Abstract
The Hippo-Yes associated protein (Yap) pathway plays an important role in organ size control by regulating cell proliferation, apoptosis, and stem cell renewal. Hippo-Yap signaling also functions at the level of cellular development in a variety of organs through its effects on cell cycle control, cell survival, cell polarity, and cell fate. Because of its important roles in normal development and homeostasis, abnormal regulation of this pathway has been shown to lead to pathological outcomes such as tissue overgrowth, tumor formation and abnormal organogenesis, including ocular-specific disorders. In this review, we summarize how normal and perturbed control of Yap signaling is implicated in ocular development and disease.
Keywords: Hippo, Yap, eye development, eye disease, retina
INTRODUCTION
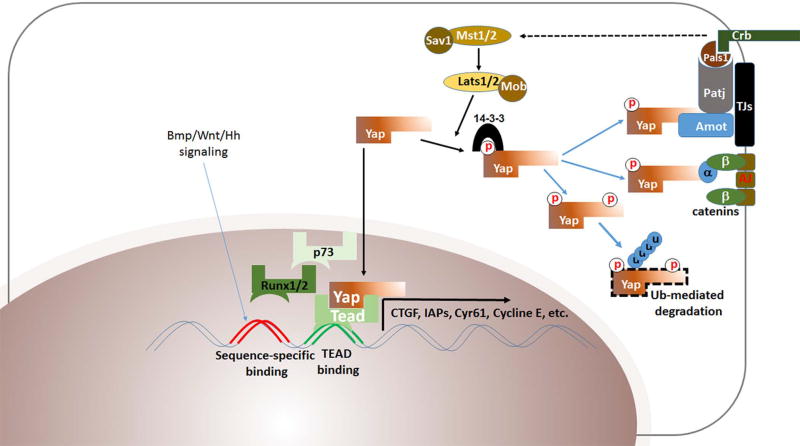
The signaling pathways mediated by protein kinase Hippo (Mst1/2 in mammals), the transcriptional coactivator Yes-associated protein (Yap; HGNC:16262) and transcriptional coactivator with PDZ-binding motif (Taz also known as WWTR1; HGNC:24042) have been recognized as important regulators of cell proliferation and differentiation, organogenesis and tissue regeneration (Edgar 2006, Pan 2007) (Fig. 1). After its functional significance as a major suppressor of tissue growth was first identified in Drosophila melanogaster, Hippo, a downstream signal transducer (Warts) and target (Yorkie) were found to have great structural and functional conservation in mammals (Halder & Johnson 2011, Hansen et al 2015, Harvey & Tapon 2007, Hilman & Gat 2011, Wang et al 2009). The upstream signals that activate/inhibit this pathway are still not completely understood, but the proposed initiators include mechanical forces, cellular stress, cellular polarity, and cell-cell contact (Dupont et al 2011, Genevet et al 2009, Gumbiner & Kim 2014, Halder et al 2012, Hamaratoglu et al 2009, Hansen et al 2015, Pan 2007, Zhao et al 2011a). Upon activation of the Hippo pathway, the serine-threonine kinase cascade involving Mammalian STE20-like protein kinase (Mst1/2 kinases) phosphorylates and activates a second class of kinases, large tumor suppressor kinases (Lats1/2). The Lats1/2 kinases phosphorylate Yap; this phosphorylation sequesters Yap in the cytoplasm, which prevents nuclear translocation and instead signals its proteasomal degradation (Zhao et al 2010, Zhao et al 2007). Inhibition of the Hippo pathway enables unphosphorylated Yap to translocate to, and be retained in, the nucleus (Chan et al 2009, Hansen et al 2015). Within the nucleus, due to its lack of a DNA binding domain, Yap binds to sets of transcription factors. One major family identified is the TEA domain transcription factor [Tead1–4; HGNC: 11714–11717] (Cao et al 2008, Vassilev et al 2001, Zhao et al 2008). Binding to Tead causes transcription of target genes responsible for cellular proliferation and inhibition of cellular apoptosis, among them cyclins, inhibitors of apoptosis (IAP), and connective tissue growth factor (Ctgf) (Cao et al 2008, Huang et al 2005, Zhao et al 2008). Other transcription factors that interact with Yap/Taz are PPXY-motif containing proteins such as tumor suppressor p73, Smads, and Runx2. PPXY-motif interacts with the WW domain of Yap (Basu et al 2003, Strano et al 2005, Vitolo et al 2007, Zaidi et al 2004). Additionally, Tbx5, which lacks a WW domain-interacting PPXY-motif, physically interacts with transcription coactivator Taz via multiple domains, including its carboxyl-terminal sequence (Murakami et al 2005). The Hippo-Yap pathway, which we define in this review as Mst1/2-Lats1/2 signaling influencing Yap, also interacts with numerous other pathways, including Wnt, Bmp, Tgfβ, Shh, and Notch (Alarcon et al 2009, Barry & Camargo 2013, Heallen et al 2011, Lai & Yang 2013, Lin et al 2012, Morgan et al 2013, Nishio et al 2013, Piccolo et al 2014, Rayon et al 2014, Varelas et al 2010, Varelas et al 2008, Zhou et al 2011). The diversity of these pathways highlights the impact of Yap on multiple biological processes and the complexity of its regulatory pathways. Because the role of the Hippo-Yap signaling pathway during development has been particularly well studied during embryogenesis of the mouse eye, we will briefly highlight the complex processes that occur during embryogenesis of mouse ocular tissues. Ocular development is a complex process that depends on various signaling pathways. The Hippo-Yap signaling pathway affects multiple stages of embryonic eye formation.
Figure 1.
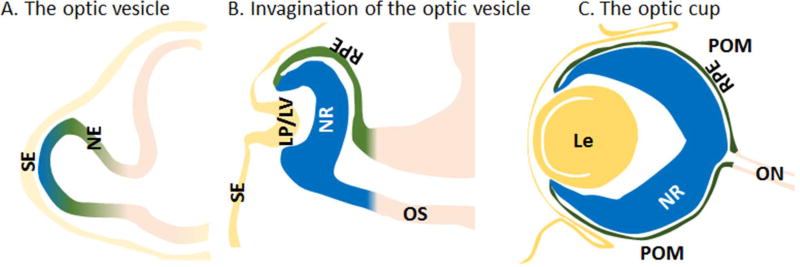
Ocular development begins at embryonic day (E) 8.5–9 in the mouse, when the optic vesicle (OV) evaginates from the diencephalon (Chow & Lang 2001, Fuhrmann 2010, Graw 2010) (Fig. 2). The lens placode forms concurrently on the overlying surface ectoderm and, at E10, begins to invaginate to form the lens vesicle along with the OV forming the optic cup (OC). The subsequent division of the OC into two main layers is facilitated by the antagonistic signaling relationship between transcription factors Vsx2 and Mitf (Nguyen & Arnheiter 2000). The outer layer forms the non-neuronal, single-layered retinal pigment epithelium (RPE), which nourishes retinal photoreceptors (Martinez-Morales et al 2004). The inner layer is made up of retinal progenitor cells (RPCs), which proliferate and differentiate into the seven different cell types (including both neuronal and glial cells) that ultimately comprise the multilayered neural retina. Retinal differentiation begins at E12 and is primarily completed when the eyelids open two weeks after birth (Graw 2010). At this stage, the retina comprises three layers: the ganglion cell layer, which contains retinal ganglion cells; the inner nuclear layer, which contains amacrine, Muller glia, bipolar, and horizontal cells; and the outer nuclear layer, which contains photoreceptor cells.
Figure 2.
The Hippo-Yap signaling pathway has key functions in both organism development and disease. During normal development, for example, the major roles played by Hippo-Yap signaling in organ size determination include: regulating cell numbers via cell proliferation and apoptosis in a cell density-dependent manner (Dong et al 2007, Huang et al 2005, Zhao et al 2007); embryonic trophectoderm cell fate specification in the preimplanted mouse embryo (Nishioka et al 2009); post-mitotic cell fate specification (Jukam et al 2013); tissue-specific regeneration of progenitor cells (Wang et al 2017); mechanotransduction (Dupont et al 2011, Halder et al 2012, Morgan et al 2013); and integration of apical and basal signaling (Elbediwy et al 2016). Perturbed regulation of the Hippo-Yap pathway can contribute to pathological tissue overgrowth and tumor progression, which result in unchecked proliferation and evasion of apoptosis, two major aspects of cancer development (Steinhardt et al 2008, Zhao et al 2007). Yap also has the potential to serve as a marker for malignancies because it is upregulated in a variety of tumors, including colon cancer, hepatocellular carcinoma, ovarian cancer (Cai & Xu 2013, Rosenbluh et al 2012, Tschaharganeh et al 2013, Zhang et al 2014), pancreatic adenocarcinoma and uveal melanoma (UM) (Yu et al 2014a). Abnormal regulation of the Hippo-Yap signaling pathway may also be associated with diseases other than cancer, including ocular-specific disorders such as ocular colobomas, Sveinsson chorioretinal atrophy (SCRA; OMIM #108985), and retinal degeneration (Fossdal et al 2004, Hamon et al 2017, Kitagawa 2007, Oatts et al 2017, Williamson et al 2014). Disorders such as these have made Yap expression in ocular tissue a particularly promising target for innovative therapies.
