Abstract
Salivary glands are responsible for maintaining the health of the oral cavity and are routinely damaged by therapeutic radiation for head and neck cancer as well as by autoimmune diseases such as Sjögren’s syndrome. Regenerative approaches based on the reactivation of endogenous stem cells or the transplant of exogenous stem cells hold substantial promise in restoring the structure and function of these organs to improve patient quality of life. However, these approaches have been hampered by a lack of knowledge on the identity of salivary stem cell populations and their regulators. In this review we discuss our current knowledge on salivary stem cells and their regulators during organ development, homeostasis and regeneration. As increasing evidence in other systems suggests that progenitor cells may be a source of cancer, we also review whether these same salivary stem cells may also be cancer initiating cells.
Introduction
Salivary glands (SGs) are one of numerous exocrine organs that have evolved to allow terrestrial living. Although their gross anatomy can vary dramatically across species, the complex serous-mucous liquid produced (saliva) plays an important and often essential role in survival through its impact on diet, for example, mice die within days after major gland removal. Although functional salivary glands are not required for human survival, SG dysfunction that arises from genetic anomalies (e.g., LADD or ASLG syndromes), or damage from surgery, therapeutic radiation for head and neck cancer (Frank et al., 1965; Valdez et al., 1993), or autoimmune diseases such as Sjögren’s syndrome (Azuma et al., 1997; Patel and Shahane, 2014; Stewart et al., 2008) impairs oral health, resulting in a myriad of symptoms including mastication and swallowing difficulties (Dusek et al., 1996; Hamlet et al., 1997; Tolentino Ede et al., 2011), speech impairment (Rhodus et al., 1995), mucosal alterations, oral infection (Azizi and Rezaei, 2009; Brown et al., 1975; Davies et al., 2006) and accelerated tooth decay (Lu et al., 2014). Despite these detrimental and life-long effects, current therapies are limited to secretagogues and artificial saliva, with no long-term solutions to restore salivary gland function. Consistent with the need to develop regenerative strategies, there has been increasing focus on the identification of stem cell populations and their regulators for the repair or regeneration of injured salivary tissue. Here we aim to provide a perspective on what is currently known about the identity and regulation of salivary stem cells during organ development and adult regeneration. Due to the lack of knowledge on salivary cancers, and the increasing evidence in other systems that progenitor cells may be a source of such neoplasms, we also discuss whether these same salivary stem cells may be the initiators of salivary cancers.
Salivary Gland Structure and Function
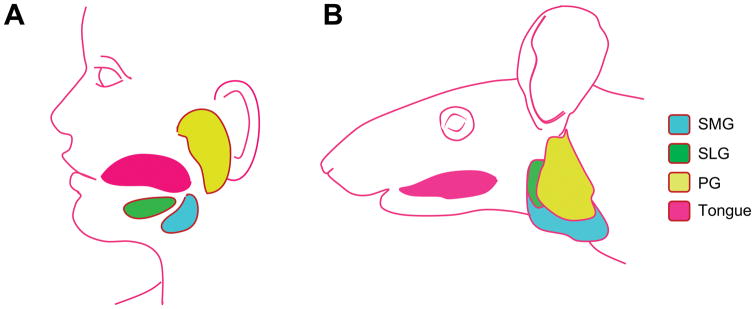
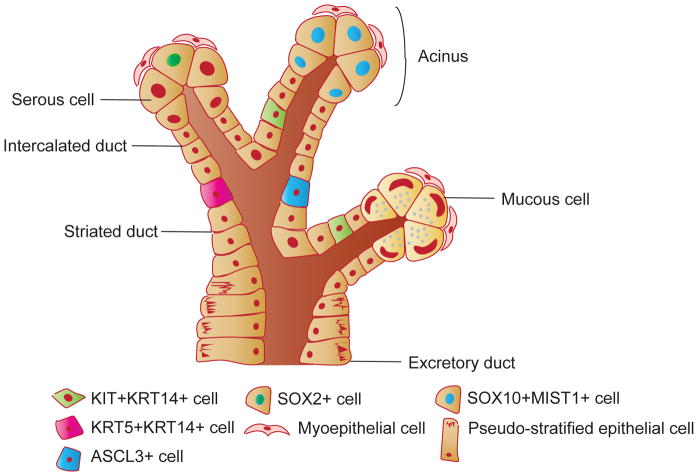
Mammalian saliva is primarily secreted by 3 pairs of major salivary glands (parotid (PG), submandibular (SMG) and sublingual (SLG)) and over 1000 minor glands. In humans and mice the SMG, SLG and PG produce >90% of the total saliva secreted into the oral cavity (Miletich, 2010). In humans, the PG is the largest of the three glands and is located inferior and anterior to the ear; the SMGs are located alongside the mandible posterior to the tongue, and the SLGs lie beneath the oral mucosa anterior to the tongue (Figure 1A). The saliva producing secretory units of the gland, acinar cells, are located at the end of the ductal network and are either of a serous or mucous cell type. In humans, acinar cells of the PG are serous, the SMG has both serous and mucous and the SLG is predominantly composed of mucous acini (Martinez-Madrigal and Micheau, 1989). The different acinar cell types can be easily distinguished from each other at the histological level: serous cells are pyramidal with a large round central nucleus, and mucous cells are columnar and contain granules, displacing the flattened nuclei to the basal membrane. Surrounding the acini are myoepithelial cells that are thought to aid in saliva secretion by constricting the acini in response to neuronal cues (Segawa et al., 1995). Once secreted from the acinar cell, saliva travels through a ductal network consisting of intercalated ducts (the smallest ducts composed of simple cuboidal epithelium), striated (simple columnar epithelium), and excretory ducts (pseudo-stratified columnar epithelium) into the oral cavity (Miletich, 2010). Mice have the same three major pairs of salivary glands, located in a similar location (Figure 1B). Mice differ from humans in that the SMG has an additional ductal network that connects the intercalated ducts to the striated ducts and is the main source of growth stimulatory molecules such as nerve growth factor and epidermal growth factor (Gresik et al., 1980; Schenck et al., 2017).
Figure 1.
Schematic to show the localisation of the three major salivary glands in humans (A) and mice B).
To produce the large quantities of saliva required each day ((0.5–1L per day in humans (Melvin et al., 2005)) the SGs need extensive vascularization (water is derived from the plasma) and innervation. For the PGs, capillaries derived from the external carotid artery wrap around the serous acini while the SMGs and SLGs are vascularised by the submental and sublingual arteries, branches of the lingual and facial arteries. Saliva secretion is primarily controlled by parasympathetic, and to a much lesser extent, sympathetic nerves of the autonomic nervous system (Proctor and Carpenter, 2007). The PGs receive parasympathetic innervation from the glossopharyngeal nerve IX via the otic ganglion, while the SMGs and SLGs are innervated by the chorda tympani via the parasympathetic submandibular ganglion (Holsinger and Bui, 2007). Parasympathetic nerves activate both cholinergic and non-cholinergic receptors to drive salivary flow by increasing water transfer. Sympathetic nerves, which travel from the superior cervical nuclei in the brain stem to innervate all three major SG, regulate protein secretion and thereby the viscosity of saliva. It is important to note that parasympathetic innervation is not only required for organ function, but is also necessary for tissue maintenance. Removal of the parasympathetic nerves (parasympathectomy) results in glandular atrophy in humans and rodents (Peronace et al., 1964; Snell and Garrett, 1958; Wells and Peronace, 1967), which can be reversed if the tissue is reinnervated (Carpenter et al., 2009). Although sympathetic nerves are not essential for tissue maintenance (Proctor and Asking, 1989), activation of beta-adrenergic receptors promotes cell proliferation and hypertrophy (Hand and Ho, 1985; Johnson, 1984) and has been demonstrated to promote regeneration of salivary tissue (Boshell and Pennington, 1980), suggesting that these nerves play may yet play a role in tissue homeostasis.
Salivary gland development
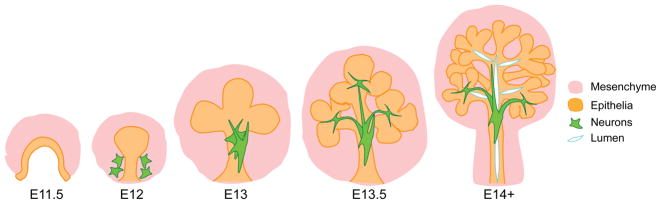
As for most mammalian glandular organs, the acinar-ductal network of the salivary glands is formed through the fundamental process of epithelial branching morphogenesis. This process, which was first described in the salivary gland by Elio Borghese in 1950 (Borghese, 1950), involves extensive rounds of epithelial tissue expansion, cleft formation, cell differentiation and lumenization that, unlike the lung, occurs in a non-stereotypical fashion (Figure 2). As most studies to date describe the murine SMG, this review will focus on this gland, however, we remind the reader that the PG and SLG differ greatly in position, size and acinar composition, suggesting potential differences in progenitor cell types and mechanisms regulating cell maturation.
Figure 2.
Schematic of salivary gland development in the fetal mouse.
The SMG initiates as an invagination of the oral epithelium into a condensing neural crest-derived mesenchyme after embryonic day (E) 11.5. Subsequent SMG development can be divided into 3 major phases: establishment of a relatively undifferentiated branched structure consisting of acinar and ductal precursors (E12–E14; Figure 2) followed by terminal differentiation into secretory cell types (E15–E17.5) and ending in functional maturation (post-natal day (P) 0 – 4 weeks). The first phase consists of an early round of branching, beginning at E12, in which a single epithelial end bud (pre-acini) undergoes multiple rounds of bud and duct formation to establish a tubular network by E15 that consists of KRT19+ duct cells and AQP5+ pre-acinar cells (myoepithelial cells have not emerged from the epithelium). By E16, alpha smooth muscle actin (αSMA)+ myoepithelial cells have emerged and acinar cells have begun to express a master regulator of secretion, MIST1 (BHLHA15), as well as early secretory proteins (e.g., parotid secretory protein (PSP)). After birth the tissue continues to undergo extensive epithelial branching and continued maturation (e.g., production of amylase) to form functional mucous and serous acinar cells, ducts and myoepithelial cells capable of producing the levels of saliva required for life (reviewed in Patel et al., 2006); Figure 3).
Figure 3.
Schematic of the structure of the salivary gland and the localization of known progenitor cells.
Not surprisingly, these complex morphogenic and differentiation events are controlled by multiple signalling networks, including those derived from epithelial-mesenchyme, nerve-epithelial and more recently endothelial-epithelial interactions. These interactions are formed at the beginnings of organogenesis: the E11.5 epithelium invaginates into a condensing neural crest-derived mesenchyme containing a discontinuous CD31+ endothelial plexus and recruits SOX10+ neural precursors to form a post-ganglionic parasympathetic ganglion (Figure 2). During the next 36 h the endothelium becomes continuous and axons extend from the ganglion and travel along the developing ductal system to envelope newly forming end buds, thus forming an integrated organ system (Coughlin, 1975; Knosp et al., 2015; Knox et al., 2010; Kwon et al., 2017). As would be expected from such a heterogeneous structure, many pathways are involved in regulating epithelial branching, including those mediated by growth factors such as FGF, EGF, WNT, Hedgehog, and EDA (reviewed in Mattingly et al., 2015) and neurotransmitters acetylcholine (ACh) (Knox et al., 2010) and vasoactive intestinal protein (VIP) (Nedvetsky et al., 2014). Although we know a great deal about the impact of these signalling pathways in controlling morphogenesis, we are only beginning to understand their impact on progenitor/stem cell behavior. Here we will review those pathways known to regulate progenitor cells and point the reader to excellent reviews on pathways regulating tissue morphogenesis (Kwon and Larsen, 2015; Mattingly et al., 2015; Patel and Hoffman, 2014).
1. Progenitor cell markers in developing and adult SG
Genetic lineage tracing has become the gold standard technique for identifying stem and progenitor cells in a plethora of developing and adult organs. By permanently labeling a specific cell and all its subsequent progeny (differentiated or not), we are able to identify initiating cells that contribute to the tissue during development, homeostasis and after injury. Here we define these initiating cells as progenitors, as unlike multipotent stem cells in the early embryo, current evidence indicates that their differentiation in the SG is limited. As only a few genetic lineage-tracing studies have been conducted thus far, we also describe potential progenitor populations based on markers identified to be present on progenitor cells in other glandular/epithelial organs via this technique. See Table 1 for the list of genes discussed below.
Table 1.
