Abstract
Pain is a common symptom amongst patients with cancer. Adequate pain assessment and management is critical to improve the quality of life and health outcomes in this population. In this review we provide a framework for safely and effectively managing cancer-related pain by summarizing the evidence for the importance of controlling pain, the barriers to adequate pain management, strategies to assess and manage cancer-related pain, how to manage pain in patients at risk of substance use disorder and considerations when managing pain in a survivorship population.
Keywords: Pain management, cancer, opioid, adjuvant, pain assessment, addiction
INTRODUCTION
A recent review of 40 years of literature revealed that 64% of patients with advanced or metastatic cancer report pain; 59% of patients currently receiving anti-cancer treatment report pain and one-third of patients have pain even after completing curative treatment.1 While in some areas of the world the major barrier to pain control is adequate access to opioids,2 even in areas where opioids are available, pain remains prevalent in patients with cancer and has a significant impact on clinical outcomes. The presence and severity of pain has important clinical implications, for pain as a variable contributing to health-related quality of life (HRQOL) factor provides prognostic information for survival.3,4 In addition, the experience of pain can influence patient outcomes either positively or negatively. Poor communication between providers and patients regarding pain control can decrease patient satisfaction.5 Poor pain control is also associated with more psychological distress and decreased social activities and social support.6 Inversely, increased symptom monitoring and patient self-reporting of pain has been shown to improve HRQOL, decrease unexpected health care utilization, and improve adherence to anti-neoplastic treatment.7 In spite of understanding the influence of pain on clinical outcomes, pain is often under-treated in patients with cancer. Studies examining the frequency and quality of pain management show room for improvement – a systematic review revealed that despite a 25% decrease in under-treatment of cancer pain between 2007 and 2013, approximately one-third of patients living with cancer still have pain that is inadequately treated.8 Although the prevalence of pain varies by malignancy and disease stage,1 studies have shown no significant difference in pain severity between solid and hematologic malignancies,9,10 reflecting that the burden of pain is not limited to specific subsets of patients living with cancer but remains widespread. Consequently, all clinicians caring for patients with cancer must know how to effectively manage pain. Given the prevalence and impact of pain, it is vital to understand the principles of pain management and the barriers that prevent these strategies from being effectively implemented.
BARRIERS TO ADEQUATE PAIN MANAGEMENT
Categories of barriers include: (1) societal attitudes towards pain management, (2) system and regulatory barriers, (3) clinician barriers, (4) patient barriers, and (5) racial and socioeconomic disparities in the assessment and management of pain.
-
Societal changes: While not specific to cancer-related pain, the societal changes that occurred and are subsequently outlined have still impacted patients with both chronic non-malignant pain and those living with cancer-related pain. In the 1980s and early 1990s, literature explored whether doctors were adequately treating pain,11 how patients living with chronic pain viewed their physicians’ treatment of them,12 and if analgesics should be recommended for patients with chronic pain.13 In 1996, there was a paradigm shift when the American Pain Society began referring to pain as the “5th Vital Sign” and physicians were encouraged to measure and aggressively treat pain.14 By 1999, the Veterans Health Administration implemented an initiative focusing on “Pain as the 5th Vital Sign” and began requiring documentation of a numeric pain score in all clinical encounters.15 This shift in viewing pain as an objective sign (rather than a subjective symptom) and asking clinicians to focus on decreasing a patient’s numeric pain score could have been accompanied by an increase in multidisciplinary pain practices. However, poor reimbursement for these clinics under fee-for-service models, an increase in managed care, rising financial pressures in medical centers, and a focus on procedure-based interventions for physicians’ pain management training all encumbered the growth of these valuable practices. The absence of multidisciplinary pain clinics, clinicians’ limited understanding of the difference between acute and chronic pain, and two small studies reporting low addiction risk led clinicians to use opioids more liberally to manage pain regardless of its etiology.16 Since that time, data has shown that 50–70% of patients with chronic non-malignant pain will not respond to opioids, and there is limited data examining the safety or efficacy of using opioids for greater than 4 months in patients with chronic non-malignant pain.17 By 2006, data showed that measuring pain as a vital sign did not change the quality of pain management.15 There was a concomitant recognition that chronic non-malignant pain management should focus on quality of life and functional outcomes rather than solely on the numeric pain score. Realizing that these outcomes may not be achieved through long-term opioid use,18,19 there was a recent pendulum swing back towards less opioid prescribing and in 2016 the American Medical Association removed their recommendation for monitoring pain as a vital sign.20
However, the approach to pain management that started in the 1990s triggered an increase in opioid use and the United States is still witnessing the downstream consequences of the widespread prescribing that started more than 15 years ago. Between 1992 and 2003, the number of Americans abusing controlled prescription drugs doubled from 7.5 million to 15.1 million,21 and from 2000 to 2010 rates of accidental prescription opioid overdose increased almost fourfold.22 The overdose epidemic is complicated and spans prescribed opioids (most of which were illegally obtained from a friend/relative),23 illicit drugs,24 and opioids used in combination with other drugs.25 This opioid epidemic led to regulatory changes – many states now have mandated Prescription Drug Monitoring Programs (PDMPs)26 to prevent “doctor shopping” for opioids and in 2012 the Federal Drug Administration (FDA) began requiring that manufacturers of long-acting opioids fund continuing medical education (CME) for prescribers, including mandating prescriber education in opioid management and substance use assessment.27 Moreover, the FDA recently requested that reformulated Opana Extended Release (ER)® (oxymorphone hydrochloride) be removed from the market due to concerns that the benefits of the drug may no longer outweigh its risks.28 While patients living with cancer are not the primary population afflicted with opioid misuse, the current societal landscape has far-reaching effects on patients with cancer-related pain and exacerbates the barriers to adequate pain management. In addition, oncologists must be mindful of how clinical practice guidelines for managing chronic non-malignant pain differ from those guiding management of cancer-related pain when considering appropriate pain management strategies in the cancer survivorship population, as patients with ongoing pain in the absence of active neoplastic disease may be managed similar to patients with chronic pain in the absence of a history of cancer.
System and Regulatory Barriers: In the wake of the societal changes towards opioid use and the current opioid epidemic, a number of new requirements seek to provide transparency on use of prescription controlled substances via development of PDMPs, increased prior authorization requirements, and increasing focus on clinician education. PDMPs can provide valuable information to clinicians on whether a patient has filled controlled substances in the past, which medications have been filled, and which clinicians have been prescribing for the patient. As of August 2017, all 50 states, the District of Columbia, and Guam have PDMPs, though the PDMP in Missouri is not state-wide.29 Additionally, multiple health insurers have also incorporated more prior authorization requirements for opioids. Earlier this year, Cigna announced changes that will limit the quantity of immediate-release opioids allowed for new prescriptions and require prior authorization for any long-acting opioid not used for the management of sickle cell disease, cancer, or hospice care.30 Interestingly, this follows the same company’s move several months earlier to end requiring prior authorization for opioid addiction treatment.31 CVS health through its pharmacy benefit manager, CVS Caremark, has enacted similar restrictions. While patients with cancer - especially those receiving palliative care or hospice care - are often exempt from more stringent authorization requirements, these requirements nonetheless create a trickle-down effect of increasing paperwork and phone calls to speak to a representative and explain the clinical justification for the prescribed opioid in a patient with cancer. Finally, although there is a movement to teach physicians-in-training how to manage pain, educational initiatives often focus on identifying aberrant drug-seeking behaviors and understanding management of non-malignant pain.32 This is quite different from teaching physicians how to safely and effectively use opioids to manage pain secondary to active malignant disease.
Clinician Barriers: Lack of pain management specialists, reluctance to prescribe opioids, and perceived regulatory constraints are often cited as barriers, but one of the most pervasive barriers is inadequate provider knowledge regarding pain management.33–35 Lack of knowledge is widespread amongst physicians. Residents in training lack confidence in their ability to manage pain, and confidence does not improve as they progress through training.36 This is unsurprising considering that little formal time is devoted to pain management education for physicians throughout their training and career. Pain management has not been part of the common program requirements set forth by the Accreditation Council for Graduate Medical Education,37 and a survey of oncologists in the United States revealed that the median time they devoted to pain management education annually was approximately 1.3 hours.35 Physicians do not always understand common principles of pain management (such as equianalgesic dose titration of opioids), nor do they easily grasp the differences between pseudoaddiction, tolerance, physiologic dependence, and substance use disorder33 (Table 1) which are critical components of understanding opioid use. Interestingly, a national survey of oncologists revealed a potential unawareness of their knowledge gap with discordance between how oncologists view their ability to manage pain compared with how they responded to a sample clinical case.35 Additional physician surveys have shown that providers wish for more guidance on how to assess and properly treat pain with opioids and how to counsel patients about opioid safety.27
Patient Barriers: There are several patient-related barriers to pain management. Patients may be concerned about the meaning of the pain and whether it signifies end-of-life care or disease progression.33 Patients may also be concerned with how their medical team views the presence of pain or the need for an opioid analgesic,26 which may exacerbate either an underlying concern about reporting pain or a pre-existing reluctance to take opioids. There are also financial barriers that can influence access to opioids for many patients.33 Clinicians must consider the cost of medications that are prescribed. Both market and covered prices of opioids have increased by 5–9% per year,43 significantly outpacing inflation. This is an added concern for patients when the total cost of oncologic treatment can be burdensome and increases the risk of personal bankruptcy in patients with cancer.44
Health Care Disparities: Within the United States there are racial and ethnic disparities in the assessment and management of pain with significant variation in access to opioids.45 Additionally, the role of implicit bias among clinicians may further impede minority patients’ ability to obtain adequate pain management.46,47 Minority patients have higher rates of severe pain at the time of cancer diagnosis when compared to whites yet are less likely to receive appropriate pain assessment and management. Providers are more likely to underestimate pain in minorities – two-thirds of Hispanic patients and nearly 75% of African-American patients have a level of pain that is underestimated by their providers.48 African American patients are less likely than Caucasians to be administered or prescribed opioids45,49 and even when opioids are prescribed, many patients still report severe, under-treated pain.48 While these discrepancies remain present across all socioeconomic strata, opioids are generally prescribed more frequently to patients of higher socioeconomic status compared with patients of lower socioeconomic status.50 Even when adequate analgesics are prescribed, there are still community-based barriers to filling the prescription. A study conducted over 15 years ago demonstrated that only 25% of pharmacies in predominately nonwhite neighborhoods carried opioids to treat patients in severe pain, compared with 72% of pharmacies in predominantly white neighborhoods.51 Subsequent studies have not shown progress in eliminating this disparity - in 2005 a study of Michigan pharmacies showed that those in minority zip codes were over 50 times less likely to carry adequate opioids compared with pharmacies in white neighborhoods. While this finding was present regardless of income, the pharmacies in lower income areas were less likely to carry sufficient opioids.52 These “pharmacy deserts”, where there are fewer pharmacies in segregated minority communities53 and the pharmacies that do exist are less likely to carry prescription analgesics,54 worsen access to opioids and hinder adequate pain management. Despite multiple policy initiatives in the past 15 years to attempt to eliminate some of these disparities55 this remains a serious issue nationwide. As a result of the regulatory, legal, educational, and culture barriers limiting appropriate use of opioids to treat pain, it is even more vital that clinicians caring for vulnerable, seriously ill patients understand how to properly assess pain and develop a safe and effective treatment plan.
