Abstract
Bone metastasis, or the development of secondary tumors within the bone of cancer patients, is a debilitating and incurable disease. Despite its morbidity, the biology of bone metastasis represents one of the most complex and intriguing of all oncogenic processes. This complexity derives from the intricately organized bone microenvironment in which the various stages of hematopoiesis, osteogenesis, and osteolysis are jointly regulated but spatially restricted. Disseminated tumor cells (DTCs) from various common malignancies such as breast, prostate, lung, and kidney cancers or myeloma are uniquely primed to subvert these endogenous bone stromal elements to grow into pathological osteolytic or osteoblastic lesions. This colonization process can be separated into three key steps: seeding, dormancy, and outgrowth. Targeting the processes of dormancy and initial outgrowth offers the most therapeutic promise. Here, we discuss the concepts of the bone metastasis niche, from controlling tumor-cell survival to growth into clinically detectable disease.
The most immediate and pressing concern on receiving a cancer diagnosis is to determine the extent to which it has spread—a process known as cancer metastasis. Metastasis to any organ presents a life-threatening and often incurable disease, yet among the different organs that host metastatic disease, the biology of bone metastasis is perhaps the most exceptional. The first unique aspect is the “reverse hematopoiesis” that takes place; during normal hematopoiesis, hematopoietic stem cells (HSCs) mature into the variety of cell types that form the blood and enter into circulation. During the systemic spread of cancer, this process appears to run in reverse wherein the spongy tissue of the red marrow can collect disseminated tumor cells (DTCs) from nearly any type of cancer (Aguirre-Ghiso 2007) and the nurturing niche that normally hosts HSCs is hijacked by the cancer cells (Shiozawa et al. 2011). This idea is supported by the broad clinical observation that bone metastasis only forms in sites that host hematopoietically active red marrow (Kricun 1985). A second unique feature of bone metastasis is the subversion of the biological processes that are responsible for the structure of bone, both osteolysis and osteogenesis. Considerable research efforts have focused on the unique ability of certain cancers to grow in bone by destroying the calcified matrix while others appear to grow via generating new boney tissue (Mundy 2002).
Research efforts aimed at discovering new therapies for patients with bone metastasis have almost exclusively focused on three mechanisms that can be thought of as the minimum essential requirements for forming bone metastasis. These are (1) seeding to the bone, (2) survival via dormancy, and (3) eventual outgrowth into osteolytic or -genic tumors. These efforts have led to the approval of two therapies that attenuate cancer-induced osteolysis as well as diagnostic tools that detect dormant cancer cells in the bone marrow (Esposito and Kang 2014). However, a cure for this pathology cannot be achieved until the fundamental drivers of bone metastasis formation are discovered, particularly the drivers of survival during dormancy or exit from this state. Given the clinical importance of bone metastasis, the current review will focus not only on the well-established mechanisms of bone metastasis but also areas of study that are likely to yield new clinical strategies to treat bone metastasis.
CHARACTERISTICS OF PRIMARY TUMORS THAT FORM BONE METASTASIS
The unique predilection of certain tumors to form bone metastasis while others cannot, despite sharing similar circulatory patterns and tumor-cell deposition in the bone matrix, is indicative that either the cell of origin or stromal influence at the primary tumor is essential for bone metastasis. There is also ample evidence to support the idea that inducers of cellular plasticity, cancer stemness, and the epithelial-to-mesenchymal transition (EMT) primes certain cells for bone metastasis (Kang and Pantel 2013). An illustration of the importance of the cell of origin is that healthy kidney tissue normally expresses high levels of the calcium-sensing receptor (CsR) to control calcium homeostasis in blood. Analysis of renal-cell carcinoma (RCC) patients showed a strong correlation of CsR with bone metastasis, and calcium addition to RCC cells was mitogenic in cells from patients with bone metastasis (Joeckel et al. 2014). Supporting the idea that stromal influences precede bone metastasis, a study revealed that cancer-associated fibroblasts secrete CXCL12, thereby priming the tumor cells for metastasis to organs rich in CXCL12 via selection for high SRC activity (Zhang et al. 2013). Finally, support for the necessity of stemness/EMT is shown by the finding that embryonic miR409 correlated with higher Gleason score and EMT/stemness gene signatures in prostate cancer tumors (Josson et al. 2014) or that a loss of PTEN and gain of RAS/MAPK (mitogen-activated protein kinase) signaling in prostate cancer induced an EMT that resulted in bone metastasis with 100% penetrance (Mulholland et al. 2012).
Even within cancer types with a strong propensity to develop bone metastasis, different clinical subtypes manifest bone metastasis to vastly different degrees, thereby showing that neither stromal influence nor site of origin can fully predict the mechanisms of bone metastasis. The most notable example is breast cancer bone metastasis, in which a 15-year cumulative assessment of 1357 patients with metastatic disease showed that while bone is the most common specific site of relapse for all but one subtype, bone metastasis is observed in 71% of patients with estrogen receptor–positive (ER+) tumors and 47% of patients with estrogen receptor–negative (ER−) tumors (Kennecke et al. 2010). Furthermore, patients with ER− tumors tend to manifest overt metastatic disease within 5 years of diagnosis, whereas those with ER+ tumors experience a steady rate of presentation up to 10 years after (Kennecke et al. 2010). Although this observation indicates that bone metastasis is of paramount concern to patients with all breast cancer subtypes, it indicates that the mechanisms of bone metastasis differ greatly even within one type of cancer.
METASTATIC DISSEMINATION TO BONE
Passive Shedding to the Bone and Vascular Entry
Although specialized seeding mechanisms may be required for brain metastasis (Valiente et al. 2014), lung (Gupta et al. 2007; Padua et al. 2008), and other organs, multiple lines of evidence support the idea that tumor seeding to the bone marrow may not require specialized processes but is rather the result of passive entry. This may be in part because of the fact that the bone sinusoids have a discontinuous endothelium to facilitate the passage of hematopoietic and other cells (Oghiso and Matsuoka 1979), in contrast to the tight cell–cell junctions and continuous endothelium of the lung and other organs. In a landmark study, bone metastasis–competent DTCs were detected in the bone marrow of mice harboring only atypical ductal hyperplasia or ductal carcinoma in situ (DCIS) (Hüsemann et al. 2008). Although metastasis is typically characterized as the final step of primary tumor growth, this study shows that primary tumors and metastatic lesions can develop in parallel rather than in sequence; a hypothesis that is further supported by the fact that DTC status is not correlated to tumor size (Hüsemann et al. 2008). This finding was clinically validated by the detection of DTCs in the bone marrow of patients diagnosed only with breast DCIS (Sänger et al. 2011; Banys et al. 2012) or localized prostate cancer (Melchior et al. 1997).
Even further supporting the idea that tumor seeding into the bone marrow is not a selective process, 32% of colorectal cancer patients harbored cytokeratin-positive bone marrow smears after radical resection, yet colorectal cancer rarely forms bone lesions, and none of the patients in this study developed clinical bone metastases despite relapsing at other sites (Lindemann et al. 1992). Despite this evidence that DTCs do not always develop into bone metastases (Melchior et al. 1997), meta-analyses of all studies of prognostic indicators of breast cancer relapse established the presence of cytokeratin-detectable DTCs as a highly significant predictor of relapse to bone (Braun et al. 2005). The integration of these multiple lines of evidence therefore indicates that passive dissemination of tumor cells to the bone marrow is the early step in forming bone metastasis, but is not the critical driving event of bone metastasis formation.
