Abstract
Immune checkpoint inhibitors (ICIs) are becoming a common and important cancer therapy. ICIs are associated with a unique category of side effects, termed immune-related adverse events (irAEs). We herein report the case of a 72-year-old man with postoperative recurrence of lung squamous cell carcinoma who was treated with nivolumab and who developed proteinuria and a worsening kidney function. A kidney biopsy revealed IgA nephropathy. After drug withdrawal, the proteinuria improved and the deterioration of the patient's renal function was halted. Although renal irAEs are considered to be rare and glomerulonephritis is not typical presentation, physicians need to pay more attention to renal irAEs and glomerular injury.
Keywords: nivolumab, onconephrology, immune-related adverse events (irAEs), glomerular injury, IgA nephropathy
Introduction
Cancer immunotherapy is becoming a common therapeutic option for cancer patients. Anti-programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) antibodies have already been clinically approved and have improved the prognosis of cancer patients (1). At this time, over 20 types of immune checkpoint inhibitor (ICI) have been used in various stages of clinical trials.
In comparison to conventional cytotoxic chemotherapy, the frequency of adverse events, including hematologic toxicity, is low in patients treated with ICIs. ICIs are associated with a unique category of side effects termed immune-related adverse events (irAEs) (2). It is thought that irAEs occur due to the activation of self-reactive T cells, which are usually manageable. Furthermore, it has been reported that they are not associated with inferior cancer outcomes (3). It is known that irAEs affect many organs, including gastrointestinal, endocrine, and respiratory systems, and an early diagnosis and intervention is crucial (4). According to previous reports, renal irAEs are less frequent than other organ involvement and glomerulonephritis is not typical (5). Thus, the reporting of cases is very important for the safe advancement of treatment.
In this report, we present a case of nivolumab-associated mesangial proliferative glomerulonephritis (IgA nephropathy), which has not previously been reported.
Case Report
A 72-year-old man was referred to our nephrology clinic for the evaluation of an elevated serum creatinine (Scr) level (1.35 mg/dL) and new onset proteinuria.
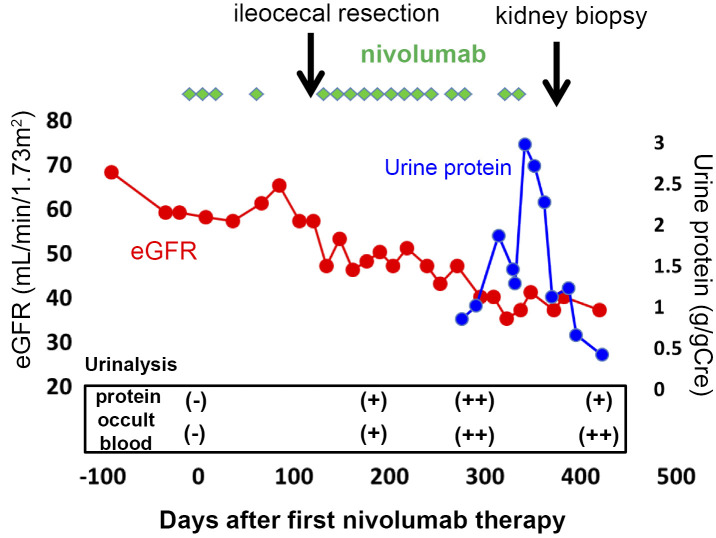
With regard to the patient's history, no urinalysis abnormalities or kidney dysfunction had ever been pointed out, and urinalyses performed at 12 and 10 months before the first nivolumab treatment were negative for hematuria and proteinuria. Ten months previously, he had been started on intravenous (IV) nivolumab therapy (3 mg/kg every other week) because of the postoperative recurrence of lung squamous cell carcinoma (cT3N2M0 stage IIIA). Although nivolumab was effective, the course was complicated by recurrent diverticulitis in the right cecum on two occasions, for which he underwent ileocecal resection. A histopathological examination revealed the infiltration of neutrophil in the serosal surface and nivolumab-related colitis was considered to be unlikely. Nivolumab was resumed. Six months after the first nivolumab treatment, the patient's serum creatinine level began to increase and proteinuria was noted. The patient's serum creatinine level increased from 0.84 to 1.35 mg/dL [estimated glomerular filtration rate (eGFR); 69 mL/min/1.73 m2 to 48 mL/min/1.73 m2] and proteinuria (0.67 g/gCre) developed over a 3-month period prior to the patient's referral (Fig. 1). He had been on telmisartan and amlodipine for hypertension, and loxoprofen for chronic pain. His blood pressure was controlled to approximately 120/80 mmHg with this regimen.