In this review, we first summarize the expression and subcellular localization of Yap and Tead family transcriptional factors during mammalian ocular development. We then consider functional studies of Hippo-Yap pathway that have used diverse gene knockout (KO) and knockdown approaches. Finally, we discuss canonical and non-canonical functions of the Hippo-Yap pathway during normal ocular development and diseases.
EXPRESSION AND SUBCELLULAR LOCALIZATION OF YAP AND INTERACTING PROTEINS IN DEVELOPING OCULAR TISSUES
(1) Yap shows a dynamic expression pattern and diverse subcellular localization during ocular development
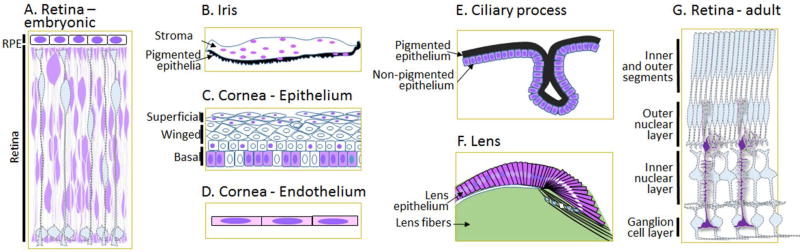
Yap is weakly expressed in the lens placode, neural retina, RPE and surface ectoderm of the developing OV (Kim et al 2016). At the OC stage, Yap is exclusively expressed in the outer neuroblastic layer (ONBL) of the neural retina where RPCs are enriched, but it is absent in the inner neuroblastic layer (INBL) where post-mitotic cells reside. Yap is also expressed in other ocular tissues, including the lens epithelium, ciliary body, iris, extrinsic muscles, RPE, corneal epithelium, and periocular mesenchymal cells (Fig. 3).
Figure 3.
Yap subcellular localization within ocular tissues follows three principal patterns (Fig. 3). (1) Yap is enriched (or nearly exclusive) in the nucleus. This pattern is seen in subsets of the corneal epithelium (mainly in the squamous cells) and endothelium (Hamon et al 2017). In the RPE, subcellular localization of Yap changes during mouse development. Yap is nuclear at E10.5 but becomes cytoplasmic at subsequent embryonic stages. In the mature RPE, Yap remains in the nucleus (Kim et al 2016, Moon et al 2018). It is also characteristic of various types of tumor cells, including human UMs, although normal melanocytes do not exhibit nuclear accumulation of Yap (Feng et al 2014, Yu et al 2014a). 2) Yap is localized in all subcellular compartments, including the nucleus, soma, processes, and apical and basal endfeet. This pattern of ubiquitous distribution appears, for example, in RPCs, MGCs, lens epithelium, and non-pigmented ciliary epithelial cells (Hamon et al 2017, Kim et al 2016). (3) Yap is located predominantly in the cytosol and excluded from the nucleus, as in transient cell-cycle exiting cells in the equators of the lens (Song et al 2014). These unique, cell-type specific localization patterns within ocular tissues strongly suggest that Yap plays novel roles beyond its oncogenic function mediated by localization in the nucleus.
(2) Yap interacts with apical junction-associated proteins
The movement of Yap between the cytoplasm and nucleus highlights the dynamic features of this protein. While nuclear translocation is facilitated when upstream kinase signaling is absent, the fate of cytoplasmic Yap is further regulated. The first way is through degradation of abundant pYap by an ubiquitin-mediated mechanism (Zhao et al 2011b). The second is by phosphorylation at Ser 127, which facilitates Yap’s interaction and stabilization with protein 14-3-3 that sequesters it in the cytoplasm (Zhao et al 2011b).
It is intriguing that cytoplasmic Yap and pYap associate with junction proteins, although the features and function of this interaction are not fully understood. Several lines of evidence from in vitro and in vivo studies support the idea that the association plays a regulatory role in nuclear translocation of Yap and the novel notion that Yap is engaged in junctional stability. First, angiomotin (Amot) tight junction-associated protein directly interacts with Yap and prevents cellular proliferation (Zhao et al 2011a). However, interaction with Amot has also been reported to facilitate nuclear translocation of Yap in other contexts (Hong 2013, Moleirinho et al 2017, Yi et al 2013). Second, physical interaction between Yap and Crumbs polarity complex proteins, including Amot, Pals1, PatJ, and Lin7C, was demonstrated by co-immunoprecipitation assay in cultured HEK293 cells (Varelas et al 2010). Third, a comprehensive, unbiased Yap interactome study by co-immunoprecipitation followed by mass spectrometry also identified many junctional proteins, including α-catenin, Par3, Pals1, and Amot (Kohli et al 2014).
One mode of regulation mediated by interaction between Yap and junction-associated protein is control of Yap’s subcellular localization. For example, αE-catenin promotes Yap’s cytoplasmic sequestration by binding with Yap; Yap is constitutively nuclear in αE-catenin-null cells (Silvis et al 2011). In addition, α-catenin controls the activity and phosphorylation of Yap by regulating Yap’s interaction with 14-3-3 and PP2A phosphatase (Schlegelmilch et al 2011). Recent results showing destabilized junctional complex proteins and laminar organization suggest Yap’s critical role in tissue organization. Importantly, Yap mutant studies have indicated that Yap may contribute to assembling and/or maintaining an apical polarity complex within epithelial cells and providing structural organization (Kim et al 2016, Song et al 2014). It has been proposed that, by localizing at the apical junction of developing ocular tissues, including the retina and lens, Yap acts as a novel regulator of cell-cell adhesion. Genetic mutant studies have revealed disruption of the apical junction and structural integrity in both Yap mutant retinas and lenses. These disturbances of cellular polarity and adhesion result in compromised cellular and nuclear shape, rosette formation, and laminar disorganization followed by cellular degeneration. Yap’s apical junctional association may therefore play a novel role in stabilizing cell-cell adhesion, particularly during ocular growth and development. Alternatively, absent transcriptional activation of Yap and/or altered genetic interaction between mutations of Yap and unknown genes may also affect junctional phenotype.
(3) Yap’s transcriptional partners in ocular tissues
In the absence of upstream kinase signaling, cytoplasmic Yap translocates into the nucleus, where it does not bind to DNA directly but forms a transcriptionally active complex with transcription factors such as Tead (Vassilev et al 2001). Mammalian genomes encode four TEA domain family DNA binding transcription factors (Tead1–4) lacking a transactivation domain. These proteins share a common TEA DNA binding domain and have different expression patterns in various tissues and developmental stages (Kaneko & DePamphilis 1998).
While most adult tissues express at least one Tead gene (Kaneko et al 1997, Yockey et al 1996), a variety of developing mammalian ocular tissues express Tead1–4 (genepaint.org). At E14.5, Tead1 transcripts are weakly distributed in the mouse eye while Tead4 is nearly absent (genepaint.org). During the same developmental stage, Tead2 is highly expressed in the ONBL of the retina and enriched on the basal side where S-phase cells are located. Tead2 is also expressed in lens epithelial cells, but not in lens fibers, cornea, or periocular mesenchyme. At this stage, Tead3 is highly enriched in INBL, which includes retinal post-mitotic cells, and is present in the ONBL, cornea, and periocular mesenchyme (genepaint.org). Considering that stimulation of nuclear Yap signaling is normally detected in cells that are actively proliferating, such as cancer cells, Tead3’s strong enrichment in the post-mitotic cells suggests a novel, non-canonical Yap function during differentiation of retinal neurons. For further insight into Yap function, knockout mutants of Tead 1 and 2 were characterized during early development (Sawada et al 2008). Tead1−/− mice died at/around E11.5 while Tead2−/− mice did not show any overt abnormalities and maintained fertility. However, Tead1−/−; Tead2−/− mice died at/around E9.5 and displayed severe growth defects and morphological phenotypes. Ocular phenotype was not described.
