Abstract
We have performed three distinct plasma enhanced chemical vapor deposition (PECVD) procedures that can be widely and consistently used in commercially available plasma systems to modify the surface of hydrocarbon-based biomaterials such as polypropylene. In particular, we have evaluated the feasibility of these procedures to provide consistent and stable charged substrates to perform layer by layer (LbL) coatings. Surface characterization of both plasma and LbL coatings were done using X-ray photoelectron spectroscopy (XPS), attenuated total reflection – Fourier transform infrared spectroscopy (ATR-FTIR), contact angle measurements and surface staining. Results showed successful surface grafting of functional groups in all plasma procedures that led to increased hydrophilicity and uniform LbL coatings with different efficiencies.
Keywords: plasma, polypropylene, maleic anhydride, allylamine, corona
Introduction
Significant advances in the surface modification of materials has been made over the last decades and is increasingly becoming a common component in the design of biomaterials and medical devices. In early application, surface chemistry was modified in devices involved in blood-contact applications (e.g. catheters, tubing, dialysis equipment) to prevent clot formation1–5. Surface modification has also been used in multiple other applications to provide medical devices with enhanced wettability and antifouling properties as well as to enable grafting of bioactive agents for drug delivery applications6–10. More recently, surface properties of biomaterials have been investigated as a key component driving the early-stage interactions of the host response at the tissue-implant interface and as a determinant of downstream performance of implanted medical devices11–14. These studies have demonstrate that modifying surface properties of medical devices has the potential to tailor the interactions at the tissue-implant interface, without changes in the underlying properties of the biomaterial including geometry, mechanical performance and functionality of the implants.
The chemical inertness of plastics such as polyethylene, polypropylene and polytetrafluoroethylene (Teflon) have made surface modification of these materials more difficult than other biomaterials. Techniques involving standard wet reactions have shown low yield and damage under harsh chemical conditions, ultimately altering the structure and functionality of biomaterials15–17. Wet-based surface modification generally involves multiple steps that make the overall process slow, dangerous, costly and non-environment friendly. On the other hand, recent plasma irradiation methods have been developed to allow gentle, environment friendly, controllable and effective surface modification in multiple biomaterials.
Multiple monomers are currently available for surface grafting of materials using plasma irradiation, enabling modification chemical properties such as reactivity, charge and hydrophilicity. Extensive studies have been done to assess the effects surface modification of biomaterials using a wide range of oxygen containing monomers on the interaction of cells and tissues at the biomaterial interface9,18–20. Amine-based monomers are also a common oxygen-free alternative for grafting molecules such as allylamine and acrylamides5,21–24 and mixtures of these monomers to deposit co-polymerized films on the surface of the materials have also been investigated25–28. These one-step methods alter the surface of biomaterials at a depth of a few hundred angstroms to nano-meters while preserving the underlying biomaterial bulk properties.
In the present work, we describe the development of enhanced plasma chemical vapor deposition (PECVD) procedures used to modify the surface of polypropylene implants with multiple common chemical entities (e.g. carboxylic acid, amine and hydroxyl groups) suitable for a wide range of chemical and crosslinking applications such as molecule grafting, surface charge and increased hydrophilicity. The methods presented can be adapted for commercially available plasma machines, using any gas, liquid or solid monomer of choice. We evaluated the efficacy of these PECVD procedures to create optimal charged polypropylene substrates for Layer by Layer (LbL) coatings, as a charged substrate is required for cyclic electrostatic deposition of polyelectrolyte films with opposite charge. Due to the negligible wettability, charge and chemical reactive moieties in the surface of plastic biomaterials, the use of the LbL technique has largely been limited to natively charged materials. Therefore, the presented plasma irradiation methods have the potential to expand the applications of LbL coatings to multiple other medical devices.
Materials and methods
Materials
A polypropylene mesh, Gynemesh® PS (Ethicon, Somerville, NJ) was used. Maleic anhydride, chondroitin sulfate B, chitosan (low molecular weight, deacetylation degree 85%) were purchased from Sigma Aldrich (St. Louis, MO).
Cleaning procedure for polypropylene mesh substrate
Polypropylene (PP) meshes were cleaned using a 1:1 acetone:isopropanol mixture under sonication for 30 seconds and then air dried prior to irradiation. Immediately before any PECVD treatment, cleaned polypropylene meshes were irradiated with argon plasma at 600W for 20 seconds, keeping the gas flow at 35 mL/min and a steady state pressure of 250 mTorr (50 mTorr initial pressure) using an Ion 40 Gas Plasma System (PVA Tepla America, Inc).