Bona fide and putative salivary gland progenitors
| Progenitor cell marker | Lineages traced in developing/adult organs | Phenotype from gene ablation/mutation |
|---|---|---|
| ASCL3 | SG - Constitutively active Cre labels duct and acinar cells (Arany, et al., 2011; Bullard, et al., 2008; Rugel-Stahl, et al. 2012) | SG - No phenotype in Ascl3-deficient SG (Arany, et al., 2011) |
| Olfactory epithelium – gives rise to microvillar cells and Bowman’s gland (Yoshida, et al., 2001; Weng, et al. 2016) | Olfactory epithelium - Ascl3-deficient mouse lacks the non-neuronal microvillar and Bowman’s gland support cells (Weng, et al. 2016) | |
| KIT | SG – duct cells (adult reported; Emmerson et al., 2018). | SG - Kit-deficient E14 SG (Kitw/w) has reduced epithelial branching (Lombaert, et al., 2013) |
|
Olfactory epithelium (Goss, et al. 2015) Lymphatics (Stanczuk, et al. 2015) |
Kidney - reductions in ureteric bud branching and nephrons (Kitw/w and via inhibition of c-kit tyrosine phosphorylation; Schmidt-Ott, et al., 2006) | |
| KRT5 | SG - acini, ducts, myoepithelial cells of fetal gland (adult not reported; Knox, et al., 2010) | SG - No Krt5-deficient SG studies reported |
|
Lacrimal gland – duct and myoepithelial cells in adult gland (fetal not reported; Farmer et al. 2017) Developing trachea and lung airway epithelia (Rock, et al. 2009) |
Skin – Krt5(−/−) die shortly after birth and exhibit skin blistering arising from basal cell cytolysis (Peters et al. 2001) Human mutations in KRT5 and KRT14 cause Epidermolysis bullosa simplex (Peters et al. 2001) |
|
| KRT14 | SG – acini (fetal only), ducts, myoepithelial cells (fetal and adult) (Lombaert, et al. 2013; Patel, et al., 2014; Kwak, et al. 2016) | SG - No Krt14-deficient SG studies reported |
|
Skin (Mascre, et al. 2012) Cornea – epithelium (Di Girolamo, et al. 2015) Developing trachea and lung (Rock, et al. 2009) |
Skin – Krt14(−/−) does not lead to the ablation of a basal cell cytoskeleton (Krt15 compensation mechanism; Peters et al. 2001) Human mutations in KRT5 and KRT14 cause Epidermolysis bullosa simplex (Peters et al. 2001) |
|
| KRT15 | SG – not reported | SG - No Krt15-deficient SG studies reported |
| Hair follicle – bulge and secondary hair germ (Ito, et al. 2005; Wang, et al., 2011; Morris, et al. 2004) | No Krt15-deficient studies reported | |
| KRT19 | SG – not reported | SG - No Krt19-deficient SG studies reported |
|
Exocrine pancreas, liver – duct cells (Means, et al., 2008) Stomach, intestine (Means, et al., 2008) |
No Krt19-deficient studies reported | |
| LGR4/LGR5/LGR6 | SG – not reported | SG – Lgr5 KO – fusion of tongue to floor of oral cavity, SG phenotype not reported (Morita, et al. 2004) |
|
Ovary (Ng, et al. 2014) Kidney nephron (Barker, et al. 2012) Skin and hair follicle (Jaks, et al. 2008) Intestine (Barker and Cleavers, 2010) Stomach (Barker, et al. 2010) |
Kidney - dilated kidney tubules and ectatic Bowman’s spaces in Lgr4 KO (Kinzel, et al. 2014) Skin – reduced basal cell proliferation and hair follicles in Lgr4 KO (Kinzel, et al. 2014). No effect on epidermal repair in Lgr6 KO (Jiang, et al. 2017) Intestine – loss of stem cells in Lgr4 KO (Kinzel, et al. 2014) and gastrointestinal tract dilation (Morita, et al. 2004) Lgr4 and Lgr5 KO are perinatal lethal (Kinzel, et al. 2014; Morita, et al. 2004) |
|
| P63 | SG – not reported | SG - aplasia in KO (Yang A, et al., 1999) |
|
Prostate (fetal) (Pignon, et al. 2013) Bladder (fetal) (Pignon, et al. 2013) Colorectal epithelium (fetal) (Pignon, et al. 2013) |
Skin – absence of squamous epithelia and derivatives in KO (Yang A, et al., 1999; Senoo, et al. 2007) Limb – truncations in KO (Yang A, et al., 1999) Craniofacial - defects in KO (Yang A, et al., 1999) Mammary and lacrimal glands – absent in KO (Yang A, et al., 1999) |
|
| PAX6 | SG – not reported | SG - abnormal development in the KO (Jaskoll, T. et al., 2002) |
|
Cornea and lens (Lin, et al. 2016) Limbs (fetal) (Li, et al. 2015) |
Eye – impaired retina, lacrimal gland and eye development in the KO (Remez, et al. 2017; Marenkova, et al. 2000) | |
| SOX2 | SG – fetal; acini, ducts (Arnold, et al., 2011; Emmerson et al. 2017), adult; acini only (Arnold, et al., 2011; Emmerson et al. 2018) |
SG – (fetal) reduced epithelial branching in conditional KO (K14CreERT2; Sox2fl/fl; Emmerson et al. 2017) SG – (adult) loss of acini in conditional KO (Sox2CreERT2; Sox2fl/fl and Sox2CreERT2; R26DTA; Emmerson et al. 2018) |
|
Stomach (Arnold, et al., 2011) Cervix (Arnold, et al., 2011) Anus (Arnold, et al., 2011) Testes (Arnold, et al., 2011) Lens (Arnold, et al., 2011) Eosophagus (Arnold, et al., 2011) |
Dermal papilla of hair follicle – no phenotype in conditional KO (K14Cre; Sox2fl/fl; Lesko, et al. 2013) Merkel cells – decreased number in conditional KO (K14Cre; Sox2fl/fl; Lesko, et al. 2013) |
|
| SOX9 | SG – fetal; acini, ducts (Chatzeli, et al. 2017) | SG - reduced branching the KO (Krt14CreERT2; Sox9fl/fl) |
|
Liver (Furuyama, et al. 2011) Exocrine pancreas (Furuyama, et al. 2011; Seymour, et al. 2007) Intestine (duodenum) (Furuyama, et al. 2011) Mammary gland (Malhotra, et al. 2014) Lung (Rockich, et al. 2013; Chang, et al. 2013) Tendon (Soeda, et al. 2010) |
Lacrimal gland – branching defect in conditional KO (Sox9fl/fl; Le-Cre+;
Chen, et al. 2014) Harderian and meibomian glands - reduced acini and loss of epithelia in conditional KO (Sox9fl/fl; Le-Cre+; Chen, et al. 2014) Skin - missing hair in eyelids and facial skin in conditional KO (Sox9fl/fl; Le-Cre+; Chen, et al. 2014) |
|
| SOX10 | SG – not reported | SG - No Sox10-deficient SG studies reported |
| No lineage tracing studies reported |
Lacrimal gland – reduced acini and branching defect in conditional KO (Sox10fl/fl; Le-Cre+;
Chen, et al. 2014) Harderian glands - reduced acini and branching defect in conditional KO (Sox10fl/fl; Le-Cre+; Chen, et al. 2014) |
A) Progenitor markers in Developing SG
Intermediate filaments: Keratin-5, 14, 15 and 19
Basal epithelial cells marked by the acidic cytokeratins KRT5 and 14 have been shown to mark progenitor cells of numerous epithelial tissues including skin, cornea, developing trachea, lung airway epithelia, bladder and salivary glands (Colopy et al., 2014; Cotsarelis et al., 1989; Di Girolamo et al., 2015; Knox et al., 2010; Lombaert et al., 2013; Moll et al., 1982; Peters et al., 2001; Rock et al., 2009). In the SG, genetic lineage tracing using inducible and non- inducible Krt14 or Krt5 promoters, have demonstrated that the KRT14+/KRT5+ cells of the invaginating oral epithelium contribute extensively to acinar, ductal and myoepithelial cells (Knox et al., 2010; Lombaert et al., 2013). These cells - and consequently SG morphogenesis - have been shown to be regulated by a number of signaling pathways including those of the HIPPO and retinoic acid (RA) family. YAP, a negative regulator of the HIPPO pathway involved in organ size and cell proliferation (Wu et al., 2003), is enriched in the nucleus of KRT14+ cells in the ducts during early stages of SG development (E13.5). Ablation of Yap, and thus activation of the HIPPO pathway, in KRT5+/KRT14+ cells before SG initiation reduces the production of Epiregulin, an ErbB receptor ligand which is involved in cell fate control (Gregorieff et al., 2015) and is required for KRT5+/KRT14+ cell expansion, thereby perturbing epithelial branching and duct formation (Szymaniak et al., 2017). In addition, restriction of nuclear Yap localization, via deletion of Lats1 and Lats2, results in the expansion of KRT5+/KRT14+ cells, aberrant enlargement of ducts and reduced end bud formation, further illustrating the requirement of controlled YAP signaling during lineage formation and epithelial morphogenesis in the developing SG (Szymaniak et al., 2017). Similar to the HIPPO pathway, the RA pathway also regulates proliferation of KRT14+ progenitors. RA signaling occurs in early SMG development (from E10.5), where it plays a role in maintaining SG progenitor cells as well as epithelial morphogenesis. RA-deficient mice exhibit SMG developmental delay (Wright et al., 2015) and similarly, blocking RA signaling in isolated epithelia with the pan-RAR antagonist BMS 493 results in reduced branching morphogenesis (Wright et al., 2015) and repressed cell proliferation (Abashev et al., 2017). Using RAR isoform-specific agonists and inhibitors DeSantis, et al. demonstrated isoform-specific roles for retinoic acid receptor (RAR) signaling in maintenance of KRT14+ cells, where RARγ is necessary, but not sufficient, to maintain KRT5+ cells, whereas RARα agonism reduces the number of KRT5+ cells and promotes differentiation (DeSantis et al., 2017).
As expression of KRT14 and KRT5 segregates during development, with the majority of KRT14+ cells in E13 end buds being deficient in KRT5 (Lombaert et al., 2013) (Figure 3), it remains unclear if cells that co-express KRT5 and 14 or those that solely express one but not the other keratin continue to contribute to the different epithelial lineages. Given recent studies showing cells solely expressing KRT5+ or KRT14+ cells but not both are regulated by different mechanisms, these cells likely differ in their progenitor cell properties. Maintenance of KRT5+ but not KRT14+ basal cells is dependent on parasympathetic nerves: acetylcholine secreted by the nerves activates muscarinic/EGFR signaling to promote KRT5+ cell self-renewal (Knox et al., 2010). Intriguingly, KRT5+ cells themselves initiate and maintain their own innervation by producing WNTs that act on neural precursors to promote ganglion formation at the primary duct, a location rich in these cells (Knosp et al., 2015). In contrast, Lombaert and co-workers demonstrated that KRT14+ cells in the end buds expand in response to FGFR2b signaling but are not depleted by the absence of innervation (Lombaert et al., 2013). Thus, multiple mechanisms including nerves, FGF, retinoic acid and Hippo signaling regulate KRT5+ and KRT14+ cells.
In addition to KRT5 and KRT14, two other keratins, KRT15 and KRT19, have also been postulated to mark progenitors in the SG due to their known status as progenitors in other epithelial/glandular organs. KRT15 marks a progenitor cell of the hair follicle (Ito et al., 2005; Lyle et al., 1998; Morris et al., 2004; Wang et al., 2011) and is expressed in the ductal region of the developing SG, similar to KRT5 (Knox et al., 2010; Lombaert et al., 2011). Although genetic lineage tracing of KRT19+ cells has not been performed in the SG, studies in the developing exocrine pancreas and liver reveal that KRT19+ cells contribute exclusively to the ductal compartment (Means et al., 2008), suggesting that these cells are specified to produce ductal cells in epithelial organs. Consistent with this outcome, KRT19 expression in the SG is also limited to the presumptive ducts of the developing SG and the luminal ductal cells of adult SG. In developing SG, inhibition or ablation of EGFR or inhibition of the vasoactive intestinal peptide (VIP)/PKA pathway depletes KRT19+ cell numbers and results in abnormal duct morphogenesis (Jaskoll and Melnick, 1999; Knox et al., 2010; Nedvetsky et al., 2014), supporting the idea that KRT19+ cells are crucial for efficient duct development.
Growth factor receptors: KIT and LGR4/5/6
A number of growth factor receptors and co-receptors have been shown to mark progenitors in developing organs. One of the most well-characterized is KIT, a receptor tyrosine kinase encoded by the oncogene c-kit that functions in multiple cellular processes including cell proliferation, differentiation, cell survival and migration through interaction with its ligand Stem Cell Factor (SCF) (Lennartsson and Ronnstrand, 2012). KIT was originally identified as a marker of hematopoietic stem cells (Shiohara et al., 1993) and was subsequently used as a marker of progenitor cells in other developing organ systems including the kidney (Schmidt-Ott et al., 2006) and salivary gland (Lombaert et al., 2013). However, despite an allele for genetically tracing KIT+ cells being readily available (van Berlo et al., 2014), to date, only the developing lymphatics (Stanczuk et al., 2015) and olfactory epithelium (Goss et al., 2015) have been reported to be derived, at least in part, from KIT+ cells. Support for KIT as a marker of progenitors in the SG is derived from its expression by a subset of KRT14+ progenitors, as well as studies showing reduced epithelial branching of E14 SG from mice deficient in Kit (Kitw/w) (Lombaert et al., 2013). Indeed, the expression pattern of KIT is similar to KRT14 in that it is expressed in both pre-acini and presumptive ductal structures and becomes restricted to the end buds by E14 (Lombaert et al., 2013; Wang et al., 2014). Given the requirement for FGF10/FGFR2b in the invagination and expansion of the primordial epithelium, it comes as no surprise that KIT+ and KRT14+ cells are both regulated by FGF10/FGFR2b signaling (Lombaert et al., 2013; Patel et al., 2014). A recent study has implicated mesenchymal-epithelial communication and epigenetic control via the miRNA miR-133b-3p and DIP2B in expansion of this KIT+K14+ population during organogenesis. Exosome transport of microRNA between the mesenchyme and epithelium is essential for SMG development and knockdown of the mature mesenchymal microRNA miR-133b-3p resulted in decreased end-bud morphogenesis and reduced proliferation of KIT+ progenitor cells. miR-133b-3p downregulates the target gene Dip2b in KIT+ progenitor cells, subsequently influencing cell cycle, and thus acts as a epigenetic regulator of KIT+K14/K5- progenitor cell expansion during SG morphogenesis (Hayashi et al., 2017). However, despite this overlap, a population of KIT+ cells remains KRT14-negative in the pre-acinar cells of the SMG (Lombaert et al., 2013), suggesting that KIT and KRT14 diverge to mark distinct progenitor populations in the SG. In support of this segregation, an inverse expression profile to Krt14 was apparent for Kit expression following RA inhibition, where BMS 493 reduces expression of Kit in isolated epithelia explants (Abashev et al., 2017). In addition, KRT14 but not KIT is expressed by emerging SMA+ myoepithelial cells, which in the lacrimal gland give rise only to themselves (Farmer et al., 2017).
The developing SG also expresses a number of other putative progenitor markers that are components of pathways important to salivary gland development. This includes the Leucine-rich repeat containing G protein-coupled receptors (LGR4, 5 and 6), components of the WNT signaling pathway, where WNT functions in SG duct development and gangliogenesis (Knosp et al., 2015; Patel et al., 2011). LGR5 marks progenitor cells in the developing kidney, where they contribute to nephron formation (Barker et al., 2012) and the embryonic ovaries where they give rise to the ovary surface epithelium (Ng et al., 2014). Genetic lineage tracing experiments have demonstrated that LGR5+ cells can maintain all cell lineages of the hair follicle (HF) and generate an entire new follicle (Jaks et al., 2008) and contribute to the intestinal epithelium (Barker and Clevers, 2010) and the stomach (Barker et al., 2010). In the SG, LGR5 expression is enriched in the primary duct of the developing SMG (similar to KRT5 and SOX2), as well as in the mesenchyme (Salivary Gland Atlas, NIDCR). Although it is not known whether LGR5 marks a progenitor population in the SG, SOX2 and LGR5 co-localize in the mouse pylorus (Arnold et al., 2011) and human minor salivary gland mesenchymal stem cells (HMSGMSCs) that possess self-renewal and multipotent ability express LGR5 (Lu et al., 2015). Although WNT signaling is a regulator of SG development and is crucial to maintain SG stem cell-containing organoids in culture (Maimets et al., 2016), whether LGR5+ cells or LGR5 function are required for SG development has not been reported. However, the mild to normal phenotypes observed in other organs of the Lgr5-deficient mouse model suggest that LGR5 itself is not essential to organism development (Kinzel et al., 2014; Morita et al., 2004). The closely related receptor LGR4 is essential for renal development (Kato et al., 2006) and is expressed throughout the developing and adult salivary gland epithelium and mesenchyme (Salivary Gland Atlas, NIDCR; Van Schoore et al., 2005). Ablation of Lgr4 results in a loss of the crypt cells of the intestine (de Lau et al., 2011), impaired intestinal, kidney and skin development (Kato et al., 2006; Kinzel et al., 2014; Mohri et al., 2008; Mohri et al., 2011) and a failure to generate intestinal organoids in culture, a phenotype exacerbated when in combination with Lgr5 knockout (de Lau et al., 2011). LGR6+ cells mark stem cells in the hair follicle that contribute to the skin lineages (Snippert et al., 2010), the nail and are essential for digit tip regeneration (Lehoczky and Tabin, 2015). However, deletion of Lgr6 demonstrates that LGR6+ cells are dispensable for epidermal repair (Jiang et al., 2017).