Intriguingly, a novel missense mutation in a conserved amino acid in the C-terminal domain of the Tead1, putative Yap binding region, has been proposed to cause an ocular disorder, SCRA (Fossdal et al 2004). Overexpression in the optic vesicle of dominant negative Tead1a, which is incapable of binding to Yap, induced RPE loss in zebrafish, similar to that in human SCRA patients with Tead1 mutation (Jonasson et al 2007a, Jonasson et al 2007b, Miesfeld et al 2015). Although ocular mouse mutant studies using Tead1–4 have not yet been performed, the relatively strong expression in multiple ocular tissues suggests that at least Tead2 and 3 serve multiple functions in the developing eye.
Since Yap partners with multiple transcriptional factors, including Tead, Runx1/2 and p73, the Tead mutant phenotype may represent a subset of that of Yap mutant.
(4) Selective Yap-Tead transcriptional activity in developing ocular tissue
Yap/Taz-responsive reporter constructs provide powerful tools for identifying cells and tissues where Yap-Tead mediated transcription is active (Mahoney et al 2005), especially during embryonic tissue development. To monitor the dynamics of Hippo-Yap signaling during development in the eye, Yap-Tead reporters have been used in vivo, including 8xGTIIC in chick embryos (Kim et al 2016) and 4xGTIIC in zebrafish (Miesfeld & Link 2014). In both zebrafish and chick embryos, reporter activity was strong in RPE, weak in neuroepithelium.
YAP AND TAZ SHOW CONTEXT-DEPENDENT FUNCTIONAL OVERLAP
Both Yap and Taz possess multiple protein-protein interaction domains, including Tead-binding and transactivation domains. Both also have coiled-coil, WW-rich domains and PDZ binding motifs at their C-termini (Kanai et al 2000). Structural comparisons between these paralogs in mice (and humans) have revealed (1) an approximately 54% (54%) overall protein sequence similarity and 39% (40%) overall protein sequence identity (ClustalW2.com) and (2) that the transcriptional activator function of both transcription coactivators depends highly on the TEA family of transcriptional factors (Tead1–4), (Kanai et al 2000).
Given this similarity, it is highly likely that the two paralogs function redundantly or compensate for loss of one another during development and disease pathogenesis. Several lines of evidence support the importance of their compensatory interactions, which may be context-dependent. First, the two paralogs have been reported to act synergistically in tumorigenesis and metastasis. A retrospective cohort study using immunohistochemistry, small interfering RNA (siRNA) transfection, and multivariate statistical analysis revealed a functional relationship between Yap and Taz in colorectal cancer (CRC) (Wang et al 2013): patients who overexpressed both Yap and Taz had a lower overall survival than those who overexpressed either Yap or Taz alone. Moreover, cells transfected with both Yap and Taz siRNAs exhibited a larger reduction in proliferation, migration, and invasion than cells transfected with either Yap or Taz siRNA alone, which highlighted the potential synergy between Yap and Taz in growth and spread of CRC cells (Wang et al 2013). Yap and Taz, however, have been found to function redundantly in the intestinal epithelium of mice: KO of a single gene did not produce a phenotype but KO of both Yap and Taz inhibited crypt cell proliferation and differentiation into goblet cells (Imajo et al 2015). KO studies in hepatocellular carcinoma cells, colon cancer cells, and healthy fibroblast cells also noted the ability of Yap to compensate for Taz ablation and rescue its proliferative function at least in part (Hayashi et al 2015). In addition, whereas deletion of both Yap and Taz in mouse epicardium resulted in cardiomyopathy, impaired coronary vasculature development, and lethality, Yap compensation produced viable mice following removal of Taz alone (Singh et al 2016).
Studies of ocular development in zebrafish provide further evidence of the compensatory interaction: loss of RPE was amplified in Yap mutant embryos when a Taz allele was also mutated. Similarly, although Yap is recognized as the principal regulatory transcription cofactor for determining RPE fate, mutant studies have highlighted the ability of Taz to compensate for Yap ablation in RPE genesis as in the myelination of peripheral nerves by Schwann cells (Grove et al 2017, Miesfeld et al 2015). However, the potential compensatory interaction between Yap and Taz need to be determined in mouse eye. The modes of interaction between Yap and Taz therefore, may be context- and tissue-dependent in both developmental and pathological conditions.
MUTANT MODELS OF YAP
A number of Yap-mutant animal models have been used to investigate the functions of Yap during different developmental stages (Table 1). One of the earliest Yap mutants was a Yap KO mouse (Morin-Kensicki et al 2006). Loss of Yap activity disrupted embryogenesis at E8.5 and resulted in failure of yolk sac vascular plexus formation and chorioallantoic attachment, shortened body axis, and caudal dysgenesis. These results revealed the critical importance of Yap in early stages of development. Conditional gene knockdown using RNA interference (RNAi) or conditional knockout (CKO) technologies have been used subsequently to ablate Yap activity in ocular progenitors in vivo. Zhang et al. showed that inhibition of Yap in late-stage RPCs by RNAi decreased proliferation and increased neuronal differentiation, which indicated Yap’s pivotal functions in regulating proliferation and cell cycle exit during retinal development (Zhang et al 2012). Nestin-Cre mediated Yap gene ablation techniques have also been used to generate a Yap CKO that eliminated Yap in developing lens epithelial cells and permitted analysis of Yap function in lens development and growth. This work demonstrated that Yap is critical for normal lens development, timely differentiation of lens progenitor cells, and maintenance of lens epithelial cell polarity and survival (Song et al 2014). Similarly, Rx-Cre was used to conditionally remove Yap in the early OV to study its function in the descendant ocular tissues, mainly RPE and retina. Ablation of Yap in early primordial retinal tissue led to defective retinal and RPE laminal organization, destabilization of apical complex proteins in the retina, increased apoptosis of retinal cells, mildly compromised RPC proliferation, altered cell cycle progression, rosette formation and, ultimately, retinal degeneration. RPE also transdifferentiated into retinal tissue in Yap CKO using Rx-Cre (Kim et al 2016). Yap’s essential function in RPE was similarly documented in zebrafish in which double mutants of Yap and Taz showed agenesis of RPE in addition to the coloboma phenotype (Miesfeld et al 2015). Overexpression of wild type (WT) or constitutively active Yap in zebrafish led to ectopic pigmentation in the retina (Miesfeld et al 2015). These results from mouse and zebrafish indicate that Yap and Taz are necessary and sufficient for RPE fate determination in OV cells.
Table 1.
Summary of Hippo-Yap pathway mutants and ocular phenotypes
| Animal | Mutation | Ocular tissue | Phenotype | References | |
|---|---|---|---|---|---|
| Yap−/− | mouse | Targeted germ-line knock-out | Embryo | Developmental arrest at E8.5: shortened body axis, convoluted anterior neuroepithelium, caudal dysgenesis, and failed york sac vaculogenesis. | (Morin-Kensicki et al., 2006) |
| Yap RNAi | mouse | Knock-down by RNA interference | Retina | Decreased proliferation of the retinal progenitor cells and concomitant increase of differentiation; proneural bHLH-mediated inhibition of Yap mRNA | (Zhang et al., 2012) |
| Yap cKO | mouse | Conditional knock-out with Nestin-Cre | Developing lens | Precocious differentiation of lens epithelial cells; disrupted lens epithelial cell polarity; reduced cell survival | (Song et al., 2014) |
| Yao/Taz double KO | zebrafish | Targeted germ-line knock-out | Retina and RPE | Agenesis of RPE; coloboma | (Miesfeld et al., 2015) |
| Yap cKO | mouse | Conditional knock-out with Rx-Cre | Retina and RPE | RPE to retinal transdifferentiation; mildly reduced proliferation of the retinal progenitor cells; disruption of polarity complex; increased apoptosis; laminar disorganization | (Kim et al., 2016) |
| Yap/Taz dKO | mouse | Conditional, inducible knock-out with VE-cadherin-ERT2 | Retinal vessels | Delayed vessel growth and hyper-pruned vascular network; blunt-end, aneurysm-like morphology at the tip of the ECs with fewer and dysmorphic filopodia; | (Kim et al., 2017) |
| Lats1/2 dKO | mouse | Conditional, inducible knock-out with VE-cadherin-ERT2 | Retinal vessels | Dense and hyperplastic vascular network with reduced radial length; increased ECs and filopodia | (Kim et al., 2017) |
| siYap | mouse | Knock-down by short interfering RNA | Retinal vessels | Vascular density and the number of branching decreased | (Choi et al., 2015) |
| Yap/Taz dKO | mouse | Conditional, inducible knock-out with Pdgfb-iCreERT2 | Vascular endothelial cells | Reduced vascular density; decreased retinal vascular field; impaired retinal vessel sprouting; reduced vascular branches | (Sakabe et al., 2017) |
| Lats1/2 dKO | mouse | Conditional, inducible knock-out with Pdgfb-iCreERT2 | Vascular endothelial cells | Defective vascular extension and filopodia formation; increased branch points and ECs; decreased vascular area and tip cells; dense hyperplasic vasculature | (Sakabe et al., 2017) |
More recently, Hippo-Yap pathway genes were also genetically manipulated in retinal endothelial cells. In one set of studies, Yap/Taz double knockout (DKO) mice were generated by combining flox alleles with inducible Cre lines such as VE-cadherin-CreERT2 or Pdgfb-iCreERT2 (Kim et al 2017, Sakabe et al 2017). Using another approach, knockdown in the retinal vasculature was achieved by injecting short-interfering RNAs (siYap). These studies revealed a critical requirement for, and functional redundancy between, Yap/Taz in coordinating proliferation of vascular endothelial cells, vascular branching and barrier formation (Choi et al 2015, Kim et al 2017, Sakabe et al 2017).
