Abstract
Diabetic retinopathy is a leading cause of vision loss. Treatment options for early retinopathy are sparse. Exercise protects dying photoreceptors in models of retinal degeneration, thereby preserving vision. We tested the protective effects of exercise on retinal and cognitive deficits in a Type 1 diabetes model and determined whether the TrkB pathway mediates this effect.
Hyperglycemia was induced in Long Evans rats via streptozotocin injection (STZ; 100mg/kg). Following confirmed hyperglycemia, both control and diabetic rats underwent treadmill exercise for 30 minutes, 5 days/week at 0m/min (inactive groups) or 15m/min (active groups) for 8 weeks. A TrkB receptor antagonist (ANA-12), or vehicle, was injected 2.5h before exercise training. We measured spatial frequency and contrast sensitivity using optokinetic tracking biweekly post-STZ; retinal function using electroretinography at 4 and 8 weeks, and cognitive function and exploratory behavior using Y-maze at 8 weeks. Retinal neurotrophin-4 was measured using ELISA.
Compared with non-diabetic controls, diabetic rats showed significantly reduced spatial frequency and contrast sensitivity, delayed electroretinogram oscillatory potential and flicker implicit times, and reduced cognitive function and exploratory behavior. Exercise interventions significantly delayed the appearance of all deficits, except for exploratory behavior. Treatment with ANA-12 significantly reduced this protection, suggesting a TrkB-mediated mechanism. Despite this, no changes in retinal neurotrohin-4 were observed with diabetes or exercise.
Exercise protected against early visual and cognitive dysfunction in diabetic rats, suggesting that exercise interventions started after hyperglycemia diagnosis may be a beneficial treatment. The translational potential is high given that exercise treatment is non-invasive, patient controlled, and inexpensive.
Keywords: Diabetes, diabetic retinopathy, exercise, TrkB, neurotrophin-4
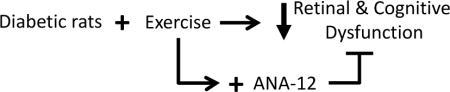
Graphical abstract
STZ-induced diabetic rats undergoing treadmill exercise show reduced visual and retinal dysfunction in early stage disease. Cognitive dysfunction occurred after detection of retinal deficits, but still benefited from the exercise intervention. Treatment with the TrkB antagonist, ANA-12, blocked the protective effects of exercise on retinal and cognitive dysfunction, suggesting that exercise activates a TrkB pathway signaling mechanism.
INTRODUCTION
Diabetes currently affects over 25 million U.S. citizens (CDC 2011 National Diabetes Fact Sheet), and its prevalence is predicted to increase by 35% by 2025 (King et al., 1998). Diabetes is characterized by hyperglycemia, hyperlipidemia, and accompanying inflammation that cause pathologic changes in circulation, leading to vascular damage in the brain, heart, nerves, kidney, and retina (Beisswenger, 1976; Pasquier et al., 2006; Wessels et al., 2006a). One of the most common complications is diabetic retinopathy, which is the leading cause of blindness in working age adults and is characterized by macular edema, retinal ischemia, and abnormal neovascularization (Klein, 2007). Early signs of diabetic retinopathy are found in prediabetic patients (Chen et al., 2012), and diabetic retinopathy may be associated with greater amounts of cerebral ischemia, cortical atrophy, and cognitive deficits (Ferguson et al., 2003; Wessels et al., 2006b; Ding et al., 2010; Haan et al., 2012), suggesting a role for early diabetic retinopathy as a marker or predictor for other diabetic complications. Currently, the leading treatment options for diabetic retinopathy are laser therapy and intravitreal injections of anti-VEGF (vascular endothelial growth factor). Unfortunately both treatments are relatively invasive and are only applicable to later stage diabetic retinopathy, when vascular pathology and loss of visual function are already advanced (Frank, 2004; Waisbourd et al., 2011). Increasing rates of diabetes combined with a lack of treatment options available until the retinopathy has progressed to its most severe stages highlight the urgent need for new therapies.
Exercise provides protection and promotes neuroregeneration in a variety of neurodegenerative diseases and injury models, and in aging (Speisman et al., 2013; Svensson et al., 2015). In animal models, exercise enhances axon regeneration and reduces neuronal death after peripheral nerve transection (Wood et al., 2012), improves performance on learning and memory tasks (van Praag et al., 1999; Anderson et al., 2000; Radak et al., 2001), and promotes neurogenesis in the central nervous system (van Praag et al., 1999). Exercise also reduces clinical symptoms in amyotrophic lateral sclerosis patients (McCrate & Kaspar, 2008), improves cognitive function in Alzheimer’s patients (Vreugdenhil et al., 2012; Groot et al., 2015), improves motor rehabilitation in stroke patients (Mang et al., 2013), and improves cognitive function in healthy adults (Ploughman, 2008; Chaddock et al., 2010; Erickson et al., 2011; Voss et al., 2013b). In diabetic patients, exercise reduces insulin resistance, ameliorates microvascular dysfunction, and reduces symptoms of neuropathy (Cooper et al., 2016; Billinger et al., 2017; Nuhu & Maharaj, 2017; Sjoberg et al., 2017).
Previously, our group demonstrated that exercise reduces retinal function deficits and photoreceptor cell death in both light-induced (Lawson et al., 2014) and inherited models (Hanif et al., 2015) of retinal degeneration. Exercise also protects against intraocular pressure injury specifically in aged mice (12 months) (Chrysostomou et al., 2014), reduces oxidative stress in the retinas of aged mice (22 months) (Kim et al., 2015), and reduces apoptosis in the diabetic rat retina (Kim et al., 2013). The TrkB (tropomyosin receptor kinase B) pathway may be mediating this protective effect in the retina and other neural tissues (Voss et al., 2013a; Lawson et al., 2014; Leckie et al., 2014; Marosi & Mattson, 2014; Hanif et al., 2015; Chrysostomou et al., 2016). After exercise, local increases occur in neurotrophin-4 (NT-4) (Skup et al., 2002; Cote et al., 2011; Chung et al., 2013) and local and systemic increases occur in brain derived neurotrophic factor (BDNF) (Ploughman et al., 2007; Griffin et al., 2011), the two ligands with the greatest affinity for the TrkB receptor (Barbacid, 1994). To confirm TrkB pathway involvement in exercise protection of retinal degeneration, we treated exercised animals with ANA-12, a TrkB inhibitor that crosses the blood-brain barrier when given systemically and prevents signal transduction and downstream processes (Cazorla et al., 2011a; Cazorla et al., 2011b). We found that blocking the TrkB receptor prevents exercise protection in both inherited and toxic bright light retinal degeneration models (Lawson et al., 2014; Hanif et al., 2015). Our work also showed that animals given exercise treatment after exposure to toxic bright light had higher levels of BDNF in serum, retina, and hippocampus (Lawson et al., 2014).
While exercise has been shown to have protective effects in the retina and in diabetes, the effects of exercise on retinal and visual function in diabetes have not been studied. Here, we tested the protective effects of treadmill exercise treatment in the streptozotocin (STZ) rat model of Type I diabetes. Rats were confirmed to have hyperglycemia prior to exercise treatment to model the clinical scenario of treating a patient newly diagnosed with diabetes. We hypothesized that exercise would reduce early visual and retinal deficits, as measured by optomotor response (OMR) and electroretinogram (ERG), and early cognitive deficits, as measured by Y-maze. We further hypothesized that because the TrkB pathway mediates the protective effect of exercise in diabetes and in other models of retinal disease, treatment with the TrkB inhibitor ANA-12 would diminish or even prevent this protective effect.
MATERIALS AND METHODS
Animals, diabetes induction, and exercise
Healthy adult male Long Evans rats (325-350 g; Charles River, Wilmington, MA; n = 120) were housed in shoe-box style cages on a 12:12 light:dark cycle (light onset at 6:00 AM) with chow and water provided ad libitum. Rats were run in sets of 8-12, but Y-maze was not performed on every set of rats. All procedures were approved by the Atlanta Veterans Affairs Institutional Animal Care and Use Committee and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). During the course of the experiment, 9 diabetic rats died or had to be sacrificed early, 2 control rats died, 6 diabetic rats reverted to non-diabetic status, 2 diabetic rats were excluded from analysis because they had abdominal absesses, 1 control rat was excluded from analysis due to abnormal ERG function, and 1 control rat was excluded because it was completely unresponsive in OMR testing. Note: both control rat deaths occurred during anesthesia, and we have had no control rat deaths since changing our reversal agent from yohimbine to antisedan (atipamezole).
At approximately 9 weeks of age, hyperglycemia was induced with a single intravenous injection of STZ (100 mg/kg; Sigma-Aldrich, Inc., Milwaukee, WI) dissolved in citrate buffer (pH 4.0). STZ is a toxin that selectively destroys pancreatic beta cells – the cells that produce insulin to regulate blood glucose levels – making it useful in creating animal models of Type I diabetes. Diabetes mellitus (DM) was defined as two successive daily blood glucose levels higher than 250 mg/dL (freestyle handheld blood glucose meter from tail-prick blood), measured 2-3 days after STZ injection. Body weights and blood glucose were monitored twice per week. If body weight decrease approached a 10% loss, diabetic rats were treated with small pellets of sustained-release subcutaneous insulin (Linplant; Linshin Canada, Scarborough, ON, Canada) at a dose sufficient to prevent excessive weight loss and catabolic response, but not enough to control hyperglycemia (Thule et al., 2006).
Rats confirmed to be diabetic were assigned to one of four groups: inactive+vehicle, active+vehicle, inactive+ANA-12, and active+ANA-12. Non-diabetic control rats were assigned to the same four groups, although significant differences among control groups were not observed for any of the functional parameters studied. Thus, the control groups have been combined and are referred to as “non-diabetic controls”).
Exercise treatment started 3 days post-STZ injection after hyperglycemia was confirmed and continued to 8 weeks post-STZ when rats were euthanized via decapitation (due to future analysis for catecholamines in the brain). Active rats were exercised on rodent treadmills with shock detection (Exer-3/6; Columbus Instruments, Columbus, OH) five times per week for 30 minutes at a rate of 15 m/min. Rats received a maximum of 10 shocks from an electrified grid (1 Hz, 0.46 mA) if they stepped off the moving belt during exercise sessions. Rats learned to run on the treadmill very easily, and rarely received shocks after the first week. Inactive rats followed an identical regimen, with the exception that their treadmills were stationary (Acclimation Cage-2; Columbus Instruments, Columbus, OH).
