Abstract
We herein report the case of a 37-year-old man with both pheochromocytoma and visceral fat accumulation and describe the sequential changes in his adiponectin levels throughout the clinical course from catecholamine crisis until the follow-up for adrenalectomy. His adiponectin level decreased during catecholamine crisis and increased after adrenalectomy. However, his adiponectin level decreased again at two years postoperatively when his visceral fat area greatly increased. This case suggests that catecholamines and visceral fat volume may affect adiponectin metabolism in subjects with pheochromocytoma, which may precipitate cardiovascular complications in this endocrine disease.
Keywords: pheochromocytoma, visceral fat area, adiponectin
Introduction
Pheochromocytoma is a catecholamine-producing-tumor that is usually derived from the adrenal medulla (1). Catecholamine crisis is a life-threatening condition in this disease because of the excessive secretion of catecholamines from the tumor, which presents spontaneously or as the result of several inducing factors (2). Patients with pheochromocytoma are usually lean because of adipose tissue lipolysis by catecholamines. However, recent lifestyle changes have increased the number of obese individuals, including those with pheochromocytoma.
Adiponectin is an adipocyte-derived plasma protein with anti-diabetic, anti-inflammatory, anti-atherogenic, and anti-cardiomyopathic properties (3). Adiponectin levels are regulated by various factors and altered in different conditions. The levels have been found to be decreased in subjects with abdominal obesity, a central feature of metabolic syndrome (3). Although catecholamines are metabolic regulators of adipose tissue, their effects on adiponectin remain unclear. Furthermore, the adiponectin levels of patients with pheochromocytoma are controversial, with reports of their being increased (4), decreased (5), or unchanged (6). To date, there are no studies investigating the relationship between the adiponectin levels and visceral fat area (VFA) in pheochromocytoma patients.
We herein report a patient with both abdominal obesity and pheochromocytoma and describe the sequential changes in the adiponectin levels as well as the VFA throughout the clinical course, from catecholamine crisis to recovery.
Case Report
A 37-year-old man was diagnosed with hypertension and obesity at an annual health checkup, followed by further examinations in which a left adrenal tumor was revealed. Subsequently, he was referred to our hospital for endocrinological evaluations and was scheduled to be hospitalized. His height and body weight were 168 cm and 91 kg [body mass index (BMI): 32.2 kg/m2], respectively. Five days later, he presented to the emergency room of our hospital with a complaint of general fatigue and left abdominal pain. He had severe hypertension with a blood pressure of 234/120 mmHg and exhibited sinus tachycardia of 146 beats per minute. He was admitted to the intensive care unit with a diagnosis of catecholamine crisis, probably due to pheochromocytoma. We started antihypertensive therapy using phentolamine, nicardipine, and doxazosin, which kept his blood pressure and heart rate under control.
His white blood cell count on admission increased with a slight rise in C-reactive protein (Table). The plasma glucose level increased over 200 mg/dL, despite a normal HbA1c value. Other routine laboratory test data, including findings for the liver function, renal function, and electrolyte levels, were normal. The diagnosis of pheochromocytoma was confirmed by the marked elevation of catecholamines and their metabolites in the plasma and urine (Table).
Table.
Laboratory Tests on Admission.