YAP FUNCTION IN OCULAR DEVELOPMENT
(1) Control of cell proliferation and retinogenesis
Gain and loss of function studies showed that Yap provides timely regulation of retinal cell differentiation by promoting proliferation of RPCs while inhibiting cell cycle exit. Yap is also negatively regulated by pro-neural basic-helix-loop-helix (bHLH) proteins such as Neurog2 and Ascl1. For example, bHLH proteins can indirectly inhibit Yap function by downregulation of Yap mRNA or by post-translational inhibition due to activating Lats1/2 kinases. This inhibition of Yap can reduce proliferation of RPC and increase cell cycle exit and neuronal differentiation (Zhang et al 2012). These results demonstrated a mutually antagonistic interaction between Yap and pro-neural genes that coordinates the timely exit of RPC to produce retinal neurons. Similarly, in Yap-ablated developing lens epithelial cells, BrdU (+) progenitor pools were severely reduced and a CDK inhibitor such as p57, and a factor promoting cell cycle exit such as Prox1, were ectopically upregulated (Song et al 2014). Interestingly, the fraction of S-phase cells in Yap-deficient mouse retinas was only slightly reduced compared to WT retinas (Kim et al 2016). However, Yap-deficient RPCs show an overall delayed progression through the cell cycle, additional evidence of Yap’s critical function in normal cell cycle progression. These findings received further support from studies of the amphibian retina in which Yap knockdown markedly reduced the proportion of time that retinal stem cells spent in S-phase, which ultimately increased DNA damage and cell death (Cabochette et al 2015). In summary, these results suggest that Yap plays a vital role in regulating progenitor proliferation and neurogenesis in the retina.
(2) Anti-apoptosis
One class of Yap/Taz-Tead transcription target genes includes anti-apoptotic genes such as Ctgf, c-myc, Sox4, Mcl1, and inhibitors of apoptosis such as survivin/BIRC5 and BIRC2 (Dong et al 2007, Hong & Guan 2012, Tian et al 2015, Zhao et al 2008). Yap’s upregulation of these anti-apoptotic genes may indicate a critical role in the survival of ocular cells (Hong & Guan 2012). Consistent with this notion, both INBL and ONBL of Yap-deficient developing retinas showed a dramatic increase in dying cells, as determined by an increase of cell death markers such as activated cleaved caspase 3 (Kim et al 2016). In addition, a CKO of Yap in the developing mouse lens reduced the lens epithelial progenitor cell (LEC) pool, which was partially due to increased apoptosis of LECs (Song et al 2014). These results highlight the possibility that Yap serves an anti-apoptotic function in ocular tissues, as in pancreatic beta cells, podocytes, and colorectal cancer cells (Campbell et al 2013, Wang et al 2013, Yuan et al 2016).
(3) Regional fate determination
In addition to its transcriptional functions regulating cell proliferation and survival, Yap also plays an important role in determining the fate of cells such as epidermal stem cells (Totaro et al 2017) and of regions such as the early developing ocular tissues of vertebrates. In zebrafish, Yap/Taz-Tead activity is critical for OV progenitors to become RPE and depends on nuclear localization of Yap as well as Yap’s interaction with Tead (Miesfeld et al 2015). Therefore, Yap-deficient zebrafish lose a subset of RPE cells, while Taz-deficient zebrafish develop normal RPE. Additional loss of Taz in Yap-deficient zebrafish causes a larger loss of RPE cell types than Yap deficiency alone, suggesting functional compensation by Taz in Yap mutant (Miesfeld et al 2015). Complementary to this result, expression of WT or constitutively active Yap (YapS87A) in RPCs led to ectopic pigmentation in addition to disrupted lamination. Intriguingly, in mouse Yap mutant eyes, RPE, normally a cuboidal epithelial sheet consisting of a single layer, transdifferentiates into multilayered pseudostratified epithelial cells that express retinal markers, including Chx10 and Tubulin beta III, rather than the RPE markers Ezrin and Mitf, as in WT (Kim et al 2016). These findings indicate that Yap plays a novel role in determining regional RPE fate during OV/OC development. Interestingly, Yorkie, a homolog of Yap in Drosophila, contributes to determining the fate map of the fly eye by similarly exerting an instructive role in non-neural RPE territory in the developing OV. The inactivation of Yorkie thus led the peripodial epithelium (non-neural) to transdifferentiate into disc proper (neural retina) (Zhang et al 2011). Therefore, the function of Yap or Yorkie in instructing the regional fate of the non-neural tissue in developing epithelium is evolutionarily conserved between vertebrates and invertebrates.
(4) Photoreceptor cell differentiation
Evidence demonstrating the role of the Hippo-Yap signaling pathway in zebrafish embryogenesis has shed light on its importance in retinal cell differentiation during development. First, a knockdown of zebrafish Mst2, a homolog of Hippo kinase, resulted in abnormal eye development and retinal pigmentation (Asaoka et al 2014). Second, similar phenotypes were subsequently observed in zebrafish when constitutively active Yap (Yap5SA) was overexpressed by mutating five serine residues to alanine to produce phosphorylation-defective Yap (Asaoka et al 2014). Microarray and gene ontology analyses of these Yap5SA-injected embryos revealed downregulation of photoreceptor transcription factors such as Crx, Nr2e3 and Otx5, and of genes involved in phototransduction such as rhodopsin (Asaoka et al 2014). In addition, co-immunoprecipitation analysis showed a physical interaction between Yap and retina-specific transcription factor Rx1; functional assays demonstrated that this physical interaction enabled Yap to prevent the Rx1-mediated transactivation of the rhodopsin and Otx5/Crx genes. Thus, instead of promoting proliferation of RPCs, Yap inhibited photoreceptor cell differentiation by preventing the transcription of genes downstream of Rx, such as the rhodopsin and transcription factors Otx and Crx that are crucial for photoreceptor cell differentiation. It has been suggested that Yap’s binding with TEAD (Yap binding domain with TEAD-binding domain) promotes transcription of downstream target genes, whereas Yap’s binding with Rx1 (WW1 & 2 with PPXY) represses downstream genes involved in photoreceptor differentiation. The widespread expression of Rx1 in retinal progenitor cells suggests that Yap may function as a general inhibitor of retinal neurogenesis.
(5) Cell polarity, junctional integrity, cellular and laminar organization
In addition to its well-studied growth control function, Hippo-Yap signaling has been implicated in regulating cell polarity and size of the apical domain in Drosophila (Enderle & McNeill 2013, Genevet & Tapon 2011, Grifoni et al 2013, Grzeschik et al 2010, Hamaratoglu et al 2009, Robinson et al 2010, Yang et al 2015). Imaginal discs deficient in Hippo or Warts showed an increased apical domain and enhanced localization of apical polarity determinants including Crumbs, which was independent of tissue growth regulation by Crumbs (Chen et al 2010, Genevet et al 2009, Ling et al 2010). In columnar epithelium, the effect is mediated by Crumbs –dependent phosphorylation of Yap/Taz by activating Mst1/1-Lats1/2 signaling. Therefore, apical polarity complex proteins are putative upstream regulators of Hippo-Yap pathway (Elbediwy et al 2016).
Ablation of Yap in developing mouse lens and retina disrupted cellular and laminar organization (Kim et al 2016, Song et al 2014). Yap-deficient lens epithelial cells lost their normal columnar appearance and oval nuclear shapes concomitant with reduction and disorganization of apical junctional proteins. Instead of their normal appearance, lens epithelial cells assumed a flattened shape similar to that of squamous cells. Nuclei were also severely flattened. Additionally, developing retinas devoid of Yap showed disrupted apical junctions in the embryonic counterpart of the outer limiting membrane. As a result, retinal epithelia demonstrated areas of folding or rosette formation, and ultimately lost their laminar arrangement, indicating Yap’s crucial role in maintaining cellular, nuclear and laminar organization. Similar findings appeared in other central nervous system tissues such as the ventricular layer of the developing midbrain (Park et al 2016). In the absence of Yap, progenitor cells in the developing aqueduct lost their polarized shape and showed rounding of their nuclei in addition to disrupted junctions.
