Summary
In Bacteria, ribosomes may bind to the nascent RNA emerging from the transcribing RNA polymerase and initiate translation. Transcription-translation coupling plays diverse roles in cellular physiology, including attenuation control, mRNA surveillance, and maintenance of genome integrity. While the existence of coupling is broadly accepted, its mechanism and ubiquity are debated. Structural evidence supports mutually exclusive modes of RNA polymerase-ribosome contacts. In a model based on nuclear magnetic resonance data, NusG binds to a ribosomal protein S10 and acts as an adapter between RNA polymerase and the 30S subunit. Recent single-particle cryo electron microscopy analyses of RNA polymerase bound to 30S and 70S ribosomes revealed extensive, and very distinct, contacts which are incompatible with bridging by NusG. In this issue of Molecular Microbiology, Saxena et al. provide the first evidence for NusG-mediated coupling in vivo. Their results demonstrate that Escherichia coli NusG interacts with the 70S ribosomes through a previously established interface and that these interactions are required for survival when translation elongation is hindered to weaken coupling. Future studies will address a likely possibility that distinct bridging mechanisms underpin context-dependent coupling in the cell.
Keywords: NusG, S10, ribosomes, RNA polymerase, transcription/translation coupling
Graphical abstract
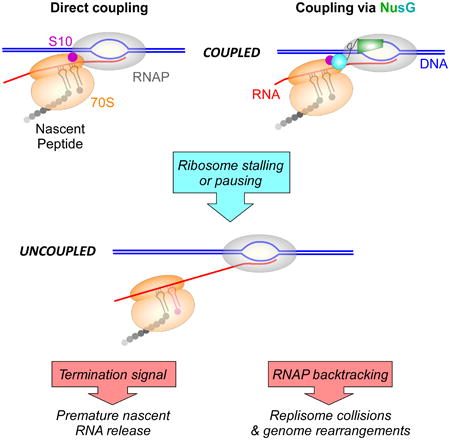
Maintenance and accurate transmission of genetic information rely on quality control mechanisms. RNA synthesis by RNA polymerase (RNAP) provides global surveillance of the genome. Transcribing RNAP scans the DNA and, upon encountering a stalling lesion in the template DNA strand, triggers transcription-coupled DNA repair (Spivak, 2016). Since DNA damage is not limited to coding DNA regions, pervasive bi-directional transcription would be necessary for efficient genome-wide error detection. Indeed, recent studies revealed that synthesis of noncoding and antisense RNAs is widespread across all life domains, giving rise to numerous useless and potentially inhibitory transcripts. In Escherichia coli, synthesis of antisense and other untranslated RNAs is inhibited by a transcription termination factor Rho (Peters et al., 2012). E. coli Rho is an abundant and essential ATP-driven motor that binds to the nascent RNA, translocates along the RNA chain toward the elongating RNAP, and triggers transcription termination (Mitra et al., 2017). Acting alone at optimal RNA sequences or together with a transcription elongation factor NusG at suboptimal sites (Peters et al., 2012, Valabhoju et al., 2016), Rho could induce premature termination of any unprotected RNA. Complete synthesis of an RNA chain thus requires that Rho action is inhibited, either by antitermination factors or by a translating ribosome.
