Abstract
Quantification of cardiac T1 relaxation time holds great potential for the detection of various cardiac diseases. However, due to both cardiac and respiratory motion, only one 2D T1 map can be acquired in one breath-hold with most current techniques, which limits its application for whole heart evaluation in routine clinical practice. In this study, an ECG-triggered 3D Look-Locker method was developed for cardiac T1 measurement. Fast 3D data acquisition was achieved with a spoiled gradient-echo sequence in combination with a stack-of-spirals trajectory and through-time non-Cartesian GRAPPA acceleration. The effects of different MR parameters on T1 quantification with the proposed technique were first examined by simulating data acquisition and T1 map reconstruction using Bloch equation simulations. Accuracy was evaluated in studies with both phantoms and healthy subjects. These results show that there was close agreement between the proposed technique and the reference method for a large range of T1 values in phantom experiments. In vivo studies further demonstrate that rapid cardiac T1 mapping for 12 3D partitions (spatial resolution, 2×2×8 mm3) can be achieved in a single breath-hold of ~12 sec. The mean T1 values of myocardial tissue and blood obtained from normal volunteers at 3T was 1311±66 ms and 1890±159 ms, respectively. In conclusion, a 3D T1 mapping technique was developed using a non-Cartesian parallel imaging technique, which enables fast and accurate T1 mapping of cardiac tissues in a single short breath-hold.
Keywords: T1 mapping, cardiac imaging, Look-Locker method, non-Cartesian parallel imaging
Graphical Abstract
In this study, a 3D cardiac T1 mapping technique was developed using a spoiled gradient-echo sequence in combination with a stack-of-spirals trajectory and through-time non-Cartesian GRAPPA acceleration. The method was evaluated with both phantom experiments and human studies and the in vivo results demonstrate that rapid cardiac T1 mapping for 12 3D partitions (spatial resolution, 2×2×8 mm3) can be achieved in a single breath-hold of ~12 sec.

INTRODUCTION
Cardiac T1 mapping holds great potential for the detection of various cardiac diseases, including acute and chronic myocardial infarction,1 edema,2 cardiac amyloidosis,3 and myocardial fibrosis.4 Quantification of T1 relaxation times before and after contrast administration also enables the measurement of extracellular volume fraction (ECV), a parameter that represents the percentage of extracellular space within the myocardial tissue.5 Quantification of ECV has been demonstrated as a valuable tool for the assessment of diffuse myocardial fibrosis, which is difficult to detect by conventional late gadolinium enhancement methods.6,7
In practice, due to both cardiac and respiratory motion, measurement of T1 values in the heart can be extremely challenging. The modified Look-Locker imaging (MOLLI) technique is the most commonly used approach for myocardial T1 mapping.8 It uses a single-shot balanced steady state free-precession (bSSFP) pulse sequence to acquire multiple T1-weighted images at end-diastole, which can then be used to calculate a 2D T1 map in a single slice of the heart. The whole acquisition typically requires 17 heartbeats using a standard 3-3-5 approach, which include three inversion-recovery cycles and each contains 3, 3 and 5 ECG-triggered images. Recently, several variants of the MOLLI method, including ShMOLLI, SASHA, and SAPPHIRE, have been proposed to further improve the performance of MOLLI in scan duration, accuracy, precision and reproducibility.9–11 Despite these advances, only one T1 map covering a single slice can be acquired during one breath-hold with these techniques. However, a major goal for cardiac T1 mapping and ECV measurement is to assess fibrosis, which may be highly inhomogeneous in patients with chronic myocardial infarctions, myocarditis, or hypertrophic cardiomyopathy. In such patients who may have only regional abnormalities, acquiring a few 2D slices may not provide a complete depiction of the health of myocardial tissue, potentially leading to inappropriate diagnosis and therapeutic management.12,13 Volumetric T1 mapping techniques that can provide whole heart coverage are highly desirable for routine clinical applications.
To date, several 3D T1 mapping methods have been proposed for cardiac imaging.14–18 For example, a fast spiral readout has been used to develop high-resolution, volumetric T1 mapping for the mouse heart.19 However, most techniques still require multiple long breath-holds or successful respiratory triggering to acquire a limited number of slices (typically 5~6). Recently, a rapid 3D T1 mapping method for the abdomen has been developed, which uses the Look-Locker technique in combination with the through-time spiral GRAPPA acceleration technique.20 In this study, this mapping technique has been expanded to enable rapid volumetric T1 mapping for the heart. The 3D spiral GRAPPA T1 mapping method was evaluated with phantom experiments and in vivo cardiac studies. Compared to other existing techniques, high data sampling efficiency can be achieved with the proposed technique, which uses both a spiral readout and a Look-Locker-type acquisition. In addition, a high acceleration factor is enabled through the use of the through-time spiral GRAPPA method, which has been shown to preserve image quality due to the use of geometry-specific GRAPPA weights. These features together enable an ultrafast data acquisition scheme for volumetric cardiac T1 mapping within a single breath-hold.
METHODS
3D cardiac T1 mapping
MRI experiments were performed on a Siemens 3T Skyra scanner using 34 receive channels (18 channels from the body surface array and 16 channels from the spine array). A sampling algorithm similar to a short version of MOLLI was adopted for the proposed 3D T1 measurement.21 The scan was divided into two segments (two inversion recovery periods, each of four heartbeats) with a time delay between segments (Figure 1). A nonselective adiabatic inversion pulse (duration, 10.2 ms) was applied at the beginning of each segment to invert the magnetization. Both inversion pulses were ECG-triggered, and a variable trigger delay (TD) was applied before the inversion pulse to achieve more uniform coverage of the data collection along the T1 relaxation curve. In the first segment, data acquisition started immediately after the inversion RF pulse, while in the second segment a time delay (200~300 ms depending on the heart rate) was applied before the data acquisition to ensure all the data were obtained during mid- to end-diastole.