Maleic anhydride - PECVD treatment of polypropylene meshes
An adapted plasma irradiation procedure based on a previously developed microwave plasma procedure with solid maleic anhydride as a monomer was used to obtain a negatively charged surface with carboxylic acid groups7. In the present work, we have adapted this method to perform PECVD using solid maleic anhydride on more widely available standard plasma systems. Maleic anhydride powder (1.5 g) was placed into a glass plate inside of the reaction chamber. Cleaned 1 cm2 pieces of PP mesh were then placed around the plate to a distance of 8.5 cm. After an initial pressure of 50 mTorr was reached inside of the reaction chamber, maleic anhydride plasma irradiation was performed for 30 seconds at 600W, an argon gas flow of 35 mL/min and a steady state pressure of 250 mTorr. Finally, in order to remove the physisorbed maleic anhydride, hydrolyze the surface-bound anhydrides and produce carboxylic acid groups (negatively charged at physiological pH), PP meshes were rinsed for 30 minutes with milli-Q water and then boiled for 20 minutes in fresh milli-Q water.
Allylamine - PECVD treatment of polypropylene meshes
Allylamine was used to provide amine groups and a positive charge in the surface of polypropylene. Cleaned 1 cm2 pieces of PP mesh were placed inside the reaction chamber. After an initial pressure of 50 mTorr was reached, allylamine plasma irradiation was performed for 5 minutes at 300W, an allylamine gas flow of 180 mL/min, a steady state pressure of 350 mTorr, a 20% cycle duty and a frequency of 150 hertz. Finally, in order to remove the physisorbed allylamine, PP meshes were rinsed for 30 minutes with milli-Q water and then boiled for 20 minutes in fresh milli-Q water.
Oxygen - PECVD treatment of polypropylene meshes
Oxygen was used to provide a negatively charged corona of both hydroxyl and carboxylic acid groups in the surface of polypropylene. Cleaned 1 cm2 pieces of PP mesh were placed inside the reaction chamber. After an initial pressure of 50 mTorr was reached, oxygen plasma irradiation was performed for 30 seconds at 300W, an oxygen gas flow of 100 mL/min, and a steady state pressure of 420 mTorr. Finally, in order to remove the physisorbed oxygen by products, PP meshes were rinsed for 30 minutes with milli-Q water and then boiled for 20 minutes in fresh milli-Q water.
Layer by Layer coating of PECVD treated polypropylene meshes
In order to assess the feasibility of each PECVD treatment to create a stable and functional charged surface on polypropylene substrates, a Layer by Layer (LbL) film deposition procedure was performed as previously described6. Briefly, chitosan was chosen as polycation and chondroitin sulfate B as polyanion. Chitosan was dissolved in 0.5 % acetic acid and chondroitin sulfate B in milli-Q water. Both polyelectrolites were prepared at a concentration of 2 mg/mL. Each layer deposition is performed by dipping the PECVD treated polypropylene substrate into the polyelectrolyte solution, incubated 10 minutes at room temperature, then samples are washed 3 times (10, 20 and 30 seconds) in milli-Q water and air dried (a short burst of pressurized clean air). Air drying is an optional step intended to remove more efficiently the excess of polymer solutions, reduce solution cross-contamination, and according to our studies does not impact the coating deposition. This procedure was repeated alternately for each polyelectrolyte in cycles until a core coating of 10 bilayers (PP−[CH/DS]10 or PP+[DS/CH]10) was achieved. After coating, meshes were lyophilized and stored at 4°C.
Surface characterization
Elemental composition of the mesh surface (6 mesh samples each group) was performed using an X-ray photoelectron spectroscopy (XPS), using an ESCALAB 250Xi, Thermo Scientific (Pittsburgh, PA). To identify the overall composition of the surface an initial survey of 10 scans was obtained and for detailed elemental information spectra of 25 scans were obtained for Carbon, Oxygen, Nitrogen and Sulfur. Data acquisition was performed using constant analyzer energy of 50 eV, dwell time of 10 milliseconds, 0.1 eV step size and an X-ray spot size of 200 μm. Carbon peak was used as reference, with a value of 284 eV for adventitious carbon. Spectra data was analyzed using Avantage software, Thermo Scientific.
Additionally, meshes were analyzed under Fourier transform infrared spectroscopy with attenuated total reflectance (ATR-FTIR) using a Bruker Vertex 70 (Billerica, MA) equipped with a germanium ATR crystal at a resolution of 1 cm−1, 2 mm of aperture, 32 scans and processed by OPUS software to adjust the baseline, to smooth spectra and to remove H2O and CO2 peaks due to environmental noise.
Finally, an alcian blue staining was performed on whole samples to stain the GAG components and reveal the LbL coating. A 1% alcian blue solution was made in 3% acetic acid and adjusted to pH 2.5. Coated meshes and controls were re-hydrated in distilled water and then immersed into the alcian blue solution for 30 minutes at RT. Then meshes were washed in running tap water for 5 minutes and rinsed 5 minutes in distilled water. Images were taken using a standard optical camera.