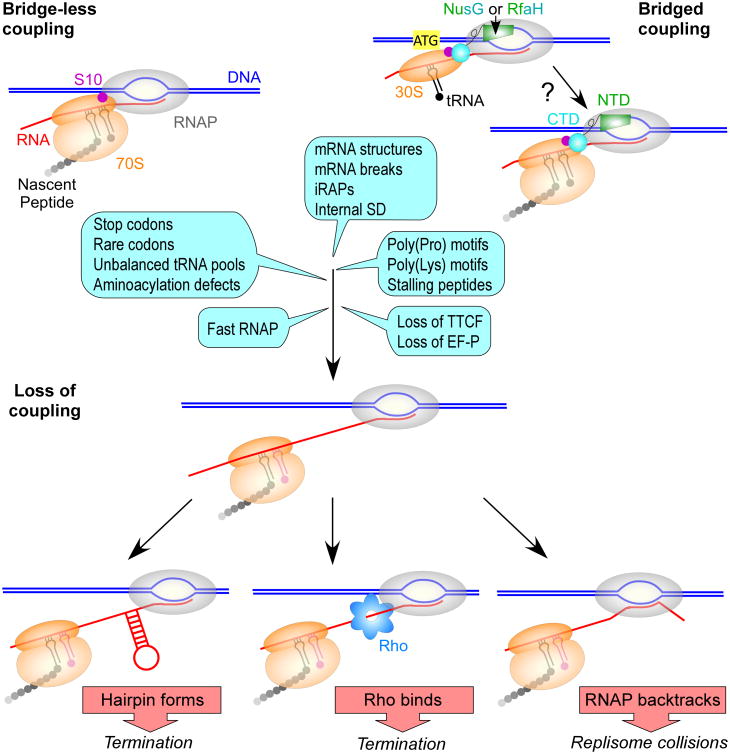
In Bacteria and Archaea, which lack well-defined cellular compartments that separate RNAP from ribosomes, a newly transcribed RNA can be immediately bound by a ribosome, coupling transcription to translation (McGary & Nudler, 2013). The pivotal role of the first (pioneer) round of translation in mRNA quality control has been long recognized. RNAs with aberrant nonsense codons or splicing defects are detected by the lead ribosome and eventually targeted for degradation. In eukaryotes, where RNAs are translated post-transcriptionally following their export from the nucleus, the pioneer round and steady-state translation employ different sets of translation factors (Maquat et al., 2010). Dedicated cap-binding proteins that initiate the pioneer round of translation and target flawed mRNAs for nonsense-mediated decay are replaced with eIF4, which supports subsequent rounds of translation. The pioneer round of translation plays an analogous role in Bacteria to ensure that only translatable mRNAs are completely synthesized. Rho efficiently silences poorly translated RNAs, such as those encoded by bacteriophages and other horizontally acquired genes (Mitra et al., 2017). A closely trailing lead ribosome, a distinguishing feature of the pioneer round, physically insulates the elongating RNAP from Rho and favors pause-free RNA synthesis. Transcription-translation coupling is utilized to monitor the mRNA quality and to determine if cellular conditions are favorable for translation (Figure 1). If coupling is disrupted, e.g., due to unchecked ribosome stalling at a polyproline stretch (Elgamal et al., 2016), at a sense codon when a cognate aminoacylated tRNA is limiting (Roche & Sauer, 1999), or upon premature ribosome release at an early nonsense codon (Richardson, 1991), the defective RNA is released from RNAP and lost.
Figure 1.
Causes and consequences of uncoupling. RNA and protein syntheses could be coupled by direct contacts between the RNAP and the ribosome (left) or by an accessory transcription-translation coupling factor (TTCF), such as NusG or RfaH (right). Recruitment of the 30S subunit to an SD-less RNA followed by ribosome scanning and preinitiation complex assembly at AUG has been proposed for RfaH (Burmann et al., 2012); these bridging contacts may be maintained throughout elongation. Conditions that slow (or stall) the ribosome or accelerate RNAP would lead to uncoupling (examples are shown as blue callouts; see Rodnina, 2016 and references therein), with only some pause events associated with the lack of tRNA in the A-site (magenta). Once coupling is lost, the nascent RNA can fold into secondary structures (Roland et al., 1985) and recruit RNAP-binding proteins, such as Rho (Richardson, 1991). A terminator hairpin or Rho would induce RNA release from RNAP. In the absence of the leading ribosome or secondary structures, RNAP can backtrack, turning into a formidable block to replication (Dutta et al., 2011).
Coupling has been directly observed by electron microscopy (Miller et al., 1970) and inferred from reports that the rates of RNAP and ribosome are closely matched and change in unison when translation is perturbed (Proshkin et al., 2010, Landick et al., 1985). Observations that E. coli NusG can interact with RNAP via its N-terminal domain (NTD) (Mooney et al., 2009b) and with a ribosomal protein S10 (a.k.a. NusE) (Burmann et al., 2010) or Rho (Mooney et al., 2009b) via its C-terminal domain (CTD) led to an elegant model in which the RNAP-bound NusG uses its flexibly connected CTD to bind Rho to facilitate termination or elicit antitermination through interactions with S10, either as a component of a multi-partite antitermination complex or as part of the trailing ribosome (Burmann et al., 2010). Although the simultaneous RNAP-NusG-ribosome bridging contacts have not been demonstrated experimentally, a single-particle cryo-EM structure of a complete bacteriophage λ N antitermination complex (Said et al., 2017) showed that the NusG-CTD can interact with S10 when the NusG-NTD is engaged by the E. coli transcription elongation complex (EC). The bridged model is also supported by observations that E. coli RfaH, a specialized paralog of NusG that binds to the same sites on RNAP and S10, activates translation initiation in vivo (Burmann et al., 2012), presumably by recruiting the 30S subunit to mRNA through direct protein-protein contacts; RfaH target mRNAs lack Shine-Dalgarno (SD) sequences and thus cannot initiate translation via canonical base pairing between 16S rRNA and the SD element.