Maintenance within the Bone
On the other hand, numerous molecular mechanisms have been described that promote residency within the bone, potentially through chemotaxis to specialized bone niches. Among the characterized interactions, that between stromal-cell-derived factor 1 (SDF-1)/CXCL12 and CXCR4 is the best studied, both in bone metastasis and HSC function (Teicher and Fricker 2010). Early studies showed that breast cancer cells typically express both CXCR4 and CCR7, while their respective ligands, SDF-1 and CCL21, are expressed in sites that commonly host breast cancer metastases (Muller et al. 2001). Furthermore, CXCR4 is one of the most enriched genes following in vivo selection for highly bone-metastatic breast cancer variants (Kang et al. 2003). This mechanism is shared with HSCs as transgenic expression of CXCR4 ex vivo promotes better engraftment of HSCs into the bone marrow (Brenner et al. 2004; Kahn et al. 2004). Furthermore, granulocyte colony-stimulating factor (G-CSF)/granulocyte macrophage CSF (GM-CSF) is the standard treatment used to mobilize HSCs into the blood for collection and transplantation, which occurs via specific down-regulation of SDF-1 within human bone marrow (Petit et al. 2002). In a parallel manner, prostate cancer cells compete with HSCs for the same niche, and treatment with G-CSF can mobilize these prostate cells into circulation (Shiozawa et al. 2011). Treatment with AMD3100, a CXCR4 antagonist, can also mobilize acute myeloid leukemia (AML) cells from the bone into the blood where they become more sensitive to chemotherapy (Nervi et al. 2009). Further studies show that inhibition of the CXCR4 interaction between cancer cells and the stroma sensitizes these cells to standard chemotherapy (Domanska et al. 2012), pointing toward the pleiotropic role of this interaction. More targeted studies of the interaction show that specific deletion of SDF-1 in vascular endothelial cells of the bone marrow is sufficient to impede T-cell acute lymphoblastic leukemia growth, implying that an SDF-1-producing vascular niche is critical to bone metastasis progression (Pitt et al. 2015).
Pharmacologic inhibition of platelet-derived growth factor receptor (PDGFR) on bone marrow–derived cells with sunitinib has also implicated PDGFR in homing or maintenance within bone, but the exact mechanism is unclear (Catena et al. 2010). The functions of receptor activator of nuclear factor (NF)-κB (RANK) and its ligand (RANKL) also extend beyond their canonical role in osteoclastogenesis (covered below) as RANK is expressed by hormone-responsive epithelial cancers, namely, breast and prostate cancer. This expression was instructive in guiding migration along RANKL gradients in vitro, and growth of nonosteolytic bone metastases were slowed by osteoprotegerin (OPG) treatment in vivo (Jones et al. 2006). Therefore, cancer cells expressing RANK may be attracted to sites of active osteolysis where high concentrations of RANKL and other fostering stromal components exist.
SURVIVAL AND DORMANCY IN THE BONE NICHE
Clinical Evidence for Dormancy
Of all the anatomical compartments in which DTCs have been detected, the bone marrow appears to be the only site that harbors minimal residual disease from nearly every cancer type (Aguirre-Ghiso 2007), yet the majority of these cancer types will never develop bone metastases. For those cancer types that do often develop bone metastases, such as prostate or breast cancer, DTCs in the bone marrow are detected at much higher rates than metastatic disease develops (Harbeck et al. 1994; Melchior et al. 1997). This observation indicates that, although many cancer types are competent to enter a dormant state in the bone, the control of and emergence from dormancy may be the keystone variable in predicting metastatic relapse.
The duration of dormancy is widely variable; breast cancer patients diagnosed with luminal A/B tumors experience a steady probability of metastatic relapse to bone for 10 years following diagnosis, whereas those with the more aggressive triple-negative breast cancer (TNBC) subtypes develop bone metastases within 5 years of diagnosis (Kennecke et al. 2010). Further complicating the study of dormancy, it is unclear whether metastatic latency is controlled by cell-autonomous programs or by the bone metastasis “niche” (Ren et al. 2015). Understanding the molecular basis of this dormancy as well as the factors influencing the exit from dormancy will be instrumental in lowering the rate of bone metastasis–associated deaths.
Dormancy and Survival-Inducing Cellular Programs in HSCs and Cancer
Several cell-autonomous means of DTC survival during dormancy have emerged, most relating to various types of stress responses. Early reports provided evidence that early DTCs found in the bone marrow of breast cancer patients showed a high enrichment of mesenchymal-like CD44+/CD24– cells compared with the primary site (Balic et al. 2006), therefore indicating that EMT is a critical process for early dissemination and survival. Further supporting this idea, data from Malladi and colleagues (2016) has shown that early DTCs show a mesenchymal phenotype and maintain high Dickkopf-1 (DKK1) levels to inhibit active Wnt signaling and thereby evade immune surveillance. These DTCs are also resistant to conventional cytotoxic therapy, as shown by positive cytokeratin staining in bone marrow aspirates of advanced breast cancer patients after standard-of-care chemotherapy (Braun et al. 2000). A combined clinical and experimental analysis showed that Src expressed by latent breast cancer cells is a central positive regulator of DTC survival in response to the stromal environment (Zhang et al. 2013).
Stromal Control of Dormancy in the HSC and Bone Metastasis Niche
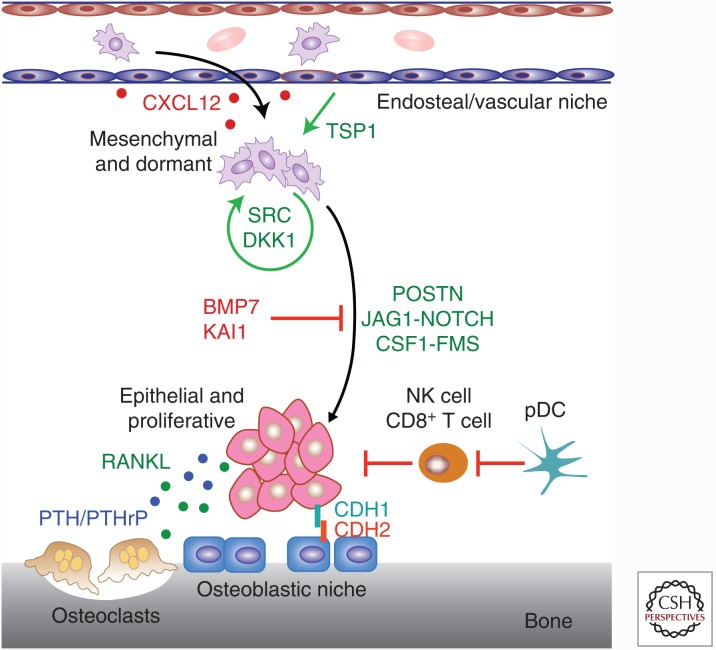
Although bone metastatic tumor cells have been ascribed with a few cell-autonomous means of maintaining dormancy during the bone metastatic process, much more evidence indicates that the metastatic “soil” controls dormancy through both context- and temporally dependent cues from the surrounding stroma. This idea is exemplified by a study of breast cancer dormancy in multiple organ sites wherein dormancy was maintained by vascular contact and communication via thrombospondin-1, but sprouting of neovasculature released transforming growth factor (TGF)-β and periostin, which stimulated proliferation of dormant cells (Ghajar et al. 2013). Other studies have found that the stromal compartment induces the p38 stress response via secretion of bone morphogenetic protein (BMP)7, which is bound by BMPR2 found on prostate cancer cells. This result was interpreted as an innate host-defense mechanism (Kobayashi et al. 2011). A third model described a situation in which high TGF-β2 concentrations in the bone caused tumor dormancy while lower concentrations in the lung were permissive to growth (Bragado et al. 2013). A similar mechanism was found wherein KAI1/CD82 is inversely correlated with prostate cancer bone metastases, and mechanistic analysis revealed that its binding to Duffy antigen receptor for chemokines (DARC) in the bone marrow causes p21-induced dormancy (Bandyopadhyay et al. 2006). In cases in which the stroma enforces a state of dormancy within the tumor cells, such as fibroblast growth factor (FGF)-induced growth arrest, adhesion to extracellular matrix proteins such as fibronectin (FN1) may be instructive in maintaining cell survival via integrin β1 binding (Korah et al. 2004). Supporting the necessity of adhesion to the niche components, one study showed that heterotypic adherence junctions between tumor-cell E-cadherin (CDH1) and osteoblast N-cadherin (CDH2) are critical to the early colonization of breast cancer cells (Fig. 1) (Wang et al. 2015).
