Abstract
Background
Frailty is a geriatric syndrome characterized by weakness and weight loss, and is associated with adverse health outcomes. It is often an unmeasured confounder in pharmacoepidemiologic and comparative effectiveness studies using administrative claims data.
Methods
Among the Atherosclerosis Risk in Communities (ARIC) Study Visit 5 participants (2011–2013; n=3146), we conducted a validation study to compare a Medicare claims-based algorithm of dependency in activities of daily living (or dependency) developed as a proxy for frailty with a reference standard measure of phenotypic frailty. We applied the algorithm to the ARIC participants’ claims data to generate a predicted probability of dependency. Using the claims-based algorithm, we estimated the c-statistic for predicting phenotypic frailty. We further categorized participants by their predicted probability of dependency (<5%, 5% – <20%, and ≥ 20%) and estimated associations with difficulties in physical abilities, falls, and mortality.
Results
The claims-based algorithm showed good discrimination of phenotypic frailty (c-statistic=0.71, 95% confidence interval (CI) 0.67, 0.74). Participants classified with a high predicted probability of dependency (≥20%) had higher prevalence of falls and difficulty in physical ability, and a greater risk of one-year all-cause mortality (hazard ratio=5.7 (95% CI: 2.5, 13) than participants classified with a low predicted probability (<5%). Sensitivity and specificity varied across predicted probability of dependency thresholds.
Conclusion
The Medicare claims-based algorithm showed good discrimination of phenotypic frailty and high predictive ability with adverse health outcomes. This algorithm can be used in future Medicare claims analyses to reduce confounding by frailty and improve study validity.
Keywords: frailty, Medicare, pharmacoepidemiology, Confounding, comparative effectiveness research
Introduction
Frailty is a geriatric syndrome, operationalized as a phenotype by Fried et al. using measures of unintentional weight loss and exhaustion, and performance in grip strength, walking speed, and physical activity metrics,1 and is associated with adverse health outcomes.1–6 While the frailty phenotype has been measured in several population-based cohort studies,2–6 it is not captured in administrative claims databases, including Medicare, often used to evaluate the effectiveness of medical interventions on health outcomes in older adults. Findings from studies using claims data suggest that exaggerated mortality reductions associated with lipid-lowering drugs7,8 and influenza vaccination9 are likely due, in part, to unmeasured confounding by frailty. This confounding can result when preventive care is withheld from frail older adults near the end of life.10 Increasingly, efforts to identify frailty proxies using claims data have been developed to improve estimation of medical intervention effects in older adults.11–13
As an initial step to measure frailty in claims data, Faurot et al. developed and internally validated an algorithm to predict dependency in activities of daily living (or dependency), a proxy for frailty, using the 2006 Medicare Current Beneficiary Survey.14 The algorithm reported excellent discrimination (c-statistic=0.84) and predictive validity. Participants with higher predicted probabilities of dependency had higher mortality compared to those with lower probabilities.14 Our objective was to validate the Medicare claims-based algorithm against a reference standard, the Fried frailty phenotype, derived from the Atherosclerosis Risk in Communities (ARIC) Study cohort data. Reducing confounding by frailty would improve the validity of treatment effect estimates from some pharmacoepidemiologic and comparative effectiveness studies using Medicare data.
Methods
Study Design
We conducted analyses using data from the fifth examination of the ARIC Study (2011–2013) and linked Medicare fee-for-service (FFS) claims. The Medicare claims-based algorithm drew upon medical claims for diagnoses and services from the year prior to ARIC Visit 5, and was compared to the frailty phenotype assessed at Visit 5.
Study Population
The ARIC Study is a population-based cohort of randomly selected participants from defined populations within four US communities (Washington County, MD; Minneapolis, MN; Jackson, MS (African Americans only); Forsyth County, NC).15 At baseline (1987–1989) 15,792 men and women (45–64 years) were enrolled. Four additional examinations and annual (semi-annual from 2012) telephone interviews have been conducted. Medicare claims were linked to eligible participants.16 Among the 6,538 Visit 5 participants, we excluded those not continuously enrolled in Medicare Parts A and B FFS one year prior to Visit 5 (n=3,181) and those with missing frailty information (n=191). Asian or American Indian/Alaskan Indian participants (n=11) and African-American participants in Minneapolis and Washington County (n=9) were excluded due to small numbers.