To explore the role of TrkB pathway signaling in exercise protection, rats were given intraperitoneal injections of either vehicle (VEH) (1% DMSO, 16.5% CremophorEL; 16.5% ethanol, 66% Dulbecco’s PBS, pH 7.4) or ANA-12 in vehicle (0.2 mg/kg body weight; Sigma-Aldrich Inc., Milwaukee, WI), which readily crosses the blood-brain barrier and selectively inhibits the TrkB receptor (Cazorla et al., 2011a). Based on previously characterized pharmacokinetics of ANA-12 (Cazorla et al., 2011a), rats were injected 2.5 hours before each exercise session to align peak TrkB inhibition with exercise.
Animals received visual acuity and contrast sensitivity testing one week prior to the beginning of the experiment, and were tested at 2, 4, 6 and 8 weeks afterward in the same manner. Additionally, animals underwent ERG recordings at the 4 and 8 week time points to assess retinal function and Y-maze at the 8 week time point to assess cognitive function and exploratory behavior. A subset of animals (n = 12/group) received Y-maze at 4, 6, and 8 weeks in conjunction with OMR testing in order to determine the temporal appearance of visual vs. cognitive dysfunction. All animals were given ear tags for IDs that did not reveal their designated group. While the diabetic and control rats could be differentiated easily by their weights, whether the rats received exercise or ANA-12 treatment was hidden from the tester.
Assessment of Visual Function – Optomotor Response
Visual function was tested using the virtual optokinetic system (OptoMotry system; Cerebral Mechanics, Lethbridge, AB, Canada), as previously described (Douglas et al., 2005). Briefly, an unrestrained rat was placed on a platform at the center of a virtual-reality chamber composed of four computer monitors that display vertical sine wave gratings rotating at 12 deg/s. A video camera positioned above the animal was used to watch in real time for the presence or absence of reflexive head movements (tracking) in response to the projected gratings rotating in the same direction. The experimenter manually tracked the head of the rat to align the center of the virtual cylinder with the viewing position of the rat. During visual acuity assessment, the grating started at a spatial frequency of 0.042 cyc/deg with 100% contrast. The acuity threshold was determined automatically by the OMR software using a staircase paradigm based on observations of head-tracking reflexes. Similarly, the contrast sensitivity threshold was determined by reducing the contrast of the black and white gradients from 100% in a staircase paradigm until animal head-tracking movements were no longer observed. Contrast sensitivity was measured at the spatial frequency of 0.064 cyc/deg for the study. This was the spatial frequency that elicited the maximum sensitivity obtained from the rats at baseline when a contrast sensitivity curve was assessed across five spatial frequencies, as previously described (Aung et al., 2013). Contrast sensitivity was calculated as a reciprocal of the Michelson contrast from the screen’s luminance (i.e. [maximum + minimum]/[maximum-minimum]), as previously described (Prusky et al., 2006). Spatial frequency and contrast sensitivity thresholds from 0 to 8 weeks were normalized to control rat values and each rat’s individual baseline. For optomotor response, the following animal numbers were tested: non-diabetic control (n = 35-37), DM+inactive+VEH (n = 16-17), DM+active+VEH (n = 16-19), DM+inactive+ANA (n = 10), and DM+active+ANA (n = 9-11). Numbers vary due to some animals not being tested at all timepoints.
Assessment of Retinal Function – Electroretinogram (ERG)
Rats were dark-adapted overnight and then prepared under dim red illumination, as previously described (Ciavatta et al., 2009). Briefly, rats were anesthetized (ketamine [60 mg/kg] and xylazine [7.5 mg/kg]), and the corneal surface anesthetized (0.5% tetracaine HCl) and pupils dilated (1% tropicamide). Electrical responses were recorded to flash stimuli presented in order of increasing luminance using a signal-averaging system (UTAS BigShot; LKC Technologies, Gaithersburg, MD) via a gold loop electrode contacting the cornea. ERG stimuli consisted of a 6-step protocol of flash stimuli. Five sequential dark-adapted responses were recorded (scotopic: −3.0 to 2.1 log cd s/m2) to isolate rod-dominated and mixed rod and cone responses. Then, rats were exposed to a steady background adapting field (30 cd/m2) for 10 min to saturate the rod photoreceptors and isolate cone pathway function. After the light-adaptation period, animals were presented with 2.0 log cd s/m2 flicker stimuli at 6 Hz in the presence of the background light. Responses were recorded using the same signal averaging system (UTAS bigShot; LKC Technologies). After testing, rats received yohimbine (2.1 mg/kg) to reverse the effects of xylazine and to prevent corneal ulcers (Turner & Albassam, 2005).
ERG data were analyzed offline. For dark adapted ERG responses, amplitudes and implicit times were measured for a-waves, b-waves, and oscillatory potentials (OPs). OPs were digitally filtered using the ERG system software (75-500 Hz; EM Version 8.1.2, 2008; LKC Technologies). For light adapted ERG responses, flicker response amplitudes and implicit times were measured from the trough of the signal after the flash onset to the peak. Oscillatory potentials (OPs) were analyzed for two flash stimuli: −1.9 and 0.7 log cd s/m2, which we have previously shown to distinguish rod vs. rod-cone pathway dysfunction in early diabetes, respectively (Aung et al., 2013; Pardue et al., 2014). OP implicit times were reported normalized to control values at each time point. For ERG, the following animal numbers were tested: non-diabetic control (n = 32-40), DM+inactive+VEH (n = 20-23), DM+active+VEH (n = 17-21), DM+inactive+ANA (n = 10), and DM+active+ANA (n = 9-10). Numbers vary due to some animals not being tested at all timepoints.
Analysis of cognitive and exploratory behavior – Y-maze
The Y-maze (San Diego Instruments, San Diego, CA) was used to assess spatial working memory, based on the methods of Maurice and colleagues (Maurice et al., 1995). Briefly, each rat was placed in one arm of the Y-maze and permitted to explore the entire maze freely for 8 minutes. The series of arm entries was monitored visually and recorded. One correct alternation was defined as entering the 3 arms consecutively (for example, the pattern ABCACB would have 3 correct alternations – ABC, BCA, and ACB). The percentage of correct spatial alternation was calculated as the number of correct alternations/(total number of arm entries −2) × 100. Additionally, the total number of arm entries was recorded as a measure of exploratory behavior. For Y-maze, the following animal numbers were tested: non-diabetic control (n = 24), DM+inactive+VEH (n = 20), DM+active+VEH (n = 7), DM+inactive+ANA (n = 7), and DM+active+ANA (n = 7).
Analysis of NT-4 levels in the retina – ELISA (enzyme-linked immunoabsorbent assay)
Rats were euthanized via decapitation at 8 weeks post-STZ, and their retinas were removed and snap frozen. To perform tissue lysate extraction, individual retinas were re-suspended in approximately 10 weight/volume ratio of acid-extraction buffer containing protease inhibitors (50 mmol/L sodium acetate, 1 mol/L NaCl, 0.1% Triton X-100, pH 4, Roche Complete Mini Protease Inhibitors, Catalog Number 11836153001) and sonicated using an ultrasonic processor. Homogenates were kept on ice for 30 minutes; sonication and inclubation on ice was repeated. The homogenates were then centrifuged for 30 minutes at 15,000 g and 4⁰C and the supernatant was transferred to a new tube. Total protein concentration was measured using a BCA protein assay kit (Pierce BCA Protein Assay Kit, Catalog Number PI23225) and the absorbance was measured at 562 nm using BioTek Synergy 2 plate reader.
A commercial ELISA kit was used to assess the NT-4 levels in retina samples [Biosensis Neurotrophin 4/5 Rapid ELISA Kit: Human, Monkey, Rat, and Mouse (2 Plates), Catalog Number BEK-2211-2P]. NT-4 standards (100 μL of 15.6 – 1000 pg/mL) and retina samples (100 μL, diluted 1:2) were run in triplicates according to the manufacturer’s instructions. The absorbance was measured at 450 nm using a BioTek Synergy 2 plate reader. The sample concentration was determined from the standard concentration and absorbance measurements with a four-parameter non-linear regression using Gen5 software. Results were expressed as concentration of NT-4 (pg/mg). Note: ELISA results were added to this study many months after the initial tissue harvesting, and thus, not all retinal samples had detectable levels of protein. Retinal numbers were as follows: non-diabetic controls (n = 11), DM+inactive+VEH (n = 12), DM+active+VEH (n = 8), DM+inactive+ANA (n = 8), and DM+active+ANA (n = 8).
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). Weight, OMR, and ERG were analyzed using a two-way repeated measures ANOVA followed by Holms-Sidak post-hoc comparisons (SigmaPlot version X; Systat Software, Inc.; San Jose, CA). Blood glucose and Y-maze (both spatial alternation and exploratory behavior) were analyzed using a one-way ANOVA followed by Holms-Sidak tests for individual comparisons. Chi-square was used to compared the categorical data on survival, reversion to non-diabetic state and need for insulin. Non-diabetic control groups were combined when no statistical differences were found between groups for any of our functional measures, including OMR (contrast sensitivity and spatial frequency), ERG (oscillatory potentials and flicker), y-maze (spatial alternation and exploratory behavior), and blood glucose (p = 0.706 for contrast sensitivity, our most sensitive measure). Animals numbers (n) are occasionally reported as a range because some animals did not survive for the full experiment.
