Abstract
Hedgehog (Hh) pathway signaling is crucial for the maintenance of blood cell progenitors in the lymph gland hematopoietic organ present in Drosophila third instar larvae. Previous studies from our lab have likewise shown the importance of the mir-7 and bag of marbles (bam) genes in maintaining the progenitor state. Thus we sought to investigate a possible interaction between the Hh pathway and mir-7/bam in the prohemocyte population within this hematopoietic tissue. Gain of function mir-7 was able to rescue a blood cell progenitor depletion phenotype caused by Patched (Ptc) inhibition of Hh pathway signaling in these cells. Similarly, expression of a dominant/negative version of Ptc was able to rescue the severe reduction of prohemocytes due to bam loss of function. Furthermore, we demonstrated that Suppressor of fused [Su(fu)], another known inhibitor of Hh signaling, likely serves as a translational repression target of the mir-7 miRNA. Our results suggest the mir-7/bam combination regulates the Hh signaling network through repression of Su(fu) to maintain hemocyte progenitors in the larval lymph gland.
Keywords: Bag of marbles, Drosophila blood cell progenitors, Hedgehog signaling pathway, mir-7 miRNA, Patched, Suppressor of fused
1 / INTRODUCTION
Drosophila has emerged as an excellent model system for the study of hematopoiesis. Two distinct waves of blood cell formation exist during Drosophila development (Evans, Hartenstein, & Banerjee, 2003). The first wave occurs in the embryonic head mesoderm. Generated hemocytes are contributed to the larval form and persist in groups under the larval cuticle. The second wave occurs in the larval lymph glands, which are formed during embryogenesis and eventually degenerate during metamorphosis, releasing large numbers of mature blood cells that persist into the adult animal.
The lymph glands present in third instar larvae are composed of several pairs of lobes. The anterior, primary lobes consist of three distinct cellular domains: the medullary zone (MZ), cortical zone (CZ) and posterior signaling center (PSC). The MZ is composed of blood cell progenitors, thought to function as hematopoietic stem-like cells. The CZ includes mature hemocytes such a plasmatocytes, crystal cells and lamellocytes. The PSC secretes multiple signaling molecules such as Unpaired-3, Serrate, Hedgehog (Hh) and Pvf to control progenitor cell maintenance versus blood cell differentiation onset (Jung et al., 2005; Lebestky, Jung, & Banerjee, 2003; Mandal et al., 2007; Mondal et al., 2011; Tokusumi et al., 2010).
The MZ cellular domain, initially marked with a domeless (dome) reporter, has now been characterized by many additional molecular markers expressed therein from multiple signaling pathway components and other genes (Benmimoun et al., 2012, 2015; Dragojilovic-Munther & Martinez-Agosto, 2012, 2013; Gao, Wu, & Fossett, 2011, 2013; Gao et al., 2016; Jung et al., 2005; Mandal et al., 2007; Mondal et al., 2014; Morin-Poulard et al., 2016; Oyallon et al., 2016; Shim, Mukherjee, & Banerjee, 2012; Sinenko et al., 2009; Tokusumi et al., 2011, 2017). The hh pathway is one important signaling pathway used to maintain blood cell progenitor quiescence (Mandal et al., 2007). The secreted Hh ligand binds to the receptor protein Patched (Ptc), preventing Ptc inhibition of Smoothened whose normal function is to activate the downstream transcriptional effector Cubitus interruptus (Ci) (Osterlund & Kogerman, 2006). The Ci protein is also regulated by other factors including the Suppressor of fused [Su(fu)] protein. Hh is expressed in PSC cells, which serves as the source for ligand communication with cells of the MZ. In the absence of hh function, or the inhibition of other positively-acting components of the Hh network, MZ cell quiescence is lost and the progenitor cells enter blood cell differentiation pathways (Giordani et al., 2016; Mandal et al., 2007).
Previously, we demonstrated that the small regulatory RNA mir-7 genetically interacts with the bag of marbles (bam) gene to maintain the lymph gland MZ prohemocyte population through the inhibition of the Yan pro-blood cell differentiation transcription factor (Tokusumi et al., 2011). In this study, we show that mir-7 and bam genetically interact with components of the hh pathway in the maintenance of hematopoietic stem-like progenitors. Mechanistically, mir-7 appears to function in the repression of Su(fu) protein expression, allowing for active hh pathway signaling in MZ cells and the maintenance of the blood cell progenitor state.