Figure 1.
The balance between dormancy and outgrowth of bone-resident cancer cells. Multiple lines of research suggest that dormant cancer cells within the bone maintain a prosurvival mesenchymal state characterized by high thrombospondin-1 (TSP1), transforming growth factor (TGF)-β/bone morphogenetic protein (BMP) signaling and SRC activity, as well as Dickkopf-1 (DKK1)-suppressed Wnt activity. On stimulation by various stromal signaling and adhesion interactions, such as POSTN, JAG1–NOTCH, E-cadherin (CDH1)–N-cadherin (CDH2), and colony-stimulating factor 1 (CSF1)–FMS, these cells enter a proliferative and epithelial state that is vulnerable to immune surveillance as well as cytotoxic agents. The processes underlying this transition remain poorly described but are thought to rely on key factors from both the endosteal niche and the osteoblastic niche. Transit and homing between these two niches appears to be regulated by high CXCL12 levels at the endosteal site and high receptor activator of nuclear factor (NF)-κB ligand (RANKL) or parathyroid hormone–related protein (PTHrP) levels at the osteoblastic site. NK, Natural killer; pDC, plasmacytoid dendritic cell.
Although the molecular cues that control tumor-cell dormancy remain largely uncharacterized, a greater body of evidence describes the regulation of HSC dormancy. At the center of this research is a complete description of the HSC niche in both the active and quiescent state (Morrison and Scadden 2014). A series of experiments seeking to understand the physical components of the HSC niche and how those influence HSC maintenance and expansion has uncovered a critical role for osteoblasts (Zhang et al. 2003). Specifically, HSCs and osteoblasts physically interact in the bone niche at the endosteal surface, and treatment with parathyroid hormone (PTH) stimulates HSC expansion in what is assumed to be an osteoblast-dependent manner (Adams et al. 2007). Follow-up studies have since established that HSCs and osteoblastic cells are colocalized at the endosteal surface, where osteoblast stimulation with PTH induces Jagged1 expression, which in turn activates Notch signaling in HSCs to promote their proliferation (Calvi et al. 2003). Physical contact between HSC and osteoblast cells has also been shown to maintain HSC quiescence via Tie2 and ANGPTL interaction (Arai et al. 2004). In addition to osteoblasts, endothelial cells are the other major component of the HSC niche, with two studies showing that SDF-1 and stem-cell factor (SCF) expressed by endothelial cells and perivascular stromal cells are both essential for HSC maintenance (Ding et al. 2012; Ding and Morrison 2013). Another study showed that E-selectin expressed by endothelial cells in the vascular niche were bound to HSCs and induced proliferation and/or differentiation (Winkler et al. 2012). Other components of the HSC niche have also been described to a lesser extent. One study showed that mesenchymal stem cells (MSCs) are an essential regulatory element of the HSC niche as Nestin depletion concomitantly reduces HSC residency (Méndez-Ferrer et al. 2010). On the other hand, the matrix protein osteopontin (OPN) is a negative regular of HSC pools (Stier et al. 2005).
The application of these findings to the bone metastasis field has already yielded new therapeutic opportunities and showed that bone metastatic cells mimic HSCs to a large extent. For example, interactions between Jagged1–Notch1, SDF-1–CXCR4, and GM–CSF–FMS are at the center of our current understanding of bone metastasis. Therefore, additional research into the applicability of these other uncharacterized interactions, such as the role of Tie2, SCF, or E-selectin, may yield valuable insights into the regulation of bone metastatic cells in the HSC niche (Fig. 1).
Role of the Immune System in Dormancy
Avoidance of immune surveillance in early bone lesions is an essential but overlooked component of bone metastasis. Limited experiments conducted thus far indicate that the immune system limits bone metastasis colonization and enforces dormancy. Despite the observable immune privilege of the bone marrow HSC niche (Fujisaki et al. 2011), CD8+ T cells actively control proliferation and enforce dormancy of lymphoma cells (Muller et al. 1998). Clinical analysis of bone marrow aspirates from breast cancer patients found the highest proportions of CD56+ CD8+ T cells and memory CD4+ T cells in patient marrow samples harboring DTCs compared with either tumor-bearing but DTC-negative patients or healthy donors (Feuerer et al. 2001). More recent studies have shown that dormant DTCs express DKK1 to suppress Wnt signaling and therefore proliferation-associated antigens, providing a mechanism of immune escape from natural killer cells (Malladi et al. 2016). Further research has also shown that interferon-induced genes are strongly silenced in patients with spine metastasis (Bidwell et al. 2012). At the same time, components of the adaptive immune system have also shown important roles in establishing immune suppression and facilitating bone metastasis. For example, infiltrating plasmacytoid dendritic cells (pDCs) establish a sustained T helper (Th)2 response that suppresses CD8+ T-cell function and promotes regulatory T-cell (Treg) maturation. In turn, depletion of pDCs slows bone metastasis progression by promoting the expansion of cytolytic CD8+ T cells (Sawant et al. 2012).
FROM COLONIZATION TO THE VICIOUS CYCLE OF METASTATIC EXPANSION IN BONE
Triggering Outgrowth
The events taking place between the seeding on DTCs in the bone marrow and the emergence of clinically detectable bone lesions are the least characterized yet most important molecular interactions of the bone metastatic cascade. This deficiency can be traced to the fact that no bona fide experimental models of bone metastasis dormancy exist, particularly when mouse models that cause death within 3–6 weeks are compared with the multiyear pathogenesis observed in patients. Limited studies performed thus far have implicated several interactions in driving tumor outgrowth, but the discovered pathways are by no means exclusive, and research into tumor dormancy will revolutionize our understanding of metastasis in the coming years.
From the limited work that has been performed, three major developmental signaling programs appear to be the master regulators of emergence from bone metastasis dormancy: TGF-β, Wnt, and Notch. Multiple studies have shown that the TGF-β signaling pathway is active throughout the full course of bone metastasis, but its role in dormancy exit was revealed by the finding that pharmacologic inhibition was only effective in lessening bone metastasis burden when treatment commenced 3 days postinjection compared with 21 days postinjection (Korpal et al. 2009). This study further suggested that TGF-β signaling, tumor-cell growth, and osteoclastogenesis are functionally related as bisphosphonate treatment, by blocking osteoclastic bone resorption, also reduced TGF-β activity by preventing release and activation of TGF-β from the mineralized bone matrix (Korpal et al. 2009). Wnt signaling has been implicated but not proven to be a driver in bone metastasis, as Wnt suppression via DKK1 is critical to maintaining metastatic dormancy, suggesting that Wnt activation is an early event in the eventual outgrowth of metastatic lesions (Malladi et al. 2016). Of the three implicated signaling pathways, Notch activation appears to be the most important in early colonization events as well as advanced osteolysis (Zayzafoon et al. 2004; Sethi et al. 2011).
Broader transcriptional identity is also important to the early events of bone metastasis; considerable evidence suggests that EMT and stemness are essential to early DTC survival (Kang and Pantel 2013; Malladi et al. 2016). Yet, markers of epithelial cells are used to detect bone micrometastases (Mansi et al. 1987); thus, a mesenchymal-to-epithelial transition (MET) must accompany the outgrowth of metastatic lesions. Given that multiple developmental pathways are closely related to the balance between EMT/MET and stemness (Thiery and Sleeman 2006), considerable work must be performed to understand how these signaling pathways instruct tumor-cell identity.
Beyond the major signaling programs, numerous adhesion proteins and chemokine signaling programs are implicated in dormancy exit. Tumor-cell integrin α4β1 was one of the original adhesion proteins found to be important for bone metastasis (Matsuura et al. 1996), and its engagement to VCAM1 expressed by stromal cells stimulates osteoclast cells (Michigami et al. 2000). Studies have since found integrin α4β1 to be expressed on multiple stromal components of the bone with varying functions. For example, the interaction between NF-κB-induced VCAM1 on dormant breast cancer cells and integrin α4β1 expressed on monocytes can regulate the exit from dormancy by elevating local osteoclast activity (Lu et al. 2011). Regardless of the expression patterns, depletion of α4 reduces myeloma-associated osteolysis (Mori et al. 2004), whereas attachment to the bone matrix has been observed in multiple bone metastasis models via integrin α2β1 (Hall et al. 2006).