Predicted probabilities of dependency
The Medicare claims-based model developed by Faurot et al. was based on a logistic regression model with 20 claims for conditions, symptoms, and medical equipment predictive of dependency (Table 1), in addition to age, sex, and race.14 To apply the claims-based model to data for ARIC participants, we first defined the 20 dependency indicators using the freely available SAS macro containing International Classification of Diseases, 9th Edition, Clinical Modification (ICD-9) diagnosis and procedure codes, and Current Procedural Terminology (CPT) and Healthcare Common Procedure Coding System (HCPCS) codes.14,17 Claims were extracted for the year prior to Visit 5 from the carrier, inpatient, outpatient, durable medical equipment, home health agency, hospice, and skilled nursing facility files. Next, for each participant, we multiplied the original dependency model coefficients by the 20 indicators and demographic variables derived in our study dataset and summed these values to calculate the log odds of dependency, which was further converted to a predicted probability. We grouped participants by their predicted probability of dependency using categories defined by Faurot et al. (<5%, 5% – <20%, and ≥ 20%).14
Table 1.
Demographic characteristics and prevalence of claims-based predictors of dependency in activities of daily living by predicted probability and phenotypic frailty, ARIC participants (n=3146) (2010–2013).
| Overall | Predicted probability of dependency | Phenotypic frailty | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| <5% | 5%–<20% | ≥ 20% | Robust | Pre-frail | Frail | ||
| N=3146 | N=2162 | N=841 | N=143 | N=1345 | N=1585 | N=216 | |
| Frailty components; N (%) | |||||||
| Unintentional weight loss | 669 (21) | 357 (17) | 255 (30) | 57 (43) | 0 (0.0) | 540 (35) | 129 (60) |
| Exhaustion | 296 (9) | 140 (7) | 120 (14) | 36 (26) | 0 (0.0) | 214 (14) | 82 (38) |
| Low physical activity | 481 (16) | 289 (14) | 163 (20) | 29 (25) | 0 (0.0) | 345 (23) | 136 (63) |
| Low grip strength | 820 (27) | 479 (23) | 295 (38) | 46 (48) | 0 (0.0) | 637 (44) | 183 (86) |
| Slow walk speed | 473 (16) | 217 (10) | 200 (26) | 56 (61) | 0 (0.0) | 306 (21) | 167 (79) |
| Age group; N (%) | |||||||
| 65–69 | 311 (10) | 270 (13) | 39 (5) | 2 (1) | 181 (14) | 125 (8) | 5 (2) |
| 70–74 | 1123 (36) | 907 (42) | 190 (23) | 26 (18) | 583 (43) | 483 (31) | 57 (26) |
| 75–79 | 866 (28) | 635 (29) | 200 (24) | 31 (22) | 367 (27) | 450 (28) | 49 (23) |
| 80–84 | 619 (20) | 316 (15) | 259 (31) | 44 (31) | 173 (13) | 375 (24) | 71 (33) |
| ≥ 85 | 227 (7) | 34 (2) | 153 (18) | 40 (28) | 41 (3) | 152 (10) | 34 (16) |
| White; N (%) | 2457 (78) | 1769 (82) | 594 (71) | 94 (66) | 1069 (80) | 1211 (76) | 177 (82) |
| Female; N (%) | 1872 (60) | 1212 (56) | 559 (67) | 101 (71) | 755 (56) | 971 (61) | 146 (68) |
| Claims; N (%) | |||||||
| Arthritis | 1400 (45) | 758 (35) | 527 (63) | 115 (80) | 523 (39) | 749 (47) | 128 (59) |
| Bladder Dysfunction | 254 (8) | 98 (5) | 116 (14) | 40 (28) | 86 (6) | 140 (9) | 28 (13) |
| Stroke/brain injury | 116 (4) | 19 (1) | 61 (7) | 36 (25) | 23 (2) | 75 (5) | 18 (8) |
| Skin Ulcer | 67 (2) | 5 (0) | 36 (4) | 26 (18) | 7 (1) | 47 (3) | 13 (6) |
| Dementia | 202 (6) | 22 (1) | 109 (13) | 71 (50) | 40 (3) | 134 (9) | 28 (13) |