2 / RESULTS AND DISCUSSION
2.1 / The mir-7 and bam genes regulate hh signaling to maintain prohemocytes in Drosophila larval lymph glands
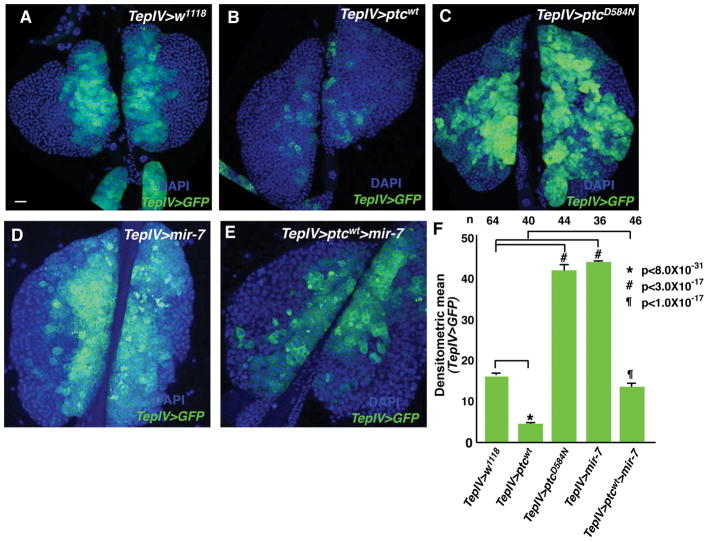
The hh pathway plays a crucial role in maintaining blood cell precursors populating the MZ domain (Mandal et al., 2007). An MZ-specific Gal4 driver, TepIV-Gal4, was used to express the wild type ptc gene in lymph glands, with the result being a strong decrease in TepIV-GFP positive cells (Figure 1B, F). In contrast, expression of a dominant negative version of Ptc (ptcD584N) induced a copious number of blood cell progenitors in the lymph glands (Figure 1C, F). These results agreed with previous MZ cell phenotypes elicited in hh mutant and Ci gain of function analyses (Mandal et al., 2007). Recently, two groups demonstrated that the PSC is not required for MZ cell maintenance and Hh signaling may not be necessary for this process (Benmimoun et al., 2015; Oyallon et al., 2016). However, our results suggested the Hh signaling pathway clearly plays a role in maintaining the MZ prohemocyte population.
Figure 1.
Medullary zone cell reduction due to ptc expression and inhibition of hh pathway signaling is rescued by mir-7 expression. (A) TepIV>w1118 serves as a wild type control. (B) TepIV > ptcwt lymph glands. (C) TepIV-driven dominant negative form ptcD584N lymph glands. (C) TepIV>mir-7 lymph glands. (D) TepIV-driven co-expression of mir-7 and ptcwt lymph glands. In all panels, blue corresponds to DAPI staining and green indicates TepIV>UAS-GFP expression marking MZ cells. Bar in (A) indicates 20μm. All lymph gland images are at the same magnification. (F) Relative values of densitometric scans of TepIV>GFP expression in the various genetic backgrounds. P-values indicate significance differences.
We previously demonstrated that the mir-7 miRNA is expressed in MZ cells and is required therein to maintain this population in a pluripotent progenitor state through its repression of Yan (Tokusumi et al., 2011). Intriguingly, mir-7 gain of function led to an expanded MZ cell population, similar to the TepIV-Gal4>ptcD584N result (Figure 1D, F). It is known that miRNAs can have multiple targets to regulate gene expression for tissue and organ homeostasis. We hypothesized that mir-7 could control not only Yan expression, but also hh pathway signaling in the maintenance of the progenitor population. To address this possibility, we co-expressed mir-7 and ptcwt in MZ cells. This co-expression led to a near normal number of MZ cells, indicating mir-7 expression can rescue the blood cell progenitor loss due to Ptc expression and Ptc inhibition of Hh signaling (Figure 1E, F). These results suggested the mir-7 miRNA may also function through its regulation of the hh genetic network in the maintenance of the prohemocyte population.