TABLE 1.
| Concept | Definition |
|---|---|
| Tolerance | Loss of drug effect with chronic dosing; requires more drug for the same analgesic effect. |
| Physiologic Dependence | Development of a withdrawal syndrome when a drug is suddenly discontinued or an antagonist is administered. |
| Pseudoaddiction | Patient exhibits distress and engages in medication-seeking behaviors because pain treatment is inadequate. Behaviors typically resolve when pain is adequately treated. |
| Substance Use Disorder | The Diagnostic and Statistical Manual of Mental Disorders-5 contains a comprehensive list of criteria for mild, moderate, and severe substance use disorder. Selected criteria include taking an opioid in larger amounts than prescribed or for longer than prescribed, a desire to decrease use but unable to do so, craving use, a decrease in social or occupational activities due to opioid use. |
| Equianalgesic dose ratio | Ratio of the dose of two opioids required to produce the same analgesic effect. |
| Total pain | Pain is not just physical but is also mental, psychological, emotional, spiritual – is the basis for the concept of “total pain” as coined by Cicely Saunders in the 1960s. |
PAIN ASSESSMENT
Multiple oncologic groups have endorsed the importance of screening for and treating pain. The National Comprehensive Cancer Network (NCCN) created guidelines on assessing and managing cancer-related pain in the adult oncologic population. A comprehensive pain assessment includes evaluating the intensity of pain, the etiology and pathophysiology of pain, and identifying what the patient identifies as a goal pain score or functional outcome.56 The Quality Oncology Practice Initiative (QOPI) highlights the importance of proper pain management strategies by including documentation of pain assessment as part of their quality metrics.57 In addition, clinicians should always ask about patterns in pain scores (i.e., highest pain score, lowest pain score, average pain score in the past week) and response to analgesic regimen so historical pain over time can be assessed rather than only focusing on the pain present at the time of the evaluation.58 This may be done through verbal history, pain diaries, or both. Most pain that is not related to an oncologic emergency (i.e., spinal cord compression, impending fracture, superior vena cava syndrome, etc.) can be effectively managed in the ambulatory setting.
Consider the meaning of the pain: Pain is a complex multidimensional experience; physical pain is influenced by many factors as reflected in the concept of “total pain”(Table 1).42 A patient’s prior experience with pain, cultural or religious attitudes towards pain, existential suffering, and patient (or family) pre-existing attitudes towards pain and the use of analgesics all influence a clinician’s ability to effectively manage pain. These factors must be understood and explored to build rapport with the patient and address underlying patient-related barriers to adequate pain management.6
Pain Assessment in the Cognitively Intact Patient: There are several ways to evaluate and measure the intensity of pain. A cognitively intact patient may be able to rate pain on the Numerical Rating Scale of 0 (no pain) to 10 (worst pain imaginable). Alternatively, some patients may use a categorical scale or visual analog scale (no pain, mild pain, moderate pain, or severe pain).56,59 Clinicians should find the scale that works for the individual patient and then use the same scale at each encounter so that changes in pain intensity can be consistently tracked over time.
Pain Assessment in Patients Not Cognitively Intact: Patients with diminished cognition may not be able to provide a verbal pain history but can still provide data that will inform and guide a management plan. Clinicians should evaluate patients and look for nonverbal signs of discomfort (i.e., agitation, irritability, restlessness, grimacing, or confusion). Patients who are verbal but have impaired memory may not be able to provide a reliable pain history for how they felt in the past, so providers need to focus on the level of pain reported during the encounter. Patients with severe cognitive impairment may not even exhibit characteristic behavioral responses to pain. In these cases, clinicians should use their judgment to consider whether a cognitively intact patient with similar disease burden would be expected to report pain, and if so the clinician should initiate a symptom treatment plan and monitor for response.60 When analgesics were empirically started and titrated in a study of patients with impaired cognition and agitation, the authors found reduction in agitation after initiation of analgesics, reinforcing that agitation may be one manner in which cognitively impaired patients display pain.61 Clinicians should not assume the absence of pain simply because the patient is unable to provide a classic verbal pain history.
Characteristics of the pain: Clarifying the characteristics of the pain helps determine the etiology and appropriate management. The classic components of the history include provoking factors, alleviating factors, associated symptoms (nausea, vomiting, constipation, etc.), radiation of pain to a different location in the body, the location of pain (and whether it correlates with disease burden on imaging or mirrors the overall clinical trajectory of the patient). The description of the pain can be used to elucidate whether it is somatic (often described as aching, stabbing, or pressure), visceral (a gnawing or cramping pain) or neuropathic (a burning, tingling, shooting pain).56 These differences are important because these pain syndromes have different treatment algorithms.
Response to current analgesic regimen: The patient’s reported response to their current analgesic regimen should further be broken down into the amount of pain relief (i.e., whether each pro re nata [PRN (as needed)] dose causes significant pain relief, moderate pain relief, or no pain relief) and the duration of analgesic effect (i.e., does each PRN dose provide pain relief for 2 hours or 4 hours?).56 A typical PRN dose is calculated at 10–20% of the total 24-hour dose of long-acting opioid (i.e., a patient using 300mg oral morphine per day via extended-release morphine may be prescribed morphine immediate release 30mg PO q1hr prn).56 A brief but directed pain history can differentiate between basal pain and incident or “breakthrough” pain. Breakthrough pain has been defined as “a transient exacerbation of pain that occurs either spontaneously, or in relation to a specific predictable or unpredictable trigger, despite relatively stable and adequately controlled background pain.”62 Breakthrough pain may occur in three scenarios: (1) The basal opioid dose may be insufficient to control total daily pain level, (2) the dose of PRN analgesic is inadequate, or (3) the patient may not be strategically using PRN analgesic prior to activities that precipitate pain. There are specific tools that have been used to assess the prevalence and severity of breakthrough pain in patients with cancer.63 Clinicians may decide whether to incorporate a formal tool for assessment or to develop a set of targeted questions in the history to elucidate presence, frequency, and severity of breakthrough pain in a patient and how it impacts their quality of life and functional status.
Use of Pain Diaries: Consider implementing pain diaries into clinical practice,56 as they provide valuable insight into adherence to analgesic regimen.64 A typical pain diary may document the frequency of medication use, time of day medications are used, any side effects, concomitant symptoms and the impact of pain on functional status. This information can be reviewed with the patient by a Registered Nurse (RN) prior to the physician visit and then used by the physician to guide changes in the analgesic regimen. Pill counts are another informative tool especially if a patient’s cognitive status precludes their ability to provide a history. Incorporating pain diaries and pill counts into routine clinical practice can reveal important information about all patients, but is particularly important when patients and caregivers may be too overwhelmed to remember several weeks of a pain history during the physician visit. Pain diaries can improve patient empowerment and involvement in pain management65 while providing the clinician with insight into the triggers for opioid use (i.e., whether the opioid is being used for physical pain or emotional pain).
PAIN MANAGEMENT
After a comprehensive pain assessment is completed, a multimodal management plan can be implemented. One of the first steps in managing pain is setting appropriate expectations for patients. The etiology of pain influences the expected outcome and improvement in intensity of pain and functional status. For example, pain from local tumor burden or an acute fracture may be expected to improve in a predictable manner as the disease is treated, while chronic neuropathy has a very different trajectory over time. Setting appropriate expectations is linked to better patient satisfaction5 and treatment adherence.