However, several recent reports argue that RNAP and ribosome may be coupled directly, rather than via NusG. In a cryo-EM structure of E. coli expressome (Kohler et al., 2017), RNAP and 70S are juxtaposed on the nascent mRNA, with no RNA spacer in between. In this structure, RNAP and 70S establish an extensive and functionally validated interface that could facilitate coupling between transcription and translation in the absence of an accessory factor. A cryo-EM structure of E. coli RNAP:30S binary complex (Demo et al., 2017) revealed RNAP-30S subunit interactions which are consistent with previous reports of RNAP contacts to ribosomal proteins S1 and S2 (Sukhodolets & Garges, 2003, Buttner et al., 1997) but very distinct from the expressome model (Kohler et al., 2017). In both structures, the residues on the interface are conserved, supporting a notion that these contacts may mediate RNAP-ribosome interactions in diverse phyla. It is possible that the 30S:RNAP and 70S:EC structures may represent interactions established at different steps of translation, e.g., during initiation and elongation. Importantly, however, in either structure NusG cannot simultaneously interact with its binding sites on the RNAP β' clamp domain (Mooney et al., 2009b) and S10 (Burmann et al., 2010) even if the flexible linker that connects the NTD and CTD of NusG was fully extended. Direct contacts observed between RNAP and the ribosome in solution (Fan et al., 2017) also support the bridge-less model.
Thus, evidence exists in support of both bridged (Burmann et al., 2010, Burmann et al., 2012) and bridge-less (Demo et al., 2017, Fan et al., 2017, Kohler et al., 2017) modes of coupling, but in each case the picture is incomplete. It is also quite possible that distinct mechanisms of coupling could be utilized in vivo in different contexts. Inspired by their previous report of direct NusG-S10 interactions (Burmann et al., 2010), Max Gottesman and colleagues sought to find out if NusG and 70S ribosome establish physical contacts that mediate coupling. In this issue of Molecular Microbiology, Saxena et al. show that E. coli NusG binds to 70S ribosomes in vivo and in vitro. Alanine substitutions of Phe165 in NusG and Met88/Asp97 in S10, the residues at the NusG-CTD:S10 interface observed by NMR (Burmann et al., 2010), abolish this association in vitro and significantly compromise it in vivo, demonstrating that it is physiologically relevant. Partial defects in vivo suggest that the NusG:70S complex could be stabilized through contacts with accessory proteins such as NusA, which binds to the NusG-CTD in the λN antitermination complex (Said et al., 2017).
Does the observed interaction reflect NusG-mediated coupling between the transcribing RNAP and translating ribosome or is it established by a free NusG? To answer this question, Saxena et al. tested the effect of NusG and S10 substitutions on survival under translation stress. A tightly-coupled lead ribosome inhibits RNAP backtracking and formation of double-stranded DNA breaks, thereby bolstering genome stability (Dutta et al., 2011). In support of the role of NusG and S10 in bridging the EC to the ribosome, substitutions of the interface residues appear to trigger uncoupling of transcription from translation, as reflected by increased sensitivity to chloramphenicol, which inhibits translation elongation, but not kasugamycin, which inhibits translation initiation. Saxena et al. also show that the detrimental effects of substitutions in nusG and nusE are specific to those at the NusG/S10 interface and are suppressed by rpoB*35, an RNAP variant that resists backtracking (Trautinger et al., 2005) and relieves the DNA break-inducing collisions with the replisome (Dutta et al., 2011). Together, these results strongly support the hypothesis that stable NusG/S10 interactions are essential for coupling.