Figure 1.
A diagram of the proposed 3D Look-Locker acquisition method. The scan was divided into two inversion-recovery periods separated by a pre-defined time delay. Both inversion pulses were ECG-triggered and a variable trigger delay (TD) was applied to achieve uniform sampling along the T1 recovery curve. While the imaging readout was only performed during diastole, the excitation pulses and all the encoding gradients were applied continuously after the inversion pulse to maintain steady-state conditions.
The MOLLI technique used for 2D cardiac T1 mapping is based on the bSSFP pulse sequence.8 However, for volumetric cardiac imaging at 3T, field inhomogeneities that are often present near the heart can lead to banding artifacts which complicate T1 quantification. In the current study, the data acquisition module was replaced with a single-shot 3D fast low angle shot (FLASH) pulse sequence, which is the same as the standard Look-Locker method.22 While the imaging data were only acquired during diastole, the excitation pulses and all the gradients were applied continuously throughout the whole inversion recovery period to maintain steady-state conditions (Figure 1).
Even though the Look-Locker method is considered to be a fast T1 mapping technique, a 17-s breath-hold is required to acquire a 2D cardiac T1 map with a Cartesian readout.8 Multiple long breath-holds would be needed for volumetric coverage in the heart, which is impractical for clinical applications. To accelerate data collection in the current study, the 3D FLASH acquisition was combined with a stack-of-spirals trajectory and through-time non-Cartesian GRAPPA.23 To meet the Nyquist criterion, a total of 48 spiral interleaves in-plane are required. To accelerate the scanning, a reduction factor of six was used in-plane (only 8 spiral interleaves were collected). Other imaging parameters included: FOV = 44×44 cm; matrix size = 224×224 for an effective in-plane resolution of 2.0 mm; number of partitions = 12; partition thickness = 8 mm; TR = 4.7 ms; TE = 0.6 ms; flip angle = 7°; partial Fourier in the partition direction = 6/8. In each heartbeat, one 3D volume of 12 partitions was acquired and the acquisition time was approximately 340 ms. As shown in Figure 1, a total of eight T1-weighted 3D volumes were obtained during one breath-hold for T1 fitting. The delay time between the inversion and the collection of the central partition was used as the effective inversion time for each 3D volume. To achieve a reliable quantification for low T1 values such as post-contrast myocardial tissue, the partition encoding was applied in a reverse order so that with partial Fourier of 6/8, a minimum TI of ~130 ms was achieved. The maximum TI was ~3500 ms and varies with the heart rate. In addition to the accelerated T1-weighted data, a 3D FLASH-based calibration scan, consisting of four fully-sampled 3D volumes (~10 seconds), was collected for use in the 3D through-time spiral GRAPPA calibration step.20 For in vivo studies, this calibration scan was acquired during free breathing without ECG gating, as suggested in the literature.20,24–26
Image reconstruction and processing
All the image reconstruction was implemented off-line using MATLAB (The Mathworks, Natick, MA) on a standalone desktop computer (Intel Xeon E3-1270 quad-core CPUs at 3.4 GHz and 16GB of RAM). The undersampled spiral data were reconstructed using 3D through-time spiral GRAPPA as described previously.20,24 In brief, the 3D through-time spiral GRAPPA reconstruction consists of two major steps. The first step is the calculation of the GRAPPA weights based on the calibration scan and the second step is the application of the calculated weights to reconstruct the missing spiral arms in the undersampled dataset. In this study, a 3×2 GRAPPA kernel was formed in the spiral readout × spiral arm directions to generate the missing k-space points from source points. Due to the nature of non-Cartesian sampling with spiral trajectory, both shape and orientation of these GRAPPA kernels vary with the sampling position in k-space (Figure 2a). To accurately calculate the GRAPPA weights for these kernel geometries, multiple occurrences of the same kernel geometry (both shape and orientation) are needed in the calibration step. For the 3D through-time spiral GRAPPA, this requirement was achieved with 1) multiple repetitions acquired through time (Figure 2b); 2) the appearance of the same GRAPPA kernel along the partition direction (Figure 2c); and 3) similar kernels along the spiral readout direction over a small segment (Figure 2d). In the current study, a k-space data segment made up of four consecutive kernels was used for the GRAPPA weight calibration. Once the GRAPPA weights have been calculated, they were applied to the portions of the accelerated data with the same geometry to reconstruct the missing points. This step was repeated until each of the missing k-space points was reconstructed. After the 3D through-time spiral GRAPPA reconstruction was completed, a non-uniform Fast Fourier Transform (NUFFT) was performed using an image reconstruction toolbox27 and multi-coil images were combined using the adaptive reconstruction technique.28 The average reconstruction time for one measurement was ~33 min, of which 29 min was spent on weight calibration and 4 min was spent on the estimation of missing points and the NUFFT.
Figure 2.
Schematic drawing for the 3D through-time spiral GRAPPA calibration. (a) Representative 3×2 GRAPPA kernels at two different k-space locations for the spiral acquisition. To accurately calculate GRAPPA weights, GRAPPA kernels with similar size and shape from three different sources were used in the calibration step, which include multiple repetitions acquired through time (b), multiple partitions within the same volume (c), and consecutive kernels in a small segment along the spiral readout direction (d). After the GRAPPA weights are calibrated, they are applied to the undersampled data in the same kernel location. Note that the kernel geometry is constant along the partition direction, which reduces the number of different kernels in the dataset and thus the number of GRAPPA weights which must be determined.