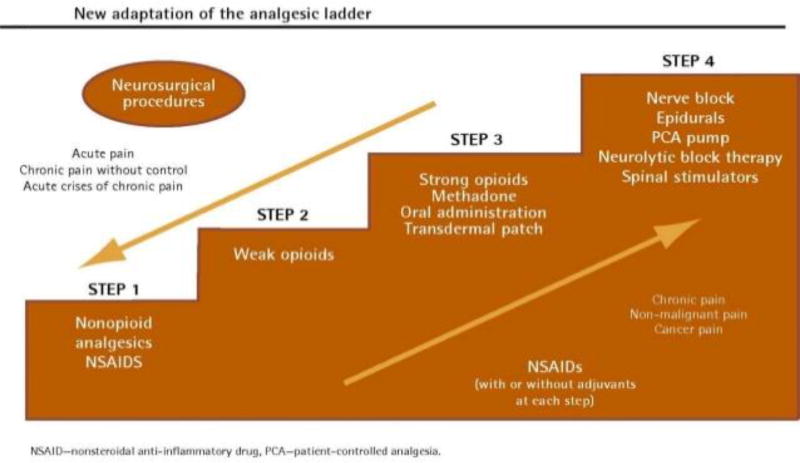
A framework for managing pain often starts with the World Health Organization (WHO) Analgesic Ladder. The WHO ladder (Figure 1) consists of a stepwise approach where the choice of analgesic is determined by the severity of pain; as the level of pain increases so does the strength of recommended analgesic. “Step 1” on the WHO ladder consists of using over-the-counter analgesics to manage pain. “Step 2” escalates to using medications traditionally considered “weak” opioids (e.g. codeine), and “Step 3” advocates for use of stronger opioids. A final “Step 4” reminds clinicians to consider use of interventions for non-pharmacologic management options for pain.66 The WHO ladder was originally developed to guide clinicians through a systemic approach pain management. While it has been found to be effective in treating cancer pain in a majority of patients, there is ongoing debate about whether these guidelines remain the optimal way of treating pain in all patients.67 Newer evidence shows that patients with moderate pain secondary to cancer are more likely to respond to low-dose morphine than they are to codeine, calling into question whether it is necessary to try “weak” Step 2 opioids prior to initiating morphine for control of moderate pain especially considering there were no differences in adverse effects between the two groups.68 While not included on the WHO ladder, adjuvant analgesics, integrative therapies, and interventions can and should be considered at any step in pain management. Finally, recent evidence suggests that interventions may be more beneficial when offered earlier in the disease trajectory rather than reserving these for when pain is considered refractory to standard pharmacologic management.69,70
Figure 1.
Modified World Health Organization Analgesic Ladder. NSAIDs indicates nonsteroidal anti-inflammatory drugs; PCA, patient-controlled analgesia. Reprinted with permission from the author from: Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician. 201055:514-517.66
There are a number of acceptable treatment options that can be offered to patients. These include over-the-counter analgesics, non-opioid prescription medications, interventions, complementary therapies, and systemic opioids.
PAIN MANAGEMENT: NON-OPIOID
Although opioids are the mainstay of moderate-to-severe cancer-related pain there are several non-opioid treatment modalities available to patients. These include both pharmacologic and non-pharmacologic strategies.
Acetaminophen: Acetaminophen can be used as a first-line treatment in patients with mild cancer pain who may not require an opioid or may be hesitant to use an opioid. Peak plasma concentrations occur in approximately 30–60 minutes and daily dose limits depend on age and underlying hepatic function.67 Acetaminophen can be used in combination with opioids; some prescription formulations contain acetaminophen plus an opioid in the same pill for ease of administration. However, a systematic review of the evidence for acetaminophen plus an opioid found no benefit to the addition of acetaminophen in four out of five studies. Of note, the study that found a benefit to acetaminophen used a daily dose of 5 grams, higher than the recommended daily dose and followed patients for only 4 days.71 Consequently, while patients may start with use of acetaminophen for mild pain, clinicians should consider promptly changing the regimen to an opioid for more optimal pain control if adequate analgesia is not achieved with acetaminophen alone. In addition, use of acetaminophen in the oncologic population is limited by hepatotoxicity, particularly in patients with liver disease as well as the need for close monitoring for fevers in patients with neutropenia.
Nonsteroidal Anti-inflammatory Drugs (NSAIDs): NSAIDS have anti-inflammatory, analgesic, and antipyretic properties. This class of medications has a maximum daily dose and multiple safety considerations (e.g., bleeding, pre-existing renal impairment, risk of precipitating renal impairment in patients with multiple myeloma, increased risk of hypertension). NSAIDs may be used alone or in combination with an opioid. There is conflicting evidence on the benefits of adding an NSAID to an opioid, with some studies showing a benefit to the combination72 while other studies have shown minimal to no difference when comparing use of an NSAID plus opioid to either class of drug being used alone.73,74
-
Adjuvant Medications: Adjuvants are medications that are used for the management of nociceptive pain and may have an additive effect when used in combination with opioids or may be used as single-agent treatment for managing neuropathic pain. Neuropathic pain affects approximately 20–40% of patients with cancer75 and more often causes sensory rather than motor damage.76 While the prevalence of chemotherapy-induced peripheral neuropathy (CIPN) varies by drug, a systematic review reported that approximately 68% of patients had CIPN within 30 days of any chemotherapy and by 6 months 30% of patients were still affected by CIPN.77 Importantly, there are no agents recommended to prevent the development of CIPN,78 thus understanding how to treat it is critical given its prevalence and chronicity in cancer patients and cancer survivors. Clinicians should understand how to maximize non-opioids and minimize long-term opioid use in patients who may live for decades with a chronic pain syndrome in the absence of anti-neoplastic disease. Two common classes of adjuvant medications include antidepressants and anticonvulsants.
-
Antidepressants: The pathophysiology of neuropathy is complex and involves receptors for norepinephrine, serotonin, opioids, and N-Methyl-D-aspartic acid (NMDA). As a result, some antidepressants with activity at these receptors can be effective in treating neuropathic pain.79
Duloxetine: Duloxetine has been shown to be superior to placebo in treating CIPN. One study showed that 59% of patients treated with duloxetine reported “any decrease” in pain compared with 38% of patients treated with placebo; the relative risk of a 30% reduction in pain was 1.96 compared to placebo. In addition, the authors found that patients with oxaliplatin-related neuropathy had more benefit than patients with taxane-related neuropathy. Secondary outcomes (decrease in pain interfering with daily function, decrease in numbness/tingling and improvement in pain-related quality of life) were better for patients treated with duloxetine.80 While it can be difficult to decrease the numerical pain score when treating neuropathy, the improvement in secondary outcomes may be clinically significant in improving quality of life for patients.
Venlafaxine: Venlafaxine has been shown to be superior to placebo in treating CIPN secondary to taxane agents and oxaliplatin. The study showed more than 75% relief of symptoms; the most striking responses were for patients who had received a taxane and reported “burning-tingling-stabbing” and patients who received oxaliplatin and reported “pain triggered by cold.”81
Tricyclic Antidepressants (TCAs): Amitriptyline is the most commonly studied TCA. A Cochrane review studying amitriptyline’s efficacy for neuropathy estimated that it may be more effective than placebo in treating neuropathic pain but may not necessarily treat neuropathic pain that is caused by anti-neoplastic treatments.82 However, the ASCO guidelines note that in light of limited treatment options, TCAs may be considered in some patients after an individualized discussion of the risks and benefits of the medication when weighed against the severity of symptom burden.78 In addition, TCAs may be helpful in patients who have multiple concomitant causes of neuropathy (for example, a patient with neuropathy from diabetes, multiple myeloma, and chemotherapy). While amitriptyline is commonly cited in studies, in clinical practice other TCAs with fewer anticholinergic side effects are often used.83
-
Anticonvulsants:
Gabapentin: The efficacy of gabapentin has been shown in a variety of non-malignant neuropathic pain states.84 Studies evaluating its effectiveness in treating CIPN show poor to no effect.85,86 In spite of this, ASCO notes that it is “reasonable” to try it in certain populations as there are limited treatment options available.76 In addition, some insurers still require documentation of a trial of gabapentin prior to approving coverage for pregabalin.
Pregabalin has been shown to be superior to gabapentin and amitriptyline in managing neuropathic cancer pain. In a randomized, double blind, placebo-controlled study, patients who were treated with pregabalin had less pain, needed less PRN morphine, and had improved functional status when compared with patients who were treated with gabapentin or amitriptyline.85
-
Radiation therapy: Radiation therapy can be an integral component of cancer pain management. Because malignancy-specific indications are part of the oncologic care plan and coordinated jointly between the medical and radiation oncologists, the details of radiation therapy indications for each specific malignancy are not discussed in detail here. Across all cancer types, approximately 50% of radiation therapy is considered to be palliative rather than curative in nature. Treatment duration is determined after considering multiple clinical factors; though there is some observed variability based on geographic region, income level, and race that parallels disparities seen in other areas of health care. Another worthwhile consideration is that studies have shown that around 20–25% of patients die within 2 weeks of completing radiation, and nearly 20% of patients who received radiation in the last 30 days of life spent more than 10 of those days receiving radiation treatment.87 Considering the time delay between delivery of radiation and improvement in symptom burden, the decision about whether to proceed with a palliative course of radiation should include a thoughtful discussion of prognosis and treatment goals.
-
Interventions: Interventions may be considered at any point in the disease trajectory, but may be especially helpful when patients have inadequate pain control with systemic analgesics, intolerable side effects, or if additional barriers towards opioids preclude adequate use. Two main categories of interventions are epidural or intrathecal administration of analgesics and nerve blocks.
Epidural/Intrathecal Analgesics: These procedures allow different classes of drugs to be administered simultaneously and permit the use of drugs that are not available in oral formulations.88 Deciding on use of these interventions requires an estimation of prognosis and assessment of risks (contraindications to spinal procedures in patients with risk of bleeding, infection, or local tumor burden) compared with the potential benefits in patients whose pain is inadequately managed. Much of the data on outcomes is based on uncontrolled studies and thus more studies are needed to evaluate the efficacy and safety of spinal opioids.89
Nerve blocks: While historically nerve blocks are Step 4 on the WHO analgesic ladder, more recent evidence shows that interventions may be more effective when considered earlier in the disease course. A randomized controlled trial of early versus later neurolytic sympathectomy for pain from an abdominal or pelvic cancer showed that patients who received the intervention earlier used less oral analgesics and reported improved pain control and QOL.70 Therefore, nerve blocks can be considered earlier in management, if appropriate.
