects.(Cheah et al, 2016; Jacobson and LaCasce 2014; Vaidya and Witzig 2014) They may require expedited, sometimes inpatient, workup and prompt delivery of chemotherapy. Clinical trials provide guidance for clinicians when they are counselling patients about their optimal treatment and prognosis at the time of diagnosis. However, trial participants are not representative of the general population of patients with lymphoma because of the strict selection criteria inherent to clinical research.(Augustin et al, 2017; Terschuren et al, 2010) For rare subtypes like PTCL, overall survival (OS) achieved in clinical trials conducted in research centres may be even less reliable when extrapolated to the population at large.(Schmitz et al, 2010; Vose et al, 2008)
In DLBCL, patients with low-risk disease can achieve event-free survival exceeding 90% after rituximab-based chemoimmunotherapy with or without radiation, and recent trials have focused on high-risk DLBCL subgroups in which outcomes remain unsatisfactory.(Lamy et al, 2018) Surprisingly, despite selection on the basis of high International Prognostic Index (IPI) or the unfavourable, non-germinal centre B cell-like (GCB) phenotype, some of these studies did not show improvements over standard of care, and the survival of enrolled patients far exceeded estimates expected from prior observations. A randomized phase 2 trial of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP), with or without bortezomib in non-GCB DLBCL reported 2-year progression-free survival (PFS) of 78%, rather than the expected 62%, and OS of 90% rather than the expected 69%.(Leonard et al, 2017) Phase 3 trials comparing R-CHOP with high-dose sequential therapy among patients with high-risk IPI resulted in OS of over 74% regardless of treatment arm.(Chiappella et al, 2017; Cortelazzo et al, 2016) There is a concern that the power of these trials to demonstrate the intended effects of novel therapies may be lowered by the selection of participants with favourable prognosis.(Nowakowski et al, 2016)
One potential factor leading to relative under-representation of high-risk patients in clinical trials is the short time from diagnosis to start of chemotherapy. Trial enrolment may require an independent review of pathology, molecular testing, or extended staging procedures during the screening period. Patients requiring urgent symptom control or systemic treatment may not tolerate the delay, and thus may selectively not participate in research. In one retrospective study, patients with DLBCL who started therapy >15 days from diagnosis had lower-risk characteristics and better 2-year event-free survival than those who were treated more urgently.(Maurer et al, 2016) It remains unclear to what extent this phenomenon affects OS estimates reported in trials, and whether it is specific to DLBCL or other histologies. Our objective was to evaluate whether time from diagnosis to treatment is independently associated with OS in the four most common aggressive NHL subtypes. Knowledge of such an association would provide a strong argument for a practical modification of eligibility criteria in clinical trials, facilitating enrolment of patients who need immediate therapy. Furthermore, it would enable a critical appraisal of survival estimates derived from trials, and provide guidance for additional stratification on the basis of time from diagnosis or need for emergency therapy, before study registration.
Methods
Patients and data source
This retrospective cohort study was approved by the Institutional Review Board at Rhode Island Hospital, and used data from the National Cancer Data Base (NCDB) Participant User File, a joint project of the Commission on Cancer of the American College of Surgeons and the American College of Surgeons.(Boffa et al, 2017) The NCDB contains over 34 million records from over 1,500 hospital cancer registries accredited by the Commission on Cancer, and captures about 84% of all newly diagnosed lymphomas in the United States (US). Data quality is evaluated through electronic cross-checks of records between different institutions and by audits of participating programs, which must provide survival follow-up for at least 90% of submitted cases during 5 years from diagnosis. The NCDB contains patients’ sociodemographic characteristics (including median income in the ZIP code of residence, linked from the US census), Charlson-Deyo comorbidity index as a measure of baseline mortality risk,(Deyo et al, 1992) stage of the lymphoma according to the American Joint Committee on Cancer (including presence of B symptoms) (Amin et al 2016), primary site, histology, human immunodeficiency virus (HIV) status, as well as application of radiation and systemic therapy during the first course of treatment.(Fallah et al, 2016) Use of chemotherapy is described as single-agent or multi-agent, without discerning specific drugs, regimens, doses or schedules. OS is the only recorded outcome, whereas cause of death, response to therapy, PFS or related outcomes are not recorded.
Variables and endpoints
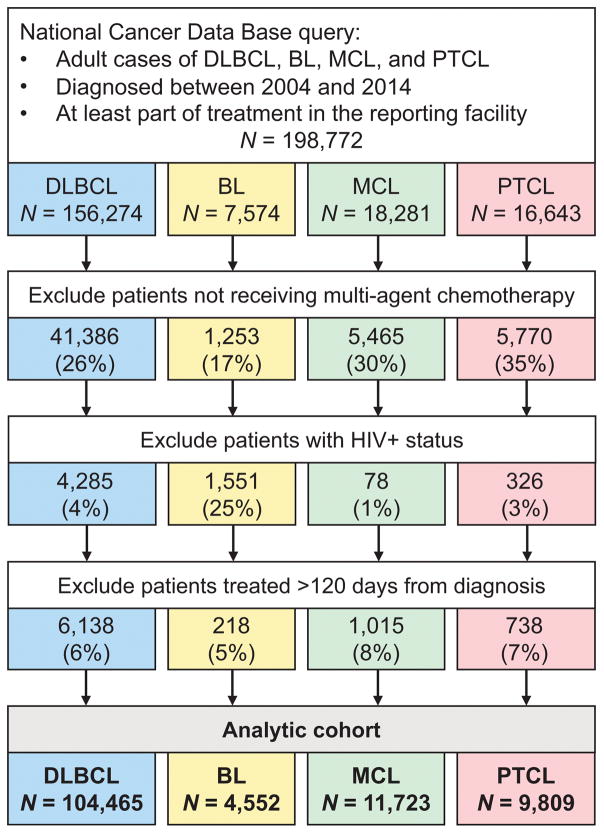
Using the World Health Organization histology codes (Swerdlow et al 2017), we selected adult patients with DLBCL (codes 9680/3, 9684/3), BL (9687/3), MCL (9673/3), and PTCL (including PTCL, not otherwise specified [9702/3], angioimmunoblastic T-cell [9705/3] and anaplastic large cell lymphoma [9714/3]), diagnosed between 2004 and 2014, who did not have an HIV infection, and who started multi-agent chemotherapy within 120 days of diagnosis (Fig. 1). We excluded patients treated entirely outside of the reporting facility, as their treatment modalities or outcomes are not recorded by the NCDB. We categorized the recorded primary site of the lymphoma as nodal, extranodal high-risk, or other extranodal. High-risk extranodal sites were defined by prior studies in DLBCL in the rituximab era, and included central nervous system, lung, liver, pancreas, gastrointestinal tract, and bone marrow.(Castillo et al, 2014; Zhou et al, 2014) OS was calculated as time from the start of chemotherapy to death or last recorded follow up. This approach avoids immortal time bias, and mimics the practice of clinical trials, which measure survival from registration rather than from the date of diagnosis.