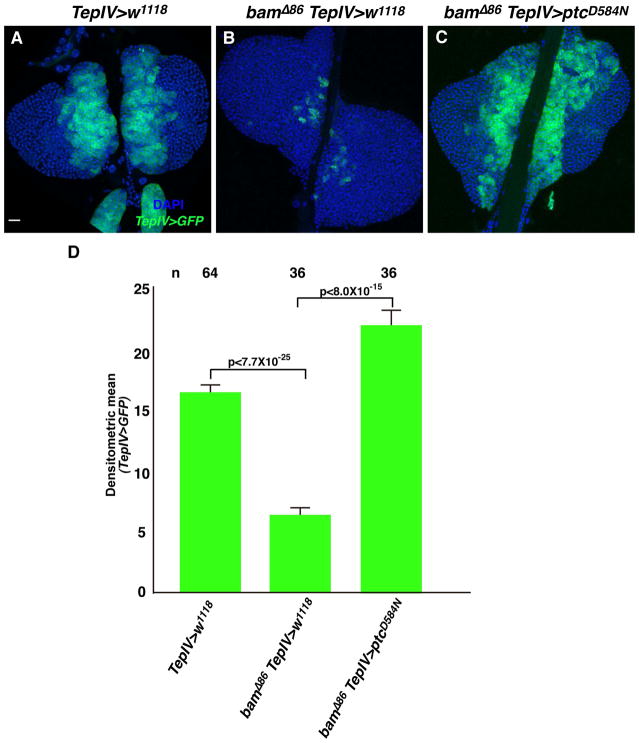
We previously reported that Bam, initially identified as a germ line differentiating factor, functionally interacts with mir-7 to control hematopoiesis in the larval lymph gland. Specifically, Bam and mir-7 act as positive regulators of hematopoietic progenitor maintenance in this developmental process (Tokusumi et al., 2011). Thus we sought to determine if bam gene function might also be involved in the regulation of progenitor cell number through an interaction with the hh pathway. As shown in a previous report, bamΔ86 null mutant lymph glands present with a greatly diminished MZ cell population (Figure 2B, D and Tokusumi et al., 2011). This MZ reduction in the bam mutant lymph glands was rescued by ptcD584N expression in MZ cells (Figure 2C, D). This finding is consistent with the mir-7 analysis, suggesting the bam and mir-7 genes functionally interact with components of the hh pathway in the maintenance of blood cell progenitors in the MZ domain of the larval lymph glands.
Figure 2.
Medullary zone cell reduction due to bam loss of function is rescued by enhanced hh pathway signaling due to dominant-negative Ptc expression. (A) w1118 lymph glands serve as a wild type control. (B) bamΔ86 null mutant lymph glands. (C) TepIV>ptcD584N expression rescues the bam loss of function phenotype of reduced MZ cells. In all panels, blue corresponds to DAPI staining and green indicates TepIV>UAS-GFP expression marking MZ cells. Bar in (A) indicates 20μm. All lymph gland images are at the same magnification. (D) Relative values of densitometric scans of TepIV>GFP expression in the various genetic backgrounds. P-values indicate significant differences.
2.2 / mir-7 can negatively regulate a member of the hh pathway, Suppressor of fused
Several targets of the mir-7 regulatory RNA have been identified in previous studies (Brennecke et al., 2005; Da Ros et al., 2013; Li & Carthew, 2005; Stark et al., 2003). We hypothesized one or more members of the hh pathway might be a target of mir-7. Therefore, we ran the search program Target Scan Fly (http://www.targetscan.org/fly_12/) to identify potential mir-7 targets amongst hh pathway genes (Kheradpour et al., 2007). The program found two candidates: interference hedgehog (ihog) and Su(fu). ihog, which encodes a Hh receptor and is an inhibitor of Hh signal transduction, has already been shown to be a target of mir-7 in imaginal discs (Da Ros et al., 2013). We examined if TepIV>ihog RNAi expression affected the MZ population, but could not detect a significant difference in MZ cell number as compared to wild type lymph glands (data not shown).