Transcription factors: ASCL3, SOX2, SOX10, SOX9, P63, PAX6
The basic helix-loop-helix transcription factor ASCL3 (achaete-scute family bHLH transcription factor 3) and its family members ASCL1 and 2 are essential determinants of cell fate and differentiation in multiple tissues (Battiste et al., 2007; van der Flier et al., 2009). ASCL3 was originally named Salivary Glands 1 (Sgn1) due to its prominent expression in a subset of striated and excretory duct cells in the adult mouse SMG (Yoshida et al., 2001). Similarly, in the developing SG expression of Ascl3 (begins at E14) is also localized to cells in the ductal regions. This location in the ducts correlates with the long-believed notion that the SG progenitors resided in the ductal compartment. Consistent with this, using a non-inducible recombinase under the control of the Ascl3 promoter (Ascl3EGFP-Cre/+) crossed to a Rosa26R reporter, Bullard and colleagues determined that ASCL3+ cells give rise to ductal and acinar cells during development (Bullard et al., 2008). However, as not all acinar and duct cells were labeled by Ascl3 induction, the authors suggested the presence of other progenitor cells that likely contribute to salivary gland development. This was shown to be the case when basal epithelial cells expressing KRT5 or KRT14 were also shown to contribute to all acinar, ductal and myoepithelial cells (Knox et al., 2010; Lombaert et al., 2013). However, whether KRT5 and KRT14 cells contribute to the synthesis of ASCL3+ cells or if this is a population of cells that arises from another progenitor cell type remains to be investigated. Furthermore, ASLC3+ cells exclusively gives rise to microvillar cells and Bowman’s glands of the olfactory epithelium (Weng et al., 2016).
Genetic lineage tracing has also identified the transcription factor SOX2, an essential regulator of pluripotency of embryonic stem cells (ESCs), as a marker of progenitor cells for the duct and acinar lineages in the developing SMG and SLG (PG not investigated (Emmerson et al., 2017)), as well as many other epithelial tissues (Arnold et al., 2011). We recently reported that despite SOX2 being expressed throughout the oral epithelium, SOX2 has an essential role in the generation of acini: genetic ablation of epithelial Sox2 prior to gland ontogenesis impairs the production of SOX10+ acini but not ducts, in part, through cell death (Emmerson et al., 2017). This lineage specificity was postulated to be mediated by direct regulation of Sox10, a known regulator of acinar cell differentiation in the lacrimal glands (Chen et al., 2014). Furthermore, we showed that SOX2 expression and SOX2+ cell proliferation is regulated by neuronal acetylcholine-muscarinic signaling, demonstrating a novel role for parasympathetic nerves and SOX2 in directing a specific lineage during SG development (Emmerson et al., 2017).
Another member of the SOX family, SOX9, marks multipotent progenitor cells in the developing pancreas, mammary gland, lung, liver, duodenum and tendons (Chang et al., 2013; Furuyama et al., 2011; Jo et al., 2014; Malhotra et al., 2014; Rockich et al., 2013; Seymour et al., 2007; Soeda et al., 2010). Similarly, SOX9+ cells have recently been shown to be a bona fide progenitor cell population that gives rise to cells of the acinar and ductal lineages during SG development (Chatzeli et al., 2017). Furthermore, conditional ablation of Sox9 using the Krt14 promoter arrested acinar and ductal morphogenesis and impaired specification of distal putative progenitors (marked by Myb and SOX10), indicating an essential role for this transcription factor in morphogenic processes and cell fate. This role is consistent with other studies showing Sox9 is required for epithelial branching in the developing lung (Chang et al., 2013; Rockich et al., 2013), kidney and ocular glands (Reginensi et al., 2011), as well as the development of the secretory acinar and myoepithelial cells of the lacrimal and harderian glands (Chen et al., 2014). Whether SOX9 continues to mark acinar progenitors and control cell fate in the SG remains to be investigated. In the pancreas SOX9+ cells become lineage restricted over time, contributing solely to the ductal lineage shortly after birth (Kopp et al., 2011), SOX9+ cells in the postnatal mammary gland give rise to estrogen receptor (ER)-negative luminal and basal cells (Malhotra et al., 2014; Wang et al., 2017) and in the hair follicle become restricted to the early bulge progeny of the outer root sheath (ORS) during the tissue growth phase known as anagen (Kadaja et al., 2014).
Although definitive lineage tracing studies have not been performed for SOX10, due to its restricted expression to the pre-acinar cells of the developing lacrimal gland (Chen et al., 2014) and salivary gland (Lombaert et al., 2013; Lombaert and Hoffman, 2010) and its requirement the production of acini and myoepithelial cells during lacrimal gland morphogenesis (Chen et al., 2014), it has been proposed as a marker of acinar progenitor cells in the developing SG. Moreover, SOX9 and SOX10 are co-expressed in these cells, where SOX9 regulates lineage outcomes in the lacrimal glands through SOX10. As SOX10 is also expressed in acinar cells of the adult SG (Ohtomo et al., 2013) (Figure 3) it is likely to be a marker of acinar progenitors throughout development and homeostasis (Lombaert et al., 2013; Lombaert and Hoffman, 2010). Consistent with the enrichment of SOX9 and SOX10 in the acini, and the essential role for FGF10/FGFR2b in acinar cell expansion, both SOX9 and SOX10 are regulated by FGF signaling (Chatzeli et al., 2017; Chen et al., 2014; Lombaert et al., 2013). Ablation of Fgf10, in the developing lacrimal gland results in a depletion of both Sox9 and Sox10 (Chen et al., 2014). In addition, Sox9 expression is severely reduced in the developmental placodes of the premature SGs of Fgf10 knockout mice (Chatzeli et al., 2017). Additional studies in the SG have also shown that SOX10 is reduced in the absence of FGF10 or KIT (Lombaert et al., 2013), indicating a common mechanism for maintenance of progenitors marked by SOX9 and SOX10 in glandular tissues.
Transformation-related protein 63 (Trp63/P63) and Paired box protein-6 (PAX6) which mark progenitors in multiple epithelial tissues, including lacrimal glands, thymus and skin (Finley et al., 2014; Senoo et al., 2007; Yang et al., 1999), are also postulated as progenitors for the SG. P63 and more specifically the NH2- terminal truncated (ΔN) p63 isoform marks basal epithelial cells and myoepithelial cells in the salivary gland (Bilal et al., 2003), as well as basal epithelial cells in the bladder, prostate (Cheng et al., 2006; Pignon et al., 2013; Signoretti et al., 2000), cornea, skin trachea and lung (Mills et al., 1999; Rock et al., 2009; Yang et al., 1998). Although not reported for all epithelial organs, genetic lineage tracing using a non-inducible Cre under the control of the ΔNp63 promoter has established p63 as a progenitor in the developing prostate, bladder and colorectal epithelium (Pignon et al., 2013). Global ablation of p63 or ΔNp63 results in an absence of all squamous epithelia and their derivatives, including the SGs, lacrimal glands and the stratified epidermis of the skin (Yang et al., 1999). These phenotypes result, at least in part, from apparent defects in stem and progenitor cells’ capacity to proliferate or survive (Pellegrini et al., 2001; Senoo et al., 2007; Yang et al., 1999).
PAX6, a protein initially found to regulate neural stem cell self-renewal and differentiation, marks progenitor cells in the developing lens, cornea and lacrimal glands (Li et al., 2015; Lin et al., 2016). PAX6 is essential for ocular organ formation as shown by the absence of eyes and lacrimal gland in Pax6−/− embryos (Hill et al., 1991) and impaired eye and lacrimal gland morphogenesis in embryos heterozygous for Pax6 (Makarenkova et al., 2000; Remez et al., 2017). Although genetic lineage tracing has not been reported in the salivary glands, PAX6+ cells have been identified in developing SG and global ablation of Pax6 results in a reduction in epithelial branching compared to wild type controls (Jaskoll et al., 2002). Whether ablation of Pax6 (or other genes) reflects their role as SG progenitors themselves or as regulators of differentiated epithelial cells requires further investigation.
B) Progenitor cell markers in the adult SG
Epithelial progenitors
KRT14, SOX2 and KIT mark lineage restricted epithelial progenitor cells
The intercalated ducts of the adult salivary glands were originally predicted to harbor a stem cell population capable of giving rise to both acini and ducts (Denny et al., 1993; Ihrler et al., 2004; Redman, 1995). However, a number of recent studies based on genetic lineage tracing have disputed this hypothesis. Kwak et al. utilized an inducible Cre under the control of the Krt14 promotor to demonstrate that cells marked by KRT14 i.e., myoepithelial cells and basal cells located in the intercalated ducts, give rise to cells of the granular convoluted tubules but not intercalated ducts, myoepithelial cells or acinar cells (Kwak et al., 2016). We also recently showed through long term genetic lineage tracing of KIT+ cells that these cells, even after 6 months, contribute solely to the intercalated duct cells and not acinar cells of the homeostatic SG (Emmerson et al., 2018). In concordance with these results, Aure et al. demonstrated that acini give rise exclusively to acini and not to the ductal system. Using a tamoxifen inducible Cre under the control of a Mist1 promoter, where MIST1 labels the acinar cell lineage in mice (Lemercier et al., 1997; Yoshida et al., 2001), they demonstrated that acinar cells are replaced during homeostasis and after injury by labeled acinar cells and not by unlabeled cells arising from the ducts. Although from this study it was postulated that acinar cell replacement is mediated by self-duplication, whether a bona fide progenitor contributes to the acinar lineage was not known. We recently reported the presence of an acinar progenitor population, marked by SOX2 (Arnold et al., 2011; Figure 3), that gives rise to differentiated MUC19+ acinar cells of the SLG during homeostasis and after injury (Emmerson et al., 2018). Furthermore, ablation of SOX2+ cells results in a striking loss of acinar cells and deletion of Sox2 impairs acinar cell replenishment after radiation-induced damage, suggesting that SOX2+ cells are the sole progenitors of the murine SLG. A recent study using the single cell colony method suggested SOX2+ cells may also be progenitors for human SG (all three major SG have a subpopulation of acini that are SOX2+ (Emmerson et al., 2018)) as these colonies expressed SOX2 and engrafted into SCID mice (Lu et al., 2015). Similar to the developing SG, SOX2+ cells and SOX2-mediated acinar cell replacement are dependent on functional parasympathetic innervation, with administration of acetylcholine muscarinic mimetics being sufficient to drive acinar cell regeneration in mice and promote SOX2 expression and the acinar lineage in human SG (Emmerson et al., 2018). As parasympathetic nerves and SOX2+ cells are diminished in irradiated human SG (Emmerson et al., 2018), tissue degeneration may be due to a loss of progenitor cells and the cues that regulate them. However, many more studies are needed to determine if these are the sole progenitors or if other subsets of progenitors exist.
Mesenchymal progenitor cells
Hematopoietic stem cell markers: SCA1, KIT, THY1 and CD49f
The mesenchyme surrounding the salivary epithelium is derived from the neural crest and likely contains progenitor cells capable of contributing to the mesenchyme and/or other cell types, however, to date no lineage tracing has been performed to confirm this. In support of this theory, a number of putative progenitor cell surface receptors have been identified in murine salivary mesenchyme, including the hematopoietic stem cell markers SCA1 (stem cell antigen 1, expressed in mouse but not human HSCs), KIT, CD49f (integrin alpha 6; (Hisatomi et al., 2004; Okumura et al., 2003) and THY1 (thymocyte antigen 1; (Matsumoto et al., 2007; Sato et al., 2007) that possess stem/progenitor cell properties. For example, murine SCA1+KIT+CD49f+ cells have the ability to clonally expand under stress conditions, suggesting they have replenishing capacity (David et al., 2008), and are able to differentiate into hepatic, pancreatic or salivary-like cells (Hisatomi et al., 2004; Okumura et al., 2003). When cultured SCA1+KIT1+ cells form salispheres that have the ability to branch (Lombaert et al., 2008). More recently, CD49f+THY1+ cells were shown to have proliferation potential, form organoids in culture and like SCA1+KIT+CD49f+ cells could also differentiate into pancreatic-like and amylase-expressing cells (Sato et al., 2007). Histatomi et al. report that SCA1+ and KIT+ cells are rare in the healthy SG but are found in clusters in the ducts following ligation injury, suggesting that they proliferate and expand under stress or injury conditions in order to replenish the injured tissue (Hisatomi et al., 2004). Indeed, when SCA1+KIT+ cells are transplanted into irradiated SGs they are able to successfully regenerate the gland, forming both acinar and ductal structures (Lombaert et al., 2008). The fact that KIT+ cells derived from SG can transdifferentiate into hepatic and pancreatic lineages (Hisatomi et al., 2004; Okumura et al., 2003) may indicate that they are truly multipotent and are, in fact, regulated by their microenvironment or niche to transdifferentiate into different lineages besides SG epithelial tissue. In addition, far fewer KIT+ cells are required to be transplanted to rescue radiation-induced damage than CD133+, CD49f+ or CD29+ cells, demonstrating their potency as a true progenitor (Nanduri et al., 2011). Furthermore, when KIT+ cells are serially transplanted they are able to expand even after several rounds of regeneration, demonstrating their ability to self-renew as a progenitor cell (Lombaert et al., 2008).
Classical mesenchymal stem cell (MSC) markers
There are a number of markers that are internationally recognized as classical MSCs, including CD24, CD29 and CD44 (Coulombel et al., 1997). A number of studies have reported the successful isolation of putative progenitor cells expressing these markers from human SG via clonal assay (Rotter et al., 2008; Schwarz and Rotter, 2012; Tatsuishi et al., 2009). Following culture the cells resemble mesenchymal stem cells (MSCs) and express classical MSC markers, including CD29, CD44 and CD90. Furthermore, these cells are able to differentiate into osteogenic, adipogenic and chondrogenic lineages (Rotter et al., 2008; Schwarz and Rotter, 2012). CD24+CD29+ cells can proliferate and expand ex vivo and, crucially, can rescue radiation-induced SG dysfunction in vivo (Nanduri et al., 2014). Interestingly, when this CD24+CD29+ population also expresses KIT, and is injected into irradiated SMG they not only recovered saliva flow but also improved tissue architecture (Nanduri et al., 2013). However, in this study the authors do not demonstrate that the isolated cells are directly incorporated into the regenerated epithelia. Indeed, since endothelial cells are sensitive to radiation and vasculature is often adversely affected by radiation therapy (Ying et al., 2007) and vascularization of the tissue was also notably improved in this study it cannot be ruled out that this CD24+CD29+KIT+ population may be, at least in part, also influencing the vasculature of the injured gland via release of VEGF, for example (Beckermann et al., 2008), and thus indirectly improving tissue regeneration.