Figure 1.
Cohort selection for the study.
BL: Burkitt lymphoma; DLBCL: diffuse large B-cell lymphoma; HIV: human immunodeficiency virus; MCL: mantle cell lymphoma; PTCL: peripheral T-cell lymphoma
Statistical analysis
In this very large dataset, we refrained from reporting any univariate statistical comparisons, as even minuscule differences between groups would result in “statistical significance”. To examine the independent association of time-to-treatment with OS, we used two analyses of survival models. In the first analysis, limited to the subset of patients with recorded IPI (17% for DLBCL, 14% for MCL, 15% for PTCL), we used Cox models stratified by the prognostic index.(The International Non-Hodgkin’s Lymphoma Prognostic Factors Project 1993) Disease-specific scores, such as the MCL IPI (MIPI) or the International PTCL Score, were not available.(Gutierrez-Garcia et al, 2011; Hoster et al, 2008) In case of BL, we used the previously described population-based prognostic index, because of uncertain value of the standard IPI.(Castillo et al, 2013) In the second analysis, we fitted a multivariable Cox model (separately for each type of lymphoma), adjusting for age (using a fractional polynomial to account for non-linearity), sex, race/ethnicity, Ann Arbor stage, presence of B-symptoms, primary site, comorbidity index, median income and, for PTCL, histological subcategory. Such a model, incorporating disease-specific risk factors, as well as sociodemographic indicators of general mortality, has been shown in DLBCL and PTCL to provide a better stratification of OS using cancer registry data than the IPI.(Olszewski et al, 2015; Petrich et al, 2015) Model sensitivity to the proportional hazard assumption was evaluated by plotting smoothed scaled Schoenfeld residuals versus time. All estimates are presented with 95% confidence intervals (95%CI). Statistical analyses were conducted using Stata/MP 15.1 (StataCorp, College Station, TX).
Results
Among the 130,549 patients in the analytic cohort (80.0% DLBCL, 3.5% BL, 9.0% MCL, 7.5% PTCL), median time from diagnosis to start of chemotherapy was 22 days (interquartile range [IQR], 11–36), ranging from 12 days in BL to 27 days in MCL, and this was unchanged between 2004 and 2014. Chemotherapy was started within 7 days of diagnosis in 15.4%, 8–14 days in 17.9%, 15–30 days in 33.5% and more than 30 days from diagnosis in 33.2%. In the largest DLBCL cohort, patients who started treatment early did not differ in age, sex, race/ethnicity, or comorbidities from those who started it later (Table I). In contrast, patients treated early had a higher proportion of unfavourable disease-related factors: advanced-stage disease, B symptoms and high-intermediate or high IPI. The same associations were seen in BL, MCL, and PTCL (Tables S1–S3). Two percent of all patients received radiation before chemotherapy, which was slightly more common in the group starting chemotherapy >30 days from diagnosis. Less than 1% of patients (N=147) had an indicator of receiving therapy on a clinical trial, and the odds of it were significantly higher among patients treated 15 days or more from diagnosis (odds ratio, 1.73; 95%CI, 1.17–2.57).
Table I.
Characteristics of DLBCL patients, stratified by time from diagnosis to start of chemotherapy.
| All patients | Time from diagnosis to chemotherapy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 0–7 days | 8–14 days | 15–30 days | >30 days | |||||||
| N | 104,465 | 15,728 | 18,903 | 35,744 | 34,090 | |||||
| Age, median (IQR); years | 66 | (55–75) | 65 | (53–75) | 65 | (54–75) | 66 | (55–75) | 66 | (56–76) |
| Sex, N (%) | ||||||||||
| Male | 56,395 | (54) | 8,489 | (54) | 10,506 | (56) | 19,307 | (54) | 18,093 | (53) |
| Female | 48,070 | (46) | 7,239 | (46) | 8,397 | (44) | 16,437 | (46) | 15,997 | (47) |
| Race / ethnicity, N (%) | ||||||||||