| Complete blood count | Reference ranges | Hormone | Reference ranges | |||
|---|---|---|---|---|---|---|
| WBC (/μL) | 15,900 | 3,500-8,500 | ACTH (pg/mL) | 17.4 | 7.2-63.3 | |
| RBC (/μL) | 517×104 | 430-570 | Cortisol μg/dL) | 20.8 | 4.0-19.3 | |
| Hb (g/dL) | 16.7 | 13.5-17.5 | TSH μIU/mL) | 0.49 | 0.35-4.94 | |
| Plt (/μL) | 32.1×104 | 12.0-38.0 | FT4 (ng/dL) | 0.97 | 0.70-1.48 | |
| Blood Chemistry | Reference ranges | Intact PTH (pg/mL) | 107 | 10-65 | ||
| Calcitonin (pg/mL) | 17.0 | 15.0-86.0 | ||||
| AST (IU/L) | 19 | 10-37 | renin activity (ng/mL/hr) | 12.3 | 0.2-2.3 | |
| ALT (IU/L) | 25 | 4-40 | Aldosteron (pg/mL) | 314 | 30-159 | |
| LDH (IU/L) | 178 | 100-211 | DHEA-S μg/dL) | 64 | 98-516 | |
| ALP (IU/L) | 247 | 98-328 | Adrenaline (ng/mL) | 3.8 | 0.0-0.17 | |
| CK (IU/L) | 52 | 60-250 | Noradorenaline (ng/mL) | 6.7 | 0.15-0.57 | |
| AMY (IU/L) | 71 | 33-120 | Dopamine (ng/mL) | 0.03 | <0.03 | |
| TP (g/dL) | 8.3 | 6.7-8.3 | urinary metanephrine (mg/day) | 11 | 0.05-0.23 | |
| Alb (g/dL) | 4.9 | 3.8-5.1 | urinary normetanephrine (mg/day) | 5.6 | 0.07-0.26 | |
| T-Bil (mg/dL) | 1.06 | 0.20-1.20 | urinary VMA (mg/day) | 49.4 | 1.5-4.3 | |
| BUN (mg/dL) | 16 | 8-20 | Urinalysis | |||
| Cre (mg/dL) | 0.63 | 0.30-0.90 | Protein | (1+) | ||
| Na (mEq/L) | 136 | 135-147 | Glucose | (1+) | ||
| K (mEq/L) | 3.4 | 3.6-5.0 | Ketone | (1+) | ||
| Cl (mEq/L) | 100 | 98-108 | Occult Blood | (2+) | ||
| Ca (mg/dL) | 9.7 | 8.2-10.2 | ||||
| glucose (mg/dL) | 239 | 60-110 | ||||
| HbA1c (%) | 5.0 | 4.3-5.8 | ||||
| CRP (mg/dL) | 0.54 | <0.3 | ||||
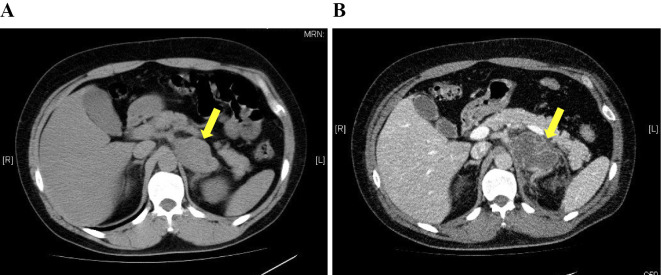
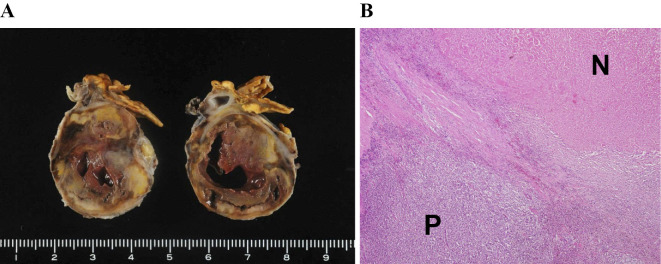
On day 8, his blood pressure level rose to 200 mmHg, with recurrence of left-sided abdominal pain. Computed tomography (CT) revealed a left adrenal tumor, inhomogeneous inside and surrounded by panniculitis, on the day of admission (Fig. 1A), and necrosis in the left adrenal tumor and peri-adrenal fat stranding were also noted (Fig. 1B). Because of the unstable blood pressure, we chose conservative therapy with α- and β-adrenoceptor blockade instead of emergency adrenalectomy. The catecholamine crisis and abdominal pain gradually resolved, and he was discharged from the hospital on day 23. On day 37, he underwent successful left adrenalectomy at the Department of Urology in our hospital. Macroscopic (Fig. 2A) and microscopic (Fig. 2B) examinations of the resected tumor revealed massive necrosis, probably due to ischemia rather than tumor necrosis. The limited viable tumor tissue revealed no malignant cells. Browning of adipocytes in the adipose tissue surrounding the tumor was not observed.