-
Integrative therapies: While some integrative therapies may not be the first-line treatment of cancer-related pain, patients may be interested in non-pharmacologic management strategies either in addition to or in lieu of pharmacologic therapy. A full listing is not included here as options may vary across medical centers, but in brief:
Acupuncture: It is estimated that up to 31% of cancer patients use acupuncture.90 The results are often conflicting as the literature includes multiple types of pain (chronic pain, neuropathic pain, post-thoracotomy pain, post-operative pain, etc.)90 and often have high risk of bias.91 One acupuncture pilot study offered 10 treatments to patients and found that numerical pain scores decreased from 6 (pre-intervention) to 3.8 (post-intervention) and there was a decrease in pain medication prescribing.92 Other reviews have shown no significant difference between acupuncture and conventional care vs. conventional care alone or between real acupuncture vs. sham acupuncture.93 Given this conflicting data, clinicians should discuss potential risks and benefits with each patient on an individualized basis.
Mindfulness: Mindfulness has been described as a practice of “cognitive control, emotional reappraisal or reduced judgment, and existential insight.”94 While some early work focused on the use of mindfulness in managing chronic non-malignant pain, its use has increasingly been studied in treating pain and non-pain symptoms in patients with cancer. Notably, considering the number and complexity of factors that influence a patient’s overall well-being and experience of pain, mindfulness could be an important tool for patients’ pain management in addition to pharmacologic options. Mindfulness also allows the patient to have some control over when and where they engage in this practice. There is emerging evidence that mindfulness can positively influence biomarkers that are associated with stress.95
Cannabis or “Medical Marijuana”: To date, 29 states in the United States, the District of Columbia, Guam, and Puerto Rico allow for a medical marijuana program.96 When discussing the use of cannabis for medicinal purposes with patients, it is important to separate the broader movement to decriminalize the recreational use of marijuana from the evidence regarding its efficacy for medicinal purposes in patients living with a serious illness. Clinicians should differentiate between plant-based phytocannabinoids and synthetic cannabis products, as the former are thought to contain multiple substances that create a synergistic entourage effect which may not be replicated in synthetic products.97 The studies evaluating the use of cannabis in treating symptoms also often include a mix of cancer and non-cancer symptoms, and many include formulations that are not available in the United States. One consistency across studies is that there is often some form of methodological flaw, including design quality and/or risk of bias. Most studies can clearly document the adverse effects; the increased risk of dizziness, nausea, fatigue, somnolence, disorientation, drowsiness, and confusion98 may be particularly important when considering the frailty, baseline symptom burden, and complicated co-morbidities of many patients receiving anti-neoplastic treatment. One study of uncontrolled cancer-related pain comparing THC with CBD (THC:CBD), THC alone, and placebo showed that THC:CBD significantly improved pain compared with placebo.99 A review published in 2017 specifically evaluated cannabinoid use in treating cancer-related pain; only 8 studies of “low to moderate quality” (which were conducted from the 1970s through 2014) were able to be included. These studies compared cannabinoids to placebo or codeine and found it was “not possible to demonstrate a clear therapeutic benefit” to using cannabinoids and that therapeutic effects were limited by adverse effects.100 In conclusion, there is a paucity of high-quality evidence on using cannabinoids to treat cancer-related pain and clinicians should thoroughly discuss the side effect profile and current lack of evidence when discussing marijuana for management of cancer-related pain.
PAIN MANAGEMENT: OPIOID
In order for clinicians to safely and effectively manage cancer-related pain with opioids it is important they understand basic opioid pharmacology, are able to titrate an immediate release or long-acting opioid, and can anticipate and treat expected side effects of opioid therapy. Sample case scenarios can be found in Table 2.
TABLE 2.
Clinical Case Examples104
| Clinical Scenario | Problem | Next Step |
|---|---|---|
| Patient is prescribed morphine 15mg PO q4hr PRN and uses 6 doses per day. Each dose reduces pain by 60% and causes no side effect; patient wakes every night to take PRN opioid. | Needs a long-acting opioid | This patient is using a total of 90mg/day of oral morphine. Extended-release morphine is available in 15mg, 30mg, 60mg, 100mg, and 200mg tablets. A clinician could start morphine extended release 45mg PO q12hr (replaces 100% of current daily use but requires prescribing both a 30mg tablet and a 15mg tablet); alternatively, a clinician could start morphine extended release 30mg PO q12hr (replaces 2/3 of current daily use but lower pill burden per day) and then reassess again in 1–2 weeks. |
| Patient is prescribed oxycodone 5mg PO q6hr PRN and uses all 4 allotted doses per day. Patient reports that each PRN dose reduces pain by 70% but pain relief lasts only 3 hours. By the time the patient is able to take the next PRN dose, the pain has escalated to an intolerable level. Patient has normal renal/hepatic function. | End-dose failure due to inappropriate dosing interval | A patient with normal renal and liver function should have a dosing interval of q4hr for an immediate release opioid. The proper regimen for this patient would be oxycodone 5mg PO q4hr, not q6hr. This patient may need addition of a long-acting opioid, but it would be appropriate to first properly prescribe the PRN regimen and then use the total daily opioid use to calculate an effective dose of an extended-release opioid. |
| Patient is prescribed morphine extended release 200mg PO q12hr and morphine immediate release 7.5mg PO q4hr PRN. Patient takes 3 PRN doses per day but reports that each PRN reduces pain from a 9/10 to an 8/10. | Inadequate dose of PRN opioid | A typical PRN opioid dose is calculated as approximately 10%-20% of the total daily opioid requirement. Thus, a patient using morphine 200mg PO q12hr (or 400mg/day) should have morphine immediate release PRN of approximately 40mg. Since morphine immediate release does not come in a 40mg tablet, it would be appropriate to start with one tablet of morphine immediate release 30mg and assess response. |
Abbreviations: PO, orally; PRN, pro re nata (as needed); q12h, every 12 hours; q4h, every 4 hours; q6h, every 6 hours.
See Goldberg & Smith 2013.102
Opioid pharmacology: The foundation of appropriate opioid management is an understanding of basic opioid pharmacology. There are three primary opioid receptors in the body – the mu, kappa, and delta receptors;101,102 genetic variation in receptors is one factor contributing to the varying response to opioids within or between individuals.103 With the exception of transmucosal immediate release fentanyl, a typical oral immediate release opioid will provide peak analgesic effect within 60–90 minutes and, in a patient with normal renal and hepatic function, should provide pain relief for approximately 4 hours.104 Helping patients understand how much pain relief they should expect with each opioid dose and preparing them for the time to peak analgesic effect can set appropriate expectations for outcomes in pain management and also teach patients to strategically use pain medication during times when it is needed most. For example, if a patient needs to participate in physical therapy or knows that a certain activity worsens pain, it is prudent to instruct the patient to use the immediate-release opioid approximately one hour before they engage in that activity so that they have maximal chance of adequate pain control at that time.
Choice of opioid in the opioid-naïve patient: In patients whose pain is not adequately controlled by over-the-counter analgesics, a clinician can either use a Step 2 opioid or may start with a Step 3 opioid (i.e., morphine, hydromorphone, or oxycodone), as per the WHO analgesic ladder. Oral immediate-release morphine, when used at appropriate starting doses in an opioid-naïve patient, may provide better relief of cancer-related pain than traditional Step 2 opioids.72 There is no significant difference between morphine, oxycodone, and hydromorphone as the first choice of opioid in moderate to severe cancer pain.72 Practical considerations such as cost, access to opioids at pharmacies, adequate insurance coverage and route of administration should be considered when choosing an opioid.
Initial Opioid Dose: Clinicians must consider the patient’s prior exposure to opioids, current medications that may interact with or augment the effect of an opioid, and end-organ function when determining the starting dose of an immediate-release opioid. Morphine is often a first-line opioid due to cost and ease of administration as it is available in multiple formulations. The initial opioid doses for opioid naive individuals are 5 mg every 4 hours as needed for morphine immediate release and oxycodone 2.5–5 mg every 4 hours as needed.105,106 A clinician may choose to err more conservatively based on the individual patient and the overall clinical picture. For example, if a patient is more concerned about potential side effects with opioid initiation and pain is not severe, a clinician may choose to start with oral oxycodone immediate release 2.5mg instead of 5mg. Of utmost importance is the prompt reassessment of the dose within 24 hours so that if it is ineffective it can be adjusted as soon as possible.
Opioid Titration: If a patient has poor pain control despite adequate access to an opioid, clinicians should obtain a focused pain history to determine the underlying issue. There are two primary ways that an immediate-release opioid can be ineffective in controlling pain. First, the dose may be inadequate. If a single dose of immediate-release opioid provides no pain relief and causes no side effects, the dose may be increased by 100%. If the dose provides moderate pain relief the dose may be increased by 50%.104 The second reason pain may be uncontrolled despite access to PRN opioid is if the dosing interval is too long and the patient has breakthrough pain from end-of-dose failure.107 The duration of action of an immediate-release opioid is typically 4 hours and this varies from 2–4 hours depending on the rate of metabolization. However, a common error in prescribing is choosing too long of a dosing interval, typically every 6 hours. When using a non-combined immediate release opioid (i.e., an opioid that is not combined with acetaminophen), a dosing interval of 6 hours is only appropriate if the patient has compromised renal or hepatic function. If a patient reports that an immediate release opioid relieves pain completely but the pain relief lasts only 2 hours, the appropriate next step is to liberalize the PRN frequency for 1–2 days to determine total daily opioid requirements before adding a long-acting opioid to the regimen. This will generally be more effective than increasing the PRN dose since the issue the patient is experiencing in this case is not inadequate pain relief with each PRN, but rather is inadequate duration of relief with each PRN. Of note, for safety reasons a patient should always reach steady state on the current opioid dose before an additional titration is completed. It takes 4–5 half-lives for a medication to reach steady state,108 thus an immediate-release reaches steady state in approximately 24 hours while an extended-release opioid takes about 2–3 days to reach steady state. The time to steady state for methadone is at least 5 days. Long-acting opioids should never be adjusted faster than the time to reach steady state.