| White non-Hispanic | 86,350 | (83) | 12,865 | (82) | 15,554 | (82) | 30,245 | (85) | 27,686 | (81) |
| White Hispanic | 6,187 | (6) | 1,041 | (7) | 1,142 | (6) | 1,801 | (5) | 2,203 | (7) |
| Black | 6,857 | (7) | 988 | (6) | 1,271 | (7) | 2,068 | (6) | 2,530 | (7) |
| Asian/Other | 4,182 | (4) | 698 | (4) | 789 | (4) | 1,332 | (4) | 1,363 | (4) |
| Unrecorded | 889 | (1) | 136 | (1) | 147 | (1) | 298 | (1) | 308 | (1) |
| Comorbidity index, N (%) | ||||||||||
| 0 | 79,546 | (76) | 11,352 | (72) | 13,953 | (74) | 27,757 | (78) | 26,484 | (78) |
| 1 | 18,388 | (18) | 3,108 | (20) | 3,518 | (19) | 6,013 | (17) | 5,749 | (17) |
| 2 | 4,686 | (5) | 860 | (6) | 1,021 | (5) | 1,421 | (4) | 1,384 | (4) |
| ≥3 | 1,845 | (2) | 408 | (3) | 411 | (2) | 553 | (2) | 473 | (1) |
| Ann Arbor stage, N (%) | ||||||||||
| 1 | 25,204 | (24) | 2,596 | (17) | 3,558 | (19) | 8,703 | (24) | 10,347 | (30) |
| 2 | 21,653 | (21) | 2,902 | (19) | 3,703 | (20) | 7,567 | (21) | 7,481 | (22) |
| 3 | 20,161 | (19) | 3,029 | (19) | 3,822 | (20) | 7,282 | (20) | 6,028 | (18) |
| 4 | 35,908 | (34) | 6,935 | (44) | 7,530 | (40) | 11,703 | (33) | 9,740 | (29) |
| Unrecorded | 1,539 | (2) | 266 | (2) | 290 | (2) | 489 | (1) | 494 | (1) |
| B symptoms, N (%) | ||||||||||
| Absent | 63,843 | (61) | 8,018 | (51) | 10,433 | (55) | 22,427 | (63) | 22,965 | (67) |
| Present | 29,963 | (29) | 6,202 | (39) | 6,576 | (35) | 9,748 | (27) | 7,437 | (22) |
| Unrecorded | 10,659 | (10) | 1,508 | (10) | 1,894 | (10) | 3,569 | (10) | 3,688 | (11) |
| Primary site, N (%) a | ||||||||||
| Nodal | 71,242 | (68) | 12,028 | (77) | 13,763 | (73) | 24,399 | (68) | 21,052 | (62) |
| Extranodal, high risk | 15,846 | (15) | 2,337 | (15) | 2,924 | (16) | 5,157 | (14) | 5,428 | (16) |
| Extranodal, other | 17,377 | (17) | 1,363 | (9) | 2,216 | (12) | 6,188 | (17) | 7,610 | (22) |
| Radiation first, N (%) | ||||||||||
| No | 102,383 | (98) | 15,534 | (99) | 18,663 | (99) | 35,231 | (99) | 32,955 | (97) |
| Yes | 2,082 | (2) | 194 | (1) | 240 | (1) | 513 | (1) | 1,135 | (3) |
| IPI, N (%) b | ||||||||||
| Low | 5,587 | (31) | 485 | (19) | 740 | (22) | 2,168 | (33) | 2,194 | (39) |
| Low-Intermediate | 4,663 | (26) | 557 | (22) | 808 | (25) | 1,735 | (26) | 1,563 | (27) |
| High-Intermediate | 4,047 | (22) | 636 | (25) | 853 | (26) | 1,466 | (22) | 1,092 | (19) |
| High | 3,973 | (22) | 912 | (35) | 902 | (27) | 1,305 | (20) | 854 | (15) |
High-risk sites included central nervous system, lung, liver, pancreas, gastrointestinal tract, and bone marrow.(Castillo et al, 2014; Olszewski et al, 2015; Zhou et al, 2014)
Missing values omitted.
IPI: International Prognostic Index; IQR: interquartile range
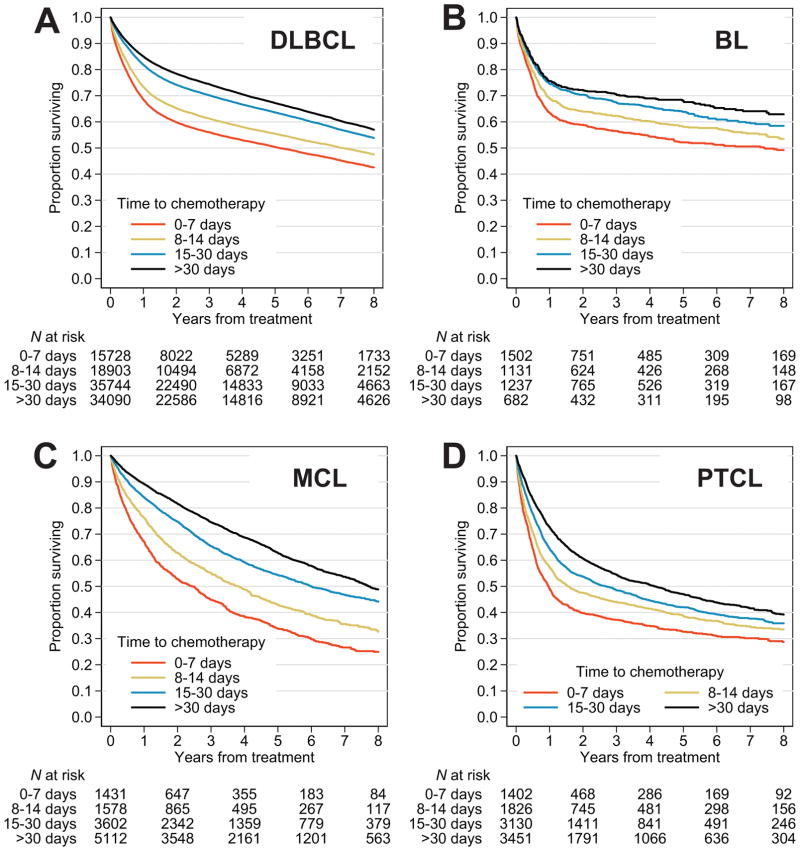
Overall survival was progressively better for patients who started chemotherapy later from diagnosis, across all histologies (Fig. 2). Three-year OS estimates between groups treated within 0–7 or >30 days from diagnosis differed by 18% in DLBCL, 14% in BL, 30% in MCL and 17% in PTCL (Table II). As an illustrative example of potential discrepancies in outcomes, when median OS was calculated for the entire DLBCL cohort, it was 8.7 years (95%CI, 8.6 to 8.9). In contrast, if patients treated within 2 weeks from diagnosis were excluded from the calculation, median OS would be reported as 9.7 years (95%CI, 9.5 to 9.9) (Table S4). Similarly, 3-year OS estimates would be reported as 67.8% (95%CI, 67.5–68.1) and 72.2% (95%CI, 71.9–72.6), respectively. Omitting patients treated within 2 weeks from diagnosis would produce consistently skewed 3-year OS estimates in BL (68.5%, compared with actual 63.0%), MCL (70.7% compared with actual 65.5%), and PTCL (51.7%, compared with actual 48.2%). Shorter survival for patients treated early was also evident when analysis was limited to the subgroup with recorded IPI (Fig. S1), or to the subgroup with high-intermediate or high IPI, except for PTCL, where no difference was observed (Fig. S2).