Numerical simulation to determine optimal acquisition parameters
Bloch equation simulations were performed in MATLAB to study the behavior of the magnetization over the course of the proposed acquisition scheme. The effects of the pulse sequence on tissues with T1 values ranging from 300 ms to 2000 ms, T2 values ranging from 25 ms to 100 ms (in 25 ms increments), heart rate from 40 beat per minute (bpm) to 100 bpm (in 20 bpm increments) and time delay from 3 s to 5 s (1 s increment) were investigated. At the beginning of each simulation, the longitudinal magnetization was set at −1 to simulate the inversion pulse. The signal for each T1 value was then calculated for approximately 4 heartbeats, following by a free T1 relaxation period determined by the pre-defined time delay (3 ~ 5 s). The longitudinal signal attained at the end of the time delay was then inverted for the preparation of the second acquisition section. Any incomplete inversion recovery due to insufficient time delay was considered in the simulations. After both acquisition sections were simulated, transverse magnetization values at eight inversion times, which are determined by specific heart rate, were used to fit the T1 values and the results were compared to the ground truth T1 values.
The effects of noise and transmit field inhomogeneity (B1) on the accuracy of T1 measurement were further investigated using Bloch equation simulations. T1 values ranging from 300 ms to 2000 ms were examined with some fixed parameters including a T2 of 50 ms, a heart rate of 60 bpm, and a time delay of 4 s. Variable levels of Gaussian white noise of 5%, 10% and 20% were added to the data and the simulations were repeated 100 times for each set of noise level and T1. B1 field inhomogeneity ranging from 0.8 to 1.2 (in 0.1 increments) was also investigated. Here, B1 field of 1.0 indicates that a nominal flip angle of 7° was applied in the simulation. Since an adiabatic pulse was used to invert magnetization, the variation in the inversion pulse was not considered in this study.
Phantom imaging
The accuracy of the proposed T1 measurement was validated using a manufactured phantom containing ten 50-ml vials with varying concentrations of GdCl3 and agarose. Reference T1 values were established using an inversion-recovery single-echo spin-echo sequence (TR = 6 s; data collected at eight inversion times of 30, 50, 100, 200, 500, 1000, 2000 and 3800 ms). The proposed T1 mapping measurement was performed using simulated ECG signals with heart rates ranging from 40 bpm to 100 bpm in 20 bpm increments. The MOLLI sequence with the standard 3-3-5 pattern (Siemens WIP 780C) was also performed at a simulated heart rate of 60 bpm as a comparison. Inversion times of 120, 200 and 280 ms were applied in the three inversion recovery periods. Other imaging parameters for the MOLLI acquisition included: FOV = 44×44 cm; matrix size = 224×224; TR = 2.24 ms; TE = 1.12 ms; flip angle = 20°; slice thickness = 8 mm; GRAPPA factor = 2; partial Fourier in the phase-encoding direction = 7/8.
In vivo studies
After phantom validation, 3D T1 mapping of the heart was performed on thirteen asymptomatic volunteers (male: female, 9:4; 29.3 ± 13.6 years) in the short-axis view at 3T. The study was approved by our institutional review board and written consent was obtained from all subjects prior to imaging. For eight of the total thirteen subjects (male: female, 5:3; 28.0 ± 15.1 years), 3D T1 measurements with the spiral sequence as well as 2D MOLLI acquisitions was performed both prior to and after contrast administration for the calculation of ECV for myocardial tissues. For each subject, 3D T1 maps were first obtained using the proposed method in a single breath-hold at end exhalation. Standard 2D MOLLI acquisitions with in-line motion correction were then performed at 3~5 different slice locations from the apex to the base of the heart as a comparison. Each MOLLI acquisition was performed in a single breath-hold and the imaging parameters were the same as applied in the phantom studies. Following the baseline measurements, a bolus injection of 0.1 mmol/kg of Gadoversetamide (OptiMark; Tyco Healthcare/Mallinckrodt, St. Louis, MO) was applied at 2.5 mL/s and 3D T1 maps were obtained with the proposed technique 15 mins after the contrast injection. A post-contrast MOLLI sequence was also performed afterwards at the mid-ventricle, followed by a free-breathing calibration scan to calculate the 3D through-time spiral GRAPPA weights. The GRAPPA weights calculated with this post-contrast calibration scan were then applied to reconstruct both pre-contrast and post-contrast undersampled images for T1 calculation. For the rest five subjects (male: female, 4:1; 31.4 ± 12.2 years), only 3D T1 maps at the baseline condition were acquired, followed directly by the calibration scan.
Data analysis
T1 maps based on the MOLLI acquisition were generated in line on the scanner. All other T1 maps were generated off-line with MATLAB after the 3D through-time spiral GRAPPA reconstruction. Voxel-by-voxel nonlinear curve fitting was performed with a three-parameter model as:29
| (1) |
and T1 values after the Look-Locker correction were calculated from the resulting parameters as:
| (2) |
In phantom experiments, T1 values were determined from a square ROI (3×3) in the center of the tubes. For in vivo studies, both endocardial and epicardial contours were traced manually by Y. C. (with five years of experience in cardiac MR imaging) on all T1 maps to extract myocardial T1 values. The blood T1 values were calculated from a ROI manually traced in the LV pool. Based on myocardial and blood T1 values acquired before and after contrast administration, ECV maps were obtained as described in the literature.5 One subject moved between the pre- and post-contrast T1 measurements which led to misalignment in the T1 maps, so the ECV maps were only obtained from seven subjects. A default value of 0.4 for hematocrit was used for all subjects as blood was not drawn for the assessment of hematocrit. A paired Student’s t test was used to compare the results obtained with the proposed technique and the MOLLI method (n = 8). In all comparisons, p values less than 0.05 were taken to indicate that the parameters measured were significantly different.