RESULTS
Diabetic rats showed reduced body weight and increased blood glucose
In order to evaluate the systemic effects of exercise on diabetes, we measured body weight and blood glucose. All four diabetic rat groups (DM+inactive+VEH, DM+active+VEH, DM+inactive+ANA, and DM+active+ANA) showed significant reductions in body weight compared with non-diabetic controls beginning at 4 weeks post-STZ (p < 0.002) and continuing to 8 weeks (p < 0.001), similar to previous studies using STZ-induced diabetes (Aung et al., 2013) [Repeated measures ANOVA interaction effect, F(28, 559) = 5.638, p < 0.001] (Fig. 1A). Weights from diabetic groups that were exercised and/or received ANA-12 did not differ significantly from each other. All four diabetic groups, regardless of exercise or ANA-12 treatments, showed blood glucose levels of >250 mg/dL beginning the first week post-STZ. Blood glucose averaged across 8 weeks was significantly higher in all four diabetic groups compared with non-diabetic controls [ANOVA, F(4, 78) = 251.778, p < 0.001](Fig. 1B) and did not show any differences with exercise and/or ANA-12 treatment.
Figure 1. Reduced weight and increased blood glucose in all four diabetic groups compared with non-diabetic controls.
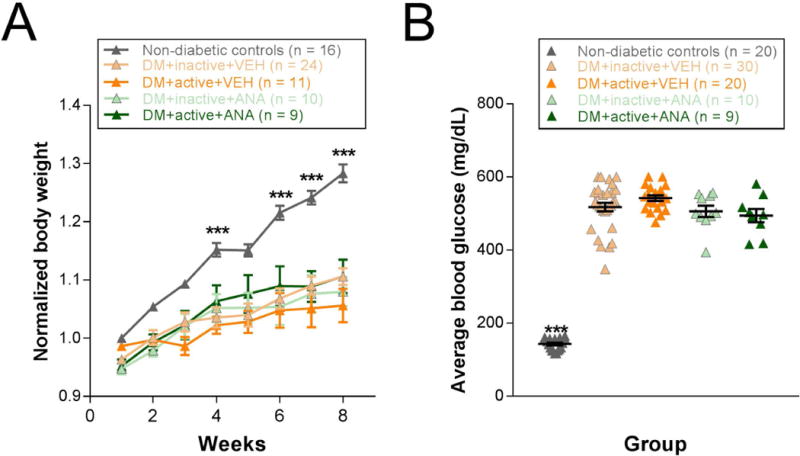
A) Weight for weeks 1-8 post-STZ. Each animal’s weights were normalized to its baseline weight, which for diabetic rats is a week 0 weight taken prior to STZ injection. B) Average blood glucose (mg/dL) at 8 weeks post-STZ. *** p < 0.001. Results expressed as means ± SEM with scatter plot.
Weights and blood glucose were not affected by exercise treatment (Fig. 1). While not significant, diabetic rats that received exercise showed a trend for lower death rates (7.69%) than DM+inactive groups (12.82%; combination of DM+inactive+VEH and DM+inactive+ANA) or DM+active+ANA (18.18%; Table 1; Chi-square = 0.08, p = 0.960) and appeared less likely to need an insulin intervention (57.69%) than DM+inactive (64.71%) or DM+active+ANA (70.00%) groups (Table 1; Chi-square = 0.70, p = 0.704). DM+active+VEH rats also showed an increased likelihood (15.38%) of reverting to a non-diabetic state after two consecutive high blood glucose readings (>250 mg/dL) than DM+inactive (5.13%) or DM+active+ANA (0%) groups (Table 1; Chi-square = 12.90, p = 0.002). This reversion usually occurred between 1-4 weeks post-STZ injection. Despite a propensity to revert to a non-diabetic state, DM+active+VEH rats that maintained hyperglycemia did not show reduced blood glucose levels compared with other diabetic groups (Fig. 1B).
Table 1. Death rates, reversion to a non-diabetic state, and need for insulin intervention in active and inactive diabetic rats.
Inactive includes DM+inactive+VEH rats.
| Inactive (n=34) | Active (n=26) | Active + ANA (n=11) | |
|---|---|---|---|
| % of rats that died/were sacrificed | 14.71% | 7.69% | 18.18% |
| % of rats that reverted to non-diabetic state* | 5.88% | 15.38% | 0.00% |
| % of rats that needed insulin | 75.86% | 57.69% | 70.00% |
Note: once diabetic rats reverted to a non-diabetic state, they were excluded from the study.
Chi-square p=0.002.
Protective effects of exercise on spatial frequency and contrast sensitivity deficits are blocked by treatment with ANA-12
Beginning at 4 weeks post-STZ, DM rats showed a significant difference in spatial frequency thresholds depending on whether they received exercise and/or ANA-12 [Two-way repeated ANOVA interaction effect F(16, 456) = 12.974, p < 0.001]. DM+inactive+VEH rats showed significant reductions in spatial frequency thresholds compared with non-diabetic controls (at 4 weeks, p = 0.004). Exercised diabetic rats (DM+active+VEH) exhibited preserved visual acuity with spatial frequency thresholds significantly higher than those of DM+inactive+VEH rats (at 8 weeks, values normalized to non-diabetic controls and each animal’s individual baseline: DM+active+VEH: 0.94±0.02; DM+inactive+VEH: 0.82±0.03; p < 0.001). Daily injections of ANA-12 blocked the protective effects of exercise such that spatial frequency thresholds of DM+active+ANA rats were statistically indistinguishable from DM+inactive+VEH thresholds between 4 and 8 weeks post-diabetic induction (at 8 weeks, values normalized to non-diabetic controls and each animal’s individual baseline: DM+inactive+VEH: 0.82±0.03; DM+active+ANA: 0.76±0.03; p = 0.076) (Fig. 2A).
Figure 2. Protective effects of exercise on spatial frequency and contrast sensitivity thresholds in diabetic rats are blocked by the TrkB antagonist, ANA-12.
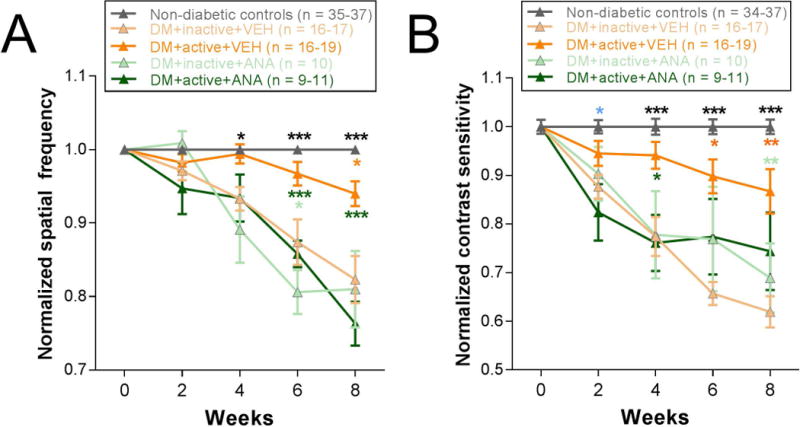
A) Spatial frequency thresholds for weeks 0 to 8 post-STZ, normalized to control rat values and each rat’s individual baseline. B) Contrast sensitivity thresholds from 0 to 8 weeks post-STZ, normalized to control rat values and each rat’s individual baseline. Black asterisks indicate comparisons of non-diabetic controls to DM+inactive+VEH, DM+inactive+ANA, and DM+active+ANA groups; green asterisks indicate comparisons of DM+active+VEH group to all other DM groups (DM+inactive+VEH, DM+inactive+ANA, and DM+active+ANA); light green asterisks indicate comparisons of DM+active+VEH to both inactive DM groups (DM+inactive+VEH and DM+inactive+ANA); orange asterisks indicate comparisons of non-diabetic controls to DM+active+VEH group; light blue asterisks indicate comparisons of non-diabetic controls to DM+active+ANA and DM+inactive+VEH groups. *** p < 0.001, ** p < 0.01, * p < 0.05. Results expressed as means ± SEM.
Similarly, beginning at 4 weeks post-STZ, DM+inactive+VEH rats showed significant reductions in contrast sensitivity compared with non-diabetic controls (Two-way repeated ANOVA interaction effect F(16, 456) = 8.179, p < 0.001; post-hoc comparison: p < 0.001). Beginning at 4 weeks, exercise preserved contrast sensitivity in diabetic rats with contrast sensitivity thresholds significantly higher in DM+active+VEH rats compared to DM+inactive+VEH (p = 0.002). ANA-12 treatment abolished this effect such that DM+active+ANA rats had contrast sensitivity thresholds significantly lower than DM+active+VEH rats (at 4 weeks; p = 0.003). In fact, DM+active+ANA contrast sensitivity thresholds were statistically equivalent to the contrast sensitivity thresholds of the DM+inactive+VEH group at all relevant time points (at 8 weeks: normalized value for DM+inactive+VEH: 0.62±0.03; DM+active+ANA: 0.74±0.08, p = 0.160) (Fig. 2B).
Protective effects of exercise on ERG OPs and flicker implicit times are blocked by treatment with ANA-12
Representative OP waveforms showed delays in inactive diabetic rats that were not observed in diabetic rats that received exercise. ANA-12 treatment blocked this protection (Fig. 3A). When quantified, OP2 implicit time (flash intensity −1.9 log cd s/m2) was significantly delayed at 4 and 8 weeks post-STZ in all 4 diabetic rat groups compared with non-diabetic controls (Fig. 3C; Two-way repeated ANOVA interaction effect F(4,191) = 3.544, p = 0.01). This difference was measureable at 4-weeks post-STZ (p < 0.001). By 8 weeks, however, diabetic rats assigned to exercise treatment showed significant improvements in OP2 implicit time delays compared with inactive rats, (DM+inactive+VEH rats 1.14±0.02 vs. DM+active+VEH 1.07±0.01, normalized to control; post hoc comparison, p < 0.001). Treatment with ANA-12 abolished this protective effect, such that DM+active+ANA rats (1.17±0.02) were statistically indistinguishable from DM+inactive+VEH rats (Fig. 3C). Amplitudes and implicit times for OP1, 3, and 4 were also analyzed and showed the same implicit time delays with diabetes and attenuation with exercise (data not shown); OP2 is shown here because it has the best signal to noise ratio.
Figure 3. Exercise protects ERG OP2 and flicker implicit times through TrkB pathway signaling.