Opioid use in end-organ dysfunction: Morphine and codeine have active metabolites that are renally cleared; the use of these opioids is contraindicated in patients with renal insufficiency or failure.109 Hydromorphone is often substituted as a first-line opioid in patients with compromised renal function, though evidence suggests that it too has active metabolites that accumulate in renal failure.110 Overall, clinicians should use lower doses of PRN opioids and use longer dosing intervals than usual (every 6–8 hours) in the presence of end-organ dysfunction. On the contrary, methadone has no active metabolites and undergoes fecal elimination thus its use is considered safe in patients with renal failure.109,111 However, the initiation and titration of methadone in managing cancer-related pain is usually deferred to a specialist given its complicated pharmacology.
Addition of an extended-release opioid: An extended-release opioid should be considered for any patient who has inadequate pain control with PRN use or who requires four or more PRNs per day regularly to control pain and maintain functional status. The dose of oral long-acting opioid is determined by calculating the total dose of PRN opioid used in a 24-hour period, dividing by two and approximating a new dose to administer in form of an extended-release opioid (e.g., morphine immediate release 15mg, used 5 times a day for a total of 75mg could be converted to long-acting morphine 30mg PO q12hr). If a long-acting opioid is not controlling pain when used as prescribed, a patient may use additional PRN doses during the day and then the clinician can adjust the long-acting opioid to desired analgesic effect. Clinicians may still need to counsel patients on strategic use of PRN opioid prior to painful activities in order to prevent pain from escalating to uncontrollable level.104
-
Opioid-related adverse effects: Side effects from opioids can be divided into two broad categories: (1) those that are normal, expected, and can be prevented or treated and (2) those that are not expected and may warrant a change in the opioid regimen. Mild nausea, constipation and mild dose-initiation sleepiness are common and expected. Setting appropriate expectations with patients at the time of the first opioid prescription can prevent patients from becoming frustrated with initial side effects and self-discontinuing the medications.
Nausea/vomiting: Opioid-induced nausea may affect up to 40% of patients with cancer.72 Clinicians may use anti-emetics if needed but these rarely need to be given on a scheduled basis. Patients should be tolerant to the nausea within a week.104
Constipation: Opioid-induced constipation has recently been defined as “a change when initiating opioid therapy from baseline bowel habits (over 7 days) that is characterized by any of the following: reduced bowel movement frequency, development or worsening of straining to pass bowel movements, a sense of incomplete rectal evacuation, or harder stool consistency.”112 Patients need to be started on a stimulant laxative when they are started on opioids. There is no strong evidence to support one agent over the other – the important step is to start a laxative concomitantly with the initiation of the opioid to prevent constipation from occurring.72 Docusate is a stool softener, and there is no benefit to using docusate in addition to senna compared with senna alone when preventing opioid-induced constipation.113
Sedation: It is important to differentiate between the slight drowsiness that is common and transient in first few days of new opioid regimen versus persistent sedation that warrants dose-reduction.72 Sedation occurs before respiratory depression and is a warning sign that the dose of opioid needs to be reduced to prevent harm. Clinicians should incorporate screening for opioid-related adverse effects, including sedation, at each encounter for patients who are on opioids and before any adjustment in an opioid regimen.
Neurologic effects: Agitation or delirium is more likely to be caused by undertreated pain61 rather than from an opioid itself. While the prevalence is not reliably reported, opioids can in rare cases cause neuroexcitation72 or urinary retention;114 these are not expected side effects and patients experiencing these may need an opioid rotation. Clinicians should recognize that these syndromes may occur and these patients should be promptly referred to a specialist for additional evaluation of the opioid regimen and alternative symptom management options.
Opioid rotation: Many clinicians receive little formal education on when or how to safely rotate opioid, including calculation of equianalgesic doses.115 Because many side effects that commonly occur with opioid initiation or dose adjustment are time-limited, an opioid rotation should not be the first-line option if a patient experiences mild side effects upon starting a new opioid. While in some cases opioid rotation is needed to improve pain control and reduce adverse effects, a recent review found Grade D evidence for opioid rotation, highlighting too many uncontrolled confounders that limit the ability to support a stronger recommendation.116 When inadequate access to opioid, intolerable side effects, poor pain control despite appropriate dose increases, unsafe drug-drug interactions, need for change in route of administration (i.e., oral to transdermal), or changes in end-organ function preclude continuing a patient on their current opioid regimen the clinician should consider an opioid rotation. When rotating a patient to a new opioid, the clinician must calculate the equianalgesic dose of the new opioid and then decrease that dose by 25–50% to account for incomplete cross-tolerance; this is recommended as a safety mechanism117 due to an individual’s variability in opioid receptors.
SCREENING FOR SUBSTANCE USE DISORDER
In light of the nationwide opioid epidemic and the potential for chronic opioid use, it is important to understand how substance use disorders can influence management of pain. Untreated substance abuse complicates pain management and limits a clinician’s ability to adequately control pain and other symptoms. In addition, an active or prior substance use disorder may influence the willingness and ability of caregivers to adequately assist with pain management in the ambulatory setting.118 Risk factors for substance use disorder include:56
Personal history of substance use disorder (alcohol, illicit drugs, or prescription drugs)
Family history of substance use disorder
Personal history of psychiatric disease (anxiety, depression, attention deficit hyperactivity disorder)
Personal history of preadolescent sexual abuse
There are a number of available tools (Table 3) that can be used to screen patients for their risk of substance use disorder. Screening tools are not intended to preclude patients from receiving treatment with opioids; the intent is to identify patients at highest risk for misuse and tailor the pharmacologic and non-pharmacologic options as needed to lower the risk of misuse and provide increased safety mechanisms and monitoring.38 While all patients should be regularly monitored for safe and appropriate opioid use, those at higher risk of opioid misuse should have more intensive monitoring as it has been showed to lower risk of misuse and medication diversion.38 Consensus guidelines also recommend the use of opioid treatment agreements. While more commonly used by pain specialists, a simple agreement may facilitate a conversation between the patient and prescribing clinician regarding expectations of both the patient (i.e., call if pain is not controlled by prescribed regimen, in-person office visit for re-evaluation prior to a refill) and the provider (i.e., believe the patient’s report of pain, offer multiple appropriate treatment modalities). Some agreements stipulate conditions under which a patient may be asked to see an additional provider, including a psychiatrist, psychologist, or addiction specialist if it is determined that these resources are necessary for safe prescribing of opioids. The purpose of the agreement is to outline expectations at the start of the prescribing relationship and facilitate an open dialogue about opioid use and safe prescribing. When incorporated into routine clinical practice they should not be perceived as punitive or used to “single out” patients presumed to be at higher risk of opioid misuse.104 Clinicians should decide what method of screening and monitoring is best suited to their individual clinical practice and patient population.
TABLE 3.
Validated Assessment Tools to Screen and Monitor for Aberrant Drug-Related Behaviors38
| Assessment Tool |
Purpose and Administration |
Validation | Concordance Index/Sensitivity and Specificity |
|---|---|---|---|
|
| |||
| Screener and Opioid Assessment Measure for Patients with Pain – Revised (SOAPP-R) | Assess the risk of opioid abuse in patients with chronic pain | Adult patients, nonmalignant pain | ≥ 17 / sensitivity: 0.83; specificity: 0.65 |
| Self-report rating scalesa | Strong predictive validity, reliability, and internal consistency | ≥ 18 / sensitivity: 0.81; specificity: 0.68 | |
| ≥ 19 / sensitivity: 0.77; specificity: 0.75 | |||
|
| |||
| Opioid Risk Tool (ORT) | Assess risk of aberrant behaviors when introduced to opioid therapy | Adult patients, nonmalignant pain | Concordance index considers sensitivity and specificity For men, c=0.82 For women, c=0.85 |
| Self-report checklista | |||
|
| |||
| Pain Medication Questionnaire (PMQ) | Assess the risk of opioid misuse | Adult patients, nonmalignant pain | ≥ 25 / Sensitivity: 0.36; specificity: 0.78 |
| Intended for use at start and throughout opioid therapy | Evidence of construct and concurrent validity | ≥ 30 / Sensitivity: 0.92; specificity: 0.80 | |
| Self-report rating scalea,b | Acceptable reliability, strong test-retest reliability | ||
|
| |||
| Prescription Drug Use Questionnaire (PDUQ) | Identify opioid abuse/dependence in patients with chronic pain | Adult patients, nonmalignant pain | ≥ 20 / Sensitivity: 0.67; specificity: 0.60 |
| Moderate reliability | |||
| Interview format and self-report questionnaire availablea,b | Strong concurrent and predictive validity | ||
|
| |||
| Addiction Behavior Checklist (ABC) | Long-term tracking of behaviors consistent with opioid abuse | Veteran population, chronic nonmalignant pain | ≥ 3 / Sensitivity: 0.87; specificity: 0.86 |
| Interview format incorporating observational datab | Strong interrater reliability and concurrent validity | ||
|
| |||
| Current Opioid Misuse Measure (COMM) | Monitor aberrant medication-related behaviors for patients already on long-term opioid therapy | Adult population, chronic nonmalignant pain | ≥ 9 / Sensitivity: 0.77; specificity 0.66 |
| Self-report rating scaleb | Strong internal consistency and test-retest reliability | ||
| Evidence of concurrent and predictive validity | |||
Tool to be used as a screener, before long-term opioid therapy.