RESULTS
Numerical simulations
The effects of time delay, heart rate and T2 on the accuracy of T1 quantification with the proposed technique were first evaluated using numerical simulations. Figure 3a shows two representative signal evolution curves simulated using the following input parameters: T2 = 50 ms; heart rate = 60 bpm; time delay = 4 s. Two different T1 values of 1400 ms and 600 ms are depicted, representing normal pre-contrast and post-contrast myocardial tissues. For a T1 value of 600 ms, the longitudinal magnetization returned to 99.98% of its pre-inversion level at the end of 4-s time delay, and the corresponding T1 value calculated based on 8 measured points was 614 ms. A slightly lower longitudinal magnetization (97.51%) was obtained with the longer T1 of 1400 ms. However, an accurate T1 measurement was still achieved (1395 ms) despite the incomplete inversion recovery.
Figure 3.
Numerical simulations of the effects of different data collection and tissue property parameters on T1 accuracy. (a) A plot showing the longitudinal magnetization for two representative T1 values of 1400 ms and 600 ms (time delay, 4 s; T2, 50 ms; heart rate, 60 bpm). At the end of the time delay, the longitudinal signal reached 97.51% and 99.98%, respectively. (b) A plot showing the effects of various time delays on the accuracy of T1 measurements. (c) A plot showing the effects of heart rate on the accuracy of T1 measurements. (d) A chart depicting the effects of differences in T2 values on the accuracy of T1 measurements. The nominal T1 value used for native myocardium, native blood, post-contrast myocardium and post-contrast blood was 1300 ms, 1900 ms, 700 ms and 300 ms, respectively. (e) Effect of Gaussian white noise on T1 accuracy. The results were averaged from 100 simulations for each setting. (f) Effect of B1 field inhomogeneity on T1 measurement with the proposed technique.
Figure 3b shows the impact of the time delay on the accuracy of T1 estimation. No correlation between T1 accuracy and time delay was observed when T1 was less than 1400 ms and a maximum 5.6% error was observed for T1 of 300 ms. For T1 higher than 1400 ms, the error in T1 measurement decreased with increased delay times and a maximum 5% error was observed with a 3-s time delay for T1 of 2000 ms. To ensure an accurate T1 measurement, a time delay of 4 s was used in the following simulations and in vivo studies.
The effect of heart rate variations on the accuracy of T1 measurements was also evaluated. All simulations were performed with a fixed 4-s time delay and 50 ms T2 relaxation time. As shown in Figure 3c, each T1 value could be quantified with an error of less than 6%, and no clear dependence of T1 measurement on heart rate was observed for a large range of heart rates from 40 bpm to 100 bpm.
Figure 3d shows the effect of T2 relaxation time on the accuracy of T1 measurement. Four categories representing T1 values from native myocardial tissue (1300 ms), native blood (1900 ms), post-contrast myocardial tissue (700 ms), and post-contrast blood (300 ms) are shown. For most of the cases except native blood (1900 ms), the T1 error increased with increasing T2 values. A maximum 6.1% error was noticed for the case with T1 of 300 ms and T2 of 100 ms (the combination of the shortest T1 and longest T2 examined).
Figure 3e shows the mean T1 error obtained from 100 simulations for each noise level. For each T1 value, an increase in mean T1 error was observed when the noise level increased. A maximum error of 6.1% was observed for 20% of noise and nominal T1 of 300 ms.
The effect of B1 field inhomogeneity on the accuracy of T1 quantification is shown in Figure 3f. The accuracy of T1 quantification is affected by transmit B1 field mainly for low T1 values (less than 1200 ms). A maximum error of 7.3% was noted for B1 of 1.2 and nominal T1 of 300 ms.
Phantom studies
The accuracy of the 3D spiral GRAPPA-based T1 measurements was evaluated in phantom studies. Figure 4 demonstrates that T1 values acquired with the proposed technique are in close agreement with the results from an IR spin echo sequence for a wide range of T1 relaxation times from 200 to 1700 ms (mean percentage difference, 2.6±4.7%). The measurement was also performed by simulating various heart rates using artificial ECG signals. A representative T1 map acquired at a simulated heart rate of 100 bpm is shown in Figure 4b. For heart rates ranging from 40 bpm to 100 bpm, the largest T1 variation among all the vials was 1.1%, which is consistent with the simulation results. The T1 measurement in phantoms was also performed with the MOLLI sequence and the results are in good agreement with the standard reference (Figures 4c&d; mean percentage difference, 3.4±2.2%). However, a consistent underestimation of T1 with the MOLLI technique was noted as compared with the reference method.
Figure 4.

T1 maps of multi-compartment phantoms acquired using the proposed 3D spiral GRAPPA Look-Locker technique with a simulated ECG of 60 bpm (a) and 100 bpm (b), the inversion-recovery single-echo spin echo method (c) and standard 3-3-5 MOLLI technique with a simulated ECG of 60 bpm (d). (e) Comparison of T1 values from ROI analysis in (a) and (d).