| Heart Failure | 287 (9) | 79 (4) | 142 (17) | 66 (46) | 71 (5) | 169 (11) | 47 (22) |
| Hypotension/Shock | 76 (2) | 18 (1) | 37 (4) | 21 (15) | 24 (2) | 44 (3) | 8 (4) |
| Lipid Abnormality | 2173 (69) | 1527 (71) | 541 (64) | 105 (73) | 921 (69) | 1098 (69) | 154 (71) |
| Paralysis | 25 (1) | 0 (0) | 5 (1) | 20 (14) | 4 (0) | 16 (1) | 5 (2) |
| Parkinson’s Disease | 30 (1) | 4 (0) | 13 (2) | 13 (9) | 7 (1) | 21 (1) | 2 (1) |
| Podiatric Care | 243 (8) | 71 (3) | 131 (16) | 41 (29) | 65 (5) | 152 (10) | 26 (12) |
| Psychiatric Illness | 454 (14) | 123 (6) | 237 (28) | 94 (66) | 124 (9) | 267 (17) | 63 (29) |
| Rehabilitation Care | 244 (8) | 124 (6) | 86 (10) | 34 (24) | 69 (5) | 138 (9) | 37 (17) |
| Cancer Screening | 1487 (47) | 1221 (57) | 240 (29) | 26 (18) | 699 (52) | 707 (45) | 81 (38) |
| Vertigo | 299 (10) | 203 (9) | 73 (9) | 23 (16) | 108 (8) | 157 (10) | 34 (16) |
| Weakness | 237 (8) | 61 (3) | 107 (13) | 69 (48) | 57 (4) | 141 (9) | 39 (18) |
| Ambulance | 237 (8) | 48 (2) | 114 (14) | 75 (53) | 58 (4) | 137 (9) | 42 (19) |
| Home Hospital Bed | 10 (0) | 0 (0) | 0 (0) | 10 (7) | 0 (0) | 9 (1) | 1 (1) |
| Home Oxygen | 59 (2) | 4 (0) | 30 (4) | 25 (18) | 14 (1) | 35 (2) | 10 (5) |
| Wheelchair | 20 (1) | 0 (0) | 2 (0) | 18 (13) | 0 (0) | 15 (1) | 5 (2) |
Abbreviations: SD=standard deviation
Sample size for frailty components varies: weight loss (N=3127), exhaustion (N=3123), physical activity (N=3042), grip strength (N=3001), walk speed (N=2984)
Frailty Phenotype (Reference Standard)
The Fried frailty phenotype was operationalized using data from ARIC Visit 5 and had high criterion and predictive validity.4 Phenotype criteria components included weight loss, low physical activity, slow walking speed, exhaustion, and low grip strength (eTable 1).4 Participants were classified as frail (three or more components), pre-frail (one or two components), and robust (no components); we collapsed the latter categories to indicate frail or non-frail (pre-frail or robust) for selected analyses.
Longitudinal Outcomes
Longitudinal outcomes were collected during semi-annual follow-up telephone interviews conducted within one year from Visit 5 and included number of falls in the past 6 months and physical ability assessments. Physical ability was measured according to standard domains (eTable 2) to ascertain the level of difficulty (no difficulty, some difficulty, and unable to do) in performing physical tasks and activities of daily living.18–21 For each domain, participants who self-reported some difficulty or unable to do the task were coded as having a difficulty in that domain.
We ascertained vital status from the Medicare enrollment data (2010–2013). We calculated survival from Visit 5 to death, end of one-year follow-up, or December 31, 2013, whichever came first.
Statistical Analysis
We examined the discriminative ability of the claims-based algorithm to predict phenotypic frailty (classified as frail vs. non-frail) by estimating the c-statistic and plotting a receiver operating characteristic (ROC) curve using the pROC R package.22 Kernel density and calibration plots were used to examine overlap and agreement between predicted probability of dependency and phenotypic frailty distributions.