Although many studies suggest that an amplification of osteolytic activity is necessary for initial outgrowth, some evidence also suggests that there is decoupling between the bone-degradation processes and the emergence from dormancy. Osteoclast-specific deletion of either β3 integrin or Src does not affect tumor-cell proliferation in the bone but does prevent osteolysis (Bakewell et al. 2003). Given that Src was previously shown to be essential for osteolysis (Boyce et al. 1992; Schwartzberg et al. 1997), this study shows that osteoclasts are not strictly required for the early outgrowth events. One potential confounding detail of this research is that inducers of dormancy exit are difficult to distinguish from generally mitogenic mechanisms within the bone marrow. For example, lypophosphatidic acid derived from platelets and bound by bone metastatic breast cancer cells causes growth (Boucharaba et al. 2004), but the relevance to dormancy is unknown.
Vicious Cycle of Osteolytic Bone Metastasis
Once metastatic cells have established a foothold within the bone niche, they become growth-limited by multiple factors—nutrients, growth factors, oxygen delivery, and physical space. For cancer types that form osteolytic metastases, such as hormone-independent breast cancer and multiple myeloma (Mundy 2002), these constraints are solved by a unique positive feedback cycle composed of tumor cells, osteoclasts, osteoblasts, and the bone matrix. This “vicious cycle of bone metastasis” has been extensively studied (Roodman 2004; Weilbaecher et al. 2011) and is targeted by the only Food and Drug Administration (FDA)-approved bone metastasis therapies: the bisphosphonates zoledronic acid and pamidronate (zometa and aredia, Novartis), the anti-RANKL antibody, denosumab (Amgen), and a radionucleotide, radium-223 (xofigo, Bayer).
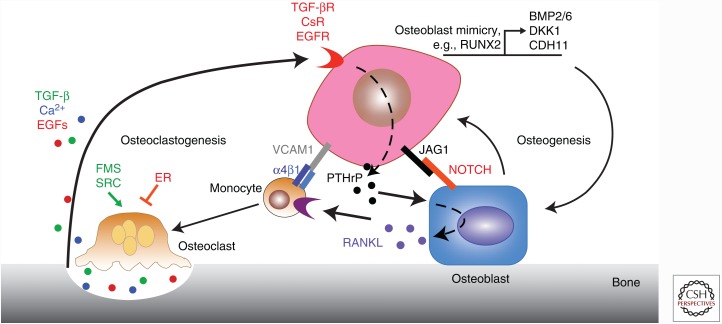
The core vicious cycle initiates when tumor cells stimulate osteoclastic bone resorption. TGF-β released from the mineralized bone matrix as a consequence of osteoclastic bone resorption binds to these bone metastatic tumor cells, which subsequently activates expression of osteolytic factors such as parathyroid hormone–related protein (PTHrP) (Yin et al. 1999) and Jagged1 (Sethi et al. 2011). Jagged1 promotes osteoclastogenesis by directly binding monocytes while PTHrP binds osteoblasts and induces the production of RANKL (TNFS11) (Boyle et al. 2003). RANKL is the major driver of osteoclast differentiation from monocytes (Anderson et al. 1997; Yasuda et al. 1998) and its binding to RANK is sufficient to induce osteoclast maturation from committed hematopoietic precursors (Lacey et al. 1998). RANKL binding to RANK induces NF-κB signaling, which is essential for osteoclast formation (Iotsova et al. 1997). Activated osteoclasts then degrade the surrounding bone matrix, resulting in the release of numerous mitogenic growth factors, such as TGF-β, which further fuels this feedforward cycle (Fig. 2) (Hauschka et al. 1986). TGF-β-induced Jagged1 further enhances this vicious cycle by stimulating the production of tumor growth–promoting interleukin (IL)-6 from osteoblasts (Fig. 2).
Figure 2.
The “vicious cycles” of osteolytic and osteogenic bone metastases. A positive-feedback cycle develops during late-stage bone metastasis in which the normal processes regulating bone homeostasis are disrupted and hijacked by tumor cells. The core process of the vicious cycle of bone metastasis involves several secreted factors. Parathyroid hormone–related protein (PTHrP) secreted by tumor cells induces receptor activator of nuclear factor (NF)-κB ligand (RANKL) secretion by osteoblasts. This stimulates the maturation of monocytes into osteoclasts, which are responsible for degrading the bone matrix. This matrix is replete with growth factors such as calcium and transforming growth factor (TGF)-β, which then bind tumor cells to induce more production of metastasis-promoting factors, such as PTHrP and Jagged1. Jagged1 directly promotes osteoclastogenesis and stimulates the production of tumor-promoting interleukin (IL)-6 from osteoblasts, leading to more bone destruction and metastatic tumor growth. This cycle is further augmented by direct interaction with monocytic or osteoblastic cells via integrin, epidermal growth factor receptor (EGFR) and other signaling pathways. Less is known about osteoblastic metastases, except that cancer cells mimic the transcriptional programs used by osteoblasts to generate various osteogenic signals. CsR, calcium-sensing receptor; ER, estrogen receptor.
Beyond this core cycle, many additional regulators and interactions have been described, both during bone metastasis and normal development (Boyle et al. 2003). One of the first discovered factors was OPG, a protein that serves as a decoy receptor for RANKL, whose administration prevents breast cancer–induced osteolysis in experimental models (Morony et al. 2001). Additional osteoclastogenesis-stimulating factors have been discovered beyond RANK–RANKL and Jagged1–Notch, the most significant of which is the CSF1–FMS interaction (Park et al. 2007).
Each core component of the vicious cycle also has pleiotropic roles in regulating osteolysis. For example, PTHrP secretion also induces CCL2 production in both endothelial and osteoblast cells of the bone marrow (Li et al. 2009). A study using mouse versus human-specific anti-CCL2 antibodies suggested that CCL2 derived both from the tumor cells and the stroma are responsible for bone metastasis progression (Loberg et al. 2007).
Several classes of proteases and extracellular matrix–modifying enzymes have been implicated in the osteolytic cascade. For example, ADAMTS1 and MMP1 were shown to release growth factors from the matrix of tumor cells, and these were in turn responsible for potentiating osteoclast signaling (Lu et al. 2009). Osteoclast-derived MMP7 has also been implicated in a similar process through solubilization of RANKL (Lynch et al. 2005). Conversely, expression of tissue-inhibitor of metalloproteases (TIMP2), or maspin, an inhibitor of uPA, prevents bone metastasis or proteolytic remodeling of bone fragments implanted with prostate cancer (Yoneda et al. 1997; Cher et al. 2003). Tumor-cell secretion of heparanase (HPSE) can distantly promote osteolytic degradation through an unknown mechanism (Kelly et al. 2005) and hypoxia induction in ER tumors has also been shown to enhance lysyl oxidase (LOX) secretion, which can independently activate the osteolytic process (Cox et al. 2015). Finally, cathepsin was implicated as an additional collagen-degrading enzyme essential for a spontaneous bone metastasis model (Withana et al. 2012).
Numerous methods to treat this vicious cycle have also emerged, in most cases derived from osteoporosis therapies. The earliest of these approaches used bisphosphonates in preclinical models, resulting in the FDA approval of pamidronate (Aredia) as the first bisphosphonate used in bone metastases for breast cancer or multiple myeloma patients. This success was followed by the approval of zoledronic acid (Zometa) to treat bone metastasis from all solid tumors or myeloma. Bisphosphonates function by binding to the mineralized bone matrix and inhibiting enzymes of the mevalonate pathway when internalized in osteoclasts. More recently, blockade of the RANKL pathway with denosumab has emerged as the most effective antiresorptive treatment for bone metastases (Esposito and Kang 2014). Another approach to prevent bone resorption is treatment with OPG, the decoy receptor for RANKL, whose administration reduces osteolysis in vitro, in vivo, and in patients (Body et al. 2003; Canon et al. 2008).