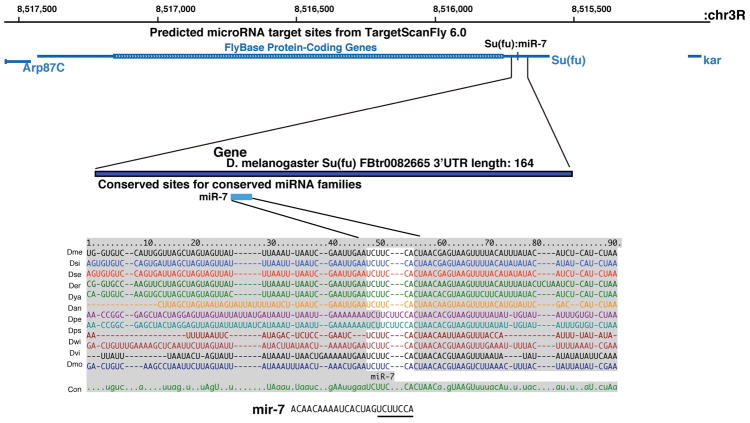
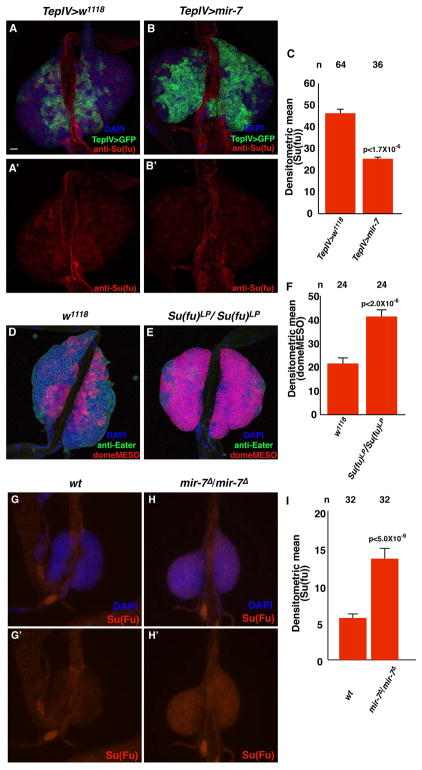
The second potential target, Su(fu), encodes a protein found in the cytoplasm wherein it binds to the Ci factor and inhibits Ci transcriptional activity. One potential binding site for mir-7 was found in the 3′ UTR of the Su(fu) gene, and this site is perfectly conserved in the Su(fu) genes of 12 sequenced Drosophila species (Figure 3). We confirmed that the Su(fu) protein was expressed in the lymph glands, including cells of the MZ domain (Figure 4A, A′). Based on this observation, we tested if mir-7 expression in hemocyte progenitors could negatively affect Su(fu) protein accumulation in these cells. TepIV-Gal4 driven expression of UAS-mir-7 significantly diminished the level of Su(fu) in MZ cells (Figure 4B, B′, C). To determine whether Su(fu) loss-of-function could affect the MZ prohemocyte population, we examined the Su(fu)LP homozygous phenotype in lymph glands. These lymph glands showed a strong increase of blood progenitor cells in parallel to a reduction of mature hemocytes (Figure 4D, 4E, 4F). To further support the hypothesis, we examined if Su(Fu) expression is affected in mir-7 mutants. At the second or the early third instar stage, there are detectable MZ cells in mir-7 mutants, although they are rapidly reduced after the mid third instar stage. We confirmed Su(Fu) expression in mir-7 mutant lymph glands was higher than the protein level detected in wild type lymph glands. These results indicated the mir-7 miRNA could negatively attenuate Su(fu) gene expression, allowing for Hh signaling and the maintenance of the MZ progenitor cells.
Figure 3.
Conservation of a consensus mir-7 binding site in the 3′UTR of the Su(fu) gene in the genomes of 12 Drosophila species.
Figure 4.