In vivo studies
The baseline T1 measurements were performed on thirteen normal subjects. The mean heart rate was 63.7±5.5 bpm during the scan and the average acquisition time for T1 mapping in 12 partitions was 11.4±0.6 sec (~12 heartbeats in a single breath-hold). Figure 5 shows a single partition depicting representative T1-weighted images generated using 3D spiral GRAPPA and the corresponding T1 map from a healthy volunteer. Examples of T1 maps from 5 partitions of the 12 collected from three additional subjects are presented in Figure 6. The heart rate varied between 55 and 70 bpm among the three subjects. For all of them, both the myocardial wall and papillary muscles are well delineated. For the left ventricular myocardium, the mean T1 value for each subject was 1359±55 ms (Figure 6a), 1332±30 ms (Figure 6b), and 1339±57 ms (Figure 6c). These results demonstrate that 3D quantitative T1 measurement from the heart can be achieved in a single breath-hold with the proposed technique. The mean T1 values of left ventricular myocardium and blood before contrast from thirteen subjects were 1311±66 ms and 1890±159 ms, respectively.
Figure 5.
Representative T1-weighted images collected using the 3D spiral trajectory and reconstructed using 3D spiral GRAPPA, and the corresponding T1 map from a healthy subject (in-plane resolution, 2.0 mm; partition thickness, 8 mm). 3D T1 maps of 12 partitions were acquired in a single breath-hold of 11.3 sec for this subject and only one T1 map is plotted here.
Figure 6.
Representative 3D T1 maps acquired using the 3D spiral GRAPPA Look-Locker method in multiple subjects. For each subject, five out of a total of 12 partitions are presented that cover the heart from base to apex.
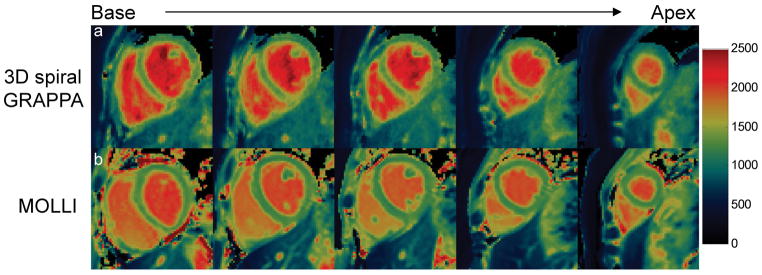
Figure 7a shows representative short-axis cardiac T1 maps from base to apex acquired using 3D spiral GRAPPA during a breath-hold scan from another volunteer. The 2D T1 maps acquired from similar positions using the MOLLI sequence are also presented in Figure 7b. Both techniques provide good definition of myocardial walls and homogenous myocardial tissues. Comparison between these two techniques revealed that the T1 values were substantially higher in both myocardial tissues and blood with the proposed technique (myocardium, 1319±44 ms; blood, 2141±100 ms) as compared to those from the MOLLI technique (myocardium, 1271±37 ms; blood, 1968±41 ms).
Figure 7.
(a) Representative 3D cardiac T1 maps from base to apex obtained from one acquisition at the baseline. (b) 2D T1 maps with MOLLI acquired with multiple breath-holds at similar locations as in (a). Consistently higher T1 values were observed for both myocardium tissues and blood with the proposed 3D technique.
Figure 8 shows representative T1-weighted images generated using the proposed 3D T1 mapping technique (Figure 8a) and the corresponding T1 map acquired 15 min after contrast administration (Figure 8b). The post-contrast MOLLI T1 map is also shown in Figure 8c. Note that banding artifacts due to field inhomogeneities can be observed in the T1 map acquired with MOLLI but not with the proposed technique. As compared with the results from MOLLI (myocardium, 614±14 ms; blood, 439±21 ms), substantially higher T1 values in both myocardium and blood were also observed after contrast administration with the proposed technique (myocardium, 688±38 ms; blood, 522±42 ms). Table 1 summarizes the results from the eight subjects with both pre- and post-contrast measurements. All the T1 values were significantly higher with the proposed technique as compared to the MOLLI measurement (p<0.05).
Figure 8.

Representative T1-weighted images (a) and the corresponding T1 map (b) acquired 15 min post contrast administration. (c) Post-contrast 2D MOLLI map from the same location. Severe banding artifacts were observed in the T1 map with MOLLI, but not the proposed technique.
Table 1.
Average T1 relaxation times (ms) in the myocardium and blood acquired at baseline and 15 min after contrast administration from 8 healthy subjects.
| Myocardium | Blood | |||
|---|---|---|---|---|
|
|
|
|||
| Native | Post-contrast | Native | Post-contrast | |
| 3D Spiral | 1309 ± 66 | 732 ± 65 | 1930 ± 193 | 600 ± 49 |
| MOLLI | 1230 ± 44* | 690 ± 57* | 1783 ± 166* | 529 ± 62* |
p < 0.05 as compared with the proposed T1 mapping technique.
Based on the pre- and post-contrast measurements, the ECV maps were calculated. Figure 9 shows representative ECV maps from the same subject using the proposed 3D T1 mapping technique (Figure 9a) and MOLLI technique (Figure 9b). The mean ECV from 7 subjects was 32.2%±5.0% and no statistically significant difference was found as compared to the MOLLI results (31.0%±5.7%; p>0.05).
Figure 9.
Representative ECV maps acquired using the proposed technique (a) and MOLLI (b) from the same volunteer. The average ECV values of myocardium are given at the bottom of each map.
DISCUSSION
In this study, rapid 3D T1 mapping was achieved by collecting accelerated spiral data and reconstructing images with through-time spiral GRAPPA. This technique was originally proposed for free-breathing 2D cardiac imaging and then found widespread use in multiple 3D applications, including breath-hold 3D cardiac perfusion imaging,25 free-breathing whole-liver perfusion imaging,24 and 3D abdominal T1 mapping.20 In this study, a 3D cardiac T1 mapping technique was developed, which allows fast and accurate T1 mapping of the heart in a single short breath-hold.