The report that the highly proliferative SCA1+KIT+CD24+ population can rescue SMG function (saliva flow) and architecture (functional acini) following radiation-induced damage with the addition of glial cell line-derived neurotrophic factor (GDNF) (Xiao et al., 2014) suggests that the niche and external cues are essential for these so-called SG MSCs to elicit their positive effects. Since cell tracing has never been performed in any such experiments it cannot be ruled out that these populations may not be contributing directly to restoration of the SG epithelium, but merely altering the niche or microenvironment or providing trophic cues to positively enhance epithelial regeneration. Since GDNF is neuroattractive and improved innervation of the SG following radiation-induced damage has been shown to improve epithelial regeneration (Knox et al., 2013) such outcomes could be the result of improvements to the niche, such as occurs during development (Ferreira and Hoffman, 2013). Indeed, a recent study demonstrated that GDNF itself does not protect SG stem cells from radiation-induced damage directly (Peng et al., 2017), suggesting that such outcomes are the result of improvements to the supporting niche. Further tracing experiments are required to unequivocally demonstrate that such isolated cells are directly regenerating damaged SG tissue. Until recently it was thought that CD34 marked hematopoietic stem cells but was a negative marker of MSCs, but recent studies have suggested that this is due to an artefact of cell culture and that a small CD34+ MSC population exists in multiple tissues (Lin et al., 2012; Sidney et al., 2014). A recent study found that CD34 is expressed by MSCs of all three of the major human SGs. Furthermore, these cells express genes involved in ERK, FGF and PDGF signaling, pathways essential to salivary gland development and regeneration, and when transplanted engraft into murine SGs (Togarrati et al., 2017). The co-expression of CD44 in these cells is in agreement with the results of Bahn et al. (2013) and both CD34 and CD44 are expressed by murine SG salispheres, the cells of which can rescue SG function following radiation injury (Banh et al., 2011; Lombaert et al., 2008).
Table 2 lists all markers used to enrich for mesenchymal salivary gland progenitor cell populations.
Table 2.
Cell surface markers used to enrich for mesenchymal salivary gland progenitor cells
Are long lived cells also progenitors?
Early studies utilized a number of methods to identify stem cells in adult mouse SGs, including a principle property of progenitor cells that they are slow-cycling and replicate infrequently, thus retaining DNA nucleotide labels such as Bromodeoxyuridine and Ethynyldeoxyuridine (BrDU and EdU). Experiments to mark these so-called label retaining cells (LRCs) have led to the discovery of progenitor populations in multiple epithelial tissues, including the skin (reviewed in Terskikh et al., 2012), sweat glands (Leung et al., 2013; Lu et al., 2012), teeth (Seidel et al., 2010), pancreas (Teng et al., 2007) and intestine (Buczacki et al., 2013). Original observations using single injections of radiolabeled thymidine into adult mice or rats (6–7 weeks of age) found LRCs to reside in intercalated ducts and excretory ducts but not acinar cells (Man et al., 2001; Zajicek et al., 1985). Other recent studies in similarly aged rodents using BrdU delivered over 4 or 7 days followed by a chase of 7–8 weeks have found LRCs throughout the gland in both acinar, ductal and myoepithelial cells, as well as the connective tissue, suggesting a larger number of cells are long lived and/or have progenitor cell-like properties than previously thought (Kim et al., 2008; Kimoto et al., 2008). Up until recently the identity of these LRCs was not known, however, a study by Chibly and colleagues determined that LRCs in both acinar and ductal structures co-localize with the embryonic SG progenitor markers KRT5, KRT14, and SOX2 protein and Kit mRNA (Chibly et al., 2014). Of these, only KRT5 has not yet been reported to contribute to the adult tissue via lineage tracing experiments. However, intriguingly, these studies contrast to a recent report using a H2BGFP mouse model showing the presence of actively dividing pools of progenitor cells in the intercalated and excretory ducts (Kwak and Ghazizadeh, 2015). As H2B can also acts in the DNA damage response (Kim et al., 2008), it remains to be determined whether these cells actively contribute to the SG.
Are proliferating salisphere cells the true stem cells of the SG?
In an effort to translate these finding toward a clinical therapeutic a number of studies have attempted to isolate SG progenitor cell populations from adult human SG, based on markers identified in murine studies (Feng et al., 2009) or based on in vitro assays, as described earlier (Jeong et al., 2013; Lu et al., 2015; Okumura et al., 2012; Rotter et al., 2008; Schwarz and Rotter, 2012; Tatsuishi et al., 2009). Feng et al. collected tissue from human PG and SMG and by optimizing conditions used for culture of murine cells were able to successfully culture human salispheres that expressed KIT. These human-derived salispheres are able to branch when transferred to a 3D matrix (Okumura et al., 2012), in a manner similar to embryonic mouse epithelial rudiments (Wei et al., 2007), suggesting stem cell-like properties. Conversely, Jeong et al. developed a human SG progenitor culture system that negated the requirement for cell sorting and surface markers, and demonstrated that the adherent cells in their culture exhibited MSC-like characteristics and could rescue acinar structure and hyposalivation in irradiated rats. Similarly, Rotter et al. used the clonal assay technique to enrich for progenitors that could differentiate into multiple lineages, demonstrating their multipotency. However, until transplantation studies are performed, we remain in the dark regarding the ability of these cells to contribute to the salivary gland in vivo.
2. Is salivary gland cancer derived from salivary progenitors?
Emerging evidence points toward the existence of cancer initiating cells (CICs) that possess multipotency and self-renewal capacity, characteristics attributed to stem cells. CICs can both initiate and maintain a tumour and are often resistant to chemotherapy, thus enabling tumour recurrence, often many years later (reviewed in Adams et al., 2013). Cancers of the SG are the most heterogeneous in humans, consisting of 24 distinct pathological sub-types (Gillespie et al., 2012) and are notoriously difficult to treat given their poor response to chemo- and radio-therapy (Laurie and Licitra, 2006). As such, despite the high survival rate in the first 5–10 years, their ability to evade treatments combined with their high recurrence rate leads to low long-term survival, implicating the possibility of a CIC population capable of being reactivated following therapy. As such, a better understanding of salivary gland malignancies and these possible CICs is essential for the generation of effective therapies.
CICs in human head and neck cancers (HNCs) were first reported in 2007 (Prince et al., 2007). These expressed CD44 and showed high tumorigenicity in NOD/SCID mice. Subsequently, expression of aldehyde dehydrogenase (ALDH), a marker originally used for CICs in the breast (Ginestier et al., 2007) was reported to be a characteristic of HNC CICs (Clay et al., 2010) and salivary CICs with high metastatic potential (Sun and Wang, 2010). More recently a population of ALDH expressing CD24+/CD44+ cells present in human head and neck squamous cell carcinomas (HNSCCs) (Han et al., 2014) and salivary gland malignant neoplasms (SGMNs) (Soave et al., 2013) was reported to exhibit stemness characteristics (Adams et al., 2015), similar to CICs of the pancreas and breast (Al-Hajj et al., 2003; Li et al., 2007). These cells were resistant to chemotherapeutic agents such as Cisplatin and, when injected into nude mice, induced large tumors (Han et al., 2014). WNT/β-catenin signalling may play a role in regulating these cells as activity correlates with expression of CD44 in SG cancers and treatment of CICs with a WNT/β-catenin active small molecule inhibitor, LF3, blocked their self-renewal capacity (Fang et al., 2016). Indeed, WNT inhibition supresses CIC stemness and induces cellular senescence in SG squamous cell carcinoma (SCC) (Ramachandran et al., 2014), whereas activation of the WNT/β-catenin pathway, via a β-catenin gain of function approach, induces a rapidly growing, aggressive phenotype (Wend et al., 2013). While these studies did not specifically investigate the association between WNT/β-catenin and CD24/CD44 expression in SG CICs, this result suggests that dysregulated WNT/β-catenin signalling may be, at least in part, responsible for the expansion of SG CICs and tumor progression. As WNT/β-catenin signalling has been reported to protect SG function during therapeutic radiation to treat head and neck cancer, presumably by preserving adult SG stem cells (Hai et al., 2012), this pathway may also protect CICs from elimination by radiotherapy. Thus, signalling pathways that control SG stem cells in the healthy gland must be carefully regulated if they are to act as a therapeutic approach and not lead to secondary tumors.
Other markers of SG progenitors have also been reported in tumour tissue; however, whether the expression of these markers specifically correlates with cellular attributes of CICs is yet to be determined. In spite of this, high expression of many of these markers is linked to metastasis and poor patient survival. An example of this is SOX2, which is often aberrantly expressed in HNCs (Dong et al., 2014; Ge et al., 2010; Lee et al., 2014; Li et al., 2014; Schrock et al., 2014) with high SOX2 expression being linked to distant metastasis in adenoid cystic carcinoma (ACC) of the salivary gland (Dai et al., 2014) and high tumour grade in salivary cancer (Sedassari et al., 2017) as well as head and neck cancers in general (Dong et al., 2014). Elevated SOX2 expression is also associated with increased resistance to chemotherapy agents, although whether this is a direct effect is uncertain (Schrock et al., 2014). However, silencing SOX2 in breast cancer cells in culture leads to increased sensitivity to the chemotherapeutic agent paclitaxel and reduction in mammosphere formation (Mukherjee et al., 2017), suggesting a direct effect of SOX2 expression on chemoresistance in cancer cells. In addition, elevated or ectopic SOX2 expression has also been associated with the progression of other cancers, including skin squamous cell carcinoma (SCC) (Boumahdi et al., 2014), glioblastoma (Annovazzi et al., 2011), laryngeal squamous cell carcinoma (LSCC) (Yang et al., 2014), bladder cancer (Zhu et al., 2017) and small-cell lung cancer (SCLC) (Hussenet et al., 2010). Interestingly, other members of the SOX family, which are expressed in salivary glands, may also be linked to cancer progression. SOX9 is overexpressed in a number of cancers, where it promotes cell proliferation and inhibits senescence (Matheu et al., 2012), while SOX10 expression appears to be involved in transcriptional programming and progression of salivary ACC (Ivanov et al., 2013; Ohtomo et al., 2013). c-Myc, alongside Sox2, Klf4 and Oct3/4 is expressed by ESCs and as such is intrinsically involved with pluripotency (Takahashi and Yamanaka, 2006). c-Myc is also highly expressed in salivary gland carcinoma, compared to healthy SG tissue (Schoenhals et al., 2009) and in conjunction with Transforming Growth Factor alpha (TGFα) promotes adenocarcinoma (Amundadottir et al., 1995). Thus, cells expressing high levels of both SOX2 and c-Myc may act as an undifferentiated cancer cell, with the ability to rapidly proliferate and metastasize.
Since SOX2 is regulated by acetylcholine derived from autonomic nerves innervating the developing and adult salivary gland (Emmerson et al., 2018; Emmerson et al., 2017), it would be of therapeutic interest to know if innervation and neuronal signalling also regulates SOX2 expression in salivary cancer. To date no studies have investigated the correlation between SOX2, innervation of salivary gland tumors and cancer progression. A number of recent studies have demonstrated the interaction between nerves and cancer cells known to overexpress SOX2 in other cancers such as those of the stomach and prostate (Magnon et al., 2013; Zhao et al., 2014) (Zhao et al., 2014). As denervation suppresses tumor progression in gastric cancer (Zhao et al., 2014), pancreatic cancer (Saloman et al., 2016) and fibrosarcoma (Lackovicova et al., 2011), nerves may provide trophic cues required for cancer development and for maintenance of SOX2, although expression of SOX2 in these denervated conditions has not been examined. Given the potential success of anti-neurogenic therapeutics in breast and gastric cancer (Hondermarck, 2012; Miknyoczki et al., 2002), modulating innervation and neuronal signalling in salivary tumors may be a therapeutic approach to reduce SOX2 expression and improve patient survival.
Since p63 is considered both a tumour protein and a progenitor cell marker one would expect a high association between p63 and SG cancer. p63 is strongly expressed in basal cell adenoma, adenoid cystic carcinoma (ACC) and polymorphous low-grade adenocarcinoma (PLGA) and these cells may represent a neoplastic population of basal cancer stem cells (Edwards et al., 2004; Emanuel et al., 2005; Sams and Gnepp, 2013). Of importance, expression of p63 may also act as a marker of prognosis: patients who survive for more than 10 years following SG cancer diagnosis exhibit a lower extent of p63 expression than those who died within 10 years (Ramer et al., 2010). In support of this, tumors expressing high levels of p63 are commonly associated with chemoresistance (Rocca et al., 2008; Zangen et al., 2005) via interference with apoptotic pathways (Mundt et al., 2010). Specifically, the NH2- terminal truncated (ΔN) p63 isoform, which lacks the TA-domain, promotes cell proliferation and tumorigenesis in head and neck SCC and downregulation of ΔNp63 via RACK1 determines the cellular response of a tumor to chemotherapy agents, such as Cisplatin (Fomenkov et al., 2004). Thus, degradation of ΔNp63 in cancer cells may provide a therapeutic approach to treat high p63 expressing cells of salivary tumors. Whether p63 marks a true CIC in the adult salivary gland remains to be determined.
High expression of KRT5 is also strongly associated with poor survival rates for SG cancers such as high-grade mucoepidermoid carcinoma, a common SG cancer (Lueck and Robinson, 2008), similar to what has been reported in breast cancer (van de Rijn et al., 2002). In addition, a recent study has demonstrated that pleomorphic adenomas are, at least in part, derived from KRT14+ SG progenitors (Ogawa et al., 2000) and multiple human SG neoplasms are positive for KRT14 (Bansal et al., 2012) implicating the presence a KRT14+ CIC. While KRT5+ cells are regulated by autonomic nerves in the developing salivary gland (Knox et al., 2013; Knox et al., 2010) there has been no correlation demonstrated between KRT5 expression and nerves or neuronal signalling in adult salivary glands or salivary cancers. However, as discussed above, the association between nerve infiltration and tumor progression has been demonstrated in other cancers including those of the head and neck (Dobrenis et al., 2015; Magnon et al., 2013; Pundavela et al., 2014), and as such modulating neuronal signalling in salivary cancer may provide therapeutic benefit by modulating multiple CICs.
KIT is expressed in multiple SG tumour types, including ACCs and monomorphic adenomas (Andreadis et al., 2006; Edwards et al., 2003; Mino et al., 2003), although there remains controversy about whether polymorphous low-grade adenocarcinomas (PLGAs) also express KIT, with some studies claiming they detect expression (Edwards et al., 2003), while others claim no expression (Meer et al., 2011; Penner et al., 2002). Perineural invasion is a reliable indicator of poor survival in numerous cancers (Beard et al., 2004; Ozaki et al., 1999) and a number of studies have demonstrated a relationship between perineural invasion and KIT expression i.e., tumor cells invading facial nerves exhibit high expression levels of KIT (Phuchareon et al., 2014; Tang et al., 2010; Youssef and Said, 2014). This phenotype is associated with poor patient prognosis (Huyett et al., 2018; Ko et al., 2007) but whether nerves regulate KIT in these tumors, and if KIT is required for perineural invasion, is not known. However, while two case reports suggested that KIT-targeted therapy could inhibit ACC progression (Alcedo et al., 2004; Faivre et al., 2005), larger phase II therapies targeting KIT activity show no efficacious impact on cancers of the head and neck, suggesting that KIT itself is not driving tumor behaviour (Bruce et al., 2005; Hotte et al., 2005; Laurie and Licitra, 2006).