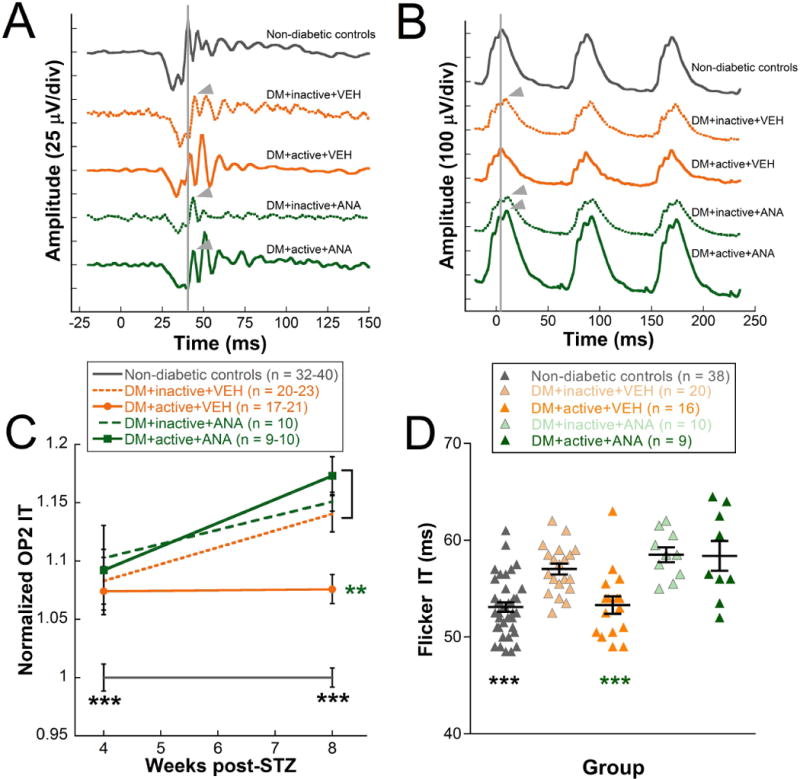
A) Representative OP waveforms at 8 weeks post-STZ in response to −1.9 log cd s/m2 flash stimuli showed OP2 delays in DM+inactive+VEH, DM+inactive+ANA, and DM+active+ANA (arrowheads) compared to non-diabetic controls and DM+active+VEH (indicated by vertical gray line). B) Representative flicker ERG waveforms from rats at 8 weeks post-STZ showed similar delays as the OPs. The vertical gray line indicates the flicker b-wave peak and the arrowheads mark delayed waves. C) OP2 implicit times for weeks 4-8 post-STZ, normalized to non-diabetic control rat values. D) Flicker ERG implicit times at 8 weeks post-STZ. Black asterisks indicate comparisons of non-diabetic controls to all diabetic groups; green asterisks indicate comparisons of DM+active+VEH group to DM+inactive+VEH, DM+inactive+ANA, and DM+active+ANA groups. *** p < 0.001, ** p < 0.01. Results expressed as means ± SEM.
At 4 weeks, ERG flicker amplitudes and implicit times were not different between the treatment groups. At 8 weeks, representative flicker waveforms showed delays in inactive diabetic rats that were not observed in diabetic rats that received exercise. ANA-12 treatment blocked this protection (Fig. 3B). When 8 week data was quantified, DM+inactive+VEH rats exhibited significant delays in ERG flicker implicit times (57.0±0.6 ms) compared to non-diabetic control rats (Fig. 3D; 53.1±0.5 ms; One-way ANOVA F(4, 92) = 12.407, p < 0.001; posthoc comparision, p < 0.001). Exercise maintained the flicker implicit times in DM+active+VEH rats, which were statistically indistinguishable from non-diabetic controls and had significantly faster flicker implicit times than DM+inactive+VEH rats (53.3±0.9 vs. 57.0±0.6 ms, p < 0.001). In contrast, active diabetic rats injected daily with ANA-12 had 8-week flicker implicit times that were indistinguishable from the inactive diabetic rats, and thus, were significantly slower than those of the DM+active+VEH group (DM+active+ANA: 58.4±1.5 ms; Fig. 3D).
A- and b-wave amplitudes and implicit times, oscillatory potential amplitudes, and flicker amplitudes were also analyzed, but the results were not significantly different between treatment groups (data not shown).
Exercise reduced cognitive function deficits in diabetic rats but did not change exploratory behavior; ANA-12 blocked protection
At 8 weeks post-STZ, inactive diabetic rats showed significant reductions in spatial alternation compared to non-diabetic controls (values normalized to non-diabetic controls: 1.01±0.03; DM+inactive+VEH: 0.85±0.04, One-way ANOVA, F (4,64) = 5.429, p < 0.001; post-hoc comparision p = 0.002). With exercise treatment, these cognitive deficits were not observed in diabetic rats (DM+active+VEH normalized values: 1.04±0.06, p = 0.007). Futhermore, treatment with ANA-12 abolished the protective effects of exercise on cognitive deficits (DM+active+ANA12 normalized values: 0.77±0.06, p = 0.021) (Fig. 4A). For exploratory behavior, diabetic groups showed reductions in number of entries on Y-maze compared with non-diabetic controls, but neither exercise nor ANA-12 injections altered the magnitude of these deficits (One-way ANOVA, F (4,64) = 4.665, p = 0.002) (Fig. 4B).
Figure 4. Beneficial effects of exercise on cognitive function deficits, but not exploratory deficits.
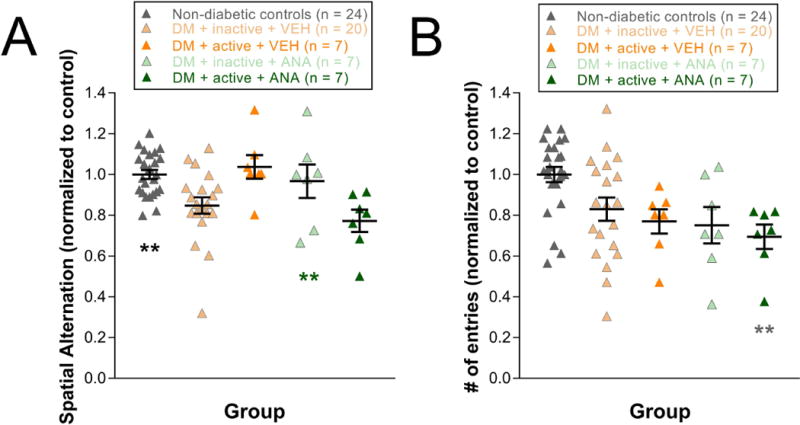
A) Cognitive function at 8 weeks post-STZ as measured by spatial alternation on Y-maze (values normalized to control). Black asterisks indicate a comparison of Non-diabetic controls to DM+inactive+VEH and DM+active+ANA groups. Green asterisks indicate a comparison of DM+active+VEH group to DM+inactive+VEH, and DM+active+ANA groups. B) Exploratory behavior at 8 weeks post-STZ as measured by # of entries (number of entries) on Y-maze (values normalized to control). Gray asterisks indicate a comparison to Non-diabetic controls. ** p < 0.01. Results expressed as means ± SEM.
Effects of diabetes and exercise on visual and cognitive deficits
In a subset of the inactive diabetic animals presented above, we performed Y-maze tests at 4 and 6 weeks, in addition to 8 weeks, so that we could compare cognitive function to 4, 6, and 8 week visual function results. The earliest significant deficits in visual function were in contrast sensitivity at 4 weeks post-STZ (Two-way repeated ANOVA Interaction effect, F(2, 61) = 13.192, p < 0.001; post-hoc comparision p = 0.046). Meanwhile, significant cognitive deficits, measured as spatial alternation on Y-maze, did not appear until 6 weeks (Two-way repeated ANOVA Main effect, F(1, 50) = 7.505, p = 0.01; post-hoc comparison p = 0.027) (Fig. 5A).
Figure 5. Effects of diabetes and exercise on visual and cognitive deficits in rats.
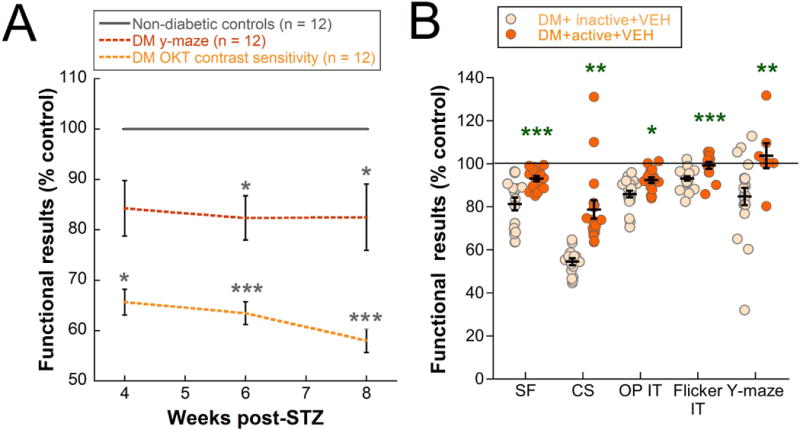
A) Diabetes resulted in deficits in cognitive function as measured by spatial alternation on Y-maze and retinal function as measured by contrast sensitivity on OMR at 4, 6, and 8 weeks (values expressed as % control). Black asterisks indicate a comparison to Non-diabetic controls. B) A summary of the protective effects of exercise on visual and cognitive deficits when comparing inactive vs. active diabetic rats (values are expressed as % control and represent 8 week data for all tests). Green asterisks indicate a comparison of DM+active+VEH group to DM+inactive+VEH group. *** p < 0.001, ** p < 0.01, * p < 0.05. SF = spatial frequency, CS = contrast sensitivity, OP IT = oscillatory potential implicit time, Flicker IT = flicker implicit time. Results expressed as means ± SEM.
Exercise interventions in diabetic rats reduced functional deficits on a variety of tests: OMR (both spatial frequency and contrast sensitivity), ERG (both oscillatory potential and flicker implicit times), and Y-maze (spatial alternation only). In Figure 5B, we compare exercise efficacy for each of these five parameters as a percent of the control values for the 8-week data in DM+inactive+VEH and DM+active+VEH rats. Contrast sensitivity showed the largest percent difference in active versus inactive animals (24%; Fig. 5B). However, Y-maze and flicker implicit time, while exhibiting smaller changes in percent difference, were completely preserved with exercise, having functional scores that were maintained at control levels (Fig. 5B).