Figure 1.
The clinical course. The eGFR (mL/min/1.73 m2) and urinary protein level (g/gCre) are shown in red and blue lines, respectively. The timing of ileocecal resection and kidney biopsy is shown with arrows.
At the first visit, a physical examination revealed no peripheral edema, no signs of fluid overload and the patient's blood pressure was controlled (116/57 mmHg). A urinalysis showed proteinuria (1.69 g/gCre), mild hematuria (5-9/HPF) and no casts. Gallium-67 scintigraphy showed no uptake in the kidney, which revealed that there was no active inflammation process.
After referral, telmisartan was switched to atenorol and loxoprofen was discontinued. However, the patient's proteinuria continued to increase and his kidney function continued to decline (Fig. 1). A kidney biopsy was performed to determine the cause of kidney dysfunction and the newly developed proteinuria. The laboratory data obtained from the renal biopsy are shown in Table.
Table.
Laboratory Data at Kidney Biopsy.
| Urinalysis | Reference range | Chemistsy | Reference range | |||||
|---|---|---|---|---|---|---|---|---|
| specific gravity | 1.016 | BUN | 14 | 8-20 | mg/dL | |||
| pH | 6.5 | SCr | 1.32 | 0.4-0.9 | mg/dL | |||
| protein | 2+ | UA | 4.6 | 2.0-7.0 | mg/dL | |||
| glucose | - | Na | 140 | 135-146 | mEq/L | |||
| protein | 1.09 | g/day | K | 4.5 | 3.5-4.8 | mEq/L | ||
| protein/gCre | 0.95 | g/gCre | Cl | 105 | 98-108 | mEq/L | ||
| gluccose | - | Ca | 8.4 | 8.8-10.1 | mg/dL | |||
| Occult blood | 2+ | P | 3.3 | 2.4-4.6 | mg/dL | |||
| RBC | 10-19 | /HPF | Mg | 2.4 | 1.6-2.3 | mg/dL | ||
| WBC | 1-4 | /HPF | T.prot | 7 | 6.5-8.2 | g/dL | ||
| CBC | Alb | 3.4 | 3.9-4.9 | g/dL | ||||
| WBC | 4.3 | 4.0-9.0 | ×103/μL | T.chol | 182 | 130-220 | mg/dL | |
| neutrophil | 62 | % | TG | 126 | 35-150 | mg/dL | ||
| eosino | 4.8 | % | LDL-C | 95 | 65-163 | mg/dL | ||
| lympho | 20.1 | % | HDL-C | 59 | 40-100 | mg/dL | ||
| mono | 10 | % | GOT | 23 | 10-35 | IU/L | ||
| RBC | 3.62 | 3.9-4.9 | ×106/μL | GPT | 22 | 5-40 | IU/L | |
| Hb | 11.5 | 11.5-14.5 | g/dL | LDH | 170 | 110-220 | IU/L | |
| Ht | 34.5 | 34-43 | % | ALP | 364 | 100-340 | IU/L | |
| Plt | 241 | 150-350 | ×103/μL | γ-GTP | 22 | 0-30 | IU/L | |
| Blood coagulation test | CK | 174 | 30-150 | IU/L | ||||
| PT | 10.7 | 10.5-13.4 | sec | Glu | 133 | mg/dL | ||
| PT-INR | 0.86 | 0.85-1.15 | Serology | |||||
| APTT | 31.9 | 24.3-35.0 | sec | IgG | 1,455 | 870-1,700 | mg/dL | |
| Fib | 605 | 174-404 | mg/dL | IgA | 507 | 110-410 | mg/dL | |
| IgM | 85 | 46-260 | mg/dL | |||||
| C3 | 102 | 73-138 | mg/dL | |||||
| C4 | 17 | 11-31 | mg/dL | |||||
| CH50 | 60 | 32-49 | U/mL | |||||
| ANA | + | - | ||||||
| HBsAg | - | - | ||||||
| HCVAb | - | - | ||||||
Kidney biopsy
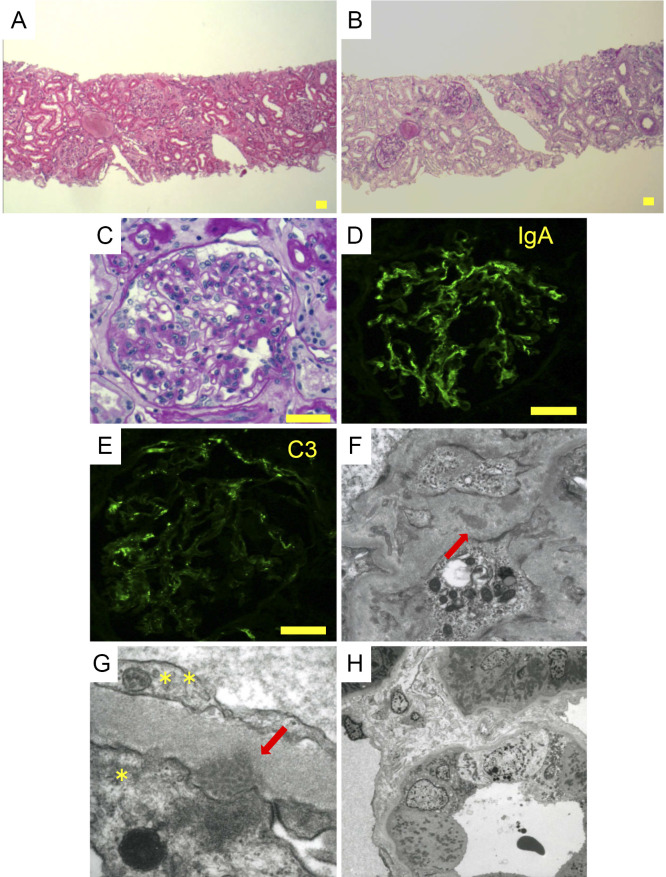