If unchecked, an intrinsic antipausing activity of the NusG-NTD (Mooney et al., 2009b) would be expected to accelerate RNAP, triggering uncoupling and inhibiting Rho-dependent termination. Likewise, a fast, pause-resistant rpoB2 RNAP allele is less sensitive to Rho and exacerbates the effects of the efp deletion, which leads to prominent ribosome stalling at polyproline motifs (Elgamal et al., 2016). The universally-conserved transcription-promoting activity of the NusG-NTD can be turned off or amplified by the mutually exclusive CTD contacts with Rho and S10. The wild-type NusG potentiates termination, presumably by facilitating Rho transition into a translocation-competent, closed-ring state (Valabhoju et al., 2016), whereas NusG F165A variant defective in binding to Rho decreases termination (Saxena et al., 2018). Conversely, NusG-S10 interactions magnify the antipausing effect in the context of ribosomal RNA or λ antitermination complexes, in which RNAP is resistant to Rho-mediated release (Mitra et al., 2017). The results presented by Saxena et al. argue that during synthesis of mRNAs, the antipausing action of the NusG-NTD is safeguarded by the NusG-CTD:S10 bridge, which maintains in-sync translocation of RNAP and 70S.
Saxena et al. show that NusG:S10 contacts that enable coupling are particularly important when the ribosome is slowed down by sub-lethal concentrations of chloramphenicol, which stalls the moving ribosomes. What physiological conditions and RNA features would be expected to increase the demand for coupling? Many different signals induce ribosome pausing (Figure 1; reviewed by Rodnina, 2016). Pseudoknots and hairpins formed in mRNA hinder ribosome translocation. Structurally diverse cis-encoded inhibitory RNA aptamers (iRAPs) have been recently proposed to uncouple transcription from translation via interactions with E. coli RNAP (Sedlyarova et al., 2017). Ribosome pausing may be induced by specific sequences of the nascent peptide in the exit tunnel, by polyproline motifs, by rare codons decoded by low-abundance tRNAs, by defects in tRNA aminoacylation, and perhaps by high-affinity RNA sequences. Ribosome pausing is subject to regulation by accessory factors and environmental conditions. Pausing at polyproline motifs is counteracted by an elongation factor P (EF-P), an activity that depends on modification of a critical Lys residue in EF-P (Elgamal et al., 2014); pausing at poly(Pro) may be induced under (yet unknown) conditions when EF-P is downregulated or its modification is inefficient. Pausing at the end of the tnaC leader peptide is modulated by free tryptophan (Martinez et al., 2014). Pausing at rare codons may be infrequent in E. coli growing under optimal nutrient-rich conditions, but becomes significant during amino acid stress or starvation when aminoacylation is limiting (Subramaniam et al., 2014). Interestingly, an E. coli strain engineered to alleviate dependence on an otherwise essential nusG cannot survive the stationary phase (Saxena et al., 2018). While the reason for this conditional lethality is not yet known, the loss of coupling could provide one explanation.
It is also possible that NusG could facilitate 30S recruitment, similarly to the mechanism proposed for RfaH. RNAP pausing at “consensus” sites overlapping SD signals could promote initiation by maintaining the SD accessibility (Larson et al., 2014). NusG binds to RNAP at sites that match the pause consensus (Belogurov et al., 2009) and may capture the 30S subunit to form a coupled translation initiation complex, perhaps aiding initiation at suboptimal SD elements.
Are NusG:S10 contacts essential for coupling in all protein-coding genes? Studies of E. coli NusG association with the transcribing RNAP make this scenario unlikely: although NusG binds to nearly all operons, it is recruited relatively late, well into a first coding region, on many operons (Mooney et al., 2009a). If coupling is crucial, alternative means should be deployed within the upstream regions ahead of the NusG arrival, mediated either by direct contacts between RNAP and ribosomes or by another bridging factor. Astonishing diversity of regulatory mechanisms that control gene expression at all stages argues against a single coupling mechanism being employed in all genes and under all conditions. Coupling could be particularly important in very long horizontally-acquired operons that have many rare codons and termination signals, such as those activated by RfaH (Burmann et al., 2012). Coupling via a highly flexible NusG-like bridge may be advantageous to accommodate transient fluctuations of relative rates of RNAP and ribosome, for example, during ribosome pausing at the protein domain boundaries (Rodnina, 2016). Directly and more rigidly coupled expressome could be optimized for action on genes with a uniform rate of synthesis.