Results
Maleic anhydride - PECVD treatment of polypropylene meshes
The PECVD treatment of polypropylene using maleic anhydride as a solid monomer (that becomes a gas under high vacuum and high electric power) is intended to graft the maleic anhydride on the surface of polypropylene, thereby producing carboxylic acids after hydrolysis. One possible reaction starts with the formation of highly reactive radicals in the surface of polypropylene7, that subsequently react with one of the arene carbons of the maleic anhydride aromatic ring to form a stable covalent bond, and then the anhydride is opened by hydrolysis, to generate two highly hydrophilic and negatively charged carboxylic acids groups (Figure 1a).
Figure 1. Maleic anhydride- PECVD treatment of polypropylene meshes.
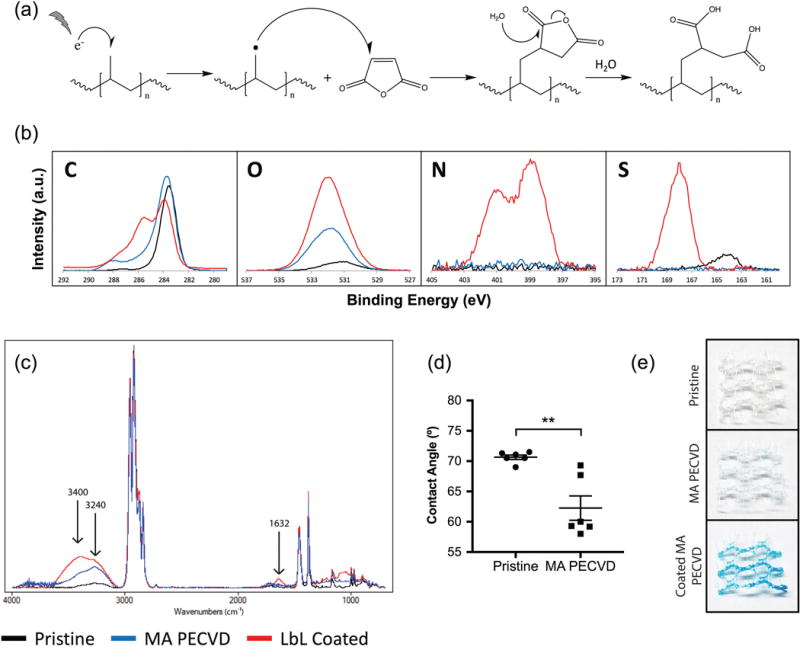
a) Proposed PECVD reaction schematics of carboxylic acid grafting using maleic anhydride as monomer. b) XPS and c) ATR-FTIR spectra of pristine (black), MA plasma-treated (blue) and LbL coated/MA plasma-treated meshes (red). d) Contact angle measurements of pristine and MA plasma-treated meshes. The graph shows the mean ± SEM and points represent each sample measurement. Statistical significance as (**) p < 0.01, using a two-tailed unpaired t-test. e) Images of 1 cm2 pieces of pristine (top), MA plasma-treated (middle) and LbL coated/MA plasma-treated (bottom) polypropylene meshes stained with alcian blue, to reveal the presence of the LbL coating components (blue color).
Attachment of carboxylic acid groups after maleic anhydride PECVD treatment of polypropylene meshes is corroborated primarily by the presence of a strong peak of oxygen (532 eV) and a carbon peak (O-C=O, 288 eV), characteristic of carboxylic acids in the XPS spectra (Figure 1b). These peaks are consistent with a significant increase in the percentage of oxygen, compared to pristine meshes (Table 1). Similarly, FTIR-ATR spectra confirmed carboxylic acid attachment by a peak at 3240 cm−1 (carboxylic acid O-H stretch, Figure 1c). Due to the limitations of FTIR-ATR sampling depth of ~1 micron, the signal of the other carboxylic peak at 1700 cm−1 (C=O stretch) is obscured by the hydrocarbon peak signals from the polypropylene bulk. In addition, there was a marked increase in hydrophilicity as shown by a significant decrease in contact angle (Figure 1d), confirming that surface properties have changed due to carboxylic acid attachment to the surface of polypropylene.
Table 1.