Figure 2.
Overall survival of patients with aggressive non-Hodgkin lymphomas, stratified by time from diagnosis to chemotherapy: (A) diffuse large B-cell lymphoma (DLBCL), (B) Burkitt lymphoma (BL), (C) mantle cell lymphoma (MCL), and (D) peripheral T-cell lymphoma (PTCL).
Table II.
Survival outcomes of patients with aggressive non-Hodgkin lymphomas, stratified by histology and time from diagnosis to start of chemotherapy.
| Lymphoma, time to treatment | 3-year OS | Models stratified by IPI a | Multivariable models b | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| % | 95% CI | N | HR | 95% CI | N | HR | 95% CI | |
| DLBCL | ||||||||
|
| ||||||||
| 0–7 days | 56.1 | (55.2–56.9) | 2,590 | 1.38 | (1.28–1.48) | 15,728 | 1.66 | (1.61–1.71) |
|
| ||||||||
| 8–14 days | 61.3 | (60.5–62.0) | 3,303 | 1.17 | (1.08–1.25) | 18,903 | 1.44 | (1.40–1.48) |
|
| ||||||||
| 15–30 days | 70.2 | (69.7–70.7) | 6,674 | 1.05 | (0.99–1.12) | 35,744 | 1.13 | (1.10–1.16) |
|
| ||||||||
| >30 days | 74.4 | (73.9–74.9) | 5,703 | Ref. | 34,090 | Ref. | ||
|
| ||||||||
| BL | ||||||||
|
| ||||||||
| 0–7 days | 56.5 | (53.9–59.1) | 1,502 | 1.42 | (1.22–1.66) | 1,502 | 1.51 | (1.29–1.77) |
|
| ||||||||
| 8–14 days | 62.3 | (59.3–65.1) | 1,131 | 1.24 | (1.06–1.46) | 1,131 | 1.29 | (1.09–1.52) |
|
| ||||||||
| 15–30 days | 67.4 | (64.6–70.0) | 1,237 | 1.11 | (0.94–1.30) | 1,237 | 1.15 | (0.97–1.35) |
|
| ||||||||
| >30 days | 70.5 | (66.8–73.8) | 682 | Ref. | 682 | Ref. | ||
|
| ||||||||
| MCL | ||||||||
|
| ||||||||
| 0–7 days | 44.8 | (42.1–47.6) | 177 | 2.23 | (1.79–2.78) | 1,431 | 2.26 | (2.08–2.46) |
|
| ||||||||
| 8–14 days | 55.2 | (52.5–57.7) | 237 | 1.56 | (1.27–1.93) | 1,578 | 1.73 | (1.59–1.88) |
|
| ||||||||
| 15–30 days | 65.4 | (63.7–67.0) | 501 | 1.18 | (0.99–1.41) | 3,602 | 1.27 | (1.18–1.36) |
|
| ||||||||
| >30 days | 74.6 | (73.2–75.8) | 723 | Ref. | 5,112 | Ref. | ||
|
| ||||||||
| PTCL | ||||||||
|
| ||||||||
| 0–7 days | 37.2 | (34.6–39.9) | 216 | 1.46 | (1.18–1.81) | 1,402 | 1.70 | (1.57–1.85) |
|
| ||||||||
| 8–14 days | 44.0 | (41.7–46.4) | 251 | 1.28 | (1.04–1.58) | 1,826 | 1.40 | (1.30–1.52) |
|
| ||||||||
| 15–30 days | 48.5 | (46.7–50.4) | 500 | 1.28 | (1.07–1.52) | 3,130 | 1.24 | (1.16–1.33) |
|
| ||||||||
| >30 days | 54.6 | (52.8–56.3) | 551 | Ref. | 3,451 | Ref. | ||
IPI for DLBCL, MCL, and PTCL population-based prognostic index for Burkitt lymphoma(Castillo et al, 2013)
Model adjusting for age, sex, race/ethnicity, stage, presence of B-symptoms, primary site, comorbidity index, and median income in the county of residence (Olszewski et al, 2015)
BL: Burkitt lymphoma; CI: confidence interval; DLBCL: diffuse large B-cell lymphoma; HR: hazard ratio; IPI: international prognostic index; MCL: mantle cell lymphoma; OS: overall survival; PTCL: peripheral T-cell lymphoma; Ref.: reference level.
Both in models stratified by the IPI, or adjusting for multiple patient- and disease-related characteristics (and fitted separately for each histology), the association between time to chemotherapy and OS was confirmed to be independent of other factors (Table II). Adjusting for the IPI, the hazard of death was higher by 38% in DLBCL, 42% in BL, 46% in PTCL, and more than twice in MCL for patients initiating chemotherapy in the first week from diagnosis compared with the reference group who started it >30 days from diagnosis. Using a test of interaction, we determined that the association between time to chemotherapy and survival was similar for patients younger or older than 65 years (P>0.05 in all lymphoma subtypes). The association was also consistent in a sensitivity analysis including only patients who survived >4 months from first treatment (Table S5).
When time from diagnosis to treatment was studied as a continuous variable, we observed that the non-linear association with hazard ratio for OS was different for DBLCL/BL/PTCL than for MCL (Fig. 3). In DLBCL, BL, and PTCL, the hazard ratio was lowest for patients initiating chemotherapy around day 45–60 from diagnosis, and increased afterwards, possibly reflecting additional unfavourable circumstances leading to treatment delay. In contrast, the risk of death dropped precipitously and then steadily decreased in MCL, suggesting a different clinical course in this lymphoma, which can sometimes be initially observed without therapy.
Figure 3.