Su(fu) is a likely target of translational repression by the mir-7 miRNA. (A, A′) Localization of Su(fu) proteins in wild type lymph glands. (B, B′) TepIV-driven mir-7 expression in MZ cells reduces the level of Su(fu) protein in lymph gland cells. Blue, green and red indicate DAPI nuclear staining, TepIV-UAS-GFP MZ cell expression and Su(fu) protein expression, respectively. Bar in (A) indicates 20μm. All lymph gland images are at the same magnification. (C) Relative values of densitometric scans of Su(fu) protein expression in the two different genetic backgrounds. MZ marker domeMESO and plasmatocyte marker anti-Eater antibody staining patterns in (D) wild type and (E) Su(fu) loss-of-function mutant Su(fu)LP lymph glands. Blue, green and red show respectively nucleus (DAPI), Eater protein and domeMESO. (F) Densitometric relative values of domeMESO expression in wild type and Su(fu)LP lymph glands. Su(fu) protein expression at the early third instar stage in (G, G′) wild type and (H, H′) mir-7 mutants. Blues and red mean DAPI and Su(fu) protein, respectively. (I) Densitometric relative values of Su(fu) expression in wild type and mir-7Δ lymph glands at the second or the early third instar stage. All p-values (C, F, and I) indicate significant differences.
2.3 / Summary
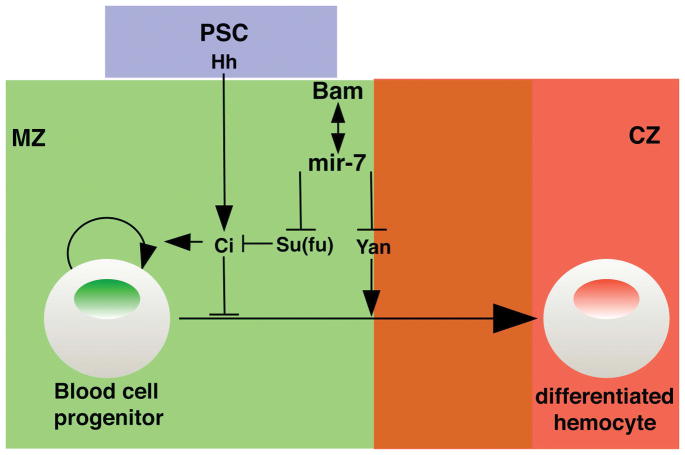
In this study, we demonstrated mir-7 can regulate the hh pathway via repression of Su(fu) expression, with the reduction of the level of Su(fu) binding to the Ci transcriptional factor allowing for enhanced Hh signaling (Figure 5). The hh pathway plays important roles in lymph gland hematopoiesis, especially prohemocyte maintenance. Without the Hh ligand, Ptc inhibits the activation of Ci, resulting in the promotion of blood cell differentiation. In previous studies, hh gene expression in PSC niche cells was shown to be stable during larval stages under various stress conditions such as animal starvation and injury. Under these conditions, blood cell progenitors are maintained in the lymph gland MZ domain, with the exception of wasp infestation (Krzemien et al., 2007; Tokusumi et al., 2012; unpublished data). A question can be raised as to how Hh signaling is fine-tuned under various stress conditions, even if the hh expression level is the same during larval stages. Previously, we suggested that mir-7 interacts with bam to maintain hematopoietic progenitor cells through their negative regulation of Yan which normally functions as a factor that promotes blood cell differentiation (Tokusumi et al., 2011). One major function of miRNAs is to attenuate gene expression to promote tissue and organ homeostasis in response to changing physiological environments (Carthew, Agbu, & Giri, 2016). Our findings implicate that the mir-7 and bam genes may be able to positively regulate Hh signaling, towards the goal of blood cell progenitor maintenance and lymph gland homeostasis in response to various stress conditions.
Figure 5.
Model of the interaction between the hh signaling pathway and mir-7/bam genes. Abbreviations: CZ, cortical zone; MZ, medullary zone; PSC, posterior signaling center.
3 / MATERIALS AND METHODS
3.1 / Drosophila strains
The following strains were used in this study: UAS-mCD8GFP, UAS-ptc, UAS-ptcD584N, bamΔ86, Su(fu)LP (Bloomington Drosophila Stock Center); TepIV-Gal4 (DGRC, Kyoto); UAS-mir-7 (Li & Carthew, 2005).