While multiple techniques have been proposed for fast T1 mapping, MOLLI is the most widely used because of its robustness and high precision in myocardial tissue characterization. However, multiple studies have shown that MOLLI provides a consistent underestimation of T1 values due to various factors including magnetization transfer (MT) effect, inversion pulse accuracy and T2 dependence.30–32 Robson et al. have showed that even though SASHA uses the same bSSFP sequence, the frequently applied saturation pulses at each heartbeat reduce the accumulation of the MT effect and therefore enable more accurate T1 quantification.32 The drawback of this technique is the low precision also due to the application of saturation pulses.33
In the current study, data acquisition was performed using the 3D FLASH sequence, which provides multiple advantages as compared to the bSSFP sequence. First, field inhomogeneities at 3T are problematic for cardiac imaging when using a bSSFP-based sequence, which leads to the well-known banding artifacts in both T1-weighted images and cardiac T1 maps. Kellman et al. have shown that even small fluctuation in B0-field can introduce substantial regional variations in myocardial T1 values with MOLLI.34 In contrast, the 3D FLASH sequence is less sensitive to the field inhomogeneities and therefore can provide more robust T1 mapping, especially at 3T (Figure 8). Secondly, as compared to a high flip-angle bSSFP sequence, the low flip-angle FLASH readout is less sensitive to the MT effect. With the proposed technique, consistently higher T1 values in pre- and post-contrast myocardium tissues were observed as compared to the results obtained using the MOLLI technique. These results matched well with the previous findings with SASHA and numerical simulations.9,30,32 Finally, continuous RF excitations were applied throughout the whole inversion recovery period, which can achieve a full tracking of the MR signal and potentially provide more accurate T1 quantification. In fact, the standard Look-Locker method was proposed with the FLASH readout.22 Even though T1 recovery is perturbed by the continuous RF excitations with the FLASH acquisition, accurate T1 measurement can be achieved by applying a correction factor (Eq. 2) given that the tissue follows the RF excitations for the whole inversion recovery period. However, such a condition is difficult to achieve with 2D cardiac T1 mapping where constant through-plane motions exist over the cardiac cycle. For this reason, a bSSFP sequence with fewer perturbations of the MR signal was used in the 2D MOLLI technique.8,35 In this study, the 3D acquisition scheme allows the excitation of the entire volume despite the through-plane motion. Therefore, the proposed technique can provide accurate results with a 3D FLASH readout after correction (Eq. 2). The penalty of using the 3D FLASH sequence is its intrinsic low SNR as compared to bSSFP sequence. However, with a 3D acquisition and a spiral trajectory, good image quality was still achieved as shown in our in vivo results (Figures 5–9).
The baseline T1 relaxation times in the myocardium (1311±66 ms) and blood (1890±159 ms) acquired with the proposed 3D method are consistent with previous findings using MOLLI (myocardium, 1315±39 ms; blood, 2020±129 ms) and shMOLLI (myocardium, 1286±59 ms; blood, 2074±112 ms) at 3T.21,36 In both of these studies, the T1 underestimation with the MOLLI technique was corrected based on the results of phantom experiments. Additionally, because a similar percentage increase in both myocardial T1 (5.8%) and blood T1 (7.5%) values was observed as compared to those from MOLLI, the ECV value obtained with the proposed 3D method is close to the MOLLI results and the values from both techniques are consistent with the numbers reported in the literature.7,21,36
In the spiral GRAPPA reconstruction, fully sampled dataset from multiple time points were used to calibrate the GRAPPA weights. While substantial signal intensity changes and motion exist in the calibration data as a whole, the points within a single kernel are collected close to each other in time to avoid the inconsistencies which could arise in the weights due to signal intensity changes within the GRAPPA kernel. Each GRAPPA kernel only includes seven consecutive spiral readouts (for a reduction factor of 6), which in this study are collected over a period of ~33 ms. Early studies have demonstrated that when multiple repetitions with different underlying images (due to contrast changes or respiratory/cardiac motion) are used for GRAPPA weight calibration, the weights provide information about the coil sensitivities, and do not contain information about the image content or contrasts23,37. Thus, the through-time GRAPPA reconstruction is appropriate for applications such as T1 mapping which require images with different T1-weightings to be collected rapidly.
In the current study, the acquisition time of 340 ms for one 3D volume is substantially longer than that of the MOLLI technique (~200 ms for one 2D image). Even though robust T1 measurement was achieved with healthy subjects in this study, further acceleration in the acquisition speed would be beneficial for patient scans and will be explored by applying Cartesian GRAPPA in the partition direction in the future.38 This long acquisition window also limits our spatial resolution for T1 quantification. Some blurring in both T1-weighted images and T1 maps were noticed in our in vivo studies, which is likely due to either the long acquisition window or partial volume effects with an 8-mm partition thickness. Imaging acceleration in the partition direction can also be applied to improve the spatial resolution and reduce the acquisition window in the future. Free-breathing imaging approaches, when successful respiratory triggering is achieved, have the freedom to obtain higher spatial resolutions at the cost of longer scan times.15 Additionally, previous studies have demonstrated the importance of image registration for accurate T1 mapping39 and such registration has been implemented in the MOLLI technique used in this study. No image registration was performed with the proposed 3D spiral GRAPPA technique, although undesired cardiac or respiratory motion could affect T1 quantification (Figure S1). On the other hand, with greater coverage in the true 3D acquisition, more accurate and reliable registration is expected with the proposed technique as compared to the 2D acquisition and this subject will be pursued in future studies.