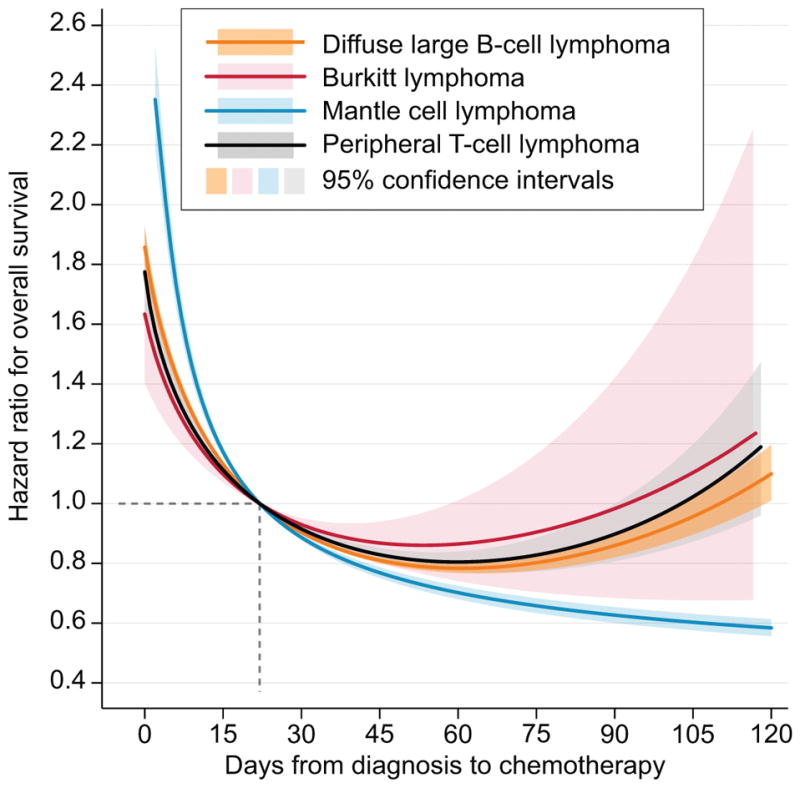
Hazard ratio for death in aggressive non-Hodgkin lymphomas according to time from diagnosis to start of chemotherapy; non-linear relationship was modelled using a fractional polynomial scaled so that patients with time-to-treatment of 22 days had the reference hazard ratio of 1.0; separate models were fitted for each histology; shaded areas indicate corresponding 95% confidence intervals.
Discussion
This retrospective observational study, which included most aggressive lymphomas diagnosed in the US between 2004 and 2014, identified time from diagnosis to chemotherapy as a risk factor independent of other clinical and socio-demographic characteristics, including the IPI. The 15% of patients who started chemotherapy within the first week from diagnosis had more unfavourable disease-related characteristics and significantly worse survival than those whose treatment started later. This phenomenon was consistent in all studied histologies, and particularly pronounced in MCL. Furthermore, OS calculated while excluding recipients of early chemotherapy were consistently too optimistic, even when limited to the subgroup with high-intermediate or high IPI. Patients starting therapy within 2 weeks from diagnosis were also less likely to participate in a clinical trial.
Our findings suggest that time to treatment may serve as a surrogate for lymphoma aggressiveness that remains uncaptured by the IPI, even though the index incorporates performance status, clinical stage and serum lactate dehydrogenase level. The residual high-risk biology may manifest as severe systemic symptoms, obstruction of vital organs, malignant hypercalcaemia or tumour lysis syndrome requiring urgent therapy. Time to treatment is rarely reported in clinical trials or observational studies. It may be a barrier in clinical research, leading to under-representation of high-risk patients and biased survival estimates. Failure to understand how time to treatment correlates with prognosis in aggressive NHL may explain some confusing observations, discrepancies between retrospective and prospective studies, or failure to enrol target high-risk populations. Some pragmatic trials allow registration of patients after one cycle of cytoreductive chemotherapy, but most protocols accept only subjects with untreated lymphoma. As a result, trial participants systematically differ from the population to which their results are then extrapolated. Prior research suggested that this discrepancy was largely due to exclusion of subjects who are older or have comorbidities, but did not analyse how treatment urgency affects enrolment.(Augustin et al, 2017; Cherubini et al, 2013; Stone et al, 1994; Terschuren et al, 2010) Recently, the American Society of Clinical Oncology has advocated for inclusion of patients with central nervous system involvement, well-controlled HIV infection or prior malignancies, as ways to broaden trial enrolment and generalizability.(Kim et al, 2017) In our NCDB data, early treatment initiation was also associated with a significantly lower likelihood of receiving trial therapy. Therefore, in aggressive NHL, accommodating patients who present with high urgency to treat is an additional important opportunity to improve external validity of clinical trials.
The importance of time to treatment in NHL may also reside in its hypothetical correlation with both known and unknown high-risk molecular features. As an example, retrospective observational cohorts have identified up to 12% of DLBCL cases harbouring concurrent MYC and BCL2 and/or BCL6 rearrangements (double- or triple-hit lymphomas)—a distinct, aggressive entity characterized by early dissemination, higher IPI, and significantly worse OS (less than 40% after R-CHOP).(Barrans et al, 2010; Pedersen et al, 2012) The prevalence of double-hit lymphoma appears to be much lower in prospective clinical trials. In the British multi-centre phase 3 trial of 1,080 DLBCL patients, less than 2% of lymphomas had concurrent MYC and BCL2 rearrangements, and their OS was not significantly inferior.(Cunningham et al, 2013) The German High-Grade Non-Hodgkin’s Lymphoma Study Group reported that double/triple-hit lymphomas constituted 4% of the cohort enrolled in the RICOVER-60 trial, and 8% in the R-MegaCHOEP trial.(Staiger et al, 2017) Similarly, researchers from the Groupe d’Etudes des Lymphomes de l’Adulte/Lymphoma Study Association (GELA/LYSA) found a prevalence of 5.6%, which was not independently prognostic for OS.(Copie-Bergman et al, 2015) Similar considerations may arise for ongoing and planned trials attempting to enrol non-GCB, “double-expressor”, or other high-risk DLBCL subcategories.