Retinal NT-4 levels did not change significantly with diabetes or exercise
NT-4 ELISAs were performed on retinas from diabetic rats and non-diabetic controls at 8 weeks post-STZ. No differences in NT-4 levels were observed in any treatment group relative to non-diabetic controls (Fig. 6).
Figure 6. Retinal NT-4 levels did not change significantly with diabetes or exercise.
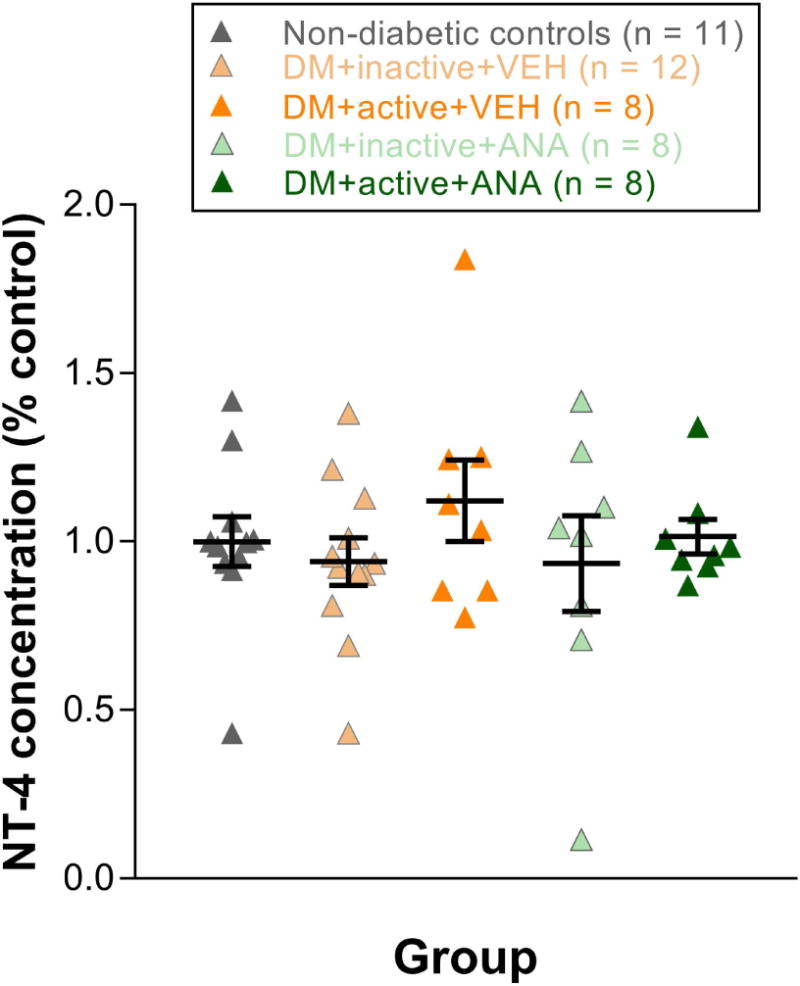
Retinal NT-4 levels at 8 weeks post-STZ. Results expressed as means ± SEM.
DISCUSSION
Exercise was found to be neuroprotective against early deficits in visual and cognitive function in diabetic rats that exhibited confirmed hyperglycemia prior to exercise treatment. The delayed appearance of retinal deficits in diabetic rats with exercise was blocked using ANA-12, an antagonist that binds to the TrkB receptor and blocks signal transduction, suggesting that the TrkB pathway is critical for exercise protection in diabetes.
Early retinal and cognitive deficits with hyperglycemia
We observed early retinal deficits in STZ-induced diabetic rats, including spatial frequency and contrast sensitivity deficits at 4 weeks post-STZ, delays in oscillatory potential implicit time at 4 weeks, and delays in flicker implicit time at 8 weeks. Similar to previous reports from our lab and others (Barber, 2003; Antonetti et al., 2006; Aung et al., 2013; Pardue et al., 2014), these functional deficits occurred early in the disease, well before acellular capillaries and pericyte dropout are observed in the retina (6-8 months) (Kern et al., 2010). While others have observed decreases in a- and b-wave responses at similar time points post-STZ (Li et al., 2002; Ly et al., 2011), in our hands, the STZ rat model does not show consistent changes in a- and b-wave amplitude or implicit time (Aung et al., 2013; Aung et al., 2014; Pardue et al., 2014). We only see consistent changes in OP implicit times, with the dim flash responses showing delays before the bright flash responses, and light-adapted flicker implicit times (Aung et al., 2013; Pardue et al., 2014).
Using a Y-maze test to assess spartial alternation, we observed early cognitive deficits with hyperglycemia at 8 weeks post-STZ. Cognitive deficits have been reported previously with diabetes, both in STZ-induced and high fat diet models (Reisi et al., 2009; Datusalia & Sharma, 2014; Klein et al., 2016; Underwood & Thompson, 2016). In order to determine whether retinal or cognitive deficits presented first, we performed OMR and Y-maze testing at 4, 6, and 8 weeks in a subset of rats. We chose to use contrast sensitivity on OMR as our measure of retinal function because it showed the greatest deficit. Deficits in contrast sensitivity appeared prior to Y-maze deficits (4 weeks vs. 6 weeks) (Fig. 5A). Additionally, deficits in contrast sensitivity were more severe than Y-maze deficits (55% vs. 84%) (Fig. 5A). Taken together, these results suggest that the retina is affected early in diabetes and could be used as an early biomarker for the extent of diabetic injury to the brain.
Exercise protected against retinal and cognitive deficits in diabetic rats
Previously, exercise treatment has been shown to be protective in a variety of retinal disease models, including light induced retinal degeneration (Lawson et al., 2014), the rd10 model of retinal degeneration (Hanif et al., 2015), intraocular pressure injury of ganglion cells (Chrysostomou et al., 2014), natural aging (Kim et al., 2015), and diabetes (Kim et al., 2013). While treadmill exercise was shown to reduce apoptosis (Kim et al., 2013) and VEGF expression (Ji et al., 2013) in the retina of STZ-induced diabetic rats, the effects of exercise on retinal function deficits were not investigated. Here, we showed that exercise treatment in STZ-induced diabetes resulted in delayed appearance of deficits in spatial frequency and contrast sensitivity as measured by OMR and reduced delays in implicit time for oscillatory potentials and flicker ERG. We focused on functional rather than histological results in this paper because visual function had yet to be investigated with respect to diabetic retinopathy and exercise and because cell death has primarily been reported after the latest time point investigated here (8 weeks) (Barber et al., 2005; Gastinger et al., 2006; Kern & Barber, 2008; Adamiec-Mroczek et al., 2015), though increases in caspase-3 and other apoptotic proteins have been reported as early as 2-4 weeks (Barber et al., 2005; Li et al., 2008; Adamiec-Mroczek et al., 2015). Interestingly, the exercise protection observed here and elsewhere in diabetic retinopathy coupled with our earlier work on the protective effects of exercise in retinal degeneration models may reflect a generalized protective mechanism to retinal neuronal dysfunction and cell loss rather than a disease-specific treatment. In these experiments, exercise did not provide complete protection from diabetic retinopathy, but rather induced signficant delays in the observed dysfunction by several weeks (Fig. 2 for spatial frequency and contrast sensitivity thresholds). It is not known from this dataset if the exercised animals would have sustained benefit with disease progression. Future studies could expose diabetic rats to exercise for a longer period (6-8 months) to determine the long-term effects.
Exercise has been shown previously to be protective against diabetes-induced cognitive deficits, as measured by Morris Water Maze (Reisi et al., 2009; Klein et al., 2016). Our finding that exercise reduced Y-maze deficits after diabetes confirmed these results. Interestingly, exercise reduced spatial alternation deficits on Y-maze, but not exploratory behavior deficits, perhaps because retinal and spatial memory deficts share a neuronal origin, while exploratory behavior deficits are more an indicator of overall systemic disease (i.e., diabetic rats not moving around as much due to general sickness behavior). It is possible that visual improvements with exercise are due to an enhancement of brain function by exercise. However, given that exercise protection in the retina (using ERG) is observed prior to exercise protection in the brain, the retinal protection appears to be, at least in part, tissue specific.
In order to determine which functional responses were most impacted by the effects of exercise, we compared exercise protection on a variety of tests. We found that while contrast sensitivity showed the greatest difference in active vs. inactive rats, flicker implicit time and Y-maze scores for active rats reached control levels (Fig. 5B). In addition, diabetic rats receiving exercise showed a significant increase in the propensity to revert to a non-diabetic state, while there was a non-significant trend towards reduced death rates and a reduced need for insulin intervention. Previous studies have demonstrated a protective effect of exercise on mortality related to cardiovascular fitness in diabetic animals (Souza et al., 2007) and people (Wei et al., 2000), as well as a reduced need for insulin use in women with gestational diabetes (Brankston et al., 2004). However, while exercise has been shown to reduce insulin resistance, hyperglycemia, and HbA1c in diabetic patients, we observed no differences in weight and blood glucose between our exercised diabetic group and any other diabetic group.
TrkB pathway signaling mediates the protective effects of exercise in both the retina and the brain
A number of studies point to the TrkB signal transduction pathway and its ligands as a mediator of exercise’s protective effects (Ploughman et al., 2007; Griffin et al., 2011; Voss et al., 2013a; Lawson et al., 2014; Leckie et al., 2014; Marosi & Mattson, 2014; Hanif et al., 2015; Chrysostomou et al., 2016). Inhibiting the TrkB pathway via ANA-12, which prevents ligands from binding to the TrkB receptor, blocked exercise’s protective effects in light-induced and hereditary models of retinal degeneration (Lawson et al., 2014; Hanif et al., 2015). Many exercise studies highlight BDNF as the protection-mediating ligand, including previous work by our group demonstrating that mice undergoing treadmill exercise following light-induced retinal degeneration exhibit higher levels of BDNF in the retina, hippocampus, and serum (Lawson et al., 2014). However, rats express higher levels of NT-4 in addition to BDNF in healthy spinal cord after treadmill exercise (Skup et al., 2002) and in injured spinal cord after bike exercise (Cote et al., 2011). Additionally, exercise protection after peripheral nerve injury is blocked in NT-4/5 knockout mice (English et al., 2011). These studies suggest that both NT-4 and BDNF could be upregulated with exercise.