We examined the bivariate relationship between the predicted probability of dependency and falls and physical ability difficulties. We compared 1-year all-cause mortality across categories of predicted probability of dependency and phenotypic frailty using Cox proportional hazard models. We standardized survival curves by deriving weights for each claims-based dependency and phenotypic frailty strata to reflect the age (65–74, 75–79, and ≥80 years) and sex distribution of the 2010 Census population aged 65 years and older. Weights were applied to Cox models to estimate adjusted hazard ratios (aHR) and 95% confidence intervals (CI) using a robust variance.
We estimated the sensitivity, specificity, positive predictive value, and negative predictive value of the claims-based algorithm relative to phenotypic frailty using a range of dichotomous cut-points from the predicted probabilities. We also computed the number of individuals who would remain in a study, if the population were restricted to those likely to be non-frail using various cut-points. We further examined how the sensitivity of the algorithm varied by age, sex, and race.
All analyses were conducted with SAS version 9.4 (Cary, North Carolina) and R version 3.3.2 and approved by the Institutional Review Boards of participating ARIC Study centers.
Results
The analytic sample included 3,146 adults; average age was 76 years, 78% were white, and 60% were female (Table 1). The prevalence of phenotypic frailty was 7%. Frail participants and those with ≥20% predicted probability of dependency had a higher prevalence of most claims-based indicators compared to non-frail participants and those with lower predicted probabilities (Table 1). We observed a low prevalence of claims for home hospital bed and wheelchair overall, which were identified in the original Faurot et al. study population as strong predictors of dependency (eTable 3).14
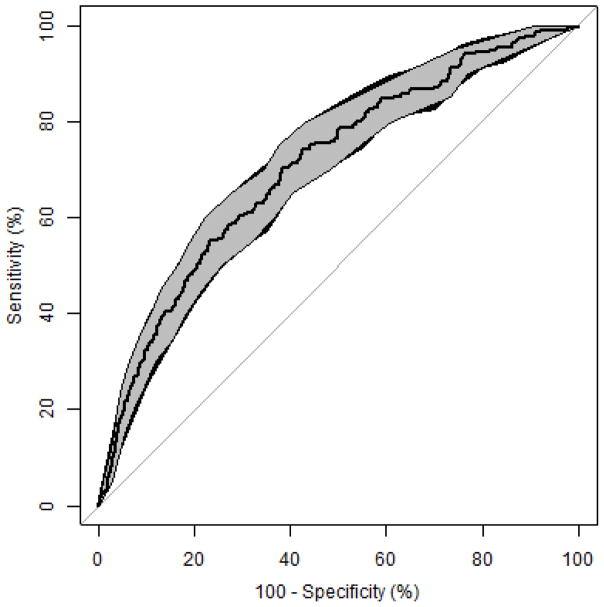
The claims-based algorithm demonstrated good discrimination of phenotypic frailty (c-statistic=0.71, 95% CI 0.67, 0.74) (Figure). The distribution of the predicted probability of dependency stratified by phenotypic frailty, suggests higher predicted probabilities for those classified as phenotypically frail (eFigure 1a). The calibration plot (eFigure 1b) shows that as the predicted probability of dependency increases, so does the proportion of participants classified as phenotypically frail, suggesting good agreement between the two measures. Among the 191 participants who did not have phenotypic frailty information measured at Visit 5, the mean predicted probability of claims-based dependency was 21% compared to 6% in those with Visit 5 frailty information. The prevalence of most claims-based indicators was higher among participants without frailty information available (eTable 4 and eFigure 2).
Figure.
Receiver operating characteristic (ROC) curve and 95% confidence interval (CI) bands for the Medicare claims-based algorithm prediction of phenotypic frailty, ARIC participants (n=3146) (2010 – 2013). Bootstrapping with 2000 replicates was used to compute 95% CIs for sensitivities and specificities using methods described by Robin et al.22 and Fawcett et al.27 The shaded grey region denotes the 95% CIs for specificities at a range of sensitivity values. The small black region denotes where the 95% CIs for sensitivities at a range of specificity values is slightly wider than the 95% CIs for specificities.
Participants classified with a predicted probability of dependency ≥20% had a higher prevalence of falls and difficulties in physical ability compared to those with lower predicted probabilities (Table 2). For example, the prevalence of falls in the last 6 months was highest for participants with ≥20% probability (33%) and lower for those with probabilities from 5% – <20% (21%) and < 5% (13%). For difficulties in physical mobility and housekeeping, participants with ≥20% probabilities reported the highest prevalence (mobility 84%, housekeeping 60%), followed by those with probabilities from 5% – <20% (mobility 66%, housekeeping 34%) and < 5% (mobility 45%, housekeeping 16%).