Is transcription-translation coupling ubiquitous in E. coli? This notion has been questioned by live super-resolution imaging, which revealed that ribosomes and RNAP are strongly segregated. More than 95% of E. coli RNAP is co-localized with the nucleoid, whereas ribosomes are densely packed near the poles, with only 10% lying within the nucleoid (Bakshi et al., 2012). Similarly, free and transcribing E. coli RNAP molecules are anti-localized to different regions of the nucleoid, particularly at high growth rates when most RNAPs are transcribing rRNA operons. Each rRNA operon is bound by up to 80 RNAP molecules, and these large arrays appear to be excluded to the periphery of the nucleoid, whilst free RNAPs are diffusing inside in search of a promoter (Stracy et al., 2015). This spatial segregation can be due to dynamic reorganization of the nucleoid triggered by active RNA synthesis or to exclusion of very large complexes from the densely packed nucleoid. Modeling suggests that differential localization of ribosomes can be explained by excluded volume effects (Castellana et al., 2016). While free ribosomes can readily diffuse inside the nucleoid, only a small fraction of 70S is present in the nucleoid; this fraction may represent 70S monosomes engaged in mRNA translation. Larger polysomes diffuse toward the cell poles within a few seconds, where they can be translated multiple times (depending on the message) for the rest of their much longer life, ∼5° min in E. coli (Bernstein et al., 2004). In this model, segregation most strongly affects mRNAs loaded with multiple ribosomes, consistent with a report that polysome arrays localize to the cell poles (Golding & Cox, 2004).
Together, these results argue that different steps of gene expression are phase separated in Bacteria despite the lack of physical barriers. Importantly, they do not rule out coupling during the pioneer round of translation. rRNA comprises more than half of newly made RNA molecules, and the bulk of transcription is independent of translation. Similarly, most translation appears to be unlinked from RNA synthesis: coupling occurs during only the first round of translation, with subsequent rounds taking place on RNAs that diffused to the peripheral ribosome-rich regions. However, since ribosomes outnumber RNAPs by a factor of ten (Bakshi et al., 2012), a small fraction of nucleoid-localized ribosomes (Bakshi et al., 2012, Castellana et al., 2016) could easily account for the pioneer round of translation. Does each and every mRNA engage in coupled translation, perhaps under conditions that promote uncoupling? Does the pioneer round of translation occur in the nucleoid, and does it license the message for steady cytoplasmic translation? Do the mechanisms that link the transcription and translation machineries differ among mRNAs and at different stages of translation? Future studies will be necessary to address these and other emerging questions.
Abbreviations
- CTD
C-terminal domain
- EC
elongation complex
- iRAP
inhibitory RNA aptamer
- NTD
N-terminal domain
- RNAP
RNA polymerase
- SD
Shine-Dalgarno
- TTCF
transcription-translation coupling factor
References
- Bakshi S, Siryaporn A, Goulian M, Weisshaar JC. Superresolution imaging of ribosomes and RNA polymerase in live Escherichia coli cells. Mol Microbiol. 2012;85:21–38. doi: 10.1111/j.1365-2958.2012.08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belogurov GA, Mooney RA, Svetlov V, Landick R, Artsimovitch I. Functional specialization of transcription elongation factors. The EMBO journal. 2009;28:112–122. doi: 10.1038/emboj.2008.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein JA, Lin PH, Cohen SN, Lin-Chao S. Global analysis of Escherichia coli RNA degradosome function using DNA microarrays. Proc Natl Acad Sci U S A. 2004;101:2758–2763. doi: 10.1073/pnas.0308747101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmann BM, Knauer SH, Sevostyanova A, Schweimer K, Mooney RA, Landick R, Artsimovitch I, Rosch P. An alpha helix to beta barrel domain switch transforms the transcription factor RfaH into a translation factor. Cell. 2012;150:291–303. doi: 10.1016/j.cell.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmann BM, Schweimer K, Luo X, Wahl MC, Stitt BL, Gottesman ME, Rosch P. A NusE:NusG complex links transcription and translation. Science. 2010;328:501–504. doi: 10.1126/science.1184953. [DOI] [PubMed] [Google Scholar]