3.2 / Tissue staining
Tissue staining methods were described previously (Tokusumi et al., 2015). We dissected lymph glands at the mid 3rd instar larval stage and fixed them with 4% paraformaldehyde in PBS for 30 min. For antibody staining, the fixed samples were blocked with a PBSTB solution consisting of 5% goat serum and 0.05% Triton X-100 in PBS, for 1 hr and then incubated with an antibody solution diluted with PBSTB overnight at 4°C. The following primary antibodies were used: mouse anti-Su(Fu) antibody (1:100, Developmental Studies Hybridoma Bank); anti-β-galactosidase (1:100, Promega), anti- Eater (1:1000, Chung & Kocks, 2011). After washing 3 times with 0.05% Triton X-100 in PBS, we incubated the samples with Alexa-555 conjugated anti-mouse antibody for 1 hr at room temperature (1:500, Thermo Fisher Scientific). After washing 3 times with 0.05% Triton X-100 in PBS, tissues were mounted in 50% glycerol in PBS. Images were captured with a Nikon A1R laser-scanning confocal microscope.
3.3 / Quantification of labeled cells in lymph glands
Densitometric means of GFP-labeled or immunostained samples were quantified with a previously described method and the values were analyzed with the Mann-Whitney’s U test for statistical analyses (Gao, Wu, & Fossett, 2009; Tokusumi et al., 2015). In all bar graphs, error bars are indicated with standard error.
Acknowledgments
Funding information
This work was supported by grant AI121985 from NIH/NIAID (to R.A.S.)
The authors would like to thank R. Carthew, the Bloomington Stock Center, and the Drosophila Genomics and Genetic Resources at Kyoto for Drosophila strains. We also thank the Notre Dame Integrated Imaging Facility for use of laser scanning confocal microscopes. This work was supported by grant AI121985 from NIH/NIAID (to R.A.S.).
References
- Benmimoun B, Polesello C, Haenlin M, Waltzer L. The EBF transcription factor Collier directly promotes Drosophila blood cell progenitor maintenance independently of the niche. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:9052–9057. doi: 10.1073/pnas.1423967112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmimoun B, Polesello C, Waltzer L, Haenlin M. Dual role for Insulin/TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development. 2012;139:1713–1717. doi: 10.1242/dev.080259. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biology. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew RW, Agbu P, Giri R. MicroRNA function in Drosophila melanogaster, Seminars in Cell and Developmental Biology. 2017;65:29–37. doi: 10.1016/j.semcdb.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YSA, Kocks C. Recognition of pathogenic microbes by the Drosophila phagocytic pattern recognition receptor Eater. Journal of Biological Chemistry. 2011;286:26524–26532. doi: 10.1074/jbc.M110.214007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Ros VG, Gutierrez-Perez I, Ferres-Marco D, Dominguez M. Dampening the signals transduced through hedgehog via microRNA miR-7 facilitates notch-induced tumourigenesis. PLoS Biology. 2013;11:e1001554. doi: 10.1371/journal.pbio.1001554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragojlovic-Munther M, Martinez-Agosto JA. Multifaceted roles of PTEN and TSC orchestrate growth and differentiation of Drosophila blood progenitors. Development. 2012;139:3752–3763. doi: 10.1242/dev.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragojlovic-Munther M, Martinez-Agosto JA. Extracellular matrix-modulated Heartless signaling in Drosophila blood progenitors regulates their differentiation via a Ras/ETS/FOG pathway and target of rapamycin function. Developmental Biology. 2013;384:313–330. doi: 10.1016/j.ydbio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Developmental Cell. 2003;5:673–690. doi: 10.1016/s1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- Gao H, Baldeosingh R, Wu X, Fossett N. The Friend of GATA transcriptional co-regulator, U-Shaped, is a downstream antagonist of Dorsal-driven prohemocyte differentiation in Drosophila. PLoS One. 2016;11:e0155372. doi: 10.1371/journal.pone.0155372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Wu X, Fossett N. Upregulation of the Drosophila Friend of GATA gene U-shaped by JAK/STAT signaling maintains lymph gland prohemocyte potency. Molecular and Cellular Biology. 2009;29:6086–6096. doi: 10.1128/MCB.00244-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Wu X, Fossett N. Odd-skipped maintains prohemocyte potency and blocks blood cell development in Drosophila. Genesis. 2011;49:105–116. doi: 10.1002/dvg.