Figure 1.
At the time of admission, abdominal CT imaging showed a left adrenal tumor, inhomogeneous inside and surrounded by panniculitis [diameter 65 mm (arrow)] and visceral fat area of 153 cm2 (A). On day 8, CT showed necrosis in the left adrenal tumor (arrow) and periadrenal fat stranding (B).
Figure 2.
Macroscopic examinations of the resected tumor revealed massive necrosis, probably due to ischemia rather than tumor necrosis (A). Hematoxylin and Eosin staining (×100) showed necrotic tissue (N) and viable pheochromocytoma tissue (P). Limited viable tumor tissue revealed no malignant cells (B).
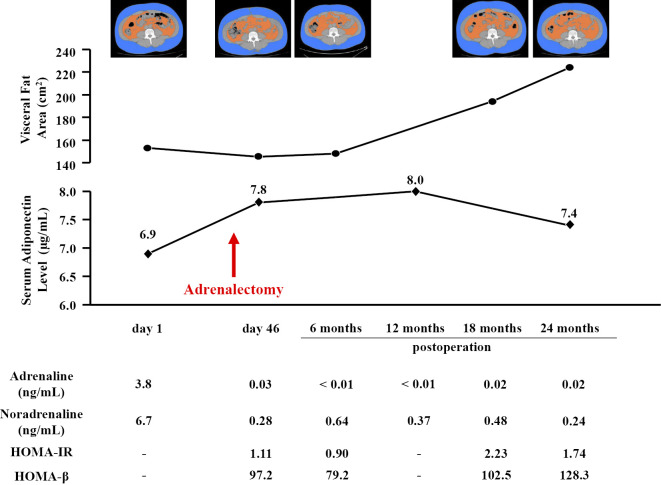
The VFA was measured by CT at the umbilical level using the FatScan software program (N2system, Osaka, Japan) (7). The serum adiponectin levels were measured using an enzyme-linked immunosorbent assay kit (Otsuka Pharmaceutical, Tokyo, Japan) (8). On the day of admission at the time of the catecholamine crisis, his VFA was 153 cm2, and his serum adiponectin level was 6.9 μg/mL (Fig. 3). After adrenalectomy, his VFA decreased slightly, and his adiponectin level increased to 7.8 μg/mL. The VFA at 6 months postoperatively remained unchanged at 148 cm2, and the adiponectin level at 12 months postoperatively also did not increase (8.0 μg/mL). At 2 years postoperatively, the VFA increased to 224 cm2, and the serum adiponectin level decreased to 7.4 μg/mL (Fig. 3).
Figure 3.
The time course of the visceral fat area and serum adiponectin level. The visceral fat area (orange-colored area) was measured on CT cross-sectional scans obtained at the umbilical level in the spine position. Homeostasis model assessment of insulin resistance (HOMA-IR) = FPG (mg/dL) × fasting IRI (μU/mL)/405. Homeostasis model assessment of β-cell function (HOMA-β) = 360 × fasting IRI (μU/mL)/(FPG-63).
Homeostasis model assessment of insulin resistance (HOMA-IR), a marker of insulin resistance, decreased after adrenalectomy and increased at two years postoperatively (Fig. 3). Homeostasis model assessment of β-cell function (HOMA-β), a marker of insulin secretion, increased with the increase in HOMA-IR.
Discussion
In this case report, we describe changes in the serum adiponectin levels and VFA throughout the clinical course from catecholamine crisis to the postoperative follow-up period. To our knowledge, there have been no such reports in the literature. Furthermore, our patient was obese with a marked accumulation of visceral fat. Most patients with pheochromocytoma are lean and rarely develop visceral fat obesity (9), as catecholamines induce lipolysis. The effects of catecholamines are greater in visceral fat than in subcutaneous fat, possibly due to different quantities of β-adrenergic receptors expressed in the adipose tissue (10). However, because of recent lifestyle changes, such as the adoption of fat-rich, high-energy diets and physical inactivity, the number of obese subjects-including those with pheochromocytoma-has increased. Because both catecholamine and visceral fat volume (3) can be regulators of adipocytokine metabolism, we were very interested in the serum adiponectin levels in our obese patient with pheochromocytoma.