Tool to be used to monitor throughout opioid therapy.
Reprinted with permission from: Anghelescu DL, Ehrentraut JH, Faughnan LG. Opioid misuse and abuse: risk assessment and management in patients with cancer pain. J Natl Compr Canc Netw. 2013;11:1023-1031.38
SURVIVORSHIP
The American Society of Clinical Oncologists (ASCO) has put forth recommendations for management of pain in cancer survivors. Pain in the cancer survivor may indicate recurrent disease and can be an important indication for imaging and comprehensive evaluation. However, for patients that develop a chronic pain syndrome following completion of treatment, and in the absence of active malignant disease, clinicians need to consider all potential options for pain management. Opioids may be trialed in a subset of carefully selected patients where benefits may outweigh the risks, but clinicians must carefully select patients who have not responded to more conservative management and who continue to experience distress or functional impairment. When opioids are started as a trial, clinicians should set expectations regarding the length of trial and objective standards for improvement rather than planning to continue opioids indefinitely. Risks of adverse effects of opioids should be assessed. Clinicians should clearly understand the differences between opioid-related tolerance, physiologic dependence, abuse and addiction to minimize nonmedical use of prescription opioids and adverse consequences.119
SUMMARY/CONCLUSIONS
Inadequate pain management continues to plague patients with cancer despite multiple safe and effective options for managing pain in this population. While there are many barriers to pain management, clinicians must be armed with the knowledge to dispel myths and misconceptions related to cancer-related pain and the use of opioids in this population. Pain should be assessed at every visit, and while patients may not become completely pain-free, clinicians and patients can work together to determine a plan that will allow a patient to live an independent, functional life with a tolerable level of pain. A multimodal approach of opioids, adjuvant medications and interventional or complementary therapies may be used in conjunction with disease-directed treatment. Given current regulatory climate towards opioid use, it is more important than ever for oncology teams to proactively, safely and effectively manage pain within the framework of patients living with cancer.
-
Additional Resources include:
NCCN Clinical Practice Guidelines in Oncology: Adult Cancer Pain. Available at: https://www.nccn.org/professionals/physician_gls/PDF/pain.pdf
ASCO: Information for patients on cancer-related pain. Available at: http://www.cancer.net/navigating-cancer-care/side-effects/pain
Center to Advance Palliative Care. Contains online courses for opioid prescribing and other symptoms. CME credit available. Available at: www.capc.org
SCOPE of Pain: Safe and Competent Opioid Prescribing Education. CME credit available. Available at: https://www.scopeofpain.com/
Acknowledgments
Funding Source
Dr. Smith is supported by the American Cancer Society MRSG PCSM 13-232-01 and NCI P30CA196521.
Footnotes
Conflict of Interests
Dr. Smith has received honorarium from Teva. The other author has no conflicts of interest to disclose.
References
- 1.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Annals of oncology: official journal of the European Society for Medical Oncology. 2007 Sep;18(9):1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 2.Krakauer EL, Wenk R, Buitrago R, Jenkins P, Scholten W. Opioid inaccessibility and its human consequences: reports from the field. J Pain Palliat Care Pharmacother. 2010 Sep;24(3):239–243. doi: 10.3109/15360288.2010.501852. [DOI] [PubMed] [Google Scholar]
- 3.Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009 Sep;10(9):865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 4.Efficace F, Bottomley A, Smit EF, et al. Is a patient’s self-reported health-related quality of life a prognostic factor for survival in non-small-cell lung cancer patients? A multivariate analysis of prognostic factors of EORTC study 08975. Annals of oncology : official journal of the European Society for Medical Oncology. 2006 Nov;17(11):1698–1704. doi: 10.1093/annonc/mdl183. [DOI] [PubMed] [Google Scholar]
- 5.Beck SL, Towsley GL, Berry PH, Lindau K, Field RB, Jensen S. Core aspects of satisfaction with pain management: cancer patients’ perspectives. J Pain Symptom Manage. 2010 Jan;39(1):100–115. doi: 10.1016/j.jpainsymman.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Zaza C, Baine N. Cancer pain and psychosocial factors: a critical review of the literature. J Pain Symptom Manage. 2002 Nov;24(5):526–542. doi: 10.1016/s0885-3924(02)00497-9. [DOI] [PubMed] [Google Scholar]
- 7.Basch E, Deal AM, Kris MG, et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J Clin Oncol. 2016 Feb 20;34(6):557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014 Dec 20;32(36):4149–4154. doi: 10.1200/JCO.2014.56.0383. [DOI] [PubMed] [Google Scholar]
- 9.Fadul NA, El Osta B, Dalal S, Poulter VA, Bruera E. Comparison of symptom burden among patients referred to palliative care with hematologic malignancies versus those with solid tumors. J Palliat Med. 2008 Apr;11(3):422–427. doi: 10.1089/jpm.2007.0184. [DOI] [PubMed] [Google Scholar]
- 10.Bandieri E, Sichetti D, Luppi M, et al. Is pain in patients with haematological malignancies under-recognised? The results from Italian ECAD-O survey. Leuk Res. 2010 Dec;34(12):e334–335. doi: 10.1016/j.leukres.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Ruddick W. Do doctors undertreat pain? Bioethics. 1997 Jul-Oct;11(3–4):246–255. doi: 10.1111/1467-8519.00063. [DOI] [PubMed] [Google Scholar]
- 12.Goldman B. Chronic-pain patients must cope with chronic lack of physician understanding. Cmaj. 1991 Jun 01;144(11):1492. 1494–1495, 1497. [PMC free article] [PubMed] [Google Scholar]
- 13.Boisaubin EV. The assessment and treatment of pain in the emergency room. Clin J Pain. 1989;5(Suppl 2):S19–24. doi: 10.1097/00002508-198906002-00004. discussion S24–15. [DOI] [PubMed] [Google Scholar]
- 14.Campbell JN. APS 1995 Presidential Address. Pain Forum. 1996;5(1):85–88. [Google Scholar]
- 15.Mularski RA, White-Chu F, Overbay D, Miller L, Asch SM, Ganzini L. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med. 2006 Jun;21(6):607–612. doi: 10.1111/j.1525-1497.2006.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tompkins DA, Hobelmann JG, Compton P. Providing chronic pain management in the “Fifth Vital Sign” Era: Historical and treatment perspectives on a modern-day medical dilemma. Drug Alcohol Depend. 2017 Apr 1;173(Suppl 1):S11–S21. doi: 10.1016/j.drugalcdep.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sehgal N, Colson J, Smith HS. Chronic pain treatment with opioid analgesics: benefits versus harms of long-term therapy. Expert Rev Neurother. 2013 Nov;13(11):1201–1220. doi: 10.1586/14737175.2013.846517. [DOI] [PubMed] [Google Scholar]
- 18.Lee TH. Zero Pain Is Not the Goal. Jama. 2016 Apr 19;315(15):1575–1577. doi: 10.1001/jama.2016.1912. [DOI] [PubMed] [Google Scholar]
- 19.Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009 Jun;24(6):733–738. doi: 10.1007/s11606-009-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AMA Annual. [Accessed May 16, 2017];Report. PM&R Highlights 2016. 2016 Jun 11–15; https://www.aapmr.org/docs/default-source/advocacy/final-ama-annual-june-2016-meeting-report-copy.pdf?sfvrsn=0.
- 21.Controlled prescription drug abuse at epidemic level. J Pain Palliat Care Pharmacother. 2006;20(2):61–64. [PubMed] [Google Scholar]
- 22.Calcaterra S, Glanz J, Binswanger IA. National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999–2009. Drug Alcohol Depend. 2013 Aug 01;131(3):263–270. doi: 10.1016/j.drugalcdep.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Substance Abuse and Mental Health Services Administration (SAMHSA) [Accessed December 3, 2017];National Survey on Drug Use and Health. 2016 Sep; https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR2-2015/NSDUH-FFR2-2015.htm.
- 24.Centers for Disease Control and Prevention. [Accessed December 3, 2017];Drug Overdose Death Data. 2016 Dec; https://www.cdc.gov/drugoverdose/data/statedeaths.html.
- 25.Kandel DB, Hu MC, Griesler P, Wall M. Increases from 2002 to 2015 in prescription opioid overdose deaths in combination with other substances. Drug Alcohol Depend. 2017 Sep 1;178:501–511. doi: 10.1016/j.drugalcdep.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brady KT, McCauley JL, Back SE. Prescription Opioid Misuse, Abuse, and Treatment in the United States: An Update. Am J Psychiatry. 2016 Jan;173(1):18–26. doi: 10.1176/appi.ajp.2015.15020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cushman PA, Liebschutz JM, Hodgkin JG, et al. What do providers want to know about opioid prescribing? A qualitative analysis of their questions. Subst Abus. 2017 Apr-Jun;38(2):222–229. doi: 10.1080/08897077.2017.1296525. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Food & Drug Administration. [Accessed July 19, 2017];FDA News Release: FDA requests removal of Opana ER for risks related to abuse. 2017 Jun 8; https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm562401.htm.
- 29.Prescription Drug Monitoring Program. [Accessed December 3, 2017];Training and Technical Assistance Center. 2017 Aug 24; http://www.pdmpassist.org/pdf/PDMP_Program_Status_20170824.pdf.
- 30.Healthcare Finance. [Accessed July 20, 2017];Cigna to implement prior authorization policy for opioid prescriptions. 2017 Apr 11; http://www.healthcarefinancenews.com/news/cigna-implement-prior-authorization-policy-opioid-prescriptions.
- 31.Castellucci M. Modern Healthcare. [Accessed July 20, 2017];Cigna ends prior authorization policy for opioid addiction treatment. 2016 Oct 21; http://www.modernhealthcare.com/article/20161021/NEWS/161029981.