Systematic under-enrolment of high-risk patients may further pose a problem for interpretation of smaller single-arm trials. Our analysis illustrates how such an exclusion can bias OS estimates. Not only in high-grade DLBCL, but also in BL,(Corazzelli et al, 2012; Dunleavy et al, 2013) MCL,(Ruan et al, 2015; Visco et al, 2013) and even PTCL,(Dupuis et al, 2015; Maeda et al, 2017) many phase 2 trials assert OS or even PFS of 70% or higher. Such trials can strongly influence clinical practice, but clinicians should be mindful of selection factors that produce these very favourable outcomes. Apart from traditional prognostic factors, like the IPI, reporting average time from diagnosis to treatment would provide insight into expected mortality risk of participants. Investigators targeting high-risk subgroups or histologies should minimize undesirable exclusion of patients requiring urgent therapy. Potential measures include allowing registration within a specified timeframe after one initial cycle of chemotherapy, or allowing for a cytoreductive “pre-phase” when necessary. Registration and screening procedures could also be streamlined to minimize repetition of tests or performing evaluations that significantly delay treatment. Enrolling subjects closer to diagnosis will become even more important in trials that involve molecular subtyping, as relevant testing further extends the pre-treatment period. Similarly, trials evaluating adoptive immunotherapy using chimeric antigen receptor-derived T cells encounter the challenge of prolonged period from enrollment to therapeutic intervention, effectively selecting out patients in need of urgent therapy.
We note several limitations of our analysis. The NCDB does not record details of chemotherapy regimens, so we could not examine differences between treatments, their delivery or early complications. However, we could identify patients who received “pre-phase” chemotherapy or palliative steroids. A substantial proportion of patients did not have a record of multi-agent therapy. This may be due to inclusion of patients who were very old or who experienced early fatal complications, but may also suggest some under-ascertainment of therapies in the NCDB. Furthermore, we excluded the 6% of patients who were not treated within 120 days of diagnosis, because these rare outliers either represent diagnostic errors, or had extremely unusual circumstances not identifiable from the data. For the same reason, we grouped all patients treated between 30–120 days as one category. Disease-specific prognostic factors (like Ki67 expression in MCL) and important lymphoma-related endpoints, like the PFS, were unavailable. Only a minority of patients had recorded IPI, although the sheer number of cases provided sufficient power for an IPI-stratified analysis, and the results were consistent in alternative multivariable models. For other missing data, we used the indicator variable method in the models, which is less efficient than multiple imputation approaches. Finally, further research will need to examine whether time from diagnosis to treatment correlates with other known prognostic factors, such as the cell of origin, immunohistochemical or molecular markers.
In conclusion, our study provides a strong rationale for modifying the enrolment criteria in clinical trials of aggressive NHL to accommodate patients requiring urgent therapy, and thus produce less biased results while retaining power to demonstrate intended treatment effect size. Haematologists should consider the 14–30% survival difference between patients starting therapy within 0–7 or >30 days from diagnosis when counselling patients on the basis of survival estimates reported in the literature. Finally, aggressive NHL presenting with severe symptoms that warrant urgent therapy should be examined with regard to their underlying biology. Their unfavourable prognosis might be potentially overcome with specific management strategies, which remain to be investigated.
Supplementary Material
Acknowledgments
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Funding: This work was supported by the American Cancer Society [grant number 128608-RSGI-15-211-01-CPHPS], and the National Institute of General Medical Sciences at National Institutes of Health [grant number U54GM115677].
Footnotes
Authorship contributions
A.J.O., T.O., and J.L.R. designed the research and wrote the paper. A.J.O. analysed data.
Conflict-of-interest disclosure: A.J.O. reports research funding from Genentech and Spectrum Pharmaceuticals; J.L.R. reports consultancy for Teva.
References
- Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR, editors. AJCC cancer staging manual. Springer Science+Business Media; New York, NY: 2016. [Google Scholar]
- Augustin A, Le Gouill S, Gressin R, Bertaut A, Monnereau A, Woronoff AS, Tretarre B, Delafosse P, Troussard X, Moreau A, Hermine O, Maynadie M. Survival benefit of mantle cell lymphoma patients enrolled in clinical trials; a joint study from the LYSA group and French cancer registries. J Cancer Res Clin Oncol. 2017 doi: 10.1007/s00432-00017-02529-00439. [DOI] [PubMed] [Google Scholar]
- Barrans S, Crouch S, Smith A, Turner K, Owen R, Patmore R, Roman E, Jack A. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol. 2010;28:3360–3365. doi: 10.1200/JCO.2009.26.3947. [DOI] [PubMed] [Google Scholar]
- Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, Thoburn K, Gress D, McKellar DP, Shulman LN, Facktor MA, Winchester DP. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. 2017;3:1722–1728. doi: 10.1001/jamaoncol.2016.6905. [DOI] [PubMed] [Google Scholar]