Table 2.
Longitudinal outcomes reported by claims-based algorithm group, ARIC participants (n=3077) (2010 – 2013)a.
| Predicted probability of dependency | |||||
|---|---|---|---|---|---|
| <5% | 5% – < 20% | ≥ 20% | Overall | ||
| missing | N=2124 | N=818 | N=135 | N=3077 | |
| Fall in the last 6 monthsb; N (%) | 295 | 251 (13) | 151 (21) | 29 (33) | 431 (16) |
| Difficulty with physical mobilityb; N (%) | 292 | 889 (45) | 479 (66) | 75 (84) | 1443 (52) |
| Difficulty with housekeepingb; N (%) | 308 | 318 (16) | 241 (34) | 53 (60) | 612 (22) |
| Difficulty with meal preparationb; N (%) | 292 | 85 (4) | 78 (11) | 30 (34) | 193 (7) |
| Difficulty with self-careb; N (%) | 292 | 90 (5) | 74 (10) | 26 (29) | 190 (7) |
| Difficulty with finance managingb; N (%) | 379 | 26 (1) | 37 (6) | 12 (16) | 75 (3) |
Abbreviations: SD=standard deviation
Falls and physical abilities are measured with the semi-annual follow-up interview. Participants who completed this interview prior to Visit 5 were excluded from this analysis to ensure the predicted probability of dependency measurement occurred prior to the falls and physical abilities measurement (N=69).
Questions asked within one year after Visit 5 with the semi-annual follow-up interview.
We observed higher 1-year mortality for participants with ≥20% predicted probability of dependency and those classified as phenotypically frail compared to those with <5% predicted probability of dependency and phenotypically robust (eTable 5, eFigure 3a and b). In adjusted models, participants with predicted probabilities ≥20% and those with 5% – <20% had an aHR of death within one year of 5.7 (95% CI 2.5, 13) and 3.6 (95% CI 1.7, 7.7) compared to participants with < 5% probabilities. Similarly, participants classified as phenotypically frail and pre-frail had aHRs for death of 6.5 (95% CI 1.7, 25) and 3.4 (95% CI 1.4, 7.8), respectively, compared to robust participants.
Using a 20% cut-point for the predicted probability of dependency to identify phenotypically frail participants (216 out of 3136 total), sensitivity was 13% (95% CI 9%, 18%) with 29 true positives. Specificity was 96% (95% CI 95%, 97%), with 2816 true negatives (Table 3). Lowering the predicted probability cut-point increased sensitivity and decreased specificity. At a predicted probability of 4%, the sensitivity of phenotypic frailty was 71% (95% CI 65%, 77%) and the specificity was 60% (95% CI 58%, 61%). For researchers wishing to optimize sensitivity to control confounding via restriction of analyses to non-frail adults, the 4% probability cut-point would only include 58% of the ARIC sample. Sensitivity was higher among older compared to younger participants but did not vary not by race or sex (eTable 6).
Table 3.
Sensitivity, specificity, positive predictive value, and negative predictive value for a range of dependency probability cut-points and resulting sample size if cut-points were used to restrict to non-frail participants, ARIC participants (n=3146) (2010–2013).
| Cut-point | TP | TN | FP | FN | Se | Sp | PPV | NPV | Restriction to non-fraila | |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| N | %, (95% CI) | N | % of sample | |||||||
| 20% | 29 | 2816 | 114 | 187 | 13 (9, 18) | 96 (95, 97) | 20 (14, 27) | 94 (93, 95) | 3003 | 95% |
| 10% | 70 | 2632 | 298 | 146 | 32 (26, 39) | 90 (89, 91) | 19 (15, 23) | 95 (94, 96) | 2778 | 88% |
| 5% | 129 | 2075 | 855 | 87 | 60 (53, 66) | 71 (69, 72) | 13 (11, 15) | 96 (95, 97) | 2162 | 69% |
| 4% | 153 | 1749 | 1181 | 63 | 71 (65, 77) | 60 (58, 61) | 11 (10, 13) | 97 (96, 97) | 1812 | 58% |
| 3% | 179 | 1248 | 1682 | 37 | 83 (78, 88) | 43 (41, 44) | 10 (8, 11) | 97 (96, 98) | 1285 | 41% |
Abbreviations: CI=confidence interval, FN=false negative, FP=false positive, NPV=negative predict value, PPV=positive predictive value, Se=sensitivity, Sp=specificity, TN=true negative, TP=true positive
Restriction to non-frail participant sample size calculated as true negatives + false negatives as classified by the dependency probability cut-point. The percentage of the sample restricted is calculated as those identified as non-frail by the claims-based algorithm (true negatives + false negatives) divided by ARIC sample size of 3,146.