(6) Retinal vascular development
Recent studies show that Yap/Taz are also involved in the proliferation and migration of vascular endothelial cells (ECs) during retinal angiogenesis, vascular barrier formation and maturation (Kim et al 2017, Sakabe et al 2017). These retinal vascular ECs show dynamic patterns of expression and localization similar to those of other ocular tissues. Yap is predominantly but diffusely localized in the cytoplasm and nuclei of ECs, whereas Taz is primarily distributed in the nuclei. Yap is localized in the nuclei of ECs in the frontal vascular region of the retinal vessels and at the branching points of invading retinal vessels. While nuclear Yap controls the proliferation of ECs via activation of Myc signaling (transcriptional), cytoplasmic Yap (possibly pYap) promotes EC migration by activating components of the actin cytoskeleton such as Rho family GTPase CDC42 (non-transcriptional). Thus deletion of Lats1/2 was found to cause cell migration defects (Sakabe et al 2017). The failure to maintain the integrity of tight and adherens junctions in Yap/Taz-deficient ECs was exemplified by disrupted ZO-1, Claudin-5, and VE-cadherin, suggesting a critical requirement in the formation and maturation of the blood-retinal barrier (Choi et al 2015, Kim et al 2017, Sakabe et al 2017).
DYSFUNCTIONAL YAP SIGNALING IN OCULAR DISEASES (TABLE 2)
Table 2.
Summary of human ocular disorders with altered Hippo-Yap pathway
| Nature of mutation | Biochemical alteration of the pathway | References | |
|---|---|---|---|
| Coloboma and optic fissure closure defect | Non-sense mutations in Yap gene (370C>T; 1066G>T) | Truncation of TEAD-binding domain | (Oatts et al., 2017, Williamson et al., 2014) |
| Sveinsson's chorioretinal atophy (SCRA) | Missense mutation in TEAD1 | Inefficient binding with Yap | (Fossdal et al., 2004, Kitagawa, 2007) |
| Uveal melanoma (UM) | Mutations in guanine nucleotide binding protein subunit alpha-Q (GNAQ) and alpha 11 (GNA11) | Increased Yap activity | (Feng et al., 2014, Lyubasyuk et al., 2015, Yu et al., 2014a) |
| Neurofibromatosis 2 (NF2) | Mutations in NF2 gene coding merlin protein | Increased Yap activity | (Guerrant et al., 2016, Wiley et al., 2010, Zhang et al., 2010) |
| Retinal degeneration | Retinal degeneration 10 (rd10) | Increased Yap activity in the Muller glia | (Hamon et al., 2017) |
(1) Coloboma and optic fissure closure defect
Yap KO mouse shows embryonic lethality at E8.5, indicating the critical requirement for Yap activity during early embryogenesis (Morin-Kensicki et al 2006). Human Yap mutations are therefore thought to be difficult to find because of their potential fetal lethality. However, a rare dominant allele of Yap was identified in human patients exhibiting ocular defects. One such disease is ocular coloboma, which results from the inability of the optic fissure to fully close during ocular development, and affects optic structures such as the lens, retina, choroid, ciliary body, iris, and cornea (Chang et al 2006, Gregory-Evans et al 2004, Oatts et al 2017). Whole exome sequencing analysis of two families with autosomal-dominant inheritance of coloboma revealed two novel heterozygous nonsense mutations in the Yap gene. One of these two nonsense mutations, c.370C>T, was specifically identified within the Tead-binding domain, and the other, c.1066G>T, within the transactivation domain of the Yap gene (Williamson et al 2014). Specifically characterized as heterozygous loss-of-function Yap mutations segregating with the optic fissure closure defect phenotype of coloboma, these mutations may be related to the incomplete penetrance of the disease (Oatts et al 2017, Williamson et al 2014).
Consistent with the observation of Yap loss-of-function mutations in humans, Yap zebrafish mutants (Yapn113/n113 mutants, and, to a lesser degree, Yap−/−) also exhibited coloboma (Miesfeld et al 2015). This mutation in the splice acceptor site of intron 4 resulted in premature truncation at the beginning of the transactivation domain. Injections of wild-type Yap mRNA rescued the coloboma phenotype in the zebrafish embryos, confirming the Yap mutation as the cause of the ocular phenotype. These results demonstrate that Yap’s role in choroid fissure closure is conserved across species and that it is important for preventing coloboma in both zebrafish and humans (Miesfeld et al 2015).
(2) Sveinsson chorioretinal atrophy (SCRA)
Ocular diseases are associated not only with Yap-specific mutations, but with mutations in regulators of the Hippo-Yap pathway. SCRA, an eye disorder causing bilateral chorioretinal degeneration, is genetically linked to a missense mutation in the gene encoding Tead1 (Kitagawa 2007). In mice this mutation causes the interaction between the C-terminal domain of Tead1 and Yap/Taz cofactors to be lost, and reduces transcriptional activity of Tead1 in the presence of Yap/Taz (Komuro et al 2003). While its precise contribution to pathogenesis in SCRA is unclear, it has been suggested that lack of Yap/Taz-Tead1 may cause RPE pathology because RPE and choroidal loss are followed by photoreceptor loss (Liu et al 2010).
(3) Uveal melanoma (UM)
Another ocular disorder related to defects in components of the Hippo-Yap signaling pathway is UM, a non-cutaneous melanoma that is one of the most common intraocular defects in adults (Amaro et al 2017). It is primarily caused by mutations in guanine nucleotide-binding protein subunit alpha-Q (GNAQ gene), which encodes for Gαq, and in guanine nucleotide binding protein subunit alpha 11 (GNA11 gene), which encodes Gα11; both are alpha subunits of the heterotrimeric G proteins that play a role in transmembrane signaling systems (Markby et al 1993). Yap activity increases in mutated mice and, when Yap is knocked down, tumor growth of UM mice is blocked (Lyubasyuk et al 2015, Yu et al 2014b). Thus, Yap seems to play a critical oncogenic role in mutant mice with UM.
(4) Neurofibromatosis 2 (NF2)
NF2 is a rare genetic disorder characterized by formation of benign tumors in the nervous system. About half of NF2 patients develop posterior subcapsular cataracts, which cause clouding of the lens and, along with retinal hamartomas and epiretinal membrane, result in progressive visual impairment (Baser et al 1999, Kaiser-Kupfer et al 1989). Nf2 encodes for a tumor suppressor that acts as an activator of the Hippo kinases and therefore downregulates Yap’s transcription coactivator function (Hamaratoglu et al 2009, Zhao et al 2007). Mouse mutants with lens-specific ablation of Nf2 developed a phenotype mimicking posterior subcapsular cataracts (Giovannini et al 2000, McLaughlin et al 2007, Wiley et al 2010, Zhang et al 2010). In these mice, lens fiber cells fail to exit the cell cycle and retain their progenitor status, which results in a poorly differentiated lens with cellular polarity defects (Wiley et al 2010). Intriguingly, reducing Yap expression by introduction of Yap heterozygote suppressed the cataracts and disorganized lens phenotype caused by Nf2 loss (Zhang et al 2010). Lens-specific Yap deletion induced a phenotype opposite to that of Nf2 mutants, which strengthens the notion that NF2 is a major upstream inhibitor of Yap in lens (Song et al 2014). Yap-deficient lens epithelial cells prematurely initiated lens fiber cell differentiation by upregulating cell cycle inhibitor gene Prox1. Together, these results suggest that suppression of Yap activity by Nf2 in the transition zone where lens epithelial cells exit the cell cycle, is crucial for timely control of lens fiber differentiation. In developing mouse eye at mid-gestation, NF2 is mainly expressed in the RPE and ciliary margin, which expand slowly, whereas its expression is diminished in the highly proliferative retina (Moon et al 2018). Postnatally, expression is maintained in the pigmented cells, including RPE and pigmented ciliary epithelium. As in the developing lens, NF2 ablation in the RPE and retina leads to increased RPE and retinal progenitor cell populations, suggesting that NF2 plays a crucial role in controlling ocular progenitor pools by inhibiting cell proliferation.
(5) Retinal degeneration
Yap has been implicated in different stages of retinal degeneration. One important study explored the activity of Hippo-Yap signaling components in degenerating retinas by comparing Yap and Tead protein expression levels in retinal degeneration 10 (rd10) mice and WT mice (Hamon et al 2017). It demonstrated that both Yap and Tead1 are normally expressed in adult Muller glial cells and that, interestingly, the number of Muller cells undergoing gliosis in the retina increases near the beginning of retinal degeneration at P13; this increase in Muller cell gliosis is directly correlated with increased Yap transcription and protein levels. Furthermore, Tead1 and Yap/Tead target genes, Ctgf and Cyr61, are also upregulated in response to photoreceptor degeneration after P20 in rd10 mouse lines and after P13 in rd1 mouse lines (Hamon et al 2017). Yap-dependent gliosis in the degenerating retina therefore may provide a framework for elucidating the unknown roles of the Hippo-Yap signaling pathway in diverse pathological and retinal degenerations.