Atomic percentages of plasma irradiated meshes determined via X-ray electron spectroscopy (XPS) elemental analysis. Values represent the mean of six samples ± SD.
| Carbon | Oxygen | Nitrogen | ||||
|---|---|---|---|---|---|---|
| Atomic % | SD | Atomic % | SD | Atomic % | SD | |
| Polypropylene | 89.1 | 4.7 | 9.2 | 4.6 | 1.2 | 0.3 |
| MA PECVD | 76.4 | 3.6 | 20.8 | 3.2 | 2.7 | 1.0 |
| Allylamine PECVD | 77.6 | 4.4 | 12.0 | 2.6 | 10.1 | 3.0 |
| Oxygen PECVD | 79.8 | 5.9 | 17.2 | 3.2 | 2.4 | 1.0 |
The formation of a thin, conformal and uniform coating by means of LbL deposition of both chitosan (polycation) and chondroitin sulfate B (polyanion) is demonstrated by the presence of a uniform blue staining after alcian blue staining (Figure 1e), a stain which reveals the presence of glycosaminoglycans (such as chitosan and chondroitin sulfate B) and absent in both pristine and plasma treated polypropylene. In addition, we have demonstrated that the coating contains both chitosan and chondroitin sulfate B due to the formation of additional peaks in the XPS spectra (Figure 1b), including:
Additional carbon peaks (C-O-C at 286 eV and O-C=O at 288 eV) due to the presence of the acetyl groups and carboxylic acids from the glycosaminoglycan sugar chains of both chitosan and chondroitin sulfate B.
Two nitrogen peaks at 399 eV (amines) and 401 eV (protonated amines), due specifically to the presence of chitosan in the coating.
A sulfur peak at 168 eV, due to the presence of sulfate groups specifically found in chondroitin sulfate B.
XPS also showed that the presence of these peaks was associated with increased percentages in oxygen, nitrogen and sulfur, compared both pristine mesh (Table 2). The FTIR spectra was consistent with these results as confirmed by peaks at 1632 cm−1 (C=O stretch), 3240 cm−1 (O-H stretch) and 3400 cm−1 (N-H stretch), all components present in both GAG polyelectrolytes.
Table 2.
Atomic percentages of coated plasma irradiated meshes determined via X-ray electron spectroscopy (XPS) elemental analysis. Values represent the mean of six samples ± SD.
| Carbon | Oxygen | Nitrogen | Sulfur | |||||
|---|---|---|---|---|---|---|---|---|
| At. % | SD | At. % | SD | At. % | SD | At. % | SD | |
| Polypropylene | 89.1 | 4.7 | 9.2 | 4.6 | 1.2 | 0.3 | 0.5 | 0.5 |
| Coated MA PECVD | 63.8 | 5.8 | 29.1 | 4.4 | 5.1 | 1.0 | 2.0 | 0.3 |
| Coated Allylamine PECVD | 62.8 | 4.9 | 29.3 | 4.3 | 5.8 | 0.9 | 2.0 | 0.4 |
| Coated Oxygen PECVD | 65.6 | 9 | 27.8 | 8 | 4.8 | 1.0 | 1.9 | 0.7 |
Allylamine - PECVD treatment of polypropylene meshes
The PECVD treatment of polypropylene using allylamine gas as monomer is intended to produce primary amine groups in the surface of polypropylene. The production of multiple radical forms29 in the surface of polypropylene would lead to the formation of a stable covalent bond by a nucleophilic attack on the alkene carbon of allylamine (Figure 2a). The result of this proposed reaction scheme would provide highly polar and positively charged amine groups to the surface of polypropylene.
Figure 2. Allylamine- PECVD treatment of polypropylene meshes.

a) Proposed PECVD reaction schematics of amine grafting using allylamine as monomer. b) XPS and c) ATR-FTIR spectra of pristine (black), allylamine plasma-treated (blue) and LbL coated/allylamine plasma-treated meshes (red). d) Contact angle measurements of pristine and allylamine plasma-treated meshes. The graph shows the mean ± SEM and points represent each sample measurement. Statistical significance as (**) p < 0.01, using a two-tailed unpaired t-test. e) Images of 1 cm2 pieces of pristine (top), allylamine plasma-treated (middle) and LbL coated/allylamine plasma-treated (bottom) polypropylene meshes stained with alcian blue, to reveal the presence of the LbL coating components (blue color).
Attachment of amine groups after allylamine PECVD treatment of polypropylene meshes was corroborated mainly by the presence of a strong peak (399 eV) of nitrogen in the XPS spectrum (Figure 2b), accompanied by an increase in the percentage of nitrogen (Table 1). ATR-FTIR spectra showed a peak at 3300 cm−1, indicative of primary amines, due to N-H stretch absorption (Figure 2c). All other elements in the XPS spectra and peaks in the FTIR remained unchanged. The plasma treatment also increased hydrophilicity of the polypropylene surface as revealed by a decreased in contact angle (Figure 2d), serving as confirmation that surface properties of polypropylene were altered due to the presence of amine groups.