In this study, we investigated TrkB pathway activation and NT-4 in the diabetic retina with and without exercise treatment. Treating active diabetic rats with the TrkB inhibitor, ANA-12, abolished the protective effects of exercise on both retinal and cognitive function. Specifically, active diabetic rats that received ANA-12 showed no benefit to spatial frequency and contrast sensitivity decreased thresholds, oscillatory potential and flicker implicit time delays, or spatial alternation deficits on Y-maze. In sum, these results suggest that TrkB signaling plays a role in mediating the protective effects of exercise in the diabetic retina and brain and demonstrates that ANA-12 can block the effects of exercise on both vision and cognition simultaneously.
It is interesting to speculate on the precise mechanism by which the TrkB pathway is mediating exercise protection in the retina. TrkB receptor expression has been localized to a number of retinal layers, including the retinal pigmented epithelium, outer plexiform layer, inner nuclear layer, inner plexiform layer, retinal ganglion cell layer, and in Müller cells in rodents exposed to light damage and dopaminergic amacrine cells (Cellerino & Kohler, 1997; Asai et al., 2007; Saito et al., 2009). TrkB’s truncated isoform, which is also capable of acting as a signaling receptor, has been observed in Müller cells of normal rats as well (Berk et al., 2015). Additionally, the TrKB inhibitor ANA-12 has been shown to reduce phosphorylated TrkB, phosphorylated ERK1/2, and Akt signaling in the retina, demonstrating that TrkB signaling occurs in the retina (Mazzaro et al., 2016; Daly et al., 2017).
NT-4 is widely expressed in the retina and has been identified in the inner and outer nuclear layers (Ghazi-Nouri et al., 2003), retinal ganglion cells (Ghazi-Nouri et al., 2008), photoreceptor outer segments (Ghazi-Nouri et al., 2008), and in Müller cells during proliferative vitreoretinopathy (Ghazi-Nouri et al., 2008). Additionally, NT-4 was shown to rescue neurons in retinal explants from STZ diabetic rats (Oshitari et al., 2011; Oshitari et al., 2014), and both NT-4 and BDNF protect against retinal ganglion cell death in an optic nerve transection model when administered intraocularly (Peinado-Ramon et al., 1996).
However, NT-4 levels did not show significant differences in diabetic animals, regardless of whether animals were given exercise treatment or ANA-12 (Fig. 6). These results suggest that the TrkB signaling underlying the protective effects of exercise on the retina may involve other neurotrophins besides NT-4.
Ligands other than NT-4 (BDNF, neurotrophin-3, nerve growth factor) are also capable of activating the TrkB receptor and could be involved in mediating the protective effects of exercise (Reichardt, 2006). The protective effects of exercise are pleitropic. In injury models outside the retina, exercise’s effects have been shown to affect neurotrophins differently in different tissue types (Keeler et al., 2012) and even different cell types (Skup et al., 2002). For example, when looking at exercise’s effects on the spinal cord, NT-4 expression was increased more in astrocytes and BDNF expression was increased more in neurons (Skup et al., 2002). Expression patterns of neurotrophins also differ based on injury type (Omura et al., 2005) and exercise type (Cote et al., 2011). Future research could investigate precisely where the TrkB pathway and neurotrophic factors are acting in the retina and how these actions are changing with different diseases and with exercise treatment.
Translation of exercise as a treatment in clinical diabetes
Exercise is an exciting treatment option for diabetic patients because it is non-invasive, can be started early on in the disease, and causes changes in retinal, visual, and cognitive function. In this study, exercise treatment was begun in rats shortly after the onset of diabetes. The fact that we used a treatment prevention protocol (exercise begins after confirmed hyperglycemia) as opposed to a preconditioning protocol (exercise begins prior to diabetes induction) is a critical distinction that makes these experiments more clinically relevant. Future studies should investigate the effects of implementing an exercise program at later time points (for example, after clear visual and retinal deficits have occurred, but prior to any vascular pathology), which would make this research even more applicable to clinical diabetes.
The exercise paradigm used here would be very manageable for patients: 30 minutes of exercise, 5 times a week at a rate of 15 m/min. The speed used here appears to translate to a purposeful walking speed in humans. If patients were informed that even walking has a substantial protective effect - that they aren’t expected to immediately shift from a sedentary lifestyle to running marathons - they might be more inclined to incorporate an exercise program into their lifestyle and treatment plan. Indirect evidence has shown that glycemic control and a healthy lifestyle (which includes exercise) decreases the risk of retinopathy (Loprinzi et al., 2014; Aiello et al., 2015; Bener et al., 2016). Research on exercise in diabetic and prediabetic patients is already being conducted to investigate insulin resistance, cognitive function, and conversion from prediabetes to diabetes, among other conditions (Anderson-Hanley et al., 2012; Fiocco et al., 2013; Davy et al., 2017; Nuhu & Maharaj, 2017; Rowan et al., 2017; Sjoberg et al., 2017). These studies would benefit from adding retinal pathology and visual function to the parameters investigated, as the retina may provide a window into some of the earliest functional deficits in diabetes as well as the earliest protection with exercise.
Conclusions
Exercise reduced early retinal and visual dysfunction in a rat model of Type I diabetes when implemented after the onset of hyperglycemia. Exercise also reduced deficits in cognitive function but did not change exploratory behavior. Exercise protection for retinal, visual, and cognitive function is blocked with ANA-12, suggesting a role for TrkB pathway signaling in mediating exercise’s protective effects in both the retina and the brain. Changes in retinal NT-4 were not observed with diabetes or exercise, suggesting other TrkB ligands may be involved. Future research is needed to define Trk-B pathway involvement in exercise-mediated protection in diabetes.
Acknowledgments
This work was supported by the National Institutes of Health [grant numbers P30 EY006360, R01 EY014026, and T32 EY007092-27 ]; the Rehabilitation R&D Service of the Department of Veterans Affairs (Merit Award I01RX000951 and Research Career Scientist Award to MTP), Research to Prevent Blindness, and the Abraham J. and Phyllis Katz Foundation.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- CS
Contrast sensitivity
- DM
Diabetes mellitus
- ELISA
Enzyme-linked immunoabsorbent assay
- ERG
Electroretinogram
- IT
Implicit Time
- NT-4
Neurotrophin-4
- OMR
Optomotor response
- OP
Oscillatory potential
- SF
Spatial frequency
- STZ
Streptozotocin
- TrkB
Tropomyosin receptor kinase B
- VEGF
Vascular endothelial growth factor
- VEH
Vehicle
Footnotes
DR. RACHAEL ALLEN (Orcid ID : 0000-0002-7216-6646)
Conflict of Interest
None of the authors have any interest or relationship, financial or otherwise, which might be perceived as influencing the objectivity of the author.
Author contributions
All authors contributed substantiatively to this manuscript, including designing the study (Allen, Hanif, Gogniat, Prall, Boatright, Pardue), running the experiments and collecting the data (Allen, Hanif, Gogniat, Prall, Haider, Aung, Prunty, Mees, Coulter), analyzing the data and interpreting the results (Allen, Hanif, Gogniat, Prall, Haider, Aung, Prunty, Mees, Coulter, Motz, Boatright, Pardue), writing the manuscript and crafting the figures (Allen, Hanif, Gogniat, Haider, Coulter, Motz, Pardue), and reading and editing the paper (all authors).
Data Accessibility
All data generated in this study has been stored on local VA computers and backed up on local VA servers. Data sets will be shared directly with interested parties after a formal written request is received by the corresponding author (Dr. Pardue).