Conclusions and Future Directions
The characterization of multiple distinct SG progenitor populations has now introduced the need to understand the balance and relative contribution of these different populations to SG homeostasis and regeneration as well as the impact of disease on their function. For example, different progenitors may differ in their self-renewal and regenerative capacity and understanding this will be crucial to the generation and application of regenerative strategies. Moreover, it is unclear whether a single progenitor cell type gives rise to a single cell type or whether these cells are derived from more than one progenitor. In the prostate, for example, luminal cells give rise to luminal cells whereas basal cells produce themselves as well as luminal cells (Ousset et al., 2012), thus providing a level of redundancy that benefits damage responses in the case that the luminal cell is compromised. Similarly, in the homeostatic intestine, fast cycling LGR5+ cells produce epithelial cells of the villi but should this cell be ablated a quiescent BMI1+ cell is able to compensate, feeding the villi with new daughter cells (Yan et al., 2012). Another option for tissue repair is the de-differentiation of terminally differentiated cells into stem cell-like cells, as occurs in the pancreas and in the regenerating digit tip of rodents. In the inflamed pancreas, terminally differentiated acinar cells de-differentiate towards the ductal lineage, resulting in cells that express both acinar and ductal markers that are thought to regenerate the tissue (Liu et al., 2016). In the case of the regenerating digit, the epithelial cells are derived from a non-epithelial progenitor cell, that is mature Schwann cells at the damage site are transformed into a more primitive lineage that can repopulate the epithelium (Johnston et al., 2016). Whether such methods contribute to the homeostasis and regeneration of the salivary gland (murine or human) or if such mechanisms are utilized by CICs remains to be discovered. However, given the diversity of salivary cancers that have been identified to date, we speculate that a number of these cell programs may be employed both during cancer initiation as well as progression. In summary, we are beginning to unfurl the identity and regulation of progenitors in the salivary gland, which will without doubt lead to a better understanding of tissue homeostasis, repair and disease.
Acknowledgments
Funding information: Funded by the National Institute of Dental and Craniofacial Research (5R01DE024188).
References
- Abashev TM, Metzler MA, Wright DM, Sandell LL. Retinoic acid signaling regulates Krt5 and Krt14 independently of stem cell markers in submandibular salivary gland epithelium. Dev Dyn. 2017;246:135–147. doi: 10.1002/dvdy.24476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A, Warner K, Nor JE. Salivary gland cancer stem cells. Oral Oncol. 2013;49:845–853. doi: 10.1016/j.oraloncology.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A, Warner K, Pearson AT, Zhang Z, Kim HS, Mochizuki D, Basura G, Helman J, Mantesso A, Castilho RM, Wicha MS, Nor JE. ALDH/CD44 identifies uniquely tumorigenic cancer stem cells in salivary gland mucoepidermoid carcinomas. Oncotarget. 2015;6:26633–26650. doi: 10.18632/oncotarget.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcedo JC, Fabrega JM, Arosemena JR, Urrutia A. Imatinib mesylate as treatment for adenoid cystic carcinoma of the salivary glands: report of two successfully treated cases. Head Neck. 2004;26:829–831. doi: 10.1002/hed.20094. [DOI] [PubMed] [Google Scholar]
- Amundadottir LT, Johnson MD, Merlino G, Smith GH, Dickson RB. Synergistic interaction of transforming growth factor alpha and c-myc in mouse mammary and salivary gland tumorigenesis. Cell Growth Differ. 1995;6:737–748. [PubMed] [Google Scholar]
- Andreadis D, Epivatianos A, Poulopoulos A, Nomikos A, Papazoglou G, Antoniades D, Barbatis C. Detection of C-KIT (CD117) molecule in benign and malignant salivary gland tumours. Oral Oncol. 2006;42:57–65. doi: 10.1016/j.oraloncology.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Annovazzi L, Mellai M, Caldera V, Valente G, Schiffer D. SOX2 expression and amplification in gliomas and glioma cell lines. Cancer Genomics Proteomics. 2011;8:139–147. [PubMed] [Google Scholar]
- Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi A, Rezaei M. Prevalence of Candida species in the oral cavity of patients undergoing head and neck radiotherapy. J Dent Res Dent Clin Dent Prospects. 2009;3:78–81. doi: 10.5681/joddd.2009.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma M, Motegi K, Aota K, Hayashi Y, Sato M. Role of cytokines in the destruction of acinar structure in Sjogren’s syndrome salivary glands. Lab Invest. 1997;77:269–280. [PubMed] [Google Scholar]
- Banh A, Xiao N, Cao H, Chen CH, Kuo P, Krakow T, Bavan B, Khong B, Yao M, Ha C, Kaplan MJ, Sirjani D, Jensen K, Kong CS, Mochly-Rosen D, Koong AC, Le QT. A novel aldehyde dehydrogenase-3 activator leads to adult salivary stem cell enrichment in vivo. Clin Cancer Res. 2011;17:7265–7272. doi: 10.1158/1078-0432.CCR-11-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal AK, Bindal R, Kapoor C, Vaidya S, Singh HP. Current concepts in diagnosis of unusual salivary gland tumors. Dent Res J (Isfahan) 2012;9:S9–s19. [PMC free article] [PubMed] [Google Scholar]
- Barker N, Clevers H. Lineage tracing in the intestinal epithelium. Curr Protoc Stem Cell Biol. 2010;Chapter 5(Unit5A.4) doi: 10.1002/9780470151808.sc05a04s13. [DOI] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Barker N, Rookmaaker MB, Kujala P, Ng A, Leushacke M, Snippert H, van de Wetering M, Tan S, Van Es JH, Huch M, Poulsom R, Verhaar MC, Peters PJ, Clevers H. Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep. 2012;2:540–552. doi: 10.1016/j.celrep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, Mandyam CD, Eisch AJ, Miyoshi G, Johnson JE. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134:285–293. doi: 10.1242/dev.02727. [DOI] [PubMed] [Google Scholar]
- Beard CJ, Chen MH, Cote K, Loffredo M, Renshaw AA, Hurwitz M, D’Amico AV. Perineural invasion is associated with increased relapse after external beam radiotherapy for men with low-risk prostate cancer and may be a marker for occult, high-grade cancer. Int J Radiat Oncol Biol Phys. 2004;58:19–24. doi: 10.1016/s0360-3016(03)01433-0. [DOI] [PubMed] [Google Scholar]
- Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, Salnikov AV, Moldenhauer G, Wagner W, Diehlmann A, Saffrich R, Schubert M, Ho AD, Giese N, Buchler MW, Friess H, Buchler P, Herr I. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99:622–631. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal H, Handra-Luca A, Bertrand JC, Fouret PJ. P63 is expressed in basal and myoepithelial cells of human normal and tumor salivary gland tissues. J Histochem Cytochem. 2003;51:133–139. doi: 10.1177/002215540305100201. [DOI] [PubMed] [Google Scholar]
- Borghese E. The development in vitro of the submandibular and sublingual glands of Mus musculus. J Anat. 1950;84:287–302. [PMC free article] [PubMed] [Google Scholar]
- Boshell JL, Pennington C. Histological observations on the effects of isoproterenol on regenerating submandibular glands of the rat. Cell Tissue Res. 1980;213:411–416. doi: 10.1007/BF00237887. [DOI] [PubMed] [Google Scholar]
- Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, Brohee S, Salmon I, Dubois C, del Marmol V, Fuks F, Beck B, Blanpain C. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- Brown LR, Dreizen S, Handler S, Johnston DA. Effect of radiation-induced xerostomia on human oral microflora. J Dent Res. 1975;54:740–750. doi: 10.1177/00220345750540040801. [DOI] [PubMed] [Google Scholar]
- Bruce IA, Slevin NJ, Homer JJ, McGown AT, Ward TH. Synergistic effects of imatinib (STI 571) in combination with chemotherapeutic drugs in head and neck cancer. Anticancer Drugs. 2005;16:719–726. doi: 10.1097/01.cad.0000168392.04676.bb. [DOI] [PubMed] [Google Scholar]
- Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- Bullard T, Koek L, Roztocil E, Kingsley PD, Mirels L, Ovitt CE. Ascl3 expression marks a progenitor population of both acinar and ductal cells in mouse salivary glands. Dev Biol. 2008;320:72–78. doi: 10.1016/j.ydbio.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter GH, Khosravani N, Ekstrom J, Osailan SM, Paterson KP, Proctor GB. Altered plasticity of the parasympathetic innervation in the recovering rat submandibular gland following extensive atrophy. Exp Physiol. 2009;94:213–219. doi: 10.1113/expphysiol.2008.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DR, Martinez Alanis D, Miller RK, Ji H, Akiyama H, McCrea PD, Chen J. Lung epithelial branching program antagonizes alveolar differentiation. Proc Natl Acad Sci U S A. 2013;110:18042–18051. doi: 10.1073/pnas.1311760110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzeli L, Gaete M, Tucker AS. Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development. Development. 2017;144:2294–2305. doi: 10.1242/dev.146019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Huang J, Liu Y, Dattilo LK, Huh SH, Ornitz D, Beebe DC. FGF signaling activates a Sox9-Sox10 pathway for the formation and branching morphogenesis of mouse ocular glands. Development. 2014;141:2691–2701. doi: 10.1242/dev.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Jacobs WB, Zhang JJ, Moro A, Park JH, Kushida M, Qiu W, Mills AA, Kim PC. DeltaNp63 plays an anti-apoptotic role in ventral bladder development. Development. 2006;133:4783–4792. doi: 10.1242/dev.02621. [DOI] [PubMed] [Google Scholar]
- Chibly AM, Querin L, Harris Z, Limesand KH. Label-retaining cells in the adult murine salivary glands possess characteristics of adult progenitor cells. PLoS One. 2014;9:e107893. doi: 10.1371/journal.pone.0107893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32:1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colopy SA, Bjorling DE, Mulligan WA, Bushman W. A population of progenitor cells in the basal and intermediate layers of the murine bladder urothelium contributes to urothelial development and regeneration. Dev Dyn. 2014;243:988–998. doi: 10.1002/dvdy.24143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Coughlin MD. Early development of parasympathetic nerves in the mouse submandibular gland. Dev Biol. 1975;43:123–139. doi: 10.1016/0012-1606(75)90136-0. [DOI] [PubMed] [Google Scholar]
- Coulombel L, Auffray I, Gaugler MH, Rosemblatt M. Expression and function of integrins on hematopoietic progenitor cells. Acta Haematol. 1997;97:13–21. doi: 10.1159/000203655. [DOI] [PubMed] [Google Scholar]
- Dai W, Tan X, Sun C, Zhou Q. High expression of SOX2 is associated with poor prognosis in patients with salivary gland adenoid cystic carcinoma. Int J Mol Sci. 2014;15:8393–8406. doi: 10.3390/ijms15058393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, Shai E, Aframian DJ, Palmon A. Isolation and cultivation of integrin alpha(6)beta(1)-expressing salivary gland graft cells: a model for use with an artificial salivary gland. Tissue Eng Part A. 2008;14:331–337. doi: 10.1089/tea.2007.0122. [DOI] [PubMed] [Google Scholar]
- Davies AN, Brailsford SR, Beighton D. Oral candidosis in patients with advanced cancer. Oral Oncol. 2006;42:698–702. doi: 10.1016/j.oraloncology.2005.11.010. [DOI] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- Denny PC, Chai Y, Klauser DK, Denny PA. Parenchymal cell proliferation and mechanisms for maintenance of granular duct and acinar cell populations in adult male mouse submandibular gland. Anat Rec. 1993;235:475–485. doi: 10.1002/ar.1092350316. [DOI] [PubMed] [Google Scholar]
- DeSantis KA, Stabell AR, Spitzer DC, O’Keefe KJ, Nelson DA, Larsen M. RARalpha and RARgamma reciprocally control K5(+) progenitor cell expansion in developing salivary glands. Organogenesis. 2017;13:125–140. doi: 10.1080/15476278.2017.1358336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo N, Bobba S, Raviraj V, Delic NC, Slapetova I, Nicovich PR, Halliday GM, Wakefield D, Whan R, Lyons JG. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells. 2015;33:157–169. doi: 10.1002/stem.1769. [DOI] [PubMed] [Google Scholar]
- Dobrenis K, Gauthier LR, Barroca V, Magnon C. Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development. Int J Cancer. 2015;136:982–988. doi: 10.1002/ijc.29046. [DOI] [PubMed] [Google Scholar]
- Dong Z, Liu G, Huang B, Sun J, Wu D. Prognostic significance of SOX2 in head and neck cancer: a meta-analysis. Int J Clin Exp Med. 2014;7:5010–5020. [PMC free article] [PubMed] [Google Scholar]
- Dusek M, Simmons J, Buschang PH, al-Hashimi I. Masticatory function in patients with xerostomia. Gerodontology. 1996;13:3–8. doi: 10.1111/j.1741-2358.1996.tb00144.x. [DOI] [PubMed] [Google Scholar]
- Edwards PC, Bhuiya T, Kelsch RD. C-kit expression in the salivary gland neoplasms adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, and monomorphic adenoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:586–593. doi: 10.1067/moe.2003.31. [DOI] [PubMed] [Google Scholar]
- Edwards PC, Bhuiya T, Kelsch RD. Assessment of p63 expression in the salivary gland neoplasms adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, and basal cell and canalicular adenomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:613–619. doi: 10.1016/S1079210403005742. [DOI] [PubMed] [Google Scholar]
- Emanuel P, Wang B, Wu M, Burstein DE. p63 Immunohistochemistry in the distinction of adenoid cystic carcinoma from basaloid squamous cell carcinoma. Mod Pathol. 2005;18:645–650. doi: 10.1038/modpathol.3800329. [DOI] [PubMed] [Google Scholar]