CONCLUSIONS AND FUTURE DIRECTIONS
Although studies of Hippo-Yap signaling have primarily focused on tumorigenesis and organ size control, the eye has provided a distinctive opportunity to understand cell and tissue context-dependent functions of Yap signaling during development and disease. One advantage, for example, is that the eye comprises the neural retina and non-neural RPE, which are derived from the neural tube, as well as the lens and cornea, which originate in the ectoderm.
Although the abundant evidence now available clearly establishes the importance of Hippo-Yap signaling in ocular development and diseases, the upstream modulators, partnering transcription factors and downstream target genes of Yap in the eye remain largely unaddressed. For example, little is known about the identity and function of the upstream regulators of Yap, such as Hippo (Mst1/2) and Warts (Lats1/2), or about the role of regulation by mechanical signaling in developing tissues. DNA sequence-specific transcription factors interacting with Yap in the nucleus of ocular cells have also not been extensively investigated. It is highly plausible that Tead1–4 participate as primary transcription factors interacting with Yap in ocular progenitors. However, expression of Tead1–4 during development is not limited to progenitors and partially overlaps with Yap expression in differentiated cells. Therefore, it is possible that Yap interacts with other ocular tissue-specific transcription factors in a context-specific manner; one known interacting transcription factor is Rx1, a regulator of photoreceptor differentiation (Asaoka et al 2014). Furthermore, there is only scant information about downstream targets (effectors). It is especially important to understand the constellation of Yap’s transcription target genes that mediate RPE specification and photoreceptor differentiation. It is widely accepted that the molecular signaling cascades initiated by Wnt, Bmp, and Hh interact with and influence Hippo-Yap signaling in non-ocular tissues during normal development, in diseases and after injury. In the eye, however, crosstalk between Hippo-Yap and other signaling pathways has not been explored, although in developing RPE, for example, Wnt, Bmp and Hh signaling are required for RPE fate and maintenance (Fuhrmann et al 2014). Therefore, study of the interactions between Hippo-Yap and these other signaling pathways is expected to reveal how crucial multifactorial regulatory pathways determine region-specific fates of OV, neural retina and RPE.
One category of proteins that interact with Yap in the cytoplasm is junction-associated proteins such as α-catenin, Crumbs complex proteins and Amot. Understanding the specific mode of protein-protein interaction between Yap and junctional proteins will greatly facilitate understanding the roles of Yap in extra-nuclear compartments and in the assembly and/or maintenance of apical epithelial junctions.
Finally, Hippo-Yap activity can be altered by pharmacological treatment (Johnson & Halder 2014, Oku et al 2015). For example, chemical agents such as verteporfin (VP) limit the amplification of tissue overgrowth and the proliferation of human cancers by interfering with the Yap-Tead complex (Al-Moujahed et al 2017, Feng et al 2016, Liu-Chittenden et al 2012). Thus VP was shown to be effective in inhibiting growth of human ocular tumors resulting from Yap activation, such as retinoblastoma and UM (Brodowska et al 2014, Liu-Chittenden et al 2012, Lyubasyuk et al 2015). While the efficacy of inhibitors of Yap-Tead has been demonstrated, identification of pharmacological agents that activate Yap will provide an important framework for treating ocular diseases caused by loss of function of Yap or of its interacting proteins such as Tead1.
Key findings.
Dynamic spatiotemporal Yap expression and subcellular localization suggest diverse functions in ocular tissues.
Yap activity exerts not only its canonical function mediating progenitor proliferation and survival, but also non-canonical functions related to cell polarity and fate determination.
Perturbations of Hippo-Yap signaling result in diverse ocular disorders.
Acknowledgments
The authors thank Dr. Alan Tessler for critical reading of the manuscript. S.K is funded by the National Institute of Health (R56NS104038-01) and is supported by research grants by Shriners Hospitals for Children (86100).
References
- Al-Moujahed A, Brodowska K, Stryjewski TP, Efstathiou NE, Vasilikos I, et al. Verteporfin inhibits growth of human glioma in vitro without light activation. Scientific reports. 2017;7:7602. doi: 10.1038/s41598-017-07632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon C, Zaromytidou AI, Xi Q, Gao S, Yu J, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139:757–69. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro A, Gangemi R, Piaggio F, Angelini G, Barisione G, et al. The biology of uveal melanoma. Cancer metastasis reviews. 2017;36:109–40. doi: 10.1007/s10555-017-9663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka Y, Hata S, Namae M, Furutani-Seiki M, Nishina H. The Hippo pathway controls a switch between retinal progenitor cell proliferation and photoreceptor cell differentiation in zebrafish. PloS one. 2014;9:e97365. doi: 10.1371/journal.pone.0097365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry ER, Camargo FD. The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Current opinion in cell biology. 2013;25:247–53. doi: 10.1016/j.ceb.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Baser ME, Kluwe L, Mautner VF. Germ-line NF2 mutations and disease severity in neurofibromatosis type 2 patients with retinal abnormalities. American journal of human genetics. 1999;64:1230–3. doi: 10.1086/302338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Molecular cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- Brodowska K, Al-Moujahed A, Marmalidou A, Meyer Zu Horste M, Cichy J, et al. The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation. Experimental eye research. 2014;124:67–73. doi: 10.1016/j.exer.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabochette P, Vega-Lopez G, Bitard J, Parain K, Chemouny R, et al. YAP controls retinal stem cell DNA replication timing and genomic stability. eLife. 2015;4:e08488. doi: 10.7554/eLife.08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Xu Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell communication and signaling : CCS. 2013;11:31. doi: 10.1186/1478-811X-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KN, Wong JS, Gupta R, Asanuma K, Sudol M, et al. Yes-associated protein (YAP) promotes cell survival by inhibiting proapoptotic dendrin signaling. The Journal of biological chemistry. 2013;288:17057–62. doi: 10.1074/jbc.C113.457390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes & development. 2008;22:3320–34. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Loo LS, Chong YF, Huang C, Hong W. TEADs mediate nuclear retention of TAZ to promote oncogenic transformation. The Journal of biological chemistry. 2009;284:14347–58. doi: 10.1074/jbc.M901568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Blain D, Bertuzzi S, Brooks BP. Uveal coloboma: clinical and basic science update. Current opinion in ophthalmology. 2006;17:447–70. doi: 10.1097/01.icu.0000243020.82380.f6. [DOI] [PubMed] [Google Scholar]
- Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, et al. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15810–5. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Zhang H, Park H, Choi KS, Lee HW, et al. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nature communications. 2015;6:6943. doi: 10.1038/ncomms7943. [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annual review of cell and developmental biology. 2001;17:255–96. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124:267–73. doi: 10.1016/j.cell.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Elbediwy A, Vincent-Mistiaen ZI, Thompson BJ. YAP and TAZ in epithelial stem cells: A sensor for cell polarity, mechanical forces and tissue damage. BioEssays : news and reviews in molecular, cellular and developmental biology. 2016;38:644–53. doi: 10.1002/bies.201600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enderle L, McNeill H. Hippo gains weight: added insights and complexity to pathway control. Science signaling. 2013;6:re7. doi: 10.1126/scisignal.2004208. [DOI] [PubMed] [Google Scholar]
- Feng J, Gou J, Jia J, Yi T, Cui T, Li Z. Verteporfin, a suppressor of YAP-TEAD complex, presents promising antitumor properties on ovarian cancer. OncoTargets and therapy. 2016;9:5371–81. doi: 10.2147/OTT.S109979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer cell. 2014;25:831–45. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossdal R, Jonasson F, Kristjansdottir GT, Kong A, Stefansson H, et al. A novel TEAD1 mutation is the causative allele in Sveinsson's chorioretinal atrophy (helicoid peripapillary chorioretinal degeneration) Human molecular genetics. 2004;13:975–81. doi: 10.1093/hmg/ddh106. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S. Eye morphogenesis and patterning of the optic vesicle. Current topics in developmental biology. 2010;93:61–84. doi: 10.1016/B978-0-12-385044-7.00003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S, Zou C, Levine EM. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Experimental eye research. 2014;123:141–50. doi: 10.1016/j.exer.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevet A, Polesello C, Blight K, Robertson F, Collinson LM, et al. The Hippo pathway regulates apical-domain size independently of its growth-control function. Journal of cell science. 2009;122:2360–70. doi: 10.1242/jcs.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevet A, Tapon N. The Hippo pathway and apico-basal cell polarity. The Biochemical journal. 2011;436:213–24. doi: 10.1042/BJ20110217. [DOI] [PubMed] [Google Scholar]
- Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, et al. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes & development. 2000;14:1617–30. [PMC free article] [PubMed] [Google Scholar]
- Graw J. Eye development. Current topics in developmental biology. 2010;90:343–86. doi: 10.1016/S0070-2153(10)90010-0. [DOI] [PubMed] [Google Scholar]
- Gregory-Evans CY, Vieira H, Dalton R, Adams GG, Salt A, Gregory-Evans K. Ocular coloboma and high myopia with Hirschsprung disease associated with a novel ZFHX1B missense mutation and trisomy 21. American journal of medical genetics. Part A. 2004;131:86–90. doi: 10.1002/ajmg.a.30312. [DOI] [PubMed] [Google Scholar]
- Grifoni D, Froldi F, Pession A. Connecting epithelial polarity, proliferation and cancer in Drosophila: the many faces of lgl loss of function. The International journal of developmental biology. 2013;57:677–87. doi: 10.1387/ijdb.130285dg. [DOI] [PubMed] [Google Scholar]
- Grove M, Kim H, Santerre M, Krupka AJ, Han SB, et al. YAP/TAZ initiate and maintain Schwann cell myelination. eLife. 2017;6 doi: 10.7554/eLife.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Current biology : CB. 2010;20:573–81. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM, Kim NG. The Hippo-YAP signaling pathway and contact inhibition of growth. Journal of cell science. 2014;127:709–17. doi: 10.1242/jcs.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nature reviews. Molecular cell biology. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Gajewski K, Sansores-Garcia L, Morrison C, Tao C, Halder G. The Hippo tumor-suppressor pathway regulates apical-domain size in parallel to tissue growth. Journal of cell science. 2009;122:2351–9. doi: 10.1242/jcs.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon A, Masson C, Bitard J, Gieser L, Roger JE, Perron M. Retinal Degeneration Triggers the Activation of YAP/TEAD in Reactive Muller Cells. Investigative ophthalmology & visual science. 2017;58:1941–53. doi: 10.1167/iovs.16-21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CG, Moroishi T, Guan KL. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends in cell biology. 2015;25:499–513. doi: 10.1016/j.tcb.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nature reviews. Cancer. 2007;7:182–91. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Higashi T, Yokoyama N, Kaida T, Sakamoto K, et al. An Imbalance in TAZ and YAP Expression in Hepatocellular Carcinoma Confers Cancer Stem Cell-like Behaviors Contributing to Disease Progression. Cancer research. 2015;75:4985–97. doi: 10.1158/0008-5472.CAN-15-0291. [DOI] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–61. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilman D, Gat U. The evolutionary history of YAP and the hippo/YAP pathway. Molecular biology and evolution. 2011;28:2403–17. doi: 10.1093/molbev/msr065. [DOI] [PubMed] [Google Scholar]
- Hong W. Angiomotin'g YAP into the nucleus for cell proliferation and cancer development. Science signaling. 2013;6:pe27. doi: 10.1126/scisignal.2004573. [DOI] [PubMed] [Google Scholar]
- Hong W, Guan KL. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Seminars in cell & developmental biology. 2012;23:785–93. doi: 10.1016/j.semcdb.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–34. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nature cell biology. 2015;17:7–19. doi: 10.1038/ncb3084. [DOI] [PubMed] [Google Scholar]
- Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nature reviews. Drug discovery. 2014;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson F, Hardarson S, Olafsson BM, Klintworth GK. Sveinsson chorioretinal atrophy/helicoid peripapillary chorioretinal degeneration: first histopathology report. Ophthalmology. 2007a;114:1541–6. doi: 10.1016/j.ophtha.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Jonasson F, Sander B, Eysteinsson T, Jorgensen T, Klintworth GK. Sveinsson chorioretinal atrophy: the mildest changes are located in the photoreceptor outer segment/retinal pigment epithelium junction. Acta ophthalmologica Scandinavica. 2007b;85:862–7. doi: 10.1111/j.1600-0420.2007.01002.x. [DOI] [PubMed] [Google Scholar]
- Jukam D, Xie B, Rister J, Terrell D, Charlton-Perkins M, et al. Opposite feedbacks in the Hippo pathway for growth control and neural fate. Science. 2013;342:1238016. doi: 10.1126/science.1238016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser-Kupfer MI, Freidlin V, Datiles MB, Edwards PA, Sherman JL, et al. The association of posterior capsular lens opacities with bilateral acoustic neuromas in patients with neurofibromatosis type 2. Archives of ophthalmology. 1989;107:541–4. doi: 10.1001/archopht.1989.01070010555030. [DOI] [PubMed] [Google Scholar]
- Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. The EMBO journal. 2000;19:6778–91. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko KJ, Cullinan EB, Latham KE, DePamphilis ML. Transcription factor mTEAD-2 is selectively expressed at the beginning of zygotic gene expression in the mouse. Development. 1997;124:1963–73. doi: 10.1242/dev.124.10.1963. [DOI] [PubMed] [Google Scholar]
- Kaneko KJ, DePamphilis ML. Regulation of gene expression at the beginning of mammalian development and the TEAD family of transcription factors. Developmental genetics. 1998;22:43–55. doi: 10.1002/(SICI)1520-6408(1998)22:1<43::AID-DVG5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim YH, Kim J, Park DY, Bae H, et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. The Journal of clinical investigation. 2017;127:3441–61. doi: 10.1172/JCI93825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Park R, Lee JH, Shin J, Nickas J, et al. Yap is essential for retinal progenitor cell cycle progression and RPE cell fate acquisition in the developing mouse eye. Developmental biology. 2016;419:336–47. doi: 10.1016/j.ydbio.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M. A Sveinsson's chorioretinal atrophy-associated missense mutation in mouse Tead1 affects its interaction with the co-factors YAP and TAZ. Biochemical and biophysical research communications. 2007;361:1022–6. doi: 10.1016/j.bbrc.2007.07.129. [DOI] [PubMed] [Google Scholar]
- Kohli P, Bartram MP, Habbig S, Pahmeyer C, Lamkemeyer T, et al. Label-free quantitative proteomic analysis of the YAP/TAZ interactome. American journal of physiology. Cell physiology. 2014;306:C805–18. doi: 10.1152/ajpcell.00339.2013. [DOI] [PubMed] [Google Scholar]
- Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. The Journal of biological chemistry. 2003;278:33334–41. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- Lai D, Yang X. BMP4 is a novel transcriptional target and mediator of mammary cell migration downstream of the Hippo pathway component TAZ. Cellular signalling. 2013;25:1720–8. doi: 10.1016/j.cellsig.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Lin YT, Ding JY, Li MY, Yeh TS, Wang TW, Yu JY. YAP regulates neuronal differentiation through Sonic hedgehog signaling pathway. Experimental cell research. 2012;318:1877–88. doi: 10.1016/j.yexcr.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Ling C, Zheng Y, Yin F, Yu J, Huang J, et al. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10532–7. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes & development. 2012;26:1300–5. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Xin Y, Ye F, Wang W, Lu Q, et al. Taz-tead1 links cell-cell contact to zeb1 expression, proliferation, and dedifferentiation in retinal pigment epithelial cells. Investigative ophthalmology & visual science. 2010;51:3372–8. doi: 10.1167/iovs.09-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubasyuk V, Ouyang H, Yu FX, Guan KL, Zhang K. YAP inhibition blocks uveal melanogenesis driven by GNAQ or GNA11 mutations. Molecular & cellular oncology. 2015;2:e970957. doi: 10.4161/23723548.2014.970957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney WM, Jr, Hong JH, Yaffe MB, Farrance IK. The transcriptional co-activator TAZ interacts differentially with transcriptional enhancer factor-1 (TEF-1) family members. The Biochemical journal. 2005;388:217–25. doi: 10.1042/BJ20041434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markby DW, Onrust R, Bourne HR. Separate GTP binding and GTPase activating domains of a G alpha subunit. Science. 1993;262:1895–901. doi: 10.1126/science.8266082. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Rodrigo I, Bovolenta P. Eye development: a view from the retina pigmented epithelium. BioEssays : news and reviews in molecular, cellular and developmental biology. 2004;26:766–77. doi: 10.1002/bies.20064. [DOI] [PubMed] [Google Scholar]
- McLaughlin ME, Pepin SM, Maccollin M, Choopong P, Lessell S. Ocular pathologic findings of neurofibromatosis type 2. Archives of ophthalmology. 2007;125:389–94. doi: 10.1001/archopht.125.3.389. [DOI] [PubMed] [Google Scholar]