The kidney biopsy specimen contained 27 glomeruli, of which 14 showed global sclerosis. The remaining glomeruli showed diffuse mesangial matrix expansion and cell proliferation. There was no crescent formation or endocapillary proliferation. There was no active interstitial inflammation. Immunofluorescence staining showed C3 and IgA deposition on mesangial areas. Staining for IgM, IgG, C4, and C1q was negative (Fig. 2A-E). Electron-microscopy demonstrated a small amount of high electron-dense deposits in the subepithelial and mesangial regions. The subepithelial structures showed a tubular microstructure. Tubular epithelial cells had no major abnormalities, and there was mild inflammatory cell infiltration in the interstitium. There was no endothelial cell swelling or subendothelial edema (Fig. 2F-H).
Figure 2.
Light microscopy of the kidney biopsy specimen. Hematoxylin and Eosin staining (A) and Periodic acid-Schiff staining showed (B,C) global sclerosis, diffuse global mesangial expansion, and the interstitial infiltration of inflammatory cells (consistent with sclerotic glomeruli). (D,E) Immunofluorescence revealed IgA and C3 deposition in the mesangial area, Scale bar: 50 μm. Electron microscopy of the kidney biopsy specimen. (F) Small highly electron-dense deposits (arrow) were observed in the mesangial and subepithelial region (×10,000). (G) The subepithelial structures (arrow) showed a tubular microstructure. *: Epithelial cell (podocyte), **: Endothelial cell, (×25,000). (H) The tubular structure had no major abnormalities, and a small amount of inflammatory cell infiltration (×1,500).
Diagnosis
The patient was diagnosed with IgA nephropathy.
Clinical follow up
After the kidney biopsy, nivolumab treatment was discontinued. The eGFR stabilized and the urinary protein excretion markedly decreased (0.24 g/gCre) and was maintained for 4 months, suggesting complete remission had been achieved. At this time there was no evidence of lung cancer recurrence (Fig. 1).
Discussion
The development of ICIs has revolutionized the treatment of a variety of cancers and ICIs have been approved for a number of types of cancer. ICIs enhance antitumor immunity by blocking co-inhibitory molecules that are expressed on both T cells and tumor cells. The PD-1-blocking antibody nivolumab is approved by the Food and Drug Administration (FDA) for the treatment of metastatic melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma, and classical Hodgkin's lymphoma (1, 6). Lung cancer is the leading cause of cancer-related death around the world; NSCLC accounts for approximately 85% of lung cancers in United States (7). In Japan, advanced NSCLC affects approximately 80,000 people per year. As seen in this case, ICIs dramatically altered the therapeutic strategy for advanced NSCLC. Thus, it is important to recognize ICI-related kidney toxicities. In early clinical trials, nivolumab was thought to be safe, with respect to the kidneys, and kidney disorders following ICI therapy are rare (1, 8, 9). The highest rate of kidney toxicities to be reported was 4% in one phase II lung cancer trial. In that trial, patients were treated with nivolumab (3 mg/kg) every two weeks, similarly to the present case (8, 10). However, recent studies have suggested that the incidence of kidney toxicities might be higher than previously reported (6). The accumulation of case reports is considered to be necessary to fully understand the incidence of ICI-related renal toxicity.