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Wu X, Fossett N. Drosophila E-cadherin functions in hematopoietic progenitors to maintain multipotency and block differentiation. PloS One. 2013;8:e74684. doi: 10.1371/journal.pone.0074684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordani G, Barraco M, Giangrande A, Martinelli G, Guadagnuolo V, Simonetti G, Perini G, Bernardoni R. The human Smoothened inhibitor PF-04449913 induces exit from quiescence and loss of multipotent Drosophila hematopoietic progenitor cells. Oncotarget. 2016;7:55313–55327. doi: 10.18632/oncotarget.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Kheradpour P, Stark A, Roy S, Kellis M. Reliable prediction of regulator targets using 12 Drosophila genomes. Genome Res. 2007;17:1919–1931. doi: 10.1101/gr.7090407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzemień J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–328. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- Lebestky T, Jung SH, Banerjee U. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes and Development. 2003;17:348–353. doi: 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Carthew RW. A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell. 2005;123:1267–1277. doi: 10.1016/j.cell.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, Banerjee U. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147:1589–1600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal BC, Shim J, Evans CJ, Banerjee U. Pvr expression regulators in equilibrium signal control and maintenance of Drosophila blood progenitors. eLife. 2014;3:e03626. doi: 10.7554/eLife.03626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Poulard I, Sharma A, Louradour I, Vanzo N, Vincent A, Crozatier M. Vascular control of the Drosophila haematopoietic microenvironment by Slit/Robo signalling. Nature Communications. 2016;7:11634. doi: 10.1038/ncomms11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund T, Kogerman P. Hedgehog signalling: how to get from Smo to Ci and Gli. Trends in Cell Biology. 2006;16:176–180. doi: 10.1016/j.tcb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Oyallon J, Vanzo N, Krzemien J, Morin-Poulard I, Vincent A, Crozatier M. Two independent functions of Collier/Early B Cell Factor in the control of Drosophila blood cell homeostasis. PLoS One. 2016;11:e0148978. doi: 10.1371/journal.pone.0148978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Mukherjee T, Banerjee U. Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nature Cell Biology. 2012;14:394–400. doi: 10.1038/ncb2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko SA, Mandal L, Martinez-Agosto JA, Banerjee U. Dual role of wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Developmental Cell. 2009;16:756–763. doi: 10.1016/j.devcel.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark A, Brennecke J, Russell RB, Cohen SM. Identification of Drosophila MicroRNA targets. PLoS Biology. 2003;1:E60. doi: 10.1371/journal.pbio.0000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi T, Tokusumi Y, Brahier MS, Lam L, Stoller-Conrad J, Kroeger PT, Schulz RA. Screening and analysis of Janelia FlyLight Project Enhancer-Gal4 strains identifies multiple gene enhancers active during hematopoiesis in normal and wasp-challenged Drosophila larvae. G3: Genes|Genomes|Genetics. 2017;7:437–448. doi: 10.1534/g3.116.034439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi T, Tokusumi Y, Hopkins DW, Schulz RA. Bag of Marbles controls the size and organization of the Drosophila hematopoietic niche through interactions with the Insulin-like growth factor pathway and Retinoblastoma-family protein. Development. 2015;142:2261–2267. doi: 10.1242/dev.121798. [DOI] [PubMed] [Google Scholar]
- Tokusumi T, Tokusumi Y, Hopkins DW, Shoue DA, Corona L, Schulz RA. Germ line differentiation factor Bag of Marbles is a regulator of hematopoietic progenitor maintenance during Drosophila hematopoiesis. Development. 2011;138:3879–3884. doi: 10.1242/dev.069336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi Y, Tokusumi T, Shoue DA, Schulz RA. Gene regulatory networks controlling hematopoietic progenitor niche cell production and differentiation in the Drosophila lymph gland. PLoS One. 2012;7:e41604. doi: 10.1371/journal.pone.0041604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi Y, Tokusumi T, Stoller-Conrad J, Schulz RA. Serpent, suppressor of hairless and U-shaped are crucial regulators of hedgehog niche expression and prohemocyte maintenance during Drosophila larval hematopoiesis. Development. 2010;137:3561–3568. doi: 10.1242/dev.053728. [DOI] [PMC free article] [PubMed] [Google Scholar]