Our patient had a lower serum adiponectin level during the catecholamine crisis than after adrenalectomy. Elenkova et al. similarly reported that adiponectin levels were lower in subjects with pheochromocytoma than in healthy controls, and the levels increased after adrenalectomy despite postoperative weight gain (4). Conversely, Isobe et al. reported that adiponectin levels were significantly higher in patients with pheochromocytoma who dominantly produce noradrenaline than in healthy controls (5). They speculated that noradrenaline might promote adiponectin production, thereby leading to protection against diabetes in this type of pheochromocytoma. However, our patient had a different type of pheochromocytoma that equally produced adrenaline and noradrenaline. Conversely, Bosanska et al. reported that adiponectin levels remained unchanged after adrenalectomy, as the normalization of catecholamines was canceled because of postoperative weight gain (6). Recent in vitro studies have demonstrated that the expression of adiponectin mRNAs is downregulated by β-adrenergic stimuli (11, 12) and that adiponectin release from isolated rat adipocytes was suppressed by high-dose adrenaline (13). Intratumoral hemorrhaging is a precipitating factor of catecholamine crisis (2), and the adrenaline level in our patient actually showed a 22-fold increase from the upper limit of the normal range. Thus, excessive catecholamine during the crisis seems to be responsible for the reduced level of serum adiponectin in our patient.
Physical inactivity and overeating in our patient gradually increased the visceral fat volume after adrenalectomy, and his adiponectin level decreased again two years postoperatively. A negative correlation has been shown between serum adiponectin levels and the VFA in healthy people (3, 14). Inflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-6, might be intermediary factors for this correlation; however, the details of the mechanism remain unclear. Adiponectin is an adipocyte-derived plasma protein with anti-inflammatory, anti-atherogenic, anti-diabetic, and anti-cardiomyopathic properties. Serum adiponectin levels have been found to be reduced in subjects with metabolic syndrome, which is associated with increased risks of cardiovascular disease and diabetes (3). Some reports have shown a high incidence of atherosclerotic complications in patients with pheochromocytoma (15, 16). Furthermore, various types of cardiomyopathies are known to develop frequently in pheochromocytoma (17). These cardiovascular complications in pheochromocytoma may be precipitated by not only catecholamine excess but also hypoadiponectinemia; however, further studies are required.
Plasma adiponectin levels have been reported to be 5-10 μg/mL in healthy subjects (8) and <4 μg/mL in subjects at high risk for diabetes or cardiovascular disease (18, 19). The adiponectin levels in our patient were not markedly low, despite his obesity. However, adiponectin levels vary considerably among individuals, and our patient may have had certain features protecting him from adiponectin decrease. Although the absolute values of adiponectin may not have been pathological in this particular patient, determining whether or not adiponectin levels can be changed by not only the visceral fat volume but also catecholamine status in pheochromocytoma patients would be a meaningful finding.
In this case report, we were unable to measure other adipocytokines, such as leptin and resistin. One report described a reduction in plasma leptin in a patient with pheochromocytoma (20), whereas another report suggested no changes (6). Wocial et al. reported that acute catecholamine excess affects the plasma leptin level, while chronic catecholamine excess does not (21). Further investigation into possible changes in leptin during the clinical course in patients with pheochromocytoma is required.
In conclusion, we described the sequential changes in serum adiponectin levels in a patient with both pheochromocytoma and visceral fat accumulation. The serum adiponectin level was low during catecholamine crisis and increased after adrenalectomy. However, it decreased again two years postoperatively when the visceral fat accumulation increased because of an unhealthy lifestyle. Adiponectin metabolism is altered by catecholamines and the visceral fat volume in pheochromocytoma, which may affect the pathophysiological condition in this endocrine disease.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Dr. Tadashi Nakamura and Mr. Tohru Yoshizumi for the measurement of the visceral fat area, Dr. Norikazu Maeda for the measurement of the serum adiponectin level, and all of the medical staff of Toyonaka Municipal Hospital, particularly those in the intensive care unit, for their helpful support.