Layer by layer coating of allylamine-plasma treated polypropylene resulted in a uniform and conformal coating distributed through the entire surface of polypropylene, as revealed by the blue colored surface present in the coated mesh only (Figure 2e). We also corroborated the components of the coating using XPS, showing increased percentage in oxygen, nitrogen and sulfur (Table 2), but also the presence of chitosan by two nitrogen peaks (amines at 399 eV and protonated amines at 401 eV) and chondroitin sulfate B due to a sulfur peak at 168 eV (Figure 2b). Similarly, FTIR spectra revealed a broad peak of amine groups at 3300 cm−1, and a peak at 1632 cm−1 (C=O stretch), coming from the amine groups and carbonyl groups present in the polyelectrolyte GAG chains, respectively (Figure 2c).
Oxygen - PECVD treatment of polypropylene meshes
Commonly proposed oxygen plasma reaction schemes start with the formation of radicals in the surface of polypropylene9, 17, 18, that subsequently react with oxygen gas, leading to multiple downstream reactions that generate numerous oxygen species such as hydroxyl, carboxylic acid and aldehyde groups; as known as corona (Figure 3a). The formation of this corona after oxygen plasma, is shown by presence of different oxygen species such as carboxylic acid carbons (288 eV), O-C-O carbons (286 eV) and oxygen (531 eV) in the XPS spectra (Figure 3b), as well as an increased percentage in oxygen, compared to pristine mesh (Table 1). The FTIR spectra was consistent with these findings as shown by a peak at 3400 cm −1 (O-H stretch). The C=O stretch peak was not detected due to signal dilution by the polypropylene bulk. Also, these changes were associated with an increased hydrophilicity, demonstrated by a decreased contact angle (Figure 3d).
Figure 3. Oxygen- PECVD treatment of polypropylene meshes.
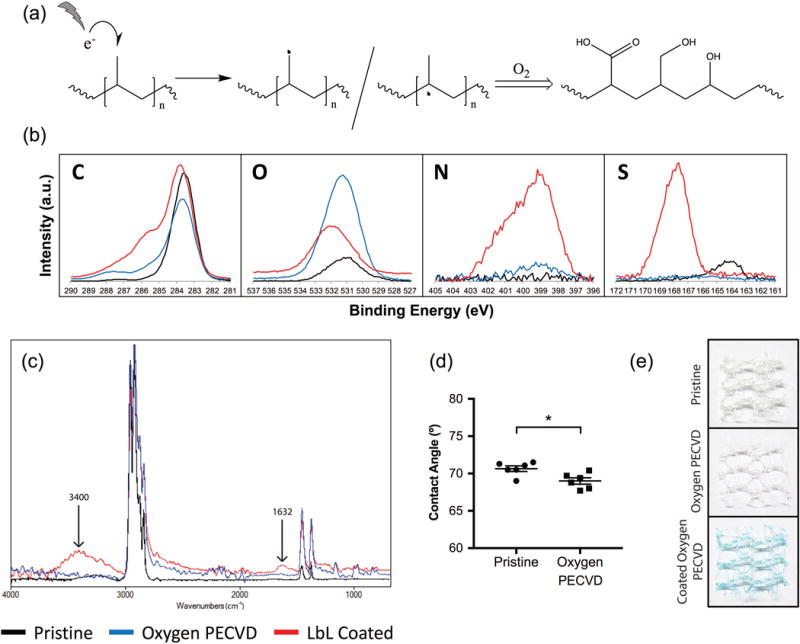
a) Proposed PECVD reaction schematics of the formation of a corona using oxygen gas. b) XPS and c) ATR-FTIR spectra of pristine (black), oxygen plasma-treated (blue) and LbL coated/oxygen plasma-treated meshes (red). d) Contact angle measurements of pristine and oxygen plasma-treated meshes. The graph shows the mean ± SEM and points represent each sample measurement. Statistical significance as (*) p < 0.05, using a two-tailed unpaired t-test. e) Images of 1 cm2 pieces of pristine (top), oxygen plasma-treated (middle) and LbL coated/oxygen plasma-treated (bottom) polypropylene meshes stained with alcian blue, to reveal the presence of the LbL coating components (blue color).
Even though the corona contains multiple non-charged oxygen species, LbL coating of oxygen plasma-treated polypropylene is possible, although weaker compared to both maleic anhydride- and allylamine- plasma. Alcian blue staining confirmed the presence of a uniform and conformal coating, as visualized by a light blue coloration of the coated polypropylene surface which were not present in all other conditions (Figure 3e). The presence of chitosan in the coating was revealed by two nitrogen peaks at 399 eV (amine) and 401 eV (protonated amines) in the XPS spectra (Figure 3b), accompanied by an increased percentage in nitrogen (Table 2). The presence of chondroitin sulfate B was corroborated by a sulfur peak at 168 eV and an increased sulfur percentage. Similarly, the FTIR spectra reveal a broad peak of amine groups at 3300 cm−1, and a peak at 1632 cm−1 (C=O stretch), which is consistent with the XPS findings.