- Emmerson E, May AJ, Berthoin L, Cruz-Pacheco N, Nathan S, Mattingly AJ, Chang JL, Ryan WR, Tward AD, Knox SM. Salivary glands regenerate after radiation injury through SOX2-mediated secretory cell replacement. EMBO Mol Med. 2018 doi: 10.15252/emmm.201708051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson E, May AJ, Nathan S, Cruz-Pacheco N, Lizama CO, Maliskova L, Zovein AC, Shen Y, Muench MO, Knox SM. SOX2 regulates acinar cell development in the salivary gland. Elife. 2017:6. doi: 10.7554/eLife.26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre S, Raymond E, Casiraghi O, Temam S, Berthaud P. Imatinib mesylate can induce objective response in progressing, highly expressing KIT adenoid cystic carcinoma of the salivary glands. J Clin Oncol. 2005;23:6271–6273. doi: 10.1200/JCO.2005.01.6055. author reply 6273–6274. [DOI] [PubMed] [Google Scholar]
- Fang L, Zhu Q, Neuenschwander M, Specker E, Wulf-Goldenberg A, Weis WI, von Kries JP, Birchmeier W. A Small-Molecule Antagonist of the beta-Catenin/TCF4 Interaction Blocks the Self-Renewal of Cancer Stem Cells and Suppresses Tumorigenesis. Cancer Res. 2016;76:891–901. doi: 10.1158/0008-5472.CAN-15-1519. [DOI] [PubMed] [Google Scholar]
- Farmer D, Nathan S, Finley J, Yu KS, Emmerson E, Byrnes L, Sneddon J, McManus M, Tward A, Knox S. Defining epithelial cell dynamics and lineage relationships in the developing lacrimal gland. Development. 2017 doi: 10.1242/dev.150789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, van der Zwaag M, Stokman MA, van Os R, Coppes RP. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol. 2009;92:466–471. doi: 10.1016/j.radonc.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Ferreira JN, Hoffman MP. Interactions between developing nerves and salivary glands. Organogenesis. 2013;9:199–205. doi: 10.4161/org.25224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JK, Farmer D, Emmerson E, Cruz Pacheco N, Knox SM. Manipulating the murine lacrimal gland. J Vis Exp. 2014:e51970. doi: 10.3791/51970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomenkov A, Zangen R, Huang YP, Osada M, Guo Z, Fomenkov T, Trink B, Sidransky D, Ratovitski EA. RACK1 and stratifin target DeltaNp63alpha for a proteasome degradation in head and neck squamous cell carcinoma cells upon DNA damage. Cell Cycle. 2004;3:1285–1295. doi: 10.4161/cc.3.10.1155. [DOI] [PubMed] [Google Scholar]
- Frank RM, Herdly J, Philippe E. ACQUIRED DENTAL DEFECTS AND SALIVARY GLAND LESIONS AFTER IRRADIATION FOR CARCINOMA. J Am Dent Assoc. 1965;70:868–883. doi: 10.14219/jada.archive.1965.0220. [DOI] [PubMed] [Google Scholar]
- Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- Ge N, Lin HX, Xiao XS, Guo L, Xu HM, Wang X, Jin T, Cai XY, Liang Y, Hu WH, Kang T. Prognostic significance of Oct4 and Sox2 expression in hypopharyngeal squamous cell carcinoma. J Transl Med. 2010;8:94. doi: 10.1186/1479-5876-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie MB, Albergotti WG, Eisele DW. Recurrent salivary gland cancer. Curr Treat Options Oncol. 2012;13:58–70. doi: 10.1007/s11864-011-0174-0. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss GM, Chaudhari N, Hare JM, Nwojo R, Seidler B, Saur D, Goldstein BJ. Differentiation potential of individual olfactory c-Kit+ progenitors determined via multicolor lineage tracing. Dev Neurobiol. 2015 doi: 10.1002/dneu.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- Gresik EW, Chung KW, Barka T, Schenkein I. Immunocytochemical localization of nerve growth factor, submandibular glands of Tfm/Y mice. Am J Anat. 1980;158:247–250. doi: 10.1002/aja.1001580212. [DOI] [PubMed] [Google Scholar]
- Hai B, Yang Z, Shangguan L, Zhao Y, Boyer A, Liu F. Concurrent transient activation of Wnt/beta-catenin pathway prevents radiation damage to salivary glands. Int J Radiat Oncol Biol Phys. 2012;83:e109–116. doi: 10.1016/j.ijrobp.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlet S, Faull J, Klein B, Aref A, Fontanesi J, Stachler R, Shamsa F, Jones L, Simpson M. Mastication and swallowing in patients with postirradiation xerostomia. Int J Radiat Oncol Biol Phys. 1997;37:789–796. doi: 10.1016/s0360-3016(96)00604-9. [DOI] [PubMed] [Google Scholar]
- Han J, Fujisawa T, Husain SR, Puri RK. Identification and characterization of cancer stem cells in human head and neck squamous cell carcinoma. BMC Cancer. 2014;14:173. doi: 10.1186/1471-2407-14-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand AR, Ho B. Mitosis and hypertrophy of intercalated duct cells and endothelial cells in the isoproterenol-treated rat parotid gland. J Dent Res. 1985;64:1031–1038. doi: 10.1177/00220345850640080201. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Lombaert IM, Hauser BR, Patel VN, Hoffman MP. Exosomal MicroRNA Transport from Salivary Mesenchyme Regulates Epithelial Progenitor Expansion during Organogenesis. Dev Cell. 2017;40:95–103. doi: 10.1016/j.devcel.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Hisatomi Y, Okumura K, Nakamura K, Matsumoto S, Satoh A, Nagano K, Yamamoto T, Endo F. Flow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic lineages. Hepatology. 2004;39:667–675. doi: 10.1002/hep.20063. [DOI] [PubMed] [Google Scholar]
- Holsinger FC, Bui DT. Salivary Gland Disorders. Springer; 2007. Anatomy, Function, and Evaluation of the Salivary Glands; pp. 1–16. [Google Scholar]
- Hondermarck H. Neurotrophins and their receptors in breast cancer. Cytokine Growth Factor Rev. 2012;23:357–365. doi: 10.1016/j.cytogfr.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Hotte SJ, Winquist EW, Lamont E, MacKenzie M, Vokes E, Chen EX, Brown S, Pond GR, Murgo A, Siu LL. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a Princess Margaret Hospital phase II consortium study. J Clin Oncol. 2005;23:585–590. doi: 10.1200/JCO.2005.06.125. [DOI] [PubMed] [Google Scholar]
- Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembele D, Martinet N, Thibault C, Huelsken J, Brambilla E, du Manoir S. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One. 2010;5:e8960. doi: 10.1371/journal.pone.0008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyett P, Duvvuri U, Ferris RL, Johnson JT, Schaitkin BM, Kim S. Perineural Invasion in Parotid Gland Malignancies. Otolaryngol Head Neck Surg. 2018 doi: 10.1177/0194599817751888. 194599817751888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrler S, Blasenbreu-Vogt S, Sendelhofert A, Rossle M, Harrison JD, Lohrs U. Regeneration in chronic sialadenitis: an analysis of proliferation and apoptosis based on double immunohistochemical labelling. Virchows Arch. 2004;444:356–361. doi: 10.1007/s00428-003-0964-2. [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Ivanov SV, Panaccione A, Nonaka D, Prasad ML, Boyd KL, Brown B, Guo Y, Sewell A, Yarbrough WG. Diagnostic SOX10 gene signatures in salivary adenoid cystic and breast basal-like carcinomas. Br J Cancer. 2013;109:444–451. doi: 10.1038/bjc.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Jaskoll T, Melnick M. Submandibular gland morphogenesis: stage-specific expression of TGF-alpha/EGF, IGF, TGF-beta, TNF, and IL-6 signal transduction in normal embryonic mice and the phenotypic effects of TGF-beta2, TGF-beta3, and EGF-r null mutations. Anat Rec. 1999;256:252–268. doi: 10.1002/(SICI)1097-0185(19991101)256:3<252::AID-AR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Jaskoll T, Zhou YM, Chai Y, Makarenkova HP, Collinson JM, West JD, Hajihosseini MK, Lee J, Melnick M. Embryonic submandibular gland morphogenesis: stage-specific protein localization of FGFs, BMPs, Pax6 and Pax9 in normal mice and abnormal SMG phenotypes in FgfR2-IIIc(+/Delta), BMP7(−/−) and Pax6(−/−) mice. Cells Tissues Organs. 2002;170:83–98. doi: 10.1159/000046183. [DOI] [PubMed] [Google Scholar]
- Jeong J, Baek H, Kim YJ, Choi Y, Lee H, Lee E, Kim ES, Hah JH, Kwon TK, Choi IJ, Kwon H. Human salivary gland stem cells ameliorate hyposalivation of radiation-damaged rat salivary glands. Exp Mol Med. 2013;45:e58. doi: 10.1038/emm.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Wang L, Chen CP, Li MQ, Liao XH. Lgr6 is dispensable for epidermal cell proliferation and wound repair. Exp Dermatol. 2017;26:105–107. doi: 10.1111/exd.13187. [DOI] [PubMed] [Google Scholar]
- Jo A, Denduluri S, Zhang B, Wang Z, Yin L, Yan Z, Kang R, Shi LL, Mok J, Lee MJ, Haydon RC. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2014;1:149–161. doi: 10.1016/j.gendis.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DA. Changes in rat parotid salivary proteins associated with liquid diet-induced gland atrophy and isoproterenol-induced gland enlargement. Arch Oral Biol. 1984;29:215–221. doi: 10.1016/0003-9969(84)90058-x. [DOI] [PubMed] [Google Scholar]
- Johnston AP, Yuzwa SA, Carr MJ, Mahmud N, Storer MA, Krause MP, Jones K, Paul S, Kaplan DR, Miller FD. Dedifferentiated Schwann Cell Precursors Secreting Paracrine Factors Are Required for Regeneration of the Mammalian Digit Tip. Cell Stem Cell. 2016;19:433–448. doi: 10.1016/j.stem.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Kadaja M, Keyes BE, Lin M, Pasolli HA, Genander M, Polak L, Stokes N, Zheng D, Fuchs E. SOX9: a stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev. 2014;28:328–341. doi: 10.1101/gad.233247.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Matsubara M, Matsuo T, Mohri Y, Kazama I, Hatano R, Umezawa A, Nishimori K. Leucine-rich repeat-containing G protein-coupled receptor-4 (LGR4, Gpr48) is essential for renal development in mice. Nephron Exp Nephrol. 2006;104:e63–75. doi: 10.1159/000093999. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Kwon HJ, Shinozaki N, Hashimoto S, Shimono M, Cho SW, Jung HS. Comparative analysis of ABCG2-expressing and label-retaining cells in mouse submandibular gland. Cell Tissue Res. 2008;334:47–53. doi: 10.1007/s00441-008-0667-8. [DOI] [PubMed] [Google Scholar]
- Kimoto M, Yura Y, Kishino M, Toyosawa S, Ogawa Y. Label-retaining cells in the rat submandibular gland. J Histochem Cytochem. 2008;56:15–24. doi: 10.1369/jhc.7A7269.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzel B, Pikiolek M, Orsini V, Sprunger J, Isken A, Zietzling S, Desplanches M, Dubost V, Breustedt D, Valdez R, Liu D, Theil D, Muller M, Dietrich B, Bouwmeester T, Ruffner H, Tchorz JS. Functional roles of Lgr4 and Lgr5 in embryonic gut, kidney and skin development in mice. Dev Biol. 2014;390:181–190. doi: 10.1016/j.ydbio.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Knosp WM, Knox SM, Lombaert IM, Haddox CL, Patel VN, Hoffman MP. Submandibular parasympathetic gangliogenesis requires sprouty-dependent wnt signals from epithelial progenitors. Dev Cell. 2015;32:667–677. doi: 10.1016/j.devcel.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, Wilson AJ, Hoffman MP. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun. 2013;4:1494. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science. 2010;329:1645–1647. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YH, Lee MA, Hong YS, Lee KS, Jung CK, Kim YS, Sun DI, Kim BS, Kim MS, Kang JH. Prognostic factors affecting the clinical outcome of adenoid cystic carcinoma of the head and neck. Jpn J Clin Oncol. 2007;37:805–811. doi: 10.1093/jjco/hym119. [DOI] [PubMed] [Google Scholar]
- Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak M, Alston N, Ghazizadeh S. Identification of Stem Cells in the Secretory Complex of Salivary Glands. J Dent Res. 2016;95:776–783. doi: 10.1177/0022034516634664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak M, Ghazizadeh S. Analysis of histone H2BGFP retention in mouse submandibular gland reveals actively dividing stem cell populations. Stem Cells Dev. 2015;24:565–574. doi: 10.1089/scd.2014.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HR, Larsen M. The contribution of specific cell subpopulations to submandibular salivary gland branching morphogenesis. Curr Opin Genet Dev. 2015;32:47–54. doi: 10.1016/j.gde.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HR, Nelson DA, DeSantis KA, Morrissey JM, Larsen M. Endothelial cell regulation of salivary gland epithelial patterning. Development. 2017;144:211–220. doi: 10.1242/dev.142497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackovicova L, Banovska L, Bundzikova J, Janega P, Bizik J, Kiss A, Mravec B. Chemical sympathectomy suppresses fibrosarcoma development and improves survival of tumor-bearing rats. Neoplasma. 2011;58:424–429. doi: 10.4149/neo_2011_05_424. [DOI] [PubMed] [Google Scholar]
- Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. J Clin Oncol. 2006;24:2673–2678. doi: 10.1200/JCO.2005.05.3025. [DOI] [PubMed] [Google Scholar]
- Lee SH, Oh SY, Do SI, Lee HJ, Kang HJ, Rho YS, Bae WJ, Lim YC. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br J Cancer. 2014;111:2122–2130. doi: 10.1038/bjc.2014.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoczky JA, Tabin CJ. Lgr6 marks nail stem cells and is required for digit tip regeneration. Proc Natl Acad Sci U S A. 2015;112:13249–13254. doi: 10.1073/pnas.1518874112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemercier C, To RQ, Swanson BJ, Lyons GE, Konieczny SF. Mist1: a novel basic helix-loop-helix transcription factor exhibits a developmentally regulated expression pattern. Dev Biol. 1997;182:101–113. doi: 10.1006/dbio.1996.8454. [DOI] [PubMed] [Google Scholar]
- Lennartsson J, Ronnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92:1619–1649. doi: 10.1152/physrev.00046.2011. [DOI] [PubMed] [Google Scholar]
- Leung Y, Kandyba E, Chen YB, Ruffins S, Kobielak K. Label retaining cells (LRCs) with myoepithelial characteristic from the proximal acinar region define stem cells in the sweat gland. PLoS One. 2013;8:e74174. doi: 10.1371/journal.pone.0074174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Li G, Xu F, Zhu J, Krawczyk M, Zhang Y, Yuan J, Patel S, Wang Y, Lin Y, Zhang M, Cai H, Chen D, Zhang M, Cao G, Yeh E, Lin D, Su Q, Li WW, Sen GL, Afshari N, Chen S, Maas RL, Fu XD, Zhang K, Liu Y, Ouyang H. Transcription Factor PAX6 (Paired Box 6) Controls Limbal Stem Cell Lineage in Development and Disease. J Biol Chem. 2015;290:20448–20454. doi: 10.1074/jbc.M115.662940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li B, Wang R, Huang D, Jin W, Yang S. SOX2 as prognostic factor in head and neck cancer: a systematic review and meta-analysis. Acta Otolaryngol. 2014;134:1101–1108. doi: 10.3109/00016489.2014.913311. [DOI] [PubMed] [Google Scholar]