References
- 1. Zelinka T, Eisenhofer G, Pacak K. Pheochromocytoma as a catecholamine producing tumor implications for clinical practice. Stress 10: 195-203, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Savage MW, Mah PM, Weetman AP, Newell-Price J. Endocrine emergencies. Postgrad Med J 80: 506-515, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Funahashi T, Matsuzawa Y. Adiponectin and the cardiometabolic syndrome: an epidemiological perspective. Best Pract Res Clin Endocrinol Metab 28: 93-106, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Elenkova A, Matrozova J, Zacharieva S, et al. Adiponectin - A possible factor in the pathogenesis of carbohydrate metabolism disturbances in patients with pheochromocytoma. Cytokine 50: 306-310, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Isobe K, Fu L, Tatsuno I, et al. Adiponectin and adiponectin receptors in human pheochromocytoma. J Atheroscler Thromb 16: 442-447, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Bosanska L, Petrak O, Zelinka T, Mraz M, Widimsky J Jr, Haluzik M. The effect of pheochromocytoma treatment on subclinical inflammation and endocrine function of adipose tissue. Physiol Res 58: 319-325, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Ryo M, Maeda K, Onda T, et al. A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care 28: 451-453, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79-83, 1999. [DOI] [PubMed] [Google Scholar]
- 9. Lee RE, Rousseau P. Pheochromocytoma and obesity. J Clin Endocrinol Metab 27: 1050-1052, 1967. [DOI] [PubMed] [Google Scholar]
- 10. Hoffstedt J, Arner P, Hellers G, Lönnqvist F. Variation in adrenergic regulation of lipolysis between omental and subcutaneous adipocytes from obese and non-obese men. J Lipid Res 38: 795-804, 1997. [PubMed] [Google Scholar]
- 11. Fasshauer M, Klein J, Neumann S, at al. Adiponectin gene expression is inhibited by beta-adrenergic stimulation via protein kinase A in3T3-L1 adipocytes. FEBS Lett 507: 142-146, 2001. [DOI] [PubMed] [Google Scholar]
- 12. Fu L, Isobe K, Zeng Q, et al. The effects of β3-adrenoceptor agonist CL-316,243 on adiponectin, adiponectin receptors and tumor necrosis factor-alpha expressions in adipose tissues of obese diabetic KKAy mice. Eur J Pharmacol 584: 202-206, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Szkudelski T, Nogowski L, Szkudelska K. Short-term regulation of adiponectin secretion in rat adipocytes. Physiol Res 60: 521-530, 2011. [DOI] [PubMed] [Google Scholar]
- 14. Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941-946, 2001. [DOI] [PubMed] [Google Scholar]
- 15. Galetta F, Franzoni F, Bernini G, et al. Cardiovascular complications in patients with pheochromocytoma: a mini-review. Biomed Pharmacother 64: 505-509, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Zelinka T, Petrák P, Turková H, et al. High incidence of cardiovascular complications in pheochromocytoma. Horm Metab Res 44: 379-384, 2012. [DOI] [PubMed] [Google Scholar]
- 17. Ferreira VM, Marcelino M, Piechnik SK, et al. Pheochromocytoma is characterized by catecholamine-mediated myocarditis, focal and diffuse myocardial fibrosis, and myocardial dysfunction. J Am Coll Cardiol 67: 2364-2374, 2016. [DOI] [PubMed] [Google Scholar]
- 18. Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 23: 85-89, 2003. [DOI] [PubMed] [Google Scholar]
- 19. Ryo M, Nakamura T, Kihara S, et al. Adiponectin as a biomarker of the metabolic syndrome. Circ J 68: 975-981, 2004. [DOI] [PubMed] [Google Scholar]
- 20. Sakane N, Yoshida T, Mizutani T, et al. Serum leptin levels in a patient with pheochromocytoma. J Clin Endocrinol Metab 83: 1400, 1998. [DOI] [PubMed] [Google Scholar]
- 21. Wocial B, Ignatowska-Switalska H, Berent H, et al. Do catecholamines influence the level of plasma leptin in patients with phaeochromocytoma? Br J Biomed Sci 59: 141-144, 2002. [PubMed] [Google Scholar]