Beyond the direct targeting of osteoclasts, subsequent studies have also suggested that the bisphosphonates may have direct tumor-killing properties, albeit at concentrations much higher than those observed in vivo. It was therefore suggested that local concentrations of bisphosphonate may kill tumor cells in areas of active osteolysis while also providing a rationale for the lack of efficacy on primary tumors (Hiraga et al. 2001). Other discoveries have found that pharmacologic blockade of both TGF-β and hypoxia-inducible factor 1α (HIF1α) were additively effective in treating bone metastasis while genetic ablation of these pathways in tumor cells showed redundancy (Dunn et al. 2009). Multiple studies have also developed kinase inhibitors of the FMS receptor, which show promise in treating osteolytic bone metastasis (Murray et al. 2003; Ohno et al. 2006). Additionally, the well-characterized cycle of bone degradation does offer a silver lining in that it offers multiple means of progression monitoring, particularly in clinical trials. For example, N-telopeptide of collagen (NTX) can be used as a biochemical marker to monitor the efficacy of antiresorptive agents (Body et al. 2003).
Recently, α-emitting radionucleotides, such as radium-223, have been developed to treat osteoblastic bone metastases from prostate cancer. This agent targets bone-forming activity and has been shown to improve overall survival in prostate cancer patients with bone metastases (Parker et al. 2013; Hoskin et al. 2014; Sartor et al. 2014). Preclinical data indicate that such therapy may also be effective in treating osteolytic bone metastases (Suominen et al. 2013).
Osteoblastic Metastasis
In comparison to the well-researched mechanisms of osteolytic metastasis, the molecular processes that give rise to osteoblastic metastasis are relatively unexplored. One clear finding is that osteolytic activity is still unbalanced in osteoblastic metastases; NTX is highest in prostate cancer patients with osteoblastic disease, compared with patients with osteolytic bone metastases (such as breast cancer). Furthermore, increased NTX predicts higher overall morbidity (Coleman et al. 2005) and death in patients with prostate cancer (Brown et al. 2005). In fact, NTX was a more significant predictor of death than prostate-specific antigen (PSA) in this setting (Brown et al. 2005). Several osteoblast-exclusive signaling programs have also been discovered. One mechanism that increases bone mineralization via increased osteoblast activity is secretion of BMP2 or BMP6 by metastatic prostate cancer cells (Dai 2005). Another interesting clue giving insight into the pathogenesis of osteoblastic bone metastasis is the well-validated role of RUNX2 in promoting both breast and prostate bone metastasis (Akech et al. 2010; Baniwal et al. 2010). RUNX2 is a transcription factor found in mature osteoblasts (Lee et al. 2000) that was shown to exert pleiotropic roles necessary for the metastatic process (Baniwal et al. 2010). Osteoblast cadherin (CDH11) was also validated as an important stromal interaction protein in prostate cancer (Chu et al. 2008), further supporting the idea that osteoblastic cancer cells mimic osteoblast-expression programs. Studies have also suggested that DKK1 is the decisive factor in determining the balance between tumor-induced osteolysis versus osteogenesis. In one study, DKK1 depletion promoted exclusively osteolytic lesions while supplementation converted to exclusively osteoblastic metastasis (Hall et al. 2005). However, the reverse scenario was observed in multiple myeloma patients, in which DKK1 inhibited osteoblast maturation (Tian et al. 2003).
This process of osteolytic destruction or osteogenesis does not only engage and affect the balance of osteoblast and osteoclast cells. Recent findings have also established that the vicious cycle is responsible for suppressing HSC differentiation into each of the different lineages (Bruns et al. 2012) as well as affecting the number and quality of hematopoietic progenitor cells (HPCs) (Colmone et al. 2008). This HSC/HPC suppression was attributed to increased TGF-β signaling that reduced the plasticity of HSCs and HPCs by broadly changing adhesion and other niche interactions (Bruns et al. 2012).
Resistance to Current Therapies
Bone metastases show peculiar resistance mechanisms that were not anticipated a priori; but are rather caused by cross talk between the metastatic cells and the bone stroma. At the center of this is the interaction between steroid hormones, osteoblasts/osteoclasts, and tumor cells.
Despite broad use of bisphosphonates as highly effective bone metastasis therapies, an early meta-analysis of bisphosphonate treatment concluded that bisphosphonate treatment did not significantly benefit patients (Ha and Li 2007). A second meta-analysis performed a decade later deconvoluted this result by showing that bisphosphonate treatment is only effective in preventing skeletal-related events in patients who were postmenopausal when treatment began (Coleman et al. 2015). This was experimentally validated by a study showing that zoledronate slowed osteolysis only in ovariectomized mice compared with sham when injected with a triple-negative breast cancer line, thereby mimicking the postmenopausal state and showing that zoledronate is only effective in low-estrogen individuals (Ottewell et al. 2014a). Many of these observations can be explained by the finding that free estrogen binding to ERα causes apoptosis in osteoclasts in females (Nakamura et al. 2007), therefore indicating that estrogen and bisphosphonates may act redundantly. However, free estrogen or androgens are mitogenic for breast cancer and prostate cancer cells, and depletion of free steroid levels is a mainline therapy for most patients (Dowsett et al. 2015). Thus, a paradox emerges wherein aromatase inhibitors reduce free estrogen levels and therefore breast cancer cell growth yet contribute to increased osteoclast activity (Smith and Dowsett 2003). In this setting, complete estrogen deprivation with ovariectomy and aromatase inhibitors increases bone resorption and bone loss, thereby stimulating tumor growth in bone of triple-negative breast cancer. This effect was blocked by zoledronic acid (Wright et al. 2017). This paradox is not constrained to ER+ breast cancer as androgen deprivation similarly promotes prostate cancer bone metastasis in an experimental system (Schneider et al. 2005; Ottewell et al. 2014b). Few mechanistic studies have analyzed the relationship behind hormone status and bone metastasis. Thus far, these studies have only shown higher expression on RANKL on bone marrow–derived cells from menopausal women versus premenopausal (Eghbali-Fatourechi et al. 2003), and that animals with experimentally induced osteoporosis by ovariectomy or mir-34 knockout suffer from increased bone metastasis (Krzeszinski et al. 2014). Collectively, these data suggest that increased osteoclastic bone resorption by hormone-deprivation therapy (both androgen and estrogen) can induce a high bone turnover state that may fuel tumor growth in bone. Thus, it is very important to monitor skeletal health and prevent bone loss when treating cancer patients with these hormonal therapies.
Numerous interactions that have been studied in models of bone metastasis have also resulted in late-stage clinical trial failures. For example, a phase 3 trial of an endothelin A (ET-1) antagonist in metastatic prostate cancer showed no effect on bone metastatic progression (Carducci et al. 2007), despite broad experimental proof for the role of ET-1 in promoting both osteoblast and prostate cancer growth (Nelson et al. 1995; Yin et al. 2003). This was in part due to the fact that the phase 3 trial was designed with overall survival as a primary end point, rather than using a bone-specific end point, such as bone metastases development. Animal studies clearly showed that ET-1 blockade had bone-specific effects, rather than effects on overall survival. Therefore, the primary end points of the phase 3 trial were not consistent with mechanisms derived from animal models and underscore the fact that clinical trial end points should be chosen based on mechanisms derived from preclinical studies. Interestingly, a secondary effect of therapeutic inhibitors of the inhibitor of apoptosis (IAP) proteins showed that, whereas these may be effective in killing tumor cells, these therapies also stimulate the NF-κB pathway in osteoclasts, thereby promoting bone metastasis (Yang et al. 2012) and highlighting the importance of considering metastasis-related side effects of new cancer-targeting therapies.