- Lin CS, Ning H, Lin G, Lue TF. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy. 2012;14:1159–1163. doi: 10.3109/14653249.2012.729817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Ouyang H, Zhu J, Huang S, Liu Z, Chen S, Cao G, Li G, Signer RA, Xu Y, Chung C, Zhang Y, Lin D, Patel S, Wu F, Cai H, Hou J, Wen C, Jafari M, Liu X, Luo L, Zhu J, Qiu A, Hou R, Chen B, Chen J, Granet D, Heichel C, Shang F, Li X, Krawczyk M, Skowronska-Krawczyk D, Wang Y, Shi W, Chen D, Zhong Z, Zhong S, Zhang L, Chen S, Morrison SJ, Maas RL, Zhang K, Liu Y. Lens regeneration using endogenous stem cells with gain of visual function. Nature. 2016;531:323–328. doi: 10.1038/nature17181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Akanuma N, Liu C, Naji A, Halff GA, Washburn WK, Sun L, Wang P. TGF-beta1 promotes acinar to ductal metaplasia of human pancreatic acinar cells. Sci Rep. 2016;6:30904. doi: 10.1038/srep30904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IM, Abrams SR, Li L, Eswarakumar VP, Sethi AJ, Witt RL, Hoffman MP. Combined KIT and FGFR2b signaling regulates epithelial progenitor expansion during organogenesis. Stem Cell Reports. 2013;1:604–619. doi: 10.1016/j.stemcr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G, Coppes RP. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IM, Knox SM, Hoffman MP. Salivary gland progenitor cell biology provides a rationale for therapeutic salivary gland regeneration. Oral Dis. 2011;17:445–449. doi: 10.1111/j.1601-0825.2010.01783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IMA, Hoffman MP. Epithelial Stem/Progenitor Cells in the Embryonic Mouse Submandibular Gland. In: Tucker AS, Miletich I, editors. Salivary Glands, Development, Adaptations and Disease. Basel: Karger; 2010. pp. 90–106. [Google Scholar]
- Lu CP, Polak L, Rocha AS, Pasolli HA, Chen SC, Sharma N, Blanpain C, Fuchs E. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Li Y, Du MJ, Zhang C, Zhang XY, Tong HZ, Liu L, Han TL, Li WD, Yan L, Yin NB, Li HD, Zhao ZM. Characterization of a Self-renewing and Multi-potent Cell Population Isolated from Human Minor Salivary Glands. Sci Rep. 2015;5:10106. doi: 10.1038/srep10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MC, Jheng CH, Tsai TY, Koo M, Lai NS. Increased dental visits in patients prior to diagnosis of primary Sjogren’s syndrome: a population-based study in Taiwan. Rheumatol Int. 2014;34:1555–1561. doi: 10.1007/s00296-014-3003-5. [DOI] [PubMed] [Google Scholar]
- Lueck NE, Robinson RA. High levels of expression of cytokeratin 5 are strongly correlated with poor survival in higher grades of mucoepidermoid carcinoma. J Clin Pathol. 2008;61:837–840. doi: 10.1136/jcp.2008.055988. [DOI] [PubMed] [Google Scholar]
- Lyle S, Christofidou-Solomidou M, Liu Y, Elder DE, Albelda S, Cotsarelis G. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J Cell Sci. 1998;111( Pt 21):3179–3188. doi: 10.1242/jcs.111.21.3179. [DOI] [PubMed] [Google Scholar]
- Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- Maimets M, Rocchi C, Bron R, Pringle S, Kuipers J, Giepmans BN, Vries RG, Clevers H, de Haan G, van Os R, Coppes RP. Long-Term In Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals. Stem Cell Reports. 2016;6:150–162. doi: 10.1016/j.stemcr.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenkova HP, Ito M, Govindarajan V, Faber SC, Sun L, McMahon G, Overbeek PA, Lang RA. FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development. 2000;127:2563–2572. doi: 10.1242/dev.127.12.2563. [DOI] [PubMed] [Google Scholar]
- Malhotra GK, Zhao X, Edwards E, Kopp JL, Naramura M, Sander M, Band H, Band V. The role of Sox9 in mouse mammary gland development and maintenance of mammary stem and luminal progenitor cells. BMC Dev Biol. 2014;14:47. doi: 10.1186/s12861-014-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man YG, Ball WD, Marchetti L, Hand AR. Contributions of intercalated duct cells to the normal parenchyma of submandibular glands of adult rats. Anat Rec. 2001;263:202–214. doi: 10.1002/ar.1098. [DOI] [PubMed] [Google Scholar]
- Martinez-Madrigal F, Micheau C. Histology of the major salivary glands. Am J Surg Pathol. 1989;13:879–899. doi: 10.1097/00000478-198910000-00008. [DOI] [PubMed] [Google Scholar]
- Matheu A, Collado M, Wise C, Manterola L, Cekaite L, Tye AJ, Canamero M, Bujanda L, Schedl A, Cheah KS, Skotheim RI, Lothe RA, Lopez de Munain A, Briscoe J, Serrano M, Lovell-Badge R. Oncogenicity of the developmental transcription factor Sox9. Cancer Res. 2012;72:1301–1315. doi: 10.1158/0008-5472.CAN-11-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Okumura K, Ogata A, Hisatomi Y, Sato A, Hattori K, Matsumoto M, Kaji Y, Takahashi M, Yamamoto T, Nakamura K, Endo F. Isolation of tissue progenitor cells from duct-ligated salivary glands of swine. Cloning Stem Cells. 2007;9:176–190. doi: 10.1089/clo.2006.0022. [DOI] [PubMed] [Google Scholar]
- Mattingly A, Finley JK, Knox SM. Salivary gland development and disease. Wiley Interdiscip Rev Dev Biol. 2015 doi: 10.1002/wdev.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means AL, Xu Y, Zhao A, Ray KC, Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008;46:318–323. doi: 10.1002/dvg.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meer S, Singh S, Altini M. c-kit and bcl-2 Are Not Useful Markers in Differentiating Adenoid Cystic Carcinoma from Polymorphous Low-Grade Adenocarcinoma. ISRN Pathology. 2011;2011:6. [Google Scholar]
- Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- Miknyoczki SJ, Wan W, Chang H, Dobrzanski P, Ruggeri BA, Dionne CA, Buchkovich K. The neurotrophin-trk receptor axes are critical for the growth and progression of human prostatic carcinoma and pancreatic ductal adenocarcinoma xenografts in nude mice. Clin Cancer Res. 2002;8:1924–1931. [PubMed] [Google Scholar]
- Miletich I. Introduction to Salivary Glands: Structure, Function and Embryonic Development. In: Tucker AS, Miletich I, editors. Salivary Glands. Development, Adaptations and Disease: Front Oral Biol. Basel: Karger; 2010. pp. 1–20. [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Mino M, Pilch BZ, Faquin WC. Expression of KIT (CD117) in neoplasms of the head and neck: an ancillary marker for adenoid cystic carcinoma. Mod Pathol. 2003;16:1224–1231. doi: 10.1097/01.MP.0000096046.42833.C7. [DOI] [PubMed] [Google Scholar]
- Mohri Y, Kato S, Umezawa A, Okuyama R, Nishimori K. Impaired hair placode formation with reduced expression of hair follicle-related genes in mice lacking Lgr4. Dev Dyn. 2008;237:2235–2242. doi: 10.1002/dvdy.21639. [DOI] [PubMed] [Google Scholar]
- Mohri Y, Oyama K, Akamatsu A, Kato S, Nishimori K. Lgr4-deficient mice showed premature differentiation of ureteric bud with reduced expression of Wnt effector Lef1 and Gata3. Dev Dyn. 2011;240:1626–1634. doi: 10.1002/dvdy.22651. [DOI] [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Morita H, Mazerbourg S, Bouley DM, Luo CW, Kawamura K, Kuwabara Y, Baribault H, Tian H, Hsueh AJ. Neonatal lethality of LGR5 null mice is associated with ankyloglossia and gastrointestinal distension. Mol Cell Biol. 2004;24:9736–9743. doi: 10.1128/MCB.24.22.9736-9743.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Gupta A, Chattopadhyay D, Chatterji U. Modulation of SOX2 expression delineates an end-point for paclitaxel-effectiveness in breast cancer stem cells. Sci Rep. 2017;7:9170. doi: 10.1038/s41598-017-08971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt HM, Stremmel W, Melino G, Krammer PH, Schilling T, Muller M. Dominant negative (DeltaN) p63alpha induces drug resistance in hepatocellular carcinoma by interference with apoptosis signaling pathways. Biochem Biophys Res Commun. 2010;396:335–341. doi: 10.1016/j.bbrc.2010.04.093. [DOI] [PubMed] [Google Scholar]
- Nanduri LS, Baanstra M, Faber H, Rocchi C, Zwart E, de Haan G, van Os R, Coppes RP. Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Reports. 2014;3:957–964. doi: 10.1016/j.stemcr.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri LS, Lombaert IM, van der Zwaag M, Faber H, Brunsting JF, van Os RP, Coppes RP. Salisphere derived c-Kit cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother Oncol. 2013 doi: 10.1016/j.radonc.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Nanduri LS, Maimets M, Pringle SA, van der Zwaag M, van Os RP, Coppes RP. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol. 2011;99:367–372. doi: 10.1016/j.radonc.2011.05.085. [DOI] [PubMed] [Google Scholar]
- Nedvetsky PI, Emmerson E, Finley JK, Ettinger A, Cruz-Pacheco N, Prochazka J, Haddox CL, Northrup E, Hodges C, Mostov KE, Hoffman MP, Knox SM. Parasympathetic innervation regulates tubulogenesis in the developing salivary gland. Dev Cell. 2014;30:449–462. doi: 10.1016/j.devcel.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng A, Tan S, Singh G, Rizk P, Swathi Y, Tan TZ, Huang RY, Leushacke M, Barker N. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat Cell Biol. 2014;16:745–757. doi: 10.1038/ncb3000. [DOI] [PubMed] [Google Scholar]
- NIDCR. Salivary Gland Atlas. http://sgmap.nidcr.nih.gov/sgmap/sgexp.html.
- Ogawa Y, Toyosawa S, Ishida T, Ijuhin N. Keratin 14 immunoreactive cells in pleomorphic adenomas and adenoid cystic carcinomas of salivary glands. Virchows Arch. 2000;437:58–68. doi: 10.1007/s004280000186. [DOI] [PubMed] [Google Scholar]
- Ohtomo R, Mori T, Shibata S, Tsuta K, Maeshima AM, Akazawa C, Watabe Y, Honda K, Yamada T, Yoshimoto S, Asai M, Okano H, Kanai Y, Tsuda H. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol. 2013;26:1041–1050. doi: 10.1038/modpathol.2013.54. [DOI] [PubMed] [Google Scholar]
- Okumura K, Nakamura K, Hisatomi Y, Nagano K, Tanaka Y, Terada K, Sugiyama T, Umeyama K, Matsumoto K, Yamamoto T, Endo F. Salivary gland progenitor cells induced by duct ligation differentiate into hepatic and pancreatic lineages. Hepatology. 2003;38:104–113. doi: 10.1053/jhep.2003.50259. [DOI] [PubMed] [Google Scholar]
- Okumura K, Shinohara M, Endo F. Capability of tissue stem cells to organize into salivary rudiments. Stem Cells Int. 2012;2012:502136. doi: 10.1155/2012/502136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousset M, Van Keymeulen A, Bouvencourt G, Sharma N, Achouri Y, Simons BD, Blanpain C. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat Cell Biol. 2012;14:1131–1138. doi: 10.1038/ncb2600. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Hiraoka T, Mizumoto R, Matsuno S, Matsumoto Y, Nakayama T, Tsunoda T, Suzuki T, Monden M, Saitoh Y, Yamauchi H, Ogata Y. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today. 1999;29:16–22. doi: 10.1007/BF02482964. [DOI] [PubMed] [Google Scholar]
- Patel N, Sharpe PT, Miletich I. Coordination of epithelial branching and salivary gland lumen formation by Wnt and FGF signals. Dev Biol. 2011;358:156–167. doi: 10.1016/j.ydbio.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Patel R, Shahane A. The epidemiology of Sjogren’s syndrome. Clin Epidemiol. 2014;6:247–255. doi: 10.2147/CLEP.S47399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VN, Hoffman MP. Salivary gland development: a template for regeneration. Semin Cell Dev Biol. 2014;25–26:52–60. doi: 10.1016/j.semcdb.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VN, Lombaert IM, Cowherd SN, Shworak NW, Xu Y, Liu J, Hoffman MP. Hs3st3-modified heparan sulfate controls KIT+ progenitor expansion by regulating 3-O-sulfotransferases. Dev Cell. 2014;29:662–673. doi: 10.1016/j.devcel.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74:349–364. doi: 10.1111/j.1432-0436.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Varendi K, Maimets M, Andressoo JO, Coppes RP. Role of glial-cell-derived neurotrophic factor in salivary gland stem cell response to irradiation. LID - S0167-8140(17)32467-2 [pii] LID. Radiother Oncol. 2017;124:448–454. doi: 10.1016/j.radonc.2017.07.008. [DOI] [PubMed] [Google Scholar]
- Penner CR, Folpe AL, Budnick SD. C-kit expression distinguishes salivary gland adenoid cystic carcinoma from polymorphous low-grade adenocarcinoma. Mod Pathol. 2002;15:687–691. doi: 10.1097/01.MP.0000018973.17736.F8. [DOI] [PubMed] [Google Scholar]
- Peronace AA, Davison TA, Houssay AB, Perec J. ALTERATIONS IN SUBMANDIBULAR AND RETROLINGUAL GLANDS FOLLOWING PARASYMPATHETIC DENERVATION IN RATS. Anat Rec. 1964;150:25–33. doi: 10.1002/ar.1091500104. [DOI] [PubMed] [Google Scholar]
- Peters B, Kirfel J, Bussow H, Vidal M, Magin TM. Complete cytolysis and neonatal lethality in keratin 5 knockout mice reveal its fundamental role in skin integrity and in epidermolysis bullosa simplex. Mol Biol Cell. 2001;12:1775–1789. doi: 10.1091/mbc.12.6.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuchareon J, van Zante A, Overdevest JB, McCormick F, Eisele DW, Tetsu O. c-Kit Expression is Rate-Limiting for Stem Cell Factor-Mediated Disease Progression in Adenoid Cystic Carcinoma of the Salivary Glands. Transl Oncol. 2014;7:537–545. doi: 10.1016/j.tranon.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignon JC, Grisanzio C, Geng Y, Song J, Shivdasani RA, Signoretti S. p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proc Natl Acad Sci U S A. 2013;110:8105–8110. doi: 10.1073/pnas.1221216110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor GB, Asking B. A comparison between changes in rat parotid protein composition 1 and 12 weeks following surgical sympathectomy. Q J Exp Physiol. 1989;74:835–840. doi: 10.1113/expphysiol.1989.sp003353. [DOI] [PubMed] [Google Scholar]
- Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Pundavela J, Demont Y, Jobling P, Lincz LF, Roselli S, Thorne RF, Bond D, Bradshaw RA, Walker MM, Hondermarck H. ProNGF correlates with Gleason score and is a potential driver of nerve infiltration in prostate cancer. Am J Pathol. 2014;184:3156–3162. doi: 10.1016/j.ajpath.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Ramachandran I, Ganapathy V, Gillies E, Fonseca I, Sureban SM, Houchen CW, Reis A, Queimado L. Wnt inhibitory factor 1 suppresses cancer stemness and induces cellular senescence. Cell Death Dis. 2014;5:e1246. doi: 10.1038/cddis.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer N, Wu H, Sabo E, Ramer Y, Emanuel P, Orta L, Burstein DE. Prognostic value of quantitative p63 immunostaining in adenoid cystic carcinoma of salivary gland assessed by computerized image analysis. Cancer. 2010;116:77–83. doi: 10.1002/cncr.24657. [DOI] [PubMed] [Google Scholar]