- Miesfeld JB, Gestri G, Clark BS, Flinn MA, Poole RJ, et al. Yap and Taz regulate retinal pigment epithelial cell fate. Development. 2015;142:3021–32. doi: 10.1242/dev.119008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld JB, Link BA. Establishment of transgenic lines to monitor and manipulate Yap/Taz-Tead activity in zebrafish reveals both evolutionarily conserved and divergent functions of the Hippo pathway. Mechanisms of development. 2014;133:177–88. doi: 10.1016/j.mod.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moleirinho S, Hoxha S, Mandati V, Curtale G, Troutman S, et al. Regulation of localization and function of the transcriptional co-activator YAP by angiomotin. eLife. 2017;6 doi: 10.7554/eLife.23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KH, Kim HT, Lee D, Rao MB, Levine EM, et al. Differential Expression of NF2 in Neuroepithelial Compartments Is Necessary for Mammalian Eye Development. Developmental cell. 2018;44:13–28. e3. doi: 10.1016/j.devcel.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JT, Murphy CJ, Russell P. What do mechanotransduction, Hippo, Wnt, and TGFbeta have in common? YAP and TAZ as key orchestrating molecules in ocular health and disease. Experimental eye research. 2013;115:1–12. doi: 10.1016/j.exer.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Molecular and cellular biology. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Nakagawa M, Olson EN, Nakagawa O. A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt-Oram syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18034–9. doi: 10.1073/pnas.0509109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development. 2000;127:3581–91. doi: 10.1242/dev.127.16.3581. [DOI] [PubMed] [Google Scholar]
- Nishio M, Otsubo K, Maehama T, Mimori K, Suzuki A. Capturing the mammalian Hippo: elucidating its role in cancer. Cancer science. 2013;104:1271–7. doi: 10.1111/cas.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Developmental cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Oatts JT, Hull S, Michaelides M, Arno G, Webster AR, Moore AT. Novel heterozygous mutation in YAP1 in a family with isolated ocular colobomas. Ophthalmic genetics. 2017;38:281–83. doi: 10.1080/13816810.2016.1188122. [DOI] [PubMed] [Google Scholar]
- Oku Y, Nishiya N, Shito T, Yamamoto R, Yamamoto Y, et al. Small molecules inhibiting the nuclear localization of YAP/TAZ for chemotherapeutics and chemosensitizers against breast cancers. FEBS open bio. 2015;5:542–9. doi: 10.1016/j.fob.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. Hippo signaling in organ size control. Genes & development. 2007;21:886–97. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- Park R, Moon UY, Park JY, Hughes LJ, Johnson RL, et al. Yap is required for ependymal integrity and is suppressed in LPA-induced hydrocephalus. Nature communications. 2016;7:10329. doi: 10.1038/ncomms10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiological reviews. 2014;94:1287–312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- Rayon T, Menchero S, Nieto A, Xenopoulos P, Crespo M, et al. Notch and hippo converge on Cdx2 to specify the trophectoderm lineage in the mouse blastocyst. Developmental cell. 2014;30:410–22. doi: 10.1016/j.devcel.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Current biology : CB. 2010;20:582–90. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–73. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakabe M, Fan J, Odaka Y, Liu N, Hassan A, et al. YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:10918–23. doi: 10.1073/pnas.1704030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada A, Kiyonari H, Ukita K, Nishioka N, Imuta Y, Sasaki H. Redundant roles of Tead1 and Tead2 in notochord development and the regulation of cell proliferation and survival. Molecular and cellular biology. 2008;28:3177–89. doi: 10.1128/MCB.01759-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–95. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvis MR, Kreger BT, Lien WH, Klezovitch O, Rudakova GM, et al. alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Science signaling. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Ramesh S, Cibi DM, Yun LS, Li J, et al. Hippo Signaling Mediators Yap and Taz Are Required in the Epicardium for Coronary Vasculature Development. Cell reports. 2016;15:1384–93. doi: 10.1016/j.celrep.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Park R, Kim JY, Hughes L, Lu L, et al. Dual function of Yap in the regulation of lens progenitor cells and cellular polarity. Developmental biology. 2014;386:281–90. doi: 10.1016/j.ydbio.2013.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, et al. Expression of Yes-associated protein in common solid tumors. Human pathology. 2008;39:1582–9. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Molecular cell. 2005;18:447–59. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Tian T, Li A, Lu H, Luo R, Zhang M, Li Z. TAZ promotes temozolomide resistance by upregulating MCL-1 in human glioma cells. Biochemical and biophysical research communications. 2015;463:638–43. doi: 10.1016/j.bbrc.2015.05.115. [DOI] [PubMed] [Google Scholar]
- Totaro A, Castellan M, Battilana G, Zanconato F, Azzolin L, et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nature communications. 2017;8:15206. doi: 10.1038/ncomms15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530–42. e12. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Developmental cell. 2010;18:579–91. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nature cell biology. 2008;10:837–48. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes & development. 2001;15:1229–41. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitolo MI, Anglin IE, Mahoney WM, Jr, Renoud KJ, Gartenhaus RB, et al. The RUNX2 transcription factor cooperates with the YES-associated protein, YAP65, to promote cell transformation. Cancer biology & therapy. 2007;6:856–63. doi: 10.4161/cbt.6.6.4241. [DOI] [PubMed] [Google Scholar]
- Wang K, Degerny C, Xu M, Yang XJ. YAP, TAZ, and Yorkie: a conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2009;87:77–91. doi: 10.1139/O08-114. [DOI] [PubMed] [Google Scholar]
- Wang L, Shi S, Guo Z, Zhang X, Han S, et al. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PloS one. 2013;8:e65539. doi: 10.1371/journal.pone.0065539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang Y, Yu A, Yu FX. The Hippo pathway in tissue homeostasis and regeneration. Protein & cell. 2017;8:349–59. doi: 10.1007/s13238-017-0371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley LA, Dattilo LK, Kang KB, Giovannini M, Beebe DC. The tumor suppressor merlin is required for cell cycle exit, terminal differentiation, and cell polarity in the developing murine lens. Investigative ophthalmology & visual science. 2010;51:3611–8. doi: 10.1167/iovs.09-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson KA, Rainger J, Floyd JA, Ansari M, Meynert A, et al. Heterozygous loss-of-function mutations in YAP1 cause both isolated and syndromic optic fissure closure defects. American journal of human genetics. 2014;94:295–302. doi: 10.1016/j.ajhg.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, Graves HK, Moya IM, Tao C, Hamaratoglu F, et al. Differential regulation of the Hippo pathway by adherens junctions and apical-basal cell polarity modules. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:1785–90. doi: 10.1073/pnas.1420850112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C, Shen Z, Stemmer-Rachamimov A, Dawany N, Troutman S, et al. The p130 isoform of angiomotin is required for Yap-mediated hepatic epithelial cell proliferation and tumorigenesis. Science signaling. 2013;6:ra77. doi: 10.1126/scisignal.2004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey CE, Smith G, Izumo S, Shimizu N. cDNA cloning and characterization of murine transcriptional enhancer factor-1-related protein 1, a transcription factor that binds to the M-CAT motif. The Journal of biological chemistry. 1996;271:3727–36. doi: 10.1074/jbc.271.7.3727. [DOI] [PubMed] [Google Scholar]
- Yu FX, Luo J, Mo JS, Liu G, Kim YC, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer cell. 2014a;25:822–30. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhang K, Guan KL. YAP as oncotarget in uveal melanoma. Oncoscience. 2014b;1:480–1. doi: 10.18632/oncoscience.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T, Rafizadeh S, Azizi Z, Lupse B, Gorrepati KD, et al. Proproliferative and antiapoptotic action of exogenously introduced YAP in pancreatic beta cells. JCI insight. 2016;1:e86326. doi: 10.1172/jci.insight.86326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi SK, Sullivan AJ, Medina R, Ito Y, van Wijnen AJ, et al. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. The EMB journal. 2004;23:790–9. doi: 10.1038/sj.emboj.7600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Deo M, Thompson RC, Uhler MD, Turner DL. Negative regulation of Yap during neuronal differentiation. Developmental biology. 2012;361:103–15. doi: 10.1016/j.ydbio.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Developmental cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhou Q, Pignoni F. Yki/YAP, Sd/TEAD and Hth/MEIS control tissue specification in the Drosophila eye disc epithelium. PloS one. 2011;6:e22278. doi: 10.1371/journal.pone.0022278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Science signaling. 2014;7:ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lu Q, Wang LH, Liu CY, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes & development. 2011a;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes & development. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nature cell biology. 2011b;13:877–83. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & development. 2007;21:2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes & development. 2008;22:1962–71. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Zhang Y, Wu H, Barry E, Yin Y, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E1312–20. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]