Based on the numerical simulations, a trend of increase in T1 error was observed with higher T2 values (Figure 3d), while a previous study with MOLLI has shown larger differences (>5%) with lower T2 values (<30 ms).30 This discrepancy is likely due to different selections of readout sequence. As compared to the bSSFP sequence applied in MOLLI, the 3D FLASH sequence used in this study is less sensitive to low T2 values, which is desired for post-contrast measurements. However, it is more challenging to achieve complete spoiling of long T2 components with FLASH. Our simulation and phantom results also suggest that the T1 accuracy is not strongly dependent on heart rate (Figures 3, 4 and S2). However, cardiac motion was not simulated in these phantom studies. In fact, with the current acquisition window of 340 ms, the heart rate would be expected to influence T1 accuracy, especially in patients with a fast heart rate. In the simulation study, the influence of imperfect inversion on T1 accuracy was not examined, but the same conclusion obtained from previous studies is expected to be valid with the proposed technique.31
There are several limitations in the current study. Firstly, the acquisition window of 340 ms is long which not only increases the sensitivity to cardiac motion, but also introduces considerable contrast variation along the z direction in k-space. Further acceleration in the image acquisition is needed to reduce this time window. Secondly, similar to other 3D acquisitions, the accuracy of T1 measurement in the partitions at the edges of the slab (two at each end for the current study) is influenced by the slice profile of the RF pulse. The accuracy of the T1 maps in these slices could to be improved in the future by including the slice profile correction in the data analysis. Finally, as compared to the results acquired with MOLLI, inhomogeneous T1 maps in the left ventricular blood pool was noticed with the proposed technique in the current study. Both MOLLI and the proposed technique quantify T1 using signal evolution acquired from multiple heart beats, so both are sensitive to flow artifacts. While the underlying mechanism for the inhomogeneous blood T1 is not clear, it is likely due to different excitation pulses between MOLLI (2D slice-selective) and the proposed method (3D volumetric excitation covering the whole left ventricle).
In conclusion, a 3D T1 mapping technique was developed using a non-Cartesian parallel imaging technique, which enables fast and accurate T1 quantification of cardiac tissues in a single short breath-hold of ~12 sec.
Supplementary Material
Acknowledgments
Grant Support: Siemens Healthineers and NIH grants R01HL094557, 1R01DK098503, 1R01EB017219, 5R01EB016728, and 2KL2TR000440.
Abbreviations used
- bSSFP
balanced steady state free-precession
- ECV
extracellular volume fraction
- FLASH
single-shot 3D fast low angle shot
- IR
inversion recovery
- MOLLI
modified Look-Locker imaging
- MT
magnetization transfer
- NUFFT
a non-uniform Fast Fourier Transform
- ROI
region-of-interest
- SAPPHIRE
saturation pulse prepared heart-rate-independent inversion recovery
- SASHA
saturation recovery single-shot acquisition
- ShMOLLI
shortened modified Look-Locker inversion recovery
References
- 1.Messroghli DR, Walters K, Plein S, et al. Myocardial T1 mapping: application to patients with acute and chronic myocardial infarction. Magn Reson Med. 2007;58(1):34–40. doi: 10.1002/mrm.21272. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira VM, Piechnik SK, Dall’Armellina E, et al. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14(1):42. doi: 10.1186/1532-429X-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karamitsos TD, Piechnik SK, Banypersad SM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6(4):488–497. doi: 10.1016/j.jcmg.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52(19):1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 5.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson. 2012;14(1):64. doi: 10.1186/1532-429X-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flett AS, Hayward MP, Ashworth MT, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: Preliminary validation in humans. Circulation. 2010;122(2):138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 7.Kellman P, Wilson JR, Xue H, et al. Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. J Cardiovasc Magn Reson. 2012;14(1):64. doi: 10.1186/1532-429X-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52(1):141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 9.Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, Thompson RB. Saturation recovery single-shot acquisition (SASHA) for myocardial T1 mapping. Magn Reson Med. 2014;71(6):2082–2095. doi: 10.1002/mrm.24878. [DOI] [PubMed] [Google Scholar]
- 10.Weingärtner S, Akçakaya M, Basha T, et al. Combined saturation/inversion recovery sequences for improved evaluation of scar and diffuse fibrosis in patients with arrhythmia or heart rate variability. Magn Reson Med. 2014;71(3):1024–1034. doi: 10.1002/mrm.24761. [DOI] [PubMed] [Google Scholar]
- 11.Piechnik SK, Ferreira VM, Dall’Armellina E, et al. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12(1):69. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57(8):891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schelbert EB, Messroghli DR. State of the art: clinical applications of cardiac T1 mapping. Radiology. 2016;278(3):658–676. doi: 10.1148/radiol.2016141802. [DOI] [PubMed] [Google Scholar]
- 14.Coniglio A, Di Renzi P, Vilches Freixas G, et al. Multiple 3D inversion recovery imaging for volume T1 mapping of the heart. Magn Reson Med. 2013;69(1):163–170. doi: 10.1002/mrm.24248. [DOI] [PubMed] [Google Scholar]