- Buttner K, Pich A, Neubauer P, Schmid R, Bahl H, Hecker M. Copurification of ribosomal protein S2 and DNA-dependent RNA polymerase from heat-shocked cells of Bacillus subtilis. J Basic Microbiol. 1997;37:3–9. [PubMed] [Google Scholar]
- Castellana M, Hsin-Jung Li S, Wingreen NS. Spatial organization of bacterial transcription and translation. Proc Natl Acad Sci U S A. 2016;113:9286–9291. doi: 10.1073/pnas.1604995113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo G, Rasouly A, Vasilyev N, Svetlov V, Loveland AB, Diaz-Avalos R, Grigorieff N, Nudler E, Korostelev AA. Structure of RNA polymerase bound to ribosomal 30S subunit. Elife. 2017;6 doi: 10.7554/eLife.28560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D, Shatalin K, Epshtein V, Gottesman ME, Nudler E. Linking RNA polymerase backtracking to genome instability in E. coli. Cell. 2011;146:533–543. doi: 10.1016/j.cell.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgamal S, Artsimovitch I, Ibba M. Maintenance of Transcription-Translation Coupling by Elongation Factor P. MBio. 2016;7 doi: 10.1128/mBio.01373-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgamal S, Katz A, Hersch SJ, Newsom D, White P, Navarre WW, Ibba M. EF-P dependent pauses integrate proximal and distal signals during translation. PLoS Genet. 2014;10:e1004553. doi: 10.1371/journal.pgen.1004553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Conn AB, Williams PB, Diggs S, Hahm J, Gamper HB, Jr, Hou YM, O'Leary SE, Wang Y, Blaha GM. Transcription-translation coupling: direct interactions of RNA polymerase with ribosomes and ribosomal subunits. Nucleic Acids Res. 2017;45:11043–11055. doi: 10.1093/nar/gkx719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding I, Cox EC. RNA dynamics in live Escherichia coli cells. Proc Natl Acad Sci U S A. 2004;101:11310–11315. doi: 10.1073/pnas.0404443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler R, Mooney RA, Mills DJ, Landick R, Cramer P. Architecture of a transcribing-translating expressome. Science. 2017;356:194–197. doi: 10.1126/science.aal3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landick R, Carey J, Yanofsky C. Translation activates the paused transcription complex and restores transcription of the trp operon leader region. Proc Natl Acad Sci U S A. 1985;82:4663–4667. doi: 10.1073/pnas.82.14.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MH, Mooney RA, Peters JM, Windgassen T, Nayak D, Gross CA, Block SM, Greenleaf WJ, Landick R, Weissman JS. A pause sequence enriched at translation start sites drives transcription dynamics in vivo. Science. 2014;344:1042–1047. doi: 10.1126/science.1251871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat LE, Tarn WY, Isken O. The pioneer round of translation: features and functions. Cell. 2010;142:368–374. doi: 10.1016/j.cell.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez AK, Gordon E, Sengupta A, Shirole N, Klepacki D, Martinez-Garriga B, Brown LM, Benedik MJ, Yanofsky C, Mankin AS, Vazquez-Laslop N, Sachs MS, Cruz-Vera LR. Interactions of the TnaC nascent peptide with rRNA in the exit tunnel enable the ribosome to respond to free tryptophan. Nucleic Acids Res. 2014;42:1245–1256. doi: 10.1093/nar/gkt923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Gary K, Nudler E. RNA polymerase and the ribosome: the close relationship. Curr Opin Microbiol. 2013;16:112–117. doi: 10.1016/j.mib.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OL, Jr, Hamkalo BA, Thomas CA., Jr Visualization of bacterial genes in action. Science. 1970;169:392–395. doi: 10.1126/science.169.3943.392. [DOI] [PubMed] [Google Scholar]
- Mitra P, Ghosh G, Hafeezunnisa M, Sen R. Rho Protein: Roles and Mechanisms. Annu Rev Microbiol. 2017;71:687–709. doi: 10.1146/annurev-micro-030117-020432. [DOI] [PubMed] [Google Scholar]