References
- Adamiec-Mroczek J, Zajac-Pytrus H, Misiuk-Hojlo M. Caspase-Dependent Apoptosis of Retinal Ganglion Cells During the Development of Diabetic Retinopathy. Adv Clin Exp Med. 2015;24:531–535. doi: 10.17219/acem/31805. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Sun W, Das A, Gangaputra S, Kiss S, Klein R, Cleary PA, Lachin JM, Nathan DM. Intensive diabetes therapy and ocular surgery in type 1 diabetes. N Engl J Med. 2015;372:1722–1733. doi: 10.1056/NEJMoa1409463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson-Hanley C, Arciero PJ, Westen SC, Nimon J, Zimmerman E. Neuropsychological benefits of stationary bike exercise and a cybercycle exergame for older adults with diabetes: an exploratory analysis. J Diabetes Sci Technol. 2012;6:849–857. doi: 10.1177/193229681200600416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, Robinson JK. Exercise influences spatial learning in the radial arm maze. Physiol Behav. 2000;70:425–429. doi: 10.1016/s0031-9384(00)00282-1. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IA. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- Asai N, Abe T, Saito T, Sato H, Ishiguro S, Nishida K. Temporal and spatial differences in expression of TrkB isoforms in rat retina during constant light exposure. Exp Eye Res. 2007;85:346–355. doi: 10.1016/j.exer.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Aung MH, Kim MK, Olson DE, Thule PM, Pardue MT. Early visual deficits in streptozotocin-induced diabetic long evans rats. Invest Ophthalmol Vis Sci. 2013;54:1370–1377. doi: 10.1167/iovs.12-10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MH, Park HN, Han MK, Obertone TS, Abey J, Aseem F, Thule PM, Iuvone PM, Pardue MT. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci. 2014;34:726–736. doi: 10.1523/JNEUROSCI.3483-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:283–290. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- Beisswenger PJ. Neuropathic and vascular complications occurring in diabetes. Postgrad Med. 1976;59:169–174. doi: 10.1080/00325481.1976.11716534. [DOI] [PubMed] [Google Scholar]
- Bener A, Al-Hamaq AO, Kurtulus EM, Abdullatef WK, Zirie M. The role of vitamin D, obesity and physical exercise in regulation of glycemia in Type 2 Diabetes Mellitus patients. Diabetes Metab Syndr. 2016;10:198–204. doi: 10.1016/j.dsx.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Berk BA, Vogler S, Pannicke T, Kuhrt H, Garcia TB, Wiedemann P, Reichenbach A, Seeger J, Bringmann A. Brain-derived neurotrophic factor inhibits osmotic swelling of rat retinal glial (Muller) and bipolar cells by activation of basic fibroblast growth factor signaling. Neuroscience. 2015;295:175–186. doi: 10.1016/j.neuroscience.2015.03.037. [DOI] [PubMed] [Google Scholar]
- Billinger SA, Sisante JV, Alqahtani AS, Pasnoor M, Kluding PM. Aerobic exercise improves measures of vascular health in diabetic peripheral neuropathy. Int J Neurosci. 2017;127:80–85. doi: 10.3109/00207454.2016.1144056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brankston GN, Mitchell BF, Ryan EA, Okun NB. Resistance exercise decreases the need for insulin in overweight women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;190:188–193. doi: 10.1016/s0002-9378(03)00951-7. [DOI] [PubMed] [Google Scholar]
- Cazorla M, Arrang JM, Premont J. Pharmacological characterization of six trkB antibodies reveals a novel class of functional agents for the study of the BDNF receptor. Br J Pharmacol. 2011a;162:947–960. doi: 10.1111/j.1476-5381.2010.01094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest. 2011b;121:1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellerino A, Kohler K. Brain-derived neurotrophic factor/neurotrophin-4 receptor TrkB is localized on ganglion cells and dopaminergic amacrine cells in the vertebrate retina. J Comp Neurol. 1997;386:149–160. [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, Pontifex MB, Raine LB, Konkel A, Hillman CH, Cohen NJ, Kramer AF. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhao Y, Zhou Z, Zhang X, Li Q, Bai L, Zhang M. Prevalence and risk factors of diabetic retinopathy in Chongqing pre-diabetes patients. Eye (Lond) 2012;26:816–820. doi: 10.1038/eye.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysostomou V, Galic S, van Wijngaarden P, Trounce IA, Steinberg GR, Crowston JG. Exercise reverses age-related vulnerability of the retina to injury by preventing complement-mediated synapse elimination via a BDNF-dependent pathway. Aging Cell. 2016 doi: 10.1111/acel.12512. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysostomou V, Kezic JM, Trounce IA, Crowston JG. Forced exercise protects the aged optic nerve against intraocular pressure injury. Neurobiol Aging. 2014;35:1722–1725. doi: 10.1016/j.neurobiolaging.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Chung JY, Kim MW, Bang MS, Kim M. Increased expression of neurotrophin 4 following focal cerebral ischemia in adult rat brain with treadmill exercise. PLoS One. 2013;8:e52461. doi: 10.1371/journal.pone.0052461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavatta VT, Kim M, Wong P, Nickerson JM, Shuler RK, Jr, McLean GY, Pardue MT. Retinal expression of Fgf2 in RCS rats with subretinal microphotodiode array. Invest Ophthalmol Vis Sci. 2009;50:4523–4530. doi: 10.1167/iovs.08-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Kluding PM, Wright DE. Emerging Relationships between Exercise, Sensory Nerves, and Neuropathic Pain. Front Neurosci. 2016;10:372. doi: 10.3389/fnins.2016.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, Azzam GA, Lemay MA, Zhukareva V, Houle JD. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma. 2011;28:299–309. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C, Shine L, Heffernan T, Deeti S, Reynolds AL, O’Connor JJ, Dillon ET, Duffy DJ, Kolch W, Cagney G, Kennedy BN. A Brain-Derived Neurotrophic Factor Mimetic Is Sufficient to Restore Cone Photoreceptor Visual Function in an Inherited Blindness Model. Sci Rep. 2017;7:11320. doi: 10.1038/s41598-017-11513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datusalia AK, Sharma SS. Amelioration of diabetes-induced cognitive deficits by GSK-3beta inhibition is attributed to modulation of neurotransmitters and neuroinflammation. Mol Neurobiol. 2014;50:390–405. doi: 10.1007/s12035-014-8632-x. [DOI] [PubMed] [Google Scholar]
- Davy BM, Winett RA, Savla J, Marinik EL, Baugh ME, Flack KD, Halliday TM, Kelleher SA, Winett SG, Williams DM, Boshra S. Resist diabetes: A randomized clinical trial for resistance training maintenance in adults with prediabetes. PLoS One. 2017;12:e0172610. doi: 10.1371/journal.pone.0172610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Strachan MW, Reynolds RM, Frier BM, Deary IJ, Fowkes FG, Lee AJ, McKnight J, Halpin P, Swa K, Price JF. Diabetic retinopathy and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59:2883–2889. doi: 10.2337/db10-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci. 2005;22:677–684. doi: 10.1017/S0952523805225166. [DOI] [PubMed] [Google Scholar]
- English AW, Cucoranu D, Mulligan A, Rodriguez JA, Sabatier MJ. Neurotrophin-4/5 is implicated in the enhancement of axon regeneration produced by treadmill training following peripheral nerve injury. Eur J Neurosci. 2011;33:2265–2271. doi: 10.1111/j.1460-9568.2011.07724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, Deary IJ, Frier BM. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes. 2003;52:149–156. doi: 10.2337/diabetes.52.1.149. [DOI] [PubMed] [Google Scholar]
- Fiocco AJ, Scarcello S, Marzolini S, Chan A, Oh P, Proulx G, Greenwood C. The effects of an exercise and lifestyle intervention program on cardiovascular, metabolic factors and cognitive performance in middle-aged adults with type II diabetes: a pilot study. Can J Diabetes. 2013;37:214–219. doi: 10.1016/j.jcjd.2013.03.369. [DOI] [PubMed] [Google Scholar]
- Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- Gastinger MJ, Singh RS, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest Ophthalmol Vis Sci. 2006;47:3143–3150. doi: 10.1167/iovs.05-1376. [DOI] [PubMed] [Google Scholar]
- Ghazi-Nouri SM, Assi A, Limb GA, Scott RA, von Bussmann K, Humphrey I, Luthert PJ, Charteris DG. Laser photocoagulation alters the pattern of staining for neurotrophin-4, GFAP, and CD68 in human retina. Br J Ophthalmol. 2003;87:488–492. doi: 10.1136/bjo.87.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi-Nouri SM, Ellis JS, Moss S, Limb GA, Charteris DG. Expression and localisation of BDNF, NT4 and TrkB in proliferative vitreoretinopathy. Exp Eye Res. 2008;86:819–827. doi: 10.1016/j.exer.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Griffin EW, Mullally S, Foley C, Warmington SA, O’Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Groot C, Hooghiemstra AM, Raijmakers PG, van Berckel BN, Scheltens P, Scherder EJ, van der Flier WM, Ossenkoppele R. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res Rev. 2015;25:13–23. doi: 10.1016/j.arr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Haan M, Espeland MA, Klein BE, Casanova R, Gaussoin SA, Jackson RD, Millen AE, Resnick SM, Rossouw JE, Shumaker SA, Wallace R, Yaffe K. Cognitive function and retinal and ischemic brain changes: the Women’s Health Initiative. Neurology. 2012;78:942–949. doi: 10.1212/WNL.0b013e31824d9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanif AM, Lawson EC, Prunty M, Gogniat M, Aung MH, Chakraborty R, Boatright JH, Pardue MT. Neuroprotective Effects of Voluntary Exercise in an Inherited Retinal Degeneration Mouse Model. Invest Ophthalmol Vis Sci. 2015;56:6839–6846. doi: 10.1167/iovs.15-16792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji ES, Ko IG, Cho JW, Davis RW, Hwang GY, Jee YS, Lim BV. Treadmill exercise inhibits apoptotic neuronal cell death with suppressed vascular endothelial growth factor expression in the retinas of the diabetic rats. J Exerc Rehabil. 2013;9:348–353. doi: 10.12965/jer.130043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler BE, Liu G, Siegfried RN, Zhukareva V, Murray M, Houle JD. Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury. Brain Res. 2012;1438:8–21. doi: 10.1016/j.brainres.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008;586:4401–4408. doi: 10.1113/jphysiol.2008.156695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern TS, Miller CM, Tang J, Du Y, Ball SL, Berti-Matera L. Comparison of three strains of diabetic rats with respect to the rate at which retinopathy and tactile allodynia develop. Mol Vis. 2010;16:1629–1639. [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Park S, Chun Y, Song W, Kim HJ, Kim J. Treadmill Exercise Attenuates Retinal Oxidative Stress in Naturally-Aged Mice: An Immunohistochemical Study. Int J Mol Sci. 2015;16:21008–21020. doi: 10.3390/ijms160921008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Jung SY, Kim CJ, Sung YH, Kim JD. Treadmill exercise ameliorates apoptotic cell death in the retinas of diabetic rats. Mol Med Rep. 2013;7:1745–1750. doi: 10.3892/mmr.2013.1439. [DOI] [PubMed] [Google Scholar]