- Redman RS. Proliferative activity by cell type in the developing rat parotid gland. Anat Rec. 1995;241:529–540. doi: 10.1002/ar.1092410411. [DOI] [PubMed] [Google Scholar]
- Reginensi A, Clarkson M, Neirijnck Y, Lu B, Ohyama T, Groves AK, Sock E, Wegner M, Costantini F, Chaboissier MC, Schedl A. SOX9 controls epithelial branching by activating RET effector genes during kidney development. Hum Mol Genet. 2011;20:1143–1153. doi: 10.1093/hmg/ddq558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remez LA, Onishi A, Menuchin-Lasowski Y, Biran A, Blackshaw S, Wahlin KJ, Zack DJ, Ashery-Padan R. Pax6 is essential for the generation of late-born retinal neurons and for inhibition of photoreceptor-fate during late stages of retinogenesis. Dev Biol. 2017;432:140–150. doi: 10.1016/j.ydbio.2017.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodus NL, Moller K, Colby S, Bereuter J. Articulatory speech performance in patients with salivary gland dysfunction: a pilot study. Quintessence Int. 1995;26:805–810. [PubMed] [Google Scholar]
- Rocca A, Viale G, Gelber RD, Bottiglieri L, Gelber S, Pruneri G, Ghisini R, Balduzzi A, Pietri E, D’Alessandro C, Goldhirsch A, Colleoni M. Pathologic complete remission rate after cisplatin-based primary chemotherapy in breast cancer: correlation with p63 expression. Cancer Chemother Pharmacol. 2008;61:965–971. doi: 10.1007/s00280-007-0551-3. [DOI] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockich BE, Hrycaj SM, Shih HP, Nagy MS, Ferguson MA, Kopp JL, Sander M, Wellik DM, Spence JR. Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc Natl Acad Sci U S A. 2013;110:E4456–4464. doi: 10.1073/pnas.1311847110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter N, Oder J, Schlenke P, Lindner U, Bohrnsen F, Kramer J, Rohwedel J, Huss R, Brandau S, Wollenberg B, Lang S. Isolation and characterization of adult stem cells from human salivary glands. Stem Cells Dev. 2008;17:509–518. doi: 10.1089/scd.2007.0180. [DOI] [PubMed] [Google Scholar]
- Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA, Rhim AD, Davis BM. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A. 2016;113:3078–3083. doi: 10.1073/pnas.1512603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams RN, Gnepp DR. P63 expression can be used in differential diagnosis of salivary gland acinic cell and mucoepidermoid carcinomas. Head Neck Pathol. 2013;7:64–68. doi: 10.1007/s12105-012-0403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Okumura K, Matsumoto S, Hattori K, Hattori S, Shinohara M, Endo F. Isolation, tissue localization, and cellular characterization of progenitors derived from adult human salivary glands. Cloning Stem Cells. 2007;9:191–205. doi: 10.1089/clo.2006.0054. [DOI] [PubMed] [Google Scholar]
- Schenck K, Schreurs O, Hayashi K, Helgeland K. The Role of Nerve Growth Factor (NGF) and Its Precursor Forms in Oral Wound Healing. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Chen X, Paragas N, Levinson RS, Mendelsohn CL, Barasch J. c-kit delineates a distinct domain of progenitors in the developing kidney. Dev Biol. 2006;299:238–249. doi: 10.1016/j.ydbio.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Schoenhals M, Kassambara A, De Vos J, Hose D, Moreaux J, Klein B. Embryonic stem cell markers expression in cancers. Biochem Biophys Res Commun. 2009;383:157–162. doi: 10.1016/j.bbrc.2009.02.156. [DOI] [PubMed] [Google Scholar]
- Schrock A, Bode M, Goke FJ, Bareiss PM, Schairer R, Wang H, Weichert W, Franzen A, Kirsten R, van Bremen T, Queisser A, Kristiansen G, Heasley L, Bootz F, Lengerke C, Perner S. Expression and role of the embryonic protein SOX2 in head and neck squamous cell carcinoma. Carcinogenesis. 2014;35:1636–1642. doi: 10.1093/carcin/bgu094. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Rotter N. Human salivary gland stem cells: isolation, propagation, and characterization. Methods Mol Biol. 2012;879:403–442. doi: 10.1007/978-1-61779-815-3_25. [DOI] [PubMed] [Google Scholar]
- Sedassari BT, Rodrigues M, Conceicao TS, Mariano FV, Alves VAF, Nunes FD, Altemani A, de Sousa S. Increased SOX2 expression in salivary gland carcinoma ex pleomorphic adenoma progression: an association with adverse outcome. Virchows Arch. 2017;471:775–784. doi: 10.1007/s00428-017-2220-1. [DOI] [PubMed] [Google Scholar]
- Segawa A, Shoi N, Yamashina S. Function of myoepithelial cells in salivary secretion: reevaluation of the expulsion theory. Kaibogaku Zasshi. 1995;70:330–337. [PubMed] [Google Scholar]
- Seidel K, Ahn CP, Lyons D, Nee A, Ting K, Brownell I, Cao T, Carano RA, Curran T, Schober M, Fuchs E, Joyner A, Martin GR, de Sauvage FJ, Klein OD. Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development. 2010;137:3753–3761. doi: 10.1242/dev.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiohara M, Koike K, Kubo T, Amano Y, Takagi M, Muraoka K, Nakao J, Nakahata T, Komiyama A. Possible role of stem cell factor as a serum factor: monoclonal anti-c-kit antibody abrogates interleukin-6-dependent colony growth in serum-containing culture. Exp Hematol. 1993;21:907–912. [PubMed] [Google Scholar]
- Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32:1380–1389. doi: 10.1002/stem.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, Yang A, Montironi R, McKeon F, Loda M. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000;157:1769–1775. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell RS, Garrett JR. The effect of postganglionic sympathectomy on the structure of the submandibular and major sublingual salivary glands of the rat. Z Zellforsch Mikrosk Anat. 1958;48:639–652. doi: 10.1007/BF00398652. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, Stange DE, Toftgard R, Clevers H. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- Soave DF, Oliveira da Costa JP, da Silveira GG, Ianez RC, de Oliveira LR, Lourenco SV, Ribeiro-Silva A. CD44/CD24 immunophenotypes on clinicopathologic features of salivary glands malignant neoplasms. Diagn Pathol. 2013;8:29. doi: 10.1186/1746-1596-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda T, Deng JM, de Crombrugghe B, Behringer RR, Nakamura T, Akiyama H. Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis. 2010;48:635–644. doi: 10.1002/dvg.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczuk L, Martinez-Corral I, Ulvmar MH, Zhang Y, Lavina B, Fruttiger M, Adams RH, Saur D, Betsholtz C, Ortega S, Alitalo K, Graupera M, Makinen T. cKit Lineage Hemogenic Endothelium-Derived Cells Contribute to Mesenteric Lymphatic Vessels. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.02.026. [DOI] [PubMed] [Google Scholar]
- Stewart CM, Berg KM, Cha S, Reeves WH. Salivary dysfunction and quality of life in Sjogren syndrome: a critical oral-systemic connection. J Am Dent Assoc. 2008;139:291–299. doi: 10.14219/jada.archive.2008.0158. quiz 358–299. [DOI] [PubMed] [Google Scholar]
- Sun S, Wang Z. ALDH high adenoid cystic carcinoma cells display cancer stem cell properties and are responsible for mediating metastasis. Biochem Biophys Res Commun. 2010;396:843–848. doi: 10.1016/j.bbrc.2010.04.170. [DOI] [PubMed] [Google Scholar]
- Szymaniak AD, Mi R, McCarthy SE, Gower AC, Reynolds TL, Mingueneau M, Kukuruzinska M, Varelas X. The Hippo pathway effector YAP is an essential regulator of ductal progenitor patterning in the mouse submandibular gland. Elife. 2017:6. doi: 10.7554/eLife.23499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tang Y, Liang X, Zheng M, Zhu Z, Zhu G, Yang J, Chen Y. Expression of c-kit and Slug correlates with invasion and metastasis of salivary adenoid cystic carcinoma. Oral Oncol. 2010;46:311–316. doi: 10.1016/j.oraloncology.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Tatsuishi Y, Hirota M, Kishi T, Adachi M, Fukui T, Mitsudo K, Aoki S, Matsui Y, Omura S, Taniguchi H, Tohnai I. Human salivary gland stem/progenitor cells remain dormant even after irradiation. Int J Mol Med. 2009;24:361–366. doi: 10.3892/ijmm_00000240. [DOI] [PubMed] [Google Scholar]
- Teng C, Guo Y, Zhang H, Zhang H, Ding M, Deng H. Identification and characterization of label-retaining cells in mouse pancreas. Differentiation. 2007;75:702–712. doi: 10.1111/j.1432-0436.2007.00170.x. [DOI] [PubMed] [Google Scholar]
- Terskikh VV, Vasiliev AV, Vorotelyak EA. Label retaining cells and cutaneous stem cells. Stem Cell Rev. 2012;8:414–425. doi: 10.1007/s12015-011-9299-6. [DOI] [PubMed] [Google Scholar]
- Togarrati PP, Sasaki RT, Abdel-Mohsen M, Dinglasan N, Deng X, Desai S, Emmerson E, Yee E, Ryan WR, da Silva MCP, Knox SM, Pillai SK, Muench MO. Identification and characterization of a rich population of CD34+ mesenchymal stem/stromal cells in human parotid, sublingual and submandibular glands. Sci Rep. 2017;7:3484. doi: 10.1038/s41598-017-03681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tolentino ES, Centurion BS, Ferreira LH, Souza AP, Damante JH, Rubira-Bullen IR. Oral adverse effects of head and neck radiotherapy: literature review and suggestion of a clinical oral care guideline for irradiated patients. J Appl Oral Sci. 2011;19:448–454. doi: 10.1590/S1678-77572011000500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez IH, Atkinson JC, Ship JA, Fox PC. Major salivary gland function in patients with radiation-induced xerostomia: flow rates and sialochemistry. Int J Radiat Oncol Biol Phys. 1993;25:41–47. doi: 10.1016/0360-3016(93)90143-j. [DOI] [PubMed] [Google Scholar]
- van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn M, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J, Torhorst J, Sauter G, Zuber M, Kochli OR, Mross F, Dieterich H, Seitz R, Ross D, Botstein D, Brown P. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol. 2002;161:1991–1996. doi: 10.1016/S0002-9440(10)64476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M, Clevers H. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Van Schoore G, Mendive F, Pochet R, Vassart G. Expression pattern of the orphan receptor LGR4/GPR48 gene in the mouse. Histochem Cell Biol. 2005;124:35–50. doi: 10.1007/s00418-005-0002-3. [DOI] [PubMed] [Google Scholar]
- Wang C, Christin JR, Oktay MH, Guo W. Lineage-Biased Stem Cells Maintain Estrogen-Receptor-Positive and -Negative Mouse Mammary Luminal Lineages. Cell Rep. 2017;18:2825–2835. doi: 10.1016/j.celrep.2017.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GY, Wang J, Mancianti ML, Epstein EH., Jr Basal cell carcinomas arise from hair follicle stem cells in Ptch1(+/−) mice. Cancer Cell. 2011;19:114–124. doi: 10.1016/j.ccr.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Larsen M, Hoffman MP, Yamada KM. Self-organization and branching morphogenesis of primary salivary epithelial cells. Tissue Eng. 2007;13:721–735. doi: 10.1089/ten.2006.0123. [DOI] [PubMed] [Google Scholar]
- Wells H, Peronace AA. Functional hypertrophy and atrophy of the salivary glands of rats. Am J Physiol. 1967;212:247–251. doi: 10.1152/ajplegacy.1967.212.2.247. [DOI] [PubMed] [Google Scholar]
- Wend P, Fang L, Zhu Q, Schipper JH, Loddenkemper C, Kosel F, Brinkmann V, Eckert K, Hindersin S, Holland JD, Lehr S, Kahn M, Ziebold U, Birchmeier W. Wnt/beta-catenin signalling induces MLL to create epigenetic changes in salivary gland tumours. Embo j. 2013;32:1977–1989. doi: 10.1038/emboj.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng PL, Vinjamuri M, Ovitt CE. Ascl3 transcription factor marks a distinct progenitor lineage for non-neuronal support cells in the olfactory epithelium. Sci Rep. 2016;6:38199. doi: 10.1038/srep38199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DM, Buenger DE, Abashev TM, Lindeman RP, Ding J, Sandell LL. Retinoic acid regulates embryonic development of mammalian submandibular salivary glands. Dev Biol. 2015;407:57–67. doi: 10.1016/j.ydbio.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- Xiao N, Lin Y, Cao H, Sirjani D, Giaccia AJ, Koong AC, Kong CS, Diehn M, Le QT. Neurotrophic factor GDNF promotes survival of salivary stem cells. J Clin Invest. 2014;124:3364–3377. doi: 10.1172/JCI74096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Yang N, Hui L, Wang Y, Yang H, Jiang X. SOX2 promotes the migration and invasion of laryngeal cancer cells by induction of MMP-2 via the PI3K/Akt/mTOR pathway. Oncol Rep. 2014;31:2651–2659. doi: 10.3892/or.2014.3120. [DOI] [PubMed] [Google Scholar]
- Ying M, Wu VW, Kwong DL. Comparison of sonographic appearance of normal and postradiotherapy parotid glands: a preliminary study. Ultrasound Med Biol. 2007;33:1244–1250. doi: 10.1016/j.ultrasmedbio.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ohbo K, Takakura A, Takebayashi H, Okada T, Abe K, Nabeshima Y. Sgn1, a basic helix-loop-helix transcription factor delineates the salivary gland duct cell lineage in mice. Dev Biol. 2001;240:517–530. doi: 10.1006/dbio.2001.0473. [DOI] [PubMed] [Google Scholar]
- Youssef NS, Said AM. Immunohistochemical expression of CD117 and vascular endothelial growth factor in retinoblastoma: possible targets of new therapies. Int J Clin Exp Pathol. 2014;7:5725–5737. [PMC free article] [PubMed] [Google Scholar]
- Zajicek G, Yagil C, Michaeli Y. The streaming submandibular gland. Anat Rec. 1985;213:150–158. doi: 10.1002/ar.1092130206. [DOI] [PubMed] [Google Scholar]
- Zangen R, Ratovitski E, Sidransky D. DeltaNp63alpha levels correlate with clinical tumor response to cisplatin. Cell Cycle. 2005;4:1313–1315. doi: 10.4161/cc.4.10.2066. [DOI] [PubMed] [Google Scholar]
- Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, Flatberg A, Johannessen H, Friedman RA, Renz BW, Sandvik AK, Beisvag V, Tomita H, Hara A, Quante M, Li Z, Gershon MD, Kaneko K, Fox JG, Wang TC, Chen D. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Qian W, Zhang H, Liang Y, Wu M, Zhang Y, Zhang X, Gao Q, Li Y. SOX2 Is a Marker for Stem-like Tumor Cells in Bladder Cancer. Stem Cell Reports. 2017;9:429–437. doi: 10.1016/j.stemcr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]