- 32.Alford DP, Carney BL, Brett B, Parish SJ, Jackson AH. Improving Residents’ Safe Opioid Prescribing for Chronic Pain Using an Objective Structured Clinical Examination. J Grad Med Educ. 2016 Jul;8(3):390–397. doi: 10.4300/JGME-D-15-00273.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon JH. Overcoming barriers in cancer pain management. J Clin Oncol. 2014 Jun 01;32(16):1727–1733. doi: 10.1200/JCO.2013.52.4827. [DOI] [PubMed] [Google Scholar]
- 34.Rhiner MI, von Gunten CF. Cancer breakthrough pain in the presence of cancer-related chronic pain: fact versus perceptions of health-care providers and patients. J Support Oncol. 2010 Nov-Dec;8(6):232–238. doi: 10.1016/j.suponc.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists’ attitudes and practice in cancer pain management: a national survey. J Clin Oncol. 2011 Dec 20;29(36):4769–4775. doi: 10.1200/JCO.2011.35.0561. [DOI] [PubMed] [Google Scholar]
- 36.Chen JT, Fagan MJ, Diaz JA, Reinert SE. Is treating chronic pain torture? Internal medicine residents’ experience with patients with chronic nonmalignant pain. Teach Learn Med. 2007 Spring;19(2):101–105. doi: 10.1080/10401330701332144. [DOI] [PubMed] [Google Scholar]
- 37.Yanni LM, McKinney-Ketchum JL, Harrington SB, et al. Preparation, confidence, and attitudes about chronic noncancer pain in graduate medical education. J Grad Med Educ. 2010 Jun;2(2):260–268. doi: 10.4300/JGME-D-10-00006.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anghelescu DL, Ehrentraut JH, Faughnan LG. Opioid misuse and abuse: risk assessment and management in patients with cancer pain. J Natl Compr Canc Netw. 2013 Aug;11(8):1023–1031. doi: 10.6004/jnccn.2013.0120. [DOI] [PubMed] [Google Scholar]
- 39.Weissman DE. Fast Facts and Concepts #68: Is it Pain or Addiction? [Accessed July 20, 2017];Palliative Care Network of Wisconsin. 2009 https://www.mypcnow.org/blank-xn3i1.
- 40.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: 2013. [Google Scholar]
- 41.Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. a critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001 Aug;22(2):672–687. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 42.Clark D. ‘Total pain’, disciplinary power and the body in the work of Cicely Saunders, 1958–1967. Soc Sci Med. 1999 Sep;49(6):727–736. doi: 10.1016/s0277-9536(99)00098-2. [DOI] [PubMed] [Google Scholar]
- 43.Craig BM, Strassels SA. Out-of-pocket prices of opioid analgesics in the United States, 1999–2004. Pain Med. 2010 Feb;11(2):240–247. doi: 10.1111/j.1526-4637.2009.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013 Jun;32(6):1143–1152. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cintron A, Morrison RS. Pain and ethnicity in the United States: A systematic review. J Palliat Med. 2006 Dec;9(6):1454–1473. doi: 10.1089/jpm.2006.9.1454. [DOI] [PubMed] [Google Scholar]
- 46.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain. 2009 Dec;10(12):1187–1204. doi: 10.1016/j.jpain.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016 Apr 19;113(16):4296–4301. doi: 10.1073/pnas.1516047113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson KO, Mendoza TR, Valero V, et al. Minority cancer patients and their providers: pain management attitudes and practice. Cancer. 2000 Apr 15;88(8):1929–1938. [PubMed] [Google Scholar]
- 49.Dickason RM, Chauhan V, Mor A, et al. Racial differences in opiate administration for pain relief at an academic emergency department. West J Emerg Med. 2015 May;16(3):372–380. doi: 10.5811/westjem.2015.3.23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joynt M, Train MK, Robbins BW, Halterman JS, Caiola E, Fortuna RJ. The impact of neighborhood socioeconomic status and race on the prescribing of opioids in emergency departments throughout the United States. J Gen Intern Med. 2013 Dec;28(12):1604–1610. doi: 10.1007/s11606-013-2516-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrison RS, Wallenstein S, Natale DK, Senzel RS, Huang LL. “We don’t carry that”--failure of pharmacies in predominantly nonwhite neighborhoods to stock opioid analgesics. N Engl J Med. 2000 Apr 06;342(14):1023–1026. doi: 10.1056/NEJM200004063421406. [DOI] [PubMed] [Google Scholar]
- 52.Green CR, Ndao-Brumblay SK, West B, Washington T. Differences in prescription opioid analgesic availability: comparing minority and white pharmacies across Michigan. J Pain. 2005 Oct;6(10):689–699. doi: 10.1016/j.jpain.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Qato DM, Daviglus ML, Wilder J, Lee T, Qato D, Lambert B. ‘Pharmacy deserts’ are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood) 2014 Nov;33(11):1958–1965. doi: 10.1377/hlthaff.2013.1397. [DOI] [PubMed] [Google Scholar]
- 54.Amstislavski P, Matthews A, Sheffield S, Maroko AR, Weedon J. Medication deserts: survey of neighborhood disparities in availability of prescription medications. Int J Health Geogr. 2012 Nov 09;11:48. doi: 10.1186/1476-072X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meghani SH, Polomano RC, Tait RC, Vallerand AH, Anderson KO, Gallagher RM. Advancing a national agenda to eliminate disparities in pain care: directions for health policy, education, practice, and research. Pain Med. 2012 Jan;13(1):5–28. doi: 10.1111/j.1526-4637.2011.01289.x. [DOI] [PubMed] [Google Scholar]
- 56.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) [Accessed March 6, 2017];Adult Cancer Pain (Version 2.2016) https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf.
- 57.Campion FX, Larson LR, Kadlubek PJ, Earle CC, Neuss MN. Advancing performance measurement in oncology: quality oncology practice initiative participation and quality outcomes. J Oncol Pract. 2011 May;7(3 Suppl):31s–35s. doi: 10.1200/JOP.2011.000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jensen MP, Castarlenas E, Tome-Pires C, de la Vega R, Sanchez-Rodriguez E, Miro J. The Number of Ratings Needed for Valid Pain Assessment in Clinical Trials: Replication and Extension. Pain Med. 2015 Sep;16(9):1764–1772. doi: 10.1111/pme.12823. [DOI] [PubMed] [Google Scholar]
- 59.Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008 Jul;101(1):17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 60.Chai E, Meier D, Morris J, Goldhirsch S, editors. Geriatric Palliative Care: A Practical Guide for Clinicians. New York: Oxford University Press; 2014. [Google Scholar]
- 61.Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. Bmj. 2011 Jul 15;343:d4065. doi: 10.1136/bmj.d4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G. The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain. 2009 Apr;13(4):331–338. doi: 10.1016/j.ejpain.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 63.Webber K, Davies AN, Zeppetella G, Cowie MR. Development and validation of the breakthrough pain assessment tool (BAT) in cancer patients. J Pain Symptom Manage. 2014 Oct;48(4):619–631. doi: 10.1016/j.jpainsymman.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 64.Miaskowski C, Dodd MJ, West C, et al. Lack of adherence with the analgesic regimen: a significant barrier to effective cancer pain management. J Clin Oncol. 2001 Dec 01;19(23):4275–4279. doi: 10.1200/JCO.2001.19.23.4275. [DOI] [PubMed] [Google Scholar]
- 65.Lovell MR, Luckett T, Boyle FM, Phillips J, Agar M, Davidson PM. Patient education, coaching, and self-management for cancer pain. J Clin Oncol. 2014 Jun 01;32(16):1712–1720. doi: 10.1200/JCO.2013.52.4850. [DOI] [PubMed] [Google Scholar]
- 66.World Health Organization. Cancer Pain Relief. 2. Geneva: WHO; 1996. [Google Scholar]
- 67.Vardy J, Agar M. Nonopioid drugs in the treatment of cancer pain. J Clin Oncol. 2014 Jun 01;32(16):1677–1690. doi: 10.1200/JCO.2013.52.8356. [DOI] [PubMed] [Google Scholar]
- 68.Bandieri E, Romero M, Ripamonti CI, et al. Randomized Trial of Low-Dose Morphine Versus Weak Opioids in Moderate Cancer Pain. J Clin Oncol. 2016 Feb 01;34(5):436–442. doi: 10.1200/JCO.2015.61.0733. [DOI] [PubMed] [Google Scholar]
- 69.de Oliveira R, dos Reis MP, Prado WA. The effects of early or late neurolytic sympathetic plexus block on the management of abdominal or pelvic cancer pain. Pain. 2004 Jul;110(1–2):400–408. doi: 10.1016/j.pain.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 70.Amr YM, Makharita MY. Neurolytic sympathectomy in the management of cancer pain-time effect: a prospective, randomized multicenter study. J Pain Symptom Manage. 2014 Nov;48(5):944–956. doi: 10.1016/j.jpainsymman.2014.01.015. e942. [DOI] [PubMed] [Google Scholar]
- 71.Nabal M, Librada S, Redondo MJ, Pigni A, Brunelli C, Caraceni A. The role of paracetamol and nonsteroidal anti-inflammatory drugs in addition to WHO Step III opioids in the control of pain in advanced cancer. A systematic review of the literature. Palliat Med. 2012 Jun;26(4):305–312. doi: 10.1177/0269216311428528. [DOI] [PubMed] [Google Scholar]
- 72.Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012 Feb;13(2):e58–68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
- 73.Amy P, Abernathy AK, David C Currow. When should nonsteroidal antiinflammatories be used to manage pain? In: Goldstein Nathan E, RSM, editors. Evidence-based practice of palliative medicine. Philadelphia, PA: Elsevier; 2013. pp. 49–53. [Google Scholar]
- 74.McNicol E, Strassels S, Goudas L, Lau J, Carr D. Nonsteroidal anti-inflammatory drugs, alone or combined with opioids, for cancer pain: a systematic review. J Clin Oncol. 2004 May 15;22(10):1975–1992. doi: 10.1200/JCO.2004.10.524. [DOI] [PubMed] [Google Scholar]