Discussion
In this validation study of a Medicare claims-based algorithm predicting dependency, we found good discrimination and high predictive validity compared with a reference standard measure of phenotypic frailty. We observed study participants with higher predicted probabilities of dependency to self-report more difficulty in physical abilities and to have higher 1-year mortality compared to participants with lower predicted probabilities.
Our results support using the algorithm to control confounding by frailty in studies relying solely on Medicare claims data. This algorithm can be used to reduce confounding by: (1) adjustment, using the 20 individual claims-based predictors or the predicted probability of dependency as a continuous variable, (2) restriction to non-frail individuals using a specific probability cut-point, or (3) adjustment using a specific probability cut-point and sensitivity and specificity estimates with quantitative bias analysis. Restriction of analyses to non-frail populations identified through the claims-based algorithm is a straightforward, and therefore tempting, approach to mitigate confounding by frailty. However, gains in confounding control from this approach must be weighed against potential losses to sample size and generalizability. In contrast, the use of a dichotomous probability cut-point together with bias analysis would retain all study participants and facilitate examination of variation in estimates across a range of bias parameters.
Limitations of this study should also be considered. The population of older adults in the ARIC Study is not representative of the general US population. While the prevalence of frailty in the ARIC cohort (7%) is comparable to other older adult cohorts,1 it is much lower than the 15% prevalence reported in the National Health and Aging Trends Study, a nationally representative population.23 This discrepancy may be due to better health in persons able to participate in research studies requiring in-person clinic visits. As the claims-based algorithm was developed using the in-home interview data from Medicare Current Beneficiary Survey, its performance might be improved in study populations that have included more frail individuals than the ARIC cohort. Furthermore, disentangling age-associated from comorbidity-associated frailty is challenging and an area of ongoing research.24–26 The claims-based algorithm includes a variety of comorbid conditions associated with frailty; 14 thus, it may be more likely to identify frail adults with comorbidity rather than those without comorbidity.
Medicare claims data are an important resource for evaluating the use and effects of medical interventions on health outcomes in older adults. These results support the use of the claims-based algorithm to assess and reduce unmeasured confounding by frailty in pharmacoepidemiologic and comparative effectiveness studies using Medicare claims data.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institutes of Health: K12CA120780 (JLL) R01/56 AG023178 (TS, MJF).
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Statement of data and code availability: The ARIC Study cohort data are available to researchers who request it through the ARIC Study Coordinating Center. SAS code for all published analyses are available upon request.
Conflicts of interest: Dr. Lund was supported by a PhRMA Foundation Research Starter Award to the Department of Epidemiology at the University of North Carolina at Chapel Hill (UNC). Dr. Stürmer receives investigator-initiated research funding as the Principal Investigator (R01/56 AG023178) and as Co-Investigator (R01 CA174453; R01 HL118255, R21-HD080214) from the National Institutes of Health. He also receives salary support as Director of the Comparative Effectiveness Research Strategic Initiative, NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR001111) and as Director of the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck) and research support from pharmaceutical companies (Amgen, AstraZeneca) to the Department of Epidemiology at UNC. Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca, and Novo Nordisk. Dr. Jonsson Funk receives investigator-initiated research funding as the Principal Investigator (R01 HL118255) and as Co-Investigator (R01/56 AG023178) from the National Institutes of Health. She also receives salary support as Core Faculty of the Comparative Effectiveness Research Strategic Initiative, NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR001111) and from the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck) and research support AstraZeneca to the Department of Epidemiology at UNC. MJF is a member of the Scientific Steering Committee (SSC) for a post-approval safety study funded by GSK. All compensation for services provided on the SSC is invoiced by and paid to UNC Chapel Hill. Dr. Jonsson Funk does not accept personal compensation of any kind from any pharmaceutical company. There are no other conflicts to report.