- Castillo JJ, Winer ES, Olszewski AJ. Population-based prognostic factors for survival in patients with Burkitt lymphoma: an analysis from the Surveillance, Epidemiology, and End Results database. Cancer. 2013;119:3672–3679. doi: 10.1002/cncr.28264. [DOI] [PubMed] [Google Scholar]
- Castillo JJ, Winer ES, Olszewski AJ. Sites of extranodal involvement are prognostic in patients with diffuse large B-cell lymphoma in the rituximab era: an analysis of the Surveillance, Epidemiology and End Results database. Am J Hematol. 2014;89:310–314. doi: 10.1002/ajh.23638. [DOI] [PubMed] [Google Scholar]
- Cheah CY, Seymour JF, Wang ML. Mantle Cell Lymphoma. J Clin Oncol. 2016;34:1256–1269. doi: 10.1200/JCO.2015.63.5904. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Pierri F, Gasperini B, Zengarini E, Cerenzia A, Bonifacio E, Falcinelli F, Lattanzio F. Are ongoing trials on hematologic malignancies still excluding older subjects? Haematologica. 2013;98:997–1000. doi: 10.3324/haematol.2013.087601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappella A, Martelli M, Angelucci E, Brusamolino E, Evangelista A, Carella AM, Stelitano C, Rossi G, Balzarotti M, Merli F, Gaidano G, Pavone V, Rigacci L, Zaja F, D’Arco A, Cascavilla N, Russo E, Castellino A, Gotti M, Congiu AG, Cabras MG, Tucci A, Agostinelli C, Ciccone G, Pileri SA, Vitolo U. Rituximab-dose-dense chemotherapy with or without high-dose chemotherapy plus autologous stem-cell transplantation in high-risk diffuse large B-cell lymphoma (DLCL04): final results of a multicentre, open-label, randomised, controlled, phase 3 study. Lancet Oncol. 2017;18:1076–1088. doi: 10.1016/S1470-2045(17)30444-8. [DOI] [PubMed] [Google Scholar]
- Copie-Bergman C, Cuilliere-Dartigues P, Baia M, Briere J, Delarue R, Canioni D, Salles G, Parrens M, Belhadj K, Fabiani B, Recher C, Petrella T, Ketterer N, Peyrade F, Haioun C, Nagel I, Siebert R, Jardin F, Leroy K, Jais JP, Tilly H, Molina TJ, Gaulard P. MYC-IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: a GELA/LYSA study. Blood. 2015;126:2466–2474. doi: 10.1182/blood-2015-05-647602. [DOI] [PubMed] [Google Scholar]
- Corazzelli G, Frigeri F, Russo F, Frairia C, Arcamone M, Esposito G, De Chiara A, Morelli E, Capobianco G, Becchimanzi C, Volzone F, Saggese M, Marcacci G, De Filippi R, Vitolo U, Pinto A. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and ‘unclassifiable’ highly aggressive B-cell lymphoma. Br J Haematol. 2012;156:234–244. doi: 10.1111/j.1365-2141.2011.08947.x. [DOI] [PubMed] [Google Scholar]
- Cortelazzo S, Tarella C, Gianni AM, Ladetto M, Barbui AM, Rossi A, Gritti G, Corradini P, Di Nicola M, Patti C, Mule A, Zanni M, Zoli V, Billio A, Piccin A, Negri G, Castellino C, Di Raimondo F, Ferreri AJ, Benedetti F, La Nasa G, Gini G, Trentin L, Frezzato M, Flenghi L, Falorio S, Chilosi M, Bruna R, Tabanelli V, Pileri S, Masciulli A, Delaini F, Boschini C, Rambaldi A. Randomized Trial Comparing R-CHOP Versus High-Dose Sequential Chemotherapy in High-Risk Patients With Diffuse Large B-Cell Lymphomas. J Clin Oncol. 2016;34:4015–4022. doi: 10.1200/JCO.2016.67.2980. [DOI] [PubMed] [Google Scholar]
- Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, Pocock C, Ardeshna KM, Radford JA, McMillan A, Davies J, Turner D, Kruger A, Johnson P, Gambell J, Linch D. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381:1817–1826. doi: 10.1016/S0140-6736(13)60313-X. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, Widemann B, Staudt LM, Jaffe ES, Little RF, Wilson WH. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. 2013;369:1915–1925. doi: 10.1056/NEJMoa1308392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J, Morschhauser F, Ghesquieres H, Tilly H, Casasnovas O, Thieblemont C, Ribrag V, Bossard C, Le Bras F, Bachy E, Hivert B, Nicolas-Virelizier E, Jardin F, Bastie JN, Amorim S, Lazarovici J, Martin A, Coiffier B. Combination of romidepsin with cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated patients with peripheral T-cell lymphoma: a non-randomised, phase 1b/2 study. Lancet Haematol. 2015;2:e160–165. doi: 10.1016/S2352-3026(15)00023-X. [DOI] [PubMed] [Google Scholar]
- Fallah J, Qunaj L, Olszewski AJ. Therapy and outcomes of primary central nervous system lymphoma in the United States: analysis of the National Cancer Database. Blood Advances. 2016;1:112–121. doi: 10.1182/bloodadvances.2016000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Garcia G, Garcia-Herrera A, Cardesa T, Martinez A, Villamor N, Ghita G, Martinez-Trillos A, Colomo L, Setoain X, Rodriguez S, Gine E, Campo E, Lopez-Guillermo A. Comparison of four prognostic scores in peripheral T-cell lymphoma. Ann Oncol. 2011;22:397–404. doi: 10.1093/annonc/mdq359. [DOI] [PubMed] [Google Scholar]
- Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, Pfreundschuh M, Reiser M, Metzner B, Einsele H, Peter N, Jung W, Wormann B, Ludwig WD, Duhrsen U, Eimermacher H, Wandt H, Hasford J, Hiddemann W, Unterhalt M German Low Grade Lymphoma Study G, European Mantle Cell Lymphoma N. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- Jacobson C, LaCasce A. How I treat Burkitt lymphoma in adults. Blood. 2014;124:2913–2920. doi: 10.1182/blood-2014-06-538504. [DOI] [PubMed] [Google Scholar]