- 75.Bennett MI, Rayment C, Hjermstad M, Aass N, Caraceni A, Kaasa S. Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain. 2012 Feb;153(2):359–365. doi: 10.1016/j.pain.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 76.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014 Jun 20;32(18):1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 77.Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014 Dec;155(12):2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 78.Accordino MK, Neugut AI, Hershman DL. Cardiac effects of anticancer therapy in the elderly. J Clin Oncol. 2014 Aug 20;32(24):2654–2661. doi: 10.1200/JCO.2013.55.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010 Aug;9(8):807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 80.Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. Jama. 2013 Apr 03;309(13):1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kus T, Aktas G, Alpak G, et al. Efficacy of venlafaxine for the relief of taxane and oxaliplatin-induced acute neurotoxicity: a single-center retrospective case-control study. Support Care Cancer. 2016 May;24(5):2085–2091. doi: 10.1007/s00520-015-3009-x. [DOI] [PubMed] [Google Scholar]
- 82.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015 Jul 06;(7):CD008242. doi: 10.1002/14651858.CD008242.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nelson JC. Tricyclic and Tetracyclic Drugs. In: Schatzberg AF, Nemeroff CB, editors. The American Psychiatric Association Publishing Textbook of Psychopharmacology. Fifth. Arlington, VA: American Psychiatric Publishing; 2017. [Google Scholar]
- 84.Wiffen PJ, Derry S, Bell RF, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017 Jun 9;6:CD007938. doi: 10.1002/14651858.CD007938.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mishra S, Bhatnagar S, Goyal GN, Rana SP, Upadhya SP. A comparative efficacy of amitriptyline, gabapentin, and pregabalin in neuropathic cancer pain: a prospective randomized double-blind placebo-controlled study. Am J Hosp Palliat Care. 2012 May;29(3):177–182. doi: 10.1177/1049909111412539. [DOI] [PubMed] [Google Scholar]
- 86.Rao RD, Michalak JC, Sloan JA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007 Nov 01;110(9):2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 87.Murphy JD, Nelson LM, Chang DT, Mell LK, Le QT. Patterns of care in palliative radiotherapy: a population-based study. Journal of oncology practice. 2013 Sep;9(5):e220–227. doi: 10.1200/JOP.2012.000835. [DOI] [PubMed] [Google Scholar]
- 88.Smith BTBaTJ. When should epidural or intrathecal opioid infusions and pumps be considered for pain management? In: Nathan E, Goldstein RSM, editors. Evidenced-Based Practice of Palliative Medicine. Philadelphia: Elsevier; 2013. pp. 93–98. [Google Scholar]
- 89.Kurita GP, Kaasa S, Sjogren P. Spinal opioids in adult patients with cancer pain: a systematic review: a European Palliative Care Research Collaborative (EPCRC) opioid guidelines project. Palliat Med. 2011 Jul;25(5):560–577. doi: 10.1177/0269216310386279. [DOI] [PubMed] [Google Scholar]
- 90.Lu W, Dean-Clower E, Doherty-Gilman A, Rosenthal DS. The value of acupuncture in cancer care. Hematol Oncol Clin North Am. 2008 Aug;22(4):631–648. doi: 10.1016/j.hoc.2008.04.005. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garcia MK, McQuade J, Haddad R, et al. Systematic review of acupuncture in cancer care: a synthesis of the evidence. J Clin Oncol. 2013 Mar 01;31(7):952–960. doi: 10.1200/JCO.2012.43.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garcia MK, Driver L, Haddad R, et al. Acupuncture for treatment of uncontrolled pain in cancer patients: a pragmatic pilot study. Integr Cancer Ther. 2014 Mar;13(2):133–140. doi: 10.1177/1534735413510558. [DOI] [PubMed] [Google Scholar]
- 93.Wu X, Chung VC, Hui EP, et al. Effectiveness of acupuncture and related therapies for palliative care of cancer: overview of systematic reviews. Sci Rep. 2015 Nov 26;5:16776. doi: 10.1038/srep16776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeidan F, Grant JA, Brown CA, McHaffie JG, Coghill RC. Mindfulness meditation-related pain relief: evidence for unique brain mechanisms in the regulation of pain. Neurosci Lett. 2012 Jun 29;520(2):165–173. doi: 10.1016/j.neulet.2012.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rouleau CR, Garland SN, Carlson LE. The impact of mindfulness-based interventions on symptom burden, positive psychological outcomes, and biomarkers in cancer patients. Cancer Manag Res. 2015;7:121–131. doi: 10.2147/CMAR.S64165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.National Conference of State Legislatures. [Accessed December 2, 2017];State Medical Marijuana Laws. 2017 http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx.
- 97.Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011 Aug;163(7):1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. Jama. 2015 Jun 23–30;313(24):2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 99.Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010 Feb;39(2):167–179. doi: 10.1016/j.jpainsymman.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 100.Tateo S. State of the evidence: Cannabinoids and cancer pain-A systematic review. J Am Assoc Nurse Pract. 2017 Feb;29(2):94–103. doi: 10.1002/2327-6924.12422. [DOI] [PubMed] [Google Scholar]
- 101.Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain. 2002 Jul-Aug;18(4 Suppl):S3–13. doi: 10.1097/00002508-200207001-00002. [DOI] [PubMed] [Google Scholar]
- 102.Pathan H, Williams J. Basic opioid pharmacology: an update. Br J Pain. 2012 Feb;6(1):11–16. doi: 10.1177/2049463712438493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hajj A, Khabbaz L, Laplanche JL, Peoc’h K. Pharmacogenetics of opiates in clinical practice: the visible tip of the iceberg. Pharmacogenomics. 2013 Apr;14(5):575–585. doi: 10.2217/pgs.13.13. [DOI] [PubMed] [Google Scholar]
- 104.Goldberg GR, Smith CB. How should opioids be started and titrated in routine outpatient settings? In: Goldstein NE, Morrison RS, editors. Evidence-Based Practice of Palliative Medicine. Philadelphia, PA: Elsevier Inc; 2013. pp. 2–7. [Google Scholar]
- 105.Mercadante S, Porzio G, Ferrera P, et al. Low morphine doses in opioid-naive cancer patients with pain. J Pain Symptom Manage. 2006 Mar;31(3):242–247. doi: 10.1016/j.jpainsymman.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 106.Ripamonti CI, Campa T, Fagnoni E, et al. Normal-release oral morphine starting dose in cancer patients with pain. Clin J Pain. 2009 Jun;25(5):386–390. doi: 10.1097/AJP.0b013e3181929b4f. [DOI] [PubMed] [Google Scholar]
- 107.Hjermstad MJ, Kaasa S, Caraceni A, et al. Characteristics of breakthrough cancer pain and its influence on quality of life in an international cohort of patients with cancer. BMJ Support Palliat Care. 2016 Sep;6(3):344–352. doi: 10.1136/bmjspcare-2015-000887. [DOI] [PubMed] [Google Scholar]
- 108.Hernandez MA, Rathinavelu A. Basic Pharmacology: Understanding Drug Actions and Reactions. CRC Press; 2017. [Accessed December 29, 2017]. [Google Scholar]
- 109.Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004 Nov;28(5):497–504. doi: 10.1016/j.jpainsymman.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 110.Lee KA, Ganta N, Horton JR, Chai E. Evidence for Neurotoxicity Due to Morphine or Hydromorphone Use in Renal Impairment: A Systematic Review. J Palliat Med. 2016 Nov;19(11):1179–1187. doi: 10.1089/jpm.2016.0101. [DOI] [PubMed] [Google Scholar]
- 111.Bruera E, Sweeney C. Methadone use in cancer patients with pain: a review. J Palliat Med. 2002 Feb;5(1):127–138. doi: 10.1089/10966210252785097. [DOI] [PubMed] [Google Scholar]
- 112.Camilleri M, Drossman DA, Becker G, Webster LR, Davies AN, Mawe GM. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil. 2014 Oct;26(10):1386–1395. doi: 10.1111/nmo.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013 Jan;45(1):2–13. doi: 10.1016/j.jpainsymman.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 114.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008 Mar;11(2 Suppl):S105–120. [PubMed] [Google Scholar]
- 115.Webster LR, Fine PG. Review and critique of opioid rotation practices and associated risks of toxicity. Pain Med. 2012 Apr;13(4):562–570. doi: 10.1111/j.1526-4637.2012.01357.x. [DOI] [PubMed] [Google Scholar]
- 116.Dale O, Moksnes K, Kaasa S. European Palliative Care Research Collaborative pain guidelines: opioid switching to improve analgesia or reduce side effects. A systematic review. Palliat Med. 2011 Jul;25(5):494–503. doi: 10.1177/0269216310384902. [DOI] [PubMed] [Google Scholar]
- 117.Fine PG, Portenoy RK. Establishing “best practices” for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage. 2009 Sep;38(3):418–425. doi: 10.1016/j.jpainsymman.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Passik SD, Theobald DE. Managing addiction in advanced cancer patients: why bother? J Pain Symptom Manage. 2000 Mar;19(3):229–234. doi: 10.1016/s0885-3924(00)00109-3. [DOI] [PubMed] [Google Scholar]
- 119.Paice JA, Lacchetti C, Bruera E. Management of Chronic Pain in Survivors of Adult Cancers: ASCO Clinical Practice Guideline Summary. Journal of oncology practice. 2016 Aug;12(8):757–762. [Google Scholar]