CONCLUSIONS AND FUTURE DIRECTIONS
Research into the molecular mechanisms of bone metastasis has significantly enhanced our understanding of the disease, both in patients and experimental models. This has refined and redirected focus from studying bone seeding to understanding that survival via dormancy and the exit from dormancy are also crucial processes to study. Unfortunately, this has also revealed a limitation in the divergence between clinical and experimental studies—current mouse models of bone metastasis have not been developed that accurately replicate the natural history of clinical progression. Owing to the lack of good models of dormancy and the challenge in developing financially viable clinical trials relevant to the prevention of bone metastasis, no therapies have been developed to target dormancy per se. This is particularly important, as targeting of dormancy might be the most optimal method to decrease the overall incidence of bone metastasis. In future efforts to develop relevant mouse models of bone metastasis seeding, dormancy, and exit, we suggest that a closer relationship be made between HSC and bone metastasis research, with discoveries from one field applied to the other.
In addition to this shortcoming in the experimental research, considerable progress must also be made in clinical research. Given that breast and prostate cancer patients are the most severely affected by bone metastasis, a more thorough understanding of how estrogen and androgen interact with both bone-resident tumor cells and endogenous stromal cells during hormone therapies must be achieved. In addition, a greater emphasis should be placed on obtaining matched primary tumor and bone metastases specimens from patients to understand how these cells evolve during metastasis and treatment progression. Finally, if therapeutic discoveries from dormancy research are to be tested, a cost-efficient framework for prophylactic clinical trials, including biomarkers to identify high-risk patients and alternative bone metastasis-specific end points that faithfully predict future long-term outcomes, will need to be developed and put into clinical practice.
ACKNOWLEDGMENTS
This work is supported by fellowships from the National Institutes of Health (NIH) (F31CA 192461) and the New Jersey Commission on Cancer Research (NJCCR) to M.E., grants from the Susan G. Komen Foundation (SAC 160067), Brewster Foundation, Department of Defense (BC123187), and the NIH (R01CA 141062) to Y.K., and U01CA143057 to T.G.
Footnotes
Editors: Gerard Karsenty and David T. Scadden
Additional Perspectives on Bone: A Regulator of Physiology available at www.perspectivesinmedicine.org
REFERENCES
- Adams GB, Martin RP, Alley IR, Chabner KT, Cohen KS, Calvi LM, Kronenberg HM, Scadden DT. 2007. Therapeutic targeting of a stem cell niche. Nat Biotechnol 25: 238–243. [DOI] [PubMed] [Google Scholar]
- Aguirre-Ghiso JA. 2007. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 7: 834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akech J, Wixted JJ, Bedard K, van der Deen M, Hussain S, Guise TA, van Wijnen AJ, Stein JL, Languino LR, Altieri DC, et al. 2010. Runx2 association with progression of prostate cancer in patients: Mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene 29: 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. 1997. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390: 175–179. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. 2004. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118: 149–161. [DOI] [PubMed] [Google Scholar]
- Bakewell SJ, Nestor P, Prasad S, Tomasson MH, Dowland N, Mehrotra M, Scarborough R, Kanter J, Abe K, Phillips D, et al. 2003. Platelet and osteoclast β3 integrins are critical for bone metastasis. Proc Natl Acad Sci 100: 14205–14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, Datar RH, Cote RJ. 2006. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res 12: 5615–5621. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Zhan R, Chaudhuri A, Watabe M, Pai SK, Hirota S, Hosobe S, Tsukada T, Miura K, Takano Y, et al. 2006. Interaction of KAI1 on tumor cells with DARC on vascular endothelium leads to metastasis suppression. Nat Med 12: 933–938. [DOI] [PubMed] [Google Scholar]
- Baniwal SK, Khalid O, Gabet Y, Shah RR, Purcell DJ, Mav D, Kohn-Gabet AE, Shi Y, Coetzee GA, Frenkel B. 2010. Runx2 transcriptome of prostate cancer cells: Insights into invasiveness and bone metastasis. Mol Cancer 9: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banys M, Gruber I, Krawczyk N, Becker S, Kurth R, Wallwiener D, Jakubowska J, Hoffmann J, Rothmund R, Staebler A, et al. 2012. Hematogenous and lymphatic tumor cell dissemination may be detected in patients diagnosed with ductal carcinoma in situ of the breast. Breast Cancer Res Treat 131: 801–808. [DOI] [PubMed] [Google Scholar]
- Bidwell BN, Slaney CY, Withana NP, Forster S, Cao Y, Loi S, Andrews D, Mikeska T, Mangan NE, Samarajiwa SA, et al. 2012. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med 18: 1224–1231. [DOI] [PubMed] [Google Scholar]
- Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP, Harousseau JL, Lipton A, Mariette X, Williams CD, et al. 2003. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer 97: 887–892. [DOI] [PubMed] [Google Scholar]
- Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O. 2004. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest 114: 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. 1992. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest 90: 1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. 2003. Osteoclast differentiation and activation. Nature 423: 337–342. [DOI] [PubMed] [Google Scholar]
- Bragado P, Estrada Y, Parikh F, Krause S, Capobianco C, Farina HG, Schewe DM, Aguirre-Ghiso JA. 2013. TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat Cell Biol 15: 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Kentenich C, Janni W, Hepp F, de Waal J, Willgeroth F, Sommer H, Pantel K. 2000. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol 18: 80–86. [DOI] [PubMed] [Google Scholar]
- Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, et al. 2005. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353: 793–802. [DOI] [PubMed] [Google Scholar]
- Brenner S, Whiting-Theobald N, Kawai T, Linton GF, Rudikoff AG, Choi U, Ryser MF, Murphy PM, Sechler JMG, Malech HL. 2004. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cells 22: 1128–1133. [DOI] [PubMed] [Google Scholar]
- Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, Lee KA, Zheng M, Hei YJ, Coleman RE. 2005. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst 97: 59–69. [DOI] [PubMed] [Google Scholar]
- Bruns I, Cadeddu RP, Brueckmann I, Frobel J, Geyh S, Bust S, Fischer JC, Roels F, Wilk CM, Schildberg FA, et al. 2012. Multiple myeloma-related deregulation of bone marrow-derived CD34+ hematopoietic stem and progenitor cells. Blood 120: 2620–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. 2003. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846. [DOI] [PubMed] [Google Scholar]
- Canon JR, Roudier M, Bryant R, Morony S, Stolina M, Kostenuik PJ, Dougall WC. 2008. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis 25: 119–129. [DOI] [PubMed] [Google Scholar]
- Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, Sleep DJ, Isaacson JD, Nelson JB. 2007. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer 110: 1959–1966. [DOI] [PubMed] [Google Scholar]
- Catena R, Luis-Ravelo D, Anton I, Zandueta C, Salazar-Colocho P, Larzabal L, Calvo A, Lecanda F. 2010. PDGFR signaling blockade in marrow stroma impairs lung cancer bone metastasis. Cancer Res 71: 164–174. [DOI] [PubMed] [Google Scholar]
- Cher ML, Biliran HR Jr, Bhagat S, Meng Y, Che M, Lockett J, Abrams J, Fridman R, Zachareas M, Sheng S. 2003. Maspin expression inhibits osteolysis, tumor growth, and angiogenesis in a model of prostate cancer bone metastasis. Proc Natl Acad Sci 100: 7847–7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Cheng CJ, Ye X, Lee YC, Zurita AJ, Chen DT, Yu-Lee LY, Zhang S, Yeh ET, Hu MCT, et al. 2008. Cadherin-11 promotes the metastasis of prostate cancer cells to bone. Mol Cancer Res 6: 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M, Saad F, Zheng M, Hei YJ, Seaman J, et al. 2005. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23: 4925–4935. [DOI] [PubMed] [Google Scholar]
- Coleman R, Powles T, Paterson A, Gnant M, Anderson S, Diel I, Gralow J, von Minckwitz G, Moebus V, Bergh J, et al. 2015. Adjuvant bisphosphonate treatment in early breast cancer: Meta-analyses of individual patient data from randomised trials. Lancet 386: 1353–1361. [DOI] [PubMed] [Google Scholar]
- Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. 2008. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 322: 1861–1865. [DOI] [PubMed] [Google Scholar]