- Kim ES, Bruinooge SS, Roberts S, Ison G, Lin NU, Gore L, Uldrick TS, Lichtman SM, Roach N, Beaver JA, Sridhara R, Hesketh PJ, Denicoff AM, Garrett-Mayer E, Rubin E, Multani P, Prowell TM, Schenkel C, Kozak M, Allen J, Sigal E, Schilsky RL. Broadening Eligibility Criteria to Make Clinical Trials More Representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol. 2017;35:3737–3744. doi: 10.1200/JCO.2017.73.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy T, Damaj G, Soubeyran P, Gyan E, Cartron G, Bouabdallah K, Gressin R, Cornillon J, Banos A, Le Du K, Benchalal M, Moles MP, Le Gouill S, Fleury J, Godmer P, Maisonneuve H, Deconinck E, Houot R, Laribi K, Marolleau JP, Tournilhac O, Branger B, Devillers A, Vuillez JP, Fest T, Colombat P, Costes V, Szablewski V, Bene MC, Delwail V, Group L. R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood. 2018;131:174–181. doi: 10.1182/blood-2017-07-793984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JP, Kolibaba KS, Reeves JA, Tulpule A, Flinn IW, Kolevska T, Robles R, Flowers CR, Collins R, DiBella NJ, Papish SW, Venugopal P, Horodner A, Tabatabai A, Hajdenberg J, Park J, Neuwirth R, Mulligan G, Suryanarayan K, Esseltine DL, de Vos S. Randomized Phase II Study of R-CHOP With or Without Bortezomib in Previously Untreated Patients With Non-Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2017;35:3538–3546. doi: 10.1200/JCO.2017.73.2784. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Nishimori H, Yoshida I, Hiramatsu Y, Uno M, Masaki Y, Sunami K, Masunari T, Nawa Y, Yamane H, Gomyo H, Takahashi T, Yano T, Matsuo K, Ohshima K, Nakamura S, Yoshino T, Tanimoto M. Dose-adjusted EPOCH chemotherapy for untreated peripheral T-cell lymphomas: a multicenter phase II trial of West-JHOG PTCL0707. Haematologica. 2017;102:2097–2103. doi: 10.3324/haematol.2017.167742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer MJ, Link BK, Habermann TM, Thompson CA, Allmer C, Johnston PB, Micallef I, Inwards DJ, Farooq U, Macon WR, Syrbu S, Feldman AL, Slager SL, Weiner GJ, Ansell S, Cerhan JR, Witzig TE, Nowakowski GS. Time from Diagnosis to Initiation of Treatment of DLBCL and Implication for Potential Selection Bias in Clinical Trials. Blood. 2016;128:3034–3034. [Google Scholar]
- Nowakowski GS, Blum KA, Kahl BS, Friedberg JW, Baizer L, Little RF, Maloney DG, Sehn LH, Williams ME, Wilson WH, Leonard JP, Smith SM. Beyond RCHOP: A Blueprint for Diffuse Large B Cell Lymphoma Research. J Natl Cancer Inst. 2016;108:djw257. doi: 10.1093/jnci/djw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski AJ, Winer ES, Castillo JJ. Validation of clinical prognostic indices for diffuse large B-cell lymphoma in the National Cancer Data Base. Cancer Causes Control. 2015;26:1163–1172. doi: 10.1007/s10552-015-0610-8. [DOI] [PubMed] [Google Scholar]
- Pedersen MO, Gang AO, Poulsen TS, Knudsen H, Lauritzen AF, Nielsen SL, Gang UO, Norgaard P. Double-hit BCL2/MYC translocations in a consecutive cohort of patients with large B-cell lymphoma - a single centre’s experience. Eur J Haematol. 2012;89:63–71. doi: 10.1111/j.1600-0609.2012.01787.x. [DOI] [PubMed] [Google Scholar]
- Petrich AM, Helenowski IB, Bryan LJ, Rozell SA, Galamaga R, Nabhan C. Factors predicting survival in peripheral T-cell lymphoma in the USA: a population-based analysis of 8802 patients in the modern era. Br J Haematol. 2015;168:708–718. doi: 10.1111/bjh.13202. [DOI] [PubMed] [Google Scholar]
- Ruan J, Martin P, Shah B, Schuster SJ, Smith SM, Furman RR, Christos P, Rodriguez A, Svoboda J, Lewis J, Katz O, Coleman M, Leonard JP. Lenalidomide plus Rituximab as Initial Treatment for Mantle-Cell Lymphoma. N Engl J Med. 2015;373:1835–1844. doi: 10.1056/NEJMoa1505237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Trumper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, Peter N, Loeffler M, Rosenwald A, Pfreundschuh M. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418–3425. doi: 10.1182/blood-2010-02-270785. [DOI] [PubMed] [Google Scholar]
- Staiger AM, Ziepert M, Horn H, Scott DW, Barth TFE, Bernd HW, Feller AC, Klapper W, Szczepanowski M, Hummel M, Stein H, Lenze D, Hansmann ML, Hartmann S, Moller P, Cogliatti S, Lenz G, Trumper L, Loffler M, Schmitz N, Pfreundschuh M, Rosenwald A, Ott G for the German High-Grade Lymphoma Study Group. Clinical Impact of the Cell-of-Origin Classification and the MYC/ BCL2 Dual Expresser Status in Diffuse Large B-Cell Lymphoma Treated Within Prospective Clinical Trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2017;35:2515–2526. doi: 10.1200/JCO.2016.70.3660. [DOI] [PubMed] [Google Scholar]
- Stone JM, Page FJ, Laidlaw CR, Cooper I. Selection of patients for randomised trials: a study based on the MACOP-B vs CHOP in NHL study. Aust N Z J Med. 1994;24:536–540. doi: 10.1111/j.1445-5994.1994.tb01754.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber D, Hasserjian R, Le Beau M, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. Vol. 2. International Agency for Research on Cancer; Lyon, France: 2017. Revised. [Google Scholar]
- Terschuren C, Gierer S, Brillant C, Paulus U, Loffler M, Hoffmann W. Are patients with Hodgkin lymphoma and high-grade non-Hodgkin lymphoma in clinical therapy optimization protocols representative of these groups of patients in Germany? Ann Oncol. 2010;21:2045–2051. doi: 10.1093/annonc/mdq214. [DOI] [PubMed] [Google Scholar]
- The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- Vaidya R, Witzig TE. Prognostic factors for diffuse large B-cell lymphoma in the R(X)CHOP era. Ann Oncol. 2014;25:2124–2133. doi: 10.1093/annonc/mdu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visco C, Finotto S, Zambello R, Paolini R, Menin A, Zanotti R, Zaja F, Semenzato G, Pizzolo G, D’Amore ES, Rodeghiero F. Combination of rituximab, bendamustine, and cytarabine for patients with mantle-cell non-Hodgkin lymphoma ineligible for intensive regimens or autologous transplantation. J Clin Oncol. 2013;31:1442–1449. doi: 10.1200/JCO.2012.45.9842. [DOI] [PubMed] [Google Scholar]
- Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA, Rodriguez MA, Nademanee A, Kaminski MS, Czuczman MS, Millenson M, Niland J, Gascoyne RD, Connors JM, Friedberg JW, Winter JN. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837–842. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.