References
- 1.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321–30. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 3.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–6. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 4.Kucharska-Newton AM, Palta P, Burgard S, Griswold ME, Lund JL, Capistrant BD, Kritchevsky SB, Bandeen-Roche K, Windham BG. Operationalizing frailty in the Atherosclerosis Risk in Communities Study Cohort. J Gerontol A Biol Sci Med Sci. 2016 doi: 10.1093/gerona/glw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ensrud KE, Ewing SK, Cawthon PM, Fink HA, Taylor BC, Cauley JA, Dam TT, Marshall LM, Orwoll ES, Cummings SR. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57(3):492–8. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiely DK, Cupples LA, Lipsitz LA. Validation and comparison of two frailty indexes: The MOBILIZE Boston Study. J Am Geriatr Soc. 2009;57(9):1532–9. doi: 10.1111/j.1532-5415.2009.02394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glynn RJ, Knight EL, Levin R, Avorn J. Paradoxical relations of drug treatment with mortality in older persons. Epidemiology. 2001;12(6):682–9. doi: 10.1097/00001648-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Glynn RJ, Schneeweiss S, Wang PS, Levin R, Avorn J. Selective prescribing led to overestimation of the benefits of lipid-lowering drugs. J Clin Epidemiol. 2006;59(8):819–28. doi: 10.1016/j.jclinepi.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Jackson ML, Yu O, Nelson JC, Naleway A, Belongia EA, Baxter R, Narwaney K, Jacobsen SJ, Shay DK, Jackson LA. Further evidence for bias in observational studies of influenza vaccine effectiveness: the 2009 influenza A(H1N1) pandemic. Am J Epidemiol. 2013;178(8):1327–36. doi: 10.1093/aje/kwt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sturmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution--a simulation study. Am J Epidemiol. 2010;172(7):843–54. doi: 10.1093/aje/kwq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidoff AJ, Zuckerman IH, Pandya N, Hendrick F, Ke X, Hurria A, Lichtman SM, Hussain A, Weiner JP, Edelman MJ. A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4(2):157–65. doi: 10.1016/j.jgo.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidoff AJ, Gardner LD, Zuckerman IH, Hendrick F, Ke X, Edelman MJ. Validation of disability status, a claims-based measure of functional status for cancer treatment and outcomes studies. Med Care. 2014;52(6):500–10. doi: 10.1097/MLR.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segal JB, Chang HY, Du Y, JDW, MCC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care. 2017;55(7):716–722. doi: 10.1097/MLR.0000000000000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faurot KR, Jonsson Funk M, Pate V, Brookhart MA, Patrick A, Hanson LC, Castillo WC, Sturmer T. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24(1):59–66. doi: 10.1002/pds.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 16.Kucharska-Newton AM, Heiss G, Ni H, Stearns SC, Puccinelli-Ortega N, Wruck LM, Chambless L. Identification of heart failure events in Medicare claims: The Atherosclerosis Risk in Communities (ARIC) Study. J Card Fail. 2016;22(1):48–55. doi: 10.1016/j.cardfail.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. [Accessed January 20, 2017];SAS macro for predicting frailty using claims data. http://sph.unc.edu/epid/harry-guess-research-community/
- 18.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54(4):439–67. [PubMed] [Google Scholar]
- 19.Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21(4):556–9. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- 20.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 21.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86. [PubMed] [Google Scholar]
- 22.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Muller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, Xue QL, Walston JD, Kasper JD. Frailty in older adults: A nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427–34. doi: 10.1093/gerona/glv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 25.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–7. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villacampa-Fernandez P, Navarro-Pardo E, Tarin JJ, Cano A. Frailty and multimorbidity: Two related yet different concepts. Maturitas. 2017;95:31–35. doi: 10.1016/j.maturitas.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Fawcett T. An introduction to ROC analysis. Pattern Recognition Letters. 2006;27(8):861–874. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.