- Cox TR, Rumney RM, Schoof EM, Perryman L, Hoye AM, Agrawal A, Bird D, Latif NA, Forrest H, Evans HR, et al. 2015. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522: 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dai J. 2005. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res 65: 8274–8285. [DOI] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. 2013. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495: 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. 2012. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481: 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanska UM, Timmer-Bosscha H, Nagengast WB, Oude Munnink TH, Kruizinga RC, Ananias HJ, Kliphuis NM, Huls G, De Vries EG, de Jong IJ, et al. 2012. CXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia 14: 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett M, Forbes JF, Bradley R, Ingle J, Aihara T, Bliss J, Boccardo F, Coates A, Coombes RC, Cuzick J, et al. 2015. Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 386: 1341–1352. [DOI] [PubMed] [Google Scholar]
- Dunn LK, Mohammad KS, Fournier PG, McKenna CR, Davis HW, Niewolna M, Peng XH, Chirgwin JM, Guise TA. 2009. Hypoxia and TGF-β drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS ONE 4: e6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. 2003. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest 111: 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M, Kang Y. 2014. Targeting tumor–stromal interactions in bone metastasis. Pharmacol Ther 141: 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M, Rocha M, Bai L, Umansky V, Solomayer EF, Bastert G, Diel IJ, Schirrmacher V. 2001. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int J Cancer 92: 96–105. [PubMed] [Google Scholar]
- Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Lo Celso C, Tsuyuzaki H, et al. 2011. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 474: 216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DYR, et al. 2013. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15: 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, Gomis RR, Manova-Todorova K, Massague J. 2007. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 446: 765–770. [DOI] [PubMed] [Google Scholar]
- Ha TC, Li H. 2007. Meta-analysis of clodronate and breast cancer survival. Br J Cancer 96: 1796–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. 2005. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res 65: 7554–7560. [DOI] [PubMed] [Google Scholar]
- Hall CL, Dai J, van Golen KL, Keller ET, Long MW. 2006. Type I collagen receptor (α2β1) signaling promotes the growth of human prostate cancer cells within the bone. Cancer Res 66: 8648–8654. [DOI] [PubMed] [Google Scholar]
- Harbeck N, Untch M, Pache L, Eiermann W. 1994. Tumour cell detection in the bone marrow of breast cancer patients at primary therapy: Results of a 3-year median follow-up. Br J Cancer 69: 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE, Klagsbrun M. 1986. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-sepharose. J Biol Chem 261: 12665–12674. [PubMed] [Google Scholar]
- Hiraga T, Williams PJ, Mundy GR, Yoneda T. 2001. The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Cancer Res 61: 4418–4424. [PubMed] [Google Scholar]
- Hoskin P, Sartor O, O’Sullivan JM, Johannessen DC, Helle SI, Logue J, Bottomley D, Nilsson S, Vogelzang NJ, Fang F, et al. 2014. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: A prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol 15: 1397–1406. [DOI] [PubMed] [Google Scholar]
- Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G, et al. 2008. Systemic spread is an early step in breast cancer. Cancer Cell 13: 58–68. [DOI] [PubMed] [Google Scholar]
- Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. 1997. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med 3: 1285–1289. [DOI] [PubMed] [Google Scholar]
- Joeckel E, Haber T, Prawitt D, Junker K, Hampel C, Thuroff JW, Roos FC, Brenner W. 2014. High calcium concentration in bones promotes bone metastasis in renal cell carcinomas expressing calcium-sensing receptor. Mol Cancer 13: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, et al. 2006. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440: 692–696. [DOI] [PubMed] [Google Scholar]
- Josson S, Gururajan M, Hu P, Shao C, Chu GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, et al. 2014. miR-409-3p/-5p promotes tumorigenesis, epithelial-to-mesenchymal transition, and bone metastasis of human prostate cancer. Clin Cancer Res 20: 4636–4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, Hardan I, Deutsch V, Gazit Z, Gazit D, et al. 2004. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood 103: 2942–2949. [DOI] [PubMed] [Google Scholar]
- Kang Y, Pantel K. 2013. Tumor cell dissemination: Emerging biological insights from animal models and cancer patients. Cancer Cell 23: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. 2003. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3: 537–549. [DOI] [PubMed] [Google Scholar]
- Kelly T, Suva LJ, Huang Y, Macleod V, Miao HQ, Walker RC, Sanderson RD. 2005. Expression of heparanase by primary breast tumors promotes bone resorption in the absence of detectable bone metastases. Cancer Res 65: 5778–5784. [DOI] [PubMed] [Google Scholar]
- Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. 2010. Metastatic behavior of breast cancer subtypes. J Clin Oncol 28: 3271–3277. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, et al. 2011. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med 208: 2641–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korah R, Boots M, Wieder R. 2004. Integrin α5β1 promotes survival of growth-arrested breast cancer cells: An in vitro paradigm for breast cancer dormancy in bone marrow. Cancer Res 64: 4514–4522. [DOI] [PubMed] [Google Scholar]
- Korpal M, Yan J, Lu X, Xu S, Lerit DA, Kang Y. 2009. Imaging transforming growth factor-β signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat Med 15: 960–966. [DOI] [PubMed] [Google Scholar]
- Kricun ME. 1985. Red-yellow marrow conversion: Its effect on the location of some solitary bone lesions. Skeletal Radiol 14: 10–19. [DOI] [PubMed] [Google Scholar]
- Krzeszinski JY, Wei W, Huynh H, Jin Z, Wang X, Chang TC, Xie XJ, He L, Mangala LS, Lopez-Berestein G, et al. 2014. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature 512: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. 1998. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93: 165–176. [DOI] [PubMed] [Google Scholar]
- Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM, et al. 2000. Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol 20: 8783–8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Loberg R, Liao J, Ying C, Snyder LA, Pienta KJ, McCauley LK. 2009. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res 69: 1685–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann F, Schlimok G, Dirschedl P, Witte J, Riethmuller G. 1992. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet 340: 685–689. [DOI] [PubMed] [Google Scholar]
- Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wojno K, Snyder LA, Yan L, Pienta KJ. 2007. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res 67: 9417–9424. [DOI] [PubMed] [Google Scholar]
- Lu X, Wang Q, Hu G, Van Poznak C, Fleisher M, Reiss M, Massague J, Kang Y. 2009. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev 23: 1882–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, Yan J, Hua Y, Tiede Benjamin J, Lu X, et al. 2011. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell 20: 701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, Vargo-Gogola TC, Begtrup JL, Peterson TE, Fingleton B, et al. 2005. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell 7: 485–496. [DOI] [PubMed] [Google Scholar]
- Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, de Stanchina E, Massague J. 2016. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165: 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansi JL, Berger U, Easton D, McDonnell T, Redding WH, Gazet JC, McKinna A, Powles TJ, Coombes RC. 1987. Micrometastases in bone marrow in patients with primary breast cancer: Evaluation as an early predictor of bone metastases. Br Med J (Clin Res Ed) 295: 1093–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura N, Puzon-McLaughlin W, Irie A, Morikawa Y, Kakudo K, Takada Y. 1996. Induction of experimental bone metastasis in mice by transfection of integrin α4β1 into tumor cells. Am J Pathol 148: 55–61. [PMC free article] [PubMed] [Google Scholar]
- Melchior SW, Corey E, Ellis WJ, Ross AA, Layton TJ, Oswin MM, Lange PH, Vessella RL. 1997. Early tumor cell dissemination in patients with clinically localized carcinoma of the prostate. Clin Cancer Res 3: 249–256. [PubMed] [Google Scholar]
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, MacArthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. 2010. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466: 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michigami T, Shimizu N, Williams PJ, Niewolna M, Dallas SL, Mundy GR, Yoneda T. 2000. Cell–cell contact between marrow stromal cells and myeloma cells via VCAM-1 and α4β1-integrin enhances production of osteoclast-stimulating activity. Blood 96: 1953–1960. [PubMed] [Google Scholar]
- Mori Y, Shimizu N, Dallas M, Niewolna M, Story B, Williams PJ, Mundy GR, Yoneda T. 2004. Anti-α4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood 104: 2149–2154. [DOI] [PubMed] [Google Scholar]