Discussion
Polypropylene and other hydrocarbon-based/plastic biomaterials are commonly used in medical devices due to desirable mechanical properties and durability. However, surface properties might not be adequate for some applications due to high hydrophobicity and chemical inertness of these biomaterials. In particular, the absence of surface charge makes these materials not suitable for coating using layer by layer deposition, where a charged template is required for polyelectrolyte deposition. This is consistent with elemental and chemical characterization of polypropylene by XPS and FTIR, but also the higher contact angle. When untreated polypropylene undergoes the layer by layer procedure, it is unable to get wet, floats in the surface of the polyelectrolyte solutions and therefore the coating process cannot be effectively performed. In addition, an observed contact angle of approximately 70 degrees is lower than expected for medical grade polypropylene, suggesting the presence of other components in the mesh biomaterial. We have indeed detected small traces of oxygen in pristine polypropylene in the present study regardless of the cleaning procedure. Likely, this is due to the presence of additives used in the manufacturing process. This finding was also corroborated by an XPS depth profiling, that showed that traces of oxygen were still present in the bulk of the polypropylene material. Sulfur content is not detected in the elemental survey, but an extremely low signal is shown when using high-resolution scanning of sulfur, likely due to environmental contamination, showing a different and weak peak at 164 eV (versus the typical highly defined peak at 168 eV). This will likely alter the contact angle measurements to a small degree, but the changes in surface tension due to each of the plasma treatments were always more significant.
We have proposed and evaluated three distinct PECVD procedures that can be used widely and consistently in commercially available plasma systems to modify the surface of hydrocarbon-based biomaterials such as polypropylene. In addition, we have evaluated the feasibility of these procedures to provide a consistent and stable charged substrate to perform a layer by layer coating, which is mediated by alternated deposition of monolayers of two oppositely charged polyelectrolytes onto the surface of a charged substrate. First, negatively charged carboxylic acids were grafted in the surface of polypropylene using solid maleic anhydride. Positively charged amine groups were grafted using allylamine gas as monomer. Also, an oxygen PECVD treatment was done to provide a corona, consisting in multiple oxygen-derived functional groups.
Surface characterization demonstrated that surface modification was successful in all three procedures. As shown by contact angle measurements (Figure 1d, 2d, 3d), they also presented increased hydrophilicity compared to non-treated polypropylene. Maleic anhydride plasma treated polypropylene having the most hydrophilic surface, followed by allylamine plasma. The presence of charged groups in both maleic anhydride and allylamine plasma treated polypropylene are likely decreasing surface tension in a higher extension compared to oxygen plasma and are therefore associated with increased hydrophilicity. Also, maleic anhydride plasma has a stronger effect on surface tension likely due to the increased charge density generated by the two carboxylic acids, compared to single grafting of amine groups result of the allylamine plasma.
Coating formation through layer by layer deposition in all plasma treated polypropylene groups was demonstrated by the presence of a uniform coating, as well as the specific presence of chitosan and chondroitin sulfate B. Maleic anhydride plasma-treated polypropylene presented a stronger blue coloration after the coating process and staining compared to the other plasma procedures. This suggests a denser coating and a more optimal charge and density of the resulting carboxylic acids in the surface of polypropylene for layer by layer deposition mediated by electrostatic charges. On the other hand, allylamine plasma treatment produces a positively charged surface on polypropylene that can also be used for subsequent layer by layer deposition. Coating deposition of allylamine plasma-treated polypropylene was observed to be qualitatively lower compared to maleic anhydride plasma; however, density of the grafted amine groups using this method could likely be increased by increasing either monomer gas flow (180 mL/min) and electric power (300 W). Although methods cannot be compared due to different settings and gases, these values are relatively high and not significantly different from maleic anhydride plasma. Regardless, maleic anhydride plasma seems to be more suitable for the production of a more dense grafting and higher charge. In particular, this may be due to the generation of two negatively charged carboxylic acids in contrast to single positively charged amine groups. For that reason, maleic anhydride might be limited in some applications where a lower grafting density or increased hydrophilicity but less surface charge is required.
Layer by layer coating of oxygen plasma is still possible even though the presence of charged groups is lower, but other interactions such as hydrogen bonds are likely participating in the process. The coating was shown to be present and corroborated by XPS, FTIR and alcian blue. However, compared to the other plasma procedures, the intensity of the blue was weaker and some of the peaks in the XPS were less defined or did not have a strong signal. Oxygen plasma was successful but it is likely more appropriate for applications where there is need for increased hydrophilicity. It is not recommended to use this procedure to perform a layer by layer coating, due to the lack of a consistent charge in the surface, but also not recommended for functionalization of other moieties using crosslinking due to non-consistent and heterogeneous functional groups in the surface, as the lack of defined functional groups would lead to a poor design and ineffective crosslinking reactions.