- 15.Weingärtner S, Akçakaya M, Roujol S, et al. Free-breathing post-contrast three-dimensional T1 mapping: Volumetric assessment of myocardial T1 values. Magn Reson Med. 2014;222:214–222. doi: 10.1002/mrm.25124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weingärtner S, Roujol S, Akçakaya M, Basha TA, Nezafat R. Free-breathing multislice native myocardial T1 mapping using the slice-interleaved T1 (STONE) sequence. Magn Reson Med. 2015;74:115–124. doi: 10.1002/mrm.25387. [DOI] [PubMed] [Google Scholar]
- 17.Clique H, Cheng HLM, Marie PY, Felblinger J, Beaumont M. 3D myocardial T1 mapping at 3T using variable flip angle method: Pilot study. Magn Reson Med. 2014;71:823–829. doi: 10.1002/mrm.24688. [DOI] [PubMed] [Google Scholar]
- 18.Kvernby S, Warntjes MJB, Haraldsson H, Carlhäll C-J, Engvall J, Ebbers T. Simultaneous three-dimensional myocardial T1 and T2 mapping in one breath hold with 3D-QALAS. J Cardiovasc Magn Reson. 2014;16:1–14. doi: 10.1186/s12968-014-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castets CR, Ribot EJ, Lefrancois W, et al. Fast and robust 3D T1 mapping using spiral encoding and steady RF excitation at 7T: Application to cardiac manganese enhanced MRI (MEMRI) in mice. NMR Biomed. 2015;28(7):881–889. doi: 10.1002/nbm.3327. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Lee GR, Aandal G, et al. Rapid volumetric T1 mapping of the abdomen using three-dimensional through-time spiral GRAPPA. Magn Reson Med. 2016;75:1457–1465. doi: 10.1002/mrm.25693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JJ, Liu S, Nacif MS, et al. Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson. 2011;13(1):75. doi: 10.1186/1532-429X-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haase A, Matthaei D, Bartkowski R, Duhmke E, Leibfritz D. Inversion recovery snapshot FLASH MR imaging. J Comput Assist Tomogr. 1989;13(6):1036–1040. doi: 10.1097/00004728-198911000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Seiberlich N, Lee G, Ehses P, Duerk JL, Gilkeson R, Griswold M. Improved temporal resolution in cardiac imaging using through-time spiral GRAPPA. Magn Reson Med. 2011;66(6):1682–1688. doi: 10.1002/mrm.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Lee GR, Wright KL, Griswold MA, Seiberlich N, Gulani V. Free-breathing liver perfusion imaging using 3D through-time spiral GRAPPA acceleration. Invest Radiol. 2015;50:367–375. doi: 10.1097/RLI.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barkauskas KJ, Rajiah P, Ashwath R, et al. Quantification of left ventricular functional parameter values using 3D spiral bSSFP and through-time Non-Cartesian GRAPPA. J Cardiovasc Magn Reson. 2014;16(1):65. doi: 10.1186/s12968-014-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright KL, Chen Y, Saybasili H, Griswold MA, Seiberlich N, Gulani V. Quantitative high-resolution renal perfusion imaging using 3-dimensional through-time radial generalized autocalibrating partially parallel acquisition. Invest Radiol. 2014;49(10):666–674. doi: 10.1097/RLI.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fessler JA. On NUFFT-based gridding for non-Cartesian MRI. J Magn Reson. 2007;188(2):191–195. doi: 10.1016/j.jmr.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array NMR imagery. Magn Reson Med. 2000;690:682–690. doi: 10.1002/(sici)1522-2594(200005)43:5<682::aid-mrm10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 29.Deichmann R. Fast high-resolution T1 mapping of the human brain. Magn Reson Med. 2005;54(1):20–27. doi: 10.1002/mrm.20552. [DOI] [PubMed] [Google Scholar]
- 30.Gai ND, Stehning C, Nacif M, Bluemke DA. Modified Look-Locker T1 evaluation using Bloch simulations: Human and phantom validation. Magn Reson Med. 2013;69:329–336. doi: 10.1002/mrm.24251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kellman P, Herzka DA, Hansen MS. Adiabatic inversion pulses for myocardial T1 mapping. Magn Reson Med. 2014;71:1428–1434. doi: 10.1002/mrm.24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robson MD, Piechnik SK, Tunnicliffe EM, Neubauer S. T1 measurements in the human myocardium: The effects of magnetization transfer on the SASHA and MOLLI sequences. Magn Reson Med. 2013;70(3):664–670. doi: 10.1002/mrm.24867. [DOI] [PubMed] [Google Scholar]
- 33.Molli C, Kellman P, Manning WJ, Thompson RB. Reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. 2014;272(3):683–689. doi: 10.1148/radiol.14140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kellman P, Herzka DA, Arai AE, Hansen MS. Influence of off-resonance in myocardial T1-mapping using SSFP based MOLLI method. J Cardiovasc Magn Reson. 2013;15(1):63. doi: 10.1186/1532-429X-15-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheffler K, Hennig J. T1 quantification with inversion recovery TrueFISP. Magn Reson Med. 2001;45(4):720–723. doi: 10.1002/mrm.1097. [DOI] [PubMed] [Google Scholar]
- 36.Kawel N, Nacif M, Zavodni A, et al. T1 mapping of the myocardium: intra-individual assessment of post-contrast T1 time evolution and extracellular volume fraction at 3T for Gd-DTPA and Gd-BOPTA. J Cardiovasc Magn Reson. 2012;14(1):26. doi: 10.1186/1532-429X-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright KL, Hamilton JI, Griswold MA, Gulani V, Seiberlich N. Non-Cartesian parallel imaging reconstruction. J Magn Reson Imaging. 2014;40(5):1022–1040. doi: 10.1002/jmri.24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton JI, Wright KL, Barkauskas KJ, Gulani V, Seiberlich N. Through-time radial GRAPPA with in-plane and through-plane acceleration. Int Soc Magn Reson Med. 2013:p2631. [Google Scholar]
- 39.Xue H, Shah S, Greiser A, et al. Motion correction for myocardial T1 mapping using image registration with synthetic image estimation. Magn Reson Med. 2012;67:1644–1655. doi: 10.1002/mrm.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.