As previously reported, two different types of immune-related kidney injury have been reported: acute tubulointerstitial nephritis and glomerular disease (11). Most cases of renal irAE present as acute tubulointerstitial nephritis (6). In the largest case series of renal irAE, Cortazar et al. described the characteristics of the clinical presentation of ICI-related acute tubulointerstitial nephritis (12). Patients developed acute kidney injury (AKI) without any symptoms and the knowledge of the timing of the onset of AKI was not considered to be helpful and pyuria was the only abnormality that was frequently observed in urinalyses. Thus, the close monitoring of laboratory values and the inclusion of renal irAE in the differential diagnosis is important for the early detection of renal irAE (1, 4). The pathological findings of irAE are indistinguishable from other drug-induced acute interstitial nephritis, where they both show T-cell-dominant infiltration of the renal interstitium; occasional plasma cells and eosinophils are present concomitantly (4).
The other disease type is immune complex glomerulonephritis. There are few reports of patients presenting with glomerular disease (5, 13). However, a previous report showed that PD-1 knockout mice developed immune complex glomerulonephritis (14). These findings suggested that the PD-1 signaling pathway is important for minimizing T-cell-mediated renal inflammation. Furthermore, recent studies have shown that galactose-deficient IgA molecules and anti-glycan antibodies play a role in immune complex formation in patients with IgA nephropathy (15). Although it may be difficult to prove a causal relationship between ICI use and IgA nephropathy, we suspect that nivolumab played a role in the pathogenesis of IgA nephropathy in the present case. The patient did not have disease-precipitating events (i.e., upper respiratory infection) prior to the onset of disease, and we observed a decrease in proteinuria and an improvement in serum creatinine level after drug withdrawal. We suspect that in PD-1 blockade by nivolumab, the pathological production of IgA may be enhanced by the activation of self-reactive (or anti-glycan) follicular helper T cells or by the reactivation of autoreactive memory B cells. IgA nephropathy is thought to be associated with mucosal immune system dysfunction and it has been suggested that intestinal immunity contributes to the development of IgA nephropathy. A recent study showed the possibility that a novel targeted-release formulation of budesonide, which is designed to deliver the drug to the distal ileum, could be a specific treatment for IgA nephropathy (16). We need to consider the possibility that activated intestinal immunity may trigger the onset of IgA nephropathy because recurrent diverticulitis occurred in the present case. However, the clinical course showed that the development of kidney dysfunction and hematuria occurred after the resection of the cecum. Thus, we concluded that intestinal immunity did not affect this case.
To the best of our knowledge, this is the third report of biopsy-proven immune complex glomerulonephritis following ICI treatment (5, 13). Similar cases may increase in the future and the accumulation of cases may have implications in the management of renal irAE.
Steroid therapy is becoming a standard treatment for renal irAEs in patients with acute tubulointerstitial nephritis (17). In our case, we did not treat the patient with steroids because the examination of the kidney biopsy specimen did not show evidence of acute pathology (i.e., cellular crescents, glomerular tuft necrosis or endocapillary proliferation). Nivolumab treatment was withdrawn. At the time of writing, the patient remains free from lung cancer recurrence and his proteinuria is improving (0.24 g/gCre).
In summary, we reported a newly diagnosed case of IgA nephropathy following nivolumab treatment. As ICIs will be used more frequently, the close monitoring of the kidney function and proteinuria, as well as consideration of early biopsy and timely intervention are highly important.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank Dr. George Meyer, MD, Clinical Professor of Medicine at the University of California at Davis for the critical reading our manuscript and English language editing.
References
- 1. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2: 1346-1353, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 27: 559-574, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol 33: 3193-3198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murakami N, Motwani S, Riella LV. Renal complications of immune checkpoint blockade. Curr Probl Cancer 41: 100-110, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jung K, Zeng X, Bilusic M. Nivolumab-associated acute glomerulonephritis: a case report and literature review. BMC nephrology 17: 188, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wanchoo R, Karam S, Uppal NN, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol 45: 160-169, 2017. [DOI] [PubMed] [Google Scholar]
- 7. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83: 584-594, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373: 123-135, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PloS One 8: e53745, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 16: 257-265, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Izzedine H, Mateus C, Boutros C, et al. Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant 32: 936-942, 2017. [DOI] [PubMed] [Google Scholar]
- 12. Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638-647, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med 361: 211-212, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11: 141-151, 1999. [DOI] [PubMed] [Google Scholar]
- 15. Suzuki H, Kiryluk K, Novak J, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795-1803, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fellstrom BC, Barratt J, Cook H, et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet 389: 2117-2127, 2017. [DOI] [PubMed] [Google Scholar]
- 17. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44: 51-60, 2016. [DOI] [PubMed] [Google Scholar]