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- Klein C, Jonas W, Iggena D, Empl L, Rivalan M, Wiedmer P, Spranger J, Hellweg R, Winter Y, Steiner B. Exercise prevents high-fat diet-induced impairment of flexible memory expression in the water maze and modulates adult hippocampal neurogenesis in mice. Neurobiol Learn Mem. 2016;131:26–35. doi: 10.1016/j.nlm.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Lawson EC, Han MK, Sellers JT, Chrenek MA, Hanif A, Gogniat MA, Boatright JH, Pardue MT. Aerobic exercise protects retinal function and structure from light-induced retinal degeneration. J Neurosci. 2014;34:2406–2412. doi: 10.1523/JNEUROSCI.2062-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, Phillips SM, Gothe NP, Mailey E, Vieira-Potter VJ, Martin SA, Pence BD, Lin M, Parasuraman R, Greenwood PM, Fryxell KJ, Woods JA, McAuley E, Kramer AF, Erickson KI. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci. 2014;8:985. doi: 10.3389/fnhum.2014.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zemel E, Miller B, Perlman I. Early retinal damage in experimental diabetes: electroretinographical and morphological observations. Exp Eye Res. 2002;74:615–625. doi: 10.1006/exer.2002.1170. [DOI] [PubMed] [Google Scholar]
- Li YH, Zhuo YH, Lu L, Chen LY, Huang XH, Zhang JL, Li SY, Wang XG. Caspase-dependent retinal ganglion cell apoptosis in the rat model of acute diabetes. Chin Med J (Engl) 2008;121:2566–2571. [PubMed] [Google Scholar]
- Loprinzi PD, Brodowicz GR, Sengupta S, Solomon SD, Ramulu PY. Accelerometer-assessed physical activity and diabetic retinopathy in the United States. JAMA Ophthalmol. 2014;132:1017–1019. doi: 10.1001/jamaophthalmol.2014.402. [DOI] [PubMed] [Google Scholar]
- Ly A, Yee P, Vessey KA, Phipps JA, Jobling AI, Fletcher EL. Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Invest Ophthalmol Vis Sci. 2011;52:9316–9326. doi: 10.1167/iovs.11-7879. [DOI] [PubMed] [Google Scholar]
- Mang CS, Campbell KL, Ross CJ, Boyd LA. Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 2013;93:1707–1716. doi: 10.2522/ptj.20130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Bayle J, Privat A. Learning impairment following acute administration of the calcium channel antagonist nimodipine in mice. Behav Pharmacol. 1995;6:167–175. [PubMed] [Google Scholar]
- Mazzaro N, Barini E, Spillantini MG, Goedert M, Medini P, Gasparini L. Tau-Driven Neuronal and Neurotrophic Dysfunction in a Mouse Model of Early Tauopathy. J Neurosci. 2016;36:2086–2100. doi: 10.1523/JNEUROSCI.0774-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrate ME, Kaspar BK. Physical activity and neuroprotection in amyotrophic lateral sclerosis. Neuromolecular Med. 2008;10:108–117. doi: 10.1007/s12017-008-8030-5. [DOI] [PubMed] [Google Scholar]
- Nuhu JM, Maharaj SS. Influence of a mini trampoline rebound exercise program on insulin resistance, lipid profile and central obesity in individuals with type 2 diabetes. J Sports Med Phys Fitness. 2017 doi: 10.23736/S0022-4707.17.07120-1. in press. [DOI] [PubMed] [Google Scholar]
- Omura T, Sano M, Omura K, Hasegawa T, Doi M, Sawada T, Nagano A. Different expressions of BDNF, NT3, and NT4 in muscle and nerve after various types of peripheral nerve injuries. J Peripher Nerv Syst. 2005;10:293–300. doi: 10.1111/j.1085-9489.2005.10307.x. [DOI] [PubMed] [Google Scholar]
- Oshitari T, Bikbova G, Yamamoto S. Increased expression of phosphorylated c-Jun and phosphorylated c-Jun N-terminal kinase associated with neuronal cell death in diabetic and high glucose exposed rat retinas. Brain Res Bull. 2014;101:18–25. doi: 10.1016/j.brainresbull.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Oshitari T, Yoshida-Hata N, Yamamoto S. Effect of neurotrophin-4 on endoplasmic reticulum stress-related neuronal apoptosis in diabetic and high glucose exposed rat retinas. Neurosci Lett. 2011;501:102–106. doi: 10.1016/j.neulet.2011.06.057. [DOI] [PubMed] [Google Scholar]
- Pardue MT, Barnes CS, Kim MK, Aung MH, Amarnath R, Olson DE, Thule PM. Rodent Hyperglycemia-Induced Inner Retinal Deficits are Mirrored in Human Diabetes. Transl Vis Sci Technol. 2014;3:6. doi: 10.1167/tvst.3.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier F, Boulogne A, Leys D, Fontaine P. Diabetes mellitus and dementia. Diabetes Metab. 2006;32:403–414. doi: 10.1016/s1262-3636(07)70298-7. [DOI] [PubMed] [Google Scholar]
- Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:489–500. [PubMed] [Google Scholar]
- Ploughman M. Exercise is brain food: the effects of physical activity on cognitive function. Dev Neurorehabil. 2008;11:236–240. doi: 10.1080/17518420801997007. [DOI] [PubMed] [Google Scholar]
- Ploughman M, Granter-Button S, Chernenko G, Attwood Z, Tucker BA, Mearow KM, Corbett D. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Res. 2007;1150:207–216. doi: 10.1016/j.brainres.2007.02.065. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Douglas RM. Enhancement of vision by monocular deprivation in adult mice. J Neurosci. 2006;26:11554–11561. doi: 10.1523/JNEUROSCI.3396-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Kaneko T, Tahara S, Nakamoto H, Pucsok J, Sasvari M, Nyakas C, Goto S. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem Int. 2001;38:17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisi P, Alaei H, Babri S, Sharifi MR, Mohaddes G. Effects of treadmill running on spatial learning and memory in streptozotocin-induced diabetic rats. Neurosci Lett. 2009;455:79–83. doi: 10.1016/j.neulet.2009.03.052. [DOI] [PubMed] [Google Scholar]
- Rowan CP, Riddell MC, Gledhill N, Jamnik VK. Aerobic Exercise Training Modalities and Prediabetes Risk Reduction. Med Sci Sports Exerc. 2017;49:403–412. doi: 10.1249/MSS.0000000000001135. [DOI] [PubMed] [Google Scholar]
- Saito T, Abe T, Wakusawa R, Sato H, Asai H, Tokita-Ishikawa Y, Nishida K. TrkB-T1 receptors on Muller cells play critical role in brain-derived neurotrophic factor-mediated photoreceptor protection against phototoxicity. Curr Eye Res. 2009;34:580–588. doi: 10.1080/02713680902972358. [DOI] [PubMed] [Google Scholar]
- Sjoberg KA, Frosig C, Kjobsted R, Sylow L, Kleinert M, Betik AC, Shaw CS, Kiens B, Wojtaszewski JFP, Rattigan S, Richter EA, McConell GK. Exercise Increases Human Skeletal Muscle Insulin Sensitivity via Coordinated Increases in Microvascular Perfusion and Molecular Signaling. Diabetes. 2017;66:1501–1510. doi: 10.2337/db16-1327. [DOI] [PubMed] [Google Scholar]
- Skup M, Dwornik A, Macias M, Sulejczak D, Wiater M, Czarkowska-Bauch J. Long-term locomotor training up-regulates TrkB(FL) receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin 4 with different topographies of expression in oligodendroglia and neurons in the spinal cord. Exp Neurol. 2002;176:289–307. doi: 10.1006/exnr.2002.7943. [DOI] [PubMed] [Google Scholar]
- Souza SB, Flues K, Paulini J, Mostarda C, Rodrigues B, Souza LE, Irigoyen MC, De Angelis K. Role of exercise training in cardiovascular autonomic dysfunction and mortality in diabetic ovariectomized rats. Hypertension. 2007;50:786–791. doi: 10.1161/HYPERTENSIONAHA.107.095000. [DOI] [PubMed] [Google Scholar]
- Speisman RB, Kumar A, Rani A, Foster TC, Ormerod BK. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav Immun. 2013;28:25–43. doi: 10.1016/j.bbi.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M, Lexell J, Deierborg T. Effects of Physical Exercise on Neuroinflammation, Neuroplasticity, Neurodegeneration, and Behavior: What We Can Learn From Animal Models in Clinical Settings. Neurorehabil Neural Repair. 2015;29:577–589. doi: 10.1177/1545968314562108. [DOI] [PubMed] [Google Scholar]
- Thule PM, Campbell AG, Kleinhenz DJ, Olson DE, Boutwell JJ, Sutliff RL, Hart CM. Hepatic insulin gene therapy prevents deterioration of vascular function and improves adipocytokine profile in STZ-diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:E114–E122. doi: 10.1152/ajpendo.00134.2005. [DOI] [PubMed] [Google Scholar]
- Turner PV, Albassam MA. Susceptibility of rats to corneal lesions after injectable anesthesia. Comp Med. 2005;55:175–182. [PubMed] [Google Scholar]
- Underwood EL, Thompson LT. A High-Fat Diet Causes Impairment in Hippocampal Memory and Sex-Dependent Alterations in Peripheral Metabolism. Neural Plast. 2016;2016:7385314. doi: 10.1155/2016/7385314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, Szabo A, Phillips SM, Wojcicki TR, Mailey EL, Olson EA, Gothe N, Vieira-Potter VJ, Martin SA, Pence BD, Cook MD, Woods JA, McAuley E, Kramer AF. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013a;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Szabo AN, Mailey EL, Wojcicki TR, White SM, Gothe N, McAuley E, Sutton BP, Kramer AF. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp. 2013b;34:2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil A, Cannell J, Davies A, Razay G. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: a randomized controlled trial. Scand J Caring Sci. 2012;26:12–19. doi: 10.1111/j.1471-6712.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- Waisbourd M, Goldstein M, Loewenstein A. Treatment of diabetic retinopathy with anti-VEGF drugs. Acta Ophthalmol. 2011;89:203–207. doi: 10.1111/j.1755-3768.2010.02010.x. [DOI] [PubMed] [Google Scholar]
- Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132:605–611. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- Wessels AM, Rombouts SA, Simsek S, Kuijer JP, Kostense PJ, Barkhof F, Scheltens P, Snoek FJ, Heine RJ. Microvascular disease in type 1 diabetes alters brain activation: a functional magnetic resonance imaging study. Diabetes. 2006a;55:334–340. doi: 10.2337/diabetes.55.02.06.db05-0680. [DOI] [PubMed] [Google Scholar]
- Wessels AM, Simsek S, Remijnse PL, Veltman DJ, Biessels GJ, Barkhof F, Scheltens P, Snoek FJ, Heine RJ, Rombouts SA. Voxel-based morphometry demonstrates reduced grey matter density on brain MRI in patients with diabetic retinopathy. Diabetologia. 2006b;49:2474–2480. doi: 10.1007/s00125-006-0283-7. [DOI] [PubMed] [Google Scholar]
- Wood K, Wilhelm JC, Sabatier MJ, Liu K, Gu J, English AW. Sex differences in the effectiveness of treadmill training in enhancing axon regeneration in injured peripheral nerves. Dev Neurobiol. 2012;72:688–698. doi: 10.1002/dneu.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]