Conclusions
Polypropylene can effectively be surface-treated using conventional plasma methods to provide increased hydrophilicity, chemical functionality and surface charge, that can be likely extrapolated to other hydrocarbon/plastic biomaterials (e.g. PTFE, PET, PS, PE, etc.) with similar outcomes. Maleic anhydride PECVD treatment generates highly dense and negatively charged carboxylic acids, best suitable for layer by layer deposition, but also useful to increase hydrophilicity and provide functional reactive groups for molecule grafting. Allylamine PECVD treatment produces positively charged amine groups and was demonstrated to be a useful method to increase hydrophilicity, perform layer by layer deposition, and molecule grafting, due to uniformity and reactivity of the amine functional groups in the surface and potentially higher control of graft density. Finally, oxygen plasma results in a corona that consists in heterogeneous oxygen-derived species, with and without charge. Therefore, oxygen plasma is not the most suitable method for both layer by layer deposition and molecule grafting, but remains common and recommended method to increase surface hydrophilicity.
Acknowledgments
This work was supported by National Institutes of Health awards K12HD043441 and R21GM107882. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding source had no involvement in the study design, collection of data, analysis of data, interpretation of data, writing of the report, or the decision to submit for publication.
References
- 1.Vasudev MC, Anderson KD, Bunning TJ, Tsukruk VV, Naik RR. Exploration of plasma-enhanced chemical vapor deposition as a method for thin-film fabrication with biological applications. ACS Appl Mater Interfaces. 2013;5(10):3983–94. doi: 10.1021/am302989x. [DOI] [PubMed] [Google Scholar]
- 2.Ding X, Yang C, Lim TP, Hsu LY, Engler AC, Hedrick JL, Yang YY. Antibacterial and antifouling catheter coatings using surface grafted PEG-b-cationic polycarbonate diblock copolymers. Biomaterials. 2012;33(28):6593–603. doi: 10.1016/j.biomaterials.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Garfinkle AM, Hoffman AS, Ratner BD, Reynolds LO, Hanson SR. Effects of a tetrafluoroethylene glow discharge on patency of small diameter dacron vascular grafts. Trans Am Soc Artif Intern Organs. 1984;30:432–9. [PubMed] [Google Scholar]
- 4.Lopez GP, Ratner BD, Tidwell CD, Haycox CL, Rapoza RJ, Horbett TA. Glow discharge plasma deposition of tetraethylene glycol dimethyl ether for fouling-resistant biomaterial surfaces. J Biomed Mater Res. 1992;26(4):415–39. doi: 10.1002/jbm.820260402. [DOI] [PubMed] [Google Scholar]
- 5.Tseng DY, Edelman ER. Effects of amide and amine plasma‐treated ePTFE vascular grafts on endothelial cell lining in an artificial circulatory system. Journal of Biomedical Materials Research Part A. 1998;42(2):188–198. doi: 10.1002/(sici)1097-4636(199811)42:2<188::aid-jbm4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Diaz Blanco C, Ortner A, Dimitrov R, Navarro A, Mendoza E, Tzanov T. Building an antifouling zwitterionic coating on urinary catheters using an enzymatically triggered bottom-up approach. ACS Appl Mater Interfaces. 2014;6(14):11385–93. doi: 10.1021/am501961b. [DOI] [PubMed] [Google Scholar]
- 7.Aumsuwan N, Ye SH, Wagner WR, Urban MW. Covalent attachment of multilayers on poly(tetrafluoroethylene) surfaces. Langmuir. 2011;27(17):11106–10. doi: 10.1021/la201957a. [DOI] [PubMed] [Google Scholar]
- 8.Arazawa DT, Oh HI, Ye SH, Johnson CA, Jr, Woolley JR, Wagner WR, Federspiel WJ. Immobilized Carbonic Anhydrase on Hollow Fiber Membranes Accelerates CO(2) Removal from Blood. J Memb Sci. 2012;404–404:25–31. doi: 10.1016/j.memsci.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratner BD. Plasma deposition for biomedical applications: a brief review. J Biomater Sci Polym Ed. 1992;4(1):3–11. [PubMed] [Google Scholar]