- Morony S, Capparelli C, Sarosi I, Lacey DL, Dunstan CR, Kostenuik PJ. 2001. Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res 61: 4432–4436. [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT. 2014. The bone marrow niche for haematopoietic stem cells. Nature 505: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, Gleave M, Wu H. 2012. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res 72: 1878–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Gounari F, Prifti S, Hacker HJ, Schirrmacher V, Khazaie K. 1998. EblacZ tumor dormancy in bone marrow and lymph nodes: Active control of proliferating tumor cells by CD8+ immune T cells. Cancer Res 58: 5439–5446. [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. 2001. Involvement of chemokine receptors in breast cancer metastasis. Nature 410: 50–56. [DOI] [PubMed] [Google Scholar]
- Mundy GR. 2002. Metastasis: Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer 2: 584–593. [DOI] [PubMed] [Google Scholar]
- Murray LJ, Abrams TJ, Long KR, Ngai TJ, Olson LM, Hong W, Keast PK, Brassard JA, O’Farrell AM, Cherrington JM, et al. 2003. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis 20: 757–766. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, et al. 2007. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130: 811–823. [DOI] [PubMed] [Google Scholar]
- Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, Simons JW. 1995. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med 1: 944–949. [DOI] [PubMed] [Google Scholar]
- Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, Prior JL, Piwnica-Worms D, Bridger G, Ley TJ, et al. 2009. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood 113: 6206–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oghiso Y, Matsuoka O. 1979. Distribution of colloidal carbon in lymph nodes of mice injected by different routes. Jpn J Exp Med 49: 223–234. [PubMed] [Google Scholar]
- Ohno H, Kubo K, Murooka H, Kobayashi Y, Nishitoba T, Shibuya M, Yoneda T, Isoe T. 2006. A c-fms tyrosine kinase inhibitor, Ki20227, suppresses osteoclast differentiation and osteolytic bone destruction in a bone metastasis model. Mol Cancer Ther 5: 2634–2643. [DOI] [PubMed] [Google Scholar]
- Ottewell PD, Wang N, Brown HK, Reeves KJ, Fowles CA, Croucher PI, Eaton CL, Holen I. 2014a. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin Cancer Res 20: 2922–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottewell PD, Wang N, Meek J, Fowles CA, Croucher PI, Eaton CL, Holen I. 2014b. Castration-induced bone loss triggers growth of disseminated prostate cancer cells in bone. Endocr Relat Cancer 21: 769–781. [DOI] [PubMed] [Google Scholar]
- Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J. 2008. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133: 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, et al. 2007. NF-κB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med 13: 62–69. [DOI] [PubMed] [Google Scholar]
- Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, Chodacki A, Wiechno P, Logue J, Seke M, et al. 2013. α Emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369: 213–223. [DOI] [PubMed] [Google Scholar]
- Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, et al. 2002. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol 3: 687–694. [DOI] [PubMed] [Google Scholar]
- Pitt LA, Tikhonova AN, Hu H, Trimarchi T, King B, Gong Y, Sanchez-Martin M, Tsirigos A, Littman DR, Ferrando AA, et al. 2015. CXCL12-producing vascular endothelial niches control acute T cell leukemia maintenance. Cancer Cell 27: 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Esposito M, Kang Y. 2015. Bone metastasis and the metastatic niche. J Mol Med (Berl) 93: 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman GD. 2004. Mechanisms of bone metastasis. N Engl J Med 350: 1655–1664. [DOI] [PubMed] [Google Scholar]
- Sänger N, Effenberger KE, Riethdorf S, Van Haasteren V, Gauwerky J, Wiegratz I, Strebhardt K, Kaufmann M, Pantel K. 2011. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int J Cancer 129: 2522–2526. [DOI] [PubMed] [Google Scholar]
- Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, Chodacki A, Wiechno P, Logue J, et al. 2014. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: Results from a phase 3, double-blind, randomised trial. Lancet Oncol 15: 738–746. [DOI] [PubMed] [Google Scholar]
- Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, Ponnazhagan S. 2012. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol 189: 4258–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, McCauley LK. 2005. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology 146: 1727–1736. [DOI] [PubMed] [Google Scholar]
- Schwartzberg PL, Xing L, Hoffmann O, Lowell CA, Garrett L, Boyce BF, Varmus HE. 1997. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src–/– mutant mice. Genes Dev 11: 2835–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi N, Dai X, Winter CG, Kang Y. 2011. Tumor-derived Jagged1 promotes osteolytic bone metastasis of breast cancer by engaging Notch signaling in bone cells. Cancer Cell 19: 192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, et al. 2011. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 121: 1298–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IE, Dowsett M. 2003. Aromatase inhibitors in breast cancer. N Engl J Med 348: 2431–2442. [DOI] [PubMed] [Google Scholar]
- Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, et al. 2005. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med 201: 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suominen MI, Rissanen JP, Kakonen R, Fagerlund KM, Alhoniemi E, Mumberg D, Ziegelbauer K, Halleen JM, Kakonen SM, Scholz A. 2013. Survival benefit with radium-223 dichloride in a mouse model of breast cancer bone metastasis. J Natl Cancer Inst 105: 908–916. [DOI] [PubMed] [Google Scholar]
- Teicher BA, Fricker SP. 2010. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res 16: 2927–2931. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. 2006. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol 7: 131–142. [DOI] [PubMed] [Google Scholar]
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD Jr. 2003. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349: 2483–2494. [DOI] [PubMed] [Google Scholar]
- Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, et al. 2014. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156: 1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu C, Gao X, Welte T, Muscarella AM, Tian L, Zhao H, Zhao Z, Du S, Tao J, et al. 2015. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27: 193–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilbaecher KN, Guise TA, McCauley LK. 2011. Cancer to bone: A fatal attraction. Nat Rev Cancer 11: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, Levesque JP. 2012. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self-renewal and chemoresistance. Nat Med 18: 1651–1657. [DOI] [PubMed] [Google Scholar]
- Withana NP, Blum G, Sameni M, Slaney C, Anbalagan A, Olive MB, Bidwell BN, Edgington L, Wang L, Moin K, et al. 2012. Cathepsin B inhibition limits bone metastasis in breast cancer. Cancer Res 72: 1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright LE, Harhash AA, Kozlow WM, Waning DL, Regan JN, She Y, John SK, Murthy S, Niewolna M, Marks AR, et al. 2017. Aromatase inhibitor-induced bone loss increases the progression of estrogen receptor-negative breast cancer in bone and exacerbates muscle weakness in vivo. Oncotarget 8: 8406–8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Davis JL, Zeng R, Vora P, Su X, Collins LI, Vangveravong S, Mach RH, Piwnica-Worms D, Weilbaecher KN, et al. 2012. Antagonism of inhibitor of apoptosis proteins increases bone metastasis via unexpected osteoclast activation. Cancer Discov 3: 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. 1998. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci 95: 3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. 1999. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 103: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JJ, Mohammad KS, Kakonen SM, Harris S, Wu-Wong JR, Wessale JL, Padley RJ, Garrett IR, Chirgwin JM, Guise TA. 2003. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci 100: 10954–10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T, Sasaki A, Dunstan C, Williams PJ, Bauss F, De Clerck YA, Mundy GR. 1997. Inhibition of osteolytic bone metastasis of breast cancer by combined treatment with the bisphosphonate ibandronate and tissue inhibitor of the matrix metalloproteinase-2. J Clin Invest 99: 2509–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayzafoon M, Abdulkadir SA, McDonald JM. 2004. Notch signaling and ERK activation are important for the osteomimetic properties of prostate cancer bone metastatic cell lines. J Biol Chem 279: 3662–3670. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. 2003. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425: 836–841. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Jin X, Malladi S, Zou Y, Wen YH, Brogi E, Smid M, Foekens JA, Massague J. 2013. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154: 1060–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]