- Mooney RA, Davis SE, Peters JM, Rowland JL, Ansari AZ, Landick R. Regulator trafficking on bacterial transcription units in vivo. Mol Cell. 2009a;33:97–108. doi: 10.1016/j.molcel.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RA, Schweimer K, Rosch P, Gottesman M, Landick R. Two structurally independent domains of E. coli NusG create regulatory plasticity via distinct interactions with RNA polymerase and regulators. J Mol Biol. 2009b;391:341–358. doi: 10.1016/j.jmb.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Mooney RA, Grass JA, Jessen ED, Tran F, Landick R. Rho and NusG suppress pervasive antisense transcription in Escherichia coli. Genes Dev. 2012;26:2621–2633. doi: 10.1101/gad.196741.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proshkin S, Rahmouni AR, Mironov A, Nudler E. Cooperation between translating ribosomes and RNA polymerase in transcription elongation. Science. 2010;328:504–508. doi: 10.1126/science.1184939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JP. Preventing the synthesis of unused transcripts by Rho factor. Cell. 1991;64:1047–1049. doi: 10.1016/0092-8674(91)90257-y. [DOI] [PubMed] [Google Scholar]
- Roche ED, Sauer RT. SsrA-mediated peptide tagging caused by rare codons and tRNA scarcity. EMBO J. 1999;18:4579–4589. doi: 10.1093/emboj/18.16.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnina MV. The ribosome in action: Tuning of translational efficiency and protein folding. Protein Sci. 2016;25:1390–1406. doi: 10.1002/pro.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland KL, Powell FE, Turnbough CL. Role of translation and attenuation in the control of pyrBI operon expression in Escherichia coli K-12. J Bacteriol. 1985;163:991–999. doi: 10.1128/jb.163.3.991-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said N, Krupp F, Anedchenko E, Santos KF, Dybkov O, Huang YH, Lee CT, Loll B, Behrmann E, Burger J, Mielke T, Loerke J, Urlaub H, Spahn CMT, Weber G, Wahl MC. Structural basis for lambdaN-dependent processive transcription antitermination. Nat Microbiol. 2017;2:17062. doi: 10.1038/nmicrobiol.2017.62. [DOI] [PubMed] [Google Scholar]
- Saxena S, Myka KK, Washburn R, Costantino N, Court DL, Gottesman ME. Escherichia coli transcription factor NusG binds to 70S ribosomes. Mol Microbiol. 2018 doi: 10.1111/mmi.13953. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlyarova N, Rescheneder P, Magan A, Popitsch N, Rziha N, Bilusic I, Epshtein V, Zimmermann B, Lybecker M, Sedlyarov V, Schroeder R, Nudler E. Natural RNA Polymerase Aptamers Regulate Transcription in E. coli. Mol Cell. 2017;67:30–43 e36. doi: 10.1016/j.molcel.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak G. Transcription-coupled repair: an update. Arch Toxicol. 2016;90:2583–2594. doi: 10.1007/s00204-016-1820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracy M, Lesterlin C, Garza de Leon F, Uphoff S, Zawadzki P, Kapanidis AN. Live-cell superresolution microscopy reveals the organization of RNA polymerase in the bacterial nucleoid. Proc Natl Acad Sci U S A. 2015;112:E4390–4399. doi: 10.1073/pnas.1507592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam AR, Zid BM, O'Shea EK. An integrated approach reveals regulatory controls on bacterial translation elongation. Cell. 2014;159:1200–1211. doi: 10.1016/j.cell.2014.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhodolets MV, Garges S. Interaction of Escherichia coli RNA polymerase with the ribosomal protein S1 and the Sm-like ATPase Hfq. Biochemistry. 2003;42:8022–8034. doi: 10.1021/bi020638i. [DOI] [PubMed] [Google Scholar]
- Trautinger BW, Jaktaji RP, Rusakova E, Lloyd RG. RNA polymerase modulators and DNA repair activities resolve conflicts between DNA replication and transcription. Mol Cell. 2005;19:247–258. doi: 10.1016/j.molcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Valabhoju V, Agrawal S, Sen R. Molecular Basis of NusG-mediated Regulation of Rho-dependent Transcription Termination in Bacteria. J Biol Chem. 2016;291:22386–22403. doi: 10.1074/jbc.M116.745364. [DOI] [PMC free article] [PubMed] [Google Scholar]