- 10.Kwok CS, Horbett TA, Ratner BD. Design of infection-resistant antibiotic-releasing polymers. II. Controlled release of antibiotics through a plasma-deposited thin film barrier. J Control Release. 1999;62(3):301–11. doi: 10.1016/s0168-3659(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 11.Hachim D, LoPresti ST, Yates CC, Brown BN. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials. 2017;112:95–107. doi: 10.1016/j.biomaterials.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown BN, Badylak SF. Expanded applications, shifting paradigms and an improved understanding of host-biomaterial interactions. Acta Biomater. 2013;9(2):4948–55. doi: 10.1016/j.actbio.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Brown BN, Sicari BM, Badylak SF. Rethinking regenerative medicine: a macrophage-centered approach. Front Immunol. 2014;5:510. doi: 10.3389/fimmu.2014.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33(15):3792–802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi M, Kale N, Lal R, Rao VR, Mukherji S. A novel dry method for surface modification of SU-8 for immobilization of biomolecules in Bio-MEMS. Biosensors & Bioelectronics. 2007;22(11):2429–2435. doi: 10.1016/j.bios.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 16.Tseng WS, Tseng CY, Chuang PK, Lo AY, Kuo CT. A High Efficiency Surface Modification Process for Multiwalled Carbon Nanotubes by Electron Cyclotron Resonance Plasma. Journal of Physical Chemistry C. 2008;112(47):18431–18436. [Google Scholar]
- 17.Penn L, Wang H. Chemical modification of polymer surfaces: a review. Polymers for Advanced Technologies. 1994;5(12):809–817. [Google Scholar]
- 18.Ertel SI, Ratner BD, Horbett TA. Radiofrequency plasma deposition of oxygen-containing films on polystyrene and poly(ethylene terephthalate) substrates improves endothelial cell growth. J Biomed Mater Res. 1990;24(12):1637–59. doi: 10.1002/jbm.820241207. [DOI] [PubMed] [Google Scholar]
- 19.Chilkoti A, Schmierer AE, Perez-Luna VH, Ratner BD. Investigating the relationship between surface chemistry and endothelial cell growth: partial least-squares regression of the static secondary ion mass spectra of oxygen-containing plasma-deposited films. Anal Chem. 1995;67(17):2883–91. doi: 10.1021/ac00113a024. [DOI] [PubMed] [Google Scholar]
- 20.Cohn D, Hoffman AS, Ratner BD. Radiation‐grafted polymers for biomaterial applications. I. 2‐hydroxyethyl methacrylate: Ethyl methacrylate grafting onto low density polyethylene films. Journal of applied polymer science. 1984;29(8):2645–2663. [Google Scholar]
- 21.Griesser HJ, Chatelier RC, Gengenbach TR, Johnson G, Steele JG. Growth of human cells on plasma polymers: putative role of amine and amide groups. J Biomater Sci Polym Ed. 1994;5(6):531–54. doi: 10.1163/156856294x00194. [DOI] [PubMed] [Google Scholar]
- 22.Vasilev K, Cook J, Griesser HJ. Antibacterial surfaces for biomedical devices. Expert Rev Med Devices. 2009;6(5):553–67. doi: 10.1586/erd.09.36. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Park H, Jung D, Kim S. Protein immobilization on plasma-polymerized ethylenediamine-coated glass slides. Anal Biochem. 2003;313(1):41–5. doi: 10.1016/s0003-2697(02)00563-8. [DOI] [PubMed] [Google Scholar]
- 24.Vasilev K, Griesser SS, Griesser HJ. Antibacterial surfaces and coatings produced by plasma techniques. Plasma Processes and Polymers. 2011;8(11):1010–1023. [Google Scholar]
- 25.Beck AJ, Whittle JD, Bullett NA, Eves P, Mac Neil S, McArthur SL, Shard AG. Plasma co‐polymerisation of two strongly interacting monomers: Acrylic acid and allylamine. Plasma processes and polymers. 2005;2(8):641–649. [Google Scholar]
- 26.Sakata J, Yamamoto M, Tajima I. Plasma polymerization of mixed monomer gases. Journal of Polymer Science Part A: Polymer Chemistry. 1988;26(7):1721–1731. [Google Scholar]
- 27.Jiang H, Grant J, Tullis S, Eyink K, Fleitz P, Bunning T. The growth and chemical structure of thin photonic films formed from plasma copolymerization: I. Effect of monomer feed ratio. Polymer. 2004;45(25):8475–8483. [Google Scholar]
- 28.Li Z, Gillon X, Diallo M, Houssiau L, Pireaux J-J. Styrene and methyl methacrylate copolymer synthesized by RF inductively coupled plasma. IOP Publishing; 2011. p 012020. [Google Scholar]
- 29.Choukourov A, Biederman H, Slavinska D, Hanley L, Grinevich A, Boldyryeva H, Mackova A. Mechanistic studies of plasma polymerization of allylamine. J Phys Chem B. 2005;109(48):23086–23095. doi: 10.1021/jp0535691. [DOI] [PubMed] [Google Scholar]


